Abstract
During pregnancy, exposure to nicotine and other compounds in cigarette smoke increases the risk of the sudden infant death syndrome (SIDS) two- to fivefold. Serotonergic (5-HT) abnormalities are found, in infants who die of SIDS, in regions of the medulla oblongata known to modulate cardiorespiratory function. Using a baboon model, we tested the hypothesis that prenatal exposure to nicotine alters 5-HT receptor and/or transporter binding in the fetal medullary 5-HT system in association with cardiorespiratory dysfunction. At 87 (mean) days gestation (dg), mothers were continuously infused with saline (n = 5) or nicotine (n = 5) at 0.5 mg/h. Fetuses were surgically instrumented at 129 dg for cardiorespiratory monitoring. Cesarean section delivery and retrieval of fetal medulla were performed at 161 (mean) dg for autoradiographic analyses of nicotinic and 5-HT receptor and transporter binding. In nicotine-exposed fetuses, high-frequency heart rate variability was increased 55%, possibly reflecting increases in the parasympathetic control of heart rate. This effect was more pronounced with greater levels of fetal breathing and age. These changes in heart rate variability were associated with increased 5-HT1A receptor binding in the raphé obscurus (P = 0.04) and increased nicotinic receptor binding in the raphé obscurus and vagal complex (P < 0.05) in the nicotine-exposed animals compared with controls (n = 6). The shift in autonomic balance in the fetal primate toward parasympathetic predominance with chronic exposure to nicotine may be related, in part, to abnormal 5-HT-nicotine alterations in the raphé obscurus. Thus increased risk for SIDS due to maternal smoking may be partly related to the effects of nicotine on 5-HT and/or nicotinic receptors.
Keywords: nicotinic receptor, serotonin receptor, parasympathetic activity, raphé obscurus, cardiorespiratory function
the sudden infant death syndrome (SIDS) is the sudden death of an infant under 12 mo of age that remains unexplained by a complete autopsy and death scene investigation (66); typically, a seemingly healthy infant is found dead after a sleep period. SIDS is the leading cause of postneonatal infant mortality in the United States, with an overall rate of 0.53/1,000 (44). Before the national sleep campaign instituted in the 1990s, the leading modifiable risk factor for SIDS was prone sleep position, which increased risk three- to fivefold (66). As the contribution of prone position diminished, the proportion of SIDS cases that were exposed in utero to nicotine in cigarette smoke increased, reaching as high as 90% (4). This change made maternal smoking during pregnancy the major modifiable risk factor for SIDS. The mechanism(s) by which prenatal cigarette smoke exposure increases the risk for SIDS is unknown, but is likely to involve cardiorespiratory dysfunction and/or arousal deficits, as exposure to maternal smoking is associated with decreased heart rate variability (69), reduced arousability (26), and increased obstructive apnea (31) in the postnatal infant. Consequently, prenatal exposure to the multiple components of cigarette smoke may disrupt the development of cardiorespiratory and arousal pathways, many of which are located in the brain stem (31, 37, 51). Brain stem neurotransmitter abnormalities are found in SIDS infants, particularly in the serotonergic (5-HT) system in the medulla oblongata (36, 50, 52). This system is involved in homeostatic modulation of cardiorespiratory activity and arousal (33, 35, 36, 50, 52). Abnormalities in the 5-HT system include reductions in 5-HT1A receptor binding in the raphé obscurus and arcuate nucleus, the latter site the putative homolog of respiratory chemosensitive fields at the ventral medullary surface in experimental animals (54), as well as age-related alterations in binding in the gigantocellularis, paragigantocellularis lateralis, and intermediate reticular zone (36, 50, 52). These nuclei contain 5-HT cell bodies and are components of the 5-HT system. 5-HT abnormalities in this network in infants who die of SIDS may contribute to a failure to respond to life-threatening physiological stressors, e.g., hypoxia, hypercarbia, asphyxia, or hypotension during a critical period of development. SIDS infants exposed to maternal cigarette smoke during pregnancy also demonstrate altered nicotinic acetylcholine receptor (nAChR) binding in mesopontine regions related to arousal (13, 46). Given that nicotine is a major neurotoxic component of cigarette smoke and is a potent agonist to nAChRs, the increased risk for SIDS due to maternal smoking during pregnancy may be related, at least in part, to the effects of nicotine on the 5-HT system and/or nAChRs.
To elucidate potential mechanisms underlying these effects, we studied nicotine exposure in a pregnant baboon model and focused on interrelationships between markers of autonomic control of fetal cardiorespiratory activity with markers of the medullary 5-HT network and brain stem nAChRs. We tested the following hypotheses: 1) exposure during pregnancy to clinically relevant concentrations of nicotine results in comparable levels in the fetus; 2) prenatal exposure to nicotine alters fetal cardiovascular and fetal breathing activity; 3) 5-HT cells express nAChRs, thereby indicating a site whereby exogenous nicotine can directly affect 5-HT neurons; and 4) nicotine exposure alters 5-HT receptor, 5-HT transporter, and/or nAChR binding in the medullary 5-HT system. These hypotheses were tested in a nonhuman primate model because of similarities to humans with regard to the physiology of pregnancy, placental function, and brain/neurobehavioral development (18, 38, 63).
MATERIALS AND METHODS
Animals
A colony of individually caged female and male baboons (Papio papio) was maintained at the Institute of Comparative Medicine, Columbia University, New York City, NY. All animal care and experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committee of Columbia University. The animal studies adhered to the guidelines of the American Association for Accreditation of Laboratory Animal Care and US Department of Agriculture. Animals were exposed to a 12:12-h light-dark cycle, fed twice daily with high-protein primate chow, supplemented with fruits and vegetables, and given free access to fresh water. Females were placed with a male during the receptive period of their cycle for up to 5 days. The date of conception was defined as the midpoint of breeding and then used to calculate all subsequent days of gestation (dg) where term gestation was ∼180 dg. After ultrasound confirmation of pregnancy, animals were assigned to receive either nicotine or saline.
Surgical procedures and tethering.
Long-term study of pregnant baboons was accomplished with a lightweight backpack and tethering system to which the animals were preconditioned. Description of this system, animal maintenance, breeding, surgery, and postoperative care have been reported previously in detail (61). All surgical procedures were done under general anesthesia (isoflurane 0.5–3% with nitrous oxide-oxygen, 60:40%) using sterile techniques. At ∼85 dg, maternal baboons with clinical signs of a normal pregnancy and ultrasonic evidence of normal fetal growth were implanted with catheters into the maternal femoral artery and vein for saline or drug administration and sampling of maternal blood. Subsequently, at ∼130 dg, fetuses were prepared surgically, for catheterization of the amniotic fluid cavity, the fetal jugular vein, carotid artery, and trachea, and for placement of electrocardiographic (ECG) leads. Catheters and leads were exteriorized, passed into a maternal backpack, and attached to a flexible tether cable for access to fetal blood samples and recording of cardiorespiratory signals. The entire system rotated around a swivel, permitting mothers to move freely with continuous monitoring of the fetus. Maternal and fetal vascular catheters were infused continuously. For the first 2–4 days postoperatively, cefazolin was administered for antibacterial prophylaxis and morphine for analgesia. Samples of arterial blood (0.5 ml) for acid-base and blood gases (Radiometer, ABL-30) were taken daily (initially) to evaluate fetal well-being.
Nicotine exposure.
Beginning after maternal catheter placement at 86 ± 3 dg, five pregnancies were exposed to nicotine by maternal intravenous infusion, which continued for a mean of 71 days (range 62–80 days). This gestational age was chosen to initiate infusions in the second trimester of pregnancy to reduce the risk of spontaneous abortion (first 10–12 wk) and to avoid the embryological period. Solutions were prepared by adding nicotine tartrate (N5260, Sigma Aldrich, St. Louis, MO) to 1 liter of normal saline and were infused at 0.5 mg/h nicotine with precalibrated peristaltic infusion pumps (P720, Instech Laboratories, Plymouth Meeting, PA). The infusion bags were weighed before and after drug delivery to confirm infusion rates; blood was sampled from mother and fetus for nicotine concentrations. A comparable five control pregnancies were infused with saline alone. All fetuses were killed following cesarean section delivery at ∼161 dg (range 156–165 dg). At delivery, the weight of the placenta and fetal body, brain, and liver were recorded to access fetal growth compared with prior norms. Due to the difficulty in accrual of fetal baboon instrumented tissues, an additional noninstrumented control fetus was delivered at the same gestation. All measures of fetal characteristics taken at delivery of this fetus were equivalent to the fetuses of the 10 infused pregnancies. Postmortem and receptor binding data from evaluation of the brain from this fetus were included with those of the saline-infused group for a total of n = 6 controls, as the results were essentially the same and reached significant differences from the exposed group, with or without this additional control (for comparison, see Tables 2 and 4).
Table 2.
Growth measurements of the nicotine-exposed fetal baboons compared with controls
| Measure | Control Fetuses | Nicotine-Exposed Fetuses | Unadjusted P Value | FDR Corrected P Value | FDR Corrected P Value* |
|---|---|---|---|---|---|
| n | 6 | 5 | 5 controls | ||
| Postconceptional age (age at sacrifice), days | 161.70±1.15 | 160.20±2.04 | 0.53 | 0.74 | 0.74 |
| Placental weight, g | 265.50±28.89 | 230.40±9.17 | 0.29 | 0.73 | 0.74 |
| Maternal weight, kg | 16.93±1.18 | 16.21±1.12 | 0.67 | 0.74 | 0.93 |
| Fetal brain weight, g | 58.62±5.13 | 73.50±2.76 | 0.05 | 0.35 | 0.35 |
| Fetal weight, g | 680.40±50.26 | 629.80±26.78 | 0.43 | 0.74 | 0.74 |
| Fetal brain-to-body weight ratio | 0.09±0.01 | 0.11±<0.01 | 0.07† | 0.35 | 0.35 |
| Fetal body-to-placental weight ratio | 2.63±0.18 | 2.74±0.11 | 0.65 | 0.74 | 0.93 |
| Fetal liver weight, g | 29.92±6.54 | 25.16±2.00 | 0.51 | 0.74 | 0.74 |
| Fetal liver-to-brain weight ratio | 0.04±0.01 | 0.04±<0.01 | 0.83 | 0.83 | 0.74 |
| Female | 3/6 (50%) | 5/5 (100%) | 0.18 | 0.60 | 0.74 |
Values are means ± SE; n, no. of animals. FDR, false discovery rate. P < 0.05 is considered significant. Unadjusted P values are from t-tests, and the last column gives the FDR correction for multiple t-tests.
Wilcoxon Rank Sum used instead of t-test (see materials and methods).
P value is following FDR correction, with removal of the unoperated control fetus that did not receive saline infusions compared with nicotine-exposed fetuses.
Table 4.
Summary of neurotransmitter measurements of selected 5-HT and nicotinic markers in medullary nuclei in the fetal baboon model of prenatal nicotine exposure
| Measure | Control Fetuses | Nicotine-Exposed Fetuses | Unadjusted P Value | FDR Corrected P Value | FDR Corrected P Value* |
|---|---|---|---|---|---|
| n | 6 | 5 | 5 controls | ||
| 5-HT1A receptor binding, fmol/mg tissue | |||||
| Dorsal motor nucleus of the vagus | 5.13±2.16 | 7.16±0.89 | 0.44 | 0.47 | 0.11 |
| Nucleus of the solitary tract | 3.18±1.18 | 7.49±1.60 | 0.05 | 0.12 | 0.11 |
| Hypoglossal nucleus | 2.30±0.93 | 4.15±0.91 | 0.19 | 0.23 | 0.15 |
| Raphé obscurus | 13.67±6.66 | 43.68±5.00 | 0.007 | 0.04 | 0.03 |
| Paragigantocellularis lateralis | 3.73±2.07 | 13.59±3.38 | 0.03 | 0.09 | 0.07 |
| Inferior olivary nucleus | 0.47±0.37 | 0.40±0.36 | 0.84† | 0.84 | 0.91 |
| 5-HT2A receptor binding, fmol/mg tissue | |||||
| Dorsal motor nucleus of the vagus | 0.54±0.13 | 1.01±0.21 | 0.08 | 0.16 | 0.11 |
| Nucleus of the solitary tract | 0.27±0.06 | 0.49±0.09 | 0.08 | 0.16 | 0.13 |
| Hypoglossal nucleus | 0.52±0.12 | 0.90±0.18 | 0.10 | 0.17 | 0.12 |
| Raphé obscurus | 0.18±0.04 | 0.32±0.06 | 0.10 | 0.17 | 0.17 |
| Paragigantocellularis lateralis | 0.25±0.07 | 0.55±0.10 | 0.03 | 0.09 | 0.11 |
| Gigantocellularis | 0.29±0.06 | 0.58±0.12 | 0.05 | 0.12 | 0.12 |
| Inferior olivary nucleus | 1.08±0.43 | 2.26±0.25 | 0.05 | 0.12 | 0.07 |
| Medial accessory olive | 0.78±0.30 | 1.45±0.24 | 0.12 | 0.19 | 0.15 |
| 5-HT transporter binding, fmol/mg tissue | |||||
| Dorsal motor nucleus of the vagus | 14.05±5.04 | 25.23±1.46 | 0.17† | 0.22 | 0.14 |
| Nucleus of the solitary tract | 11.21±3.62 | 17.71±0.86 | 0.17† | 0.22 | 0.20 |
| Hypoglossal nucleus | 16.06±3.62 | 29.41±2.83 | 0.17† | 0.22 | 0.14 |
| Raphé obscurus | 17.70±6.90 | 42.09±3.38 | 0.02 | 0.07 | 0.03 |
| Paragigantocellularis lateralis | 12.26±4.80 | 21.16±2.16 | 0.15 | 0.22 | 0.11 |
| Gigantocellularis | 12.49±4.55 | 24.46±1.62 | 0.08† | 0.16 | 0.12 |
| Inferior olivary nucleus | 6.98±2.35 | 10.53±1.22 | 0.24 | 0.28 | 0.17 |
| Medial accessory olive | 9.75±4.98 | 14.76±0.86 | 0.37 | 0.40 | 0.15 |
| α2-4, β2, or β4 nAChR binding, fmol/mg tissue | |||||
| Dorsal motor nucleus of the vagus | 19.62±2.20 | 30.83±1.49 | 0.003 | 0.030 | 0.010 |
| Nucleus of the solitary tract | 18.24±2.09 | 27.02±1.42 | 0.009 | 0.040 | 0.050 |
| Hypoglossal nucleus | 14.86±1.22 | 25.96±1.44 | 0.0002 | 0.003 | 0.009 |
| Raphé obscurus | 12.50±1.00 | 22.34±1.30 | 0.0002 | 0.003 | 0.009 |
| Paragigantocellularis lateralis | 16.35±1.09 | 29.73±1.17 | 0.008† | 0.040 | 0.090 |
| Gigantocellularis | 16.06±1.24 | 31.42±1.72 | <0.0001 | 0.003 | 0.004 |
| Inferior olivary nucleus | 29.31±3.94 | 44.49±1.60 | 0.009 | 0.040 | 0.050 |
| α7 nAChR binding, fmol/mg tissue | |||||
| Dorsal motor nucleus of the vagus | 9.61±0.83 | 5.62±1.03 | 0.02 | 0.07 | 0.09 |
| Nucleus of the solitary tract | 12.35±0.65 | 8.06±1.60 | 0.07† | 0.16 | 0.14 |
| Hypoglossal nucleus | 5.82±0.63 | 3.80±1.19 | 0.14 | 0.22 | 0.17 |
| Raphé obscurus | 4.06±0.50 | 3.38±0.57 | 0.34 | 0.38 | 0.29 |
| Paragigantocellularis lateralis | 4.76±0.75 | 3.99±0.85 | 0.52 | 0.53 | 0.45 |
| Inferior olivary nucleus | 4.36±0.43 | 2.98±0.58 | 0.09 | 0.17 | 0.12 |
Values are means ± SE; n, no. of animals. 5-HT, serotonin; nAChR, nicotinic acetylcholine receptor. P < 0.05 is considered significant. Unadjusted P values are from t-tests, and the last column gives the FDR correction for multiple t-tests.
Wilcoxon rank sum used instead of t-test (see materials and methods).
P value is following FDR correction with removal of the unoperated control fetus that did not receive saline infusions compared with nicotine-exposed fetuses.
Analysis of the fetal baboon physiological parameters.
Physiological recordings were analyzed from those records obtained from 139 to 152 dg from the 10 instrumented fetuses (saline n = 5 and nicotine-exposed n = 5). This range, from 1 wk after surgery to approximately 1 wk before delivery, was adopted to diminish the impact of surgery, the effects of prolonged tethering, and initiation of labor on the physiological data. Cardiorespiratory parameters, derived from measurement of RR intervals and fetal breaths, were quantified using techniques described in detail in past publications (45, 63). The means for each parameter were first computed on a minute-by-minute basis, and then daily averages of the ∼1,400 min each day were computed. Daily means were excluded from subsequent statistical analyses when interventions occurred (e.g., sedation for weekly physical exam). After these exclusions, statistical analyses were based on the daily averages of 11 days of data for each fetus with the 139- to 152-dg age range mentioned previously. Acid-base blood-gas determinations on all days on which physiological data were analyzed were within our laboratory's previously established norms (10, 61–63). The average gestational age for these fetal recordings was 149 days for saline controls and 148 days for nicotine exposed. The following time and frequency domain parameters of heart period variability were used as indexes of autonomic nervous system regulation of heart rate: 1) standard deviation of RR intervals (SD-RRi) for estimates of overall variability as an indirect index of both the parasympathetic and sympathetic branches; 2) root mean square of successive differences in RR intervals (RMSSD); and 3) spectral power of RR interval variability within a high-frequency band [0.5–1.5 Hz, i.e., the normal range of frequencies for fetal breathing; high-frequency heart period variability (HF-HPV)]. These latter two measures served as indexes of parasympathetic modulation of cardiac rate variability (1, 8). Fetal breathing activity (rate, variability, percent time breathing) was assessed from analyses of the fetal tracheal minus amniotic fluid pressures, as described previously (45). To evaluate differences in the maturation of cardiorespiratory function, data from each of the nicotine- and saline-treated pregnancies were summarized for two age groups: <146 and ≥146 dg. This dichotomized the data into groups that were obtained before and after the midpoint of the data collection interval (139–152 dg). Within these groups, changes in heart rate were evaluated from average daily RMSSD, and breathing activity was categorized as the percentage of time (0, 1–33, 34–67, and 68–100%) when breathing movements were present.
Measurement of nicotine and cotinine concentrations in plasma.
Following implantation of catheters, blood samples (0.6 ml) for plasma nicotine and cotinine levels were drawn into heparinized microtainers (Becton Dickinson, Franklin Lakes, NJ), separated by centrifugation, and the plasma was stored at −20°C. Plasma was pooled separately to obtain the 1-ml volume needed for analysis of either maternal or fetal concentrations. Maternal samples were divided into pre- and postfetal catheterization. Wherever possible, pooled fetal samples were matched with maternal plasma obtained at the same time points. Samples were assayed for nicotine and cotinine using gas chromatography-mass spectrometry at the Analytical Psychoanalytical Laboratories (Nathan Kline Institute).
Nicotine was assayed using an Agilent 1100 series LC-MSD with Nova-Pak C18 (30 cm × 3.9 mm, 4 μm) operated in the atmospheric pressure chemical ionization mode using mobile phase A [methanol-acetonitrile-0.02 M ammonium formate (pH 4.0 with formic acid) 32.5:32.5:35.0] and mobile phase B (acetonitrile, 9:1) at a flow rate of 0.75 ml/min. The interassay precision was determined by testing plasma controls containing 4, 40, and 80 ng/ml of nicotine on 6 separate days. The relative standard deviations were 8.6, 7.4, and 8.3%, respectively. Cotinine was assayed using an Agilent 1100 series LC-MSD with Nova-Pak C18 (30 cm × 3.9 mm, 4 μm) operated in the atmospheric pressure chemical ionization mode using methanol-1.0 mM ammonium acetate-acetic acid (85:15:0.05) as a mobile phase at a flow rate of 0.75 ml/min. The interassay precision was detected by testing plasma controls containing 4, 40, and 80 ng/ml of cotinine on 6 separate days. The relative standard deviations were 6.4, 5.9, and 6.8%, respectively.
Analysis of the fetal baboon brain stem.
The entire unfixed, frozen fetal medullae was serially sectioned at 20 μm on a motorized Leitz cryostat, mounted on glass microscopic slides, and stored at −20°C until use. A minimum of three sections from each case were stained with hematoxylin and eosin for histological analysis. The medullary nuclei were identified anatomically with reference to the stereotaxic atlas of the brain of the baboon of Davis (11). As defined by our laboratory in the human, the medullary 5-HT system is composed of 5-HT neuronal cell bodies in the raphé (raphé obscurus and raphé pallidus), extra-raphé (paragigantocellularis lateralis, gigantocellularis, intermediate reticular zone, and subtrigeminal nucleus), and ventral surface (embedded within the arcuate nucleus in humans) (34) and projects to, among other regions, the hypoglossal nucleus (upper airway patency), dorsal motor nucleus of the vagus (DMX) (preganglionic outflow of the parasympathetic system), and nucleus of the solitary tract (NTS) (autonomic sensory input). Two of the three vagal nuclei were analyzed, i.e., DMX and NTS. The nucleus ambiguous was not specifically analyzed due to its very small size and difficulties in the reproducibility of anatomic measurements; nevertheless, it was included in the boundary contours of the extra-raphé. Of note, we did not find an arcuate nucleus at the ventral medullary surface in the fetal baboon; the presence of this nucleus appears in evolutionarily more advanced animals, e.g., humans, due to the massive development of the cerebellum, a site of projection of the arcuate nucleus (H. C. Kinney, unpublished observations). One control medulla demonstrated scattered microglial nodules consistent with viral infection; a nicotine-exposed medulla demonstrated bilateral, small (1–2 μm in diameter), cavitated infarcts in the NTS (consistent with vascular underperfusion). Autoradiographic data from these cases were within the range for their group, and thus data from these cases were combined with those of the other fetuses for analysis.
Colocalization of nicotinic receptors and 5-HT neurons by immunocytochemistry.
Double-label immunofluorescence was performed as described previously (12) to determine colocalization and distribution of the α4-nAChR [which has the highest affinity to nicotine (15), guinea-pig polyclonal, 1:200, Chemicon, Billerica, MA] and 5-HT neurons (PH8, a marker for tryptophan hydroxylase, the key enzyme in the synthesis of 5-HT, murine monoclonal, 1:1,000, Chemicon). Sections were air dried for 1 h and then postfixed in 4% paraformaldehyde for 15 min, followed by 20 min in dH2O. Antigen retrieval was preformed using 10% citrate buffer (pH 6) at 195°F for 10 min, and, following phosphate-buffered saline washes (pH 7.4, 3 × 5 min), sections were incubated for 1 h in blocking buffer (4% normal goat serum and 0.1% Triton X). A cocktail mixture of antibodies diluted in blocking buffer was applied to the tissue overnight at 4°C, after which the appropriate Alexa-Fluor secondary antibodies (Invitrogen Molecular Probes, Eugene, OR) were applied for 1 h. Following phosphate-buffered saline washes (3 × 5 min), slides were air dried and coverslipped using Flouromount-GT (Electron Microscopy Sciences, Hatfield, PA). Omission of the primary antibody resulted in no staining. Slides were viewed using either fluorescein isothiocyanate or tetramethylrhodamine isothiocyanate filters using an Olympus BX51 microscope (Olympus America, Melville, NY). In this study, the α4-nAChR and 5-HT antibodies produced immunostaining patterns comparative to that of positive control tissues (rat brain) and to those reported previously in rodents (9) and humans (13, 34). Images were captured using a Photometric Cool Snap Fix camera (Photometric, Tucson, CA) and MCID Elite 6.0 program (Imaging Research, Ontario, Canada). Images were pseudocolored and merged using Adobe Photoshop 6.0 (Adobe Systems, San Jose, CA). Figures were constructed in CorelDraw Graphics Suite 12 (Corel, Ottowa, CA), including the addition of text and scale bars.
Tissue section autoradiography.
We surveyed the medulla for “markers” of nAChRs and the 5-HT system with tissue section autoradiography using the nAChR subunit-specific ligands 3H-epibatidine (α2–4, β2, β4) (30) and 125I-bungarotoxin (α7) (30) and markers of the 5-HT system, [3H]8-OH-DPAT [8-hydroxy-2-(di-n-propylamino)tertraline] (5-HT1A receptors), 125I-DOI (2,5-dimethoxy-4-iodoamphetamine) (5-HT2A receptors), and 125I-RTI-55 [3β-(4-iodophenyl)tropan-2β-carboxylic acid methyl ester] (the 5-HT transporter). All ligands were purchased from PerkinElmer (Waltham, MA). For each radioligand, the general methods of tissue autoradiography were the same. Alternating serial 20-μm cryostat sections of unfixed tissue were preincubated in buffer for 30–60 min, followed by incubation with the radioligand diluted in the same buffer. Nonspecific binding was determined in a subset of adjacent sections that were incubated with the radioligand and excess “cold” displacer. To remove unbound ligand, the sections were washed in a series of changes of buffer followed by distilled water. The sections were then dried under a stream of anhydrous air and exposed to 125I-sensitive film (Kodak BoiMax MR) or 3H-sensitive phosphoimage plates (BAS TR2025 system). To investigate heteromeric α2–4-, β2-, and β4-nAChRs, sections were incubated for 60 min at room temperature with 0.5 nM 3H-epibatidine (56 Ci/mmol) and exposed to phosphoimage plates for 3 wk. To investigate homomeric α7-nAChRs, sections were incubated for 2 h (4°C) with 1.5 nM 125I-bungarotoxin (118 Ci/mmol) and exposed to film for 1 wk. Cold displacers for nAChRs were 300 μM and 1 mM l-nicotine hydrogen (Sigma-Aldrich, St. Louis, MO), respectively. For markers of the 5-HT system, sections were incubated with either 4 nM [3H]8-OH-DPAT (120 Ci/mmol) for the 5-HT1A autoreceptor for 1 h at room temperature and exposed to phosphoimage plates for 4 wk, 86.3 pM 125I-DOI (2,200 Ci/mmol) for the 5-HT2A receptor for 60 min at room temperature and exposed to film for 1 wk, or 0.15 nM 125I-RTI-55 (2,200 Ci/mmol) for the 5-HT transporter for 90 min at room temperature and exposed to film for 9 h. Cold displacers for these ligands were 10 μM 5-HT, 10−6 M ritanserin, and 100 nM citalopram hydrobromid (Sigma-Aldrich), respectively.
Receptor binding density (expressed as the specific activity of tissue-bound ligand) was analyzed in six to eight nuclei per ligand, based on whether the boundaries of the nucleus were clearly defined. The selected nuclei were analyzed from five sections per ligand throughout the entire length of the medulla (4 specific and 1 nonspecific autoradiograms for each nucleus, 8 measurements for each paired nucleus), according to standard sampling procedures established in our laboratory (35). The MCID Elite 6 imaging system (Imaging Research, Ontario, CA) was used to perform quantitative densitometry of autoradiographs. Receptor binding density was determined in each brain stem nucleus by digitizing the boundaries of nuclei upon images of the autoradiogram displayed on the computer monitor and compared with hematoxylin and eosin counterstained sections. A set of 3H- or 125I-standards (Amersham, Piscataway, NJ) were included in each X-ray cassette to permit the conversion of the optical densities of silver grains in autoradiograms to specific activity of tissue-bound ligand to femtomoles per milligram (fmol/mg) of tissue. Calculations to equate for nonspecific binding were made, if needed. Specific activity data are presented as computer-generated mosaics with an increasing linear color step scale for each ligand.
Statistical analyses.
Before testing group differences, Shapiro Wilks tests were used to examine normality of all variables due to the small sample size. The Wilcoxon rank sum test was used in measures that failed the normality test. For normally distributed measures, t-tests were conducted. All tests were two-tailed with an α-level of 0.05; means ± SE are presented for all continuous measures. Fisher's exact tests were computed to test for associations between nicotine-exposed fetuses and controls with categorical measures (fetal sex). Group differences in physiological measures were based on data collapsed across all ages. Effects of fetal breathing activity (4 levels) and age (2 age groups) were further analyzed by ANOVA, with age and level of breathing as repeated measures within subjects. The relationship between binding and RMSSD was analyzed using Pearson correlation.
All P values were adjusted to account for multiple testing using the false discovery rate method (2); for comparison, both the unadjusted and adjusted P values are presented below. The false discovery rate method adjusts for multiple testing by controlling for the proportion of significant results that are false positives. Essentially, a corrected P value is calculated by adjusting the observed P values based on the total number of tests conducted and on the ranking of the observed P values (2). The total numbers of tests were determined by the type of measure. For instance, each marker's raw P value is adjusted by the total number of 5-HT and nicotinic marker tests conducted. This method is less stringent than the Bonferroni correction, a more commonly used method for adjustment.
RESULTS
Nicotine and cotinine levels.
The dose of nicotine delivered to the pregnant animals ranged from 0.64–0.9 mg·kg−1·day−1 and resulted in mean nicotine concentration in the mother of 14.4 ± 1.9 ng/ml (Table 1). Neither the nicotine nor the cotinine levels in fetus plasma differed from those in the maternal plasma (Table 1).
Table 1.
Nicotine dose and steady-state concentrations (ng/ml) of nicotine and cotinine in maternal and fetal plasma
| Animal No. | Nicotine Dose, mg·day−1·kg−1 | Nicotine, ng/ml |
Cotinine, ng/ml |
Fetal-to-Maternal Ratio |
|||
|---|---|---|---|---|---|---|---|
| Mother | Fetus | Mother | Fetus | Nicotine | Cotinine | ||
| 1 | 0.90 | 15.6 | 13.0 | 163 | 150 | 0.83 | 0.92 |
| 2 | 0.86 | 21.2 | 19.1 | 202 | 149 | 0.90 | 0.74 |
| 3 | 0.73 | 11.4 | 15.4 | 139 | 138 | 1.35 | 1.00 |
| 4 | 0.65 | 10.9 | 10.9 | 146 | 148 | 1.00 | 1.01 |
| 5 | 0.64 | 13.2 | 11.1 | 180 | 183 | 0.84 | 1.01 |
| Mean ± SE | 0.76±0.04 | 14.4±1.9 | 13.9±1.5 | 166±12 | 154±8 | 0.99±0.09 | 0.94±0.50 |
Parameters of fetal growth.
Fetal baboons were killed at ∼161 dg (90% gestation), which is the neurodevelopmental age equivalent of term (37–42 gestational wk) in humans (29). Maternal, placental, and fetal growth parameters were similar in both the control and nicotine-exposed groups (Table 2). Of note, there were specifically no differences in body or brain weight. However, it is important to note that this study was not powered to assess differences in these or other growth parameters known to be affected by chronic exposure to nicotine in humans and animal studies (14, 56).
Fetal physiology.
We measured heart rate and heart rate variability from fetal R-wave to R-wave intervals from the ECG, and the percentage of time the fetus spent with breathing activity and the rate of breath movements during episodes of breathing from assessment of fetal tracheal fluid pressure. Daily averages of data between 139 and 152 dg from the fetal baboons (n = 5 control and n = 5 nicotine exposed) showed no significant group difference in fetal breathing rates (Table 3). Analyses of other breathing variables indicated no significant effects of nicotine on percentage of time with fetal breathing movements, the variability in breathing rate, or the average amplitude of fetal breaths (data not shown). The mean fetal heart rate was lower in nicotine-exposed fetuses, although not significantly (Table 3); caution is warranted in interpretation of the absence of an effect due to the small sample size. Although the overall measure of heart rate variability (SD-RRi) was not altered significantly by nicotine (Table 3), a time domain measure of variability in parasympathetic activity (RMSSD) was increased 55% in the nicotine-exposed fetuses compared with controls (P = 0.04) (Table 3). This finding was supported by results from spectral analyses, which found a 47% increase in HF-HPV in the nicotine-exposed fetuses (data not shown). A secondary analysis revealed a high correlation (r = +0.96) between the time (RMSSD) and frequency (HF-HPV) domain measures of high-frequency variation in heart rate.
Table 3.
Physiological variables
| Measure | Control Fetuses | Nicotine-Exposed Fetuses | Unadjusted P Value | FDR Corrected P Value |
|---|---|---|---|---|
| n | 5 | 5 | ||
| Heart rate, beats/min | 170.3±2.5 | 161.1±0.7 | 0.12 | 0.16 |
| SD RRi, ms | 12.0±0.4 | 13.9±0.9 | 0.18* | 0.16 |
| RMSSD RRi, ms | 4.0±0.4 | 6.2±0.5 | 0.01 | 0.04 |
| Fetal breathing rate, breaths/min | 22.4±0.4 | 23.4±0.7 | 0.57 | 0.57 |
Values are means ± SE of physiological variables from groups of saline control and nicotine-exposed fetal baboons; n, no. of animals. SD RRi, SD of R-wave to R-wave intervals; RMSSD RRi, root mean squared of successive differences. P < 0.05 is considered significant. Data are first collapsed over minutes within days and then across days (139 and 152 days gestation). Unadjusted P values are from t-tests, and the last column gives the FDR correction for multiple t-tests.
Wilcoxon rank sum used instead of t-test (see materials and methods).
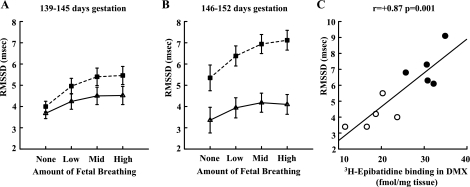
Next, we tested whether the effects of nicotine on RMSSD were affected by gestational age and/or amounts of fetal breathing (Fig. 1). Data were divided into two age groups, <146 and ≥146 dg, and four levels of fetal breathing (in percentage of time: none = 0, low = 1–33%, mid = 34–67%, high = >67%). A repeated-measures ANOVA for effects of gestational age and fetal breathing activity revealed that RMSSD was greater in the nicotine-exposed group [F(1,8) = 11.61, P = 0.009] and increased with amount of breathing [F(3,24) = 39.63, P < 0.001]. There were also significant interactions between nicotine and age [F(1,8) = 6.41, P = 0.035] and level of breathing [F(3,24) = 4.17, P = 0.016]. The effects of chronic nicotine exposure increased with both gestational age and level of fetal breathing. Similar analyses (data not shown) for heart rate indicated there was a significant three-way interaction between nicotine, age, and breathing level (P < 0.05). Post hoc t-tests indicated there were no significant effects of nicotine at any given level of breathing or age; however, at the highest level of breathing at the older ages, nicotine-exposed animals had marginally lower heart rates (P < 0.06). For SD-RRi, the only significant effect of nicotine was an interaction with breathing (P < 0.05), with SD-RRi increased in nicotine-exposed animals at the highest level of breathing (P < 0.05).
Fig. 1.
Heart rate variability. A: time domain measure of high-frequency heart period variability [root mean square of successive differences in RR intervals (RMSSD), means ± SE] at four levels of fetal breathing activity (in %time: none = 0, low = 1–33, mid = 34–67, high = >67) at 139–145 days gestation (dg). Dashed lines, nicotine-exposed fetuses (n = 5); solid lines, controls (n = 5). B: RMSSD vs. breathing at 146–152 dg. C: relationship between amount of nicotinic acetylcholine receptor (nAChR) subunit binding (3H-epibatidine) in the dorsal motor nucleus of the vagus (DMX) and RMSSD during periods of high fetal breathing activity (>67% of time) during late gestation (146–152 dg).
Distribution of 5-HT and nAChR markers in fetal baboon medulla.
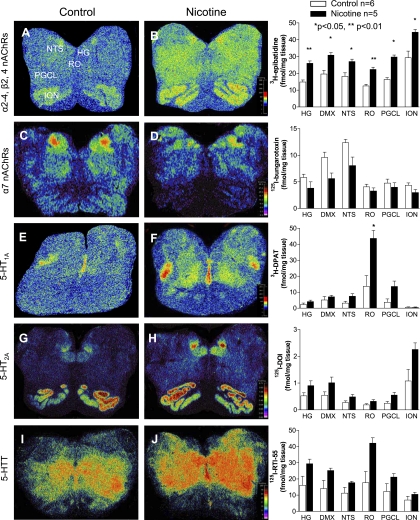
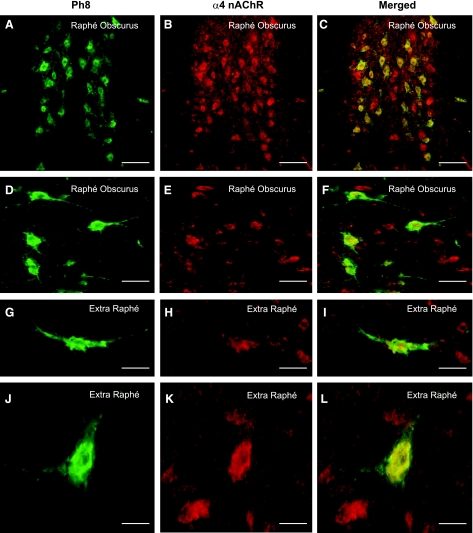
The highest binding of 5-HT1A receptors and the 5-HT transporter in the normal fetal medulla (n = 6) was in the caudal raphé, including the raphé obscurus (Fig. 2, Table 4). This distribution is homologous to that in the human neonatal and infant medulla (32, 35, 51), underscoring the relevance of the chemical anatomy of the fetal baboon to the developing human. Binding for 5-HT2A receptors and the 5-HT transporter was also high in the hypoglossal nucleus, DMX, and NTS (Fig. 2, Table 4). The ligands used for different nAChR subunits, i.e., 3H-epibatidine preferential for α2–4, β2, and β4 (30), and 125I-bungarotoxin for α7 (30), demonstrated widespread but variable binding distributions across the raphé, vagal, and other nuclei (Fig. 2, Table 4). In both the raphé (Fig. 3, C and F) and extra-raphé (paragigantocellularis lateralis) (Fig. 3, I and L), 5-HT neurons expressed α4-nAChR subunits by immunocytochemical techniques. Again, this finding mimics that in the human infant, whereby we reported the expression of nAChRs by 5-HT neurons in the same medullary regions (12).
Fig. 2.
nAChR and serotonin (5-HT) markers in the fetal baboon medulla. Pseudocolored autoradiographic images of 3H-epibatidine binding to α2–4-, β2-, and β4-nAChRs (A and B), 125I-bungrotoxin binding to α7-nAChRs (C and D), [3H]8-OH-DPAT [8-hydroxy-2-(di-n-propylamino)tertraline] binding to 5-HT1A receptors (E and F), 125I-DOI (2,5-dimethoxy-4-iodoamphetamine) binding to 5-HT2A receptors (G and H), and 125I-RTI-55 [3β-(4-iodophenyl)tropan-2 β-carboxylic acid methyl ester] binding to the 5-HT transporter (I and J) in the fetal baboon medulla with specific activity scales for control (left) and nicotine-exposed (right) fetuses with corresponding graphs of binding levels in select nuclei for each group (fmol/mg tissue). Nicotine exposure resulted in a global increase in 3H-epibatidine binding (B) and an increase in [3H]8-OH-DPAT binding in the raphé obscurus (RO) (F). HG, hypoglossal nucleus; ION, inferior olivary nucleus; NTS; nucleus of the solitary tract; PGCL, paragigantocellularis lateralis.
Fig. 3.
Colocalization of α4-nAChRs and 5-HT neurons in the fetal baboon medulla. A, D, G, and J: PH8, a marker for tryptophan hydroxylase, the key enzyme in the synthesis of 5-HT, immunopositive neurons (green). B, E, H, and K: α4 immunopositive cells (red) in the raphé obscurus (A–F) and extra-raphé (paragigantocellularis lateralis) (G–L) at 161 days of gestation (term = 180 days). C, F, I, and L: colocalization of expression is demonstrated by merging of images (yellow). A subpopulation of neurons expressing α4, which were not 5-HT, were observed in association to 5-HT cells. Scales: A–C: 115 μm; D–F: 57 μm; G–I: 30 μm; J–L: 35 μm.
Effects of prenatal nicotine exposure on 5-HT and nAChR markers.
The most robust and consistent medullary abnormality in the nicotine exposed (n = 5) compared with control fetuses (n = 6) significantly increased 5-HT1A receptor binding in the raphé obscurus (Fig. 2, Table 4, P = 0.04). There were trends for increased 5-HT1A receptor binding in the paragigantocellularis lateralis and NTS, increased 5-HT2A receptor binding in the paragigantocellularis lateralis, gigantocellularis, and inferior olive, and increased 5-HT transporter binding in the raphé obscurus. These values, unlike 5-HT1A binding in the raphé obscurus, however, did not remain significant following correction for multiple testing (Fig. 2, Table 4). We found widespread elevations in 3H-epibatidine to nAChRs throughout the medulla in the nicotine-exposed fetal baboons, including in the raphé obscurus and vagal nuclei (NTS and DMX) (Fig. 2, Table 4). In contrast, after correction for multiple testing, there were no significant differences seen for α7-nAChR subunit binding (Fig. 2, Table 4). There was, however, one trend observed at the DMX for decreased α7-nAChR binding. Finally, in further support of the relationship between effects of nicotine on receptor binding and on fetal physiology, there was a strong correlation (r = +0.87, P < 0.001) between RMSSD (vagal activity) and 3H-epibatidine nAChR binding in the DMX (Fig. 1C).
DISCUSSION
Prenatal exposure to nicotine alters the development and function of cholinergic and other neurotransmitter systems in animal models (14, 40, 56, 59). As a step towards deciphering the underlying basis of the effect of exogenous nicotine in maternal cigarette smoke upon the developing medullary 5-HT system, we examined the effects of chronic prenatal nicotine exposure from mid- to late gestation in the fetal baboon upon cardiorespiratory control and 5-HT medullary markers. The maternal nicotine and cotinine levels attained in this study are similar to afternoon levels reported in pregnant women smoking, on average, 15 cigarettes per day or wearing a 22 mg/day nicotine patch (47). We show here that nicotine crosses the primate placenta, achieving fetal levels similar to those in the mother. Parenthetically, in humans, while maternal and fetal cotinine levels are similar, fetal nicotine levels may be higher than those of the smoking mother (40). The major findings of this study in the prenatal baboon fetus are that chronic exposure to nicotine at clinically relevant concentrations alters 5-HT and nAChR binding in regions of the medulla critical to cardiorespiratory control and that these alterations are associated with abnormalities in parasympathetic function. In the following discussion, we highlight these findings in the context of the relevant biology of autonomic and respiratory control, 5-HT and nAChR in the medulla, and risk for SIDS.
Prenatal nicotine exposure and cardiorespiratory control in the fetal baboon.
Our major physiological finding is that prenatal nicotine exposure results in an increase in beat-to-beat heart rate variability, as measured by RMSSD and high-frequency spectral power in RR interval variability. Increased high-frequency beat-to-beat variability is associated with increased mean levels of parasympathetic activation and greater variability in vagal activity. Thus our finding of increased heart rate variability supports the interpretation that nicotine mediates increases in the level of fetal parasympathetic activity. Overall, the decreases in heart rate in the nicotine-exposed animals were not significant, although, at higher rates of breathing, the effect was at the P < 0.06 level, suggesting that, with more subjects, decreased heart rate would be another indication of increased parasympathetic activity. Although RMSSD appears to be a prenatal physiological marker of the biological effects of exposure to nicotine, measurements of fetal heart rate obtained clinically with the traditional ultrasound techniques do not yield a sufficiently accurate assessment of RR interval to characterize RMSSD or high-frequency beat-to-beat variability. New technologies, such as transabdominal fetal ECG or magnetocardiography, will afford the means to investigate this phenomenon in human subjects.
It has long been recognized that acute exposure of the fetus to maternal smoking decreases the amount of fetal breathing (22, 43). However, in the present study, we found no significant effects of chronic exposure to nicotine on any of the fetal breathing activity measures examined (rate, rate variability, percent time with fetal breathing). There are several possible explanations for the apparent discrepancy between previous reports and our findings. First, there may be important species differences in responses to nicotine, with the baboon being uniquely unresponsive to nicotine with regard to effects on respiratory control. In our view, a more likely explanation is in differences among studies in the ways that the fetal breathing response is tested. Unlike fetal human studies, which measured fetal breathing responses to acute exposure to maternal smoking, we investigated the effects of nicotine during chronic exposure. Moreover, studies that have demonstrated long-term effects of prenatal exposure to nicotine on respiratory control were all conducted postnatally, when the animals breathe air, and most effects are seen following a ventilatory challenge and when the offspring are no longer being exposed to high levels of nicotine. The subjects in the present study were not “breathing”, they were engaged in episodic fetal breathing activity, and spontaneous fetal activity was assessed, not the response in activity to challenges. It is possible that, if the baboons exposed to nicotine in our study had been examined during the early postnatal period, disturbances in respiratory control would have emerged, a hypothesis that warrants testing in postnatal animals.
5-HT receptor binding in the caudal raphé and parasympathetic activity.
The question arises as to the role of the medullary 5-HT system and 5-HT-nicotinic interactions in causing the bias to parasympathetic activity in the nicotine-exposed fetuses in this study. Overlap in 5-HT and nAChR binding abnormalities occurs in one site only, the raphé obscurus. This salient finding suggests the possibility that dysfunctional interactions between the 5-HT and cholinergic systems occurring at this site may be paramount in the pathogenesis of the parasympathetic abnormalities. The raphé obscurus is central to 5-HT's regulation of cardiorespiratory control via widespread projections to other brain stem sites and spinal cord (5, 27). It is implicated in the control of blood pressure (7), heart rate (23), baroreceptor reflex (23), respiration via projections to the pre-Bötzinger complex (49), chemosensitivity to carbon dioxide (53), and sleep regulation (6). It interfaces directly with the sympathetic nervous system in the control of blood pressure and heart rate via monosynaptic projections to the intermediolateral column of the spinal cord, that is, the preganglionic outflow of sympathetic drive (24, 39). Yet the raphé obscurus and 5-HT system are also heavily connected with the parasympathetic/vagal nuclei (NTS, DMX, and nucleus ambiguous), which are involved in cardiovascular regulation. Bradycardia induced in rabbits that breathe cigarette smoke, for example, is attenuated by intracisternal application of 8-OH-DPAT, a 5-HT1A receptor agonist, suggesting that 5-HT1A receptors play a role in modulating the excitability of vagal motoneurons (17). Furthermore, 5-HT released from the caudal raphé modulates the activity of the DMX (65), nucleus ambiguous, and NTS (64), and 5-HT1A and 5-HT2A receptors are heavily concentrated in the DMX and NTS in the human medulla (48, 52), as well as in the fetal baboon studied here.
In the fetal baboon, we demonstrated that 5-HT neurons in the medullary 5-HT system express nAChRs, thus providing a potential site of interaction whereby exogenous nicotine in cigarette smoke can affect the development and/or function of the 5-HT system. Furthermore, the actions of nicotine on the 5-HT system are likely to occur at not only the cell soma, but also via presynaptic modulation of 5-HT release at the synapse. In this light, it should be noted that binding abnormalities were observed in multiple nuclei critical for parasympathetic and sympathetic activity in the nicotine-exposed fetuses, and it is likely that a combination of sites and types of nAChR and/or 5-HT abnormalities leads to increased parasympathetic activity. This is supported by the observation of suggested changes in 5-HT2A receptor binding and the 5-HT transporter in the medullary 5-HT system and nAChR binding in vagal nuclei following prenatal nicotine exposure. The mechanism whereby prenatal nicotine exposure causes changes in 5-HT1A receptor binding in the medulla is unknown, but underscores the potential of this model for dissecting apart the cellular and molecular mechanisms in future studies.
Nicotinic receptor binding in the medullary 5-HT system.
We found constitutive nAChR expression in virtually all medullary nuclei, including those that are composed of 5-HT cell bodies and that mediate homeostatic control. Nicotinic receptors are pentameric, ligand-gated ion channels composed of heteromeric or homomeric subunits encoded by 9α (α2–10) and 3β (β2–4) genes (21), with α7 being the only subunit known to form homomeric receptors. They are present in the brain from early in gestation. In the human, nAChR mRNA is present by 4–6 wk (25), and thus the activation of nAChRs by nicotine may have profound effects on development. In fetal monkey brains from offspring of mothers exposed to nicotine, there is regional neuronal cell loss, decrease in cell size, and abnormal neurite outgrowth (59), the effects of which persist into the postnatal period (20, 58). Prenatal exposure to smoke or nicotine is typically associated with elevations in nAChR binding in the brain (41, 57), as was observed in this study for 3H-epibatidine. Despite this increase in binding, nAChRs are functionally desensitized with chronic nicotine exposure, i.e., there is a “functional loss” (19, 67). Because nAChRs promote the release of excitatory (glutamate) and inhibitory (GABA) neurotransmitters, as well as 5-HT, desensitization can lead to a reduction in the release of multiple transmitters (16). Nevertheless, this response appears to be subunit specific, as we found that nAChRs containing α7-subunits were not elevated following nicotine exposure in our fetal baboon model. Both the NTS and DMX were sites with the highest constitutive nAChR binding in the fetal baboon medulla, as well as abnormal 3H-epibatidine binding following nicotine exposure. Nicotinic receptors play a major role in the mediation of the function of the cardiac vagal neurons and are activated upon nicotine exposure (32). In rats, for example, prenatal nicotine exposure exacerbates postsynaptic excitatory currents in premotor cardiac vagal neurons with an alteration in the subunit composition of the nAChRs compared with unexposed rats (28).
Potential limitations of the study.
A potential limitation of this study is the small sample size due to the difficulty in obtaining neurochemical and physiological data in the same baboon pregnancy. Because these data are difficult to accrue, multiple parameters were obtained in each pregnancy. After correcting for multiple testing of primary variables, we found robust changes that underscore their potential validity. We did not, however, have sufficient power to analyze all measures available in this data set. Thus there may be other effects of nicotine not identified in this study. While we highlight our findings for 5-HT1A and nAChRs, there remain trends seen in nuclei that did not maintain significance after correcting for multiple testing. Interpretation of these trends is done cautiously, with recognition that potential significant differences warrant further study in a larger data set.
Implications of the study for SIDS.
The key finding of this study that is relevant to SIDS brain stem pathology and the increased risk for SIDS with maternal cigarette smoking during pregnancy is that prenatal exposure to nicotine is associated with alternations in 5-HT receptor binding in the raphé obscurus in the medullary 5-HT system, as well as alterations in nicotinic receptor binding throughout this system. This finding is important, because 5-HT receptor abnormalities have been found in the medullary 5-HT system of SIDS infants, with and without a history of maternal smoking during pregnancy (36, 50, 52). Based on this finding, we speculate that prenatal nicotine exposure exacerbates intrinsic 5-HT abnormalities that presumably originate in utero in SIDS by inducing combined 5-HT and nAChR abnormalities in key autonomic regions. Yet we caution that our fetal baboon model is not a model of SIDS, and thus the 5-HT and nAChR abnormalities in the fetal model do not mimic precisely those found in SIDS cases. In SIDS infants, for example, there is decreased (not increased) heart rate variability (55), reduced (not increased) 5-HT1A receptor expression in the raphé obscurus (52), and widespread abnormalities in 5-HT1A expression throughout the medulla, including the DMX and NTS (42) and not just the raphé obscurus. Furthermore, in SIDS, 5-HT transporter binding is reduced relative to overall 5-HT cell number (52), a parameter that we were unable to measure here, preventing direct comparisons. Important differences exist between SIDS cases and the fetal baboon model studied here. This study was limited to the examination of the fetus and thereby precluded examination of postnatal changes that are likely to contribute to SIDS risk, in addition to prenatal factors. The 5-HT system is dynamic in nature, especially during periods of rapid growth, i.e., the pre- and postnatal periods, and perturbations to this system, either directly or indirectly as a consequence of changes to other systems, can lead to transient or permanent changes. In addition, these effects appear dependent on the timing of the exposure and point of assessment, brain region under analysis, and sex (3, 58, 60, 68). Moreover, SIDS infants are exposed to multiple potential neurotoxins with cigarette smoking during and after pregnancy, and not just pure nicotine in the fetal period, as in this study of the fetal baboon. Furthermore, in infants dying of SIDS, consideration should be given to the contribution of other risk factors, such as alcohol consumption during pregnancy, upon the medullary 5-HT system, which often occurs in parallel with cigarette smoking; in this regard, prenatal exposure to alcohol has been shown to marginally reduce 5-HT receptor binding in the arcuate nucleus in the medullary 5-HT system in infants, including those who die of SIDS (36). Therefore, while the exact mechanisms linking maternal cigarette smoking to increased SIDS risk are yet to be determined, results from this study suggest the shift in autonomic balance in the fetal primate toward parasympathetic predominance with chronic exposure to nicotine may relate, in part, to abnormal interplay in 5-HT and cholinergic neurotransmitters that utilize 5-HT1A and nAChRs, respectively, in the raphé obscurus.
Given the above caveats, this study provides a biologically plausible mechanism for the association of increased SIDS risk with maternal smoking and nicotine exposure during pregnancy. The mechanism is based on the finding of correlations between the fetal medullary 5-HT system, particularly the raphé obscurus, with delivery of exogenous nicotine across the placenta and fetal autonomic nervous system activity. The clinical implications of these findings underscore the importance of analyses of heart rate variability in SIDS and correlations with brain stem neurotransmitter pathology. Further research should be devoted to the mechanisms whereby nicotine alters 5-HT brain stem structure/activity as a step toward a biologic basis for SIDS and increased risk in infants with 5-HT abnormalities and prenatal exposure to cigarette smoke.
GRANTS
The study was supported by National Institute of Child Health and Human Development Grant P01 HD13063 (R. I. Stark), the First Candle/SIDS Alliance, and CJ Martin Fellowship (National Health and Medical Research Council of Australia).
ACKNOWLEDGMENTS
The authors thank Richard A. Belliveau, Tung-wah Kiu, and Salha S. Daniel for assistance in this work. We also thank Dr. Thomas Cooper from Analytical Psychopharmacology Laboratories, Nathan Kline Institute, for the analysis of the plasma samples for nicotine and cotinine.
Present address of J. R. Duncan: Howard Florey Institute, University of Melbourne, Parkville, Victoria 3010, Australia.
REFERENCES
- 1. [Anon]. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 93: 1043–1065, 1996. [PubMed] [Google Scholar]
- 2. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 57: 289–300, 1995. [Google Scholar]
- 3. Benwell ME, Balfour DJ, Anderson JM. Smoking-associated changes in the serotonergic systems of discrete regions of human brain. Psychopharmacology (Berl) 102: 68–72, 1990. [DOI] [PubMed] [Google Scholar]
- 4. Blair PS, Sidebotham P, Berry PJ, Evans M, Fleming PJ. Major epidemiological changes in sudden infant death syndrome: a 20-year population-based study in the UK. Lancet 367: 314–319, 2006. [DOI] [PubMed] [Google Scholar]
- 5. Bowker RM, Westlund KN, Coulter JD. Origins of serotonergic projections to the lumbar spinal cord in the monkey using a combined retrograde transport and immunocytochemical technique. Brain Res Bull 9: 271–278, 1982. [DOI] [PubMed] [Google Scholar]
- 6. Brown JW, Sirlin EA, Benoit AM, Hoffman JM, Darnall RA. Activation of 5-HT1A receptors in medullary raphe disrupts sleep and decreases shivering during cooling in the conscious piglet. Am J Physiol Regul Integr Comp Physiol 294: R884–R894, 2008. [DOI] [PubMed] [Google Scholar]
- 7. Co W, Dantas MA, Pires JG, Futuro-Neto HA. Participation of nucleus raphe obscurus in the control of sympathetic activity in the anesthetized rat. Braz J Med Biol Res 23: 919–922, 1990. [PubMed] [Google Scholar]
- 8. Cowan MJ. Measurement of heart rate variability. West J Nurs Res 17: 32–48; discussion 101–111, 1995. [DOI] [PubMed] [Google Scholar]
- 9. Cucchiaro G, Commons KG. Alpha 4 nicotinic acetylcholine receptor subunit links cholinergic to brainstem monoaminergic neurotransmission. Synapse 49: 195–205, 2003. [DOI] [PubMed] [Google Scholar]
- 10. Daniel SS, James LS, MacCarter G, Morishima HO, Stark RI. Long-term acid-base measurements in the fetal and maternal baboon. Am J Obstet Gynecol 166: 707–712, 1992. [DOI] [PubMed] [Google Scholar]
- 11. Davis R. A Stereotaxic Atlas of the Brain of the Baboon (Papio). Austin: University of Texas Press, 1968. [Google Scholar]
- 12. Duncan JR, Paterson DS, Kinney HC. The development of nicotinic receptors in the human medulla oblongata: inter-relationship with the serotonergic system. Auton Neurosci 144: 61–75, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Duncan JR, Randall LL, Belliveau RA, Trachtenberg FL, Randall B, Habbe D, Mandell F, Welty TK, Iyasu S, Kinney HC. The effect of maternal smoking and drinking during pregnancy upon (3)h-nicotine receptor brainstem binding in infants dying of the sudden infant death syndrome: initial observations in a high risk population. Brain Pathol 18: 21–31, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dwyer JB, Broide RS, Leslie FM. Nicotine and brain development. Birth Defects Res C Embryo Today 84: 30–44, 2008. [DOI] [PubMed] [Google Scholar]
- 15. Flores CM, Rogers SW, Pabreza LA, Wolfe BB, Kellar KJ. A subtype of nicotinic cholinergic receptor in rat brain is composed of alpha 4 and beta 2 subunits and is up-regulated by chronic nicotine treatment. Mol Pharmacol 41: 31–37, 1992. [PubMed] [Google Scholar]
- 16. Fregosi RF, Pilarski JQ. Prenatal nicotine exposure and development of nicotinic and fast amino acid-mediated neurotransmission in the control of breathing. Respir Physiol Neurobiol 164: 80–86, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Futuro-Neto HA, Pires JG, Gilbey MP, Ramage AG. Evidence for the ability of central 5-HT1A receptors to modulate the vagal bradycardia induced by stimulating the upper airways of anesthetized rabbits with smoke. Brain Res 629: 349–354, 1993. [DOI] [PubMed] [Google Scholar]
- 18. Garland M, Szeto HH, Daniel SS, Tropper PJ, Myers MM, Stark RI. Zidovudine kinetics in the pregnant baboon. J Acquir Immune Defic Syndr Hum Retrovirol 11: 117–127, 1996. [DOI] [PubMed] [Google Scholar]
- 19. Gentry CL, Lukas RJ. Regulation of nicotinic acetylcholine receptor numbers and function by chronic nicotine exposure. Curr Drug Targets CNS Neurol Disord 1: 359–385, 2002. [DOI] [PubMed] [Google Scholar]
- 20. Good CH, Bay KD, Buchanan RA, McKeon KA, Skinner RD, Garcia-Rill E. Prenatal exposure to cigarette smoke affects the physiology of pedunculopontine nucleus (PPN) neurons in development. Neurotoxicol Teratol 28: 210–219, 2006. [DOI] [PubMed] [Google Scholar]
- 21. Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci 27: 482–491, 2006. [DOI] [PubMed] [Google Scholar]
- 22. Hafstrom O, Milerad J, Sandberg KL, Sundell HW. Cardiorespiratory effects of nicotine exposure during development. Respir Physiol Neurobiol 149: 325–341, 2005. [DOI] [PubMed] [Google Scholar]
- 23. Haselton JR, Winters RW, Liskowsky DR, Haselton CL, McCabe PM, Schneiderman N. Cardiovascular responses elicited by electrical and chemical stimulation of the rostral medullary raphe of the rabbit. Brain Res 453: 167–175, 1988. [DOI] [PubMed] [Google Scholar]
- 24. Helke CJ, Capuano S, Tran N, Zhuo H. Immunocytochemical studies of the 5-HT(1A) receptor in ventral medullary neurons that project to the intermediolateral cell column and contain serotonin or tyrosine hydroxylase immunoreactivity. J Comp Neurol 379: 261–270, 1997. [PubMed] [Google Scholar]
- 25. Hellstrom-Lindahl E, Court JA. Nicotinic acetylcholine receptors during prenatal development and brain pathology in human aging. Behav Brain Res 113: 159–168, 2000. [DOI] [PubMed] [Google Scholar]
- 26. Horne RS, Ferens D, Watts AM, Vitkovic J, Lacey B, Andrew S, Cranage SM, Chau B, Greaves R, Adamson TM. Effects of maternal tobacco smoking, sleeping position, and sleep state on arousal in healthy term infants. Arch Dis Child Fetal Neonatal Ed 87: F100–F105, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hornung JP. The human raphe nuclei and the serotonergic system. J Chem Neuroanat 26: 331–343, 2003. [DOI] [PubMed] [Google Scholar]
- 28. Huang ZG, Wang X, Evans C, Gold A, Bouairi E, Mendelowitz D. Prenatal nicotine exposure alters the types of nicotinic receptors that facilitate excitatory inputs to cardiac vagal neurons. J Neurophysiol 92: 2548–2554, 2004. [DOI] [PubMed] [Google Scholar]
- 29. Inder T, Neil J, Kroenke C, Dieni S, Yoder B, Rees S. Investigation of cerebral development and injury in the prematurely born primate by magnetic resonance imaging and histopathology. Dev Neurosci 27: 100–111, 2005. [DOI] [PubMed] [Google Scholar]
- 30. Jensen AA, Frolund B, Liljefors T, Krogsgaard-Larsen P. Neuronal nicotinic acetylcholine receptors: structural revelations, target identifications, and therapeutic inspirations. J Med Chem 48: 4705–4745, 2005. [DOI] [PubMed] [Google Scholar]
- 31. Kahn A, Groswasser J, Sottiaux M, Kelmanson I, Rebuffat E, Franco P, Dramaix M, Wayenberg JL. Prenatal exposure to cigarettes in infants with obstructive sleep apneas. Pediatrics 93: 778–783, 1994. [PubMed] [Google Scholar]
- 32. Kamendi H, Stephens C, Dergacheva O, Wang X, Huang ZG, Bouairi E, Gorini C, McIntosh JM, Mendelowitz D. Prenatal nicotine exposure alters the nicotinic receptor subtypes that modulate excitation of parasympathetic cardiac neurons in the nucleus ambiguus from primarily alpha3beta2 and/or alpha6betaX to alpha3beta4. Neuropharmacology 51: 60–66, 2006. [DOI] [PubMed] [Google Scholar]
- 33. Kinney HC. Abnormalities of the brainstem serotonergic system in the sudden infant death syndrome: a review. Pediatr Dev Pathol 8: 507–524, 2005. [DOI] [PubMed] [Google Scholar]
- 34. Kinney HC, Belliveau RA, Trachtenberg FL, Rava LA, Paterson DS. The development of the medullary serotonergic system in early human life. Auton Neurosci 132: 81–102, 2007. [DOI] [PubMed] [Google Scholar]
- 35. Kinney HC, Filiano JJ, White WF. Medullary serotonergic network deficiency in the sudden infant death syndrome: review of a 15-year study of a single dataset. J Neuropathol Exp Neurol 60: 228–247, 2001. [DOI] [PubMed] [Google Scholar]
- 36. Kinney HC, Randall LL, Sleeper LA, Willinger M, Belliveau RA, Zec N, Rava LA, Dominici L, Iyasu S, Randall B, Habbe D, Wilson H, Mandell F, McClain M, Welty TK. Serotonergic brainstem abnormalities in Northern Plains Indians with the sudden infant death syndrome. J Neuropathol Exp Neurol 62: 1178–1191, 2003. [DOI] [PubMed] [Google Scholar]
- 37. Lewis KW, Bosque EM. Deficient hypoxia awakening response in infants of smoking mothers: possible relationship to sudden infant death syndrome. J Pediatr 127: 691–699, 1995. [DOI] [PubMed] [Google Scholar]
- 38. Liu F, Garland M, Duan Y, Stark RI, Xu D, Dong Z, Bansal R, Peterson BS, Kangarlu A. Study of the development of fetal baboon brain using magnetic resonance imaging at 3 Tesla. Neuroimage 40: 148–159, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Loewy AD. Raphe pallidus and raphe obscurus projections to the intermediolateral cell column in the rat. Brain Res 222: 129–133, 1981. [DOI] [PubMed] [Google Scholar]
- 40. Luck W, Nau H, Hansen R, Steldinger R. Extent of nicotine and cotinine transfer to the human fetus, placenta and amniotic fluid of smoking mothers. Dev Pharmacol Ther 8: 384–395, 1985. [DOI] [PubMed] [Google Scholar]
- 41. Lv J, Mao C, Zhu L, Zhang H, Pengpeng H, Xu F, Liu Y, Zhang L, Xu Z. The effect of prenatal nicotine on expression of nicotine receptor subunits in the fetal brain. Neurotoxicology 29: 722–726, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Machaalani R, Say M, Waters KA. Serotoninergic receptor 1A in the sudden infant death syndrome brainstem medulla and associations with clinical risk factors. Acta Neuropathol (Berl) 117: 257–265, 2009. [DOI] [PubMed] [Google Scholar]
- 43. Manning F, Wyn Pugh E, Boddy K. Effect of cigarette smoking on fetal breathing movements in normal pregnancies. Br Med J 1: 552–553, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mathews TJ, MacDorman MF. Infant mortality statistics from the 2003 period linked birth/infant death data set. Natl Vital Stat Rep 54: 1–29, 2006. [PubMed] [Google Scholar]
- 45. Myers MM, Fifer W, Haiken J, Stark RI. Relationships between breathing activity and heart rate in fetal baboons. Am J Physiol Regul Integr Comp Physiol 258: R1479–R1485, 1990. [DOI] [PubMed] [Google Scholar]
- 46. Nachmanoff DB, Panigrahy A, Filiano JJ, Mandell F, Sleeper LA, Valdes-Dapena M, Krous HF, White WF, Kinney HC. Brainstem 3H-nicotine receptor binding in the sudden infant death syndrome. J Neuropathol Exp Neurol 57: 1018–1025, 1998. [DOI] [PubMed] [Google Scholar]
- 47. Ogburn PL, Jr, Hurt RD, Croghan IT, Schroeder DR, Ramin KD, Offord KP, Moyer TP. Nicotine patch use in pregnant smokers: nicotine and cotinine levels and fetal effects. Am J Obstet Gynecol 181: 736–743, 1999. [DOI] [PubMed] [Google Scholar]
- 48. Ozawa Y, Okado N. Alteration of serotonergic receptors in the brain stems of human patients with respiratory disorders. Neuropediatrics 33: 142–149, 2002. [DOI] [PubMed] [Google Scholar]
- 49. Pace RW, Mackay DD, Feldman JL, Del Negro CA. Role of persistent sodium current in mouse preBotzinger Complex neurons and respiratory rhythm generation. J Physiol 580: 485–496, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Panigrahy A, Filiano J, Sleeper LA, Mandell F, Valdes-Dapena M, Krous HF, Rava LA, Foley E, White WF, Kinney HC. Decreased serotonergic receptor binding in rhombic lip-derived regions of the medulla oblongata in the sudden infant death syndrome. J Neuropathol Exp Neurol 59: 377–384, 2000. [DOI] [PubMed] [Google Scholar]
- 51. Parslow PM, Cranage SM, Adamson TM, Harding R, Horne RS. Arousal and ventilatory responses to hypoxia in sleeping infants: effects of maternal smoking. Respir Physiol Neurobiol 140: 77–87, 2004. [DOI] [PubMed] [Google Scholar]
- 52. Paterson DS, Trachtenberg FL, Thompson EG, Belliveau RA, Beggs AH, Darnall R, Chadwick AE, Krous HF, Kinney HC. Multiple serotonergic brainstem abnormalities in sudden infant death syndrome. JAMA 296: 2124–2132, 2006. [DOI] [PubMed] [Google Scholar]
- 53. Peever JH, Necakov A, Duffin J. Nucleus raphe obscurus modulates hypoglossal output of neonatal rat in vitro transverse brain stem slices. J Appl Physiol 90: 269–279, 2001. [DOI] [PubMed] [Google Scholar]
- 54. Richerson GB, Wang W, Tiwari J, Bradley SR. Chemosensitivity of serotonergic neurons in the rostral ventral medulla. Respir Physiol 129: 175–189, 2001. [DOI] [PubMed] [Google Scholar]
- 55. Schechtman VL, Harper RM, Kluge KA, Wilson AJ, Hoffman HJ, Southall DP. Heart rate variation in normal infants and victims of the sudden infant death syndrome. Early Hum Dev 19: 167–181, 1989. [DOI] [PubMed] [Google Scholar]
- 56. Slotkin TA, MacKillop EA, Rudder CL, Ryde IT, Tate CA, Seidler FJ. Permanent, sex-selective effects of prenatal or adolescent nicotine exposure, separately or sequentially, in rat brain regions: indices of cholinergic and serotonergic synaptic function, cell signaling, and neural cell number and size at 6 months of age. Neuropsychopharmacology 32: 1082–1097, 2007. [DOI] [PubMed] [Google Scholar]
- 57. Slotkin TA, Pinkerton KE, Auman JT, Qiao D, Seidler FJ. Perinatal exposure to environmental tobacco smoke upregulates nicotinic cholinergic receptors in monkey brain. Brain Res Dev Brain Res 133: 175–179, 2002. [DOI] [PubMed] [Google Scholar]
- 58. Slotkin TA, Ryde IT, Tate CA, Seidler FJ. Lasting effects of nicotine treatment and withdrawal on serotonergic systems and cell signaling in rat brain regions: separate or sequential exposure during fetal development and adulthood. Brain Res Bull 73: 259–272, 2007. [DOI] [PubMed] [Google Scholar]
- 59. Slotkin TA, Seidler FJ, Qiao D, Aldridge JE, Tate CA, Cousins MM, Proskocil BJ, Sekhon HS, Clark JA, Lupo SL, Spindel ER. Effects of prenatal nicotine exposure on primate brain development and attempted amelioration with supplemental choline or vitamin C: neurotransmitter receptors, cell signaling and cell development biomarkers in fetal brain regions of rhesus monkeys. Neuropsychopharmacology 30: 129–144, 2005. [DOI] [PubMed] [Google Scholar]
- 60. Slotkin TA, Tate CA, Cousins MM, Seidler FJ. Prenatal nicotine exposure alters the responses to subsequent nicotine administration and withdrawal in adolescence: serotonin receptors and cell signaling. Neuropsychopharmacology 31: 2462–2475, 2006. [DOI] [PubMed] [Google Scholar]
- 61. Stark RI, Daniel SS, James LS, MacCarter G, Morishima HO, Niemann WH, Rey H, Tropper PJ, Yeh MN. Chronic instrumentation and long term investigation in the fetal and maternal baboon: tether system, conditioning procedures and surgical techniques. Lab Anim Sci 39: 25–32, 1989. [PubMed] [Google Scholar]
- 62. Stark RI, Daniel SS, Kim YI, Leung K, Rey HR, Tropper PJ. Patterns of development in fetal breathing activity in the latter third of gestation of the baboon. Early Hum Dev 32: 31–47, 1993. [DOI] [PubMed] [Google Scholar]
- 63. Stark RI, Myers MM, Daniel SS, Garland M, Kim YI. Gestational age related changes in cardiac dynamics of the fetal baboon. Early Hum Dev 53: 219–237, 1999. [DOI] [PubMed] [Google Scholar]
- 64. Thor KB, Helke CJ. Serotonin- and substance P-containing projections to the nucleus tractus solitarii of the rat. J Comp Neurol 265: 275–293, 1987. [DOI] [PubMed] [Google Scholar]
- 65. Travagli RA, Gillis RA. Effects of 5-HT alone and its interaction with TRH on neurons in rat dorsal motor nucleus of the vagus. Am J Physiol Gastrointest Liver Physiol 268: G292–G299, 1995. [DOI] [PubMed] [Google Scholar]
- 66. Willinger M, James LS, Catz C. Defining the sudden infant death syndrome (SIDS): deliberations of an expert panel convened by the National Institute of Child Health and Human Development. Pediatr Pathol 11: 677–684, 1991. [DOI] [PubMed] [Google Scholar]
- 67. Wonnacott S. The paradox of nicotinic acetylcholine receptor upregulation by nicotine. Trends Pharmacol Sci 11: 216–219, 1990. [DOI] [PubMed] [Google Scholar]
- 68. Xu Z, Seidler FJ, Ali SF, Slikker W, Jr, Slotkin TA. Fetal and adolescent nicotine administration: effects on CNS serotonergic systems. Brain Res 914: 166–178, 2001. [DOI] [PubMed] [Google Scholar]
- 69. Zeskind PS, Gingras JL. Maternal cigarette-smoking during pregnancy disrupts rhythms in fetal heart rate. J Pediatr Psychol 31: 5–14, 2006. [DOI] [PubMed] [Google Scholar]