Abstract
The circadian system is a key integrator of behavior and metabolism that synchronizes physiological processes with the rotation of the Earth on its axis. In mammals, the clock is present not only within the central pacemaker neurons of the hypothalamus, but also within extra-suprachiasmatic nucleus (SCN) regions of brain and nearly all peripheral tissues. Recent evidence suggests that the complex feedback networks that encompass both the circadian and metabolic systems are intimately intertwined and that disruption of either system leads to reciprocal disturbances in the other. We anticipate that improved understanding of the interconnections between the circadian and metabolic networks will open new windows on the treatment of sleep and metabolic disorders, including diabetes mellitus and obesity.
Keywords: metabolic syndrome, circadian rhythms, sirtuins, sleep disorders, diabetes mellitus
CLOCKS, METABOLISM, AND DISEASE
diverse organisms, ranging from unicellular fungi to plants and vertebrates, have evolved an internal molecular timekeeping mechanism to synchronize endogenous systems with the 24-h environmental light-dark cycle (hence the term circadian, which derives from circa diem, or “about a day”). In vertebrates, the circadian clock synchronizes cycles of fuel acquisition, storage, and utilization in anticipation of the daily sleep-wake cycle that corresponds with periods of fasting and feeding. While the existence of an internal clock was originally proposed based on studies of circadian flowering patterns in the mimosa plant more than 200 years ago, the molecular principles of the clock have only recently emerged with the powerful application of forward genetics in flies, plants, and mammals. Positional cloning has revealed that the cellular oscillator is programmed by a conserved transcription-translation feedback loop generated by the rhythmic and opposing action of a set of transcriptional activators and repressors (90). This endogenous timekeeping mechanism is expressed in master pacemaker neurons and nearly all peripheral tissues where it maintains synchrony of diverse processes with ∼24-h precision.
It has been recognized for years that many aspects of human health and disease exhibit marked variation across the day and night. Heart attacks, stroke, flash pulmonary edema, and hypertensive crises tend to peak at particular times of the day (17, 59). Association studies have revealed that shift workers, night workers, and sleep-deprived individuals have an increased risk of developing symptoms of the metabolic syndrome (18, 26, 39, 86). Furthermore, there is well-established rhythmicity in many metabolic processes. Lipid and carbohydrate metabolism, hormone release, blood pressure, and the production of coagulation factors all exhibit circadian oscillations. In particular, the cyclical nature of blood glucose regulation has long been established (30). In humans, blood glucose levels peak right before the onset of the activity period (4, 8), and oral glucose tolerance is impaired in the afternoon and evening compared with morning hours, coinciding with a decrease in insulin secretion and altered insulin sensitivity in the evening. Interestingly, these daily cycles of insulin secretion and sensitivity are lost in diabetic patients (7), and diurnal variations of corticosterone and locomotor activity are also abolished in diabetic rats (69, 97). While these studies indicate that there is a critical relationship between metabolic processes and the time of day, the recent availability of genetic and molecular tools has transformed our understanding of the function of the clock transcription network in these processes.
MOLECULAR BASIS OF THE CIRCADIAN OSCILLATOR
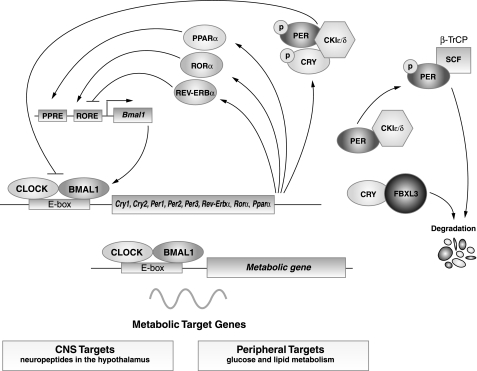
The first mammalian circadian gene Clock (circadian locomotor output cycles kaput) was discovered through an unbiased mutagenesis screen in the early 1990s (3, 40, 98). Rapid advances in the field in the ensuing years have revealed that the molecular machinery controlling circadian timekeeping consists of a core transcription/translation feedback loop that produces 24-h rhythmic patterns of gene transcription (Fig. 1). CLOCK and its partner BMAL1, both bHLH-PAS (basic helix-loop-helix–Period-Arnt-Singleminded) transcription factors, comprise the positive limb of the circadian oscillator (11, 37). In extra-suprachiasmatic nucleus (extra-SCN) tissues, neuronal PAS domain protein 2 (NPAS2) functions as a CLOCK orthologue (74). Loss-of-function mutations in Clock are compensated by Npas2 in SCN, giving rise to normal locomotor activity rhythms in Clock-deficient mice, although oscillation of clock genes within peripheral tissues is not fully compensated in the absence of CLOCK (23, 24). CLOCK is necessary and sufficient to produce circadian oscillation in neuronal cells (108). CLOCK:BMAL1 functions as a heterodimer to activate the rhythmic transcription of genes containing E-box enhancer sequences (60). The period genes Per1, Per2, and Per3 and the cryptochrome genes Cry1 and Cry2 are downstream targets of CLOCK:BMAL1 and encode components of the negative-feedback loop. On translation, the PER and CRY proteins form heterodimers that translocate to the nucleus and repress the transcriptional activity of CLOCK:BMAL1 (32, 82, 95, 111). Phosphorylation of PER and CRY proteins by casein kinase I-epsilon and -delta (CKIε/δ) and the subsequent proteasomal degradation of PERs is an important step in the generation of circadian rhythmicity (1). Recent discovery that mutation of the F-box protein FBXL3, a component of the E3 ubiquitin ligase complex, results in a period lengthening in mice suggests that steps in the degradative pathway also play a key role in generating overt rhythmicity (13, 33, 84). Other interlocking loops further modulate the function of CLOCK and BMAL1. For example, CLOCK and BMAL1 activate transcription of the orphan nuclear receptors Rev-Erbα and Rorα, which in turn comprise a short feedback loop that modulates Bmal1 transcription. REV-ERBα represses, while RORα activates, Bmal1 transcription through shared ROR-binding elements (ROREs) within the Bmal1 promoter (72).
Fig. 1.
Core circadian clock network. The core molecular clock machinery is encoded by interlocking transcription-translation feedback loop that oscillates with a 24-h periodicity. The core mammalian clock comprises transcription factors CLOCK and BMAL1, which heterodimerize to drive the transcription of downstream target genes containing E-box enhancer elements. Among these, the period (PER) and cryptochrome (CRY) proteins then multimerize and inhibit the action of the CLOCK:BMAL1 complex, resulting in a rhythmic oscillation of the core clock genes and many of their downstream targets. In addition, the CLOCK:BMAL1 heterodimer also drives the transcription of metabolic target genes in the central nervous system (CNS) and in the peripheral tissues. CKIε/δ, casein kinase I-epsilon and -delta; ROR, retinoid-related orphan receptors; RORE, ROR-binding element; PPARα, peroxisome proliferator-activated receptor-α; SCF, Skp/Cullin/F-box.
Studies of mutations in core clock genes have demonstrated their functional roles in the generation of approximately 24-h periodicity of behavior. Mice lacking Bmal1 or Per2, or mice expressing a dominant negative Clock mutation, become arrhythmic in constant darkness (3, 11, 109). However, knockout of Per1, Per3, Cry1, or Cry2 leads to either shortening or lengthening of circadian period, but not to arrhythmicity, suggesting functional redundancy among the components of the clock machinery (95, 109).
In vertebrates, the master clock mechanism was initially identified through lesioning studies in the SCN in the hypothalamus (87). The core clock machinery was later detected in other brain regions and in the periphery, including tissues important for normal cardiometabolic function (21, 107). The SCN is still considered to be the master circadian pacemaker, driving rhythmic behavior and coordinating peripheral tissue clocks. The function of most peripheral clocks remains to be defined, although recent studies have begun to elucidate the role of peripheral clocks in maintenance of energy balance and in metabolic homeostasis.
INTEGRATION OF MOLECULAR CLOCK AND METABOLIC TRANSCRIPTION NETWORKS
Understanding the mechanisms by which the molecular clock controls metabolic transcription networks, and ultimately energy homeostasis, has emerged as an exciting and challenging area. Mounting evidence suggests that various metabolic networks are under circadian control. Reportedly, 3% to 20% of all gene transcripts display a 24-h variation in mRNA levels in both the SCN and peripheral tissues. The pervasive effect of circadian regulation of gene expression in the periphery is demonstrated by the rhythmic transcription of numerous metabolic genes in liver, skeletal muscle, brown and white adipose tissue, heart, and vasculature (2, 66, 70, 78, 88, 105, 110). Among the rhythmic genes identified, many play key roles in lipid and cholesterol biosynthesis, carbohydrate metabolism and transport, oxidative phosphorylation, and detoxification pathways. The observation that key rate-limiting enzymes involved in these processes are under circadian control highlights the pervasive role of circadian clocks in normal organismal function (70). Of note, only a small subset of key metabolic genes are direct targets of the core clock genes (65, 76). Many of these targets are transcription factors or other modulators of transcription and translation that, in turn, impart rhythmicity on downstream metabolic genes (70). For example, the core circadian machinery induces daily oscillations in D-site binding protein (Dbp), a transcription factor regulating key gluconeogenic and lipogenic genes (29, 49, 76). Mutation of core circadian genes abolishes rhythmic expression and/or shifts the phase of oscillation of a large number of metabolic genes (43, 53, 67, 70).
Importantly, the period and amplitude of oscillation and the level of expression of each of these metabolic genes vary among different tissues, suggesting a physiologically meaningful role of peripheral clocks for normal cellular function. Interestingly, only a limited number of genes are rhythmically expressed in multiple peripheral tissues (88). In fact, the pattern of gene expression in each peripheral tissue seems to correlate with physiological function. For example, a large portion of rhythmic genes in the muscle show a nadir of expression in the middle of the subjective night, coinciding with the peak of physical activity (53). Many genes in fat peak in the middle of the light period, several hours after the initial bout of feeding, and are possibly associated with nutrient storage (41, 88). Rhythmic genes in the liver peak either at the beginning of day or onset of night, likely reflecting the alternating requirements for lipogenic and lipid catabolic genes across the light-dark feeding-fasting cycle (55, 70). A major question remains as to whether the rhythmic pattern of gene expression arises due to intrinsic clock expression in brain or peripheral tissue, or rather is secondary to the feeding rhythm, although studies in the ultradian vole and mice suggest that certain rhythmic patterns occur independently of the feeding rhythm (96).
A series of recent studies on nuclear hormone receptors (NHRs) further support the extensive interconnections between the circadian clock and energy metabolism. NHRs sense fat-soluble hormones, vitamins, and lipids and regulate various aspects of lipid and carbohydrate metabolism (19). Intriguingly, many NHRs, including Rev-erbα, Rorα, and Pparα, exhibit a tissue-specific 24-h pattern of gene expression and are directly regulated by the CLOCK:BMAL1 heterodimer (105). In addition to affecting metabolic gene expression, all three of these NHRs participate in circadian feedback loops and regulate the core clock genes. REV-ERBα, an inhibitor of Bmal1 expression, suppresses hepatic gluconeogenic gene transcription and affects glucose output (106). Furthermore, REV-ERBα is required for adipogenesis, adipocyte differentiation, and lipid metabolism (100). The orphan nuclear receptor RORα, a positive regulator of Bmal1, is also involved in lipogenesis and lipid storage (80). The rhythmically expressed peroxisome proliferator-activated receptor-α (PPARα) further induces Bmal1 transcription (16). Importantly, on binding to endogenous free fatty acids, PPARα also regulates transcription of genes involved in lipid and glucose metabolism (25). Furthermore, the PPAR transcriptional coactivator PGC-1α regulates adaptive energy metabolism, is rhythmically expressed, and stimulates Bmal1 transcription (50–52). Mice lacking Pgc1α display altered locomotor activity rhythm (52). In sum, the NHRs participate in the reciprocal interaction between circadian and metabolic regulatory networks.
Although the aforementioned studies demonstrate that extensive interactions exist between the core clock machinery and metabolic networks, it is not yet clear as to what factors link the regulation of metabolic and circadian systems. Research into how important metabolic sensors interact with the molecular circadian clock is an active area of investigation.
NEW ROLE FOR CHROMATIN MODIFICATION PATHWAY AND NAD
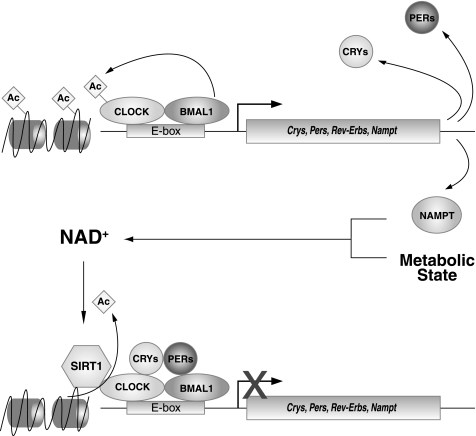
One of the early clues to explain the interdependence of circadian and metabolic processes stemmed from the finding by Rutter et al. (79) suggesting that changes in the cellular redox status of the cell, represented by nicotinamide adenine dinucleotide cofactors NAD(H) and NADP(H), regulate the transcriptional activity of CLOCK and its homolog NPAS2. First, the reduced forms of these redox cofactors enhance DNA binding of CLOCK:BMAL1 and NPAS2:BMAL1 heterodimers, while the oxidized forms inhibit binding. Second, it had been reported that the presumptive cytokine Pbef, or pre-B-cell colony enhancing factor, displayed a circadian pattern of gene expression in both liver and heart (70, 88). Interestingly, this gene was later identified as Nampt, or nicotinamide phosphoribosyltransferase, and was demonstrated to encode the rate-limiting enzyme in the NAD+ biosynthesis pathway (75). Increasing the dosage of Nampt increases both the total cellular NAD+ levels and the transcriptional activity of SIRT1, an NAD+-dependent deacetylase that has been shown to regulate both histone modification and a host of physiological processes, including glucose homeostasis, fat metabolism, insulin secretion, and apoptosis (9, 57, 75). SIRT1 catalyzes a unique reaction in which hydrolysis of NAD+ is coupled with protein deacetylation, resulting in the formation of nicotinamide, O-acetyl-ADP-ribose, and deacetylated protein substrates. Because SIRT1 relies on NAD+ for its enzymatic activity, NAD+ biosynthetic pathways play a critical role in the regulation of SIRT1 function. Of note, Nampt was one of only 37 genes that showed identical phases of peak expression in both liver and heart (88), suggesting that circadian regulation of NAMPT-mediated NAD+ biosynthesis and SIRT1 activity may represent a central core mechanism in the generation or maintenance of circadian patterns in multiple tissues.
A negative-feedback loop linking both the NAMPT-mediated NAD+ biosynthesis pathway and SIRT1 with the core circadian clock circuitry has recently been described by several groups (5, 61, 62, 73) (Fig. 2). CLOCK and BMAL1 directly regulate the transcription of Nampt, which oscillates at both the RNA and protein levels in a circadian fashion, peaking around zeitgeber (ZT) 14. The circadian oscillation of NAMPT directly translates to daily oscillations in NAD+ levels in liver, and the peak in NAMPT and NAD+ corresponds with the reported peak in SIRT1 activity around ZT15. SIRT1 then physically interacts with the positive limb of the core clock feedback loop (CLOCK and BMAL1) and is recruited to the promoters of clock target genes. Genetic and pharmacologic manipulation of the NAD+ biosynthetic pathway and SIRT1 reveal that SIRT1 negatively regulates CLOCK and BMAL1 activity. Because both NAMPT and SIRT1 are acutely sensitive to the metabolic state of the cell, the NAMPT/SIRT1/CLOCK:BMAL1 feedback loop is key to understanding how the circadian clock is able to maintain synchrony of the metabolic oscillators in peripheral tissues in response to nutritional input. Furthermore, the rhythmic production of NAD+ is also likely to play a critical role in a number of downstream metabolic pathways, as well as chromatin regulation and potentially aging.
Fig. 2.
Negative-feedback loop linking NAD+/SIRT1 with the Clock network. NAMPT, the rate-limiting enzyme in NAD biosynthesis, oscillates in a circadian pattern under the transcriptional control of the CLOCK:BMAL1 heterodimer. Alterations in NAMPT drive oscillation in NAD biosynthesis, which in turn modulates the nutrient responsive deacetylase SIRT1. SIRT1 plays an important role in energy homeostasis and has recently been discovered to function in the control of the circadian clock. SIRT1 activity, dependent on the metabolic state of the cell, inhibits CLOCK:BMAL1 activity and prevents the transcription of both circadian and metabolic downstream target genes. The CLOCK:BMAL1-NAMPT-SIRT1 pathway comprises a novel metabolic feedback that may integrate the daily cycles of activity, feeding, and energy homeostasis. Ac, acetyl.
EXPERIMENTAL GENETIC EVIDENCE FOR CROSSTALK BETWEEN CIRCADIAN AND METABOLIC TRANSCRIPTION NETWORKS
A major transformation in our understanding of peripheral clocks originated with the demonstration of cell-autonomous circadian gene oscillation in cultured fibroblasts (6). Prior to this work the prevailing model held that circadian oscillation was a unique feature of the neuron. Subsequent creation of biological reporter mice expressing luciferase driven by the promoters of cycling clock genes has enabled real-time monitoring of circadian oscillators from live cell explants of both SCN and peripheral tissues. Studies in luciferase reporter mice have demonstrated the existence of self-sustained oscillators in a variety of peripheral tissues, including liver and muscle (102, 103, 107). Bioluminescence imaging has even enabled detection of persistent circadian oscillation rhythms at the level of single cells (101). Interestingly, the period and phase of expression of key circadian genes vary between tissues of the same animal, indicating presence of local factors that modify function of the core clock loop.
Analysis of circadian mutant animals has begun to provide insight into the metabolic role of clock function in the periphery (Table 1). For example, the dominant Clock gene mutation Δ19 causes hyperlipidemia, hyperleptinemia, hyperglycemia, and hypoinsulinemia (94). In addition to altered rhythms of locomotor activity, loss of BMAL1 impairs adipogenesis, adipocyte differentiation, and hepatic carbohydrate metabolism (43, 77, 83). BMAL1 also exerts profound effects on skeletal muscle function (53). PER2 deficiency abolishes glucocorticoid rhythmicity and causes alterations in bone density (28, 104). Lack of both CRY1 and CRY2 impairs body growth, changes patterns of circulating growth hormone, and modifies expression of lipogenic and steroidogenic pathways (12). Furthermore, transgenic overexpression of mutant CRY1 results in polydipsia, polyuria, and hyperglycemia, all symptoms of diabetes mellitus (68). Mutations downstream of the core clock, the so-called clock-controlled genes, have also been shown to impact metabolism. For example, ablation of the circadian deadenylase Nocturnin, which is involved in posttranscriptional regulation of rhythmic gene expression, alters glucose tolerance, peripheral tissue insulin sensitivity, and response to high-fat diet (35).
Table 1.
Behavioral and metabolic phenotypes of circadian gene mutants
| Gene Disruption | Mutation | Circadian Phenotype | Metabolic Phenotype | Reference |
|---|---|---|---|---|
| Clock | Systemic dominant negative mutation | Arrhythmicity in DD | Hypertriglyceridemia, hypercholesterolemia, hyperglycemia, hyperleptinemia | 94 |
| Bmal1 | Systemic KO | Arrythmicity in DD | Impaired gluconeogenesis, adipogenesis, adipocyte differentiation; hyperlipidemia, glucose intolerance | 77, 83 |
| 43 | ||||
| Per2 | Systemic KO | Shortening of period | Absent glucocorticoid rhythm, absent diurnal feeding rhythm, obesity, alternations in leptin-dependent bone density | 104 |
| 28 | ||||
| Cry1 | Systemic KO | Shortening of period in DD | 99 | |
| Cry2 | Systemic KO | Lengthening of period in DD | 99 | |
| Cry1/Cry2 | Systemic double KO | Arrhythmicity in DD | Impaired body growth; feminized patterns of growth hormone and metabolic genes in liver | 12, 99 |
| Nocturnin | Systemic KO | WT | Resistance to diet-induced obesity, hepatic steatosis, impaired glucose tolerance, increased insulin sensitivity | 35 |
| Bmal1 | Liver-specific KO | WT | Resting hypoglycemia, exaggerated glucose clearance | 43 |
KO, knockout; DD, constant darkness.
Recent evidence suggests that circadian regulation of metabolic processes involves both global and cell-autonomous signals to maintain temporal coordination of feeding cycles and metabolic function. Tissue-specific circadian gene knockout and transgenic animals offer unique opportunities to further elucidate the role of cell autonomous clock function in metabolism and energy balance. Recent liver-specific knockout studies have found that Bmal1 ablation leads to alterations in glucose clearance and hypoglycemia during the rest period, as well as loss of oscillation of hepatic metabolic genes (43). Conditional liver-specific overexpression of REV-ERBα also abolishes rhythmic expression of most oscillating liver genes (42).
Transgenic overexpression of ClockΔ19 in cardiomyocytes has revealed a role of the circadian gene network in heart rate variability, contractility, and responsiveness of the heart to changes in afterload (10). Some or all of the physiological deficits in myocardial function of these mice have been linked to alterations in cardiac fuel handling. Collectively, these findings support the hypothesis that the cardiomyocyte clock enables the heart to anticipate and respond appropriately to rhythmic variations in external stimuli, such as increased workload.
It is important to note that core circadian clock components may play distinct metabolic roles in different tissues, in addition to their function in regulating circadian rhythms. For example, a muscle-specific Bmal1 rescue, which results in constitutive overexpression, revealed that BMAL1 function in the muscle is important for activity, body weight maintenance, and longevity (54). Further understanding of the role of individual tissue oscillators and their relationship to the central oscillator will provide important insight into the mechanism underlying metabolic disease phenotypes of circadian mutants.
ROLE OF SLEEP IN THE INTERACTION BETWEEN CIRCADIAN AND METABOLIC SYSTEMS
Interconnections between circadian, sleep, and metabolic systems have received increased attention in clinical studies over the past decade (please see Refs. 45 and 71 for a more comprehensive review). Cross-sectional studies have repeatedly indicated that lack of sleep is an independent risk factor for obesity and hypertension (31). Chronic short sleep duration is associated with increased BMI and incidence of type 2 diabetes (34, 36, 64, 89). Furthermore, poor sleep quality, as observed in patients with obstructive sleep apnea, is also associated with type 2 diabetes and cardiovascular disease (91). Clinical studies have found that even short periods of sleep restriction can negatively affect energy metabolism. Partial sleep loss leads to increased glucose levels, decreased insulin sensitivity, and reduced glucose tolerance (86). Short periods of reduced sleep are also associated with decreased leptin and augmented ghrelin levels, leading to increased appetite and food consumption that can potentially cause excess weight gain (85, 89).
The precise mechanisms underlying the interconnection between sleep time and energy balance are still uncertain. In addition, controversy remains concerning the role of the circadian system in sleep, although mounting evidence suggests that the SCN contributes to more than just the timing of sleep and wakefulness (27, 56). Indeed, sleep deprivation per se induces marked changes in the electrical activity of SCN neurons, suggesting that SCN also receives input from cortical structures in addition to photic input to gauge sleep phase (22). Interestingly, key clock genes such as Clock and Bmal1 also influence baseline sleep architecture and quality (44, 63). Additionally, mutations in Per2 are associated with advanced phase sleep syndrome in humans, although it is not yet known whether these mutations might exert adverse metabolic effects (92). Equally surprising is the recent observation that ablation of melanopsin, the retinal photopigment crucial for light entrainment of SCN, also impacts sleep homeostasis (93). An important output of SCN involves autonomic projections to the pineal gland where melatonin is produced (56). Recent genomewide association studies in human subjects have uncovered a strong association between variation in the melatonin type 1b receptor and glucose in humans (58), pointing toward melatonin as an additional candidate in the linkage between sleep, rhythms, and metabolic control.
Energy homeostasis, sleep, and circadian rhythmicity appear to be interrelated at multiple levels. The energy hypothesis for sleep posits a role for neuronal regeneration following the depletion of key intermediary metabolites adenosine and glycogen during periods of wakefulness (further reviewed in Ref. 81). An additional area of investigation involves the role of non-rapid eye movement (NREM) sleep as an intermediate stage during entry into torpor. Whether and how conditions that trigger torpor may affect sleep architecture is still not known (48). Of special interest will be the identification of molecular and/or hormonal signals that may coordinate sleep with energy balance and the integration of energy-sensing networks with regulation of sleep. Leptin deficient ob/ob mice and leptin-resistant db/db mice, which are severely obese and show symptoms of metabolic dysregulation, also exhibit changes in sleep architecture and diurnal rhythmicity (46, 47). Altered sleep architecture is also found in patients with type 1 diabetes (38). Similarly, high-fat feeding in mice alters both behavioral and molecular circadian function and sleep (41).
Finally, altered sleep/activity patterns can reciprocally affect the function of the central and peripheral oscillators and, ultimately, metabolism. Irregular sleep patterns or sleep loss can lead to arrhythmic exposure to light and constant resetting of the central oscillator. Extended periods of wakefulness and fragmented sleep schedule may in turn alter normal feeding patterns and desynchronize peripheral oscillators in metabolic tissues, such as liver and pancreas. Altered patterns of physical activity, such as exercise in the evening, can also lead to a phase shift of normal circadian rhythms (14, 15). Finally, nocturnal eating has separately been associated with increased body mass index (BMI) (20). Overall, dysregulation of sleep and activity can result in misalignment of the central and peripheral oscillators and desynchronization of behavior, metabolic gene expression, and hormone release. Further studies are needed to extend our understanding of the interplay between circadian systems and sleep and to unravel the neural networks and molecular signals through which these systems impact metabolic homeostasis.
CONCLUSIONS AND FUTURE DIRECTIONS
Recent advances in mammalian experimental genetics have implicated the circadian system as a key integrator of behavior and metabolism. Compelling new evidence reviewed above has uncovered that, in addition to altering activity, perturbations of normal circadian gene function may lead to impairment of metabolic health. Furthermore, energy homeostasis may be modulated by the alignment of sleep and activity behaviors with the normal timing of metabolic functions. Recent evidence indicates that both networks are integrated through a series of feedback loops, many of which are convergent with nutrient signaling pathways. Consideration of the pervasive role of circadian oscillation at the cell and molecular levels offers new opportunities in the search for mechanism-based therapies including type 2 diabetes mellitus and obesity.
ACKNOWLEDGMENTS
We thank all members of the laboratory of J. Bass for helpful discussions, and especially Dana Abrassart for help with figures.
GRANTS
This work was supported by grants from the National Institutes of Health (T32-DK-007169) to K. M. Ramsey; the National Institute on Aging (P01 AG011412); Chicago Biomedical Consortium with support from The Searle Funds at The Chicago Community Trust (C-007); Juvenile Diabetes Research Foundation (1-2008-114); and the University of Chicago Diabetes Research and Training Center (DK-20595) to J. Bass.
REFERENCES
- 1. Akashi M, Tsuchiya Y, Yoshino T, Nishida E. Control of intracellular dynamics of mammalian period proteins by casein kinase I epsilon (CKIepsilon) and CKIdelta in cultured cells. Mol Cell Biol 22: 1693–1703, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allaman-Pillet N, Roduit R, Oberson A, Abdelli S, Ruiz J, Beckmann JS, Schorderet DF, Bonny C. Circadian regulation of islet genes involved in insulin production and secretion. Mol Cell Endocrinol 226: 59–66, 2004. [DOI] [PubMed] [Google Scholar]
- 3. Antoch MP, Song EJ, Chang AM, Vitaterna MH, Zhao Y, Wilsbacher LD, Sangoram AM, King DP, Pinto LH, Takahashi JS. Functional identification of the mouse circadian Clock gene by transgenic BAC rescue. Cell 89: 655–667, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arslanian S, Ohki Y, Becker DJ, Drash AL. Demonstration of a dawn phenomenon in normal adolescents. Horm Res 34: 27–32, 1990. [DOI] [PubMed] [Google Scholar]
- 5. Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell 134: 317–328, 2008. [DOI] [PubMed] [Google Scholar]
- 6. Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell 93: 929–937, 1998. [DOI] [PubMed] [Google Scholar]
- 7. Boden G, Chen X. Effects of fatty acids and ketone bodies on basal insulin secretion in type 2 diabetes. Diabetes 48: 577–583, 1999. [DOI] [PubMed] [Google Scholar]
- 8. Bolli GB, De Feo P, De Cosmo S, Perriello G, Ventura MM, Calcinaro F, Lolli C, Campbell P, Brunetti P, Gerich JE. Demonstration of a dawn phenomenon in normal human volunteers. Diabetes 33: 1150–1153, 1984. [DOI] [PubMed] [Google Scholar]
- 9. Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol 6: 298–305, 2005. [DOI] [PubMed] [Google Scholar]
- 10. Bray MS, Shaw CA, Moore MW, Garcia RA, Zanquetta MM, Durgan DJ, Jeong WJ, Tsai JY, Bugger H, Zhang D, Rohrwasser A, Rennison JH, Dyck JR, Litwin SE, Hardin PE, Chow CW, Chandler MP, Abel ED, Young ME. Disruption of the circadian clock within the cardiomyocyte influences myocardial contractile function, metabolism, and gene expression. Am J Physiol Heart Circ Physiol 294: H1036–H1047, 2008. [DOI] [PubMed] [Google Scholar]
- 11. Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, Bradfield CA. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 103: 1009–1017, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bur IM, Cohen-Solal AM, Carmignac D, Abecassis PY, Chauvet N, Martin AO, van der Horst GT, Robinson IC, Maurel P, Mollard P, Bonnefont X. The circadian clock components CRY1 and CRY2 are necessary to sustain sex dimorphism in mouse liver metabolism. J Biol Chem 284: 9066–9073, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Busino L, Bassermann F, Maiolica A, Lee C, Nolan PM, Godinho SI, Draetta GF, Pagano M. SCFFbxl3 controls the oscillation of the circadian clock by directing the degradation of cryptochrome proteins. Science 316: 900–904, 2007. [DOI] [PubMed] [Google Scholar]
- 14. Buxton OM, Frank SA, L'Hermite-Baleriaux M, Leproult R, Turek FW, Van Cauter E. Roles of intensity and duration of nocturnal exercise in causing phase delays of human circadian rhythms. Am J Physiol Endocrinol Metab 273: E536–E542, 1997. [DOI] [PubMed] [Google Scholar]
- 15. Buxton OM, Lee CW, L'Hermite-Baleriaux M, Turek FW, Van Cauter E. Exercise elicits phase shifts and acute alterations of melatonin that vary with circadian phase. Am J Physiol Regul Integr Comp Physiol 284: R714–R724, 2003. [DOI] [PubMed] [Google Scholar]
- 16. Canaple L, Rambaud J, Dkhissi-Benyahya O, Rayet B, Tan NS, Michalik L, Delaunay F, Wahli W, Laudet V. Reciprocal regulation of brain and muscle Arnt-like protein 1 and peroxisome proliferator-activated receptor alpha defines a novel positive feedback loop in the rodent liver circadian clock. Mol Endocrinol 20: 1715–1727, 2006. [DOI] [PubMed] [Google Scholar]
- 17. Carson PA, O'Connor CM, Miller AB, Anderson S, Belkin R, Neuberg GW, Wertheimer JH, Frid D, Cropp A, Packer M. Circadian rhythm and sudden death in heart failure: results from Prospective Randomized Amlodipine Survival Trial. J Am Coll Cardiol 36: 541–546, 2000. [DOI] [PubMed] [Google Scholar]
- 18. Chaput JP, Despres JP, Bouchard C, Tremblay A. The association between sleep duration and weight gain in adults: a 6-year prospective study from the Quebec Family Study. Sleep 31: 517–523, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: opening the X-files. Science 294: 1866–1870, 2001. [DOI] [PubMed] [Google Scholar]
- 20. Colles SL, Dixon JB, O'Brien PE. Night eating syndrome and nocturnal snacking: association with obesity, binge eating and psychological distress. Int J Obes (Lond) 31: 1722–1730, 2007. [DOI] [PubMed] [Google Scholar]
- 21. Davidson AJ, London B, Block GD, Menaker M. Cardiovascular tissues contain independent circadian clocks. Clin Exp Hypertens 27: 307–311, 2005. [PubMed] [Google Scholar]
- 22. Deboer T, Vansteensel MJ, Detari L, Meijer JH. Sleep states alter activity of suprachiasmatic nucleus neurons. Nat Neurosci 6: 1086–1090, 2003. [DOI] [PubMed] [Google Scholar]
- 23. DeBruyne JP, Weaver DR, Reppert SM. CLOCK and NPAS2 have overlapping roles in the suprachiasmatic circadian clock. Nat Neurosci 10: 543–545, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. DeBruyne JP, Weaver DR, Reppert SM. Peripheral circadian oscillators require CLOCK. Curr Biol 17: R538–R539, 2007. [DOI] [PubMed] [Google Scholar]
- 25. Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev 20: 649–688, 1999. [DOI] [PubMed] [Google Scholar]
- 26. Di Lorenzo L, De Pergola G, Zocchetti C, L'Abbate N, Basso A, Pannacciulli N, Cignarelli M, Giorgino R, Soleo L. Effect of shift work on body mass index: results of a study performed in 319 glucose-tolerant men working in a Southern Italian industry. Int J Obes Relat Metab Disord 27: 1353–1358, 2003. [DOI] [PubMed] [Google Scholar]
- 27. Easton A, Meerlo P, Bergmann B, Turek FW. The suprachiasmatic nucleus regulates sleep timing and amount in mice. Sleep 27: 1307–1318, 2004. [DOI] [PubMed] [Google Scholar]
- 28. Fu L, Patel MS, Bradley A, Wagner EF, Karsenty G. The molecular clock mediates leptin-regulated bone formation. Cell 122: 803–815, 2005. [DOI] [PubMed] [Google Scholar]
- 29. Gachon F, Olela FF, Schaad O, Descombes P, Schibler U. The circadian PAR-domain basic leucine zipper transcription factors DBP, TEF, and HLF modulate basal and inducible xenobiotic detoxification. Cell Metab 4: 25–36, 2006. [DOI] [PubMed] [Google Scholar]
- 30. Gagliardino JJ, Hernandez RE, Rebolledo OR. Chronobiological aspects of blood glucose regulation: a new scope for the study of diabetes mellitus. Chronobiologia 11: 357–379, 1984. [PubMed] [Google Scholar]
- 31. Gangwisch JE, Malaspina D, Boden-Albala B, Heymsfield SB. Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep 28: 1289–1296, 2005. [DOI] [PubMed] [Google Scholar]
- 32. Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP, Takahashi JS, Weitz CJ. Role of the CLOCK protein in the mammalian circadian mechanism. Science 280: 1564–1569, 1998. [DOI] [PubMed] [Google Scholar]
- 33. Godinho SI, Maywood ES, Shaw L, Tucci V, Barnard AR, Busino L, Pagano M, Kendall R, Quwailid MM, Romero MR, O'Neill J, Chesham JE, Brooker D, Lalanne Z, Hastings MH, Nolan PM. The after-hours mutant reveals a role for Fbxl3 in determining mammalian circadian period. Science 316: 897–900, 2007. [DOI] [PubMed] [Google Scholar]
- 34. Gottlieb DJ, Punjabi NM, Newman AB, Resnick HE, Redline S, Baldwin CM, Nieto FJ. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med 165: 863–867, 2005. [DOI] [PubMed] [Google Scholar]
- 35. Green CB, Douris N, Kojima S, Strayer CA, Fogerty J, Lourim D, Keller SR, Besharse JC. Loss of Nocturnin, a circadian deadenylase, confers resistance to hepatic steatosis and diet-induced obesity. Proc Natl Acad Sci USA 104: 9888–9893, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hasler G, Buysse DJ, Klaghofer R, Gamma A, Ajdacic V, Eich D, Rossler W, Angst J. The association between short sleep duration and obesity in young adults: a 13-year prospective study. Sleep 27: 661–666, 2004. [DOI] [PubMed] [Google Scholar]
- 37. Hogenesch JB, Gu YZ, Jain S, Bradfield CA. The basic-helix-loop-helix-PAS orphan MOP3 forms transcriptionally active complexes with circadian and hypoxia factors. Proc Natl Acad Sci USA 95: 5474–5479, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jauch-Chara K, Schmid SM, Hallschmid M, Born J, Schultes B. Altered neuroendocrine sleep architecture in patients with type 1 diabetes. Diabetes Care 31: 1183–1188, 2008. [DOI] [PubMed] [Google Scholar]
- 39. Karlsson B, Knutsson A, Lindahl B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup Environ Med 58: 747–752, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. King DP, Zhao Y, Sangoram AM, Wilsbacher LD, Tanaka M, Antoch MP, Steeves TD, Vitaterna MH, Kornhauser JM, Lowrey PL, Turek FW, Takahashi JS. Positional cloning of the mouse circadian clock gene. Cell 89: 641–653, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, Turek FW, Bass J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab 6: 414–421, 2007. [DOI] [PubMed] [Google Scholar]
- 42. Kornmann B, Schaad O, Bujard H, Takahashi JS, Schibler U. System-driven and oscillator-dependent circadian transcription in mice with a conditionally active liver clock. PLoS Biol 5: e34, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lamia KA, Storch KF, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci USA 105: 15172–15177, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Laposky A, Easton A, Dugovic C, Walisser J, Bradfield C, Turek F. Deletion of the mammalian circadian clock gene BMAL1/Mop3 alters baseline sleep architecture and the response to sleep deprivation. Sleep 28: 395–409, 2005. [DOI] [PubMed] [Google Scholar]
- 45. Laposky AD, Bass J, Kohsaka A, Turek FW. Sleep and circadian rhythms: key components in the regulation of energy metabolism. FEBS Lett 582: 142–151, 2008. [DOI] [PubMed] [Google Scholar]
- 46. Laposky AD, Bradley MA, Williams DL, Bass J, Turek FW. Sleep-wake regulation is altered in leptin-resistant (db/db) genetically obese and diabetic mice. Am J Physiol Regul Integr Comp Physiol 295: R2059–R2066, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Laposky AD, Shelton J, Bass J, Dugovic C, Perrino N, Turek FW. Altered sleep regulation in leptin deficient mice. Am J Physiol Regul Integr Comp Physiol 290: R894–R903, 2006. [DOI] [PubMed] [Google Scholar]
- 48. Larkin JE, Franken P, Heller HC. Loss of circadian organization of sleep and wakefulness during hibernation. Am J Physiol Regul Integr Comp Physiol 282: R1086–R1095, 2002. [DOI] [PubMed] [Google Scholar]
- 49. Lavery DJ, Lopez-Molina L, Margueron R, Fleury-Olela F, Conquet F, Schibler U, Bonfils C. Circadian expression of the steroid 15 alpha-hydroxylase (Cyp2a4) and coumarin 7-hydroxylase (Cyp2a5) genes in mouse liver is regulated by the PAR leucine zipper transcription factor DBP. Mol Cell Biol 19: 6488–6499, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, Courtois M, Wozniak DF, Sambandam N, Bernal-Mizrachi C, Chen Z, Holloszy JO, Medeiros DM, Schmidt RE, Saffitz JE, Abel ED, Semenkovich CF, Kelly DP. PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol 3: e101, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lin J, Yang R, Tarr PT, Wu PH, Handschin C, Li S, Yang W, Pei L, Uldry M, Tontonoz P, Newgard CB, Spiegelman BM. Hyperlipidemic effects of dietary saturated fats mediated through PGC-1beta coactivation of SREBP. Cell 120: 261–273, 2005. [DOI] [PubMed] [Google Scholar]
- 52. Liu C, Li S, Liu T, Borjigin J, Lin JD. Transcriptional coactivator PGC-1alpha integrates the mammalian clock and energy metabolism. Nature 447: 477–481, 2007. [DOI] [PubMed] [Google Scholar]
- 53. McCarthy JJ, Andrews JL, McDearmon EL, Campbell KS, Barber BK, Miller BH, Walker JR, Hogenesch JB, Takahashi JS, Esser KA. Identification of the circadian transcriptome in adult mouse skeletal muscle. Physiol Genomics 31: 86–95, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. McDearmon EL, Patel KN, Ko CH, Walisser JA, Schook AC, Chong JL, Wilsbacher LD, Song EJ, Hong HK, Bradfield CA, Takahashi JS. Dissecting the functions of the mammalian clock protein BMAL1 by tissue-specific rescue in mice. Science 314: 1304–1308, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Miller BH, McDearmon EL, Panda S, Hayes KR, Zhang J, Andrews JL, Antoch MP, Walker JR, Esser KA, Hogenesch JB, Takahashi JS. Circadian and CLOCK-controlled regulation of the mouse transcriptome and cell proliferation. Proc Natl Acad Sci USA 104: 3342–3347, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Moore RY. Suprachiasmatic nucleus in sleep-wake regulation. Sleep Med 8, Suppl 3: 27–33, 2007. [DOI] [PubMed] [Google Scholar]
- 57. Moynihan KA, Imai S. Sirt1 as a key regulator orchestrating the response to caloric restriction. Drug Discov Today Dis Mech 3: 11–17, 2006. [Google Scholar]
- 58. Mulder H, Nagorny CL, Lyssenko V, Groop L. Melatonin receptors in pancreatic islets: good morning to a novel type 2 diabetes gene. Diabetologia 52: 1240–1249, 2009. [DOI] [PubMed] [Google Scholar]
- 59. Muller JE, Tofler GH, Stone PH. Circadian variation and triggers of onset of acute cardiovascular disease. Circulation 79: 733–743, 1989. [DOI] [PubMed] [Google Scholar]
- 60. Munoz E, Brewer M, Baler R. Circadian Transcription. Thinking outside the E-Box. J Biol Chem 277: 36009–36017, 2002. [DOI] [PubMed] [Google Scholar]
- 61. Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente LP, Sassone-Corsi P. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell 134: 329–340, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science 324: 654–657, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Naylor E, Bergmann BM, Krauski K, Zee PC, Takahashi JS, Vitaterna MH, Turek FW. The circadian clock mutation alters sleep homeostasis in the mouse. J Neurosci 20: 8138–8143, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Nilsson PM, Roost M, Engstrom G, Hedblad B, Berglund G. Incidence of diabetes in middle-aged men is related to sleep disturbances. Diabetes Care 27: 2464–2469, 2004. [DOI] [PubMed] [Google Scholar]
- 65. Noshiro M, Usui E, Kawamoto T, Kubo H, Fujimoto K, Furukawa M, Honma S, Makishima M, Honma K, Kato Y. Multiple mechanisms regulate circadian expression of the gene for cholesterol 7alpha-hydroxylase (Cyp7a), a key enzyme in hepatic bile acid biosynthesis. J Biol Rhythms 22: 299–311, 2007. [DOI] [PubMed] [Google Scholar]
- 66. Oishi K, Atsumi G, Sugiyama S, Kodomari I, Kasamatsu M, Machida K, Ishida N. Disrupted fat absorption attenuates obesity induced by a high-fat diet in Clock mutant mice. FEBS Lett 580: 127–130, 2006. [DOI] [PubMed] [Google Scholar]
- 67. Oishi K, Miyazaki K, Kadota K, Kikuno R, Nagase T, Atsumi G, Ohkura N, Azama T, Mesaki M, Yukimasa S, Kobayashi H, Iitaka C, Umehara T, Horikoshi M, Kudo T, Shimizu Y, Yano M, Monden M, Machida K, Matsuda J, Horie S, Todo T, Ishida N. Genome-wide expression analysis of mouse liver reveals CLOCK-regulated circadian output genes. J Biol Chem 278: 41519–41527, 2003. [DOI] [PubMed] [Google Scholar]
- 68. Okano S, Akashi M, Hayasaka K, Nakajima O. Unusual circadian locomotor activity and pathophysiology in mutant CRY1 transgenic mice. Neurosci Lett 451: 246–251, 2009. [DOI] [PubMed] [Google Scholar]
- 69. Oster MH, Castonguay TW, Keen CL, Stern JS. Circadian rhythm of corticosterone in diabetic rats. Life Sci 43: 1643–1645, 1988. [DOI] [PubMed] [Google Scholar]
- 70. Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 109: 307–320, 2002. [DOI] [PubMed] [Google Scholar]
- 71. Penev PD. Sleep deprivation and energy metabolism: to sleep, perchance to eat? Curr Opin Endocrinol Diabetes Obes 14: 374–381, 2007. [DOI] [PubMed] [Google Scholar]
- 72. Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U, Schibler U. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 110: 251–260, 2002. [DOI] [PubMed] [Google Scholar]
- 73. Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, Marcheva B, Hong HK, Chong JL, Buhr ED, Lee C, Takahashi JS, Imai SI, Bass J. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science 324: 651–654 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Reick M, Garcia JA, Dudley C, McKnight SL. NPAS2: an analog of clock operative in the mammalian forebrain. Science 293: 506–509, 2001. [DOI] [PubMed] [Google Scholar]
- 75. Revollo JR, Grimm AA, Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem 279: 50754–50763, 2004. [DOI] [PubMed] [Google Scholar]
- 76. Ripperger JA, Schibler U. Rhythmic CLOCK-BMAL1 binding to multiple E-box motifs drives circadian Dbp transcription and chromatin transitions. Nat Genet 38: 369–374, 2006. [DOI] [PubMed] [Google Scholar]
- 77. Rudic RD, McNamara P, Curtis AM, Boston RC, Panda S, Hogenesch JB, Fitzgerald GA. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol 2: e377, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Rudic RD, McNamara P, Reilly D, Grosser T, Curtis AM, Price TS, Panda S, Hogenesch JB, FitzGerald GA. Bioinformatic analysis of circadian gene oscillation in mouse aorta. Circulation 112: 2716–2724, 2005. [DOI] [PubMed] [Google Scholar]
- 79. Rutter J, Reick M, Wu LC, McKnight SL. Regulation of clock and NPAS2 DNA binding by the redox state of NAD cofactors. Science 293: 510–514, 2001. [DOI] [PubMed] [Google Scholar]
- 80. Sato TK, Panda S, Miraglia LJ, Reyes TM, Rudic RD, McNamara P, Naik KA, FitzGerald GA, Kay SA, Hogenesch JB. A functional genomics strategy reveals Rora as a component of the mammalian circadian clock. Neuron 43: 527–537, 2004. [DOI] [PubMed] [Google Scholar]
- 81. Scharf MT, Naidoo N, Zimmerman JE, Pack AI. The energy hypothesis of sleep revisited. Prog Neurobiol 86: 264–280, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Shearman LP, Jin X, Lee C, Reppert SM, Weaver DR. Targeted disruption of the mPer3 gene: subtle effects on circadian clock function. Mol Cell Biol 20: 6269–6275, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Shimba S, Ishii N, Ohta Y, Ohno T, Watabe Y, Hayashi M, Wada T, Aoyagi T, Tezuka M. Brain and muscle Arnt-like protein-1 (BMAL1), a component of the molecular clock, regulates adipogenesis. Proc Natl Acad Sci USA 102: 12071–12076, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Siepka SM, Yoo SH, Park J, Song W, Kumar V, Hu Y, Lee C, Takahashi JS. Circadian mutant Overtime reveals F-box protein FBXL3 regulation of cryptochrome and period gene expression. Cell 129: 1011–1023, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Spiegel K, Leproult R, L'Hermite-Baleriaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab 89: 5762–5771, 2004. [DOI] [PubMed] [Google Scholar]
- 86. Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet 354: 1435–1439, 1999. [DOI] [PubMed] [Google Scholar]
- 87. Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci USA 69: 1583–1586, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, Weitz CJ. Extensive and divergent circadian gene expression in liver and heart. Nature 417: 78–83, 2002. [DOI] [PubMed] [Google Scholar]
- 89. Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med 1: e62, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet 9: 764–775, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Tasali E, Mokhlesi B, Van Cauter E. Obstructive sleep apnea and type 2 diabetes: interacting epidemics. Chest 133: 496–506, 2008. [DOI] [PubMed] [Google Scholar]
- 92. Toh KL, Jones CR, He Y, Eide EJ, Hinz WA, Virshup DM, Ptacek LJ, Fu YH. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science 291: 1040–1043, 2001. [DOI] [PubMed] [Google Scholar]
- 93. Tsai JW, Hannibal J, Hagiwara G, Colas D, Ruppert E, Ruby NF, Heller HC, Franken P, Bourgin P. Melanopsin as a sleep modulator: circadian gating of the direct effects of light on sleep and altered sleep homeostasis in Opn4(-/-) mice. PLoS Biol 7: e1000125, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308: 1043–1045, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. van der Horst GT, Muijtjens M, Kobayashi K, Takano R, Kanno S, Takao M, de Wit J, Verkerk A, Eker AP, van Leenen D, Buijs R, Bootsma D, Hoeijmakers JH, Yasui A. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature 398: 627–630, 1999. [DOI] [PubMed] [Google Scholar]
- 96. van der Veen DR, Minh NL, Gos P, Arneric M, Gerkema MP, Schibler U. Impact of behavior on central and peripheral circadian clocks in the common vole Microtus arvalis, a mammal with ultradian rhythms. Proc Natl Acad Sci USA 103: 3393–3398, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Velasco A, Huerta I, Marin B. Plasma corticosterone, motor activity and metabolic circadian patterns in streptozotocin-induced diabetic rats. Chronobiol Int 5: 127–135, 1988. [DOI] [PubMed] [Google Scholar]
- 98. Vitaterna MH, King DP, Chang AM, Kornhauser JM, Lowrey PL, McDonald JD, Dove WF, Pinto LH, Turek FW, Takahashi JS. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science 264: 719–725, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Vitaterna MH, Selby CP, Todo T, Niwa H, Thompson C, Fruechte EM, Hitomi K, Thresher RJ, Ishikawa T, Miyazaki J, Takahashi JS, Sancar A. Differential regulation of mammalian period genes and circadian rhythmicity by cryptochromes 1 and 2. Proc Natl Acad Sci USA 96: 12114–12119, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wang J, Lazar MA. Bifunctional role of Rev-erbalpha in adipocyte differentiation. Mol Cell Biol 28: 2213–2220, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Welsh DK, Yoo SH, Liu AC, Takahashi JS, Kay SA. Bioluminescence imaging of individual fibroblasts reveals persistent, independently phased circadian rhythms of clock gene expression. Curr Biol 14: 2289–2295, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Wilsbacher LD, Yamazaki S, Herzog ED, Song EJ, Radcliffe LA, Abe M, Block G, Spitznagel E, Menaker M, Takahashi JS. Photic and circadian expression of luciferase in mPeriod1-luc transgenic mice in vivo. Proc Natl Acad Sci USA 99: 489–494, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science 288: 682–685, 2000. [DOI] [PubMed] [Google Scholar]
- 104. Yang S, Liu A, Weidenhammer A, Cooksey RC, McClain D, Kim MK, Aguilera G, Abel ED, Chung JH. The role of mPer2 clock gene in glucocorticoid and feeding rhythms. Endocrinology 150: 2153–2160, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Yang X, Downes M, Yu RT, Bookout AL, He W, Straume M, Mangelsdorf DJ, Evans RM. Nuclear receptor expression links the circadian clock to metabolism. Cell 126: 801–810, 2006. [DOI] [PubMed] [Google Scholar]
- 106. Yin L, Wu N, Curtin JC, Qatanani M, Szwergold NR, Reid RA, Waitt GM, Parks DJ, Pearce KH, Wisely GB, Lazar MA. Rev-erbalpha, a heme sensor that coordinates metabolic and circadian pathways. Science 318: 1786–1789, 2007. [DOI] [PubMed] [Google Scholar]
- 107. Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA 101: 5339–5346, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Zhao J, Kilman VL, Keegan KP, Peng Y, Emery P, Rosbash M, Allada R. Drosophila clock can generate ectopic circadian clocks. Cell 113: 755–766, 2003. [DOI] [PubMed] [Google Scholar]
- 109. Zheng B, Albrecht U, Kaasik K, Sage M, Lu W, Vaishnav S, Li Q, Sun ZS, Eichele G, Bradley A, Lee CC. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell 105: 683–694, 2001. [DOI] [PubMed] [Google Scholar]
- 110. Zvonic S, Ptitsyn AA, Conrad SA, Scott LK, Floyd ZE, Kilroy G, Wu X, Goh BC, Mynatt RL, Gimble JM. Characterization of peripheral circadian clocks in adipose tissues. Diabetes 55: 962–970, 2006. [DOI] [PubMed] [Google Scholar]
- 111. Zylka MJ, Shearman LP, Weaver DR, Reppert SM. Three period homologs in mammals: differential light responses in the suprachiasmatic circadian clock and oscillating transcripts outside of brain. Neuron 20: 1103–1110, 1998. [DOI] [PubMed] [Google Scholar]