Abstract
The abdominal and rib cage contributions to tidal breathing differ between rapid-eye-movement (REM) and non-NREM sleep. We hypothesized that abdominal relative contribution during NREM and REM sleep would be altered in different directions when comparing sleep on Earth with sleep in sustained microgravity (μG), due to conformational changes and differences in coupling between the rib cage and the abdominal compartment induced by weightlessness. We studied respiration during sleep in five astronauts before, during, and after two Space Shuttle missions. A total of 77 full-night (8 h) polysomnographic studies were performed; abdominal and rib cage respiratory movements were recorded using respiratory inductive plethysmography. Breath-by-breath analysis of respiration was performed for each class: awake, light sleep, deep sleep, and REM sleep. Abdominal contribution to tidal breathing increased in μG, with the first measure in space being significantly higher than preflight values, followed by a return toward preflight values. This was observed for all classes. Preflight, rib cage, and abdominal movements were found to be in phase for all but REM sleep, for which an abdominal lead was observed. The abdominal leading role during REM sleep increased while deep sleep showed the opposite behavior, the rib cage taking a leading role in-flight. In μG, the percentage of inspiratory time in the overall breath, the duty cycle (TI/TTot), decreased for all classes considered when compared with preflight, while normalized inspiratory flow, taking the awake values as reference, increased in-flight for light sleep, deep sleep, and REM. Changes in abdominal-rib cage displacements probably result from a less efficient operating point for the diaphragm and a less efficient coupling between the abdomen and the apposed portion of the rib cage in μG. However, the preservation of total ventilation suggests that short-term adaptive mechanisms of ventilatory control compensate for these mechanical changes.
Keywords: respiration, respiratory movements, abdominal/rib cage relative contribution, thoracoabdominal asynchrony
gravity plays a major role in determining chest wall mechanics, i.e., abdominal and rib cage respiratory motion. Studies on postural changes (1, 13, 36), water immersion (26, 27), exposure to transient microgravity (2, 3, 5, 8, 11, 21), and sustained microgravity (9, 10, 23, 37), as well as exposure to hypergravity (12), have highlighted different aspects of the influence of gravity in abdominal and rib cage respiratory movements. Of particular interest to the present study, during parabolic flights, where hypergravity and microgravity (μG) alternate, abdominal contribution to quiet breathing increased in μG (seated position) by almost 50% and decreased slightly (∼15%) in hypergravity (8, 21). Similar results were reported for mid (10 days) and long-term (180 days) sustained μG exposure, in seated subjects (37).
Sleep has been consistently reported as being of poor quality in microgravity, both by American astronauts and Russian cosmonauts (29, 31) yet few studies have addressed the combined action of microgravity and sleep on respiration and chest wall mechanics.
Sleep on the ground also induces major alterations in respiration, altering upper airway resistance (16, 19), changing muscular tone and thus muscle action on breathing (33), as well as by inducing changes in the control of respiration, dependent on sleep stage (28), which result in a modest decrease in functional residual capacity (FRC), a decrease that is maximal for deep sleep (sleep stages 3 and 4) (16). The impact of these changes in chest wall mechanics is not consistent between studies, some authors indicating a decrease in abdominal contribution during non-rapid eye movement (NREM) sleep (33, 35), others an increase (34); however, all these sources report a significantly higher abdominal contribution during rapid eye movement (REM) sleep when compared with awake, an effect associated with an increase in diaphragmatic EMG activity.
Thus μG produces an increased abdominal contribution to tidal breathing, with a decrease in the activity of the inspiratory rib cage muscles, as well as a less efficient coupling between the diaphragm/abdomen and the apposing portions of the rib cage. Sleep results in a significant increase in the activity of the rib cage respiratory muscles during NREM periods, and a significant decrease during REM periods (19). Therefore, we expected chest wall mechanics during NREM and REM to change differently from Earth to μG. During REM periods, as rib cage muscle activity is reduced or silent, 1 G to μG changes in chest wall mechanics would be expected to be mainly determined by the conformational changes in the rib cage and abdomen. Chest wall mechanics during NREM sleep, on the other hand, would be expected to reflect not only the conformational changes but also the μG-induced derecruitment of rib cage inspiratory muscles. Together, this derecruitment present during NREM and the decrease in coupling between the lower rib cage and the diaphragm would be expected to result in a delay in the rib cage displacement in relation to the abdominal movements.
METHODS
Data used in the present paper were obtained in the framework of the study “sleep and breathing in microgravity,” where cardiac, respiratory, and sleep-related parameters were recorded during sleep before, during, and after two space shuttle flights. Description of data collection for respiratory (10) and sleep (7) variables have been published elsewhere, and the reader is referred to those publications for a complete description. This section presents the data collection and analysis methods relevant to the present work.
Subjects and Sessions
Five astronauts, one woman and four men, participated in the study. Four subjects flew on STS-90 Neurolab (17-day mission, April 17–May 3, 1998), and one on STS-95 (10-day mission, October 29–November 7, 1998). All subjects met National Aeronautics and Space Administration (NASA) comprehensive health criteria for flight assignment, and reported no sleep disorders. All subjects were nonsmokers and had normal respiratory function with forced vital capacity (FVC) and forced expiratory volume in 1 s (FEV1) within the predicted normal range. Average age was 41.0 ± 2.7 (SD) yr, height 181 ± 13 cm, weight 79 ± 14 kg, and body mass index 24 ± 1.6 kg/m2.
Before flight, astronauts were trained extensively in application of sensors, operating the equipment and quality control of the recorded signals. The experimental protocol was approved by the Institutional Review Board of NASA Johnson Space Center, the University of California San Diego, and the Brigham and Women's Hospital. The subjects provided written informed consent before performing the protocol.
A total of 77 polysomnographic recordings (PSGs) were performed (ranging from 13 to 16 per subject), including, per subject, 9 preflight (range 6–9), 4 in flight, and 3 postflight. Each PSG lasted ∼8 h. Preflight recordings were performed in groups of two or three consecutive nights. For the four Neurolab subjects the recordings took place on days 102–101, 73–72, 45–44, and 7–6-5 before launch, and for STS-95 on days 77–76, 50–49, and 38–37 before launch. The first two recordings for each subject were considered as habituation nights and were not retained in the following analysis. In-flight recordings were also registered in batches of two consecutive nights, between days 3 and 15 of the mission for Neurolab, and between days 4 and 8 for STS-95. Postflight data collection took place on days 1, 3, and 4 after landing (corresponding to the 2nd, 4th, and 5th sleep episode after return to Earth). Subjects refrained from consuming caffeine or alcohol in the 12 h preceding each recording and were also asked to refrain from hypnotic medication.
Recordings were performed from around 11 PM to 7 AM local time, except on the first Neurolab postflight recording, where sleep started 1 h earlier on crew's request due to fatigue. A light sensor incorporated in the overall recording system allowed the determination of the exact moment of lights off and the moment when they were turned back on. All pre- and postflight recordings were performed in Johnson Space Center's crew quarters (or in a local hotel, for STS-95 only). In-flight recordings took place inside the shuttle middeck, which was equipped with four sleep compartments. These compartments are 2 m long, 0.75 m high, and wide enough for one. Cabin atmosphere during flight remained normoxic and normobaric with an increased CO2 level (by ∼0.4%). The two missions were single-shift missions, and launch/landing constraints imposed only small deviations from the normal (preflight) 24-h cycle (20 min/day for Neurolab, 35 min/day for STS-95, both shortening the normal cycle).
Polysomnography
Polysomnographic data were digitally recorded using a Vitaport-2 (TEMEC Instruments), using a sleep-monitoring system, developed for this specific experiment, and consisting of a custom-fitted sleep cap, and a computerized signal-quality assessment system. The same system and procedures were used in all pre- and postflight recordings. In-flight instrumentation was performed by a second astronaut, while on-ground instrumentation was performed by technicians.
Electroencephalogram (EEG) channels O1/A2, O2/A1, C2/A2, and C4/A1 were recorded and, together with two electrooculogram channels (left and right) and two facial EMG signals, were used for sleep stage scoring, according to the standard criteria of Rechtschaffen and Kales (25). Sleep scoring using 30-s epochs was performed for prior publication (7) and our analysis was based on those detections. Six stages were considered, non-REM sleep stages 1, 2, 3, and 4 (from lighter sleep to deeper sleep), REM sleep, and periods when the subject was awake.
For the analysis presented here, sleep stages were grouped into four classes: light sleep, including NREM sleep stages 1 and 2; deep sleep, grouping sleep stages 3 and 4; REM sleep (no distinction was made between tonic REM and phasic episodes); and awake. Events in the awake state were retained only when happening in periods of darkness. This was done to avoid standing periods, periods of movement, activity, etc., before lights out. Movements and activity were retained if occurring during lights-out period, and respiratory events were still clearly detectable in the recorded signal.
Respiratory Data
In addition to the electrophysiological recordings allowing the determination of sleep stage, measurements of interest for the present work are rib cage and abdominal motion (respiratory inductive plethysmography, 32 Hz), snoring sounds (through a microphone placed at the level of the larynx), light intensity (detector incorporated in the microphone), as well as an event marker channel. No body position sensor was used. Most of the sensors mentioned were integrated into a custom-fitted two-piece (vest and shorts) lycra body suit (Blackbottoms, Salt Lake City, UT). Rib cage and abdominal wires for the inductance plethysmography were sewn into the body suit, with the chest band at the level of the nipples and the abdominal band over the umbilicus. The vest allowed one, by adjusting shoulder straps, to adjust its torso length—in microgravity the spine lengthens—thus allowing proper location of each plethysmographic band for every gravity condition. Vest and shorts were attached to each other through velcro straps to maintain the correct band location throughout the night. Signal quality was verified before sleep onset using a laptop computer and an interface described in Ref. 4.
Data Analysis
Data were visualized using TEMEC's Vitagraph software package (TEMEC Instruments) and exported in ASCII format. These files were imported into Matlab (Mathworks). Detection of respiratory events was performed using an artificial neural network-based algorithm (32). This algorithm detects moments of onset of inspiration and expiration from the respiratory signal. These detections were visually verified for each night studied. Large body movements and other artifacts were removed from the analysis.
Rib cage and abdominal respiratory signals relative gain was determined by performing an isovolume maneuver before sleep onset. Each isovolume maneuver was visually identified in the respiratory signals and analyzed for the determination of relative abdominal and rib cage gain. No absolute calibration of volume was performed. The signal obtained from the weighted sum of rib cage and abdominal respiratory movements corresponds thus to respiratory volume measured in arbitrary units (au). Comparisons of volume from one night with the next are not possible in absolute terms; therefore volume was normalized for each night taking the awake values as reference and is therefore expressed as a percentage of awake (%Aw).
Breath-by-breath variables.
Breath period (TResp) was calculated on a breath-by-breath basis throughout the entire night as the time difference between two consecutive inspiratory onsets. Each breath was labeled with a time stamp corresponding to the time of occurrence of the first event. Inspiratory time (TI), expiratory time (TE), and the percentage of inspiratory time in the overall breath [the duty cycle (TI/TTot)] were calculated and labeled in an identical manner. Tidal volume (Vt), minute ventilation (V̇), and inspiratory flow (V̇ins = Vt/TI) were also computed based on the same detections.
The abdominal and rib cage relative contribution to breathing (ABDcont and RCcont) were also computed on a breath-by-breath basis. Relative contribution was calculated by integrating the locally (breath-by-breath) detrended abdominal and rib cage signal, divided by the integral of the volume signal for each breath.
Thoracoabdominal asynchrony (Async) was calculated once for every 30-s window. Thoracoabdominal asynchrony expresses the phase lag between abdominal and rib cage movements in percentage of breath time. Following Prisk et al. (24), asynchrony was calculated using the maximum linear correlation method, using 30-s windows synchronous with sleep staging, and a threshold of 0.75 for the acceptable correlation (below that implying an inconsistent phase relationship). All windows presenting lower r values were ignored. Asynchrony is the only variable not calculated on a breath-by-breath basis.
Statistical analysis of the effect of gravity on respiratory variables.
Data from the first pair of sleep recordings, 90 days before flight, were excluded from the data analysis to eliminate possible effects of the adaptation to the sleep instrumentation. One in-flight recording for subject B (corresponding to the 2nd recorded night) was excluded from the present analysis for technical reasons.
As sleep stages were scored using 30-s windows, each variable was averaged over 30-s windows synchronous with sleep stage scoring. Mean values for entire nights were obtained by averaging all 30-s windows of the same sleep class throughout each night, thus obtaining the overall mean value for each night. Descriptive statistics are presented as intersubject mean values ± SE.
Two-way ANOVAs were performed for each variable, and the main effects of gravity, sleep class, and the interaction between them were compared. The interaction term being negligible (P > 0.80 for all variables), and gravity and sleep class significant, post hoc analysis was performed for each sleep class individually, with gravity (timeline) as the post hoc factor. Before post hoc comparison, a Levene's test for testing the null hypothesis of identical variance was performed. When the null hypothesis was acceptable, post hoc comparison was performed using Tukey's adjustment. Whenever the null hypothesis was rejected (different variance among groups), post hoc comparison was performed using Games-Howell's adjustment, which does not require equal variances. Differences were considered significant for P < 0.05. Statistical testing was performed using SPSS 11.0.4 for Macintosh (SPSS).
RESULTS
Abdominal Contribution and Thoracoabdominal Asynchrony
Figure 1 presents the time evolution of abdominal contribution in space and on return to Earth. Comparable behavior for all classes was observed, with the first recording in space presenting a significantly higher abdominal contribution, followed by a return toward preflight values in the subsequent days. For all gravity conditions, abdominal contribution decreased from awake to light sleep, further decreased in deep sleep, while REM sleep presented the highest abdominal contribution to tidal volume. Postflight, abdominal contribution returned to preflight values (Fig. 1), for all classes. No pre- to postflight difference was observed. Two-way ANOVA showed that the interaction term (gravity × class) was negligible (P > 0.80) for abdominal contribution and all variables studied.
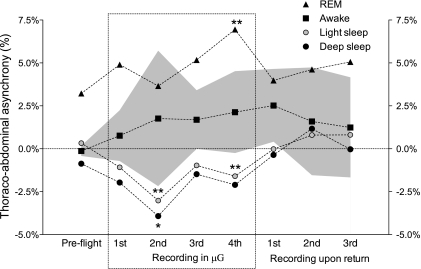
Fig. 1.
Evolution of abdominal contribution to tidal volume over time, for all classes. All preflight values were averaged. *0.05 < P < 0.1, **P < 0.05 compared with preflight. The gray area indicates the intersubject variability (SE) for the awake class. Intersubject variability for the other sleep classes considered was similar or lower. μG, microgravity; REM, rapid eye movement.
Thoracoabdominal asynchrony before flight showed that while the subjects were awake, in light sleep, and in deep sleep, the abdomen and rib cage movements were mostly in phase (Fig. 2). Asynchrony for these three classes was statistically indistinguishable from synchrony (zero phase). This was not true for REM sleep, with the abdomen leading the rib cage by 3.2% of a breath cycle preflight. Removal of gravitational load resulted in no significant change for awake, while light sleep and deep sleep presented an increased tendency of the rib cage to lead. An increase in the leading role of the abdominal compartment during REM sleep was observed, significant for the last recording in μG. On return to Earth, in-phase movements of abdomen and rib cage were observed for light and deep sleep. Asynchrony during REM decreased toward preflight values, while asynchrony in the awake class increased slightly compared with preflight.
Fig. 2.
Evolution of thoracoabdominal asynchrony over time, for all classes, expressed as percentage of breath period (TResp). Positive values correspond to an abdominal lead, and negative values to a rib cage lead. All preflight values were averaged. *0.05 < P < 0.1, **P < 0.05 compared with preflight. The gray area indicates the intersubject variability (SE) for the awake class. Variability in the remaining classes was lower.
Drive and Timing Components of Ventilation
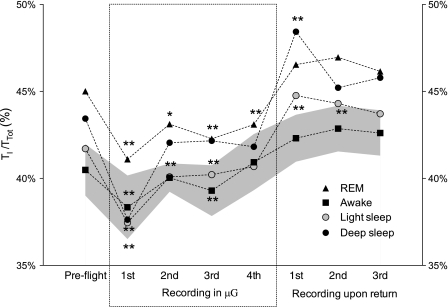
Figure 3 depicts the time evolution of duty cycle (TI/TTot). The average TI/TTot decreased for all classes in μG. Postflight, average TI/TTot was higher than preflight values. Early postflight (days 1 and 3 after return) NREM sleep, light sleep, and deep sleep presented pre- to postflight differences reaching statistical significance (Fig. 3). On earth, TI/TTot presented the lowest value while awake, increased in light sleep, further increasing in deep sleep, with REM presenting the highest values. This order was maintained in flight but not postflight, for which deep sleep and REM values were similar.
Fig. 3.
Evolution of duty cycle (TI/TTot) in time, for all classes. All preflight values were averaged. *0.05 < P < 0.1, **P < 0.05 compared with preflight. The gray area indicates the intersubject variability (SE) for the awake class. Intersubject variability for the other sleep classes was similar or smaller.
Normalized inspired flow (V̇ins), expressed as a percentage of awake values, increased in μG for NREM and REM sleep (Table 1). Postflight, only REM sleep presented a statistically significant increase of V̇ins compared with preflight. V̇ins was lower during deep sleep in all gravity conditions. Preflight light sleep and REM sleep present similar V̇ins values, while in μG, REM sleep presented a clearly higher inspiratory flow than light sleep. Inspired flow showed no change with time spent in μG for any of the sleep classes considered.
Table 1.
Tidal volume, minute ventilation, and mean inspired flow
| Preflight | μG | Postflight | |
|---|---|---|---|
| Vt, %Aw | |||
| Awake | 100.0 | 100.0 | 100.0 |
| Light sleep | 64.9±4.2 | 65.2±1.7 | 66.9±3.2 |
| Deep sleep | 63.1±4.1 | 63.1±1.8 | 67.8±2.7 |
| REM | 66.0±3.6 | 71.1±2.5* | 70.1±4.0 |
| V̇, %Aw | |||
| Awake | 100.0 | 100.0 | 100.0 |
| Light sleep | 50.4±5.6 | 59.9±2.8† | 56.1±3.5* |
| Deep sleep | 51.7±6.0 | 61.3±2.2† | 59.5±3.9† |
| REM | 57.7±7.3 | 71.9±5.0† | 64.6±6.5* |
| V̇ins, %Aw | |||
| Awake | 100.0 | 100.0 | 100.0 |
| Light sleep | 50.6±3.6 | 61.9±2.3† | 54.4±2.8‡ |
| Deep sleep | 48.9±4.4 | 60.3±2.6† | 53.4±4.1‡ |
| REM | 50.4±4.0 | 65.7±3.1† | 56.6±3.9†§ |
Values are means ± SE. Vt, tidal volume; V̇, minute ventilation; V̇ins, mean inspired flow; μG, microgravity; REM, rapid eye movement sleep. All variables were normalized and are expressed as a percentage of their awake value (%Aw).
0.05 < P < 0.1,
P < 0.05 compared with preflight;
0.05 < P < 0.1,
P < 0.05 compared with μG.
Respiratory Timing, Tidal Volume, and Minute Ventilation
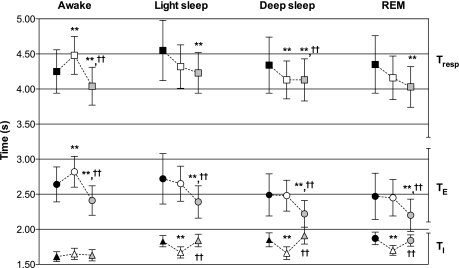
Changes in TI, TE, and TResp are represented in Fig. 4. Breathing period (TResp) increased in flight for the awake class (P < 0.05) and decreased for the remaining classes, although this decrease only reached statistical significance for deep sleep (Fig. 4). These changes in TResp were attained differently for the different classes considered. TI decreased in flight for all sleep classes (light and deep sleep and REM sleep) but not for the awake class, for which a nonsignificant increase was observed. Postflight TI was similar to preflight values, for all classes. TE changed differently: no in-flight difference for NREM and REM sleep was observed, while it significantly increased for the awake class. Postflight, TE was shorter compared with preflight, for all classes. The different directions of change of TI and TE resulted in the consistent behavior of TI/TTot presented above.
Fig. 4.
Duration of TResp (squares, top), expiratory time (TE; circles, middle), and inspiratory time (TI; triangles, bottom). Black filled symbols correspond to preflight average, white symbols to μG, while postflight data are presented in gray. **P < 0.05 compared with preflight; ††P < 0.05 compared with μG.
Vt, expressed in percentage of awake values, presented no significant change either in space or postflight for the awake and NREM classes (Table 1). A slight increase was observed for REM sleep in μG (0.05 < P < 0.1) compared with preflight. Vt was observed to be lowest in deep sleep, slightly less than in light sleep. REM Vt was higher than NREM values, while awake values were the highest.
Normalized V̇ was at its lowest during NREM sleep, was higher during REM, and highest during awake. This relation remained the same, independently of gravitational load. Normalized V̇ increased significantly during sleep in μG and remained high on return to Earth (Table 1).
Relevant Sleep Information
From previously published work on the same subjects (7, 10) we include some baseline information on sleep quality of relevance to the present work: total sleep time (TST) was 404.0 ± 11.8 min preflight and presented no significant change either in flight or postflight. The time spent in each sleep class did not present significant changes with flight timeline. Preflight, deep sleep represented 15.0 ± 1.5% of TST, light sleep 60.7 ± 6.7% of TST, and REM sleep 24.4 ± 2.6% of TST (7). The amount of arousals associated with respiratory events decreased significantly in flight, from an average of 5.5 events/h preflight to 1.8 events/h while in μG. Postflight, the number of respiratory-related arousals returned to preflight levels (10). Arterial O2 saturation preflight averaged 97% for both awake and deep sleep, increased slightly in flight in the wake periods (98.3%), and presented lower values postflight during deep sleep (95.6%).
DISCUSSION
The major findings of this study are that sleep-induced differences in abdominal and rib cage respiratory movements persist during μG, in parallel with an increase in the asynchrony between abdomen and rib cage compartments while in μG. Moreover, an initial abdominal contribution increase observed on the first week in space was followed by a return toward preflight values on the second week, an adaptation with time spent in μG that likely results from a decrease in drive to the diaphragm, or from a better coordination between the rib cage and abdominal muscles activation.
Abdominal Contribution
Abdominal contribution to tidal breathing increased significantly during the first recording in μG compared with preflight and decreased in subsequent recordings. The increase in abdominal contribution was present in all subjects (comparing the first recording performed in space to individual preflight average), and the tendency to return to preflight values was present in four of the five subjects studied. Changes observed in abdominal contribution (Fig. 1) were similar in magnitude and parallel for all sleep stages. This suggests that NREM and REM patterns of muscular activity are maintained in space, with intercostal activation during NREM sleep, and silent during REM sleep, despite the μG-induced changes in the baseline abdominal-rib cage partition.
The evolution in abdominal contribution observed in flight is the most intriguing finding presented here. Wantier et al. (37) followed two subjects on a 180-day stay on the MIR space station and reported increased abdominal contribution while in orbit (seated position) compared with preflight, yet no significant change during the duration of the mission. However, the earliest measure in space was on day 5 in orbit, so any early in-flight adaptation might have gone unnoticed.
Numerous factors potentially contribute to the observed results in μG. These include 1) the removal of weight of the abdominal contents, increasing functional residual capacity (FRC) in μG compared with supine (9), with a resulting alteration in diaphragmatic insertion angle; 2) a change in FRC (9) resulting in an altered length compensation mechanism (15); 3) a change in circulating blood volume (18); and 4) an increase in abdominal compliance in μG as reported for parabolic flight (8, 13). Our results do not allow us to assess the relative importance of these factors.
Thoracoabdominal Asynchrony
Our preflight results show in-phase abdomen and rib cage movements while awake, small abdominal lead in quiet sleep, not far from synchrony, and a clear abdominal lead during REM sleep. Although not explicitly determined, thoracoabdominal asynchrony during sleep reported for three subjects by Tusiewicz et al. (34) is consistent with our findings. As the diaphragm has a finite compliance, the abdominal and rig cage compartments are coupled (6), and this coupling is gravitationally dependent (13). Moving from the standing to the supine position results in a less effective abdominal-rib cage coupling: an identical diaphragmatic contraction is less likely to produce rib cage expansion in the supine posture (13, 34). To produce an identical RC expansion in these conditions, RC muscle activity is required. This is the case during NREM sleep: intercostal muscles are active during inspiration, simultaneously with the diaphragm. During REM sleep, the marked reduction in intercostal muscle activity makes the rib cage more passive. Following the diaphragmatic contraction, the abdomen moves first, and the rib cage only moves when the pressure differences created by the contraction are enough to force it to follow; this can explain the abdominal lead during REM.
Relative abdominal contribution and asynchrony results during REM are consistent with our initial hypothesis: a less effective diaphragm in space, subject to an increased drive and to a less efficient coupling, resulting in an increased abdominal lead. However, the NREM results are not in accordance with out initial hypothesis. We expected, following previous studies in awake subjects, that rib cage respiratory muscles' activity would be depressed in μG, and this depression would also be present during NREM sleep, thus differentiating chest wall mechanics during NREM and REM sleep in space. We observed similar amplitude, parallel variations in abdominal contribution, and opposed asynchrony variations. We are thus led to conclude that sleep-induced changes in abdominal and rib cage respiratory movements prevail over those induced by μG, despite the significant baseline change induced by weightlessness.
Respiratory Drive, Timing, Tidal Volume, and Minute Ventilation
Separating ventilation into its timing and drive components (20), the duty cycle (TI/TTot) decreased for awake and all sleep classes, while the normalized V̇ins = Vt/TI increased when comparing preflight to μG. As the increase in V̇ins outpaced the decrease in TI/TTot, relative ventilation during REM and NREM sleep is increased in μG.
When comparing relative ventilation for the different sleep stages, results for NREM and REM sleep are in accordance with other published studies (19, 30, 33). Results for tidal volume (Vt) and therefore V̇ins and minute ventilation (V̇) for the awake class are much higher than usually reported. However, previous studies selected only short portions of signal to characterize each class, while in the present work, the entire night was taken into account. The selection of “well-behaved portions” of the awake class likely resulted in selecting the most quiescent periods of the awake class in the other studies.
With the exception of a slight increase for REM sleep in μG (0.05 < P < 0.1) compared with preflight, no change in normalized Vt was present in space or on return to Earth in any of the classes considered compared with preflight. As previous studies that have compared Vt supine on earth and μG reported either a very small decrease (∼3%) in space (22) or similar Vt values for supine preflight and space (9, 23), this statement likely holds true for absolute Vt as well. Thus the increase in V̇ins reported here is likely to be the result of a real increase of respiratory drive during NREM and REM sleep in space.
Breath period increased for the awake class and decreased for the remaining classes. These changes were caused by a TI decrease and no change in TE in μG for NREM and REM sleep, while for the awake class, TE increased with no change in TI. The duration of TI increased from awake to light sleep, increased further in deep sleep, and reached its maximum during REM sleep. This order was not changed in microgravity, although the differences became statistically nonsignificant. TE in space showed no change in the NREM and REM classes, and an increase in awake. Combined, these changes result in a decreased TI/TTot in space. Reported values of TI/TTot are consistent with previous results for sleep (17, 28, 30, 33). Previous reported results on respiratory timing during sleep (breath period, TI, TE) are not consistent (17, 28, 30, 33). These results are not directly comparable to the present approach for our study used no disturbing face mask, known to alter the dynamics of respiration (14, 38), and used data from the entire sleep period.
The postflight data presented here do not suggest postflight respiratory muscle weakening, in accordance with previous results collected before, during, and after 6-mo stays on the International Space Station (23).
In conclusion, chest wall mechanics during sleep was altered by exposure to microgravity. During REM sleep, the abdominal contribution to tidal breathing and thoracoabdominal asynchrony increased, consistent with our initial assumption of a less efficient coupling between the diaphragm and the lower rib cage in μG. Abdominal contribution during NREM sleep changed in parallel and with similar magnitude, which indicates that the sleep-induced differences in chest wall mechanics persist during weightlessness, despite the reported derecruitment of rib cage respiratory muscles reported for awake subjects in μG. A rib cage tendency to lead was present for NREM sleep during spaceflight, resulting either from a less efficient diaphragm operating position, or from a less effective coupling between the two respiratory compartments. The initial abdominal contribution to tidal breathing increase was followed by a decrease toward preflight levels as the duration of exposure to μG increases, denoting a slow adaptation to the new environment. Changes in the distribution of pressure in the rib cage and abdomen in μG placed the diaphragm in a less efficient operating position compared with preflight supine, which was compensated by an increase in drive. The return to preflight values observed for abdominal contribution during sleep in space is most likely caused by a central adaptation, probably resulting in a reduction of drive to the diaphragm, or, more likely, a better coordination in the sequence of activation of rib cage and abdominal muscles improves the relative partition of respiratory effort.
GRANTS
The work of R. C. Sá was supported by a Portuguese government grant from “Fundação para a ciência e Tecnologia, 2° quadro comunitário de apoio”, (grant Praxis XXI/BD/18387/98) and by a grant from the private foundation “Fundação Calouste Gulbenkian”. R. C. Sá is currently affiliated with the Departments of Medicine and Radiology, University of California, San Diego 9500 Gilman Dr., La Jolla, CA 92093-0931, USA. The research from the “Laboratoire de Physique Biomédicale” was supported by the PRODEX program, managed by the European Space Agency in collaboration with the Belgian Federal Science Policy Office. This work was also supported by National Aeronautics and Space Administration Contracts NAS9-18764 and NAS9-19434, and National Institutes of Health Grant U01-HL-53208-01.
ACKNOWLEDGMENTS
We acknowledge the support and cooperation of the crews and support personnel of STS-90 and STS-95, and the work of our collaborators from the Brigham and Women's Hospital.
Present address for R. C. Sá: Depts. of Medicine and Radiology, Univ. of California, San Diego, 9500 Gilman Dr., La Jolla, CA 92093-0931.
Present address for M. Paiva: Respiratory Div., University Hospital Erasme, Université Libre de Bruxelles (U.L.B.), Route de Lennik 808, B-1070 Brussels, Belgium.
REFERENCES
- 1. Agostoni E, Mead J. Statics of the respiratory system. In: Handbook of Physiology. Respiration.Washington, DC:Am. Physiol. Soc., 1964, sect. 3, vol. I, chapt. 13, p. 387–410 [Google Scholar]
- 2. Bettinelli D, Kays C, Bailliart O, Capderou A, Techoueyres P, Lachaud JL, Vaida P, Miserocchi G. Effect of gravity and posture on lung mechanics. J Appl Physiol 93: 2044–2052, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Bettinelli D, Kays C, Bailliart O, Capderou A, Techoueyres P, Lachaud JL, Vaida P, Miserocchi G. Effect of gravity on chest wall mechanics. J Appl Physiol 92: 709–716, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Callini G, Essig SM, Heher D, Young LR. Effectiveness of an expert system for astronaut assistance on a sleep experiment. Aviat Space Environ Med 71: 1023–1032, 2000 [PubMed] [Google Scholar]
- 5. Dellaca RL, Bettinelli D, Kays C, Techoueyres P, Lachaud JL, Vaida P, Miserocchi G. Effect of changing the gravity vector on respiratory output and control. J Appl Physiol 97: 1219–1226, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Deschamps C, Rodarte J, Wilson T. Coupling between rib cage and abdominal compartments of the relaxed chest wall. J Appl Physiol 65: 2265–2269, 1988 [DOI] [PubMed] [Google Scholar]
- 7. Dijk DJ, Neri DF, Wyatt JK, Ronda JM, Riel E, Ritz-De Cecco A, Hughes RJ, Elliott AR, Prisk GK, West JB, Czeisler CA. Sleep, performance, circadian rhythms, and light-dark cycles during two space shuttle flights. Am J Physiol Regul Integr Comp Physiol 281: R1647–R1664, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Edyvean J, Estenne M, Paiva M, Engel LA. Lung and chest wall mechanics in microgravity. J Appl Physiol 71: 1956–1966, 1991 [DOI] [PubMed] [Google Scholar]
- 9. Elliott AR, Prisk GK, Guy HJ, West JB. Lung volumes during sustained microgravity on Spacelab SLS-1. J Appl Physiol 77: 2005–2014, 1994 [DOI] [PubMed] [Google Scholar]
- 10. Elliott AR, Shea SA, Dijk DJ, Wyatt JK, Riel E, Neri DF, Czeisler CA, West JB, Prisk GK. Microgravity reduces sleep-disordered breathing in humans. Am J Respir Crit Care Med 164: 478–485, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Estenne M, Gorini M, Van Muylem A, Ninane V, Paiva M. Rib cage shape and motion in microgravity. J Appl Physiol 73: 946–954, 1992 [DOI] [PubMed] [Google Scholar]
- 12. Estenne M, Van Muylem A, Kinnear W, Gorini M, Ninane V, Engel LA, Paiva M. Effects of increased +Gz on chest wall mechanics in humans. J Appl Physiol 78: 997–1003, 1995 [DOI] [PubMed] [Google Scholar]
- 13. Estenne M, Yernault JC, De Troyer A. Rib cage and diaphragm-abdomen compliance in humans: effects of age and posture. J Appl Physiol 59: 1842–1848, 1985 [DOI] [PubMed] [Google Scholar]
- 14. Gilbert R, Auchincloss JH, Jr, Brodsky J, Boden W. Changes in tidal volume, frequency, and ventilation induced by their measurement. J Appl Physiol 33: 252–254, 1972 [DOI] [PubMed] [Google Scholar]
- 15. Green M, Mead J, Sears T. Muscle activity during chest wall restriction and positive pressure breathing in man. Respir Physiol 35: 283–300, 1978 [DOI] [PubMed] [Google Scholar]
- 16. Hudgel DW, Devadatta P. Decrease in functional residual capacity during sleep in normal humans. J Appl Physiol 57: 1319–1322, 1984 [DOI] [PubMed] [Google Scholar]
- 17. Hudgel DW, Martin RJ, Johnson B, Hill P. Mechanics of the respiratory system and breathing pattern during sleep in normal humans. J Appl Physiol 56: 133–137, 1984 [DOI] [PubMed] [Google Scholar]
- 18. Leach CS, Alfrey CP, Suki WN, Leonard JI, Rambaut PC, Inners LD, Smith SM, Lane HW, Krauhs JM. Regulation of body fluid compartments during short-term spaceflight. J Appl Physiol 81: 105–116, 1996 [DOI] [PubMed] [Google Scholar]
- 19. Lopes JM, Tabachnik E, Muller NL, Levison H, Bryan AC. Total airway resistance and respiratory muscle activity during sleep. J Appl Physiol 54: 773–777, 1983 [DOI] [PubMed] [Google Scholar]
- 20. Milic-Emili J, Grunstein MM. Drive and timing components of ventilation. Chest 70: 131–133, 1976 [DOI] [PubMed] [Google Scholar]
- 21. Paiva M, Estenne M, Engel LA. Lung volumes, chest wall configuration, and pattern of breathing in microgravity. J Appl Physiol 67: 1542–1550, 1989 [DOI] [PubMed] [Google Scholar]
- 22. Prisk GK, Elliott AR, Guy HJ, Kosonen JM, West JB. Pulmonary gas exchange and its determinants during sustained microgravity on Spacelabs SLS-1 and SLS-2. J Appl Physiol 79: 1290–1298, 1995 [DOI] [PubMed] [Google Scholar]
- 23. Prisk GK, Fine JM, Cooper TK, West JB. Vital capacity, respiratory muscle strength, and pulmonary gas exchange during long-duration exposure to microgravity. J Appl Physiol 101: 439–447, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Prisk GK, Hammer J, Newth CJL. Techniques for measurement of thoracoabdominal asynchrony. Pediatr Pulmonol 34: 462–472, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Rechtcschaffen A, Kales A. (Editors). A Manual of Standardized Terminology, Techniques, and Scoring System for Sleep Stages of Human Subjects. Bethesda, MD: Dept. of Health, Education, and Welfare, 1968 [Google Scholar]
- 26. Reid MB, Banzett RB, Feldman HA, Mead J. Reflex compensation of spontaneous breathing when immersion changes diaphragm length. J Appl Physiol 58: 1136–1142, 1985 [DOI] [PubMed] [Google Scholar]
- 27. Reid MB, Loring SH, Banzett RB, Mead J. Passive mechanics of upright human chest wall during immersion from hips to neck. J Appl Physiol 60: 1561–1570, 1986 [DOI] [PubMed] [Google Scholar]
- 28. Rostig S, Kantelhardt JW, Penzel T, Cassel W, Peter JH, Vogelmeier C, Becker HF, Jerrentrup A. Nonrandom variability of respiration during sleep in healthy humans. Sleep 28: 411–417, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Santy PA, Kapanka H, Davis JR, Stewart DF. Analysis of sleep on Shuttle missions. Aviat Space Environ Med 59: 1094–1097, 1988 [PubMed] [Google Scholar]
- 30. Shea SA, Horner RL, Banner NR, McKenzie E, Heaton R, Yacoub MH, Guz A. The effect of human heart-lung transplantation upon breathing at rest and during sleep. Respir Physiol 72: 131–150, 1988 [DOI] [PubMed] [Google Scholar]
- 31. Stampi C. Sleep and circadian rhythms in space. J Clin Pharmacol 34: 518–534, 1994 [DOI] [PubMed] [Google Scholar]
- 32. Sá RC, Verbandt Y. Automated breath detection on long-duration signals using feedforward backpropagation artificial neural networks. IEEE Trans Biomed Eng 49: 1130–1141, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Tabachnik E, Muller NL, Bryan AC, Levison H. Changes in ventilation and chest wall mechanics during sleep in normal adolescents. J Appl Physiol 51: 557–564, 1981 [DOI] [PubMed] [Google Scholar]
- 34. Tusiewicz K, Moldofsky H, Bryan AC, Bryan MH. Mechanics of the rib cage and diaphragm during sleep. J Appl Physiol 43: 600–602, 1977 [DOI] [PubMed] [Google Scholar]
- 35. Van de Borne P, Biston P, Paiva M, Nguyen H, Linkowski P, Degaute JP. Cardiorespiratory transfer during sleep: a study in healthy young men. Am J Physiol Heart Circ Physiol 269: H952–H958, 1995 [DOI] [PubMed] [Google Scholar]
- 36. Vellody VP, Nassery M, Druz WS, Sharp JT. Effects of body position change on thoracoabdominal motion. J Appl Physiol 45: 581–589, 1978 [DOI] [PubMed] [Google Scholar]
- 37. Wantier M, Estenne M, Verbanck S, Prisk GK, Paiva M. Chest wall mechanics in sustained microgravity. J Appl Physiol 84: 2060–2065, 1998 [DOI] [PubMed] [Google Scholar]
- 38. Weissman C, Askanazi J, Milic-Emili J, Kinney JM. Effect of respiratory apparatus on respiration. J Appl Physiol 57: 475–480, 1984 [DOI] [PubMed] [Google Scholar]