Abstract
While skeletal muscle protein accretion during resistance training (RT)-mediated myofiber hypertrophy is thought to result from upregulated translation initiation signaling, this concept is based on responses to a single bout of unaccustomed resistance exercise (RE) with no measure of hypertrophy across RT. Further, aging appears to affect acute responses to RE, but whether age differences in responsiveness persist during RT leading to impaired RT adaptation is unclear. We therefore tested whether muscle protein fractional synthesis rate (FSR) and Akt/mammalian target of rapamycin (mTOR) signaling in response to unaccustomed RE differed in old vs. young adults, and whether age differences in acute responsiveness were associated with differences in muscle hypertrophy after 16 wk of RT. Fifteen old and 21 young adult subjects completed the 16-wk study. The phosphorylation states of Akt, S6K1, ribosomal protein S6 (RPS6), eukaryotic initiation factor 4E (eIF4E) binding protein (4EBP1), eIF4E, and eIF4G were all elevated (23–199%) 24 h after a bout of unaccustomed RE. A concomitant 62% increase in FSR was found in a subset (6 old, 8 young). Age × time interaction was found only for RPS6 phosphorylation (+335% in old subjects only), while there was an interaction trend (P = 0.084) for FSR (+96% in young subjects only). After 16 wk of RT, gains in muscle mass, type II myofiber size, and voluntary strength were similar in young and old subjects. In conclusion, at the level of translational signaling, we found no evidence of impaired responsiveness among older adults, and for the first time, we show that changes in translational signaling after unaccustomed RE were associated with substantial muscle protein accretion (hypertrophy) during continued RT.
Keywords: translation initiation, protein synthesis, muscle fiber, aging
the long-term net balance of skeletal muscle protein synthesis and breakdown rates significantly influences whole body protein balance and determines the state of muscle mass. A net negative balance over an extended time period, whether due to suppressed synthesis or upregulated breakdown, leads to muscle atrophy, which plays an important role in health status for a variety of disease states, including HIV/AIDS, cancer, renal failure, sepsis, diabetes, aging, bed rest, and failure to wean from the ventilator (27, 28, 41). Unlike acute muscle wasting (e.g., sepsis, burns), which is measurable in days and is caused by hypercatabolism, the slow muscle atrophy of aging (i.e., sarcopenia) is only measurable across extended periods (e.g., several years) and is thought to result, at least in part, from impaired or slowed regenerative and growth processes consequent to anabolic stimuli. It is therefore of clinical importance to manipulate synthesis rates toward the preservation and/or accretion of muscle protein in older adults.
Resistance exercise training (RT) is a well-established means of inducing muscle hypertrophy by coordinating a net gain in mixed muscle protein. This apparently results from acute upregulation of inward amino acid transport (5) leading to an elevated fractional synthetic rate (FSR) of muscle protein for as many as 48 h following each exercise bout (5, 8, 17, 48). Resistance exercise (RE) also acutely increases the fractional breakdown rate (FBR) (5, 36), but over time, net synthesis must occur for measurable hypertrophy, and the balance between FSR and FBR is dependent on feeding state (5, 34, 36) as well as training status (34, 36). RT induces hypertrophy of all myofibers and because the adaptation is preferential to type II myofibers (25), it is seemingly the ideal treatment to counteract the type II atrophy characteristic of aging sarcopenia. However, the efficacy of RT-mediated hypertrophy is highly variable across human subjects (3) and tends to decline with advancing age, particularly in old vs. young men (25, 31). This warrants a better understanding of the mechanisms regulating RT-mediated increases in FSR and eventual hypertrophy and the influence of age on these processes.
Some of the chief intracellular signaling pathways involved in skeletal muscle hypertrophy are beginning to be studied in detail. The regulation of translation initiation via the PI3K/Akt/mammalian target of rapamycin (mTOR) pathway is recognized as a significant regulator of muscle mass, and excellent reviews have been provided elsewhere (30, 39). This pathway has been shown to be upregulated in multiple hypertrophic model systems both in vitro (40) and in vivo (6). Key downstream targets of the kinase mTOR include 1) the eukaryotic initiation factor 4E (eIF4E) binding protein (4EBP1), which on phosphorylation releases its inhibition over eIF4E to promote 5′-methylguanosine cap-dependent translation initiation; and 2) p70 S6 kinase (S6K1), which is a known modulator of cell size. Characterizing alterations of these signaling processes and how they may influence muscle protein synthesis rates in response to mechanical load is important for devising effective therapeutic interventions to promote muscle hypertrophy or muscle regrowth following atrophy (e.g., aging sarcopenia).
However, to date no studies have examined the influence of aging on content/activation of proteins in this signal transduction pathway in conjunction with in vivo protein metabolism in response to acute RE, nor during long-term RT. The aims of this research were therefore to assess, in young vs. older adults, human skeletal muscle protein metabolism and translation initiation signaling in response to a single bout of unaccustomed RE and subsequent myofiber hypertrophy after a long-term RT program. We tested the following hypotheses: 1) young muscle is more responsive to unaccustomed RE than old based on a more robust increase in muscle protein FSR and upregulation of translation initiation signaling; and 2) age differences in translational signaling responsiveness to RE will lead to age differences in hypertrophy after repeated exposures during RT (16 wk × 3 days/wk). Thirty-six subjects (21 young, 15 old) were tested for responsiveness to unaccustomed RE, and all 36 then completed a 16-wk resistance training program.
METHODS
Subjects.
Thirty-six untrained but otherwise healthy adults (21 young; 15 old) were recruited from the Birmingham, Alabama, metropolitan area for participation in this research as part of a larger 16-wk exercise training clinical trial. Because a major aim of this work focused on translational signaling, we determined the young vs. old sample size based on effect sizes in prior work in which we found a significant age × time interaction in MAP kinase signaling with 17 young and 13 old adults (24). All subjects completed health history and physical activity questionnaires. Older adults passed a comprehensive physical exam conducted by a geriatrician and a diagnostic, graded exercise stress test with 12-lead ECG reviewed by a cardiologist. Subjects were free of any musculoskeletal or other disorders that might have affected their ability to complete resistance training and testing for the study. Subjects were not obese (body mass index < 30), and none had undergone knee extensor resistance training within the past 5 years. None of the subjects were being treated with exogenous testosterone or other pharmacological interventions known to influence muscle mass. The study was approved by the Institutional Review Boards of both the University of Alabama at Birmingham (UAB) and the Birmingham Veterans Affairs Medical Center. Written informed consent was obtained before participation in the research.
Unaccustomed resistance exercise bout and progressive resistance training.
Following familiarization, baseline one-repetition maximum (1RM) strength was evaluated on squat, leg press, and knee extension exercises as described (4, 33). Participants then completed 2 days of training familiarization followed by the first full RE bout, consisting of three sets at 8–12 RM with 90-s recovery between sets on each of the three exercises. This first bout represented the first session of a 3 days/wk, 16-wk progressive RT program. Resistance loads were increased when a subject could perform 12 repetitions in two of the three sets for a given exercise. 1RM strength was reevaluated after 8 and 16 wk. All training sessions were supervised by a Certified Strength and Conditioning Specialist (National Strength and Conditioning Association) and/or Certified Health Fitness Instructor (American College of Sports Medicine).
Lean mass.
Thigh lean mass and total body lean mass were determined by dual-energy X-ray absorptiometry (DEXA) using a Lunar Prodigy (model no. 8743, GE Lunar, Madison, WI) and enCORE 2002 software (version 6.10.029) according to manufacturer's instructions. Analyses at baseline and after 16 wk of RT were performed by the same technician blinded to intervention time point.
Muscle tissue collection.
Muscle tissue was removed under local anesthetic (2% lidocaine) from vastus lateralis by percutaneous needle biopsy using a 5-mm Bergstrom biopsy needle under suction as previously described (13), with any visible fat or connective tissues dissected at the bedside. Muscle tissue samples were collected at baseline, 24 h after the first full exercise bout (acute response), and 24 h after the last training bout at week 16. At baseline and week 16, ∼70 mg were mounted cross-sectionally in liquid nitrogen-cooled isopentane for subsequent histological analysis, and the remainder was weighed, divided, and snap-frozen (25–35 mg/tube) in liquid nitrogen. All tissue samples collected at the acute response time point were snap-frozen. Samples (25 mg) used for muscle protein synthesis measurements (see Muscle protein synthesis) were briefly rinsed with ice-cold saline to remove residual blood and fluid before being snap-frozen.
Myofiber size and distribution.
Using immunofluorescence microscopy techniques described previously (21, 25), myofibers positive for myosin heavy chain (MHC) type I (MHCI) and negative for MHCIIa were classified as type I, fibers positive for MHCIIa and negative for MHCI were classified as type IIa, and fibers negative for both MHCI and MHCIIa were classified as type IIx. Hybrid myofibers (e.g., coexpression of I/IIa or IIa/IIx) that were revealed by both color and intensity using this technique were excluded from analyses. Myofiber type distribution and size were determined in blinded fashion by a single analyst as described (21, 25). For cross-sectional area (CSA) measurements, each myofiber was manually traced along its laminin-stained border. Myofiber size was measured for a minimum of 50 randomly selected myofibers per type, and type distribution was assessed on an average of 1,067 and 888 myofibers at baseline and week 16, respectively. One older subject was prescribed an anticoagulant during the final few weeks of resistance training; thus a 16-wk biopsy was contraindicated. For one young subject, the histological specimen at week 16 did not satisfy our analysis criteria. Consequently, pre- and posttraining histological specimens for 34 of 36 subjects were analyzed, yielding histological data on 20 young and 14 older subjects.
Translation initiation signaling.
The content and phosphorylation of putative proteins involved in translational signaling were assessed in 35 subjects (20 young, 15 old) at baseline and 24 h after the first bout of unaccustomed resistance exercise to determine whether aging influenced the single-bout response. Standard immunoblotting was performed using established methods in our laboratory (4, 24). Muscle protein lysate was extracted from frozen muscle samples (average 30–35 mg) as detailed previously (4, 24, 25). Protein concentrations were determined using the bicinchoninic acid (BCA) technique with BSA as a standard. Samples were run on 4–12% Bis-Tris (Invitrogen) SDS-PAGE gel matrixes with 35 μg total protein loaded into each well, which was determined as ideal by preliminary experiments. Samples within subjects across time were loaded in adjacent lanes. Proteins were transferred to PVDF membranes at 100 mA for 12 h. Primary antibodies against phospho(S473)- and total Akt; phospho(S2448)- and total mTOR; phospho(T389)-, phospho(T421/S424)-, and total S6K1; phospho(S240/244)- and total ribosomal protein S6 (RPS6); phospho(T37/46)- and total 4E-BP1; phospho(S209)- and total eIF4E; and phospho(S1108)- and total eIF4G were purchased from Cell Signaling Technologies (Danvers, MA). Ideal primary antibody dilutions were determined by preliminary experiments and were 1:250 (vol/vol) for S1108-eIF4G, and 1:1,000 for all other antibodies. Horseradish peroxidase (HRP)-conjugated secondary antibody was used at 1:50,000 (wt/vol) followed by chemiluminescent detection in a Bio-Rad ChemiDoc imaging system with band densitometry performed using Bio-Rad Quantity One software (version 4.5.1). Parameters for image development in the ChemiDoc were consistent across all membranes using predefined saturation criteria for the CCD camera. Equal protein loading was verified by Ponceau S staining in most cases (not shown).
Muscle protein synthesis.
Stable isotope tracer infusion procedures were employed to assess the fractional synthetic rate (FSR) of vastus lateralis mixed muscle protein. These were performed as inpatient procedures on the UAB Pittman General Clinical Research Center (GCRC). Subjects were admitted to the GCRC the evening prior and were provided a standardized meal. The infusion protocol began at 0600 after 8–10 h of fasting. Subjects received a 5-h primed (2 μmol/kg), continuous (0.05 μmol·kg−1·min−1) infusion of l-[ring-2H5]phenylalanine (d5-PHE; Cambridge Isotope Labs) via an antecubital venous catheter for determination of FSR. Isotopic steady state in the free amino acid pools in blood and muscle, which is accomplished in 2 h, was required to calculate protein kinetics via the precursor-product method (35). d5-PHE infusion began at 0 h and continued for 5 h. At 2 h, the first biopsy was taken under local anesthetic to measure the isotopic enrichment of d5-PHE in the intracellular free and incorporated (into full-length proteins) pools. The muscle incorporated protein enrichment of d5-PHE at 2 h served as the first time point for FSR measurement, while the enrichment at 5 h served as the second time point. FSR was therefore determined as the rate of tracer incorporation from the intracellular pool into the incorporated muscle protein fraction. Free amino acids were isolated from serum, intracellular, and protein-incorporated fractions using filtered prep columns with AG 50W-X8 resin and established laboratory techniques. Measurement of d5-PHE tracer enrichment of each fraction was accomplished by gas chromatography mass spectrometry (GCMS). FSR was calculated as described previously (35) and expressed as percent per hour. FSR was assessed at baseline and 24 h after the unaccustomed resistance exercise bout in 15 (8 young, 7 old) of the 36 subjects. Postexercise FSR was not valid for one of the older subjects; thus n = 8 young and 6 old subjects.
Statistics.
Age × time repeated-measures ANOVA was used to test main effects of time and age, and age × time interactions. For acute responses to a single bout of resistance exercise, data were tested using two (age group) × two (time) repeated-measures ANOVA with baseline and acute response time points. Across 16 wk of training, data were tested via ANOVA with repeated measures as follows: 1RM strength, two (age group) × three time points (baseline, week 8, week 16); myofiber size and type distribution, two (age group) × two time points (baseline, wk 16). Tukey HSD tests were performed post hoc. Data are presented as means ± SE. Significance was accepted at P < 0.05 for all tests.
RESULTS
Acute responses to unaccustomed resistance exercise.
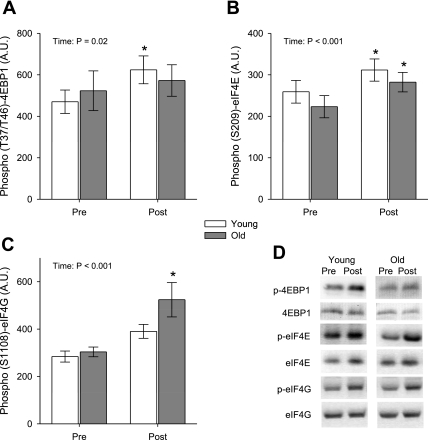
Results for the translation initiation signaling targets assessed are shown in Figs. 1 and 2. A noteworthy overall summary of the acute response translational signaling results is that only one significant age × time interaction was found (phosphorylation of RPS6). On the other hand, significant main time effects were noted for nearly all targets assessed throughout the signaling cascade, indicating that translational signaling was upregulated irrespective of age at the 24-h postexercise time point. Akt phosphorylation was increased 51% (P < 0.001, Fig. 1A) while total Akt protein content was elevated 20% (P < 0.005). Within age groups, phosphorylated Akt was increased 60% among young and 39% among old subjects with no age × time interaction (P = 0.37). No changes in total or phosphorylated mTOR were found. Downstream of mTOR, we were unable to detect measurable phosphorylation of S6K1 at T389 (data not shown), which is thought to be largely an mTOR-specific site (19), most likely due to the fasted state of the subjects. However, phosphorylation of the S6K1 autoinhibitory domain (T421/S424), which is driven primarily by ERK1/2 and p38 signaling (20, 45), was enhanced 61% overall (P < 0.05) (Fig. 1B) while total S6K1 did not change. A concomitant and robust 199% increase (P < 0.001) was found for RPS6 phosphorylation (S240/S244) overall (Fig. 1C), while total RPS6 was unchanged. An age × time interaction for RPS6 phosphorylation (P < 0.05) was driven by a significant 335% increase among old. Despite the lack of T389 phosphorylation of S6K1, increased phosphorylation was noted for another mTOR downstream target, 4EBP1. The phosphorylation state of 4EBP1 was elevated 23% (P < 0.05), an effect primarily driven by young subjects (Fig. 2A). At the level of the eukaryotic initiation factors, the 4EBP1 binding partner eIF4E was increased in both phosphorylation state (23%, P < 0.001, Fig. 2B) and total protein content (19%, P < 0.001). The magnitudes of these changes were similar among young and old subjects. Phosphorylation of the eIF4E binding partner, eIF4G, was elevated overall 53% (P < 0.001) and this response appeared greater in old subjects despite no significant (P = 0.067) interaction term (Fig. 2C). Total eIF4G content remained unchanged. Among the signaling targets studied, no age differences were seen at baseline.
Fig. 1.
Effects of an unaccustomed resistance exercise bout on phosphorylation of Akt (A), S6K1 auto-inhibitory domain (T421/S424) (B), and ribosomal protein S6 (RPS6) (C) 24 h after exercise (Post) in young and old subjects. D: representative immunoblots. Bars are means ± SE. Significant main effects and interaction (Intrxn) terms of each age × time ANOVA are shown in A–C. *Post different from before exercise (Pre) within group as determined post hoc by Tukey's honest significant difference (HSD) test, P < 0.05. AU, arbitrary units.
Fig. 2.
Effects of an unaccustomed resistance exercise bout on phosphorylation of eukaryotic initiation factor 4E (eIF4E) binding protein (4EBP1) (A), eIF4E (B), and eIF4G (C) 24 h after exercise in young and old subjects. D: representative immunoblots. Bars are means ± SE. Significant main effects of each age × time ANOVA are shown in A–C. *Post different from Pre within group as determined post hoc by Tukey's HSD test, P < 0.05.
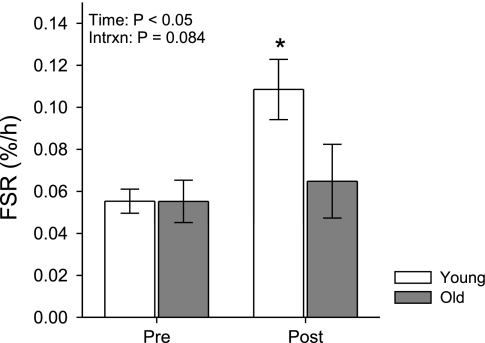
Upregulated translational signaling 24 h after the unaccustomed resistance loading bout occurred in conjunction with an overall 62% increase in FSR, which was evaluated in a subset of the subjects (n = 14; 8 young, 6 old) (Fig. 3). However, unlike the signaling results, increased FSR was driven entirely by young subjects, who experienced a robust 96% increase (P < 0.05) compared with no change among old subjects (P = 0.95). The age × time interaction term did not reach significance (P = 0.084) despite the apparent age difference in responsiveness, owing most likely to the limited sample size in the subset of subjects completing FSR studies. Baseline fasting FSR was nearly identical in the two age groups (young 0.0553 ± 0.006%/h; old 0.0552 ± 0.010%/h).
Fig. 3.
Effects of an unaccustomed resistance exercise bout on the fractional synthesis rate (FSR) of mixed muscle protein 24 h after exercise in young and old subjects. Bars are mean ± SE. Results of the age × time ANOVA are shown. *Post different from Pre within group as determined post hoc by Tukey's HSD test, P < 0.05.
Adaptations during progressive resistance training.
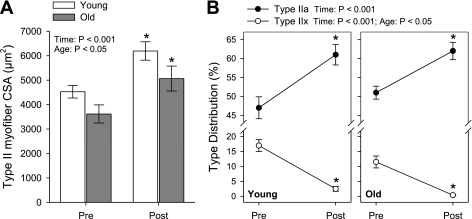
Myofiber adaptations are shown in Fig. 4. Similar to the acute response signaling results, no age × time interactions were noted for any of the myofiber adaptations to 16 wk of RT. Across all subjects, mean myofiber size increased an average of 1,345 μm2 (31%) by week 16 (P < 0.001) and this was significant among both young (1,444 μm2 or 32%, P < 0.005) and old (1,203 μm2 or 30%, P < 0.05). Hypertrophy was greatest in type II myofibers (P < 0.001) with 38% type II growth on average overall. Within age groups, type II myofiber hypertrophy was seen in both young (1,666 μm2 or 37%, P < 0.001) and old (1,447 μm2 or 40%, P < 0.001) (Fig. 4A). Type II myofibers were smaller among old subjects (main age effect, P < 0.05), indicative of type II atrophy, a classic phenotypic index of sarcopenia. Type I myofiber size also increased overall (972 μm2 or 22%, P < 0.001) (data not shown). The type IIx-to-IIa myofiber type shift typical of resistance training was significant within each age group similar to our previous reports (3, 25) (Fig. 4B). Including all subjects, the relative distribution of type IIa myofibers increased (P < 0.001) from 48.9% to 61.5%, while the distribution of type IIx myofibers dropped (P < 0.001) from 14.8% to 1.7%. Overall, fewer type IIx fibers were found in old vs. young (main age effect, P < 0.05). As expected, no change in type I myofiber distribution was found.
Fig. 4.
Effects of a 16-wk resistance training program on type II myofiber cross-sectional area (CSA) (A) and distribution of type IIa and type IIx myofibers (B) in young and old subjects. Bars are means ± SE. Results of each age × time ANOVA are shown in A and B. *Post different from Pre within group as determined post hoc by Tukey's HSD test, P < 0.05.
Table 1 displays lean mass and strength results. In agreement with myofiber hypertrophy, DEXA-determined thigh lean mass increased overall (P < 0.001) with no significant age × time interaction. Total body lean mass also increased (P < 0.005) and, as expected since the RT program focused on hip and knee extensors, the bulk of this increase was in the thigh compartment. Main time effects (P < 0.001) were noted for all three maximum voluntary dynamic (1RM) strength tests (leg press, knee extension, squat). For example, knee extension 1RM strength (Table 1) rose 44% among young and 38% in old subjects by week 16 (P < 0.001). For all three movements, the majority of the strength gains were acquired by week 8. For example, significant knee extension 1RM strength gains (P < 0.01) were noted in both young (29%) and old (23%) after the first 8 wk of RT.
Table 1.
Baseline descriptive characteristics and effects of resistance training on lean mass and strength in young and old subjects
| All Subjects (n = 36) | Young (n = 21) | Old (n = 15) | |
|---|---|---|---|
| Baseline traits | |||
| Age, yr | 27.9±1.0 | 64.4±0.9 | |
| Height, cm | 170.4±2.0 | 169.9±2.8 | |
| Weight, kg | 75.4±3.0 | 76.8±3.9 | |
| Lean mass, kg* | |||
| Baseline | 48.1±1.8 | 49.6±2.4 | 45.9±2.7 |
| 16 wk | 48.8±1.9a | 50.5±2.5a | 46.4±2.8 |
| Thigh lean mass, g* | |||
| Baseline | 11,612±518 | 12,341±660 | 10,591±783 |
| 16 wk | 12,284±565a | 13,040±742a | 11,227±822a |
| Bilateral knee extension 1RM strength, kg*†‡ | |||
| Baseline | 48.7±2.8 | 54.9±3.5 | 40.0±3.6 |
| 8 wk | 61.9±3.7a | 70.7±4.8a | 49.4±4.3a |
| 16 wk | 69.2±4.4a,b | 79.2±5.8a,b | 55.1±5.0a |
Values are means ± SE. 1RM, 1-repetition maximum.
Main time effect, P < 0.05;
main age effect, P < 0.05;
age × time interaction, P < 0.05;
different from baseline within group, P < 0.05;
different from week 8 within group, P < 0.05.
DISCUSSION
Changes in muscle protein synthesis or translation initiation signaling following a single bout of RE or a single exposure to an alternate exogenous stimulus (e.g., insulin or amino acids) are often speculated to be indexes of responsiveness that may predict whether the final desired end point of increased muscle mass would be achieved with repeated exposures. This study is the first to evaluate whether changes in both translational signaling and FSR in response to unaccustomed RE are in agreement with changes in muscle size following long-term RT, and whether aging influences these responses. We report overall that translational signaling was upregulated at multiple control points along the pathway after a single RE exposure, and that modest hypertrophy followed suit as measured after 16 wk of RT. However, contrary to our expectations, age did not influence signaling responses to RE, nor the hypertrophy adaptation to RT. Further, in a subset of participants, we report lack of agreement between FSR and translational signaling responses to RE. Each of these findings is discussed below.
With analysis of a putative translational signaling pathway, we found that all of the signaling (phosphorylation) of proteins of interest, except mTOR, were upregulated 24 h after unaccustomed RE in this group of 35 subjects. This may seem surprising given the relatively late tissue collection post-RE (24 h)—certainly we probably missed the peak activation state of some or all targets. However, FSR has been shown to be increased for up to 48 h post-RE, and indeed we have found it to be increased at 24 h. Therefore it can be reasonably presumed that at least a subset of proteins that control translation initiation remain active at this time point. Clearly these findings support RE as a relatively potent and long-lasting stimulus of translation initiation. While some have found the timeline of phosphorylation of many components of translation initiation pathways, including Akt, mTOR, and 4EBP1, to be quite transient (1, 7, 9, 12), increased phosphorylation of Akt and mTOR has also been noted as long as 48–72 h after the final bout of RE 8 wk into a RT program (29). RE-mediated phosphorylation of S6K1 has been shown to correlate with long-term muscle hypertrophy in both rodents (numerous phosphorylation sites combined) (2) and humans (T389 at 30 min postexercise) (43). Additionally, robust phosphorylation of the S6K1 autoinhibitory domain at T421/S424 has been found to occur immediately (∼30 s) after resistance exercise and persist for at least 24 h (10).
Full activation of S6K1 requires a series of sequential, differentially regulated phosphorylation steps (11, 37, 46). The autoinhibitory domain regulates access to T389 and therefore must be phosphorylated (S411/S418/T421/S424) first to open the conformation for subsequent T389 phosphorylation. Likewise in sequence, T389 phosphorylation must occur prior to loop site (T229) phosphorylation, T229 being the final activating site required for full kinase activity. T389 is considered the acute response phosphorylation site; thus it is not surprising that T389 phosphorylation occurs within 1–2 h after RE (12, 26) but returns to baseline shortly thereafter (26) and was not detected in our model 24 h after RE. Because the autoinhibitory domain must be phosphorylated first to make T389 accessible, prolonged phosphorylation of this domain would presumably facilitate full kinase activation (T389 followed by T229) with each acute stimulus such as protein feeding. In the present study we found a modest correlation (r = 0.53, P < 0.005) between the percent change in S6K1 autoinhibitory domain phosphorylation (T421/S424) 24 h after the single RE bout and percent change in mean myofiber size after 16 wk of RT. Admittedly the zero-order correlation as applied here is an overly simplistic view of a complex series of events required for successful hypertrophy; however, the positive correlation supports the notion that those individuals with prolonged S6K1 autoinhibitory domain phosphorylation following a bout of RE were better poised to respond (with sequential T389 phosphorylation) to other acute anabolic stimuli (e.g., protein feeding) during the time period between bouts of RE.
Somewhat surprising to us, we did not find a compelling effect of aging on translational signaling responsiveness to unaccustomed RE. We are aware of only one other human RE study in which such an aging effect was tested after an overnight fast (26). Kumar et al. (26) found increases in S6K1 and 4EBP1 phosphorylation in young (by combining subjects from 3 different exercise intensities) but not old subjects 1 h after RE, with no other differences noted from baseline for up to 4 h after RE. Lack of agreement between our results and those of Kumar likely results from a major difference in study design timing. Kumar detected age differences in responses 1 h postexercise, including the acute response phosphorylation site on S6K1 (T389), which returned to baseline by 2 h post-RE. Kumar et al. did not assess phosphorylation of the S6K1 autoinhibitory domain, but based on the sequential activation paradigm its phosphorylation would have been obligatory, at least in the young. That we found no age difference in long-term autoinhibitory domain phosphorylation does not rule out the possibility that aging may affect subsequent T389 phosphorylation in response to acute stimuli. On the other hand, it should not be overlooked that one putative downstream target of fully activated S6K1, RPS6, was robustly phosphorylated in old subjects and, in fact, revealed the only significant age × time interaction found among the signaling targets studied here with a greater response in old. Our S6K1 findings generally agree with those of Hornberger et al. (18), who showed using an ex vivo mouse model of passive stretch that S6K1 phosphorylation (both T421/S424 and T389) in response to mechanical strain was not different in extensor digitorum longus muscles isolated from young and old mice. There appeared to be subtle influences of age at different points along the signaling pathway (e.g., within-groups increases in Akt and 4EBP1 phosphorylation only among young and eIF4G only among old); however, the predominance of main time effects without age × time interactions in this relatively large data set leads to the general conclusion that translational signaling responsiveness to RE was unaffected by age. Our results regarding myofiber hypertrophy after 16 wk of RT fully support this conclusion.
Both age groups achieved significant and comparable myofiber hypertrophy across 16 wk of training. Myofiber hypertrophy is achieved via net protein accretion, a process that may become dependent on the addition of myonuclei if myofiber volume expands substantially due to the likelihood of a threshold or ceiling for the myonuclear domain (31, 32). We have previously shown successful but blunted resistance training-mediated myofiber hypertrophy among larger cohorts of older adults vs. young (25, 31), with the decreased efficacy among old vs. young attributed largely to failing myonuclear addition (31). In fact, via K-means cluster analysis we reported recently that the population of available satellite cells pretraining, and the propensity to add myonuclei during training, appear to be major factors in determining the magnitude of myofiber hypertrophy achieved during RT (32). In the present study, while the number of myonuclei per fiber in cross sections increased (P < 0.001) from 2.30 to 2.71, the myonuclear domain expanded (P < 0.01) from 1,862 ± 65 μm2 to 2,087 ± 72 μm2 per nucleus, indicating protein accretion at a rate in excess of myonuclear addition. No age × time interactions were noted, and the posttraining domain size just reached the theoretical threshold of ∼2,000 μm2 per nucleus that we have suggested may increase the dependency on myonuclear addition for further myofiber hypertrophy (31). These findings suggest myofiber hypertrophy resulting predominately from protein accretion is not impaired in old, and this is supported by the similar acute translational signaling responses in young and old. Any age-related attenuation of RT-mediated hypertrophy appears to be more closely linked to age effects on satellite cell activity (31), which may not have been a limiting factor in the present study based on the modest myofiber growth achieved.
Our finding of increased mixed muscle FSR after unaccustomed RE in young subjects is similar to previous reports (17, 23, 34, 47). Using FSR as the outcome measure, aging has been associated with blunted responsiveness to anabolic stimuli (16, 38, 42), leading to the speculation that repeated exposures would cause impaired adaptation in aging muscle. Sheffield-Moore et al. (42) reported that unlike young, old men were unable to increase FSR 3 h postexercise, a time point previously found to be reflective of FSR at 24 h (44). We too report a failed FSR response to unaccustomed RE in a subset of the older participants but show for the first time that this was not in agreement with the translational signaling results and did not appear to impact the eventual gains in muscle mass and myofiber hypertrophy at the conclusion of a 16-wk RT program. In fact, excluding the 22 subjects lacking FSR assessments, myofiber hypertrophy was similarly achieved (P < 0.05) in the subset of young and old with measured FSR, and in this subset acute response FSR did not correlate with the magnitude of eventual hypertrophy. We are not the first to report lack of agreement between acute translational signaling and FSR responses; however, this is the first report of such a phenomenon in response to RE. During intravenous delivery of amino acids and incrementing insulin concentrations, Greenhaff et al. (15) found a dose response for Akt (S473) and S6K1 (T389) phosphorylation while FSR failed to increase with rising insulin delivery. The authors concluded that their data do not support the commonly accepted model by which increases in FSR “follow from proportionate alterations in the activity of signaling molecules”; consequently, Greenhaff and colleagues consider this model an oversimplification (15). We agree with Greenhaff that unknowns such as the temporal relationship between phosphorylation (i.e., signaling) and anabolism (i.e., FSR), or the degree of phosphorylation indicative of true signaling activity, complicate interpretation. Without question such unknowns make data interpretation more difficult; however, based on the fasting FSR vs. fasting signaling responses seen 24 h after unaccustomed RE, our results suggest fasting FSR may not be an ideal index of responsiveness to an unaccustomed stimulus, particularly when the outcome of interest is myofiber growth following several weeks of RT.
While the findings reported herein are novel and should prove useful for future studies, we fully appreciate the limitations. First, FSR measurements were limited to a subset of participants. The FSR infusion studies were considered a “substudy,” and therefore, although we aimed to recruit 10 young and 10 old subjects into this substudy, we were unable to reach this goal. (Two additional young subjects completed the repeat FSR assessments but did not complete the hypertrophy training program, and no more older subjects volunteered for the substudy.) Second, the assessment of translational signaling was certainly not exhaustive and a more thorough analysis of S6K1 downstream mediators may prove revealing. Third, a single postexercise time point may limit data interpretation; however, we have found the 24 h time point to be quite meaningful in this and prior (3, 22, 24, 32) studies. Fourth, we recognize that the power to detect age × time interactions may have been limited by sample size. On the other hand, using similar or in some cases identical procedures and fewer subjects, we have previously found age × time interactions for both myofiber hypertrophy and cell signaling following RT (17 young vs. 13 old) (24), as well as group × time differences in fasting FSR with only five to six subjects per group following unloading (14).
In summary, contrary to our hypothesis, translational signaling responses to unaccustomed RE were not blunted in older adults, suggesting the protein synthesis machinery is relatively intact and reasonably responsive among old. Further, young and old experienced similar magnitudes of myofiber hypertrophy, which appeared largely by protein accretion based on myonuclear domain expansion. Further study is warranted to better understand the temporal relationship between translational signaling and rates of muscle protein synthesis, particularly among older adults.
ACKNOWLEDGMENTS
We are indebted to the research subjects for invaluable contributions to this work. We thank S. C. Tuggle for administering the resistance training program.
GRANTS
Funding for this work was provided by National Institute on Aging Grants R01-AG-017896 (M. M. Bamman) and F30-AG-031623 (D. L. Mayhew), a Veterans Affairs Merit Grant (M. M. Bamman), and General Clinical Research Center Grant M01-RR-00032.
REFERENCES
- 1. Atherton PJ, Babraj J, Smith K, Singh J, Rennie MJ, Wackerhage H. Selective activation of AMPK-PGC-1alpha or PKB-TSC2-mTOR signaling can explain specific adaptive responses to endurance or resistance training-like electrical muscle stimulation. FASEB J 19: 786–788, 2005. [DOI] [PubMed] [Google Scholar]
- 2. Baar K, Esser K. Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol Cell Physiol 276: C120–C127, 1999. [DOI] [PubMed] [Google Scholar]
- 3. Bamman MM, Petrella JK, Kim JS, Mayhew DL, Cross JM. Cluster analysis tests the importance of myogenic gene expression during myofiber hypertrophy in humans. J Appl Physiol 102: 2232–2239, 2007. [DOI] [PubMed] [Google Scholar]
- 4. Bamman MM, Ragan RC, Kim JS, Cross JM, Hill VJ, Tuggle SC, Allman RM. Myogenic protein expression before and after resistance loading in 26- and 64-yr-old men and women. J Appl Physiol 97: 1329–1337, 2004. [DOI] [PubMed] [Google Scholar]
- 5. Biolo G, Maggi SP, Williams BD, Tipton KD, Wolfe RR. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol Endocrinol Metab 268: E514–E520, 1995. [DOI] [PubMed] [Google Scholar]
- 6. Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3: 1014–1019, 2001. [DOI] [PubMed] [Google Scholar]
- 7. Bolster DR, Kubica N, Crozier SJ, Williamson DL, Farrell PA, Kimball SR, Jefferson LS. Immediate response of mammalian target of rapamycin (mTOR)-mediated signalling following acute resistance exercise in rat skeletal muscle. J Physiol 553: 213–220, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chesley A, MacDougall JD, Tarnopolsky MA, Atkinson SA, Smith K. Changes in human muscle protein synthesis after resistance exercise. J Appl Physiol 73: 1383–1388, 1992. [DOI] [PubMed] [Google Scholar]
- 9. Coffey VG, Zhong Z, Shield A, Canny BJ, Chibalin AV, Zierath JR, Hawley JA. Early signaling responses to divergent exercise stimuli in skeletal muscle from well-trained humans. FASEB J 20: 190–192, 2006. [DOI] [PubMed] [Google Scholar]
- 10. Deldicque L, Atherton P, Patel R, Theisen D, Nielens H, Rennie MJ, Francaux M. Decrease in Akt/PKB signalling in human skeletal muscle by resistance exercise. Eur J Appl Physiol 104: 57–65, 2008. [DOI] [PubMed] [Google Scholar]
- 11. Dennis PB, Pullen N, Pearson RB, Kozma SC, Thomas G. Phosphorylation sites in the autoinhibitory domain participate in p70(s6k) activation loop phosphorylation. J Biol Chem 273: 14845–14852, 1998. [DOI] [PubMed] [Google Scholar]
- 12. Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol 576: 613–624, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Evans W, Phinney S, Young V. Suction applied to a muscle biopsy maximizes sample size. Med Sci Sports Exerc 14: 101–102, 1982. [PubMed] [Google Scholar]
- 14. Ferrando AA, Tipton KD, Bamman MM, Wolfe RR. Resistance exercise maintains skeletal muscle protein synthesis during bed rest. J Appl Physiol 82: 807–810, 1997. [DOI] [PubMed] [Google Scholar]
- 15. Greenhaff PL, Karagounis LG, Peirce N, Simpson EJ, Hazell M, Layfield R, Wackerhage H, Smith K, Atherton P, Selby A, Rennie MJ. Disassociation between the effects of amino acids and insulin on signaling, ubiquitin ligases, and protein turnover in human muscle. Am J Physiol Endocrinol Metab 295: E595–E604, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guillet C, Prod'homme M, Balage M, Gachon P, Giraudet C, Morin L, Grizard J, Boirie Y. Impaired anabolic response of muscle protein synthesis is associated with S6K1 dysregulation in elderly humans. FASEB J 18: 1586–1587, 2004. [DOI] [PubMed] [Google Scholar]
- 17. Hasten DL, Pak-Loduca J, Obert KA, Yarasheski KE. Resistance exercise acutely increases MHC and mixed muscle protein synthesis rates in 78–84 and 23–32 yr olds. Am J Physiol Endocrinol Metab 278: E620–E626, 2000. [DOI] [PubMed] [Google Scholar]
- 18. Hornberger TA, Mateja RD, Chin ER, Andrews JL, Esser KA. Aging does not alter the mechanosensitivity of the p38, p70S6k, and JNK2 signaling pathways in skeletal muscle. J Appl Physiol 98: 1562–1566, 2005. [DOI] [PubMed] [Google Scholar]
- 19. Hornberger TA, Sukhija KB, Wang XR, Chien S. mTOR is the rapamycin-sensitive kinase that confers mechanically-induced phosphorylation of the hydrophobic motif site Thr(389) in p70(S6k). FEBS Lett 581: 4562–4566, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Iijima Y, Laser M, Shiraishi H, Willey CD, Sundaravadivel B, Xu L, McDermott PJ, Kuppuswamy D. c-Raf/MEK/ERK pathway controls protein kinase C-mediated p70S6K activation in adult cardiac muscle cells. J Biol Chem 277: 23065–23075, 2002. [DOI] [PubMed] [Google Scholar]
- 21. Kim JS, Kosek DJ, Petrella JK, Cross JM, Bamman MM. Resting and load-induced levels of myogenic gene transcripts differ between older adults with demonstrable sarcopenia and young men and women. J Appl Physiol 99: 2149–2158, 2005. [DOI] [PubMed] [Google Scholar]
- 22. Kim JS, Petrella JK, Cross JM, Bamman MM. Load-mediated downregulation of myostatin mRNA is not sufficient to promote myofiber hypertrophy in humans: a cluster analysis. J Appl Physiol 103: 1488–1495, 2007. [DOI] [PubMed] [Google Scholar]
- 23. Kim PL, Staron RS, Phillips SM. Fasted-state skeletal muscle protein synthesis after resistance exercise is altered with training. J Physiol 568: 283–290, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kosek DJ, Bamman MM. Modulation of the dystrophin-associated protein complex in response to resistance training in young and older men. J Appl Physiol 104: 1476–1484, 2008. [DOI] [PubMed] [Google Scholar]
- 25. Kosek DJ, Kim JS, Petrella JK, Cross JM, Bamman MM. Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J Appl Physiol 101: 531–544, 2006. [DOI] [PubMed] [Google Scholar]
- 26. Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, Williams J, Smith K, Seynnes O, Hiscock N, Rennie MJ. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol 587: 211–217, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, Price SR, Mitch WE, Goldberg AL. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J 18: 39–51, 2004. [DOI] [PubMed] [Google Scholar]
- 28. Lecker SH, Solomon V, Mitch WE, Goldberg AL. Muscle protein breakdown and the critical role of the ubiquitin-proteasome pathway in normal and disease states. J Nutr 129: 227S–237S, 1999. [DOI] [PubMed] [Google Scholar]
- 29. Leger B, Cartoni R, Praz M, Lamon S, Deriaz O, Crettenand A, Gobelet C, Rohmer P, Konzelmann M, Luthi F, Russell AP. Akt signalling through GSK-3beta, mTOR and Foxo1 is involved in human skeletal muscle hypertrophy and atrophy. J Physiol 576: 923–933, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nader GA. Molecular determinants of skeletal muscle mass: getting the “AKT” together. Int J Biochem Cell Biol 37: 1985–1996, 2005. [DOI] [PubMed] [Google Scholar]
- 31. Petrella JK, Kim JS, Cross JM, Kosek DJ, Bamman MM. Efficacy of myonuclear addition may explain differential myofiber growth among resistance-trained young and older men and women. Am J Physiol Endocrinol Metab 291: E937–E946, 2006. [DOI] [PubMed] [Google Scholar]
- 32. Petrella JK, Kim JS, Mayhew DL, Cross JM, Bamman MM. Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: a cluster analysis. J Appl Physiol 104: 1736–1742, 2008. [DOI] [PubMed] [Google Scholar]
- 33. Petrella JK, Kim JS, Tuggle SC, Hall SR, Bamman MM. Age differences in knee extension power, contractile velocity, and fatigability. J Appl Physiol 98: 211–220, 2005. [DOI] [PubMed] [Google Scholar]
- 34. Phillips SM, Parise G, Roy BD, Tipton KD, Wolfe RR, Tamopolsky MA. Resistance-training-induced adaptations in skeletal muscle protein turnover in the fed state. Can J Physiol Pharmacol 80: 1045–1053, 2002. [DOI] [PubMed] [Google Scholar]
- 35. Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol Endocrinol Metab 273: E99–E107, 1997. [DOI] [PubMed] [Google Scholar]
- 36. Phillips SM, Tipton KD, Ferrando AA, Wolfe RR. Resistance training reduces the acute exercise-induced increase in muscle protein turnover. Am J Physiol Endocrinol Metab 276: E118–E124, 1999. [DOI] [PubMed] [Google Scholar]
- 37. Pullen N, Thomas G. The modular phosphorylation and activation of p70s6k. FEBS Lett 410: 78–82, 1997. [DOI] [PubMed] [Google Scholar]
- 38. Rasmussen BB, Fujita S, Wolfe RR, Mittendorfer B, Roy M, Rowe VL, Volpi E. Insulin resistance of muscle protein metabolism in aging. FASEB J 20: 768–769, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rennie MJ, Wackerhage H, Spangenburg EE, Booth FW. Control of the size of the human muscle mass. Annu Rev Physiol 66: 799–828, 2004. [DOI] [PubMed] [Google Scholar]
- 40. Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol 3: 1009–1013, 2001. [DOI] [PubMed] [Google Scholar]
- 41. Shanely RA, Zergeroglu MA, Lennon SL, Sugiura T, Yimlamai T, Enns D, Belcastro A, Powers SK. Mechanical ventilation-induced diaphragmatic atrophy is associated with oxidative injury and increased proteolytic activity. Am J Respir Crit Care Med 166: 1369–1374, 2002. [DOI] [PubMed] [Google Scholar]
- 42. Sheffield-Moore M, Paddon-Jones D, Sanford AP, Rosenblatt JI, Matlock AG, Cree MG, Wolfe RR. Mixed muscle and hepatic derived plasma protein metabolism is differentially regulated in older and younger men following resistance exercise. Am J Physiol Endocrinol Metab 288: E922–E929, 2005. [DOI] [PubMed] [Google Scholar]
- 43. Terzis G, Georgiadis G, Stratakos G, Vogiatzis I, Kavouras S, Manta P, Mascher H, Blomstrand E. Resistance exercise-induced increase in muscle mass correlates with p70S6 kinase phosphorylation in human subjects. Eur J Appl Physiol 102: 145–152, 2008. [DOI] [PubMed] [Google Scholar]
- 44. Tipton KD, Borsheim E, Wolf SE, Sanford AP, Wolfe RR. Acute response of net muscle protein balance reflects 24-h balance after exercise and amino acid ingestion. Am J Physiol Endocrinol Metab 284: E76–E89, 2003. [DOI] [PubMed] [Google Scholar]
- 45. Tokuda H, Hatakeyama D, Shibata T, Akamatsu S, Oiso Y, Kozawa O. p38 MAP kinase regulates BMP-4-stimulated VEGF synthesis via p70 S6 kinase in osteoblasts. Am J Physiol Endocrinol Metab 284: E1202–E1209, 2003. [DOI] [PubMed] [Google Scholar]
- 46. Weng QP, Andrabi K, Kozlowski MT, Grove JR, Avruch J. Multiple independent inputs are required for activation of the p70 S6 kinase. Mol Cell Biol 15: 2333–2340, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yarasheski K, Zachwieja J, Bier D. Acute effects of resistance exercise on muscle protein synthesis rate in young and elderly men and women. Am J Physiol Endocrinol Metab 265: E210–E214, 1993. [DOI] [PubMed] [Google Scholar]
- 48. Yarasheski KE, Zachwieja JJ, Bier DM. Acute effects of resistance exercise on muscle protein synthesis rate in young and elderly men and women. Am J Physiol Endocrinol Metab 265: E210–E214, 1993. [DOI] [PubMed] [Google Scholar]