Abstract
This study compared the time to task failure for a submaximal fatiguing contraction in the presence and absence of a cognitive stressor in men and women. In study 1, 10 men and 10 women (22 ± 3 yr of age) performed an isometric fatiguing contraction at 20% maximal voluntary contraction force until task failure with the elbow flexor muscles during two separate sessions. Subjects performed a mental-math task during one of the fatiguing contractions that aimed to increase anxiety and stress (stressor session). Salivary cortisol and reported levels of arousal (visual analog scale for anxiety, and State-Trait Anxiety Inventory scores) were elevated during the stressor session compared with a control session for both sexes (P < 0.05). Time to task failure, however, was briefer during the stressor session compared with control (P = 0.005) but more so for the women (27.3 ± 20.1%) than the men (8.6 ± 23.1%) (P = 0.03). The briefer time to task failure was associated with target force (r2 = 0.21) and accompanied by a higher mean arterial pressure, heart rate, and rate-pressure product during the fatiguing contraction in the stressor session compared with control in women. In study 2 (11 men and 8 women, 20 ± 3 yr of age), time to task failure was similar for a fatiguing contraction with simple mental-math that did not increase stress (mental-attentiveness session) and control for both men and women. The greater change in fatigability of women than men with performance of a cognitive stressor involved initial strength and increases in indexes of sympathetic neural activity and cardiac work compared with control conditions.
Keywords: arousal, elbow flexor muscles, muscle fatigue, sympathetic nervous system
physiological responses to psychological stress are important determinants of health (25). Heightened physiological responses to stress potentially suppress the immune response (8, 31) and increase vulnerability to stress/anxiety disorders and musculoskeletal disorders (18, 38). Psychological stress is characterized by emotional responses of fear and anxiety that elicit physiological responses mediated by the neuroendocrine and sympathetic nervous systems (25). Some physiological manifestations of exposure to an acute stressor include increased blood pressure, heart rate, and cardiac output, changes in blood flow, increased sweating, and hormonal responses, including increased cortisol and catecholamine levels (7, 25). Musculoskeletal function can also be disturbed (increased restlessness, tremors, and feelings of weakness) and motor performance of precision tasks impaired (6, 7, 35, 36). For example, steadiness (quantified as the magnitude of force fluctuations) of a low-force target matching task with the hand muscles was reduced immediately after exposure to an acute stressor particularly in women and individuals who have high levels of trait anxiety (7, 35). Thus individual differences such as trait anxiety and sex can determine the physiological response to an acute stressor.
Most studies that have assessed steadiness of the hand (7, 36) measured performance immediately after exposure to an acute stressor. Many daily life activities, however, require motor performance while simultaneously performing a cognitive task that increases levels of arousal. Fatigue during a motor task can cause decrements in performance of a task that involves a cognitive and motor component (reaction time) (29, 51). The effect of a cognitive task that increases levels of arousal on motor fatigue and performed simultaneously (as occurs in many daily activities) is less explored. A recent study showed that women had a briefer time to task failure of a low-force contraction than men when the gain of the visual feedback was altered so that the task required more attention (34). Whether the high-gain condition induced increased levels of stress or acted as a distraction to influence performance is not known. The reduction in motor performance, however, is consistent with the sex differences observed in motor performance after exposure to a stressor (7, 36).
The reason for a sex difference in motor response with exposure to an acute stressor is not clear (7, 36). Some studies indicate that indexes of sympathetic neural activity (heart rate in particular) are heightened in women compared with men in response to an acute stressor (for review, see Ref. 25). Heightened sympathetic responses of women may change neuromuscular function differently compared with men by modulating skeletal muscle blood flow and contractile function (38, 50). More recently it was shown that heightened sympathetic activation in response to an acute stressor increased motor unit discharge rate and modulated contractility of human leg muscles (44). Contractile function of type II fibers (fast) can be enhanced with increased sympathetic activation but type I fibers produce less force and a reduced relaxation time (38, 44), predisposing muscles with more type I fibers to increased fatigability. The role of stress-induced increases in sympathetic activity on motor performance in men and women who potentially have different proportional areas of fiber types (19) is not understood.
The purpose of this study was to compare the time to task failure for a submaximal fatiguing contraction and indexes of sympathetic neural activity in the presence and absence of a cognitive stressor in men and women. Because women have greater sympathetic response to acute stressors (25) and slower contractile properties compared with men (20, 56), we hypothesized that 1) women would show greater reductions in time to task failure of a low-force fatiguing contraction than men in the presence of a cognitive stressor (a difficult mental-math task known to increase arousal) compared with a control condition (study 1); and 2) there would be no difference in time to task failure between the control condition and when the fatiguing contraction was performed in the presence of a simple mental-math task that did not increase arousal (mental-attentiveness task) for both men and women (study 2). Indexes of sympathetic neural activity included heart rate and mean arterial pressure (MAP) and were measured during the fatiguing contraction. Stress was induced with a cognitive stressor (a difficult mental-math task) that is established as elevating arousal levels and MAP and heart rate (4, 5, 25). Arousal was quantified using cognitive assessments of stress and anxiety and salivary free cortisol (25).
METHODS
Twenty young adults (10 men, 22 ± 4 yr of age and 10 women, 22 ± 2 yr of age) participated in study 1. A second cohort of 19 young adults (11 men, 20 ± 2 yr of age; and 8 women, 20 ± 4 yr of age) participated in study 2. No subject participated in both study 1 and study 2. All subjects were healthy with no known neurological or cardiovascular diseases and were naive to the protocol. Each subject had low-to-moderate levels of anxiety (21 to 51) for the trait score of the State-Trait Anxiety Inventory (STAI) (49) and reported no history or current mental pathology, including anxiety or depressive disorder. Each subject provided informed consent, and the protocol was approved by the institutional review board at Marquette University.
The physical activity level for each subject was assessed with a questionnaire that estimated the relative kilocalorie expenditure per week. Subjects were righthanded (0.71 ± 0.1 vs. 0.72 ± 0.2 for men and women, respectively, with a ratio of 1 indicating complete righthandedness) as estimated with the Edinburgh Handedness Inventory (37). The day of the menstrual cycle on which the experimental sessions were performed was recorded for each female participant.
Study 1
Each subject reported to the laboratory on three occasions: once for a familiarization session and then for two experimental sessions (control and stressor sessions) that were >7 days apart to perform a protocol that involved a fatiguing contraction with the elbow flexor muscles of the nondominant arm. The nondominant arm was chosen to minimize variability between subjects that may occur due to differences in activities performed with the dominant arm. In the control session, each subject performed the tasks without exposure to the cognitive stressor, i.e., without performing the difficult mental-math task. During the stressor session, each subject was required to perform 4 min of mental math before (at rest) and continually while performing a fatiguing contraction at 20% of maximal voluntary contraction (MVC) force for as long as possible (see Experimental Protocol for more detail). Before the fatiguing contraction the following variables were measured before and after performance of mental math (without contraction): anxiety and stress using the visual analog scale (VAS) and STAI and salivary cortisol levels. The order of the control and stressor sessions was counterbalanced among the subjects. All experimental sessions were performed in the afternoon because of the circadian rhythm of glucocorticoid (cortisol) production and release in the hypothalamus-pituitary-adrenalcortical (HPA) system (40, 47).
Mechanical recording of force.
Each subject was seated upright in an adjustable chair with the nondominant arm abducted slightly and the elbow resting on a padded support with the elbow joint flexed to 90°. The setup is similar to that described elsewhere (57). In brief, the hand and forearm were placed in a modified wrist-hand-thumb orthosis (Orthomerica, Newport Beach, CA), and the forearm was placed midway between pronation and supination. Elbow flexion force was measured with a transducer (JR-3 Force-Moment Sensor; JR-3, Woodland, CA) and displayed on an oscilloscope. Force was recorded on line at 500 samples/s using a Power 1401 analog-to-digital (A-D) converter and Spike 2 software (Cambridge Electronic Design, Cambridge, UK).
Electrical recordings.
EMG signals were recorded with bipolar surface electrodes (Ag-AgCl, 8-mm diameter; 16 mm between electrodes) that were placed over biceps brachii, brachioradialis, and triceps brachii muscles. Reference electrodes were placed on a bony prominence at the elbow. The EMG signal was amplified (1,000×) and band-pass filtered (13–1,000 Hz) with Coulbourn modules (Coulbourn Instruments, Allentown, PA) before being recorded directly to a computer with the Power 1401 A-D converter and Spike 2 software (CED). The EMG signals were digitized at 2,000 samples/s.
Cardiovascular measurements.
Heart rate and blood pressure were monitored during the fatiguing contractions. Both heart rate and blood pressure were monitored with an automated beat-by-beat blood pressure monitor (Finapres 2300; Ohmeda, Louisville, CO). The blood pressure cuff was placed around the middle finger of the relaxed dominant hand with the arm placed on a table adjacent to the subject at heart level. The blood pressure signal was recorded on-line to a computer with the Power 1401 A-D converter and Spike 2 software (CED) at 500 samples/s.
Cognitive assessment of arousal.
Cognitive levels of anxiety and stress were assessed throughout the protocol using VAS (23) and the state portion of the STAI questionnaire (49). Each VAS (for anxiety and another for stress) involved a 10-cm line anchored at the far left by “not at all anxious” or “not at all stressed” and at the far right by “very anxious” or “very stressed.” The right anchor corresponded to the most stressful or most anxious moment in the life of the subject. Each subject was instructed that anxiety was defined as the negative feelings regarding the immediate future, whereas stress represented the physical changes (e.g., increase in heart rate and perspiration) perceived by the subject that was above and beyond the expectation for their level of exertion (7). VAS for anxiety and stress were recorded at eight time points during the protocol: two baseline assessments before intended arousal (T1, T2); after a 2-min bout of mental math during rest (stressor session) or quiet rest (control session) (T3); after a second 2-min bout of mental math at rest (stressor session) or quiet rest (control session) (T4); immediately after the fatiguing contraction (T5); and then 5, 10, and 20 min after fatiguing contraction (T6–T8) (Fig. 1).
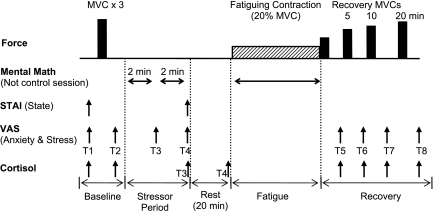
Fig. 1.
Experimental protocol. Top: order of force tasks performed by each subject with the elbow flexor muscles. Maximal voluntary contractions (MVC) (solid bars) were performed at the start and during recovery (immediately after the fatiguing contraction and at 5, 10, and 20 min of recovery). The fatiguing contraction (20% MVC) is symbolized with the hatched rectangle and was performed until task failure by each subject. Bottom: horizontal arrows show when the mental math was performed during the stressor session only. Mental math was performed at rest for 2 × 2-min bouts before the fatiguing contraction and continuously for the duration of the fatiguing contraction. In the control session, each subject sat quietly for 2 × 2 min and performed the fatiguing contraction with no mental math. The State-Trait Anxiety Inventory (STAI) questionnaire and visual analog scale (VAS) were assessed multiple times in each protocol. The timing of the VAS (T1–T8) is indicated by the arrows. In study 1, salivary cortisol samples were taken on 8 occasions [indicated by arrows and usually after each VAS with variation on samples 3 (T3) and 4 (T4) as shown]. The schematic is not to scale for time or force.
The STAI-state questionnaire involved 20 statements that required a response on a four-point Likert-type scale. Assessment of STAI was performed before and after quiet sitting (control session) and before and after 4 min (2 × 2-min bouts) of mental math (stressor session) (Fig. 1).
Hormonal assessment of arousal: salivary cortisol.
Salivary cortisol, a measure of adrenal output of free cortisol (26), was assessed during each experimental session (36). Saliva was collected using a salivette (Salimetrics LLC, State College, PA) according to manufacturer's recommendations and stored at −20°C for later analysis. Cortisol levels were measured using an enzymatic immunoassay (Salimetrics LLC) to determine levels of free cortisol. A previous study has established the reliability of this technique (41).
Eight salivary cortisol samples were collected throughout each experimental session: two baseline samples before any intended arousal (T1, T2); a sample before the fatiguing contraction that was after the mental math task (2 × 2-min bouts of mental math for the stressor session) or quiet sitting for 4 min for the control session (T3); a sample after 20 min of quiet rest during both sessions (T4); immediately after the fatiguing contraction (T5); and then 5, 10, and 20 min after the fatiguing contraction (T6–T8) (Fig. 1). Free cortisol takes 10–20 min to peak in the saliva (after onset of stress) and eventual release from the adrenal glands (26), necessitating the 20-min period before sampling.
Mental-math task (cognitive stressor).
Difficult mental math is an established psychosocial technique to induce stress (25) and was used as the cognitive stressor during the stressor session (36). Each subject was asked to perform serial subtraction from a four-digit number by 13 or 7 with 1 count required every 3 s (36). Once the subject made an error in the math or was not able to provide the correct answer within 3 s, they were asked to start the mental math again from the first number in the series. After three errors, the investigator asked the subject to begin with a new four-digit number. Each subject performed this mental math during the cognitive stressor session only and not the control session. They performed the mental math before the fatiguing contraction while at rest (2 × 2-min bouts) and then continuously during the fatiguing contraction.
Experimental protocol.
The protocol for each experimental session (control session and stressor session) involved procedures in the following order: 1) maximal voluntary contractions (MVC) of the elbow flexor muscles and elbow extensor muscles, 2) assessment of cognitive and physiological arousal before and after either quiet sitting (control session) or 4 min (2 × 2-min bouts) of mental math (stressor session), 3) performance of a fatiguing contraction at 20% MVC force, and 4) recovery MVCs and assessment of cognitive and physiological arousal immediately after the fatiguing contraction, and at 5, 10, and 20 min recovery (Fig. 1).
MVC TASK.
Each subject performed 3–4 MVC trials with the elbow flexors followed by the elbow extensor muscles at the beginning of each session, with 60-s rest between the MVC trials. When the peak forces from two of the three trials were not within 5% of each other, additional trials were performed until this was accomplished. The greatest force achieved with the elbow flexor muscles was taken as the MVC force and used as the reference to calculate the target level for the fatiguing contractions. For the elbow flexor muscles, MVCs were also performed during recovery (Fig. 1).
FATIGUING CONTRACTION.
A fatiguing contraction was performed with the elbow flexor muscles at 20% MVC force during each experimental session. The subject was required to match the vertical target force as displayed on the monitor and was verbally encouraged to sustain the force for as long as possible. The fatiguing contraction was terminated when the target force declined by 10%. To minimize the influence of transient fluctuations in motor output on the criteria for task failure, the task was terminated only after force fell below the predetermined threshold for 2.5 s of a 5-s interval. Task failure was detected automatically using a custom-designed program (Spike 2, CED) that monitored the force signal, and this time was recorded as the time to task failure. Subjects were not informed of their time to task failure until completion of their last session.
An index of perceived effort, the rating of perceived exertion (RPE), was assessed with the modified Borg 10-point scale (2). Each subject was instructed to focus the assessment of effort on the arm muscles performing the fatiguing task. The scale was anchored so that 0 represented the resting state and 10 corresponded to the strongest contraction that the arm muscles could perform. The RPE was recorded at the beginning of the contraction and every minute thereafter until task failure for the fatiguing contraction. To do so during the stressor session, the subject was interrupted and asked their RPE. Each subject was then given the last number they had verbalized before reporting their RPE during the mental-math task so as to restart the serial subtraction.
Study 2
The experimental setup and protocol for study two was similar to that outlined for study one with the exception that salivary cortisol and EMG were not monitored. After an initial familiarization, each of the 11 men and 8 women attended two experimental sessions that were >7 days apart and were counterbalanced in order among the subjects: a mental-attentiveness session and a control session. In the mental-attentiveness session, each subject was required to perform a simple mental-math task that was not aimed at increasing levels of arousal (counting backward by 1 from 50) before and throughout the fatiguing contraction. In the control session, each subject performed the tasks without performing the simple mental-math task. As in study 1, the fatiguing contraction involved maintaining a force that was equivalent to 20% MVC force for as long as possible.
Data Analysis
For each study, the torque for the MVC and submaximal contractions was calculated as the product of force and the distance between the elbow joint and the point at which the wrist was attached to the force transducer. The MVC torque was quantified as the average value over a 0.5-s interval that was centered about the peak. The maximal EMG for each muscle was determined as the RMS (root mean square) value over a 0.5-s interval about the same interval of the MVC torque measurement (study 1). The maximal EMG value of the involved muscles was then used to normalize the RMS EMG values recorded during the fatiguing contraction. For study 1, the RMS of the EMG signal of the elbow flexor muscles and triceps brachii muscles and the fluctuations in force were quantified during the fatiguing contraction at the following time intervals: the first and last 30 s of task duration, and 15 s either side of 25%, 50%, and 75% of time to task failure. The RMS EMG recorded during the fatiguing contraction for the biceps brachii, brachioradialis, and the triceps brachii was normalized to the maximal EMG value for each respective muscle. The amplitude of the force fluctuations was quantified as the coefficient of variation (CV = SD/mean × 100).
Heart rate and MAP were recorded during the fatiguing contraction and analyzed by comparing ∼15-s averages at 25% intervals. For each interval, the blood pressure signal was analyzed for the mean peaks [systolic blood pressure (SBP)], mean troughs [diastolic blood pressure (DBP)], and number of pulses per second (multiplied by 60 to determine heart rate). MAP was calculated for each epoch with the following equation: MAP = DBP + 1/3(SBP − DBP).
Statistical Analysis
Data were reported as means ± SD within the text and displayed as means ± SE in the figures. ANOVAs with repeated measures and with sex (men and women) as a between-subject factor were used to compare the various dependent variables. For both studies, repeated-measures factors included session (control and stressor or mental attentiveness), stress (before session, 2 min, 4 min, and after fatiguing task), time (0, 25, 50, 75, 100% of time to failure), and fatigue (before and after the fatiguing contraction). Specifically, the statistical designs were as follows for the dependent variables: 1) session × sex for time to task failure; 2) session × stress × sex for cortisol and VAS of stress and anxiety; 3) session × fatigue × sex for comparison of MVC; and 4) session × time × sex for RMS EMG, MAP, heart rate, RPE, and force fluctuations during the fatiguing contraction. The strength of an association is reported as the squared Pearson product-moment correlation coefficient (r2). Stepwise linear regression analysis was used to gain insight into the contribution of dependent variables to the total variation of change in time to task failure between sessions (SPSS version 17). A significance level of P < 0.05 was used to identify statistical significance.
RESULTS
Study 1: Cognitive Stressor vs. Control
Time to task failure and MVC torque.
Time to task failure was reduced during the stressor session compared with the control session (session effect, F1,18 = 10.1, P = 0.005). The reduction in time to failure was specific to the women (interaction of session × sex, F1,18 = 5.35, P = 0.03). The women had a longer time to task failure than the men for the control session (14.4 ± 8.2 vs. 8.1 ± 2.8 min, respectively) but a similar time to failure to the men for the stressor session (10.2 ± 5.6 vs. 7.4 ± 3.6 min, respectively). The difference in time to failure between the stressor and control sessions for the women was 27.3 ± 20.1% and for the men was 8.6 ± 23.1%. There was no association between the day of the menstrual cycle for the women and the time to task failure on the experimental days (sessions analyzed together and separately, P > 0.05).
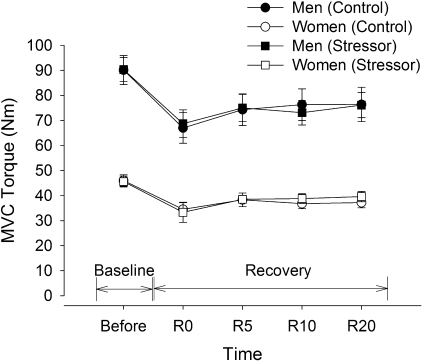
Men had twice the strength of women (sex effect, F1,18 = 60.8, P < 0.001), and MVC torque was similar across the sessions before and after the fatiguing contractions for both men and women (Fig. 2). The MVC torque was reduced for both men and women after the fatiguing contraction for the control and stressor sessions (fatigue effect, F1,18 = 88.6, P < 0.001) (Fig. 2). The relative decline in MVC torque was similar for men and women across the sessions (session × fatigue × sex, F4,15 = 1.13, P = 0.38). The reduced MVC torque recovered to 85% of initial MVC values within 5 min of task failure but remained depressed 20 min after completion of the fatiguing contraction for both men and women (baseline vs. 20-min recovery, F1,18 = 6.14, P = 0.02).
Fig. 2.
MVC torque of the elbow flexor muscles (study 1). Mean (±SE) MVC torque of the men (closed symbols) and women (open symbols) are shown before (Baseline) and after the fatiguing contraction (Recovery) during the control session (circles) and stressor session (squares). Recovery time points are indicated by R0 (within 5 s of task failure), and R5, R10, and R20, representing 5, 10 and 20 min after task failure, respectively. The men were stronger than the women (P < 0.001). Both men and women had similar reductions in MVC force during recovery for the control and stressor sessions.
Cognitive levels of stress and anxiety.
STATE STAI ANXIETY SCORES.
State STAI anxiety scores were similar at the beginning of the two experimental sessions (control 32 ± 10 vs. stressor 33 ± 9). These scores, however, increased after exposure to the 2 × 2 min of difficult mental math performed at rest before the fatiguing contraction but did not change after the quiet rest during the control session (session × stress, F1,18 = 7.36, P = 0.014). For the control session, the state STAI scores were similar at the start of the session and immediately after the 2 × 2 min of quiet rest (32 ± 10 vs. 33 ± 9, respectively). For the stressor session, state STAI scores increased after the 2 × 2 min of mental math from 33 ± 9 to 41 ± 10. Women showed higher state STAI scores than men for both sessions (effect of sex, F1,18 = 6.72, P = 0.018), but the relative increase was similar for the sexes during the stressor session (stress × sex, F1,18 = 2.23, P = 0.89).
VAS SCORES: ANXIETY.
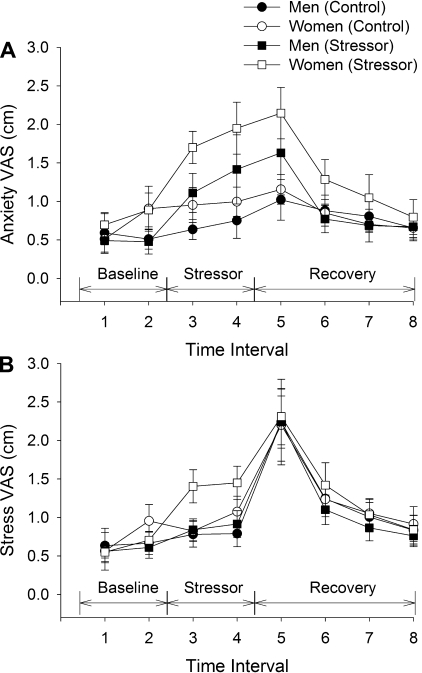
The VAS scores for anxiety (quantified on the 10-cm scale) were similar at the beginning of the control and stressor sessions (0.6 ± 0.5 vs. 0.6 ± 0.6 cm, respectively; averaged time 1 and 2, P = 0.525, Fig. 3A). There was a main effect of stress (effect of stress, F1,18 = 13.8, P = 0.002) and a session × stress interaction (F7,12 = 5.2, P = 0.006) because the VAS scores for the stressor session were elevated immediately after the 2 and 4 min of mental math and also after the fatiguing contraction compared with the control session. There was no difference between men and women for VAS scores (sex effect, F1,18 = 0.9, P = 0.35). There was also no interaction of sex and session (F1,18 = 1.77, P = 0.20), or sex and session over time (session × stress × sex, F7,12 = 0.98, P = 0.49). Thus VAS scores for anxiety were elevated similarly for the men and women during the stressor session and the elevated values lasted until 10 min recovery (baseline vs. 10-min recovery, F1,18 = 5.89, P = 0.026) with no difference at 20 min recovery.
Fig. 3.
VAS scores for anxiety (A) and stress (B) (study 1). Mean (±SE) VAS scores for men (closed symbols) and women (open symbols) are shown before (Baseline), during the stressor period at rest (Stressor), and after the fatiguing contraction (Recovery) during the control session (circles) and stressor session (squares). Time intervals are as follows: baseline (1, 2), after 2 min of mental math at rest (3), after the second bout of 2 min of mental math at rest (4), during recovery after task failure (5), and then at 5, 10, and 20 min of recovery, respectively (6, 7, 8).
VAS SCORES: STRESS.
The VAS scores for stress were similar at the beginning of the experiment for the control and stressor sessions (0.7 ± 0.4 vs. 0.6 ± 0.5 cm, respectively, Fig. 3B). There was a main effect of stress (stress effect, F1,18 = 5.53, P = 0.03) and an interaction of session × stress × sex (F1,18 = 5.0, P = 0.04) because the VAS scores for the stressor session were elevated in the women immediately after the 2 and 4 min of mental math compared with the control session. Men and women, however, had similar values at the beginning of the sessions (baseline: sex effect, F1,18 = 0.2, P = 0.66). After the fatiguing contraction the VAS scores were elevated similarly for the control and stressor sessions and for the men and women. The elevated VAS scores lasted until 10 min of recovery (F1,18 = 13.8, P = 0.002) with no difference at 20-min recovery.
Salivary cortisol.
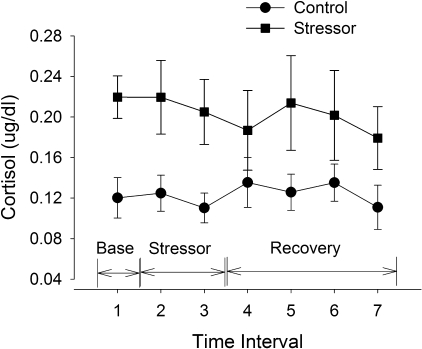
Salivary samples were not able to be analyzed for three men because of technical difficulties. Cortisol was elevated during the stressor session (Fig. 4) compared with the control session (session effect, F1,15 = 12.26, P = 0.003). There was no interaction of time and session (F1,10 = 0.59, P = 0.73) because baseline cortisol levels were elevated during the stressor session compared with the control session, most likely due to anticipation of the stressor session. There was no sex difference in cortisol levels (sex effect, F1,15 = 0.64, P = 0.44) and no interaction between session, sex, and time (F1,10 = 1.89, P = 0.19).
Fig. 4.
Free cortisol levels from saliva samples (study 1). Mean (±SE) cortisol levels are shown for men and women pooled at baseline (Base), after the mental-math stressor period at rest (Stressor), and after the fatiguing contraction (Recovery) during the control session (circles) and stressor session (squares). There were no differences between the men and women (P > 0.05) and so their values were pooled. The two baseline samples measured at the start of the sessions (before any contractions) did not differ (P > 0.05) and so were averaged for display. Time intervals are as follows: baseline (1, mean of two samples at baseline), after 2 × 2 min of mental math at rest (2), 20 min after the 2 × 2 min of mental math (3), during recovery after task failure (4), and then at 5, 10, and 20 min of recovery, respectively (5, 6, 7). There was a main effect of session (P < 0.05).
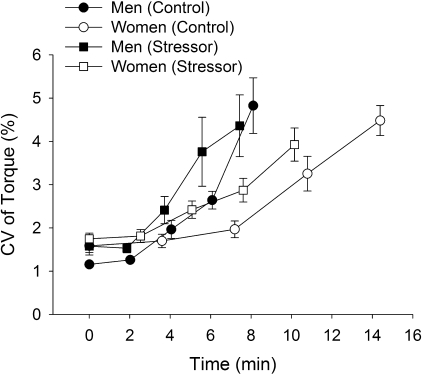
Fluctuations in torque during the fatiguing contraction.
The fluctuations in torque (CV) increased during the fatiguing contraction for the control session (first 30 s, 1.4 ± 0.4% to the last 30 s, 4.7 ± 1.6%) and stressor session (1.7 ± 0.5% to 4.2 ± 1.8%, respectively, time effect, F4,15 = 15.9, P < 0.001) (Fig. 5). The CV of torque during the fatiguing contraction in the stressor session was greater than control for the first four time intervals (up to 75% of time to failure) but not at task failure for both men and women (session × time, F4,15 = 6.5, P = 0.003). The increase in CV of torque during the stressor session compared with control was similar for the men and women (session × time × sex, F4,15 = 1.2, P = 0.34).
Fig. 5.
Fluctuations in torque during the fatiguing contraction (study 1). Values are means ± SE coefficient of variation (CV, %) of torque for men (closed symbols) and women (open symbols) during the control session (circles) and stressor session (squares). CV values during the fatiguing contraction are shown at 25% increments of the time to task failure for 30-s intervals.
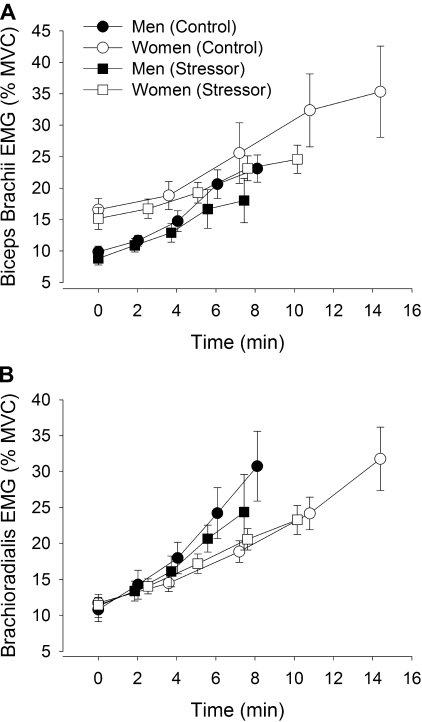
EMG activity during the fatiguing contraction.
The amplitude of the RMS EMG (%MVC) for the elbow flexor muscles, including the biceps brachii, increased during the fatiguing contractions (time effect, F4,15 = 15.7, P < 0.001, Fig. 6A). The EMG activity of the biceps brachii appeared less at the end of the fatiguing contraction during the stressor session than the control session (session × time, F4,15 = 3.0, P = 0.05). Furthermore, the amplitude of biceps brachii RMS EMG for the women was greater than the men during both sessions (sex effect, F1,18 = 8.84, P = 0 .008) with no interactions between sex, time, and session.
Fig. 6.
EMG activity of the elbow flexor muscles (study 1). Root mean square (RMS) EMG (%MVC) of the biceps brachii (A) and brachioradialis (B) for men (closed symbols) and women (open symbols) during the fatiguing contraction. The control session is represented with circles and stressor session with squares. Each data point represents means ± SE at 25% increments of the time to task failure for a 30-s interval for A and B.
The amplitude of RMS EMG of brachioradialis was similar at the beginning of the fatiguing contractions, for the stressor and control sessions (Fig. 6B). Although not statistically significant, there was a suggestion that the EMG activity was less during the stressor session compared with the control session (session effect, F1,18 = 4.1, P = 0.058). There was no interaction of session and time (F4,15 = 1.44, P = 0.27) and the rate of increase in EMG activity during the fatiguing contractions was similar for both sessions (P = 0.71). There was no difference in brachioradialis EMG activity between men and women during the fatiguing contractions (sex effect, F1,18 = 0.02, P = 0.88).
For the triceps brachii muscle, RMS EMG activity during the fatiguing contractions was similar across sessions (session effect, F1,18 = 2.22, P = 0.15) and between men and women (sex effect, F1,18 = 2.2, P = 0.16). There were no interactions (session × time × sex, F4,15 = 0.6, P = 0.66).
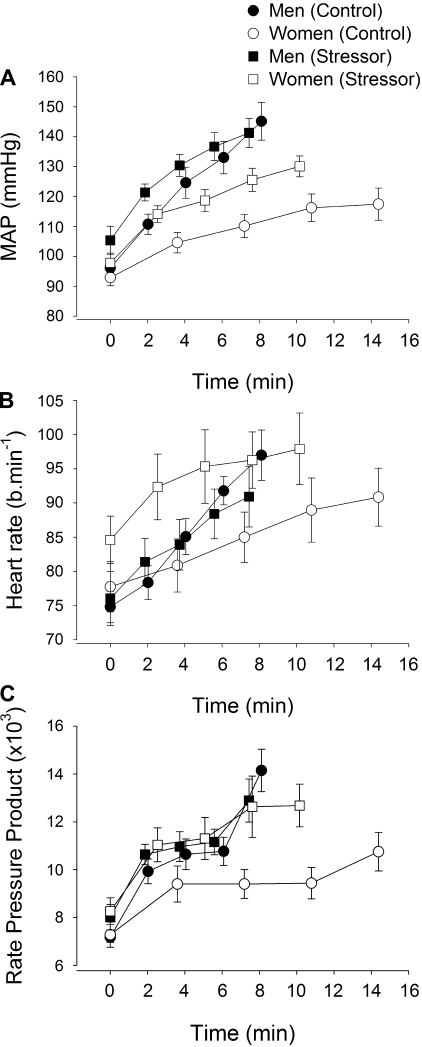
Cardiovascular measurements during the fatiguing contraction.
SBP and MAP increased during the fatiguing contractions in the control and stressor sessions for the men and women (time effect, F4,15 > 18, P < 0.001) (Fig. 7A). Because the results of SBP and MAP were similar, only MAP is reported. There was a main effect of session (F1,18 = 7.36, P = 0.01) and an interaction of session by time (F4,15 = 3.73, P = 0.027) because both the men and women increased MAP during the fatiguing contraction to higher values during the stressor session compared with the control session (Fig. 7A). Furthermore, there was a main effect of sex (P = 0.008) because the men had greater MAP than the women. The interaction of time and sex was P = 0.067, and although not significant, these results suggest that the women had a slower rate of increase in MAP than men. The sex difference was significant when the MAP was normalized to absolute contraction times for the men and women during the control (6.7 ± 4.1 vs. 2.2 ± 2.1 mmHg/min, respectively) and stressor session (6.2 ± 6.1 vs. 3.7 ± 2.4 mmHg/min, respectively).
Fig. 7.
Mean arterial pressure (MAP; A), heart rate (beats/min; B), and rate-pressure product (RPP; C) during the fatiguing contractions (study 1). The values are means ± SE at 25% increments of the time to task failure for men (closed symbols) and women (open symbols) during the control session (circles) and stressor session (squares). Averages of 15-s intervals were used for the MAP and heart rate. Rate-pressure product was the product of heart rate and MAP for the equivalent time periods as in A and B.
Heart rate also increased during both fatiguing contractions (time effect, F4,15 = 14.8, P > 0.001) (Fig. 7B). Heart rate was higher during the stressor session than the control session (session effect, F1,18 = 5.9, P = 0.026), but the greater heart rate was specific to the women. Women showed a higher heart rate at the beginning and end of the fatiguing contraction for the stressor session compared with the control session, but men had a similar heart rate during the fatiguing contraction for the control and stressor sessions (session × sex, F1,18 = 10.6, P = 0.004).
RATE-PRESSURE PRODUCT.
To understand the cardiac work we calculated the rate-pressure product (heart rate × blood pressure) (15, 53). Because the findings for the rate-pressure product were similar when calculated with SBP and MAP, only those results with MAP are reported. Rate-pressure product increased during both sessions for men and women (time effect, F1,18 = 26.0, P < 0.001) (Fig. 7C). A session × sex interaction (F1,18 = 8.9, P = 0.008) indicated that the rate-pressure product was similar for the men during the fatiguing contraction for the stressor and control sessions but differed for the women. The women had a lower rate-pressure product during the control session but values similar to the men during the fatiguing contraction in the stressor session.
RPE during the fatiguing contraction.
RPE increased during both fatiguing contractions (effect of time, F4,15 = 156, P < 0.001). RPE was similar for the men and women during the fatiguing contraction for the control and stressor session, respectively (sex effect, F1,18 = 0.50, P = 0.52). The RPE values were lower for the stressor task compared with the control task for both men and women (session × time, F4,15 = 5.8, P = 0.005) with no significant interaction of stress × time × sex (F4,15 = 0.40, P = 0.83).
Stepwise linear regression analysis found that the significant predictor for the change (relative difference) in time to task failure between the control and stressor session was the baseline maximal voluntary torque (mean of MVCs from both sessions) (r = 0.50, P < 0.05), which explained 21% of the variance (adjusted r2 = 0.21). Thus weaker individuals exhibited a greater decrement in the time to failure for the stressor session compared with control.
Study 2: Mental Attentiveness vs. Control
As for study 1, the men were stronger than the women for the mental-attentiveness and control sessions (sex effect, F1,17 = 84, P < 0.001). Before the fatiguing contraction, the MVC of the men was 82 ± 16 N·m and 36 ± 6 N·m for the women. There was no difference in MVC torque between sessions (session effect, F1,17 = 0.34, P = 0.57). MVC torque at the start of the control session was 62 ± 27 N·m and 63 ± 27 N·m at the start of the mental-attentiveness session. MVC force was similarly reduced after the fatiguing contractions across the two sessions (session × fatigue, F1,17 = 0.02, P = 0.84) and similarly reduced for the men and women (session × fatigue × sex, F1,17 = 0.06, P = 0.82). Thus MVC force was similarly decreased for men and women and after the two fatiguing contractions.
Time to task failure was similar for the mental-attentiveness session and control session (10.5 ± 6.4 vs. 10.2 ± 6.4 min, respectively; session effect, F1,17 = 0.18, P = 0.68) for both the men and women. There was a main effect of sex (F1,17 = 9.8, P = 0.006) and no interaction of task and sex (F1,17 = 0.13, P = 0.72) because the women had a longer time to task failure than men for both the mental-attentiveness session (15.1 ± 8.2 vs. 7.2 ± 1.1 min, respectively) and the control session (15.1 ± 7.9 vs. 7.7 ± 1.0 min, respectively). The time to task failure was similar between study 1 and study 2 for the control sessions for both men and women (session effect, F1,38 = 0.007, P = 0.93).
VAS scores did not differ across sessions for anxiety (session effect, F1,17 = 0.05, P = 0.83) and stress (session effect, F1,17 = 0.35, P = 0.56). There was no increase in VAS for anxiety (time effect, F8,10 =1.70, P = 0.21); however, VAS for stress increased after the fatiguing contraction (time effect, F8,10 = 5.05, P = 0.01) similarly across sessions for both men and women. Consequently, there were no interactions of time × session × sex for anxiety and stress (P > 0.50).
Heart rate and blood pressure were unable to be recorded from two subjects (1 man and 1 woman) due to technical difficulties. Heart rate and MAP during the fatiguing contractions increased (P < 0.001) but did not differ between sessions for either men or women. For heart rate there was no difference across sessions (session effect, F1,15 = 0.04, P = 0.84). Men had greater heart rates than women throughout the fatiguing contractions (sex effect, F1,15 = 10.7, P = 0.005), but there were no interactions including session × sex (F1,15 = 0.04, P = 0.85) and session × sex × time (F4,12 = 0.38, P = 0.82). Similarly, there was no difference across sessions for MAP (session effect, F1,15 = 1.18, P = 0.29). Men had greater MAP than women (sex effect, F1,15 = 8.06, P = 0.01) and also a greater rate of increase in MAP during the fatiguing contractions (sex × time, F4,12 = 4.44, P = 0.02). The increase, however, was similar for both sessions because there were no other interactions of session × sex (F1,15 = 0.51, P = 0.49) or session × sex × time (F4,12 = 0.78, P = 0.56).
DISCUSSION
This study compared the time to task failure for a submaximal fatiguing contraction in the presence and absence of a cognitive stressor (study 1) and a mental-attentiveness task (study 2) for men and women. The new findings have significant clinical and scientific implications: 1) exposure to a cognitive stressor can increase fatigability especially for women when performing low-force fatiguing contractions, which are the foundation of many daily tasks; 2) the increase in neuromuscular fatigability with a cognitive stressor was associated with initial strength of the subject and was paralleled by changes in cardiovascular responses that are indexes of sympathetic neural activity; 3) the cognitive stressor increased cardiac work (indicated by rate-pressure product) during the fatiguing contraction especially for the women; and 4) performance of a mental-attentiveness task that did not increase levels of arousal did not reduce the time to failure for men or women or alter the physiological adjustments during the fatiguing contraction compared with control.
This study demonstrated that muscle fatigability can be exacerbated during performance of a cognitive task that increases arousal (29, 51), more so in women than men. In contrast fatigability did not change when the cognitive task did not increase arousal (mental-attentiveness task). An important finding in this study was that the magnitude of muscle fatigue (reduction in MVC force) at task failure was similar across sessions and sexes despite a difference in time to failure between conditions. Furthermore, the time to task failure was similar for the women during the mental-attentiveness and control sessions. Thus women did not reach task failure prematurely compared with men in study 1 because of distraction during the cognitive stressor. Rather, the increase in arousal and not distraction from multitasking likely contributed to the 27% reduction in time to task failure for the women.
Psychological and physiological measures indicated that both men and women had increased levels of arousal to the cognitive stressor task in study 1, but not for the mental-attentiveness task in study 2. Some variables to quantify the increased arousal included salivary cortisol levels, anxiety VAS, and STAI scores. Before the fatiguing contraction, both men and women perceived the difficult mental-math task in study 1 as an acute stressor (VAS for anxiety increased for both sexes), and the women reported they had greater physiological changes in response to the stressor alone (stress VAS). These findings are consistent with studies indicating that women have greater changes in stress reactivity especially increased heart rate than men during exposure to a cognitive stressor (9, 28).
Salivary cortisol, a glucocorticoid that is mediated by the HPA axis in response to stress (26), was also elevated during the entire stressor session compared with control. The elevated levels of salivary cortisol could have been due to anticipation of the stressor session (7, 27) because each subject was aware which protocol they were conducting on each experimental day. Consequently, future studies should obtain true baseline levels on a different day at the same time of day when the subject is not participating in the experiment. Furthermore, both men and women showed similarly elevated cortisol levels during the stressor session further indicating that subjects perceived the difficult mental-math in study 1 as a stressor. These results also indicate that the sex difference in time to task failure in response to the cognitive stressor was not attributable to a difference in the HPA axis activity between the men and women.
The obvious question is, what caused the greater reductions in the time to failure for the women compared with the men when the cognitive stressor was imposed? Women can have different functional neural networks during a cognitive stressor and motor tasks compared with men (1, 52, 54). Mental stress and performance of a motor task, for example, both involve the anterior cingulate cortex, which differs in activation in men and women in regulating cardiovascular responses (54). The impact of such sex differences in functional neural networks is not understood when a cognitive stressor is imposed during a fatiguing motor task.
Under control conditions and during the mental-attentiveness task women had lower rates of rise in MAP and lower heart rates than men during the isometric contraction (11, 54) and a longer time to task failure (22, 57). Heart rate and MAP are considered indexes of sympathetic activation (4). Regulation of the sympathetic nervous system is altered in men compared with women such that women have less sympathetic excitation and greater inhibition, and this difference could involve regulation of estrogen (11, 17, 54). Because menstrual cycle phase did not correlate with time to failure or with changes in cardiovascular responses during the contractions, the acute fluctuations in estrogen during the menstrual cycle could not account for the higher cardiovascular responses in the women during the stressor session, nor the change in the time to failure with the cognitive stressor.
Initial strength was, however, associated with the reduced time to task failure of women and predicted 21% of this change. Under control conditions when an isometric contraction is sustained, the time to task failure for a fatiguing contraction and the cardiovascular responses are similar for the strength-matched men and women (21, 22). The cardiovascular responses during an isometric contraction are also regulated by feedback from group III and IV afferents in response to metabolite accumulation (pressor reflex) (33, 46) and central command. Heart rate during isometric contraction is modulated primarily by central command (13, 14, 54), which is the parallel activation of neural circuits in the brain stem and spinal cord that control motor, ventilatory, and cardiovascular function (17). The sex difference in time to task failure of a low-force sustained contraction under control conditions therefore potentially involves mechanisms that can be associated with the lower target forces exerted by women compared with men, including 1) a sex difference in perfusion pressure and a lesser reduction in stroke volume during a fatiguing contraction for the women (55); 2) a sex difference in muscle perfusion and accumulation of metabolites (21); and 3) a sex difference in fiber types resulting in a more rapid greater accumulation of metabolites in the men than the women (19). The sex differences in fatigability and cardiovascular responses during control conditions, however, were diminished with exposure to a cognitive stressor.
Although cardiovascular variables were not direct predictors of the change in time to task failure for the women, increased sympathetic activity may have been involved and secondary to the initial strength of the subject. Possible explanations include: 1) altering skeletal muscle blood flow (24, 50); and 2) reducing force and relaxation times of slow-twitch type I fibers while potentiating force of fast twitch type II fibers (38, 44). Because absolute strength can be associated with differences in blood flow and also fiber types, the association of initial strength with the change in time to task failure suggests that either of these mechanisms were possible. First, increased sympathetic activity during the fatiguing contraction in the cognitive stressor session could have altered blood flow regulation and maybe more so in the women. Increased sympathetic activation will increase vasoconstriction to inactive muscles (50). Although, active muscle becomes progressively more ischemic due to mechanical compression during a sustained isometric contraction, vasodilation can occur via local metabolites acting on vascular endothelium and smooth muscle and stimulation of β-2 receptors (via circulating epinephrine) (24). The target forces were similar across sessions for both men and women, so there were likely no differences in mechanical forces onto vasculature across sessions. The relative changes in blood flow due to sympathetic activity however, could have influenced perfusion more so in women who exerted lower target forces and who may have had greater muscle perfusion than the stronger men during control conditions. In contrast, stressful mental-math, can cause vasodilation in upper limbs via similar local vasodilator mechanisms to contraction (16, 24), although not always (53). Hence, increased muscle perfusion during a cognitive stressor could be expected to enhance rather than decrease motor performance of a fatiguing task. Although the interactions are not fully understood, the balance of vasoconstriction and vasodilation could potentially alter the net perfusion of the active muscle in women more than the men during the cognitive stressor causing a reduced time to failure.
Another potential explanation for the reduced time to failure of the women is altered contractility of type I and type II skeletal fibers when sympathetic activation is greater (38). Increased sympathetic activation can decrease fatigability, especially in fast-contracting mammalian muscle (3) and can be specific to the fiber type. Accordingly, contractile force can be potentiated in type II fast-twitch fibers but reduced in type I slow-twitch fibers in animal and human muscle (3, 44). Women demonstrate slower rates of muscle relaxation before fatigue for the elbow flexor and knee extensor muscles (20, 56), which is consistent with larger proportional area of fast-twitch fibers in the muscle of men compared with the women (39, 45, 48). A greater proportional area of type I fibers could predispose women to greater changes in muscle fatigue when sympathetic activation is increased compared with control. These findings are consistent with greater force fluctuations observed during the stressor session for the women, although men also had greater force fluctuations. Furthermore, under such conditions a greater discharge rate to achieve the same force and a greater rise in EMG activity during the cognitive stressor task may occur (44). Consequently, not all our findings support this above mechanism.
In contrast to expectations, there was a tendency for lower EMG activity during the stressor task compared with control for both the elbow flexor muscles in the women and men (study 1). The EMG activity paralleled the effort perceived by the men and women who both showed less RPE during the stressor task. Either the measures of EMG and RPE were not sensitive to the changes in performance or increased stress had differential effects on EMG recordings and RPE compared with indexes of sympathetic activation. Recruitment of additional larger motor units occurs during a fatiguing contraction despite an overall decrease in motor unit discharge rate and even cessation or intermittent discharge behavior from some motor units that are recruited as the contraction progresses (34, 43). Because of the divergent behavior of motor units during a fatiguing contraction, the detectable difference of EMG activity between the two task conditions in study 1 may have been minimal, especially given that cancellation of the EMG signal can occur (12). Although different activation strategies within a muscle can alter time to task failure for a low-intensity fatiguing contraction (10), these results indicate that indexes of sympathetic activation, EMG activity, and RPE were dissociated. Other possible explanations for the lower EMG during exposure to the cognitive stressor involve inhibitory influence of the group III and IV activation onto the motor neuron pool (30) and the differential behavior of neuromodulators in the cortex and spinal cord such as serotonin and norepinephrine (32, 42). The role of these neuromodulators during periods of increased stress in men and women and their influence on fatiguing motor tasks are not understood.
Finally, a clinically relevant finding of this study was that women had an elevated rate-pressure product during the stressor session that increased arousal (study 1) compared with the control session (Fig. 7C). Rate-pressure product is an indicator of cardiac work and myocardial oxygen consumption (15, 53). Thus any cardioprotective effect of lower cardiovascular adjustments during a low-force isometric fatiguing contraction in the women under control conditions was negated by imposing a cognitive stressor.
In summary, performance of a cognitive stressor that increased self-reported and physiological indicators of arousal resulted in a briefer time to task failure of a submaximal sustained contraction with the elbow flexor muscles, especially for young women. Consequently, the sex difference in time to failure observed during the control conditions was diminished when imposing a cognitive stressor. Despite a similar force requirement during the two tasks, the reduction in time to failure with the cognitive stressor was in part explained by initial strength and was accompanied by altered cardiovascular adjustments. In contrast, a mental-attentiveness task that did not alter arousal did not alter the time to task failure for men or women or alter the sex difference in cardiovascular responses. Thus, under stressful conditions, young women may experience increased neuromuscular fatigability and increased cardiac work during low-force fatiguing contraction that are the foundation of many daily tasks.
GRANTS
This research was supported by awards to S. K. Hunter: a Marquette University Regular Research Grant and Grant 3-T42-OH008672 from the National Institute for Occupational Safety and Health (NIOSH).
DISCLAIMER
The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of NIOSH.
REFERENCES
- 1. Bell EC, Willson MC, Wilman AH, Dave S, Silverstone PH. Males and females differ in brain activation during cognitive tasks. Neuroimage 30: 529–538, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14: 377–381, 1982 [PubMed] [Google Scholar]
- 3. Bowman W. Effects of adrenergic activators and inhibitors on skeletal muscles. In: Handbook of Experimental Pharmacology. Adrenergic Activators and Inhibitors, edited by Szekeres L. New York: Springer-Verlag, 1980, p. 47–128 [Google Scholar]
- 4. Callister R, Suwarno NO, Seals DR. Sympathetic activity is influenced by task difficulty and stress perception during mental challenge in humans. J Physiol 454: 373–387, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carter JR, Durocher JJ, Kern RP. Neural and cardiovascular responses to emotional stress in humans. Am J Physiol Regul Integr Comp Physiol 295: R1898–R1903, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Christou EA. Visual feedback attenuates force fluctuations induced by a stressor. Med Sci Sports Exerc 37: 2126–2133, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Christou EA, Jakobi JM, Critchlow A, Fleshner M, Enoka RM. The 1- to 2-Hz oscillations in muscle force are exacerbated by stress, especially in older adults. J Appl Physiol 97: 225–235, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Cohen S, Herbert TB. Health psychology: psychological factors and physical disease from the perspective of human psychoneuroimmunology. Annu Rev Psych 47: 113–142, 1996 [DOI] [PubMed] [Google Scholar]
- 9. Earle TL, Linden W, Weinberg J. Differential effects of harassment on cardiovascular and salivary cortisol stress reactivity and recovery in women and men. J Psychosom Res 46: 125–141, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Enoka RM, Duchateau J. Muscle fatigue: what, why and how it influences muscle function. J Physiol 586: 11–23, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ettinger SM, Silber DH, Collins BG, Gray KS, Sutliff G, Whisler SK, McClain JM, Smith MB, Yang QX, Sinoway LI. Influences of gender on sympathetic nerve responses to static exercise. J Appl Physiol 80: 245–251, 1996 [DOI] [PubMed] [Google Scholar]
- 12. Farina D, Merletti R, Enoka RM. The extraction of neural strategies from the surface EMG. J Appl Physiol 96: 1486–1496, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Fisher WJ, White MJ. Training-induced adaptations in the central command and peripheral reflex components of the pressor response to isometric exercise of the human triceps surae. J Physiol 520: 621–628, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gandevia SC, Hobbs SF. Cardiovascular responses to static exercise in man: central and reflex contributions. J Physiol 430: 105–117, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gobel FL, Norstrom LA, Nelson RR, Jorgensen CR, Wang Y. The rate-pressure product as an index of myocardial oxygen consumption during exercise in patients with angina pectoris. Circulation 57: 549–556, 1978 [DOI] [PubMed] [Google Scholar]
- 16. Halliwill JR, Lawler LA, Eickhoff TJ, Dietz NM, Nauss LA, Joyner MJ. Forearm sympathetic withdrawal and vasodilatation during mental stress in humans. J Physiol 504: 211–220, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hayes SG, Moya Del Pino NB, Kaufman MP. Estrogen attenuates the cardiovascular and ventilatory responses to central command in cats. J Appl Physiol 92: 1635–1641, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Holden C. Sex and the suffering brain. Science 308: 1574, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Hunter SK. Sex differences and mechanisms of task-specific muscle fatigue. Exerc Sport Sci Rev 37: 113–122, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hunter SK, Butler JE, Todd G, Gandevia SC, Taylor JL. Supraspinal fatigue does not explain the sex difference in muscle fatigue of maximal contractions. J Appl Physiol 101: 1036–1044, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Hunter SK, Critchlow A, Shin IS, Enoka RM. Fatigability of the elbow flexor muscles for a sustained submaximal contraction is similar in men and women matched for strength. J Appl Physiol 96: 195–202, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Hunter SK, Enoka RM. Sex differences in the fatigability of arm muscles depends on absolute force during isometric contractions. J Appl Physiol 91: 2686–2694, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Johnson E. Visual Analogue Scale (VAS). Am J Phys Med Rehabil 80: 717, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Joyner MJ, Halliwill JR. Sympathetic vasodilatation in human limbs. J Physiol 526: 471–480, 2000 [PubMed] [Google Scholar]
- 25. Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology 31: 151–178, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Kirschbaum C, Hellhammer D. Salivary cortisol in psychoneuroendocrine research: recent developments and applications. Psychoneuroendocrinology 19: 313–333, 1994 [DOI] [PubMed] [Google Scholar]
- 27. Kirschbaum C, Wust S, Hellhammer D. Consistent sex differences in cortisol responses to psychological stress. Psychosomatic Med 54: 648–657, 1992 [DOI] [PubMed] [Google Scholar]
- 28. Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C. Differential heart rate reactivity and recovery after psychosocial stress (TSST) in healthy children, younger adults, and elderly adults: the impact of age and gender. Int J Beh Med 11: 116–121, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Lorist MM, Kernell D, Meijman TF, Zijdewind I. Motor fatigue and cognitive task performance in humans. J Physiol 545: 313–319, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Martin PG, Smith JL, Butler JE, Gandevia SC, Taylor JL. Fatigue-sensitive afferents inhibit extensor but not flexor motoneurons in humans. J Neurosci 26: 4796–4802, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Res 886: 172–189, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Meeusen R, Watson P, Hasegawa H, Roelands B, Piacentini MF. Central fatigue: the serotonin hypothesis and beyond. Sports Med 36: 881–909, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Mitchell JHJB. Wolffe memorial lecture. Neural control of the circulation during exercise. Med Sci Sports Exerc 22: 141–154, 1990 [PubMed] [Google Scholar]
- 34. Mottram CJ, Hunter SK, Rochette L, Anderson MK, Enoka RM. Time to task failure varies with the gain of the feedback signal for women, but not for men. Exp Brain Res 174: 575–587, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Noteboom JT, Barnholt KR, Enoka RM. Activation of the arousal response and impairment of performance increase with anxiety and stressor intensity. J Appl Physiol 91: 2093–2101, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Noteboom JT, Fleshner M, Enoka RM. Activation of the arousal response can impair performance on a simple motor task. J Appl Physiol 91: 821–831, 2001 [DOI] [PubMed] [Google Scholar]
- 37. Oldfield RC. The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia 9: 97–113, 1971 [DOI] [PubMed] [Google Scholar]
- 38. Passatore M, Roatta S. Influence of sympathetic nervous system on sensorimotor function: whiplash associated disorders (WAD) as a model. Eur J Appl Physiol 98: 423–449, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Porter MM, Stuart S, Boij M, Lexell J. Capillary supply of the tibialis anterior muscle in young, healthy, and moderately active men and women. J Appl Physiol 92: 1451–1457, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Pruessner J, Wolf O, Hellhammer D, Buske-Kirschbaum A, von Auer K, Jobst S, Kaspers F, Kirschbaum C. Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sci 61: 2539–2549, 1997 [DOI] [PubMed] [Google Scholar]
- 41. Pruessner JC, Wolf OT, Hellhammer DH, Buske-Kirschbaum A, von Auer K, Jobst S, Kaspers F, Kirschbaum C. Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sci 61: 2539–2549, 1997 [DOI] [PubMed] [Google Scholar]
- 42. Rekling JC, Funk GD, Bayliss DA, Dong XW, Feldman JL. Synaptic control of motoneuronal excitability. Physiol Rev 80: 767–852, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Riley ZA, Maerz AH, Litsey JC, Enoka RM. Motor unit recruitment in human biceps brachii during sustained voluntary contractions. J Physiol 586: 2183–2193, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Roatta S, Arendt-Nielsen L, Farina D. Sympathetic-induced changes in discharge rate and spike-triggered average twitch torque of low-threshold motor units in humans. J Physiol 586: 5561–5574, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Roepstorff C, Thiele M, Hillig T, Pilegaard H, Richter EA, Wojtaszewski JF, Kiens B. Higher skeletal muscle alpha2AMPK activation and lower energy charge and fat oxidation in men than in women during submaximal exercise. J Physiol 574: 125–138, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rowell LB, O'Leary DS. Reflex control of the circulation during exercise: chemoreflexes and mechanoreflexes. J Appl Physiol 69: 407–418, 1990 [DOI] [PubMed] [Google Scholar]
- 47. Schmidt-Reinwald A, Pruessner JC, Hellhammer DH, Federenko I, Rohleder N, Schurmeyer TH, Kirschbaum C. The cortisol response to awakening in relation to different challenge tests and a 12-hour cortisol rhythm. Life Sci 64: 1653–1660, 1999 [DOI] [PubMed] [Google Scholar]
- 48. Simoneau JA, Bouchard C. Human variation in skeletal muscle fiber-type proportion and enzyme activities. Am J Physiol Endocrinol Metab 257: E567–E572, 1989 [DOI] [PubMed] [Google Scholar]
- 49. Spielberger CDGR, Lushene RE. State-Trait Anxiety Inventory Manual. Palo Alto, CA: Consulting Psychologists, 1970 [Google Scholar]
- 50. Thomas GD, Segal SS. Neural control of muscle blood flow during exercise. J Appl Physiol 97: 731–738, 2004 [DOI] [PubMed] [Google Scholar]
- 51. van Duinen H, Renken R, Maurits N, Zijdewind I. Effects of motor fatigue on human brain activity, an fMRI study. Neuroimage 35: 1438–1449, 2007 [DOI] [PubMed] [Google Scholar]
- 52. Wang J, Korczykowski M, Rao H, Fan Y, Pluta J, Gur RC, McEwen BS, Detre JA. Gender difference in neural response to psychological stress. Soc Cognitive Affective Neurosc 2: 227–239, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wasmund WL, Westerholm EC, Watenpaugh DE, Wasmund SL, Smith ML. Interactive effects of mental and physical stress on cardiovascular control. J Appl Physiol 92: 1828–1834, 2002 [DOI] [PubMed] [Google Scholar]
- 54. Wong SW, Kimmerly DS, Masse N, Menon RS, Cechetto DF, Shoemaker JK. Sex differences in forebrain and cardiovagal responses at the onset of isometric handgrip exercise: a retrospective fMRI study. J Appl Physiol 103: 1402–1411, 2007 [DOI] [PubMed] [Google Scholar]
- 55. Wright JR, McCloskey DI, Fitzpatrick RC. Effects of muscle perfusion pressure on fatigue and systemic arterial pressure in human subjects. J Appl Physiol 86: 845–851, 1999 [DOI] [PubMed] [Google Scholar]
- 56. Wust RC, Morse CI, de Haan A, Jones DA, Degens H. Sex differences in contractile properties and fatigue resistance of human skeletal muscle. Exp Physiol 93: 843–850, 2008 [DOI] [PubMed] [Google Scholar]
- 57. Yoon T, Schlinder Delap B, Griffith EE, Hunter SK. Mechanisms of fatigue differ after low- and high-force fatiguing contractions in men and women. Muscle Nerve 36: 512–524, 2007 [DOI] [PubMed] [Google Scholar]