Abstract
Although testosterone administration elicits well-documented anabolic effects on skeletal muscle mass, the enhancement of muscle regeneration after injury has not been widely examined. The purpose of this study was to determine whether anabolic steroid administration improves skeletal muscle regeneration from bupivacaine-induced injury. Male C57BL/6 mice were castrated 2 wk before muscle injury induced by an intramuscular bupivacaine injection into the tibialis anterior (TA) muscle. Control mice received an intramuscular PBS injection. Anabolic steroid [nandrolone decanoate (ND), 6 mg/kg] or sesame seed oil was administered at the time of initial injury and continued every 7 days for the study's duration. Mice were randomly assigned to one of four treatment groups for 5, 14, or 42 days of recovery, as follows: 1) control (uninjured); 2) ND only (uninjured + ND); 3) bupivacaine only (injured); or 4) bupivacaine + ND (injured + ND). TA morphology, protein, and gene expression were analyzed at 14 and 42 days after injury; protein expression was analyzed at 5 days after injury. After 14 days of recovery, the injury and injury + ND treatments induced small-diameter myofiber incidence and also decreased mean myofiber area. The increase in small-myofiber incidence was 65% greater in injury + ND muscle compared with injury alone. At 14 days, injury + ND induced a fivefold increase in muscle IGF-I mRNA expression, which was greater than injury alone. Muscle Akt activity and glycogen synthetase kinase-3β activity were also induced by injury + ND at 14 days of recovery, but not by injury alone. ND had a main effect for increasing muscle MyoD and cyclin D1 mRNA expression at 14 days. After 42 days of recovery, injury + ND increased large-diameter myofiber incidence compared with injury only. Nandrolone decanoate (ND) administration can enhance castrated mouse muscle regeneration during the recovery from bupivacaine-induced injury.
Keywords: muscle regeneration, anabolic steroids, myotoxin
testosterone is a critical regulator of skeletal muscle mass. Circulating testosterone levels are decreased by many clinically relevant conditions, including advancing age, as a side effect of cancer treatments, and in wasting diseases, such as acquired immunodeficiency syndrome (AIDS) (9, 38, 49). The ablation of circulating testosterone by castration can induce atrophy to mouse hindlimb muscles, varying in phenotype and function, and testosterone replacement can restore the mass of these muscles (4). A long-standing research question that is related to testosterone's regulation of skeletal muscle mass, but less fervently studied, is whether testosterone administration can benefit muscle regeneration from injury (52).
The effect of exogenous testosterone on muscle regeneration in the intact animal has been investigated with several types of muscle injury (6, 16, 17). Several questions related to the role of testosterone on muscle regeneration still remain, since the interpretation of these studies must account for type of injury inflicted, the outcome variables used to assess regeneration, and the type of muscle examined (6, 16, 17). Additionally, the majority of studies have not examined anabolic steroid administration in castrated or diseased male rodents that have diminished or ablated baseline testosterone levels. Exogenous testosterone administration in the form of ND to noncastrated rats has proven beneficial for gastrocnemius muscle regeneration from crush injury (6). Rat soleus muscle mass accretion in intact rats is also enhanced by anabolic steroid administration 25 days after snake venom-induced muscle injury, while fast-glycolytic extensor digitorum longus muscle mass accretion was not altered (16). Exogenous testosterone or anabolic steroid regulation of the rate and extent of muscle regeneration in castrated mice is in need of further investigation. The primarily fast-glycolytic mouse tibialis anterior (TA) muscle has been shown to be sensitive to circulating androgens and undergoes atrophy in castrated mice (4, 64). Additionally, testosterone has also been implicated in successful mouse TA regeneration from muscle graft surgery when comparing intact and castrated mice (19). Although the regulation of TA muscle regeneration from injury has been widely examined (4, 64), the impact of castration and anabolic steroid administration on these regenerative processes has not been established.
Skeletal muscle's initial regenerative response after injury has similarities to the physiological response of wound repair and requires the coordinated regulation of inflammation, extracellular matrix remodeling, and myofiber growth (2, 22). Testosterone has a documented ability to modulate the activity of immune, fibroblast, and myogenic precursor cells, which are all components of regeneration (18–20, 48, 66). Analysis of myotoxin-induced muscle injury has provided considerable insight into mechanisms that promote or limit skeletal muscle regeneration. Intramuscular injection of bupivacaine, a local anesthetic, causes extensive muscle fiber necrosis, initial muscle mass loss, and contractile force deficits, while leaving the basal lamina, nerves, and blood vessels intact (7, 40, 67). The initial phase of muscle recovery in this model is characterized by damaged tissue necrosis and an inflammatory response (58), which is then followed by satellite cell activation and proliferation (45). Morphological analysis has shown that many indexes of successful regeneration in healthy muscle can be completed within 2–3 wk of recovery from injury (34, 44). As recovery continues, muscle mass and force production return to uninjured values, and, at longer time points of recovery, bupivacaine-treated muscle can hypertrophy (42, 44). The effect of anabolic steroid administration on the regulation of regeneration in the mouse TA muscle has not been examined.
Insulin-like growth factor I (IGF-I) induced signaling can promote muscle regeneration after injury and is also a potential target for testosterone action during muscle regeneration (14, 30). IGF-I initiated signaling can stimulate muscle protein synthesis and satellite cell activity (14, 61). IGF-I overexpression also enhances recovery from myotoxin-induced injury (47, 56). Anabolic steroid administration has been shown to increase both circulating IGF-I and muscle mRNA expression of IGF-I (15, 55). Akt (protein kinase B) is a serine-threonine kinase and intracellular mediator of IGF-I/phosphatidylinositol 3-kinase signaling that can regulate muscle mass (24, 54). Akt is activated by phosphorylation at serine 473 (1), and activated Akt can phosphorylate glycogen synthetase kinase-3β (GSK-3β), inactivating this kinase, and removing inhibition of translation initiation factor eIF2b (41, 63). Although testosterone can increase Akt phosphorylation in cultured myotubes (46), anabolic steroid-induced hypertrophy in vivo can occur independent of the Akt/mammalian target of rapamycin signaling (21). Testosterone's effect on Akt and GSK-3β phosphorylation during regeneration from muscle injury is not certain.
The effect of anabolic steroid administration on bupivacaine-induced regeneration of the mouse TA muscle from castrated mice has not been investigated. The purpose of this study was to determine whether mouse TA muscle regeneration from bupivacaine-induced injury was responsive to anabolic steroid administration. We hypothesized that ND administration would increase muscle mass accretion by stimulating IGF-I signaling and small myofiber formation during the first 2 wk of the regenerative process, which will support enhanced muscle growth at 42 days after injury.
METHODS
Animals
C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME). The overall study consisted of four separate animal experiments. Study 1 examined protein expression 5 days postinjury. Study 2 examined muscle morphology, protein expression, and gene expression 14 days postinjury. Study 3 examined muscle morphology, protein expression, and gene expression 42 days postinjury. Study 4 examined muscle force production 42 days postinjury. All studies were initiated with male C57BL/6 mice that were ∼8 wk of age. All animals in the study were subject to castration surgery 1 wk after arrival at the University of South Carolina animal facility and 2 wk before the initiation of muscle injury (see Castration Surgery below). For studies 1–3, mice (n = 5–6) were randomly assigned to one of four treatment groups as follows: 1) uninjured; 2) ND only (uninjured + ND); 3) bupivacaine (injured); and 4) bupivacaine + ND (injured + ND). For study 4, animals (n = 3–6) were assigned to uninjured, injured, and injured + ND treatment groups. All animals were housed individually, kept on a 12:12-h light-dark cycle, and given ad libitum access to normal rodent chow and water for the duration of the study at the fully accredited animal care facilities at the University of South Carolina, Columbia, SC. The University of South Carolina Animal Care and Use Committee approved all procedures used in this study.
Castration Surgery
After 1 wk in the animal facility (∼9 wk of age), all mice were subjected to castration surgery. Mice were given a subcutaneous injection of ketamine/xylazine/acepromazine cocktail [1.4 ml/kg body wt (BW)]. A small incision (∼2cm) was made in the abdominal wall. Testes were located and pulled up through the incision. A cut through the epididymis was made to remove the testes. Following removal, the vas deferens were tied off, and the abdominal incision sealed with suture. All mice were given 2 wk to recover to allow a washout period for endogenous testosterone, before bupivacaine and steroid treatment (26).
Anabolic Steroid Administration
The anabolic steroid, ND (Deca-Durabolin, Organon), was used in these studies because of its long biological half-life and previous studies demonstrating an anabolic effect in rodent skeletal muscle (12, 59, 60). Fourteen days after the castration procedure, supraphysiological doses of ND (6 mg/kg BW) or sesame seed oil control were injected in the same volume intramuscularly into the hip region every 7 days, and the right and left hip were alternated each week for the duration of the study. Injection began on the day of bupivacaine injections and is referred to as day 0. Animals in the 14-day study received two injections at day 0 and day 7, while animals in the 42-day study received injections every 7 days, starting at day 0 and ending at day 35.
Bupivacaine Injection
Fourteen days after the castration procedure, on the same day as ND treatment, mice were given injections of bupivacaine or PBS in the TA muscle. A small (1 cm) incision was made in the skin overlying the distal one-third of the TA. A 25-gauge, 5/8 (0.5 × 16 mm) needle was inserted along the longitudinal axis of the muscle, and the bupivacaine was injected slowly as the needle was withdrawn. A second injection was performed ∼3 mm and at a 15° angle from the first injection. The injection volume of 0.75% bupivacaine (Marcaine HCl) for the mouse was 0.03 ml per injection. After the injection, the skin was bound with Vet-bond adhesive.
Muscle and Tibia Extraction
Mice were anesthetized with an intramuscular injection of ketamine hydrochloride (90 mg/kg BW), xylazine (7 mg/kg BW), and acepromazine (1 mg/kg BW). The TA muscles were extracted at 5, 14, and 42 days after bupivicaine injection. One side was snap frozen in liquid nitrogen and stored at −80°C for protein and gene expression analysis, and the other was cut at the midbelly, mounted in optimum cutting temperature compound (OCT), and then dropped in freezing isopentane. Once frozen, the sample was stored at −80°C for later morphological analysis. After the TA was dissected out, the tibia was removed and measured with a plastic caliper (Fine Science Tools, Foster City, CA).
Morphological Analysis
Sectioning of muscle and staining was performed the same as described previously (26, 31) and modified for the TA muscle. Cross-sectional area (CSA) analysis was done, as previously described (5, 35). Three distinct digital images from hematoxylin and eosin-stained muscle sections at a ×200 magnification were taken per muscle and digitally analyzed for muscle fiber CSA (μm2) with National Institutes of Health imaging software (Scion Image). Approximately 200 fibers were traced per sample, which, by examination of no additional change in standard deviation, was determined to be an appropriate fiber number. All fibers in the cross-sectional images were quantified, unless the sarcolemma was not intact. The classification of small and large fibers was determined by setting three standard deviations from the mean CSA for the uninjured group at both 14- and 42-day time points. The set point came to ∼3,500 μm2 to designate large fibers and 1,000 μm2 to designate small fibers at 14 days, and 3,250 μm2 to designate large fibers and 1,500 μm2 to designate small fibers at 42 days.
Force Measurements
Mice were anesthetized with a subcutaneous injection of ketamine/xylazine/acepromazine (1.4 ml/kg BW) cocktail. The distal tendon of the TA muscle was exposed through an incision on the anterior side of the ankle. The tendon was cut just before the insertion at the ankle. The tendon was tied with a 4.0 nylon suture and attached to an isometric transducer. The sciatic nerve was exposed with an incision in the hamstring region. The tibial nerve was cut just after the sciatic nerve splits into the tibial and peroneal nerves to eliminate any contraction from the gastrocnemius muscle causing background in the force data. The sciatic nerve was then cut as close to the hip bone as possible and dissected from surrounding fascia. The exposed sciatic nerve was then laid over two electrodes with a small piece of parafilm placed under the suspended nerve to prevent contact with other tissue. The nerve was kept moist with periodic treatment of mineral oil. The nerve was stimulated using a supramaximal square-wave pulse of 0.1-ms duration. Measurements were made at the length at which maximal tension was obtained during the twitch. Data were recorded for submaximal (P50 Hz; stimulating frequency of 50 Hz) and maximal (stimulation frequency of 75–150 Hz) isometric force. Specific maximal force was quantified by correcting for muscle mass.
RNA Isolation, cDNA Synthesis, and Real-time PCR
RNA isolation, cDNA synthesis, and real-time PCR were performed as previously described (32), using reagents from Applied Biosystems (Foster City, CA). Quantitative real-time PCR analysis was carried out in 25-μl reactions consisting of 2× SYBR green PCR buffer (AmpliTaq Gold DNA polymerase, buffer, dNTP mix, AmpErase UNG, MgCl2), 0.1 μl cDNA, RNase-free water, and 60 nM of each primer. The sequences for the primers were as follows: IGF-I forward, 5′-GCATTGTGGATGAGTGTTGC-3′; IGF-I reverse, 5′-GGGAGGCTCCTCCTACATTC-3′,18s forward, 5′-AAACGGCTACCACATCCAAG-3′ 18s reverse, 5′-CCCTCTTAATCATGGCCTCA-3′; skeletal α-actin forward, 5′-CTC CTA CGT GGG TGA TGA GG-3′; skeletal α-actin reverse, 5′-AGG TGT GGT GCC AGA TCT TC-3′. MyoD, cyclin D1 (FAM dye), and 18s (VIC dye) primers were purchased from Applied Biosystems gene expression assays. Samples were analyzed on an ABI 7300 Sequence Detection System. Reactions were incubated for 2 min at 50°C and 10 min at 95°C, followed by 40 cycles consisting of a 15-s denaturing step at 95°C and 1-min annealing/extending step at 60°C. Data were analyzed by ABI software using the cycle threshold (CT), which is the cycle number at which the fluorescence emission is midway between detection and saturation of the reaction. The 2−ΔΔCT method (28) was used to determine changes in gene expression between treatment groups, with the 18s CT as the correction factor.
Western Blotting
Western blot analysis was performed as previously described (35). Briefly, frozen TA muscle was homogenized in Mueller buffer and protein concentration determined by the Bradford method (10). Crude muscle homogenate (40 μg) was fractionated on 6–10% SDS-polyacrylamide gels. Gels were transferred to polyvinylidene difluoride membranes overnight. Membranes were Ponceau stained to verify equal loading of each gel. Membranes were blocked overnight in 5% milk in Tris-buffered saline with 0.1% Tween-20 (TBS-T). Primary antibodies for androgen receptor (AR) (Santa Cruz), pAkt (ser473), Akt, pGSK-3β (ser9), and GSK-3β (Cell Signaling) were diluted 1:1,000 to 1:500 in 5% milk in TBS-T, followed by 1-h incubation with membranes at room temperature. Anti-rabbit IgG horseradish-peroxidase-conjugated secondary antibodies (Cell Signaling) were incubated with the membranes at 1:2,000 dilutions for 1 h in 5% milk in TBS-T. Enhanced chemiluminescence (GE Healthcare Life Sciences, Piscataway, NJ) was used to visualize the antibody-antigen interactions. Images were digitally scanned, and blots were quantified by densitometry using scientific imaging software (Scion Image, Frederick, MD). The Ponceau-stained membranes were also digitally scanned, and the 45-kDa actin bands were quantified by densitometry and used as a protein loading correction factor for each lane.
Data Analysis
Results are reported as means ± SE. The χ2 analysis was used to determine differences in proportion of small fibers and proportion of large fibers between treatment groups. Two-way ANOVAs were used to assess main effects for injury, ND administration, or interactions between injury and ND at days 5, 14, or 42. Where significant interactions existed, Student-Newman-Keuls methods were used as post hoc analyses between treatments. A one-way ANOVA was used to determine differences in force data with Student-Newman-Keuls methods used to determine differences between groups. A P value of ≤0.05 was considered significant.
RESULTS
Body Weight and Tibia Length
Castration and anabolic steroid administration can both alter body mass and postnatal growth. There was a main effect of ND administration to increase body weight at 14 days (P = 0.009; Table 1) and 42 days (P < 0.001; Table 1), compared with uninjured controls. These differences in body weight may be related to ND offsetting body weight loss due to castration. There was no effect of bupivacaine-induced muscle injury on body weight at either 14 or 42 days. Mouse tibia lengths were used as indexes of animal size. Tibia length was not affected by 14 days of ND administration, but mice receiving 42 days of ND treatment showed a main effect of ND to increase tibia length compared with oil-injected mice. These results demonstrate that 42 days of ND administration accelerated body mass accretion and overall growth in castrated mice.
Table 1.
Body weight, tibialis anterior muscle weight, tibia length, tibialis anterior muscle weight normalized by tibia length, and mean myofiber cross-sectional area at 14 and 42 days after bupivacaine-induced injury
| Body Weight, g |
|||||||
|---|---|---|---|---|---|---|---|
| Treatment | n | Pre | Post | TA Weight, mg | Tibia, mm | TA/Tibia | CSA, μm2 |
| 14 Days of recovery from injury | |||||||
| Uninjured | 6 | 28.5±0.7 | 25.5±0.5 | 37.5±2.1 | 16.6±0.1 | 2.3±0.1 | 2,267±160 |
| Uninjured + ND | 5 | 27.4±0.9 | 28.0±1.2* | 39.6±1.7* | 16.4±0.2 | 2.4±0.1* | 2,486±94 |
| Injured | 6 | 28.2±1.0 | 25.1±0.6 | 39.5±1.7† | 16.6±0.1 | 2.4±0.1† | 1,966±140† |
| Injured + ND | 5 | 26.5±0.8 | 27.1±0.8* | 45.2±1.5*† | 16.4±0.1 | 2.7±0.1*† | 1,763±133† |
| 42 Days of recovery from injury | |||||||
| Uninjured | 5 | 26.0±0.5 | 25.4±0.8 | 42.3±1.7 | 17.0±0.1 | 2.5±0.1 | 2,400±126 |
| Uninjured + ND | 6 | 25.2±0.5 | 29.4±0.5* | 50.7±1.2* | 17.5±0.3* | 2.9±0.1* | 2,837±126* |
| Injured | 6 | 25.9±0.5 | 25.1±0.9 | 48.0±3.2† | 17.0±0.1 | 2.8±0.2† | 2,761±222† |
| Injured + ND | 6 | 25.7±0.3 | 29.8±0.4* | 61.1±2.36*† | 17.6±0.2* | 3.5±0.2*† | 3,207±155*† |
Values are means ± SE; n, no. of animals. ND, nandrolone decanoate; Pre, before; Post, after; TA, tibialis anterior; TA/tibia, TA muscle weight normalized by tibia length; CSA, cross-sectional area. Data were analyzed with a two-way ANOVA within each day of recovery.
Significant main effect of ND (P < 0.05).
Significant main effect of injury (P < 0.05).
Muscle Weight
TA muscle weight was recorded at 5, 14, and 42 days after bupivicaine-induced injury. At 5 days after injury, there was a main effect of injury to decrease muscle weight (uninjured 30.8 ± 1.5, uninjured + ND 39.1 ± 1.4, injured 26.7 ± 2.0, injured + ND 29.9 ± 2.4 mg; P = 0.003) in TA muscle weight independent of ND treatment, although there was also a main effect ND treatment to increased TA weight (P = 0.008). Both ND and injury independently increased TA weight at 14 and 42 days of recovery (Table 1). At 14 and 42 days of recovery, there was a main effect of both ND and injury to increase muscle mass. There was no significant interaction between ND and injury on TA weight at 14 (P < 0.327) or 42 days (P = 0.317) or recovery. Muscle weight corrected for tibia length was used to account for differences in overall body growth. The muscle-to-tibia length ratio showed main effect of both injury (P < 0.001; Table 1) and ND (P = 0.004; Table 1) to increase the ratio of TA weight and tibia length at 14 and 42 days of recovery.
Muscle Force
Recovery of muscle force production is an indicator of successful regeneration from injury (17). Muscle force was quantified in situ after 42 days of recovery from injury. Submaximal isometric force production at a stimulation of 50 Hz (P50) was not affected by injury at 42 days of recovery. P50 force was increased 33% (P = 0.05; Table 2) in injured muscle receiving ND compared with uninjured controls. Maximal isometric tetanic force was 40 and 22% greater in injured muscle receiving ND (P = 0.018; Table 2) compared with injured and uninjured control muscle, respectively. Although there was a 18 and 26% reduction in specific force in the injured and injured + ND groups, there was no significant change in force corrected for muscle mass among treatment groups.
Table 2.
Force measurements at Po, P50, and sP in the tibialis anterior muscle 42 days after bupivacaine-induced injury
| Treatment | n | Po, mN | P50, mN | sP, mN/g |
|---|---|---|---|---|
| Uninjured | 3 | 955±63 | 614±62 | 22,782±2,640 |
| Injured | 6 | 1,096±122 | 621±76 | 18,664±1,395 |
| Injured + ND | 6 | 1,336±90* | 818±61* | 16,906±1,388 |
Values are means ± SE; n, no. of animals. Po, maximal isometric force; P50, submaximal isometric force production at a stimulation of 50 Hz; sP, specific force (determined by mN at Po divided by muscle weight in g).
Significant difference from uninjured and injured groups (P < 0.05).
Muscle-fiber CSA
Fourteen days of recovery.
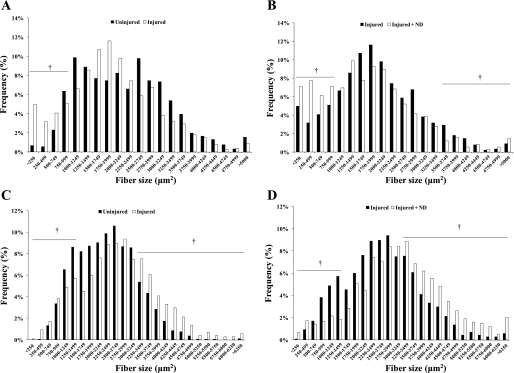
There was a main effect of injury to decreased myofiber mean CSA at 14 days of recovery (Table 1). Mean fiber area does not account for skeletal muscle shifts in overall fiber size distribution that are induced by remodeling stimuli. Muscle fibers were stratified by their CSA. At 14 days of recovery, the injured muscle demonstrated a significant increase (17 vs. 10%; P < 0.05) in the percentage of small-diameter fibers (<1,000 μm2), compared with the uninjured control muscle (Fig. 1A). Injured muscle also receiving ND had a significantly larger percentage of small-diameter fibers compared with the uninjured control (28 vs. 10%; P < 0.05) or injury without ND (28 vs. 17%; P < 0.05; Fig. 1B). Administration of nandrolone to uninjured muscle was able to increase large-diameter fibers (17 vs. 11%; P < 0.05) and decrease small-diameter fibers (4 vs. 10%; P < 0.05). However, injured muscle with nandrolone administration showed an increase in small fibers (28 vs. 4%; P < 0.05) compared with uninjured muscle with nandrolone, yet had a decrease in the percentage of large fibers (6 vs. 17%; P < 0.05).
Fig. 1.
Muscle fiber size distribution after 14 and 42 days of recovery from bupivacaine-induced injury, with or without nandrolone decanoate (ND) administration. Fiber distribution is shown for 14-day uninjured and injured (A) and injured and injured + ND (B) groups, and 42-day uninjured and injured (C) and injured and injured + ND (D) groups. †Difference in the percentage of small (<1,000 μm2) or large (>3,500 μm2) fibers, P ≤ 0.05.
Forty-two days of recovery.
There was a main effect of both ND and injury to increase mean fiber CSA after 42 days of recovery (Table 1), and there was no interaction between the treatments. Injury alone decreased the percentage of small-diameter (<1,500 μm2) fibers (17 vs. 20%; P < 0.05) and increased the large-diameter (>3,250 μm2) fibers (30 vs. 16%; P < 0.05; Fig. 1C) after 42 days of recovery (Fig. 1C). The injured muscle receiving ND had a significantly higher percentage of large-diameter fibers (47 vs. 30%; P < 0.05; Fig. 1D) and a reduction in small-diameter fibers (9 vs. 17%; P < 0.05) compared with injury alone. ND administration alone reduced the percentage of small-diameter fibers (11 vs. 20%; P < 0.05) and increased the percentage of large fibers (35 vs. 16%; P < 0.05) compared with the uninjured control muscle. The injured muscle receiving nandrolone had a similar percentage of small-diameter fibers (9% vs. 11%) compared with the uninjured muscle receiving nandrolone. However, the injured + ND group had an increase in percentage of large fibers (47 vs. 35%; P < 0.05) compared with the uninjured + ND group.
Myogenic Gene Expression and Growth-related Signaling
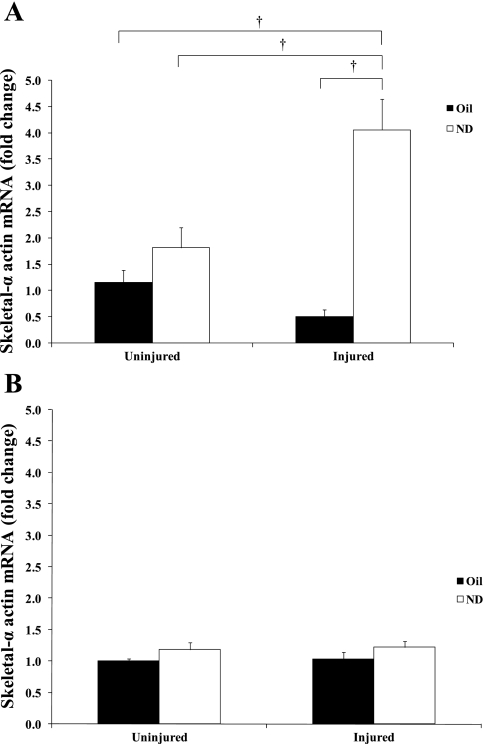
Skeletal α-actin mRNA expression.
Expression of thin-filament structural protein skeletal α-actin mRNA was quantified at 14 and 42 days of recovery (Fig. 2). At 14 days of recovery, there was an interaction between ND and injury to increase skeletal α-actin mRNA expression (P = 0.001), injured muscle receiving ND for 14 days increased skeletal α-actin mRNA expression fourfold above control levels (Fig. 2A), while injury alone did not alter skeletal α-actin expression. This ND induction of skeletal α-actin mRNA in injured muscle was also significantly greater (P = 0.011) than ND treatment without injury. At 42 days of recovery, there was a trend for a main effect of ND to increase skeletal α-actin (P = 0.066).
Fig. 2.
Skeletal α-actin gene expression at 14 (A) and 42 days (B) after bupivacaine-induced injury. All data were normalized to uninjured control. Injured groups were injected with bupivicaine. Uninjured groups were injected with PBS. Oil, solid bars; ND, open bars. Values are means ± SE. †Significant difference from injured + ND and all other groups, P ≤ 0.05.
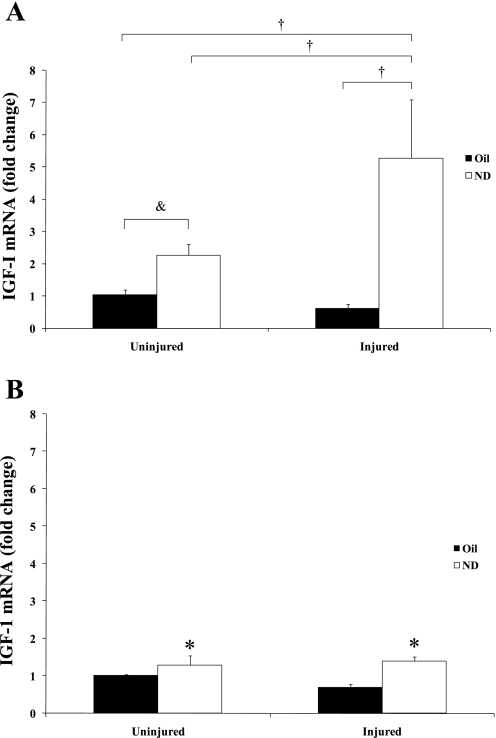
IGF-I mRNA expression.
Expression of muscle IGF-I mRNA was quantified at 14 and 42 days of recovery (Fig. 3, A and B, respectively). At 14 days of recovery, there was a significant interaction between muscle injury and ND administration that was not present at 42 days of recovery. Injured muscle receiving ND for 14 days increased muscle IGF-I mRNA expression fivefold above control levels (Fig. 3A), while injury alone did not change IGF-I gene expression at 14 days of recovery. This ND induction of IGF-I mRNA in the injured muscle was also significantly greater than ND treatment without injury. At 42 days, there was a main effect of ND to increase IGF-I mRNA expression.
Fig. 3.
IGF-I mRNA expression at 14 (A) and 42 days (B) of recovery after bupivacaine-induced injury. All data were normalized to uninjured control. Injured groups were injected with bupivicaine. Uninjured groups were injected with PBS. Oil, solid bars; ND, open bars. Values are means ± SE. &Significant difference between uninjured + ND and uninjured control; †significant difference from injured + ND; *main effect of ND: P ≤ 0.05.
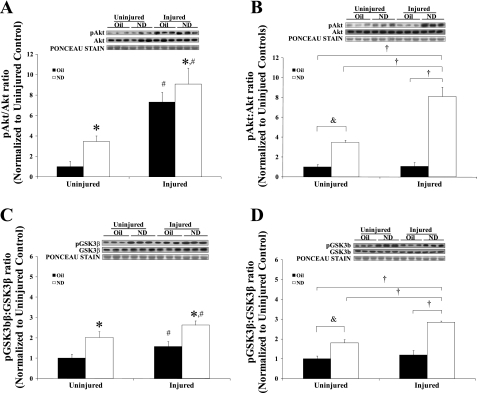
Akt activity.
The muscle expression of total and phosphorylated Akt (ser473) protein was determined at 5 and 14 days of recovery (Fig. 4, A and B, respectively). Akt activity is presented as the ratio of phosphorylated to total protein. All data are normalized to uninjured controls. There were no significant changes in total Akt protein with injury or ND administration at either recovery day. At 5 days of recovery, there was a main effect of both ND (P = 0.002) and injury (P = 0.001) to increase Akt activity. There was no interaction between injury and ND administration at 5 days of recovery. However, there was a significant interaction between injury and ND administration on Akt activity at 14 days of recovery. Injured muscle receiving ND increased Akt activity eightfold above control muscle at 14 days of recovery, whereas injury alone had no effect on Akt activity. While 14 days of ND administration increased Akt activity threefold (P = 0.001) in uninjured muscle, the induction in injured muscle receiving ND was twofold greater (P = 0.02) than the induction by ND alone.
Fig. 4.
Akt/glycogen synthetase kinase (GSK)-3β signaling at 5 and 14 days of recovery after bupivacaine-induced injury. Akt activity is shown 5 (A) and 14 days (B) after injury, and GSK-3β activity is shown 5 (C) and 14 days (D) after injury. All data were normalized to uninjured control. Injured groups were injected with bupivicaine. Uninjured groups were injected with PBS. Oil, solid bars; ND, open bars. Values are means ± SE. *Main effect of ND; #main effect of injury; &difference between uninjured + ND and uninjured control; †difference from injured + ND, P ≤ 0.05.
GSK-3β activity.
The muscle expression of total and phosphorylated GSK-3β (ser9) protein was determined at 5 and 14 days of recovery (Fig. 4, C and D, respectively). GSK-3β activity is presented as the ratio of phosphorylated to total protein. There were no significant changes in total GSK-3β protein with injury or ND administration at either recovery day.
There was a main effect of both ND (P = 0.001) and injury (P = 0.028) to increase the ratio of phosphorylated to total GSK-3β at 5 days of recovery. There was no interaction between injury and ND administration at 5 days of recovery. After 14 days of recovery, there was a significant interaction between injury and ND administration on GSK-3β activity. Injured muscle receiving ND increased the ratio of phosphorylated to total GSK-3β approximately threefold above control muscle at 14 days of recovery, while injury alone had no effect on GSK-3β activity. While 14 days of ND administration increased the ratio of phosphorylated to total GSK-3β approximately twofold (P = 0.008) in uninjured muscle, the induction in injured muscle receiving ND was significantly greater (P = 0.002) than the induction by ND alone.
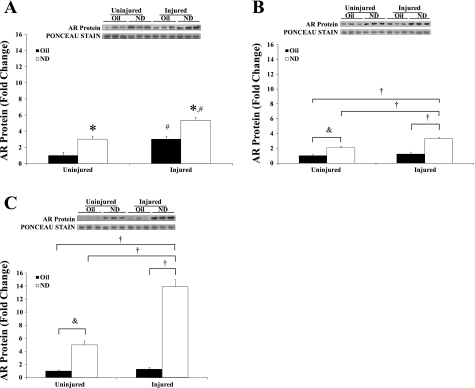
AR expression.
Muscle AR protein expression was determined at 5, 14, and 42 days of recovery (Fig. 5, A, B, and C, respectively). ND administration alone increased muscle AR protein expression two- to fivefold in uninjured muscle at 5, 14, and 42 days of administration. There was a main effect of injury at 5 days of recovery to increase AR protein expression, but no interaction between injury and ND administration. At 14 and 42 days of recovery, there were significant interactions between injury and ND administration for the induction of AR protein. Injured muscle receiving ND increased AR protein expression 3-fold above control at 14 days of recovery and 14-fold above control at 42 days of recovery. Injury alone did not have a significant effect on AR expression at either 14 or 42 days of recovery. The induction of AR expression in injured muscle receiving ND was significantly greater than the induction by ND alone at both 14 and 42 days of recovery. At 42 days of recovery (Fig. 5C), there was a threefold greater induction of AR expression in the injured muscle receiving ND compared with the ND administration alone.
Fig. 5.
Androgen receptor (AR) protein expression at 5 (A), 14 (B), and 42 days (C) after recovery from bupivacaine-induced injury. All data were normalized to uninjured control. Injured groups were injected with bupivicaine. Uninjured groups were injected with PBS. Oil, solid bars; ND, open bars. †Difference from injured + ND; &difference between uninjured + ND and uninjured control; *main effect of ND; #main effect of injury: P ≤ 0.05.
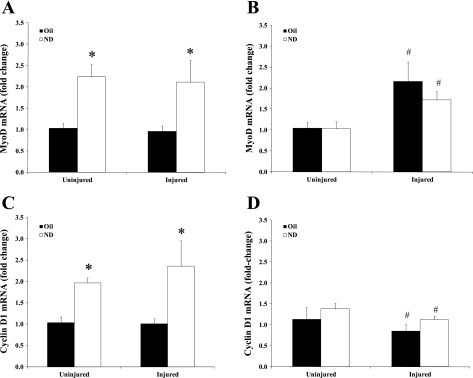
MyoD mRNA expression.
Expression of MyoD mRNA was quantified at 14 and 42 days recovery (Fig. 6, A and B, respectively). There was a main effect of ND administration to increase MyoD mRNA expression after 14 days of recovery (P < 0.001; Fig. 6A). There was no effect of injury on MyoD mRNA at day 14. After 42 days of recovery, there was a main effect of injury to increase MyoD mRNA expression, while there was no effect of ND administration (Fig. 6B).
Fig. 6.
Myogenic gene expression at 14 and 42 days after bupivacaine-induced injury. A: MyoD gene expression at 14 days of recovery after bupivacaine-induced injury. B: MyoD gene expression 42 days after injury. C: cyclin D1 gene expression after 14 days of recovery from injury. D: cyclin D1 gene expression after 42 days of recovery from injury. All data were normalized to uninjured control. Injured groups were injected with bupivicaine. Uninjured groups were injected with PBS. Oil, solid bars; ND, open bars. Values are means ± SE. *Main effect of ND; #main effect of injury: P ≤ 0.05.
Cyclin D1 mRNA expression.
Cell cycle regulator cyclin D1 mRNA expression was quantified at 14 and 42 days of recovery (Fig. 6, C and D, respectively). There was a main effect of ND administration to increase cyclin D1 mRNA expression after 14 days of recovery (P = 0.001; Fig. 6C). After 42 days of recovery, there was a main effect of injury to decrease cyclin D1 mRNA expression. ND administration did not have an effect on cyclin D1 mRNA expression after 42 days of recovery.
DISCUSSION
It is well established that testosterone and its synthetic analogs are potent regulators of skeletal muscle mass, and this has served as the basis for their therapeutic use in patients suffering from muscle-wasting diseases (8). Testosterone therapy also has the potential for aiding skeletal muscle regeneration after severe injury. Although exogenous testosterone administration has been shown to enhance rat soleus muscle mass and myosin protein accretion during regeneration from myotoxin-induced damage, this effect was suggested to be muscle phenotype specific (16). To the best of our knowledge, our study reports the novel finding that ND administration to castrated mice can accelerate myofiber growth during regeneration from injury in a primarily fast-glycolytic muscle. Although our findings provide no direct causal relationship, this response is associated with a synergistic induction of muscle IGF-I gene expression and Akt activation during the first 2 wk of recovery by the combination of injury and ND. Muscle AR expression was also synergistically induced by injury and ND and suggests that the injured muscle receiving ND was also more androgen sensitive. AR induction of target gene transcription may be important for the acceleration of muscle mass accretion by ND during recovery from injury.
Skeletal muscles have been reported to differ in their sensitivity to circulating androgen levels (3). The mouse TA is a primarily fast-glycolytic hindlimb muscle, whose mass has been shown to be affected by circulating androgen levels (4). TA muscle mass steadily declines over time in castrated mice, and testosterone replacement can reverse this mass loss (4). As little as 2 wk of ND administration significantly increased TA muscle mass and the percentage of large-diameter myofibers. Possibly related to the induction of muscle mass, ND also increased muscle sensitivity to circulating androgens. Muscle AR expression was induced two- to fivefold in uninjured muscle receiving ND administration for as short as 5 days or as long as 42 days. IGF-I is a potent regulator of skeletal muscle growth (62), and exogenous testosterone administration can induce muscle IGF-I expression (15). ND administration for 2 wk induced IGF-I expression and activated signaling through Akt and GSK-3β. The activation of phosphatidylinositol 3-kinase/Akt signaling could serve to increase the rate of protein synthesis in the uninjured muscle. Increased satellite cell activity is also thought to be an important mechanism for the anabolic action of testosterone in skeletal muscle (33, 50, 51). Although we did not directly measure satellite cell activation, gene expression of cellular regulator proteins associated with muscle growth and the activation of satellite cells were quantified. Our study demonstrates that MyoD mRNA and cyclin D1 mRNA are significantly induced by ND in uninjured muscle. The activation of satellite cells has been thought to be required to increase myonuclear number during postnatal and overload-induced muscle growth. It appears in uninjured muscle lacking an androgenic stimulus that anabolic steroids can stimulate muscle androgen sensitivity, IGF-I signaling related to protein accumulation, and myogenic cell proliferation as early as 5 days after treatment. This appears to be a potent multisignaling approach for muscle growth not requiring mechanical overload.
Castration is a potent stimulus for decreasing TA muscle mass, but our data demonstrate that testosterone was not required for restoration of muscle mass and force after bupivacaine injury. Coinciding with muscle regeneration and healing, bupivacaine- or myotoxin-induced injury also induces muscle hypertrophy after extended recovery in rodent hindlimb muscles (39, 44). Bupivacaine-induced hypertrophy of the mouse TA muscle was also not inhibited by castration. Injured muscle increased mass and had a significant shift to larger myofibers after 42 days of recovery, regardless of ND administration. These data demonstrate that the mouse TA muscle mass is sensitive to circulating androgen levels, but plasticity related to regeneration and hypertrophy after injury is retained. The question remaining is whether anabolic steroid administration enhances the rate of muscle mass accretion in the castrated mouse. Although muscle in the castrated mouse was able to successfully regenerate from injury, the present study provides evidence that anabolic steroid administration can enhance myofiber growth during injury recovery. There was a 57% increase in large-diameter fibers in injured muscle treated with ND for 42 days, compared with injury alone. Additionally, muscle peak tension was significantly increased in injured muscle treated with ND, compared with injury alone. Related to muscle mass accretion, there were significant main effects of both injury and ND administration to independently increase muscle mass after 14 and 42 days of recovery. However, due to the large effect of ND alone and variability within the regenerating muscles, there was no statistically relevant interaction between injury alone and injury plus ND at either 14 or 42 days of recovery. Mean muscle fiber CSA also demonstrated main effects of both ND and injury to independently increase myofiber area after 42 days of recovery. However, the induction of both large and small fibers with these treatments makes the examination of the myofiber size profile a more valid assessment of overall shifts than the size of muscle fibers. The increase in muscle force and percentage of large-diameter fibers shows that there was a potent interaction between bupivacaine-induced muscle regeneration processes and ND availability.
Intramuscular injection of bupivacaine has been shown to cause extensive muscle fiber necrosis and loss in muscle mass (7, 40, 67), which requires myogenesis during the initial phase of recovery to restore muscle mass (11, 27). There is evidence that processes related to the ND induction of myofiber growth in injured muscle begin early in the recovery process. Although bupivacaine injection alone increased the incidence of small-diameter fibers after 2 wk of recovery, anabolic steroid administration with injury induced a significantly greater number of small-diameter myofibers. This increase in small-diameter fibers may be the basis for sustained and accelerated ND-induced muscle enlargement throughout recovery. Skeletal α-actin gene expression, an essential sarcomeric protein, was significantly induced in injured muscle receiving ND at 14 days of recovery. The large induction of skeletal α-actin suggests that myofibers induced by regeneration and anabolic steroid administration are in an active growth state at 14 days of recovery. The present data extend these studies to demonstrate that anabolic steroid administration can accelerate the early myogenic response during regeneration from bupivacaine-induced muscle injury. The time course of myogenesis during the initial periods of recovery from bupivacaine or myotoxin-induced injury has been well described (25, 37, 64). Initially, muscle repair involves the activation of an inflammatory response and removal of damaged tissue (58). This phase is rapidly followed by satellite cell activation, which is required for myogenesis to proceed, and peaks 2–3 days post-myotoxin injury (45, 64). Subjecting regenerating muscle to gamma irradiation blocks satellite cell activity and impairs muscle regeneration (43, 64). Satellite cell replication and differentiation-related signaling are transiently increased after injury and typically returned to baseline after several days (25, 29, 64). Administration of agents that inhibit the early inflammatory response can also alter satellite cell proliferation, which will attenuate the regeneration process (36, 43, 53, 64). In rat skeletal muscle overloaded by ablation of synergists, simultaneous nandrolone administration can alter cell cycle regulator gene expression and suppress inflammatory gene expression at the onset of overload (33, 57).
Cyclin D1 and MyoD are increased the first few days after injury, and this time corresponds to peak satellite cell activity (37, 64). MyoD expression can stay elevated up to 28 days after myotoxin-induced injury (37). The induction of MyoD expression appears important for small-diameter fiber growth during muscle regeneration. The inhibition of MyoD expression during regeneration from myotoxin injury increases small-fiber incidence at 28 days of recovery and indicate a failure of the regenerative response to proceed successfully (65). The effect of anabolic steroids on myogenic gene expression during recovery from injury has not been previously examined. Androgens have a profound effect on skeletal muscle mass, in part, by activation of satellite cells (51). The satellite cell has high expression of ARs and serves as a direct target for androgen action in muscle (13). Our laboratory has previously reported ND administration increased both AR protein and cyclin D1 mRNA expression in rat hindlimb muscles (33). In our present study, ND induced muscle cyclin D1 mRNA expression after 14 days in injured and uninjured muscle. Myogenic gene expression due to injury typically returns to baseline after 2 wk (37, 64). ND administration may be widening the window of myogenic regulation during recovery and providing a mechanism for enhanced myofiber growth.
In the present study, ND induced muscle IGF-I mRNA expression, and this effect was magnified in injured muscle. IGF-I has been previously shown to enhance muscle regeneration (47, 56). Increased circulating IGF-I (47) and local overexpression of IGF-I (47, 56) in injured muscle enhanced regeneration. Growth processes induced by this IGF-I expression are likely not isolated to myogenesis, since there was no change in gene expression for MyoD or cyclin D1 at 14 days of recovery. The accentuated IGF-I expression, coupled with ND-induced expression of MyoD and cyclin D1 mRNAs, could be a potent stimulus for fiber enlargement during the entire 42-day recovery period. Downstream targets of IGF-I signaling are activated by IGF-I overexpression during muscle regeneration (47). Our findings suggest that Akt/GSK-3β signaling is activated early after injury, and this is independent of ND administration or endogenous testosterone. It has been shown that blocking testosterone will not block IGF-I expression after resistance training in men (23). However, the IGF-I measurement was taken only at 24 h after the resistance training session, and an effect of testosterone or anabolic steroids may be to prolong elevated IGF-I expression. Injury alone did not increase IGF-I gene expression at 14 days, and the corresponding activity of Akt/GSK-3β signaling was also not induced. It appears that injured muscle receiving ND has a prolonged activation of Akt activity due to an exaggerated induction of IGF-I, which may promote myogenesis and myofiber growth. At 14 days after injury, the Akt/GSK-3β pathway was only active in nandrolone-treated groups, with the highest activity seen in the injured muscle receiving ND. This enhanced signaling activity mirrors the IGF-I expression at 14 days and could be an upstream activator of the Akt/GSK-3β pathway.
In summary, these data demonstrate that ND administration can enhance myofiber growth after regeneration from injury. At 5 days after injury, protein signaling within the IGF-I pathway is activated, regardless of circulating androgens. At 14 days after injury, growth-related gene expression and protein signaling significantly increased over injured muscle not receiving ND and ND treatment in uninjured muscle. Two key mechanisms could support the anabolic steroid-induced growth at this time point: 1) the increase in IGF-I seen in injury + ND group; and 2) the increase in myogenesis observed with ND administration. The combination of the two elements could enhance fiber formation, which is supported by the large increase in small fibers seen with injury and ND. This process could set up later growth when given time to regenerate for 42 days after injury. We have shown an increase in large-diameter fibers at 42 days after injury and a reduction in small fibers with injury and ND treatment. These data have possible implications for the use of testosterone supplementation as a therapy to improve muscle regeneration in hypogonadal men. Exercise has been prescribed to preserve muscle mass in wasting conditions, including aging and disease-related cachexia, that are commonly associated with low levels of circulating testosterone. The combination of pharmacological and physical interventions may be complimentary to aid in successful recovery and maintenance of skeletal muscle in hypogonadal men.
GRANTS
This work was supported by the National Institutes of Health R03 AR051434-01A1 to James A. Carson.
ACKNOWLEDGMENTS
The authors thank Dr. Tyrone A. Washington, Marie Quig, and Dr. Raymond Thompson for technical assistance.
REFERENCES
- 1. Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol 7: 261–269, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Ambrosio F, Kadi F, Lexell J, Fitzgerald GK, Boninger ML, Huard J. The effect of muscle loading on skeletal muscle regenerative potential: an update of current research findings relating to aging and neuromuscular pathology. Am J Phys Med Rehabil 88: 145–155, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Antonio J, Wilson JD, George FW. Effects of castration and androgen treatment on androgen-receptor levels in rat skeletal muscles. J Appl Physiol 87: 2016–2019, 1999 [DOI] [PubMed] [Google Scholar]
- 4. Axell AM, MacLean HE, Plant DR, Harcourt LJ, Davis JA, Jimenez M, Handelsman DJ, Lynch GS, Zajac JD. Continuous testosterone administration prevents skeletal muscle atrophy and enhances resistance to fatigue in orchidectomized male mice. Am J Physiol Endocrinol Metab 291: E506–E516, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Baltgalvis KA, Berger FG, Pena MM, Davis JM, White JP, Carson JA. Muscle wasting and interleukin-6-induced atrogin-I expression in the cachectic Apc (−/+) mouse. Pflügers Arch 457: 989–1001, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beiner JM, Jokl P, Cholewicki J, Panjabi MM. The effect of anabolic steroids and corticosteroids on healing of muscle contusion injury. Am J Sports Med 27: 2–9, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Beitzel F, Gregorevic P, Ryall JG, Plant DR, Sillence MN, Lynch GS. Beta 2-adrenoceptor agonist fenoterol enhances functional repair of regenerating rat skeletal muscle after injury. J Appl Physiol 96: 1385–1392, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Bhasin S, Storer TW. Anabolic applications of androgens for functional limitations associated with aging and chronic illness. Front Horm Res 37: 163–182, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Bhasin S, Storer TW, Javanbakht M, Berman N, Yarasheski KE, Phillips J, Dike M, Sinha-Hikim I, Shen R, Hays RD, Beall G. Testosterone replacement and resistance exercise in HIV-infected men with weight loss and low testosterone levels. JAMA 283: 763–770, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 11. Camargo FD, Green R, Capetanaki Y, Jackson KA, Goodell MA. Single hematopoietic stem cells generate skeletal muscle through myeloid intermediates. Nat Med 9: 1520–1527, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Carson JA, Lee WJ, McClung J, Hand GA. Steroid receptor concentration in aged rat hindlimb muscle: effect of anabolic steroid administration. J Appl Physiol 93: 242–250, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Doumit ME, Cook DR, Merkel RA. Testosterone up-regulates androgen receptors and decreases differentiation of porcine myogenic satellite cells in vitro. Endocrinology 137: 1385–1394, 1996 [DOI] [PubMed] [Google Scholar]
- 14. Engert JC, Berglund EB, Rosenthal N. Proliferation precedes differentiation in IGF-I-stimulated myogenesis. J Cell Biol 135: 431–440, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ferrando AA, Sheffield-Moore M, Yeckel CW, Gilkison C, Jiang J, Achacosa A, Lieberman SA, Tipton K, Wolfe RR, Urban RJ. Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab 282: E601–E607, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Ferry A, Noirez P, Page CL, Salah IB, Daegelen D, Rieu M. Effects of anabolic/androgenic steroids on regenerating skeletal muscles in the rat. Acta Physiol Scand 166: 105–110, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Ferry A, Vignaud A, Noirez P, Bertucci W. Respective effects of anabolic/androgenic steroids and physical exercise on isometric contractile properties of regenerating skeletal muscles in the rat. Arch Physiol Biochem 108: 257–261, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Friedl R, Brunner M, Moeslinger T, Spieckermann PG. Testosterone inhibits expression of inducible nitric oxide synthase in murine macrophages. Life Sci 68: 417–429, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Grounds MD. Phagocytosis of necrotic muscle in muscle isografts is influenced by the strain, age, and sex of host mice. J Pathol 153: 71–82, 1987 [DOI] [PubMed] [Google Scholar]
- 20. Horiguchi T, Shibata MA, Ito Y, Eid NA, Abe M, Otsuki Y. Macrophage apoptosis in rat skeletal muscle treated with bupivacaine hydrochloride: possible role of MCP-1. Muscle Nerve 26: 79–86, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Hourde C, Jagerschmidt C, Clement-Lacroix P, Vignaud A, Ammann P, Butler-Browne GS, Ferry A. Androgen replacement therapy improves function in male rat muscles independently of hypertrophy and activation of Akt/mTOR pathway. Acta Physiol (Oxf) 195: 471–482, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Huard J, Li Y, Fu FH. Muscle injuries and repair: current trends in research. J Bone Joint Surg Am 84-A: 822–832, 2002 [PubMed] [Google Scholar]
- 23. Kvorning T, Andersen M, Brixen K, Schjerling P, Suetta C, Madsen K. Suppression of testosterone does not blunt mRNA expression of myoD, myogenin, IGF, myostatin or androgen receptor post strength training in humans. J Physiol 578: 579–593, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lai KM, Gonzalez M, Poueymirou WT, Kline WO, Na E, Zlotchenko E, Stitt TN, Economides AN, Yancopoulos GD, Glass DJ. Conditional activation of akt in adult skeletal muscle induces rapid hypertrophy. Mol Cell Biol 24: 9295–9304, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Launay T, Armand AS, Charbonnier F, Mira JC, Donsez E, Gallien CL, Chanoine C. Expression and neural control of myogenic regulatory factor genes during regeneration of mouse soleus. J Histochem Cytochem 49: 887–899, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Lee WJ, McClung J, Hand GA, Carson JA. Overload-induced androgen receptor expression in the aged rat hindlimb receiving nandrolone decanoate. J Appl Physiol 94: 1153–1161, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Lescaudron L, Peltekian E, Fontaine-Perus J, Paulin D, Zampieri M, Garcia L, Parrish E. Blood borne macrophages are essential for the triggering of muscle regeneration following muscle transplant. Neuromuscul Disord 9: 72–80, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[-delta delta C(T)] method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Marsh DR, Criswell DS, Carson JA, Booth FW. Myogenic regulatory factors during regeneration of skeletal muscle in young, adult, and old rats. J Appl Physiol 83: 1270–1275, 1997 [DOI] [PubMed] [Google Scholar]
- 30. Marsh DR, Criswell DS, Hamilton MT, Booth FW. Association of insulin-like growth factor mRNA expressions with muscle regeneration in young, adult, and old rats. Am J Physiol Regul Integr Comp Physiol 273: R353–R358, 1997 [DOI] [PubMed] [Google Scholar]
- 31. McClung JM, Davis JM, Wilson MA, Goldsmith EC, Carson JA. Estrogen status and skeletal muscle recovery from disuse atrophy. J Appl Physiol 100: 2012–2023, 2006 [DOI] [PubMed] [Google Scholar]
- 32. McClung JM, Lee WJ, Thompson RW, Lowe LL, Carson JA. RhoA induction by functional overload and nandrolone decanoate administration in rat skeletal muscle. Pflügers Arch 447: 345–355, 2003 [DOI] [PubMed] [Google Scholar]
- 33. McClung JM, Mehl KA, Thompson RW, Lowe LL, Carson JA. Nandrolone decanoate modulates cell cycle regulation in functionally overloaded rat soleus muscle. Am J Physiol Regul Integr Comp Physiol 288: R1543–R1552, 2005 [DOI] [PubMed] [Google Scholar]
- 34. McLoon LK, Nguyen LT, Wirtschafter J. Time course of the regenerative response in bupivacaine injured orbicularis oculi muscle. Cell Tissue Res 294: 439–447, 1998 [DOI] [PubMed] [Google Scholar]
- 35. Mehl KA, Davis JM, Berger FG, Carson JA. Myofiber degeneration/regeneration is induced in the cachectic Apc −/+ mouse. J Appl Physiol 99: 2379–2387, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Mendias CL, Tatsumi R, Allen RE. Role of cyclooxygenase-1 and -2 in satellite cell proliferation, differentiation, and fusion. Muscle Nerve 30: 497–500, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Mendler L, Zador E, Dux L, Wuytack F. mRNA levels of myogenic regulatory factors in rat slow and fast muscles regenerating from notexin-induced necrosis. Neuromuscul Disord 8: 533–541, 1998 [DOI] [PubMed] [Google Scholar]
- 38. Morley JE, Perry HM, 3rd, Kaiser FE, Kraenzle D, Jensen J, Houston K, Mattammal M, Perry HM., Jr Effects of testosterone replacement therapy in old hypogonadal males: a preliminary study. J Am Geriatr Soc 41: 149–152, 1993 [DOI] [PubMed] [Google Scholar]
- 39. Plant DR, Beitzel F, Lynch GS. Length-tension relationships are altered in regenerating muscles of the rat after bupivacaine injection. J Appl Physiol 98: 1998–2003, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Plant DR, Colarossi FE, Lynch GS. Notexin causes greater myotoxic damage and slower functional repair in mouse skeletal muscles than bupivacaine. Muscle Nerve 34: 577–585, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Rhoads RE. Signal transduction pathways that regulate eukaryotic protein synthesis. J Biol Chem 274: 30337–30340, 1999 [DOI] [PubMed] [Google Scholar]
- 42. Rosenblatt JD. A time course study of the isometric contractile properties of rat extensor digitorum longus muscle injected with bupivacaine. Comp Biochem Physiol Comp Physiol 101: 361–367, 1992 [DOI] [PubMed] [Google Scholar]
- 43. Rosenblatt JD, Parry DJ. Gamma irradiation prevents compensatory hypertrophy of overloaded mouse extensor digitorum longus muscle. J Appl Physiol 73: 2538–2543, 1992 [DOI] [PubMed] [Google Scholar]
- 44. Rosenblatt JD, Woods RI. Hypertrophy of rat extensor digitorum longus muscle injected with bupivacaine. A sequential histochemical, immunohistochemical, histological and morphometric study. J Anat 181: 11–27, 1992 [PMC free article] [PubMed] [Google Scholar]
- 45. Saito Y, Nonaka I. Initiation of satellite cell replication in bupivacaine-induced myonecrosis. Acta Neuropathol (Berl) 88: 252–257, 1994 [DOI] [PubMed] [Google Scholar]
- 46. Sato K, Iemitsu M, Aizawa K, Ajisaka R. Testosterone and DHEA activate the glucose metabolism-related signaling pathway in skeletal muscle. Am J Physiol Endocrinol Metab 294: E961–E968, 2008 [DOI] [PubMed] [Google Scholar]
- 47. Schertzer JD, Lynch GS. Comparative evaluation of IGF-I gene transfer and IGF-I protein administration for enhancing skeletal muscle regeneration after injury. Gene Ther 13: 1657–1664, 2006 [DOI] [PubMed] [Google Scholar]
- 48. Schneider CP, Schwacha MG, Samy TS, Bland KI, Chaudry IH. Androgen-mediated modulation of macrophage function after trauma-hemorrhage: central role of 5 alpha-dihydrotestosterone. J Appl Physiol 95: 104–112, 2003 [DOI] [PubMed] [Google Scholar]
- 49. Schreiber G, Ziemer M. The aging male–diagnosis and therapy of late-onset hypogonadism. J Dtsch Dermatol Ges 6: 273–279, 2008 [DOI] [PubMed] [Google Scholar]
- 50. Sinha-Hikim I, Roth SM, Lee MI, Bhasin S. Testosterone-induced muscle hypertrophy is associated with an increase in satellite cell number in healthy, young men. Am J Physiol Endocrinol Metab 285: E197–E205, 2003 [DOI] [PubMed] [Google Scholar]
- 51. Sinha-Hikim I, Taylor WE, Gonzalez-Cadavid NF, Zheng W, Bhasin S. Androgen receptor in human skeletal muscle and cultured muscle satellite cells: up-regulation by androgen treatment. J Clin Endocrinol Metab 89: 5245–5255, 2004 [DOI] [PubMed] [Google Scholar]
- 52. Sloper JC, Pegrum GD. Regeneration of crushed mammalian skeletal muscle and effects of steroids. J Pathol Bacteriol 93: 47–63, 1967 [DOI] [PubMed] [Google Scholar]
- 53. Soltow QA, Betters JL, Sellman JE, Lira VA, Long JH, Criswell DS. Ibuprofen inhibits skeletal muscle hypertrophy in rats. Med Sci Sports Exerc 38: 840–846, 2006 [DOI] [PubMed] [Google Scholar]
- 54. Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell 14: 395–403, 2004 [DOI] [PubMed] [Google Scholar]
- 55. Storer TW, Magliano L, Woodhouse L, Lee ML, Dzekov C, Dzekov J, Casaburi R, Bhasin S. Testosterone dose-dependently increases maximal voluntary strength and leg power, but does not affect fatigability or specific tension. J Clin Endocrinol Metab 88: 1478–1485, 2003 [DOI] [PubMed] [Google Scholar]
- 56. Takahashi T, Ishida K, Itoh K, Konishi Y, Yagyu KI, Tominaga A, Miyazaki JI, Yamamoto H. IGF-I gene transfer by electroporation promotes regeneration in a muscle injury model. Gene Ther 10: 612–620, 2003 [DOI] [PubMed] [Google Scholar]
- 57. Thompson RW, McClung JM, Baltgalvis KA, Davis JM, Carson JA. Modulation of overload-induced inflammation by aging and anabolic steroid administration. Exp Gerontol 41: 1136–1148, 2006 [DOI] [PubMed] [Google Scholar]
- 58. Tidball JG. Inflammatory cell response to acute muscle injury. Med Sci Sports Exerc 27: 1022–1032, 1995 [DOI] [PubMed] [Google Scholar]
- 59. Tsika RW, Herrick RE, Baldwin KM. Effect of anabolic steroids on overloaded and overloaded suspended skeletal muscle. J Appl Physiol 63: 2128–2133, 1987 [DOI] [PubMed] [Google Scholar]
- 60. Tsika RW, Herrick RE, Baldwin KM. Effect of anabolic steroids on skeletal muscle mass during hindlimb suspension. J Appl Physiol 63: 2122–2127, 1987 [DOI] [PubMed] [Google Scholar]
- 61. Vandenburgh HH, Karlisch P, Shansky J, Feldstein R. Insulin and IGF-I induce pronounced hypertrophy of skeletal myofibers in tissue culture. Am J Physiol Cell Physiol 260: C475–C484, 1991 [DOI] [PubMed] [Google Scholar]
- 62. Velloso CP. Regulation of muscle mass by growth hormone and IGF-I. Br J Pharmacol 154: 557–568, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Welsh GI, Stokes CM, Wang X, Sakaue H, Ogawa W, Kasuga M, Proud CG. Activation of translation initiation factor eIF2B by insulin requires phosphatidyl inositol 3-kinase. FEBS Lett 410: 418–422, 1997 [DOI] [PubMed] [Google Scholar]
- 64. Yan Z, Choi S, Liu X, Zhang M, Schageman JJ, Lee SY, Hart R, Lin L, Thurmond FA, Williams RS. Highly coordinated gene regulation in mouse skeletal muscle regeneration. J Biol Chem 278: 8826–8836, 2003 [DOI] [PubMed] [Google Scholar]
- 65. Zador E, Bottka S, Wuytack F. Antisense inhibition of myoD expression in regenerating rat soleus muscle is followed by an increase in the mRNA levels of myoD, myf-5 and myogenin and by a retarded regeneration. Biochim Biophys Acta 1590: 52–63, 2002 [DOI] [PubMed] [Google Scholar]
- 66. Zhang J, Pugh TD, Stebler B, Ershler WB, Keller ET. Orchiectomy increases bone marrow interleukin-6 levels in mice. Calcif Tissue Int 62: 219–226, 1998 [DOI] [PubMed] [Google Scholar]
- 67. Zink W, Graf BM. Local anesthetic myotoxicity. Reg Anesth 29: 333–340, 2004 [DOI] [PubMed] [Google Scholar]