Abstract
Exercise presents a considerable stress to the pulmonary system and ventilation-perfusion (V̇a/Q̇) heterogeneity increases with exercise, affecting the efficiency of gas exchange. In particular, prolonged heavy exercise and maximal exercise are known to increase V̇a/Q̇ heterogeneity and these changes persist into recovery. We hypothesized that the spatial heterogeneity of pulmonary perfusion would be similarly elevated after prolonged exercise. To test this, athletic subjects (n = 6, V̇o2max = 61 ml· kg−1·min−1) with exercising V̇a/Q̇ heterogeneity previously characterized by the multiple inert gas elimination technique (MIGET), performed 45 min of cycle exercise at ∼70% V̇o2max. MRI arterial spin labeling measures of pulmonary perfusion were acquired pre- and postexercise (at 20, 40, 60 min post) to quantify the spatial distribution in isogravitational (coronal) and gravitationally dependent (sagittal) planes. Regional proton density measurements allowed perfusion to be normalized for density and quantified in milliliters per minute per gram. Mean lung density did not change significantly in either plane after exercise (P = 0.19). Density-normalized perfusion increased in the sagittal plane postexercise (P = <0.01) but heterogeneity did not (all P ≥ 0.18), likely because of perfusion redistribution and vascular recruitment. Density-normalized perfusion was unchanged in the coronal plane postexercise (P = 0.66), however, perfusion heterogeneity was significantly increased as measured by the relative dispersion [RD, pre 0.62(0.07), post 0.82(0.21), P < 0.0001] and geometric standard deviation [GSD, pre 1.74(0.14), post 2.30(0.56), P < 0.005]. These changes in heterogeneity were related to the exercise-induced changes of the log standard deviation of the ventilation distribution, an MIGET index of V̇a/Q̇ heterogeneity (RD R2 = 0.68, P < 0.05, GSD, R2 = 0.55, P = 0.09). These data are consistent with but not proof of interstitial pulmonary edema as the mechanism underlying exercise-induced increases in both spatial perfusion heterogeneity and V̇a/Q̇ heterogeneity.
Keywords: functional magnetic resonance imaging, ventilation-perfusion inequality, lung density
exercise presents a considerable stress to the pulmonary system. Ventilation-perfusion (V̇a/Q̇) heterogeneity increases with exercise, affecting the efficiency of gas exchange (21, 25). The precise matching of ventilation to perfusion beyond that determined by anatomy, is primarily accomplished by regulation of pulmonary vascular and to a lesser extent airway conductances (reviewed in Ref. 45). However, little is known about how exercise effects the spatial heterogeneity of pulmonary blood flow. In humans, low-resolution imaging studies using radiolabeled tracers have shown that exercise induces gross redistribution of pulmonary blood flow, such that apical perfusion is increased (14, 35). In horses, a species noted for minimal V̇a/Q̇ heterogeneity both at rest and during exercise (19, 41), exercise causes minimal overall redistribution of pulmonary blood flow and decreases the spatial heterogeneity (4, 42). Conversely in pigs, which develop an increase in V̇a/Q̇ heterogeneity with exercise similar to humans (26), there is no significant change in spatial perfusion heterogeneity even with very intense exercise (23). Similar findings are observed in dogs, which also do not show an alteration in the spatial heterogeneity of pulmonary blood flow with exercise (37).
In humans V̇a/Q̇ heterogeneity has been shown to increase with increasing exercise intensity as well as duration (21) and is correlated with pulmonary arterial pressure (9). In addition, altered V̇a/Q̇ heterogeneity persists into recovery from exercise (40) suggesting a transient structural rather than a functional cause. Of a variety of possibilities, subclinical interstitial edema resulting from increased pulmonary arterial pressure has been suggested to be the most likely candidate (reviewed in Ref. 8). Interstitial edema has the potential to alter the architectural characteristics of small pulmonary blood vessels or airways, leading to increased V̇a/Q̇ heterogeneity through changes in resistance and compliance in these conduits (8, 21) affecting V̇a/Q̇ matching. If this is the case, it would be similarly expected that the spatial distribution of pulmonary blood flow would be also be disrupted. Therefore, we hypothesized that the spatial heterogeneity of pulmonary perfusion would be increased after exercise in keeping with well documented increases in V̇a/Q̇ heterogeneity that occur with exercise and persist into recovery.
To test this, we used a functional MRI technique known as arterial spin labeling flow-sensitive alternating inversion recovery with an extra radio frequency pulse (ASL-FAIRER; 32, 33) to measure the spatial distribution of pulmonary perfusion before and after 45 min of cycle exercise at ∼70% of V̇a/Q̇. ASL-FAIRER uses endogenous protons as a tracer and has been shown to be highly reliable (30) and sensitive to subtle changes in perfusion resulting from changes in posture (38), hypoxia (20), and normal aging (30). The application of this technique in the lung has been recently reviewed (24). The advantage of this technique is that high-resolution repeated measurements can be made in human subjects without contrast injection or ionizing radiation and is thus ideally suited to detect subtle alterations in the distribution of pulmonary perfusion following exercise.
METHODS
Subjects
This study was approved by the University of California, San Diego Human Research Protection Program. Six healthy, athletic male subjects [age = 24(4) yr, mean(SD), weight = 71.4(6.8) kg, V̇o2max 61(3) ml·kg−1·min−1] who had previously had the extent of V̇a/Q̇ heterogeneity with exercise measured using the multiple inert gas elimination technique (MIGET) were recruited. Gas exchange data from three of these subjects has been previous published as part of an unrelated study (29). The subjects participated after providing informed consent and undergoing screening with pulmonary and MRI safety questionnaires as well as a medical history.
Study Design
V̇o2max testing and determination of the ventilatory threshold to define the exercise test workload took place at a preliminary testing session at least 1 day prior to imaging. During the imaging session, subjects completed spirometry just before the baseline preexercise MRI scans. Each subject underwent MRI measurements of lung perfusion and lung proton density of the right lung in both the coronal and sagittal planes as outlined below. These imaging planes were chosen because in the supine posture, data obtained within the coronal plane are less likely to be influenced by gross redistribution of perfusion caused by elevated pulmonary arterial pressure as all of the imaged lung is within an iso-gravitational plane. Conversely in the sagittal plane, imaged lung will have a gravitationally influenced hydrostatic gradient in pulmonary arterial pressure (50) that is expected to be altered postexercise. Following these initial measurements, subjects exercised for 45 min at a workload that approximated their previously determined ventilatory threshold. Immediately following the exercise task, subjects repeated the spirometry, and then the imaging protocol was repeated at 20, 40, and 60 min postexercise.
Preliminary Testing
To determine the workload for the exercise test, each subject had their maximum oxygen consumption (V̇o2max; ParvoMedics TruMax 2400 system, Sandy, UT) measured using a progressive incremental test on a cycle ergometer (Excaliber, Quinton Instruments, Groningen, The Netherlands). V̇o2max was calculated in liters per minute and milliliters per kilogram per minute; workload (watts) and heart rate (beats/min) were also characterized during testing. From this data, the ventilatory thresholds of the subjects were determined using the V-slope method (3) and the corresponding heart rate and workload were used as the targets for determining the workload during the 45-min exercise task.
Spirometry
Pulmonary function was evaluated using spirometry (VRS-2000, S&D Instrument, Doylestown, PA). Spirometry was performed prior to and immediately following exercise (within 5 min of completion of the exercise task).
Exercise Task
Subjects exercised on a cycle ergometer (Excaliber, Quinton Instruments, Groningen, The Netherlands) for a period of 45 min. After a 5 min warm-up period that gradually increased the workload to that corresponding to their previously determined ventilatory threshold, minor adjustments in workload were made as necessary to maintain a heart rate consistent with the previously determined ventilatory threshold heart rate. This was done to ensure the subjects completed the entire exercise task. The oxygen saturation and heart rate of the subjects were monitored by pulse oximetry (Nellcor N-395, Oxismart XL, Mallinckrodt, St. Louis, MO) using a forehead sensor (RS-10). This device has been previously validated during exercise in our laboratory against direct measures of hemoglobin saturation using co-oximetry with excellent results (51).
Data Collection
Each subject was imaged by MRI (Vision 1.5T whole-body MRI scanner, Siemens Medical Systems, Erlanger, Germany) before and after exercise. Radio frequency power and gradient switching were maintained within safety guidelines for clinical magnetic resonance examinations. Subjects lay supine in the scanner with a phased-array torso coil placed over the chest. MRI compatible EKG leads for heart rate monitoring and R-R interval determination were worn by all subjects. Large gadolinium-doped (Berlex Imaging, Magnevist, 469 mg/ml gadopentetate dimeglumine, 1:5,500 dilution) water phantoms with a T1 of 1,650 ms and T2 of 976 ms were placed in the field of view for quantification. We assumed a T1 of 1,430 ms and T2 of 117 ms for human pulmonary arterial (mixed venous) blood under normal in vivo conditions (hematocrit ∼0.4 and oxygen saturation ∼75%) (44). The doped water phantom was thus used to represent a voxel entirely filled with blood and therefore represents the maximum flow that can be measured using ASL. This allows absolute quantification of pulmonary perfusion and proton density (as described below). MR measures of perfusion, proton density, and paired density images for correction of torso coil inhomogeneity (described below) were then obtained: a 15 mm imaging slice was selected in both the coronal and sagittal planes in the right lung. Data were aquired during breath holds of ∼8 s at functional residual capacity and data were obtained in triplicate at each imaging point. After quantification, the results were averaged for each time point. Following the initial imaging session (duration 45 min), the subjects exercised for 45 min on a cycle ergometer outside the scanner as described above. Subsequently, imaging was repeated in an identical fashion at 20, 40, and 60 min postexercise. This second imaging session took ∼1 h to complete, including time to landmark and set up sequences and obtain the data.
Correction for Coil Inhomogeneity
To maximize signal-to-noise ratio for measurements of pulmonary perfusion and proton density (described below), a torso coil was used, which has increased gain compared with the body coil built into the scanner, but also increased inhomogeneity. Paired density images from the torso coil and the scanner body coil were used to correct for torso coil inhomogeneity in all images of perfusion and proton density on a subject-by-subject basis as described previously (22).
Regional Pulmonary Perfusion Quantification Using Arterial Spin Labeling
A 2D-arterial spin labeling (ASL)-FAIRER sequence with a half-Fourier acquisition single-shot turbo spin-echo (HASTE) imaging scheme was used to measure regional pulmonary perfusion (5). Imaging slice characteristics included slice thickness of 15 mm, field of view of 40 × 40 cm, and a resolution of 256 × 128 pixels, yielding voxel dimensions of ∼1.5 × 3 × 15 mm (∼0.07 cm3) that were acquired in each plane. To match the voxel size of the proton density images (3 × 3 × 15 mm), the ASL images were resized during postprocessing (providing an effective resolution of 0.14 cm3) using bilinear interpolation in MatLab (The MathWorks, Natick, MA). After the subtracted ASL image was corrected for coil inhomogeneity (17), the R-R interval, inversion time (TI = 600–800 ms), echo time (TE = 36 ms), and the gadolinium-doped water phantom were used to quantify pulmonary perfusion in milliliters per minute per cubed centimeter (17, 22).
Regional Lung Proton Density Quantification
In the same imaging slice, during a separate breath hold, a Fast Low-Angle Shot (FLASH) sequence was used to acquire proton density images. Sequence parameters included repetition time (TR = 6 ms), echo time (TE = 0.9 ms), flip angle 4°, slick thickness 15 mm, and image size 128 × 128 pixels. Each image was corrected for coil inhomogeneity and then the resulting signal in each voxel was compared with that of the signal obtained from the water phantom (by definition 100% water), providing regional lung proton (water) density in grams water per cubed centimeter lung. This proton density was corrected for the rapid T2* decay of signal from the lungs using published values for T2* [1.43(0.41) ms] (15). Protons from both tissue and blood are measured by this proton density image and, for ease of understanding, will be referred to as density in the remainder of this paper.
Density-Normalized Perfusion
By dividing the ASL image of pulmonary perfusion (ml·min−1·g water−1) by the image of proton density (g water/cm3 lung), an image of density-normalized perfusion can be obtained with units of millilters per minute per gram lung (blood and tissue). To account for any small potential changes in position between scans, the perfusion and density images were registered for both translational and rotational misalignment using a mutual-information-based technique in MatLab (22, 38). The registered perfusion image was then divided by the proton density image. To the extent that the water content represents the regional lung density, this gives density-normalized perfusion in the units of milliliters per minute per gram lung.
Data Analysis
Mean density and density-normalized perfusion were calculated for the images acquired as described above. Furthermore, the mean density and density-normalized perfusion for the nondependent, middle, and dependent thirds in the sagittal plane were calculated as described previously (22). To characterize the spatial heterogeneity of density-normalized perfusion, four indexes were calculated for the right lung: 1) the relative dispersion, also called the coefficient of variation, which is defined as the standard deviation of the signal intensity divided by the mean signal. Relative dispersion provides a global measure of spatial heterogeneity where larger values indicate greater heterogeneity (16, 30); 2) the fractal dimension, which is a spatial heterogeneity index independent of image resolution. The fractal dimension measure of spatial distribution ranges in value between 1.0 and 1.5, where 1.0 is homogenous and 1.5 is spatially random (30); 3) the shape parameter; and 4) geometric standard deviation of the perfusion distribution histogram. Shape and geometric standard deviation are global measures of heterogeneity based on the lognormal distribution. This was done because studies suggest that the pulmonary vasculature exhibits a lognormal distribution and thus an appropriate measure of heterogeneity of this distribution is the geometric standard deviation (31). In addition we previously have shown that the geometric standard deviation of then pulmonary blood flow distribution has the advantage of being less affected than relative dispersion by signal from larger vessels, which may not entirely represent perfusion (17). Since exercise might reasonably be expected to alter the characteristics of the large vessels, a lognormal model distribution that included a scale and shape parameter was fit to the probability histogram of perfusion data. The geometric standard deviation was calculated for each time point as geometric standard deviation = exp(shape parameter) (17).
Statistical Analysis
Statistical analysis was performed using repeated measures ANOVA (Statview 5.0, SAS Institute, Cary, NC) for detection of statistically significant changes in the primary dependent variables (mean density, mean density-normalized perfusion) and associated measures of spatial heterogeneity (relative dispersion, the fractal dimension and log normal indexes) over time from pre- to postexercise. When overall significance was discovered, post hoc testing was performed with Fischer's protected least-squares difference. All data are presented as means(SD), with the null hypothesis rejected if P < 0.05, two-tailed.
RESULTS
General Data
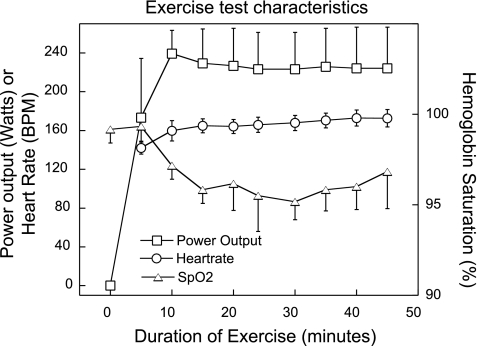
All of the subjects tolerated the study well. The general subject characteristics as well as exercise capacity and multiple inert-gas elimination data obtained during prior testing are reported in Table 1. Heart rate, power output, and oxygen saturation during exercise are given in Fig. 1. After a 5-min warm up, the subjects were able to maintain the target heart rate selected in preliminary testing that corresponded to the ventilatory threshold within 1%. As evidenced by the average V̇o2max of 61(3) ml·kg−1·min−1 [mean(SD)], the subjects were well-trained athletes. Results of spirometry performed before and immediately after exercise were within normal limits and did not show any significant changes postexercise (see Table 2).
Table 1.
Subject characteristics
| Age, yr | 24 (4) |
| Weight, kg | 71.4 (6.8) |
| V̇o2max, l/min | 4.36 (0.52) |
| V̇o2max, ml·kg−1·min−1 | 61 (3) |
| V̇o2 at VT, l/min | 3.08 (0.37) |
| Percent of V̇o2max at VT | 71 (3) |
| Max heart rate, beats/min | 191 (9) |
| Heart rate at VT, beats/min | 163 (13) |
| Rest LogSDQ̇ | 0.35 (0.06) |
| LogSDQ at 90% V̇o2max | 0.45 (0.04) |
| % Change SDQ̇ | 28 (12) |
| Rest LogSDV̇ | 0.36 (0.06) |
| LogSDV at 90% V̇o2max | 0.46 (0.07) |
| %Change LogSDV̇ | 29 (17) |
All values reported as mean(SD). Subject characteristics, including baseline, exercise capacity, and multiple inert-gas elimination data previously acquired. VT, ventilatory threshold; V̇o2max; LogSDQ̇, SD of the perfusion distribution relative to V̇a/Q̇ ratio; Log SDV̇, SD of the ventilation distribution relative to V̇a/Q̇ ratio; Percent Change SDQ̇ and SDV̇, percent change in SDQ̇ and SDQ̇ from rest to exercise at 90% of V̇o2max.
Fig. 1.
Characteristics of the exercise test. Data are mean(SD). SpO2, peripheral arterial hemoglobin oxygen saturation as measured by pulse oximetry. BPM, beats/min.
Table 2.
Spirometry
| Preexercise |
5 min Postexercise |
||||
|---|---|---|---|---|---|
| % Predicted | % Predicted | P | |||
| FVC (l) | 5.54 (0.91) | 100 (15) | 5.57 (0.96) | 101 (16) | 0.74 |
| FEV1 (l) | 4.54 (0.56) | 99 (10) | 4.60 (0.58) | 100 (12) | 0.48 |
| FEV1/FVC,% | 83 (5) | 99 (6) | 83 (5) | 100 (6) | 0.70 |
Values are reported as mean (SD), with percent of predicted values reported. Pulmonary function tests performed by spirometry before and after exercise. FVC, forced vital capacity; FEV1, forced expiratory volume in one second. No significant changes were observed in any pulmonary function measures after exercise.
Imaging Data
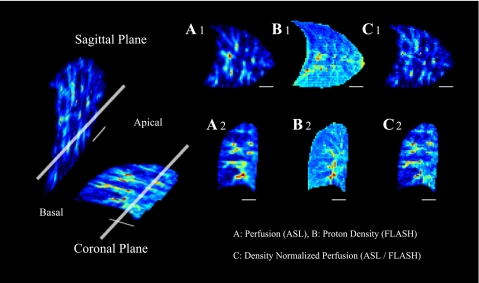
To show the orientation of the imaging planes example images of pulmonary perfusion, lung proton density, and density-normalized perfusion are shown in Fig. 2. Figure 2, top, shows the images from the sagittal plane; those in the bottom are from the coronal plane. Maps of pulmonary perfusion measured by ASL are shown in the first column (ml·min−1·cm−3 lung; Fig. 2: A1, A2). Measures of proton density (g water/cm3 lung) are illustrated by images in the second column (Fig. 2: B1, B2). The third column of images depicts distributions of density-normalized perfusion obtained by division of the respective perfusion map by density map (ml·min−1·g lung−1; Fig. 2: C1, C2).
Fig. 2.
This figure was previously published in Ref. 2. Magnetic resonance (MR) images of a representative subject in the sagittal and coronal planes of the lung. Images to the left depict the relationship of the imaging planes to one another, with the thick, long, white bar denoting the intersection of the 2 planes in the right lung. The smaller, thin, white bar illustrates a 3-cm scale on the images. Images A1, B1, and C1 are taken in the sagittal plane; the images A2, B2, C2 are from the coronal plane. Distributions of pulmonary perfusion measured by arterial spin-labeling (ASL) are shown in A1 and A2; signal intensity is proportional to perfusion (ml blood·min−1·ml lung−1). B1 and B2 depict a map of lung density using fast low-angle shot (FLASH) measures of proton density, where lung density is proportional to signal intensity in units of g water/cm3 lung. The division of the ASL perfusion images by the FLASH density images are demonstrated in C1 and C2. The signal intensity of these images reflects density-normalized pulmonary perfusion (ASL/FLASH; ml·min−1·g lung−1), which is perfusion per gram of lung water, including both blood and tissue.
Density
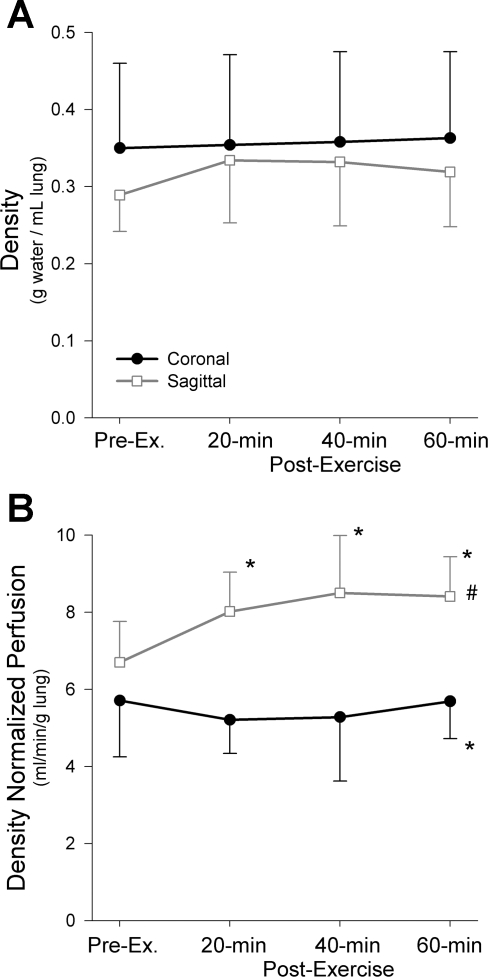
Mean lung density data from the present study are shown in Fig. 3A. Consistent with our prior work (22), the mean density was less in the nondependent than the dependent regions of the sagittal plane [nondependent, 0.21(0.05), middle, 0.32(0.07), dependent, 0.38(0.08) g water/ml lung, P < 0.0001, data not shown]. Although the mean density of the coronal plane was greater than the sagittal plane, likely because of the dependent location of the coronal slice (seen in Fig. 2), this difference was not statistically significant (Fig. 3A, P = 0.32). There were no significant differences in density postexercise in either plane [mean of both image planes preexercise 0.32(0.09), postexercise 0.34(0.09), Fig. 3A, P = 0.19].
Fig. 3.
Mean density (A) and density-normalized perfusion (B) in both the coronal and sagittal imaging planes. No significant differences in mean density were observed pre- or postexercise (P = 0.19) or between imaging planes (P = 0.32). The density-normalized perfusion in the saggital plane was significantly higher than the coronal plane (#P < 0.001). Additionally, there was a significant interaction noted between imaging plane and time postexercise (P < 0.05) accounted for by significant increases in the density-normalized perfusion postexercise at all time points (*P < 0.01) in the saggital plane, whereas the coronal density-normalized perfusion did not change significantly after exercise (P = 0.66).
Density-Normalized Perfusion
Mean values.
Mean density-normalized perfusion data are depicted in Fig. 3B. Density-normalized perfusion in the sagittal plane was significantly higher than in the coronal plane [Fig. 3B; sagittal, 7.91(1.32) ml·min−1·g−1, coronal, 5.47(1.21) ml·min−1·g−1, P < 0.001], also likely because of the posterior location of the coronal imaging slice. As density-normalized perfusion is greatest in the center of a sagittal (gravitationally influenced) lung slice (22), selection of a perpendicular coronal (dependent) lung slice below the level of maximum perfusion would be expected to show lower average density-normalized perfusion (zone 4 effect) than the sagittal slice. In keeping with our previous work (22), the density-normalized perfusion in the middle lung zone of the sagittal plane was significantly greater than either the non-dependent or the dependent zones [nondependent, 6.35(1.19), middle 8.03(1.43), dependent, 5.76(1.16) ml·min−1·g lung−1, Pnon-dependent vs. middle < 0.0005, Pmiddle vs. dependent < 0.0001, Pnon-dependent vs. dependent = 0.08, data not shown]. There was also a significant interaction between image plane and time postexercise (Fig. 3B; P = 0.02): density-normalized perfusion increased significantly after exercise in the sagittal plane at all time points [Fig. 3B; preexercise, 6.70(1.05), 20 min postexercise, 8.02(1.02), 40 min postexercise, 8.50(1.49), 60 min postexercise, 8.41(1.03) ml·min−1·g−1, P < 0.01], but the changes in the coronal plane were not statistically significant (Fig. 3B, P = 0.66).
Measures of heterogeneity.
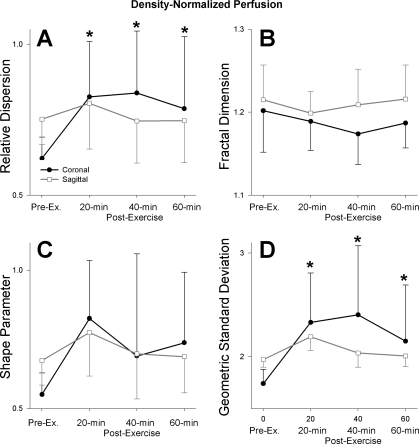
Relative dispersion, the fractal dimension, and log normal measures of heterogeneity were calculated for density-normalized perfusion. The relative dispersion of density-normalized perfusion showed a significant interaction between the imaging plane and time postexercise (Fig. 4A; P < 0.0001): there was a significant increase in the heterogeneity of density-normalized perfusion as measured by the relative dispersion in the coronal plane at all time points after exercise [Fig. 4A; preexercise 0.62(0.07), 20 min postexercise 0.82(0.18), 40 min postexercise 0.84(0.21), 60 min postexercise 0.79(0.24) ml·min−1·g lung−1; P < 0.005]. However, in the sagittal plane the relative dispersion of density-normalized perfusion did not change significantly with exercise (Fig. 4A, P = 0.56). The increased relative dispersion of the density-normalized perfusion in the coronal plane postexercise was largely a result of an increased standard deviation of the signal intensity (P < 0.05), while the mean signal remained relatively unchanged (P = 0.66). The standard deviation of the density-normalized perfusion in the sagittal plane also increased significantly after exercise; however, there was a concomitant increase in mean density normalized perfusion, leaving relative dispersion unchanged.
Fig. 4.
Effect of exercise on heterogeneity of density-normalized pulmonary perfusion as measured by relative dispersion (A), fractal dimension (B), shape parameter (C), and geometric standard deviation (D). There was a significant interaction between the imaging plane and time postexercise in the relative dispersion (A; P < 0.0001) reflecting significant increases in the coronal plane postexercise (*P < 0.005) but not in the sagittal plane (P = 0.56). The fractal dimension (B) was not significantly different between imaging planes (P = 0.14), nor was there a significant difference after exercise (P = 0.09). The shape parameter (C) was not significantly different between imaging planes (P = 0.91) or after exercise (P = 0.08). The geometric standard deviation (D) showed a significant interaction between image plane and time postexercise (P < 0.0005), which was accounted for by a significant increase in the geometric standard deviation in the coronal plane postexercise (*P < 0.005), while the changes in the sagittal plane were not significant (P = 0.18).
The fractal dimension statistic for the density-normalized perfusion data is shown in Fig. 4B. There were no significant differences in heterogeneity as measured by this index between image planes or after exercise (Fig. 4B; P = 0.14, 0.09, respectively). The shape parameter of density-normalized perfusion was also not significantly different between image planes (Fig. 4C; P = 0.91) or after exercise [Fig. 4C; preexercise 0.61(0.08), postexercise 0.74(0.21) ml·min−1·g−1, P = 0.08]. In a similar fashion to relative dispersion, the geometric standard deviation of density-normalized perfusion time by image plane interaction was significant (Fig. 4D; P < 0.005). This reflected a significant increase in this heterogeneity measure in the coronal plane postexercise at all time points [Fig. 4D; preexercise 1.74(0.14), 20 min postexercise 2.33(0.48), 40 min postexercise 2.40(0.67), 60 min postexercise 2.15(0.54), P < 0.005], but the geometric standard deviation of density-normalized perfusion in the sagittal plane did not change significantly (Fig. 4D; P = 0.18).
Comparison to Multiple Inert-Gas Elimination Data
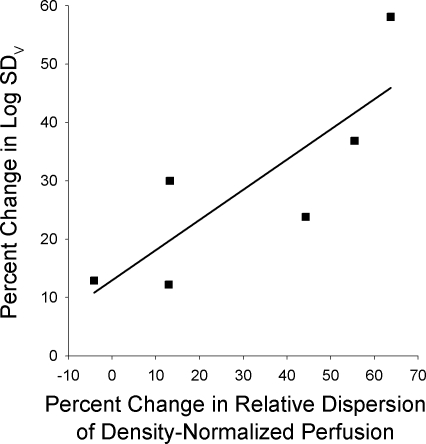
The multiple inert gas indexes of V̇A/Q̇ heterogeneity obtained in previous studies (Ref. 29 and unpublished data), the log standard deviation of the perfusion and ventilation distributions (LogSDQ̇ and LogSDV̇, respectively), were compared with the spatial perfusion heterogeneity data from the present study. The percent changes from rest to exercise at 90% of V̇o2max for LogSDQ̇ and LogSDV̇ were calculated. These were compared with the percent changes in the relative dispersion and geometric standard deviation of the density-normalized perfusion between rest and the average of the three postexercise measurements obtained in the present study. The coronal plane was chosen for this analysis to examine changes in the spatial perfusion distribution independent of any confounding effects of gravitationally based redistribution of flow. There was a significant, positive, linear relationship between the percent change in LogSDV̇ and the percent change in relative dispersion of density-normalized perfusion (Fig. 5; R2 = 0.68, P < 0.05). Additionally, there was similar relationship between the percent change in LogSDV̇ and the percent change in the geometric standard deviation of density-normalized perfusion, which did not quite reach statistical significance (not shown; R2 = 0.55, P = 0.09). There were no significant relationships observed between the LogSDQ̇ and either the relative dispersion (not shown; R2 = 0.05, P = 0.66) or the geometric standard deviation of coronal density-normalized perfusion (not shown; R2 = 0.12, P = 0.45).
Fig. 5.
Linear relationship between percent change from pre- to postexercise in the relative dispersion of density-normalized perfusion in the coronal plane vs. percent change in ventilation-perfusion heterogeneity from rest to 90% of maximal exercise. Ventilation perfusion heterogeneity was measured by the logarithm of the standard deviation of the ventilation distribution, Log SDV, from previously acquired multiple inert gas elimination technique data. There was a significant positive relationship (P < 0.05, R2 = 0.675).
DISCUSSION
This is the first study to measure the changes in the spatial heterogeneity of pulmonary perfusion in humans postexercise. We found statistically significant increases in pulmonary perfusion heterogeneity, as measured by both the relative dispersion and geometric standard deviation in the coronal (isogravitational) plane after exercise. However, similar changes were not observed in the sagittal plane, as the overall increase in heterogeneity was much smaller and variable between subjects. Mean perfusion was elevated postexercise in the sagittal but not coronal planes, suggesting that redistribution of pulmonary blood flow in the sagittal plane may have obscured more subtle findings in this plane. These data are consistent with a transient structural change occurring during exercise, such as patchy interstitial edema increasing resistance in some blood vessels.
Density-Normalized Perfusion
The distribution of density-normalized perfusion in the sagittal plane was similar to our prior studies (22, 38), showing the greatest perfusion in the middle lung section after correcting for lung density. Also, as seen in Fig. 3B, mean density-normalized perfusion was greater in the sagittal plane compared with the coronal plane, likely because of the posterior location of the coronal imaging plane, below the height where the maximal perfusion is seen in the sagittal plane. This likely reflects increased local vascular resistance in dependent lung (consistent with a zone 4 effect) as the most dependent vessels are compressed (28). In keeping with this idea, the density-normalized perfusion was unchanged in the coronal plane after exercise, whereas it was increased in the sagittal plane. These increases in the sagittal plane postexercise likely represent recruitment of additional vessels with increased pulmonary arterial pressures and redistribution of pulmonary perfusion (48). In keeping with our findings, previous studies using radiolabeled tracers have shown that light exercise causes a redistribution of pulmonary perfusion toward the apical lung (nondependent lung) in upright human subjects (14, 35, 36). These changes resolved relatively rapidly in recovery (35), but the exercise intensity and duration was much less than in the present study. Similarly animal studies have shown gross redistribution in pulmonary blood flow during exercise in some animal species (23, 37) but not others (4).
Heterogeneity of Density-Normalized Perfusion
In the coronal plane, the heterogeneity of the density-normalized perfusion, as measured by both the relative dispersion and the geometric standard deviation was increased postexercise (Fig. 4). The change in relative dispersion resulted from an increase in the standard deviation of the density-normalized signal, rather than a decrease in the overall mean signal, suggesting small-scale heterogeneity. In addition, the similar increase in geometric standard deviation is important (17). This is because the log normal distribution of pulmonary perfusion exhibits long tail characteristics, with the result that changes in the upper region of the distribution (high flow, representing large blood vessels) have little effect on the derived parameters. Geometric standard deviation and other log normal measures are thus less sensitive to flow and capacitance changes in the large blood vessels (17), than are analyses based on the normal distribution, as is the case with relative dispersion. The agreement between geometric standard deviation and relative dispersion indices in the present study indicates that the observed changes in the spatial distribution of perfusion occur primarily in the small to medium-sized blood vessels and that these changes cannot be explained by redistribution of blood volume in capacitance vessels alone. Consequently this pattern of change in pulmonary perfusion heterogeneity strongly suggests alterations in the distribution of perfusion secondary to increased resistance in some vessels, likely as a result of interstitial edema (49), discussed below.
These changes in heterogeneity of density-normalized perfusion were not seen in the sagittal (gravitationally influenced) plane. One possible reason for this is because exercise increases cardiac output and pulmonary arterial pressure, both of which will cause dilation and recruitment of blood vessels (48). Thus the distribution of perfusion is subjected to two potentially competing forces: dilation and recruitment that would decrease heterogeneity as all vessels would be perfused more uniformly, and if present, interstitial pulmonary edema that would increase heterogeneity by local compression. Dilation and recruitment would be expected to increase perfusion particularly in the nondependent portion of the lung as the hydrostatic pressures are increased. As mentioned above, mean density normalized perfusion was increased in the sagittal plane for up to an hour postexercise (Fig. 3B), and although the standard deviation was also increased the ratio of the two was not statistically significant (see below). The change in density normalized perfusion from preexercise baseline in the nondependent and middle portions of the lung averaged 1.7 and 2.0 ml·min−1·g−1, respectively, postexercise and was greater than that in dependent lung (1.3 ml·min−1·g−1) although these differences did not reach statistical significance (P = 0.14). Although we waited for 20 min after exercise to minimize the elevation in cardiac output and pulmonary arterial pressure caused by exercise, full recovery was likely prolonged (52).
Our findings contrast with microsphere studies during exercise in pigs and dogs, two species that show an increase in ventilation-perfusion inequality with exercise (26, 27). Both of these species showed no change in the heterogeneity of pulmonary perfusion as measured by the relative dispersion during exercise (23, 37). There are several possible reasons for these differences. First they may be a result of technical issues. The microsphere data have a resolution that is an order of magnitude less than that in our study, and consequently fine regional changes may be obscured. In addition the animal data were obtained during exercise, whereas ours was obtained postexercise. Pigs (23) and dogs (37) demonstrated gross redistribution of perfusion to dorsal (least dependent) regions of the lung during exercise. Thus there may be differences in the balance of the forces tending to increase heterogeneity and the effect of gross redistribution of perfusion that are different from the present study because of timing of measurements (during vs. postexercise) or because of species differences.
The fractal dimension and shape parameter indexes of density-normalized perfusion heterogeneity also did not change significantly after exercise in either plane. This is consistent with the nature of the fractal dimension measure of heterogeneity, which primarily reflects the branching structure of the lung vasculature (10). Consequently, this measure of heterogeneity might not be expected to change after exercise. In keeping with this idea, microsphere studies in artificially perfused sheep (7) and exercised unanesthetized dogs (37) have not shown a change in the fractal dimension of pulmonary perfusion with increasing cardiac output. However, in horses, there was decrease in the fractal dimension after exercise, indicating more uniform regional perfusion in the lung (42). It is possible that these are related to physiological differences between humans and horses in keeping with gas exchange measurements in the horse, a species noted for very little V̇A/Q̇ heterogeneity, even during sustained exercise (19).
Mechanisms of Ventilation-Perfusion Inequality With Exercise
Although there is considerable support for interstitial pulmonary edema as the mechanism of exercise-induced increases in V̇a/Q̇ heterogeneity (12, 26, 34, 39, 40), this has not been conclusively proven. Indeed, attempts to document the presence of lung edema as a result of exercise have proven to be difficult (discussed below and reviewed in Ref. 53). While our study does not provide direct evidence of interstitial edema after exercise, it does support this mechanism of the increase in V̇a/Q̇ heterogeneity observed with exercise. If pulmonary interstitial edema developed during sustained, heavy exercise the spatial heterogeneity of perfusion would be also expected to be increased following exercise, as was observed in our study.
In this study, changes in pulmonary perfusion heterogeneity after exercise, as measured by the relative dispersion, showed a significant positive relationship with the rest to exercise change in LogSDV̇ an index of V̇a/Q̇ heterogeneity measured by the multiple inert gas elimination technique. However, the change in LogSDQ̇ was not significantly related. These findings are expected. Somewhat counterintuitively, an increase in LogSDV̇ represents an increase in heterogeneity of perfusion and LogSDQ̇ represents an increase in ventilation heterogeneity since they are expressed relative to ventilation-perfusion ratio. This can be conceptualized by envisioning a situation where the perfusion to a lung region lung is occluded, such as by a pulmonary embolus. The ventilation-perfusion distribution will subsequently include the region of lung that is ventilated, but not perfused (high V̇a/Q̇ ratio) and the standard deviation of the ventilation distribution (the LogSDV̇) will increase, although the root cause is actually a change in perfusion. This change would be largely obscured in the LogSDQ̇ since ventilation is not affected. Since our MRI technique measures the distribution of perfusion and not of ventilation, changes in the spatial distribution of perfusion would not be expected to be related to the LogSDQ̇.
There is a substantial body of literature supporting interstitial edema as the mechanism behind exercise-induced increases in V̇a/Q̇ heterogeneity from both human and animal studies (12, 26, 39, 40, 53). For example, increased V̇a/Q̇ heterogeneity after exercise persists beyond the point at which ventilation and cardiac output normalize (40). Exercise in normobaric hypoxia causes a significant increase in V̇a/Q̇ heterogeneity, and this increase is relieved by breathing 100% oxygen (12). Since hypoxia would be predicted to increase pulmonary arterial pressures and thus capillary filtration and 100% oxygen would contrastingly decrease these, this suggests that factors that alter vascular pressures in the lung are important. Although pulmonary vascular pressures are not expected to be maximal with exercise of this intensity, they have been shown to be elevated to double that of resting values in a similar subject population performing a similar task (21). This when combined with the prolonged duration of the exercise are expected to increase extravasation of fluid from the intravascular to the extravascular space. In studies with pigs, a species that demonstrates increased V̇a/Q̇ heterogeneity during exercise, histopathologic examination of lung tissues after exercise shows increased perivascular fluid (26, 39). However, as mentioned above these animals do not show an increase in spatial heterogeneity of pulmonary perfusion during exercise (23), for reasons that are unclear.
An alternate possibility for the mechanism of increased V̇a/Q̇ heterogeneity with exercise include inequalities in airways resistance (47), which could translate into ventilation inhomogeneity when flow rates are high. In addition, increased deadspace ventilation could also affect gas mixing (46). We are unable to entirely rule out these possibilities, but there was no alteration in pulmonary function postexercise in any of our subjects to support this idea.
Lung Density
The results of the regional analysis of lung density in the sagittal (gravitationally influenced) plane were similar to those reported previously, showing a vertical gradient consistent with deformation of the lung under its own weight (22). However, we found no effects of sustained, heavy exercise on mean lung density in this study, when imaging was conducted 20 min after exercise. These findings are not necessarily inconsistent with development of interstitial pulmonary edema. Histologic examination of lung tissue in pigs after exercise demonstrates peribronchial and perivascular cuffs around both small and medium-sized airways and blood vessels (39). Interstitial edema distributed around airways thus has the potential to cause air trapping in addition to vessel compression, which could offset the effect of any additional fluid on measured lung density. Furthermore, fluid shifts between the intravascular and extravascular space may not have affected a net change in overall lung density, although interstitial edema is present. For example, Hanel et al. (13) demonstrated a 7% decrease in pulmonary blood volume postexercise, which would explain the lack of change in density after exercise, if, as expected, the volume in the interstitium was balanced by the volume lost from the intravascular space.
In keeping with the idea that postexercise interstitial edema may be subtle and difficult to detect, prior imaging studies to detect pulmonary edema after exercise have shown mixed results (reviewed in Ref. 53). For example, using computed tomography, Caillaud et al. (6) showed an increase in lung density, lung slice mass, and linear/polygonal opacities (a measure of extravascular water) in athletes following completion of a triathlon, although time from exercise to imaging ranged from 1 to 2.25 h. Conventional radiographs, using subjective radiologist-graded edema scores, have also shown increases in edema scores after exercise in both hypoxic (1) and normoxic (54) conditions. In addition, in a study using MRI and an exercise task similar to that of our study, a significant increase in pulmonary extravascular water has been shown 90 min postexercise (34). However, using computed tomography and a hypoxic exercise task, both no change (11) and a decrease in lung density (43) have been reported, although the former study examined highly trained subjects and the latter study did not recruit on the basis of aerobic fitness. Other MRI studies of athletic subjects who exercised in hypoxia and normoxia also did not show changes in lung density after exercise (18). The discrepancies in findings by various investigators may arise from differences in imaging modalities and analytical techniques and/or aerobic fitness of the subjects studied (and thus cardiac output, pulmonary arterial pressure, and driving pressure for fluid efflux). In addition the various types and duration of the exercise performed and length of recovery time postexercise before imaging (40) also add a confounding influence. Finally interstitial edema resulting from fluid shifts between the vascular and extravascular spaces (13), may not result in an overall change in density.
Study Limitations
There are several potential sources of technical error and limitations to our study that merit discussion. We have sought to minimize technical error by correcting for coil inhomogeneity and by using reference phantoms for absolute calibration. We also used consistent and multiple time points postexercise when collecting our imaging data and these errors would not be expected to change over the course of studying a single subject. Our measurement technique requires data acquisition during a breath hold, and density and perfusion images cannot be collected during the same breath hold and must be subsequently registered. Although our subjects showed reproducible breath holds, this may introduce a source of error into our measurements. Time constraints mean that we are able to sample only a portion of the right lung, a single coronal and sagittal section of right lung. The left lung is not imaged to avoid distortions or artifacts that might be caused by cardiac motion and motion of large vessels. Although we have no reason to expect that there might be differences in changes in lung density or perfusion after exercise in the left lung compared with the right, this cannot be ruled out. Additionally, our images of lung density only measure free protons (i.e., water) and, therefore, our distribution of density in the lung may be incorrect if lung tissue not represented in the MRI signal is distributed differently than water in the lung. Our subjects are imaged in supine posture, and thus the distribution of blood flow both at rest and after exercise may not reflect the distribution seen in the upright posture, to the extent that pulmonary vascular pressures and airway pressures are altered by posture.
Another issue that needs to be addressed is whether this study had adequate statistical power to support the conclusion reached, particularly in light of the small number of subjects studied. The repeated-measures design of this study, combined with a very high reliability of measurements (test-retest correlations for density were 0.99 and for relative dispersion were 0.92) offsets the potential lack of power risked by small numbers of subjects. This is evidenced by a highly significant change in perfusion heterogeneity in the coronal plane. However, we did not see a change in lung density which was 7% greater postexercise. Post hoc power calculations revealed a power of 0.99 to detect a significant difference of this magnitude with six subjects. Thus the lack of statistical significance in density is not likely related to the number of subjects. A similar increase (∼7%) was observed for the relative dispersion in the sagittal plane, and in this case the power was relatively low (0.4) to detect a significant difference of this magnitude. Twenty subjects in total would be required to elevate the power to ∼0.9 and it is possible that the changes observed in the relative dispersion in the sagittal plane might reach significance if additional subjects were studied. This lends additional support to the idea that gross redistribution of pulmonary blood flow may obscure subtle findings in the sagittal plane as the changes were much less (7% vs. 33% increase) than that seen in the coronal plane.
The MIGET measurements were not made at the same time as the imaging studies. Since a priori, we did not know if perfusion heterogeneity would increase following exercise, we chose to image subjects who had already had the extent of ventilation-perfusion inequality characterized by MIGET and did not subject them to a repeated invasive inert gas study. Previous work in our lab has shown that the measures of LogSDQ̇ and LogSDV̇ are highly reliable (R = 0.93 and 0.84, respectively) and vary ∼8% across repeated testing. This is similar to the degree of variability observed in many other physiologic variables such as V̇o2max. The imaging studies took place on average 11 mo after the MIGET studies. Since these subjects were well-trained athletes engaged in regular training, we have no reason to think that their overall physiology had changed, but we cannot exclude this as a possibility. We did not include a control group and it is possible that all individuals when exercised might show a similar increase in pulmonary blood flow heterogeneity postexercise even in the absence of changes in the MIGET-derived indexes of V̇a/Q̇ heterogeneity. However,since our subjects had a variable increase in V̇a/Q̇ heterogeneity ranging from almost none to a 60% increase in LogSDV̇, this would appear to be unlikely.
Conclusions
We examined the spatial distribution of lung density and density-normalized perfusion before and after exercise in human subjects. We found mean lung density remained constant in both sagittal and coronal planes at least 1 h after exercise, two measures of perfusion heterogeneity increased after exercise in the coronal, but not sagittal, plane. These increases in density-normalized perfusion heterogeneity correlated significantly with previously measured changes in multiple inert gas elimination measures of heterogeneity. These findings are consistent with, but not direct evidence for, interstitial edema as the mechanism of exercise-induced increases in V̇a/Q̇ heterogeneity.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute HL-081171, HL-080203, F32-HL-078128, 5-T35-HL-007491-26, and American Heart Association 0540002N.
ACKNOWLEDGMENTS
We thank our subjects for enthusiastic participation and effort.
REFERENCES
- 1. Anholm JD, Milne EN, Stark P, Bourne JC, Friedman P. Radiographic evidence of interstitial pulmonary edema after exercise at altitude. J Appl Physiol 86: 503–509, 1999. [DOI] [PubMed] [Google Scholar]
- 2. Arai TJ, Henderson AC, Dubowitz DJ, Levin DL, Friedman PJ, Buxton RB, Prisk GK, Hopkins SR. Hypoxic pulmonary vasoconstriction does not contribute to pulmonary blood flow heterogeneity in normoxia in normal supine humans. J Appl Physiol 106: 1057–1064, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol 60: 2020–2027, 1986. [DOI] [PubMed] [Google Scholar]
- 4. Bernard SL, Glenny RW, Erickson HH, Fedde MR, Polissar N, Basaraba RJ, Hlastala MP. Minimal redistribution of pulmonary blood flow with exercise in racehorses. J Appl Physiol 81: 1062–1070, 1996. [DOI] [PubMed] [Google Scholar]
- 5. Bolar DS, Levin DL, Hopkins SR, Frank LF, Liu TT, Wong EC, Buxton RB. Quantification of regional pulmonary blood flow using ASL-FAIRER. Magn Reson Med 55: 1308, 2006. [DOI] [PubMed] [Google Scholar]
- 6. Caillaud C, Serre-Cousine O, Anselme F, Capdevilla X, Prefaut C. Computerized tomography and pulmonary diffusing capacity in highly trained athletes after performing a triathlon. J Appl Physiol 79: 1226–1232, 1995. [DOI] [PubMed] [Google Scholar]
- 7. Caruthers SD, Harris TR. Effects of pulmonary blood flow on the fractal nature of flow heterogeneity in sheep lungs. J Appl Physiol 77: 1474–1479, 1994. [DOI] [PubMed] [Google Scholar]
- 8. Dempsey JA, Wagner PD. Exercise-induced arterial hypoxemia. J Appl Physiol 87: 1997–2006, 1999. [DOI] [PubMed] [Google Scholar]
- 9. Eldridge MW, Podolsky A, Richardson RS, Johnson DH, Knight DR, Johnson EC, Hopkins SR, Michimata H, Grassi B, Feiner J, Kurdak SS, Bickler PE, Wagner PD, Severinghaus JW. Pulmonary hemodynamic response to exercise in subjects with prior high-altitude pulmonary edema. J Appl Physiol 81: 911–921, 1996. [DOI] [PubMed] [Google Scholar]
- 10. Glenny RW, Robertson HT. Fractal modeling of pulmonary blood flow heterogeneity. J Appl Physiol 70: 1024–1030, 1991. [DOI] [PubMed] [Google Scholar]
- 11. Guenette JA, Sporer BC, Macnutt MJ, Coxson HO, Sheel AW, Mayo JR, McKenzie DC. Lung density is not altered following intense normobaric hypoxic interval training in competitive female cyclists. J Appl Physiol 103: 875–882, 2007. [DOI] [PubMed] [Google Scholar]
- 12. Hammond MD, Gale GE, Kapitan KS, Ries A, Wagner PD. Pulmonary gas exchange in humans during normobaric hypoxic exercise. J Appl Physiol 61: 1749–1757, 1986. [DOI] [PubMed] [Google Scholar]
- 13. Hanel B, Teunissen I, Rabol A, Warberg J, Secher NH. Restricted postexercise pulmonary diffusion capacity and central blood volume depletion. J Appl Physiol 83: 11, 1997. [DOI] [PubMed] [Google Scholar]
- 14. Harf A, Pratt T, Hughes JM. Regional distribution of VA/Q in man at rest and with exercise measured with krypton-81m. J Appl Physiol 44: 115–123, 1978. [DOI] [PubMed] [Google Scholar]
- 15. Hatabu H, Alsop D, Listerud J, Bonnet M, Gefter W. T2* and proton density measurement of normal human lung parenchyma using submillisecond echo time gradient echo magnetic resonance imaging. Eur J Radiol 29: 245–252, 1999. [DOI] [PubMed] [Google Scholar]
- 16. Henderson AC, Levin DL, Hopkins SR, Olfert IM, Buxton RB, Prisk GK. Steep head-down tilt has persisting effects on the distribution of pulmonary blood flow. J Appl Physiol 101: 583–589, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Henderson AC, Prisk GK, Levin DL, Hopkins SR, Buxton RB. Characterizing pulmonary blood flow distribution measured using arterial spin labeling. NMR Biomed. 2009. June 2 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hodges AN, Sheel AW, Mayo JR, McKenzie DC. Human lung density is not altered following normoxic and hypoxic moderate-intensity exercise: implications for transient edema. J Appl Physiol 103: 111–118, 2007. [DOI] [PubMed] [Google Scholar]
- 19. Hopkins SR, Bayly WM, Slocombe RF, Wagner H, Wagner PD. Effect of prolonged heavy exercise on pulmonary gas exchange in horses. J Appl Physiol 84: 1723–1730, 1998. [DOI] [PubMed] [Google Scholar]
- 20. Hopkins SR, Garg J, Bolar DS, Balouch J, Levin DL. Pulmonary blood flow heterogeneity during hypoxia and high-altitude pulmonary edema. Am J Respir Crit Care Med 171: 83–87, 2005. [DOI] [PubMed] [Google Scholar]
- 21. Hopkins SR, Gavin TP, Siafakas NM, Haseler LJ, Olfert IM, Wagner H, Wagner PD. Effect of prolonged, heavy exercise on pulmonary gas exchange in athletes. J Appl Physiol 85: 1523–1532, 1998. [DOI] [PubMed] [Google Scholar]
- 22. Hopkins SR, Henderson AC, Levin DL, Yamada K, Arai T, Buxton RB, Prisk GK. Vertical gradients in regional lung density and perfusion in the supine human lung: the Slinky effect. J Appl Physiol 103: 240–248, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hopkins SR, Kleinsasser A, Bernard S, Loeckinger A, Falor E, Neradilek B, Polissar NL, Hlastala MP. Hypoxia has a greater effect than exercise on the redistribution of pulmonary blood flow in swine. J Appl Physiol 103: 2112–2119, 2007. [DOI] [PubMed] [Google Scholar]
- 24. Hopkins SR, Levin DL, Emami K, Kadlecek S, Yu J, Ishii M, Rizi RR. Advances in Magnetic Resonance Imaging of Lung Physiology. J Appl Physiol 102: 1244–1254, 2007. [DOI] [PubMed] [Google Scholar]
- 25. Hopkins SR, McKenzie DC, Schoene RB, Glenny RW, Robertson HT. Pulmonary gas exchange during exercise in athletes. I Ventilation-perfusion mismatch and diffusion limitation. J Appl Physiol 77: 912–917, 1994. [DOI] [PubMed] [Google Scholar]
- 26. Hopkins SR, Stary CM, Falor E, Wagner H, Wagner PD, McKirnan MD. Pulmonary gas exchange during exercise in pigs. J Appl Physiol 86: 93–100, 1999. [DOI] [PubMed] [Google Scholar]
- 27. Hsia CC, Johnson RL, Jr, McDonough P, Dane DM, Hurst MD, Fehmel JL, Wagner HE, Wagner PD. Residence at 3,800-m altitude for 5 mo in growing dogs enhances lung diffusing capacity for oxygen that persists at least 25 years. J Appl Physiol 102: 1448–1455, 2007. [DOI] [PubMed] [Google Scholar]
- 28. Hughes JM, Glazier JB, Maloney JE, West JB. Effect of lung volume on the distribution of pulmonary blood flow in man. Respir Physiol 4: 58–72, 1968. [DOI] [PubMed] [Google Scholar]
- 29. Jonk AM, van den Berg IP, Olfert IM, Wray DW, Arai T, Hopkins SR, Wagner PD. Effect of acetazolamide on pulmonary and muscle gas exchange during normoxic and hypoxic exercise. J Physiol 579: 909–921, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Levin D, Buxton R, Spiess J, Arai T, Balouch J, Hopkins S. Effects of age on pulmonary perfusion heterogeneity measured by magnetic resonance imaging. J Appl Physiol 102: 2064–2070, 2007. [DOI] [PubMed] [Google Scholar]
- 31. Limpert E, Stahel W, Abbt M. Log-normal distributions across the sciences: keys and clues. Bioscience 51: 2010–2019, 2001. [Google Scholar]
- 32. Mai VM, Bankier AA, Prasad PV, Li W, Storey P, Edelman RR, Chen Q. MR ventilation-perfusion imaging of human lung using oxygen-enhanced and arterial spin labeling techniques. J Magn Reson Imaging 14: 574–579, 2001. [DOI] [PubMed] [Google Scholar]
- 33. Mai VM, Berr SS. MR perfusion imaging of pulmonary parenchyma using pulsed arterial spin labeling techniques: FAIRER and FAIR. J Magn Reson Imaging 9: 483–487, 1999. [DOI] [PubMed] [Google Scholar]
- 34. McKenzie DC, O'Hare TJ, Mayo J. The effect of sustained heavy exercise on the development of pulmonary edema in trained male cyclists. Respir Physiol Neurobiol 145: 209–218, 2005. [DOI] [PubMed] [Google Scholar]
- 35. Mohsenifar Z, Ross MD, Waxman A, Goldbach P, Koerner SK. Changes in distribution of lung perfusion and ventilation at rest and during maximal exercise. Chest 87: 359–362, 1985. [DOI] [PubMed] [Google Scholar]
- 36. Pande JN, Hughes JM. Regional pulmonary clearance of inhaled C15O and C15O2 in man at rest and during exercise. Clin Physiol 3: 491–501, 1983. [DOI] [PubMed] [Google Scholar]
- 37. Parker JC, Ardell JL, Hamm CR, Barman SA, Coker PJ. Regional pulmonary blood flow during rest, tilt, and exercise in unanesthetized dogs. J Appl Physiol 78: 838–846, 1995. [DOI] [PubMed] [Google Scholar]
- 38. Prisk G, Yamada K, Henderson A, Arai T, Levin D, Buxton R, Hopkins S. Pulmonary perfusion in the prone and supine postures in the normal human lung. J Appl Physiol 103: 883–894, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schaffartzik W, Arcos J, Tsukimoto K, Mathieu-Costello O, Wagner PD. Pulmonary interstitial edema in the pig after heavy exercise. J Appl Physiol 75: 2535–2540, 1993. [DOI] [PubMed] [Google Scholar]
- 40. Schaffartzik W, Poole DC, Derion T, Tsukimoto K, Hogan MC, Arcos JP, Bebout DE, Wagner PD. VA/Q distribution during heavy exercise and recovery in humans: implications for pulmonary edema. J Appl Physiol 72: 1657–1667, 1992. [DOI] [PubMed] [Google Scholar]
- 41. Seaman J, Erickson BK, Kubo K, Hiraga A, Kai M, Yamaya Y, Wagner PD. Exercise induced ventilation/perfusion inequality in the horse. Equine Vet J 27: 104–109, 1995. [DOI] [PubMed] [Google Scholar]
- 42. Sinclair SE, McKinney S, Glenny RW, Bernard SL, Hlastala MP. Exercise alters fractal dimension and spatial correlation of pulmonary blood flow in the horse. J Appl Physiol 88: 2269–2278, 2000. [DOI] [PubMed] [Google Scholar]
- 43. Snyder EM, Beck KC, Hulsebus ML, Breen JF, Hoffman EA, Johnson BD. Short-term hypoxic exposure at rest and during exercise reduces lung water in healthy humans. J Appl Physiol 101: 1623–1632, 2006. [DOI] [PubMed] [Google Scholar]
- 44. Spees WM, Yablonskiy DA, Oswood MC, Ackerman JJ. Water proton MR properties of human blood at 1.5 Tesla: magnetic susceptibility, T(1), T(2), T*(2), and non-Lorentzian signal behavior. Magn Reson Med 45: 533–542, 2001. [DOI] [PubMed] [Google Scholar]
- 45. Swenson ER, Domino KB, Hlastala MP. Physiological effects of oxygen and carbon dioxide on VA/Q heterogeneity. In: Complexity in Structure and Function in the Lung, edited by Hlastala MP, Robertson HT. New York: Marcel Dekker, 1998, p. 511–547 [Google Scholar]
- 46. Tsukimoto K, Arcos JP, Schaffartzik W, Wagner PD, West JB. Effect of common dead space on VA/Q distribution in the dog. J Appl Physiol 68: 2488–2493, 1990. [DOI] [PubMed] [Google Scholar]
- 47. Wagner PD, Gale GE, Moon RE, Torre-Bueno JR, Stolp BW, Saltzman HA. Pulmonary gas exchange in humans exercising at sea level and simulated altitude. J Appl Physiol 61: 260–270, 1986. [DOI] [PubMed] [Google Scholar]
- 48. West J. Respiratory Physiology—The Essentials. Baltimore, MD: Williams & Wilkins, 1995, p. 39–43 [Google Scholar]
- 49. West JB, Dollery CT, Heard BE. Increased pulmonary vascular resistance in the dependent zone of the isolated dog lung caused by perivascular edema. Circ Res 17: 191–206, 1965. [DOI] [PubMed] [Google Scholar]
- 50. West JB, Dollery CT, Naimark A. Distribution of blood flow in isolated lung; relation to vascular and alveolar pressures. J Appl Physiol 19: 713–724, 1964. [DOI] [PubMed] [Google Scholar]
- 51. Yamaya Y, Bogaard HJ, Wagner PD, Niizeki K, Hopkins SR. Validity of pulse oximetry during maximal exercise in normoxia, hypoxia, and hyperoxia. J Appl Physiol 92: 162–168, 2002. [DOI] [PubMed] [Google Scholar]
- 52. Yoshida T, Whipp BJ. Dynamic asymmetries of cardiac output transients in response to muscular exercise in man. J Physiol 480: 355–359, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zavorsky GS. Evidence of pulmonary oedema triggered by exercise in healthy humans and detected with various imaging techniques. Acta Physiol (Oxf) 189: 305–317, 2007. [DOI] [PubMed] [Google Scholar]
- 54. Zavorsky GS, Saul L, Decker A, Ruiz P. Radiographic evidence of pulmonary edema during high-intensity interval training in women. Respir Physiol Neurobiol 153: 181–190, 2006. [DOI] [PubMed] [Google Scholar]