Abstract
Despite recent advances in the design of hemoglobin (Hb)-based oxygen carriers (HBOCs), vasoconstriction, presumably caused by nitric oxide (NO) scavenging, vessel wall hyperoxygenation, and/or extravasation, has been identified as the principal road block hampering commercial development of HBOCs. This study was designed to analyze systemic and microvascular responses to the molecular mass and plasma concentration of tense (T)-state polymerized bovine Hb (PolybHb) solutions. Experiments were performed using the hamster window chamber model subjected to successive hypervolemic infusions of T-state PolybHb solutions. PolybHb plasma concentrations were evaluated, namely, 0.5, 1.0 and 1.5 g/dl, respectively. Infusion of PolybHb solutions with molecular mass >500 kDa elicited hypertension and vasoconstriction proportional to the plasma concentration and inversely proportional to the PolybHb cross-link density. However, two high-molecular mass PolybHb solutions, PolybHb(40:1)high PolybHb(50:1)high, did not elicit vasoconstriction at all concentrations studied, whereas PolybHb(50:1)high only elicited moderate hypertension at the highest concentration studied. In contrast, infusion of PolybHb solutions with molecular mass <500 kDa elicited significant hypertension and vasoconstriction compared with PolybHb solutions with molecular mass >500 kDa that was proportional to the plasma concentration and inversely proportional to the PolybHb cross-link density. We present promising results for highly cross-linked T-state PolybHb solutions with molecular mass >500 kDa [PolybHb(40:1)high PolybHb(50:1)high], which supports the concept that HBOC size/molecular mass influences its proximity to the vascular endothelium and molecular diffusivity. The hemodynamics of HBOC within the plasma layer surrounding the abluminal side endothelium regulates NO production and consumption, vessel oxygen flux, and extravasation. Although mechanistically attractive, neither of these hypotheses can be directly tested in vivo and will require further investigation.
Keywords: microcirculation, polymerized hemoglobin, blood substitute, hemoglobin-based oxygen carrier, functional capillary density, oxygen affinity, molecular weight, hypertension
currently, there is no hemoglobin (Hb)-based oxygen carrier (HBOC) approved for clinical use in the United States as a red blood cell (RBC) substitute (38). Worldwide, only two blood substitutes are clinically used, namely, Hemopure (Biopure, Cambridge, MA), a glutaraldehyde-polymerized bovine hemoglobin (PolybHb) in South Africa, and Peftoran (Peftoran, Moscow, Russia), a perfluorocarbon, in Russia (33). Therefore, the development of an effective RBC substitute that can maintain blood volume and deliver oxygen (O2) remains an area of national need with obvious applications in routine surgery, emergency combat care, and situations involving severe blood loss associated with trauma.
Recently tested HBOCs elicit vasoconstriction, an early event that occurs within minutes after infusion and is sustained for hours (1). Vasoconstriction is evident as a rapid rise in peripheral vascular resistance and blood pressure, commonly associated with bradycardia, and decreased cardiac output and affects perfusion to vital organs (7, 16, 23). Vasoconstriction is currently perceived to be the critical barrier hampering HBOC development (1, 37). Vasoconstriction appears to be directly linked to NO scavenging, an oversupply of O2 to the blood vessel wall, and extravasation. Regardless of the exact mechanism for vasoconstriction, the presence of acellular Hb in solution enables these various potential mechanisms of vasoconstriction (24).
These processes take place as NO and O2 traverse the RBC-free (or plasma) layer near the blood vascular-endothelium interface. NO and O2 diffusion across this interface take place due to an established concentration gradient. Therefore, the presence of acellular Hb in the RBC-free plasma layer scavenges NO, distorting the NO concentration field near the endothelial cell layer, and diverts NO from the smooth muscle. NO depletion deactivates smooth muscle guanylyl cyclase, inhibiting vascular relaxation (17). In the case of O2, the presence of acellular Hb in the RBC-free layer affects O2 transport in two ways, by augmenting the vessel wall Po2 gradient and the oxyhemoglobin (OxyHb) gradient, which therefore increases the net oxygen flux through the blood-tissue interface and causes oxygen-dependent vasoconstriction (3, 20, 26).
In this study, our aim was to determine whether systemic and microvascular vasoactive responses (hypertension, vasoconstriction, and hypoperfusion) to the infusion of tense (T)-state PolybHb are related to the molecular mass (hence, degree of polymerization) and plasma concentration of PolybHb. We hypothesized that by controlling the molecular mass of T-state PolybHb solutions, we could prevent and/or limit vasoconstriction. To investigate this hypothesis, we used the hamster window chamber model subjected to successive hypervolemic infusions of T-state PolybHb solutions with different molecular masses at 10 gHb/dl. The volume infused was calculated to reach a plasma concentration of 0.5, 1.0, and 1.5 gHb/dl, respectively.
METHODS
Materials.
Glutaraldehyde (70%), NaCl, KCl, NaOH, Na2S2O4, NaCl (USP), KCl (USP), CaCl2·2H2O (USP), NaOH (NF), sodium lactate (USP), N-acetyl-l-cysteine (USP), and NaBH4 were purchased from Sigma-Aldrich (Atlanta, GA). Sephadex G-25 resin was purchased from GE Healthcare (Piscataway, NJ). KCN, KFe(CN)6, and all other chemicals were purchased from Fisher Scientific (Pittsburgh, PA).
Hb purification.
Fresh bovine blood collected in 3.8% sodium citrate solution at a final concentration of 90:10 (vol/vol; bovine blood-sodium citrate solution) was purchased from Quad Five (Ryegate, MO). Bovine Hb (bHb) was purified from lysed bovine RBCs (bRBCs) via tangential flow filtration (13, 28). bRBCs were initially washed three times with 3 volumes of isotonic saline solution (0.9%) at 4°C. bRBCs were subsequently lysed on ice with 2 volumes of hypotonic, 3.75 mM phosphate buffer (PB) at pH 7.4 for 1 h. The lysate was then filtered through a glass chromatography column packed with glass wool to remove the majority of cell debris. Clarified bRBC lysate was then passed through 50-nm and 500-kDa hollow fiber cartridges (Spectrum Labs, Rancho Dominguez, CA) to remove additional cell debris and impurity proteins. Purified bHb was collected and concentrated on a 100-kDa hollow fiber cartridge (Spectrum Labs) to yield the precursor material for PolybHb synthesis.
Polymerization of Hb.
T-state PolybHb was synthesized according to the method previously described (27). To generate fully deoxygenated or T-state bHb, 30 g of purified bHb were diluted with PB (20 mM, pH 8.0) to yield 1,200 ml of bHb solution. The bHb solution was kept in a 2-liter glass bottle that was connected to a vacuum manifold and submerged in an ice water bath. The bHb solution was then subjected to several cycles of vacuum and argon (Ar) purging to remove the majority of O2 from the aqueous solution and the gas headspace of the bottle. After 4 h of vacuum and Ar cycling, Na2S2O4 solution (1.5 mg/ml) was titrated into the bHb solution, whereas the Po2 of the solution was simultaneously measured using a RapidLab 248 (Siemens, Malvern, PA) blood gas analyzer until the Po2 of the bHb solution attained a value of 0 mmHg. At this point, an additional 30 ml of 1.5 mg/ml Na2S2O4 solution was added to the T-state bHb solution to maintain the Po2 at 0 mmHg during and after the polymerization reaction. A 30-ml syringe with a luer lock was used to inject different amounts of glutaraldehyde preequilibrated with Ar into the sealed glass bottle under continuous stirring. The following molar ratios of glutaraldehyde to bHb were used in the polymerization reactions: 20:1, 30:1, 40:1, and 50:1.
The resulting solutions were allowed to react with glutaraldehyde at 37°C in a heated water bath for 2 h. At the end of this period, 20 ml of 2M NaBH4 in PB buffer (20 ml, pH 8.0) were injected into the glass bottle to quench the polymerization reaction. The polymerized bHb solution was then stored in the refrigerator at 4°C. The Po2 of the bHb solution before polymerization, after polymerization, and after quenching with NaBH4 was measured using a RapidLab 248 blood gas analyzer (Siemens). The Po2 of each PolybHb solution was 0 mmHg before and after polymerization and after quenching. These measurements verified that bHb was kept in the fully deoxygenated state (T state) during the entire polymerization process. All reactions were done in duplicate.
Separation of PolybHb solution.
Initially, each PolybHb solution was clarified by being passed through a glass chromatography column packed with glass wool to remove large particles. The glass wool was autoclaved at 250°C for 30 min (34) before being used to clarify the PolybHb solution, and all tubing, glassware, and plasticware were immersed in 1 M NaOH solution for more than 6 h to degrade any endotoxin present, followed by thorough rinsing with HPLC grade water (mean conductivity value 1.8 × 10−6 Ω−1·cm−1). The clarified PolybHb solution was then separated into two distinct molecular mass fractions with a 500-kDa hollow fiber cartridge (Spectrum Labs). The retentate mostly contained PolybHb molecules that were larger than 500 kDa, whereas the filtrate mostly contained PolybHb molecules that were smaller than 500 kDa. The filtrate was subsequently concentrated on a 100-kDa hollow fiber cartridge (Spectrum Labs). Therefore, two distinct molecular mass fractions of PolybHb were obtained after separation of each PolybHb mixture.
Buffer exchange of PolybHb solution.
After polymerization, PolybHb was suspended in PB buffer along with reduced glutaraldehyde and excess NaBH4. Glutaraldehyde and NaBH4 are cytotoxic (18); therefore, the PolybHb solution was buffer exchanged with a modified lactated Ringer solution [115 mM NaCl (USP), 4 mM KCl (USP), 1.4 mM CaCl2·2H2O (USP), 13 mM NaOH (NF), 27 mM sodium lactate (USP), and 2 g/l N-acetyl-l-cysteine (USP)]. The buffer exchange was conducted using an äKTA Explorer 100 system controlled by Unicorn 5.1 software (GE Healthcare). An XK 50/30 column (300-mm length, 50-mm inner diameter; GE Healthcare) was packed with 500 ml of Sephadex G-25 medium resin at room temperature. The column was balanced with modified lactated Ringer solution at a flow rate of 8 ml/min, and the PolybHb solution was injected into the XK 50/30 column via a superloop column (50 ml; GE Healthcare) at a flow rate of 5 ml/min. Sample (100 ml) was injected and then eluted with modified lactated Ringer solution. The protein concentration was detected at a wavelength of 280 nm, while the salt concentration was monitored with a conductivity detector. During the buffer exchange process, the UV signal increased as PolybHb eluted from the column, whereas the conductivity decreased when reduced glutaraldehyde and NaBH4 eluted from the column. The buffer-exchanged PolybHb solution was collected as the UV signal increased but before the conductivity signal decreased. The PolybHb fraction was concentrated with a 100-kDa hollow fiber cartridge (Spectrum Labs).
Methemoglobin level, protein concentration, and PolybHb yield.
The methemoglobin (MetHb) level of PolybHb solutions was measured via the cyanomethemoglobin method (10, 29). Total protein concentration was measured using the Bradford method (4) with the Coomassie Plus protein assay kit (Pierce Biotechnology, Rockford, IL). The yield of PolybHb was calculated using the following equation:
| (1) |
where MPolybHb is the mass of PolybHb (<500 kDa and >500 kDa) after reaction and separation, and MbHb is the total mass of bHb before polymerization.
Size exclusion chromatography coupled with multiangle static light scattering.
The absolute molecular mass distribution of PolybHb solutions was measured using a size exclusion chromatography (SEC) column (Ultrahydrogel linear column, 10 μm, 7.8 × 300 mm; Waters, Milford, MA) driven by a 1200 HPLC pump (Agilent, Santa Clara, CA) and controlled by Eclipse 2 software (Wyatt Technology, Santa Barbara, CA), connected in series to a DAWN Heleos (Wyatt Technology) light scattering photometer and an OptiLab Rex (Wyatt Technology) differential refractive index detector. The mobile phase consisted of 20 mM PB (pH 8.0), 100 parts per million Nan, and 0.2 M NaCl (Fisher Scientific) in HPLC grade water (mean conductivity value 1.8 × 10−6 Ω−1·cm−1) that was filtered through a 0.2-μm membrane filter. PolybHb solutions were diluted to 1 mg/ml with the mobile phase, and 60 all of the sample were injected into the column via a 1200 Auto sampler (Agilent). All data were collected and analyzed using Astra 5.3 software (Wyatt Technology).
SDS-PAGE.
The molecular mass distribution of PolybHb solutions was initially assessed via gel electrophoresis using a Mini-PROTEAN 3 Cell (Bio-Rad; Hercules, CA). All samples were mixed with an equal volume of sample buffer (Bio-Rad) containing 5% (vol/vol) β-mercaptoethanol and then boiled for 5 min. A 4% stacking gel with a 12% resolving gel was assembled on a minivertical gel apparatus, and each lane was loaded with 25 μg of protein. The gel was run at 120 V for ∼1 h. After electrophoresis, the gel was stained with Coomassie blue R250 (stain buffer; Bio-Rad) for 1 h and then destained with a buffer consisting of 10% acetic acid and 20% methanol. The gel was scanned on a Gel Doc XR (Bio-Rad) imaging system for further analysis.
O2-PolybHb equilibria.
The O2 affinity and cooperativity coefficients of PolybHb solutions were regressed from O2-PolybHb equilibrium curves measured on a Hemox analyzer (TCS Instruments, Southampton, PA) at 37°C. Samples were prepared by thoroughly mixing 100 μl of sample with 5 ml of Hemox buffer (pH 7.4; TCS Instruments), 20 μl of Additive-A, 10 μl of Additive-B, and 10 μl of anti-foaming agent. The PolybHb sample was allowed to equilibrate to a Po2 of 145 ± 2 mmHg using compressed air. After the sample was given sufficient time to equilibrate, the gas stream was switched to pure N2 to deoxygenate the Hb sample. The absorbance of OxyHb and deoxyhemoglobin in solution was recorded as a function of Po2 via dual-wavelength spectroscopy. O2-PolybHb equilibrium curves were fit to a four-parameter (A0, A∞, P50, n) Hill model (Eq. 2). In this model, A0 and A∞ represent the absorbance at 0 mmHg and full saturation, respectively. The cooperativity coefficient is represented by n, and the Po2 at which the Hb/PolybHb is half-saturated with O2 is represented by the O2 affinity, or P50.
| (2) |
Viscosity and colloid osmotic pressure.
The viscosity of PolybHb solutions was measured in a DV-II Plus rheometer (Brookfield Engineering Laboratories, Middleboro, MA) at a shear rate of 150 s−1, whereas the colloid osmotic pressure (COP) was measured using a 4420 colloid osmometer (Wescor, Logan, UT).
Animal preparation.
Investigations were performed in 55- to 65-g male Golden Syrian hamsters (Charles River Laboratories, Boston, MA) fitted with a dorsal skinfold window chamber. The hamster window chamber model is widely used for microvascular studies in the unanesthetized state. The complete surgical technique is described in detail elsewhere (8, 14). Arterial and venous catheters filled with a heparinized saline solution (30 IU/ml) were implanted into the carotid and jugular vessels. Catheters were tunneled under the skin, exteriorized at the dorsal side of the neck, and securely attached to the window frame. Animal handling and care followed the NIH Guide for the Care and Use of Laboratory Animals. The experimental protocol was approved by the local animal care committee.
Inclusion criteria.
The microvasculature was examined 3–4 days after the window implantation surgery, and only animals passing an established systemic and microcirculatory inclusion criteria were used. Animals were considered suitable for experiments if 1) systemic parameters were within normal range, namely, heart rate (HR) > 340 beats/min, mean arterial blood pressure (MAP) > 80 mmHg, systemic hematocrit (Hct) > 45%, and arterial O2 partial pressure (PaO2) > 50 mmHg; and 2) microscopic examination of the tissue in the chamber observed under ×650 magnification did not reveal signs of low perfusion, inflammation, edema, or bleeding.
Experimental setup.
The unanesthetized animal was placed in a restraining tube with a longitudinal slit from which the window chamber protruded and then fixed to the microscopic stage for transillumination with the intravital microscope (BX51WI; Olympus, New Hyde Park, NY). Animals were given 20 min to adjust to the tube environment before any measurements were made. The tissue image was projected onto a charge-coupled device camera (4815; COHU, San Diego, CA) connected to a videocassette recorder and viewed on a monitor. Measurements were carried out using a ×40 water-immersion objective (LUMPFL-WIR, numerical aperture 0.8; Olympus).
Test solutions.
PolybHb solution concentrations were adjusted to 10 gHb/dl when possible. PolybHb solutions were divided by the molecular mass fractions (<500 kDa and >500 kDa) and cross-link density (20:1, 30:1, 40:1, and 50:1), namely, <500 kDa: PolybHb(20:1)low, PolybHb(30:1)low, PolybHb(40:1)low, and PolybHb(50:1)low; and >500 kDa: PolybHb(20:1)high, PolybHb(30:1)high, PolybHb(40:1)high, and PolybHb(50:1)high. The biophysical properties of all PolybHb solutions are presented in Table 1.
Table 1.
Biophysical properties of PolybHb solutions
| PolyHb (10 g/dl) | Viscosity, cp | COP, mmHg | P50, mmHg | n | MetHb Level, % | Yield, % |
|---|---|---|---|---|---|---|
| <500 kDa | ||||||
| 20:1 | 1.4 | 50 | 34.6 | 1.2 | 2.5 | 48 |
| 30:1 | 1.4 | 48 | 28.6 | 1.0 | 2.0 | 29 |
| 40:1* | 1.0 | 18 | 27.7 | 0.8 | 3.6 | 4 |
| 50:1† | 1.0 | 18 | 23 | 0.8 | 2.8 | 3 |
| >500 kDa | ||||||
| 20:1 | 1.7 | 35 | 30.5 | 1.2 | 1.3 | 48 |
| 30:1 | 1.8 | 28 | 33.8 | 1.0 | 1.9 | 48 |
| 40:1 | 7.2 | 5 | 38.1 | 0.9 | 2.1 | 49 |
| 50:1 | 11.4 | 1 | 34.9 | 0.8 | 2.0 | 65 |
Values are biophysical properties of polymerized bovine hemoglobin (PolybHb) solutions, with concentrations adjusted as close as possible to 10g/dl, at low (<500 kDa) and high molecular mass (>500 kDa) and different cross-link densities (20:1, 30:1, 40:1, and 50:1). Viscosity was measured at a shear rate of 150 s−1; colloid osmotic pressure (COP) was measured at 25°C. P50, Po2 at which Hb/PolybHb is half-saturated with O2; n, cooperativity coefficient; MetHb, methemoglobin.
PolybHb (40:1)low concentration at 5.6 g/dl.
PolybHb (50:1)low concentration at 4.2 g/dl.
Hypervolemic infusion (top load) protocol.
Animals were randomly divided into eight experimental groups and assigned to a PolybHb solution. The first PolybHb infusion increased the plasma Hb concentration to 0.5 g/dl, the second PolybHb infusion targeted the plasma Hb concentration to 1.0 g/dl, and the third PolybHb infusion targeted the plasma Hb concentration to 1.5 g/dl. After each infusion, animals were allowed 20–30 min to stabilize before systemic and microvascular characterization. All infusions were completed intravenously at 100 μl/min. Infused volumes of PolybHb required to increase the plasma Hb concentration by 0.5 g/dl were estimated in each animal before each infusion, based on the animal blood volume (BV; estimated as 7% of body weight) and Hct, calculated as 0.05 × (1 − Hct) × BV; 5 min after infusion, the plasma Hb concentration was verified and increased if needed.
Experimental groups.
Experimental groups were labeled based on the type of PolybHb solution (see Test solutions). A total of 40 animals were entered into the study; all animals tolerated the entire protocol without visible signs of discomfort. Five animals were assigned to each experimental group.
Systemic parameters.
MAP and HR were recorded continuously (MP 150; Biopac System, Santa Barbara, CA). Hct was measured from centrifuged arterial blood samples taken in heparinized capillary tubes. Hb content was determined spectrophotometrically (B-Hemoglobin; Hemocue, Stockholm, Sweden).
Blood chemistry and biophysical properties.
Arterial blood was collected in heparinized glass capillaries (50 μl) and immediately analyzed for Po2, Pco2, base excess (BE), and pH (Rapidlab 248; Bayer, Norwood, MA). Blood samples for viscosity and COP measurements were withdrawn from the animal into a heparinized syringe at the end of the experiment. Blood viscosity was measured in a DV-II Plus rheometer (Brookfield Engineering Laboratories) at a shear rate of 150 s−1, and the COP of blood was measured using a 4420 colloid osmometer (Wescor).
Functional capillary density.
Functional capillaries, defined as those capillary segments that have RBC transit of at least a single RBC in a 45-s period in 10 successive microscopic fields, were assessed for a total region of 0.46 mm2. The relative change in functional capillary density (FCD) from baseline levels after each intervention is indicative of the extent of capillary perfusion (5).
Microhemodynamics.
Arteriolar and venular blood flow velocities were measured online by using the photodiode cross-correlation method (Photo Diode/Velocity Tracker model 102B; Vista Electronics, San Diego, CA). The measured centerline velocity (V) was corrected according to vessel size to obtain the mean RBC velocity (V/Rv), where Rv represents the ratio between vessel centerline velocity and vessel average blood velocity based on data obtained in glass tubes (22). According to Lipowsky and Zweifach (22), Rv = 1.6 for vessels between 15 and 90 μm in diameter, but not for larger vessels. A video image-shearing method was used to measure vessel diameter (D) (19). Blood flow (Q) was calculated from measured values as Q = π × (V/Rv)(D/2)2.
Data analysis.
Tabular results are means ± SD. Data within each group were analyzed using analysis of variance for repeated measurements (ANOVA, Kruskal-Wallis test). When appropriate, post hoc analyses were performed with the Dunn's multiple comparison test. Comparison between PolybHb solutions with different cross-link densities was analyzed using two-way ANOVA (Hb plasma concentration and cross-link density); post hoc analyses were performed with the Bonferroni post tests. Microhemodynamic data are presented as absolute values and ratios relative to baseline values. A ratio of 1.0 signifies no change from baseline, whereas lower and higher ratios are indicative of changes proportionally lower and higher than baseline (i.e., 1.5 would mean a 50% increase from the baseline level). The same vessels and capillary fields were followed so that direct comparisons to their baseline levels could be performed, allowing for more robust statistics on small sample populations. All statistics were calculated using GraphPad Prism 4.01 (GraphPad Software, San Diego, CA). Changes were considered statistically significant if P < 0.05.
RESULTS
MetHb level and yield of PolybHb solutions.
bHb was polymerized with glutaraldehyde at four different cross-link densities in the T state: 20:1, 30:1, 40:1, and 50:1. Subsequently, each PolybHb mixture was separated into two distinct molecular mass fractions: <500 kDa and >500 kDa. The biophysical properties of all eight PolybHb solutions used in this study are presented in Table 1. All PolybHb solutions exhibited low MetHb levels (<5%; Table 1). However, the four PolybHb solutions <500 kDa in molecular mass exhibited higher MetHb levels compared with the four PolybHb solutions >500 kDa in molecular mass. The yield of PolybHb solutions <500 kDa in molecular mass decreased as the cross-link density increased from 20:1 to 50:1. Conversely, the yield of PolybHb fractions >500 kDa in molecular mass increased as the cross-link density increased from 20:1 to 50:1.
Viscosity and COP of PolybHb solutions.
The solution viscosity and COP of fractionated PolybHb solutions is presented in Table 1. PolybHb fractions >500 kDa exhibited increased solution viscosity and decreased COP as the cross-link density increased. However, PolybHb fractions <500 kDa exhibited similar solution viscosities independent of cross-link density, whereas the COP decreased as the cross-link density increased.
P50 and cooperativity coefficient of PolybHb solutions.
The regressed P50 and cooperativity coefficient n of fractionated PolybHb solutions are presented in Table 1. Polymerization of bHb in the T-state resulted in a left shift in the O2-PolybHb equilibrium curve. The P50 of PolybHb solutions <500 kDa decreased slightly as the cross-link density increased. However, the P50 of PolybHb solutions >500 kDa was independent of cross-link density. The cooperativity of PolybHb solutions above and below 500 kDa decreased with increasing cross-link density. It also should be noted that PolybHb fractions above and below 500 kDa separated from the same reaction mixture possessed similar cooperativity coefficients.
Molecular mass distribution of PolybHb solutions.
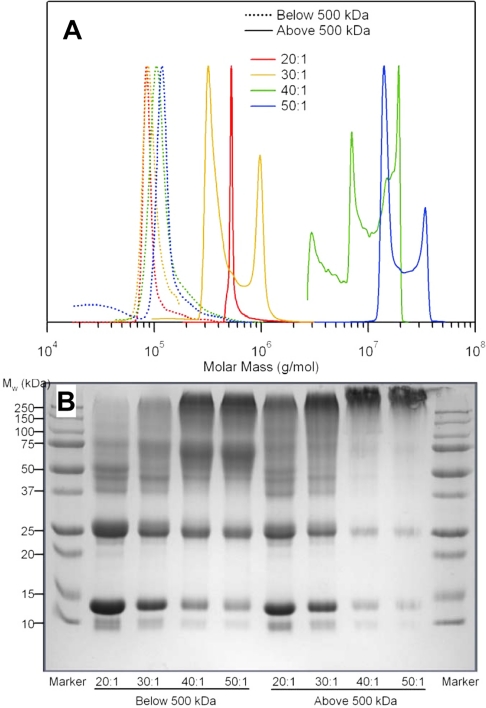
The molecular mass distribution of fractionated PolybHb solutions is presented in Fig. 1A. For PolybHb fractions with molecular mass <500 kDa, the molecular mass distribution did not increase significantly at cross-link densities ranging from 20:1 to 50:1. However, all PolybHb fractions were larger than that of a Hb tetramer. On the other hand, PolybHb fractions >500 kDa in molecular mass exhibited an increase in the molecular mass distribution as the cross-link density increased from 20:1 to 50:1, as reported previously (11, 12, 27, 40). In addition, these fractions did not show the presence of tetrameric Hb.
Fig. 1.
Molecular mass distribution (A) and SDS-PAGE (B) of low- and high-molecular-mass T-state polymerized bovine Hb (PolybHb) fractions. A: molecular mass distribution of low- and high-molecular-mass PolybHb fractions. Dotted lines represent PolybHb fractions <500 kDa in molecular mass; solid lines denote PolybHb fractions >500 kDa in molecular mass. B: SDS-PAGE of fractionated PolybHb solutions. The first and last lanes represent protein molecular mass markers, and eight sample lanes were loaded with PolybHb. The left lanes were loaded with PolybHb fractions <500 kDa in molecular mass, whereas the right lanes were loaded with PolybHb fractions >500 kDa in molecular mass. Cross-link densities ranged from 20:1 to 50:1.
SDS-PAGE of PolybHb solutions.
Figure 1B shows the SDS-PAGE of fractionated PolybHb solutions. PolybHb fractions <500 kDa at cross-link densities of 20:1 and 30:1 showed strong bands around 15 kDa, which indicates the presence of a significant fraction of unpolymerized bHb. Conversely, all 40:1 and 50:1 PolybHb solutions showed very weak bands around 15 kDa, suggesting only trace amounts of unpolymerized bHb.
Systemic parameters.
All groups were statistically similar (P > 0.40) in systemic and microcirculation parameters at baseline. Absolute values for MAP, HR, Hct, Hb, and body weight at baseline are presented in Table 2. For each PolybHb solution, the plasma Hb concentration, volume infused, Hct, total Hb, and plasma Hb levels are also presented in Table 2. The volumes of infused PolybHb(40:1)low and PolybHb(50:1)low were larger than other PolybHb solutions because of their lower concentrations. Consequently, the Hct seems to decrease more in these two groups, although no statistical significance was observed.
Table 2.
Absolute values for MAP, HR, Hct, Hb, and body weight at baseline
| <500 kDa |
>500 kDa |
|||||||
|---|---|---|---|---|---|---|---|---|
| 20:1 PolybHb(20:1)low | 30:1 PolybHb(30:1)low | 40:1 PolybHb(40:1)low | 50:1 PolybHb(50:1)low | 20:1 PolybHb(20:1)high | 30:1 PolybHb(30:1)high | 40:1 PolybHb(40:1)high | 50:1 PolybHb(50:1)high | |
| Baseline | ||||||||
| n | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| MAP, mmHg | 110±9 | 113±7 | 107±8 | 111±9 | 107±8 | 110±8 | 108±10 | 112±8 |
| HR, beats/min | 424±22 | 427±18 | 431±23 | 423±19 | 430±24 | 437±25 | 422±24 | 440±24 |
| Hct, % | 49±2 | 48±2 | 48±2 | 49±1 | 49±2 | 48±2 | 49±2 | 48±1 |
| Hb, g/dl | 15.2±0.8 | 14.8±0.7 | 15.1±1.0 | 15.2±0.5 | 15.0±0.8 | 14.7±0.6 | 14.9±0.8 | 14.7±0.8 |
| Body weight, g | 67.6±4.9 | 65.9±4.6 | 68.4±6.4 | 67.3±6.3 | 66.1±4.7 | 64.4±4.1 | 63.4±4.2 | 68.1±3.8 |
| Plasma concentration 0.5 g/dl | ||||||||
| Volume infused, ml | 0.13±0.06 | 0.13±0.04 | 0.23±0.08* | 0.30±0.14* | 0.13±0.05 | 0.12±0.06 | 0.12±0.03 | 0.13±0.05 |
| Hct, % | 46±1 | 46±2 | 45±2 | 47±1 | 47±1 | 47±2 | 48±1 | 48±1 |
| HbTotal, g/dl | 14.8±0.7 | 14.6±0.6 | 14.7±0.8 | 14.6±0.6 | 14.7±0.7 | 14.6±0.5 | 14.8±0.6 | 14.8±0.6 |
| Hbplasma, g/dl | 0.4±0.1 | 0.5±0.1 | 0.4±0.1 | 0.5±0.2 | 0.5±0.1 | 0.4±0.1 | 0.5±0.1 | 0.5±0.1 |
| Plasma concentration 1.0 g/dl | ||||||||
| Volume infused, ml | 0.28±0.11 | 0.27±0.09 | 0.47±0.11* | 0.62±0.21* | 0.26±0.09 | 0.26±0.07 | 0.25±0.09 | 0.26±0.09 |
| Hct, % | 43±2 | 43±2 | 42±2 | 42±1 | 45±2 | 43±2 | 46±1 | 46±1 |
| HbTotal, g/dl | 14.7±0.6 | 14.8±0.7 | 15.2±0.7 | 15.1±0.5 | 15.0±0.6 | 14.8±0.6 | 15.2±0.5 | 15.3±0.6 |
| Hbplasma, g/dl | 0.9±0.1 | 1.0±0.1 | 0.9±0.1 | 0.9±0.1 | 0.9±0.1 | 0.9±0.1 | 1.0±0.1 | 0.9±0.1 |
| Plasma concentration 1.5 g/dl | ||||||||
| Volume infused, ml | 0.42±0.12 | 0.40±0.10 | 0.72±0.17* | 0.92±0.22* | 0.45±0.16 | 0.49±0.15 | 0.40±0.09 | 0.41±0.09 |
| Hct, % | 43±1 | 43±1 | 40±2 | 40±1 | 45±1 | 43±1 | 45±1 | 45±1 |
| HbTotal, g/dl | 15.3±0.5 | 15.3±0.4 | 15.3±0.5 | 15.6±0.4 | 15.8±0.5 | 15.7±0.6 | 15.9±0.5 | 16.0±0.5 |
| Hbplasma, g/dl | 1.4±0.2 | 1.4±0.1 | 1.4±0.1 | 1.5±0.2 | 1.4±0.1 | 1.4±0.2 | 1.5±0.1 | 1.5±0.1 |
| Blood viscosity, cp | 3.7±0.2 | 3.7±0.2 | 3.5±0.2 | 3.5±0.1 | 3.8±0.1 | 3.8±0.2 | 4.4±0.2 | 5.0±0.3 |
| Plasma viscosity, cp | 1.1±0.1 | 1.1±0.1 | 1.2±0.1 | 1.2±0.1 | 1.1±0.2 | 1.2±0.1 | 1.6±0.2* | 2.0±0.2* |
Values are means ± SE for mean arterial blood pressure (MAP), heart rate (HR), hematocrit (Hct), hemoglobin (Hb), and body weight at baseline and various PolybHb plasma concentrations; n = no. of animals per group.
P < 0.05 compared with 20:1 and 30:1.
Changes in MAP.
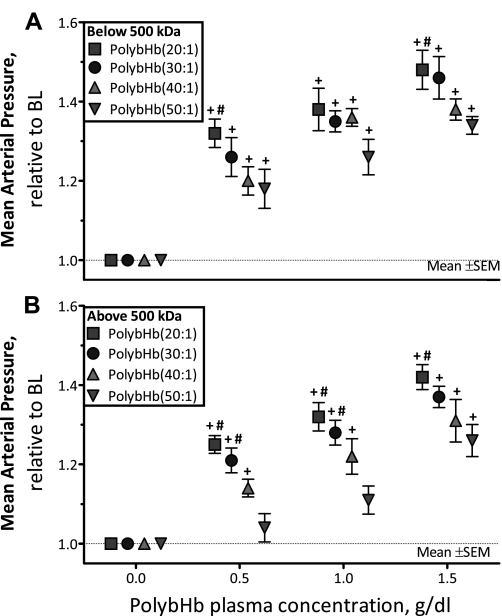
The MAP after infusion of PolybHb solutions is presented in Fig. 2: fractions <500 kDa are shown in Fig. 2A, and fractions >500 kDa are shown in Fig. 2B. PolybHb solutions below and above 500 kDa in molecular mass, independent of the degree of polymerization, showed increased hypertension that was proportional to the plasma concentration. For both PolybHb solutions below and above 500 kDa in molecular mass at a fixed plasma concentration of PolybHb, the MAP decreased with increasing cross-link density (degree of polymerization). In addition, at both a fixed plasma concentration and cross-link density of PolybHb, the PolybHb fraction <500 kDa exhibited a higher MAP compared with the PolybHb fraction >500 kDa. PolybHb(20:1)high, PolybHb(30:1)high, and PolybHb(40:1)high increased MAP from baseline at a plasma concentration of 0.5 g/dl (P < 0.05). PolybHb(50:1)high increased MAP from baseline at a plasma concentration of 1.5g/dl (P < 0.05). PolybHb(20:1)high was significantly more hypertensive compared with PolybHb(50:1)high at all concentrations tested (P < 0.05). PolybHb(30:1)high was significantly more hypertensive compared with PolybHb(50:1)high at 0.5 and 1.0 g/dl (P < 0.05).
Fig. 2.
Relative changes in mean arterial pressure (MAP) from baseline after infusion of PolybHb. A: PolybHb fractions with molecular mass <500 kDa. Baseline MAP values (mmHg) for each group were as follows: PolybHb(20:1), 110 ± 9; PolybHb(30:1), 113 ± 7; PolybHb(40:1), 107 ± 8; PolybHb(50:1), 111 ± 9. B: PolybHb fractions with molecular mass >500 kDa. Baseline MAP values (mmHg) for each group were as follows: PolybHb(20:1), 107 ± 8; PolybHb(30:1), 110 ± 8; PolybHb(40:1), 108 ± 10; PolybHb(50:1), 112 ± 8. BL, baseline level (broken line). Values are means ± SD. †P < 0.05 relative to baseline. #P < 0.05 compared with PolybHb(50:1).
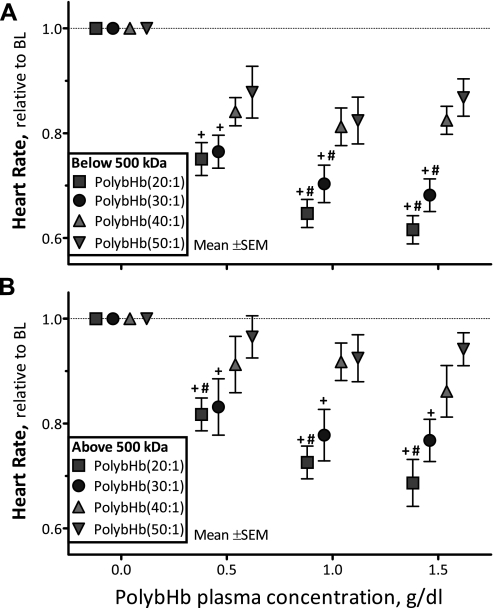
Changes in HR.
The HR after infusion of PolybHb solutions is presented in Fig. 3. PolybHb(20:1)low and PolybHb(30:1)low significantly decreased HR from baseline at all concentrations evaluated (P < 0.05); at 1.0 and 1.5 g/dl, they also induced significant bradycardia compared with PolybHb(50:1)low (P < 0.05; Fig. 3A). PolybHb(20:1)high and PolybHb(30:1)high significantly decreased HR from baseline (P < 0.05), whereas PolybHb(20:1)high induced significant bradycardia compared with PolybHb(50:1)high (P < 0.05; Fig. 3B). Conversely, PolybHb(40:1)high and PolybHb(50:1)high did not affect HR.
Fig. 3.
Relative changes from baseline in heart rate (HR) after infusion of PolybHb. A: PolybHb fractions with molecular mass <500 kDa. Baseline HR values (beats/min) for each group were as follows: PolybHb(20:1), 424 ± 22; PolybHb(30:1), 427 ± 18; PolybHb(40:1), 431 ± 23; PolybHb(50:1), 423 ± 19. B: PolybHb fractions with molecular mass >500 kDa. Baseline HR values (beats/min) for each group were as follows: PolybHb(20:1), 430 ± 24; PolybHb(30:1), 437 ± 25; PolybHb(40:1), 422 ± 24; PolybHb(50:1), 440 ± 24. Broken line represents baseline level. Values are means ± SD. †P < 0.05 relative to baseline. #P < 0.05 compared with PolybHb(50:1).
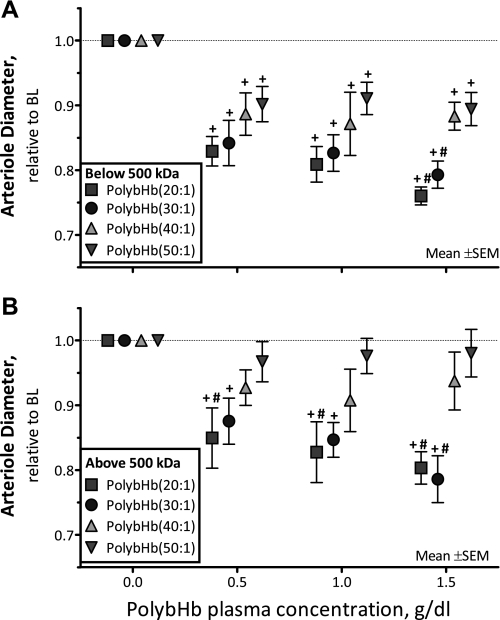
Changes in arteriolar diameter.
The arteriole diameter after infusion of PolybHb solutions is presented in Fig. 4: fractions <500 kDa are shown in Fig. 4A, and fractions >500 kDa are shown in Fig. 4B. PolybHb(20:1)low and PolybHb(30:1)low induced more vasoconstriction than PolybHb(50:1)low at 1.5 g/dl (P < 0.05). PolybHb(20:1)high and PolybHb(30:1)high decreased arteriolar diameters from baseline (P < 0.05; Fig. 4B). PolybHb(20:1)high induced significant vasoconstriction compared with PolybHb(50:1)high at all concentrations tested (P < 0.05). Conversely, PolybHb(40:1)high and PolybHb(50:1)high did not elicit arteriolar vasoconstriction.
Fig. 4.
Relative changes from baseline in arteriolar diameter after infusion of PolybHb. A: PolybHb fractions with molecular mass <500 kDa. Baseline arteriolar diameters (μm) for each group were as follows: PolybHb(20:1), 68 ± 12 (n = 28); PolybHb(30:1), 66 ± 10 (n = 31); PolybHb(40:1), 65 ± 11 (n = 26); PolybHb(50:1), 64 ± 8 (n = 25). B: PolybHb fractions with molecular mass >500 kDa. Baseline arteriolar diameters (μm) for each group were as follows: PolybHb(20:1), 67 ± 9 (n = 26); PolybHb(30:1), 68 ± 10 (n = 24); PolybHb(40:1), 64 ± 11 (n = 28); PolybHb(50:1), 66 ± 7 (n = 23). Broken line represents baseline level. Values are means ± SD; n = no. of examined vessels. †P < 0.05 relative to baseline. #P < 0.05 compared with PolybHb(50:1).
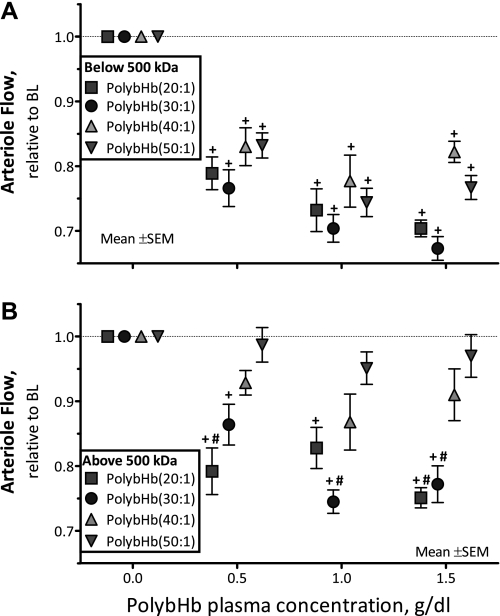
Changes in arteriolar blood flow.
Microvascular blood flow after infusion of PolybHb solutions is presented in Fig. 5. All PolybHb solutions <500 kDa significantly decreased arteriolar flows from baseline at all concentrations evaluated (P < 0.05; Fig. 5A). PolybHb(20:1)high and PolybHb(30:1)high decreased arteriolar blood flow from baseline (P < 0.05). PolybHb(20:1)high decreased arteriole blood flow compared with PolybHb(50:1)high at all concentrations evaluated (P < 0.05; Fig. 5B). At a plasma concentration of 1.5 g/dl, PolybHb(30:1)high induced a significant decrease in arteriolar blood flow compared with PolybHb(50:1)high. Conversely, PolybHb(40:1)high and PolybHb(50:1)high did not decrease arteriolar blood flow compared with baseline.
Fig. 5.
Relative changes from baseline in arteriolar blood flow after infusion of PolybHb. A: PolybHb fractions with molecular mass <500 kDa. Baseline arteriolar blood flow values (nl/s) for each group were as follows: PolybHb(20:1), 8.9 ± 3.2; PolybHb(30:1), 8.2 ± 3.8; PolybHb(40:1), 8.0 ± 4.2; PolybHb(50:1), 7.7 ± 2.9. B: PolybHb fractions with molecular mass >500 kDa. Baseline arteriolar blood flow values (nl/s) for each group were as follows: PolybHb(20:1), 8.5 ± 3.9; PolybHb(30:1), 8.5 ± 4.5; PolybHb(40:1), 7.1 ± 3.6; PolybHb(50:1), 8.3 ± 3.4. Broken line represents baseline level. Values are means ± SD. †P < 0.05 relative to baseline. #P < 0.05 compared with PolybHb(50:1).
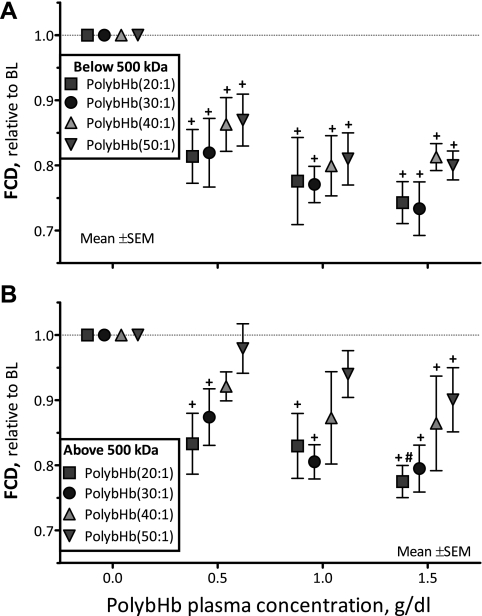
Changes in FCD.
The FCD after infusion of PolybHb solutions is presented in Fig. 6. At all concentrations evaluated, all PolybHb solutions <500 kDa significantly decreased FCD from baseline (P < 0.05; Fig. 6A). Similarly, PolybHb(20:1)high and PolybHb(30:1)high decreased FCD from baseline at all tested concentrations (P < 0.05; Fig. 6B). At a plasma concentration of 1.5 g/dl, PolybHb(20:1)high induced a significant decrease in FCD compared with PolybHb(50:1)high. At plasma concentrations of 0.5 and 1.0 g/dl, PolybHb(40:1)high and PolybHb(50:1)high did not decrease FCD compared with baseline, although at 1.5 g/dl, they decreased FCD significantly from baseline (P < 0.05).
Fig. 6.
Relative changes from baseline in functional capillary density (FCD) after infusion of PolybHb. A: PolybHb fractions with molecular mass <500 kDa. Baseline FCD values (cm−1) for each group were as follows: PolybHb(20:1), 112 ± 10; PolybHb(30:1), 104 ± 7; PolybHb(40:1), 98 ± 8; PolybHb(50:1), 102 ± 11. B: PolybHb fractions with molecular mass >500 kDa. Baseline FCD values (cm−1) for each group were as follows: PolybHb(20:1), 102 ± 11; PolybHb(30:1), 112 ± 9; PolybHb(40:1), 116 ± 11; PolybHb(50:1), 109 ± 14. Broken line represents the baseline level. †P < 0.05 relative to baseline. #P < 0.05 compared with PolybHb(50:1).
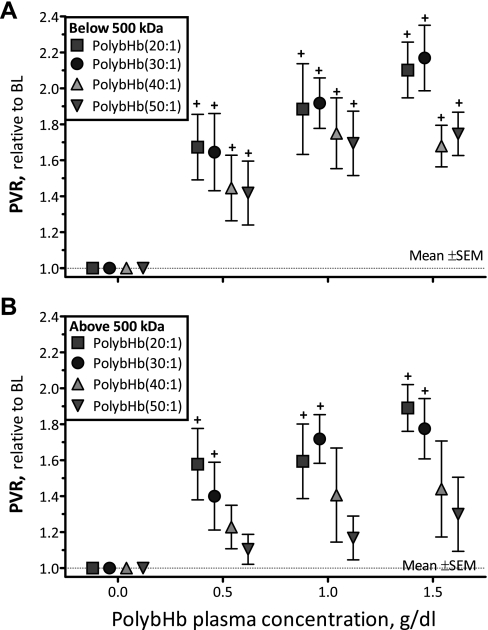
Changes in peripheral vascular resistance.
The peripheral vascular resistance (PVR) was calculated as the ratio between MAP and arteriolar blood flow. Figure 7 presents changes in PVR after infusion of PolybHb solutions. At all concentrations evaluated, all PolybHb solutions <500 kDa significantly increased PVR from baseline (P < 0.05; Fig. 7A). Similarly, PolybHb(20:1)high and PolybHb(30:1)high increased PVR from baseline at all tested concentrations (P < 0.05; Fig. 7B). Conversely, PolybHb(40:1)high and PolybHb(50:1)high did not increase PVR compared with baseline.
Fig. 7.
Relative changes from baseline in calculated peripheral vascular resistance (PVR) after infusion of PolybHb. A: PolybHb fractions with molecular mass <500 kDa. B: PolybHb fractions with molecular mass >500 kDa. Broken line represents baseline level. †P < 0.05 relative to baseline. #P < 0.05 compared with PolybHb(50:1).
DISCUSSION
Biophysical properties of T-state PolybHb solutions.
The goal of this study was examine the role PolybHb MW plays in regulating vasoconstriction. We hypothesized that by controlling the molecular mass of PolybHb solutions, we could control vasoconstriction. To test our hypothesis, we synthesized four T-state PolybHb solutions with different cross-link densities (20:1, 30:1, 40:1, and 50:1) and separated each PolybHb mixture into two distinct molecular mass fractions (<500 kDa and >500 kDa) for in vivo evaluation of systemic and microcirculatory parameters.
To minimize oxidation of the heme, bHb was polymerized in an anoxic environment (Po2 = 0 mmHg) and quenched with the strong reducing agent NaBH4. As a further precaution against auto-oxidation of the heme, fractionated PolybHb solutions were buffer exchanged against modified lactated Ringer solution containing N-acetyl-l-cysteine, an antioxidant (41), which limits heme oxidation. Compared with the high-molecular-mass PolybHb fraction, the low-molecular-mass PolybHb fraction displayed higher MetHb levels. This result is expected. Since free tetrameric Hb exists in equilibrium with αβ dimers (2), the low-molecular-mass PolybHb fraction contains more dimers in solution, which are more prone to oxidation compared with their tetrameric counterpart. This gives rise to the higher MetHb levels of low-molecular-mass PolybHbs vs. high-molecular-mass PolybHbs.
As the degree of polymerization increased, the overall yield of PolybHb decreased from its maximum value of 100%. This was partly due to the difficulty in passing the high-viscosity PolybHb solutions through the G-25 buffer exchange column. This phenomenon was more pronounced for the high-molecular-mass 40:1 and 50:1 PolybHb solutions, which resulted in a portion of the eluted sample being lost during the buffer-exchange process. It also is important to keep in mind that large PolybHb particles were removed during clarification of the PolybHb reaction mixture, postreaction, which also contributed to the decrease in overall yield of PolybHb, especially for the 40:1 and 50:1 polymerization reactions. Hence, a significant portion of PolybHb was lost during the clarification and buffer-exchange steps at the two highest PolybHb cross-link densities studied.
At low cross-link densities (20:1 and 30:1), the molecular mass distribution shows that the PolybHb fractions were polymerized primarily by intramolecular cross-links with a very minor portion possessing intermolecular cross-links. Under these conditions, the intramolecular cross-linking sites on the bHb molecule are not fully saturated with cross-linker. On the other hand, at higher cross-link densities (40:1 and 50:1), it is likely that the majority of intramolecular cross-linking sites were saturated, which facilitates subsequent intermolecular cross-linking between adjacent Hb tetramers in solution. This is supported by the observation that both the 40:1 and 50:1 high-molecular-mass PolybHb fractions showed a strong band in the SDS-PAGE above 250 kDa and an insignificant band at 15 kDa (Fig. 1B).
The polymerization process yields larger sized particles that can interact strongly with each other, especially when the PolybHb solution is concentrated via tangential flow filtration. Hence, polymerization of bHb yields high-molecular-mass fractions that display an increase in viscosity with increasing cross-link density. The low-molecular-mass fractions do not display such a trend, because the polymerized species are much smaller in size and do not interact as strongly with each other, and as the cross-link density increases, there is very little PolybHb in solution. Conversely, the COP is inversely related to particle size. Therefore, all PolybHb fractions <500 kDa in molecular mass exhibited similar COP values. However, the COP decreased as the cross-link density increased for PolybHb fractions >500 kDa in molecular mass due to the concomitant increase in molecular mass of the PolybHb molecules with increasing cross-link density.
After curve fitting of the O2-PolybHb equilibrium data to the Hill equation, the fractional saturation of the PolybHbs at a Po2 of 145 mmHg varied among the different fractionated samples. Before polymerization, the Po2 of the initial bHb solution was maintained at 0 mmHg. Therefore, all bHb molecules in solution were maintained in the low oxygen affinity T-state. After polymerization of T-state bHb, the reducing agent NaBH4 was added to the reaction to quench any remaining glutaraldehyde in solution, as well as to reduce the resulting Schiff bases. Hence, T-state PolybHb is frozen in the low oxygen affinity state via intra- and intermolecular glutaraldehyde cross-links within the bHb tetramer and between tetramers. For T-state PolybHb solutions >500 kDa, the P50 values were much higher than that of unmodified bHb (22.4–27.0 mmHg) (28) and ranged from 31.1 to 37.3 mmHg across cross-link densities ranging from 20:1 to 50:1, which was very close to the reported value of 38 mmHg for Hemopure (glutaraldehyde-cross-linked bovine Hb manufactured by Biopure) (31). The cooperativity of all PolybHb solutions is much less compared with the reported value for unmodified bHb (2.2–3.1) (28). This is caused by the glutaraldehyde cross-links present within and between the globin chains, which restrict transmission of any quaternary changes in the Hb structure to other neighboring globin chains within the Hb tetramer. This results in a significant loss of cooperative binding of O2 molecules to the Hb tetramer.
In vivo response of T-state PolybHb solutions.
The principal finding of this study is that infusion of PolybHb with molecular mass >500 kDa and higher cross-link densities [PolybHb(40:1)high, PolybHb(50:1)high] produced no vasoconstriction and less hypertension compared with those with lower cross-link densities [PolybHb(20:1)high, PolybHb(30:1)high]. In addition, infusion of PolybHb with molecular mass <500 kDa induced vasoconstriction and hypertension that was inversely proportional to the cross-link density. The plasma concentration after infusion determined the degree of vasoconstriction and hypertension induced by PolybHb solutions. Nonvasoactive PolybHb solutions [PolybHb(40:1)high, PolybHb(50:1)high] seemed to have lower plasma Hb concentration-dependent responses, i.e., arteriole diameter and flow. The observed increase in PVR after infusion of PolybHb solutions with molecular mass <500 kDa decreased the transmission of perfusion pressure to the microcirculation. The principal microvascular complication associated with low-molecular-mass PolybHb-induced vasoconstriction included a significant decrease in FCD (capillaries perfused with RBCs), a critical parameter that ensures tissue homeostasis. An important difference in PolybHb solutions with high cross-link densities and molecular mass >500 kDa is their ability to preserve microvascular perfusion even at high plasma Hb concentrations compared with PolybHb solutions with molecular mass <500 kDa.
The molecular mass and cross-link density are critical biophysical characteristics of PolybHb molecules, which directly determine viscosity, COP, and oxygen transport properties (P50 and cooperativity) and indirectly affect hypertension and vasoconstriction when infused in the circulation. The mechanism of this hypertensive effect is not completely understood and may result from more than one mechanism; our data suggest that it depends on the diffusion coefficient (size/molecular mass) and viscosity of the PolybHb in the intravascular space, which impacts NO scavenging and the facilitated transport of oxygen. Similar results were observed with PolybHb synthesized under aerobic conditions with fractions >100 kDa and cross-linking densities ranging from 5:1 to 16:1 (40). This previous study found that the extent of hypertension after infusion decreased with increasing cross-link density and decreasing tetrameric Hb content. However, the polymerization reactions were quenched with lysine, which reacts with free aldehyde groups without reducing Schiff bases in solution. Therefore, it is possible for these PolybHb preparations to degrade into free glutaraldehyde and tetrameric Hb. If this occurs, these PolybHb solutions would be highly toxic. In the present study, we have reduced Schiff bases in solution with the strong reducing agent NaBH4. This ensures that our PolybHb preparations do not degrade into free Hb and glutaraldehyde.
The size/molecular mass of the PolybHb molecule has a direct impact on its proximity to the vascular endothelium and its molecular diffusivity. Therefore, NO scavenging and vessel wall oxygen oversupply are key components to the induction of vasoconstriction. In the absence of acellular Hb in the circulation, the plasma layer adjacent to the arteriole wall regulates NO scavenging by the erythrocytes; however, after infusion of PolybHb, it becomes a sink for NO. The paradox surrounding how NO can escape PolybHb scavenging and function as the endothelium-derived relaxation factor can partially be explained by the presence of the endothelial glycocalyx. This unstirred layer surrounding the abluminal side endothelium can potentially decreases the overall NO uptake rate by PolybHb molecules. The endothelial glycocalyx was first described by Vink and Duling (36), who reported a thickness ranging from 0.4 to 0.5 μm using combined fluorescence and bright-field intravital microscopy. They found that the permeability of the intact endothelial glycocalyx to molecules depends on the size and charge of the molecule (35, 36). In our previous study (15), we showed that the intact endothelial glycocalyx is impermeable to macromolecules larger than 70 kDa and that it becomes permeable when its integrity is compromised. Similar results have been published by many research groups, through different challenges from various disease states (e.g., diabetes), oxidative stress, and target specific degradation (25). Direct in vivo evidence of NO scavenging by acellular Hb is now available, showing that the inferred NO scavenging from perivascular NO measurements is similar for a wide variety of acellular Hbs (7).
It should be noted that an additional mechanism for the vasopressor effect of acellular Hbs is enhanced by premature oxygen delivery to the resistance arterioles (24, 39). In the absence of acellular Hb, the rate of oxygen delivery in blood is limited by the diffusion of dissolved oxygen and the low solubility of oxygen in plasma. Premature delivery of oxygen in the presence of acellular Hbs is linked to the ability of OxyHb to freely diffuse through the plasma space, providing an additional pathway for oxygen transport to the tissues. In terms of Fick's law, the total oxygen flux of a solution containing acellular Hb adds facilitated diffusion to the dissolved oxygen flux (24). Reduction of the total oxygen flux can be obtained by chemically modified Hb, since the facilitated diffusion decreases by increasing molecular size. Increasing oxygen affinity also limits the magnitude of the gradient of OxyHb under high-Po2 conditions. The effect of HBOC molecular size on vasoactivity has been documented in the microcirculation (32). In this context, the properties of large PolybHb molecules are anticipated to attenuate the rate of oxygen transport from the central vessel RBC column to the vascular wall across the plasma layer.
PolybHb(20:1) and PolybHb(30:1) solutions showed strong bands in the SDS-PAGE, close to 15 kDa in molecular mass, which indicates the presence of a significant fraction of unpolymerized Hb. This can be explained by the low cross-link density, which is insufficient to completely polymerize the molecules of bHb. Under these conditions, the intramolecular cross-linking sites on the Hb tetramer are not fully saturated. For PolybHb(40:1) and PolybHb(50:1) solutions, there are very weak bands in the SDS-PAGE close to 15 kDa in molecular mass, which demonstrates that the higher cross-link density guarantees polymerization of Hb tetramers by both inter- and intramolecular cross-links. Extravasation of Hb monomers and dimers and their subsequent intercalation between the endothelium and the smooth muscle presumably acts as a sink for NO. However, the extravasation process is not clear, since the quantities of Hb molecules that are able to fit within the interstitial space between the endothelium and smooth muscle would be extremely small compared with the volume in the blood compartment. Perhaps the leading role that Hb extravasation plays is an inflammatory response due to the development of oxidative injury (9). Although the vasoactivity of small HBOC molecules could be satisfactorily explained by NO scavenging, hyperoxygenation of the arterioles, and extravasation, the vasoinactivity of PolybHb(40:1)high and PolybHb(50:1)high solutions reinforces the importance of molecular size in mitigating this response.
PolybHb(40:1)high and PolybHb(50:1)high solutions possess high solution viscosities and increased plasma viscosities, leading to a hyperviscous oxygen carrier. In this context, vessel wall shear stress and endothelial cell mechanotransduction are important factors that regulate tissue perfusion. The migration of RBCs to the axial core in vessels (Fåhraeus effect) leads to the formation of a high-viscosity, RBC-rich core and a low-viscosity, mostly RBC-free plasma layer, resulting in a nonlinear blood flow velocity profile (30). An increase in plasma viscosity directly increases vessel wall shear stress and affects total peripheral vascular resistance. The vasoinactivity of PolybHb(40:1)high and PolybHb(50:1)high solutions also could be explained in part by their increase in microcirculation shear stress, leading to the increased production of endothelium-derived autocoids, principally NO. Changes in plasma viscosity by high-viscosity plasma expanders increase vessel wall shear stress and perivascular levels of NO (6). Current mathematical models describing the NO balance in the presence of acellular Hb that include shear stress-dependent endothelial cell NO production predict that significant changes in shear stress will affect the smooth muscle NO concentration and produce minor changes in the perivascular NO concentration (21). In addition, a recent study focused on enhancing the viscosgenic properties of a commercially available polymerized bovine Hb (Oxyglobin; Biopure) demonstrated that the shear stress level influences the endothelial oxidative and inflammatory responses induced by the presence of acellular Hb (16). The increase in plasma viscosity after infusion of PolybHb(40:1)high and PolybHb(50:1)high solutions redistributes viscosity in the circulation, causing it to increase to a greater extent in the central circulation, explaining in part the increase in blood pressure measured without vasoconstriction.
In summary, our study shows a direct correlation between the hypertensive response and constriction of resistance arterioles due to the infusion of PolybHb solutions with molecular masses <500 kDa. However, hyperviscous PolybHb [PolybHb(40:1)high and PolybHb(50:1)high] solutions can increase plasma O2 carrying capacity without inducing vasoconstriction or microcirculatory disturbances. The hypervolemic infusion model used to evaluate vasoconstrictive responses induced by PolybHb solutions in this study has all the regulatory mechanisms responsible for vasoconstrictive responses fully presented. It anticipates the physiological response to PolybHb solutions more than clinically relevant conditions, such as exchange transfusion, where HBOCs would be utilized. Under anemic conditions, the amount of O2 delivered to the tissues becomes insufficient to meet their oxygen demand and consumption starts to decline, indirectly indicating onset of tissue hypoxia. At this point, allogeneic blood transfusion cannot be avoided. However, an attractive alternative to allogeneic blood transfusion can include infusion of high-molecular-mass T-state PolybHb solutions [PolybHb(40:1)high and PolybHb(50:1)high], which at 1.5 g/dl do not elicit vasoconstriction, produce moderate hypertensive responses, and can restore roughly 11% of the total blood O2 carrying capacity of nondiluted blood, preventing tissue hypoxia. In addition, because of its acellular nature, PolybHb can also increase O2 release to tissues via the process of facilitated O2 diffusion. This accomplishment provides a new therapeutic approach for transfusion medicine, the treatment of ischemia, and conditions where tissue oxygenation is affected. High-molecular mass PolybHb solutions are hyperviscous and can increase the O2 carrying capacity of blood to provide a significant advancement in the development of blood substitutes. Future research on PolybHb solutions with high molecular mass must be directed toward understanding their O2 transport characteristics. Finally, the net results of this work and further microvascular evaluation should result in the development of a universal transfusion fluid that lowers the transfusion trigger and is more efficacious and economic than blood, to save and reduce our reliance on blood transfusions.
GRANTS
This work was supported by National Institutes of Health (NIH) Bioengineering Research Partnership Grant R24-HL64395, NIH Program Project P01 HL071064, and NIH Grants R01-HL078840, R01-HL62354, R01-HL62318, and R01-HL76182.
ACKNOWLEDGMENTS
We thank Froilan P. Barra and Cynthia Walser for the surgical preparation of the animals.
REFERENCES
- 1. Alayash AI. Oxygen therapeutics: can we tame haemoglobin? Nat Rev Drug Discov 3: 152–159, 2004. [DOI] [PubMed] [Google Scholar]
- 2. Atha DH, Riggs A. Tetramer-dimer dissociation in hemoglobin and the Bohr effect. J Biol Chem 251: 5537–5543, 1976. [PubMed] [Google Scholar]
- 3. Bouwer ST, Hoofd L, Kreuzer F. Diffusion coefficients of oxygen and hemoglobin measured by facilitated oxygen diffusion through hemoglobin solutions. Biochim Biophys Acta 1338: 127–136, 1997. [DOI] [PubMed] [Google Scholar]
- 4. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976. [DOI] [PubMed] [Google Scholar]
- 5. Cabrales P, Tsai AG, Frangos JA, Briceno JC, Intaglietta M. Oxygen delivery and consumption in the microcirculation after extreme hemodilution with perfluorocarbons. Am J Physiol Heart Circ Physiol 287: H320–H330, 2004. [DOI] [PubMed] [Google Scholar]
- 6. Cabrales P, Tsai AG, Frangos JA, Intaglietta M. Role of endothelial nitric oxide in microvascular oxygen delivery and consumption. Free Radic Biol Med 39: 1229–1237, 2005. [DOI] [PubMed] [Google Scholar]
- 7. Cabrales P, Tsai AG, Intaglietta M. Nitric oxide regulation of microvascular oxygen exchange during hypoxia and hyperoxia. J Appl Physiol 100: 1181–1187, 2006. [DOI] [PubMed] [Google Scholar]
- 8. Colantuoni A, Bertuglia S, Intaglietta M. Quantitation of rhythmic diameter changes in arterial microcirculation. Am J Physiol Heart Circ Physiol 246: H508–H517, 1984. [DOI] [PubMed] [Google Scholar]
- 9. Dull RO, DeWitt BJ, Dinavahi R, Schwartz L, Hubert C, Pace N, Fronticelli C. Quantitative assessment of hemoglobin-induced endothelial barrier dysfunction. J Appl Physiol 97: 1930–1937, 2004. [DOI] [PubMed] [Google Scholar]
- 10. Eike JH, Palmer AF. Effect of Cl− and H+ on the oxygen binding properties of glutaraldehyde-polymerized bovine hemoglobin-based blood substitutes. Biotechnol Prog 20: 1543–1549, 2004. [DOI] [PubMed] [Google Scholar]
- 11. Eike JH, Palmer AF. Effect of glutaraldehyde concentration on the physical properties of polymerized hemoglobin-based oxygen carriers. Biotechnol Prog 20: 1225–1232, 2004. [DOI] [PubMed] [Google Scholar]
- 12. Eike JH, Palmer AF. Effect of NaBH4 concentration and reaction time on physical properties of glutaraldehyde-polymerized hemoglobin. Biotechnol Prog 20: 946–952, 2004. [DOI] [PubMed] [Google Scholar]
- 13. Elmer J, Harris DR, Sun G, Palmer AF. Purification of hemoglobin by tangential flow filtration with diafiltration. Biotechnol Prog. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Endrich B, Asaishi K, Götz A, Messmer K. Technical report: a new chamber technique for microvascular studies in unanaesthetized hamsters. Res Exp Med (Berl) 177: 125–134, 1980. [DOI] [PubMed] [Google Scholar]
- 15. Frietsch T, Gassmann M, Groth G, Waschke KF, Vogel J, Cabrales P, Vajkoczi P, Dorn-Beineke A, Intaglietta M, Kerger H. Excessive erythrocytosis does not elevate capillary oxygen delivery in subcutaneous mouse tissue. Microcirculation 14: 111–123, 2007. [DOI] [PubMed] [Google Scholar]
- 16. Gaucher-Di Stasio C, Paternotte E, Prin-Mathieu C, Reeder BJ, Poitevin G, Labrude P, Stoltz JF, Cooper CE, Menu P. The importance of the effect of shear stress on endothelial cells in determining the performance of hemoglobin based oxygen carriers. Biomaterials 30: 445–451, 2009. [DOI] [PubMed] [Google Scholar]
- 17. Gladwin MT, Crawford JH, Patel RP. The biochemistry of nitric oxide, nitrite, and hemoglobin: role in blood flow regulation. Free Radic Biol Med 36: 707–717, 2004. [DOI] [PubMed] [Google Scholar]
- 18. Huang-Lee LLH, Cheung DT, Nimni ME. Biochemical changes and cytotoxicity associated with the degradation of polymeric glutaraldehyde derived crosslinks. J Biomed Mater Res 24: 1185–1201, 1990. [DOI] [PubMed] [Google Scholar]
- 19. Intaglietta M, Tompkins WR. Microvascular measurements by video image shearing and splitting. Microvasc Res 5: 309–312, 1973. [DOI] [PubMed] [Google Scholar]
- 20. Johnson PC. Flow measurement techniques in the microcirculation. In: Microcirc Tech, edited by Baker CH, Nastuk WL. London: Academic, 1986, p. 149–159 [Google Scholar]
- 21. Kavdia M, Tsoukias NM, Popel AS. Model of nitric oxide diffusion in an arteriole: impact of hemoglobin-based blood substitutes. Am J Physiol Heart Circ Physiol 282: H2245–H2253, 2002. [DOI] [PubMed] [Google Scholar]
- 22. Lipowsky HH, Zweifach BW. Application of the “two-slit” photometric technique to the measurement of microvascular volumetric flow rates. Microvasc Res 15: 93–101, 1978. [DOI] [PubMed] [Google Scholar]
- 23. Matheson B, Kwansa HE, Bucci E, Rebel A, Koehler RC. Vascular response to infusions of a nonextravasating hemoglobin polymer. J Appl Physiol 93: 1479–1486, 2002. [DOI] [PubMed] [Google Scholar]
- 24. McCarthy MR, Vandegriff KD, Winslow RM. The role of facilitated diffusion in oxygen transport by cell-free hemoglobins: implications for the design of hemoglobin-based oxygen carriers. Biophys Chem 92: 103–117, 2001. [DOI] [PubMed] [Google Scholar]
- 25. Nieuwdorp M, Mooij HL, Kroon J, Atasever B, Spaan JA, Ince C, Holleman F, Diamant M, Heine RJ, Hoekstra JB, Kastelein JJ, Stroes ES, Vink H. Endothelial glycocalyx damage coincides with microalbuminuria in type 1 diabetes. Diabetes 55: 1127–1132, 2006. [DOI] [PubMed] [Google Scholar]
- 26. Nishide H, Chen XS, Tsuchida E. Facilitated oxygen transport with modified and encapsulated hemoglobins across non-flowing solution membrane. Artif Cells Blood Substit Immobil Biotechnol 25: 335–346, 1997. [DOI] [PubMed] [Google Scholar]
- 27. Palmer AF, Sun G, Harris DR. The quaternary structure of tetrameric hemoglobin regulates the oxygen affinity of polymerized hemoglobin. Biotechnol Prog. In press [DOI] [PubMed] [Google Scholar]
- 28. Palmer AF, Sun G, Harris DR. Tangential flow filtration of hemoglobin. Biotechnol Prog 25: 189–199, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Patton JN, Palmer AF. Physical properties of hemoglobin-poly(acrylamide) hydrogel-based oxygen carriers: effect of reaction pH. Langmuir 22: 2212–2221, 2006. [DOI] [PubMed] [Google Scholar]
- 30. Reinke W, Gaehtgens P, Johnson PC. Blood viscosity in small tubes: effect of shear rate, aggregation, and sedimentation. Am J Physiol Heart Circ Physiol 253: H540–H547, 1987. [DOI] [PubMed] [Google Scholar]
- 31. Tsai AG, Cabrales P, Manjula BN, Acharya SA, Winslow RM, Intaglietta M. Dissociation of local nitric oxide concentration and vasoconstriction in the presence of cell-free hemoglobin oxygen carriers. Blood 108: 3603–3610, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tsai AG, Friesenecker B, McCarthy M, Sakai H, Intaglietta M. Plasma viscosity regulates capillary perfusion during extreme hemodilution in hamster skin fold model. Am J Physiol Heart Circ Physiol 275: H2170–H2180, 1998. [DOI] [PubMed] [Google Scholar]
- 33. Tsai AG, Vandegriff KD, Intaglietta M, Winslow RM. Targeted O2 delivery by low-P50 hemoglobin: a new basis for O2 therapeutics. Am J Physiol Heart Circ Physiol 285: H1411–H1419, 2003. [DOI] [PubMed] [Google Scholar]
- 34. Tsuji K, Harrison SJ. Dry-heat destruction of lipopolysaccharide: dry-heat destruction kinetics. Appl Environ Microbiol 36: 710–714, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vink H, Duling BR. Capillary endothelial surface layer selectively reduces plasma solute distribution volume. Am J Physiol Heart Circ Physiol 278: H285–H289, 2000. [DOI] [PubMed] [Google Scholar]
- 36. Vink H, Duling BR. Identification of distinct luminal domains for macromolecules, erythrocytes, and leukocytes within mammalian capillaries. Circ Res 79: 581–589, 1996. [DOI] [PubMed] [Google Scholar]
- 37. Winslow RM. αα-Crosslinked hemoglobin: was failure predicted by preclinical testing? Vox Sang 79: 1–20, 2000. [DOI] [PubMed] [Google Scholar]
- 38. Winslow RM. New transfusion strategies: red cell substitutes. Annu Rev Med 50: 337–353, 1999. [DOI] [PubMed] [Google Scholar]
- 39. Winslow RM, Vandegriff KD. Hemoglobin Oxygen Affinity and the Design of Red Cell Substitutes. Boston: Birkhäuser, 1997, p. 167–188 [Google Scholar]
- 40. Yu B, Liu Z, Chang TM. Polyhemoglobin with different percentage of tetrameric hemoglobin and effects on vasoactivity and electrocardiogram. Artif Cells Blood Substit Immobil Biotechnol 34: 159–173, 2006. [DOI] [PubMed] [Google Scholar]
- 41. Zhang X, Banerjee A, Banks WA, Ercal N. N-Acetylcysteine amide protects against methamphetamine-induced oxidative stress and neurotoxicity in immortalized human brain endothelial cells. Brain Res 1275: 87–95, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]