Abstract
Hormone replacement therapy (HRT) is used in postmenopausal women to relieve symptoms of menopause and prevent osteoporosis. We sought to evaluate changes in mRNA expression of key myogenic factors in postmenopausal women taking and not taking HRT following a high-intensity eccentric resistance exercise. Fourteen postmenopausal women were studied and included 6 control women not using HRT (59 ± 4 years, 63 ± 17 kg) and 8 women using traditional HRT (59 ± 4 yr, 89 ± 24 kg). Both groups performed 10 sets of 10 maximal eccentric repetitions of single-leg extension on a Cybex dynamometer at 60°/s. Muscle biopsies of the vastus lateralis were obtained from the exercised leg at baseline and 4 h after the exercise bout. Gene expression was determined using RT-PCR for follistatin, forkhead box 3A (FOXO3A), muscle atrophy F-box (MAFbx), muscle ring finger-1 (MuRF-1), myogenic differentiation factor (MyoD), myogenin, myostatin, myogenic factor 5 (Myf5), and muscle regulatory factor 4 (MRF4). At rest, the HRT group expressed higher levels of MyoD, myogenin, Myf5, MRF4, and follistatin (P < 0.05). In response to eccentric exercise, follistatin, MyoD, myogenin, Myf5, and MRF4 were significantly increased (P ≤ 0.05) and FOXO3A, MAFbx, MuRF-1, and myostatin were significantly decreased in the control and HRT groups (P ≤ 0.05). Significantly greater changes in mRNA expression of follistatin, FOXO3A, MAFbx, MuRF-1, MyoD, myogenin, myostatin, Myf5, and MRF4 (p≤0.05) occurred in the HRT group than in the control group after exercise. These data suggest that postmenopausal women using HRT express higher myogenic regulatory factor gene expression, which may reflect an attempt to preserve muscle mass. Furthermore, postmenopausal women using HRT experienced a greater myogenic response to maximal eccentric exercise.
Keywords: follistatin, myostatin, myogenic differentiation factor
hormone replacement therapy (HRT) is commonly used in postmenopausal women to treat symptoms of menopause. Nevertheless, the effects of HRT on certain tissues, such as skeletal muscle, are unknown. A potential protective effect of estrogen may exist against exercise-induced skeletal muscle damage; however, the effects of HRT on myogenic gene expression after eccentric exercise in postmenopausal women is unknown. Previous studies have indicated that the response of myogenic gene expression in older women to resistance exercise is similar to that of young women (27). Additionally, estrogen and synthetic HRT may provide an anabolic effect on skeletal muscle (30, 35); thus it is important to investigate the effects of HRT use on myogenic gene expression. Quantifying changes in gene expression of myogenic regulatory factors, proteolytic genes, and growth factors are necessary to understand the effects of HRT on skeletal muscle gene expression and hypertrophy, particularly in older adults at risk of sarcopenia.
Members of the muscle regulatory factor (MRF) family, including myogenic differentiation factor (MyoD), myogenic factor 5 (Myf5), muscle regulatory factor 4 (MRF4), and myogenin, regulate muscle differentiation. Studies have indicated that MyoD and Myf5 stimulate the early onset of differentiation of myoblasts, whereas MRF4 and myogenin stimulate terminal differentiation of myoblasts (26). Proteolytic genes are involved in the ubiquitination and degradation of proteins. Exercise-responsive proteolytic genes involved in the muscle cell ubiquitin/proteolysis pathway (UPP) include muscle ring finger-1 (MuRF-1), muscle atrophy F-box (MAFbx), and forkhead box 3A (FOXO3A). MAFbx and MuRF-1 are associated with skeletal muscle atrophy (5), where FOXO3A acts as a common transcription factor (33). Muscle growth regulators also include myostatin and follistatin. Myostatin, a member of the transforming growth factor-β superfamily, is a negative regulator of myogenesis and controls the proliferation of muscle precursor cells (39). Myostatin inhibits expression of the myogenic regulatory factors (Myf5 and MyoD), thus inhibiting differentiation of skeletal muscle myoblasts (2, 39). Conversely, lower concentrations of myostatin may allow greater activation of MRFs and satellite cells responsible for muscle hypertrophy (25). Follistatin, a recognized inhibitor of myostatin (2), may be upregulated (39) by binding to the ACTIIB myostatin receptor site (19), allowing for greater muscle hypertrophy.
MRF, proteolytic, and muscle growth regulator gene expression have not been examined in postmenopausal women who are using HRT. In addition, although some studies have indicated that HRT does not influence muscle strength, muscle mass, or protect against sarcopenia (3, 16, 23, 29, 38), other studies have indicated that HRT use increases lean body mass (35) and muscle power (30). Therefore, the purpose of this investigation was to determine whether HRT potentiates a promyogenic response to maximal eccentric exercise in postmenopausal women. We hypothesized that in postmenopausal women, after a single bout of maximal eccentric exercise, HRT would enhance myogenic gene expression compared with women not taking HRT.
METHODS
Study participants.
We randomly selected 14 healthy, untrained (no regular resistance or aerobic training) postmenopausal women, 55–65 years of age, who were or were not taking HRT. Participants provided written informed consent approved by the University of Southern California Institutional Review Board. The participants must not have had spontaneous menstrual bleeding for at least 6 mo. Six served as the control group since they were not taking any form of HRT; the other eight participants were receiving HRT (active intervention group). Participants not using a particular hormone therapy must have not taken drug therapy for at least 3 mo.
Participants were recruited to the HRT group if they were using one of the following types of oral HRT for at least 3 mo: Premarin (0.3, 0.45, 0.625 mg tablet/day; Wyeth, Philadelphia, PA), Prempro (0.3, 0.45, 0.625 mg tablet/day; Wyeth), Estrace (0.5, 1.0 mg tablet/day; Warner Chilcott, Rockaway, NJ), FemHRT (0.5, 1.0 mg tablet/day; Warner Chilcott), or Menest (0.3, 0.625 mg tablet/day; King Pharmaceuticals, Bristol, TN). Premarin, Menest, and Estrace are synthetic forms of HRT containing estrogen only. Prempro and FemHRT are synthetic forms of HRT containing estrogen and progesterone. Oral forms and doses were chosen based on popularity of usage and common doses prescribed as determined by a university gynecologist. Because of a lack of research in HRT use and myogenic factors, there was no justification to limit recruitment of HRT users to either estrogen alone or estrogen-progestin forms. Additionally, we did not expect different types of HRT to affect the outcomes of the study.
Screening of participants was performed with clinical history and physical examination, as well as laboratory tests, including complete blood counts with differential, coagulation profile, prothrombin and partial thromboplastin times, fasting blood glucose, liver, and kidney chemistry panels, thyroid-stimulating hormone, lipid profile, urinalysis, ECG, and chest X-ray. Exclusion criteria included cancer within the last 5 years, diabetes or fasting blood sugar >126 mg/dl, cirrhosis or active hepatitis, uncontrolled thyroid function, chronic lung creatinine clearance <30 ml/min, alanine aminotransferase >1.5× upper limit of normal, or cardiovascular abnormalities (myocardial infarction, heart failure, or active angina within the prior 6 mo before enrollment), musculoskeletal disease or injury (rheumatoid arthritis, neuropathy, back injury, etc.) that would prevent resistance training, and use of testosterone or other anabolic therapies, heparin, or coumadin within the last 6 mo.
Body composition testing.
Total body mass, fat mass, lean mass, and percent fat were determined with a total body dual-energy X-ray absorptiometer (model DPX-IQ 2288 with Smart Scan version 4.7e; Lunar, Madison, WI) at baseline. Quality assurance was performed using a single acrylic block three times per week following the manufacturers' guidelines to confirm the accuracy and precision of the DEX dual-energy X-ray absorptiometer A system. The same experienced investigator was responsible for performing and analyzing all scans.
Physical activity assessment.
All potential participants were prescreened over the phone by the principal investigator to ensure that they were not participating in regular strenuous physical activity. Physical activity levels were assessed for each participant at baseline using the Entry Questionnaire and Physical Activity Scale (1).
Dietary analysis.
The participants completed a 3-day dietary food record and were instructed to record their dietary intake over the course of 2 weekdays and 1 weekend day. Participants confirmed their completed food record in an oral interview with a registered dietician to determine accuracy in portion size estimates and detail of food intake. Analysis of the dietary record was performed by the registered dietician using Nutritionist Pro (nutrition analysis software version 1.3, Jones and Bartlett Publishers, Sudbury, MA).
Strength testing.
Strength testing was performed using the Cybex Norm dynamometer (Cybex International, Ronkonkoma, NY) at baseline. The testing was preceded by 5 min of warm-up on a cycle ergometer. Participants received detailed instructions on the eccentric and concentric leg extension exercises and performed 10 repetitions of each exercise at maximal effort. The maximum torque of the knee extensors for eccentric and concentric leg extension was performed with the dominant leg at 60°/s. The Cybex was calibrated immediately before the exercise bout. Participants were positioned on the Cybex by visually aligning the placement of the lateral femoral condyle with the axis of rotation of the lever arm. A strap was placed distally on the dominant leg at the level of the load cell. The load cell was positioned 3 cm proximal to the talocural joint. During maximal loading, a shoulder harness, hip restraint, and thigh strap (exercised leg) were used to limit excessive movement and secure the participant to the device. The settings were recorded to ensure exact placement for each exercise bout.
The tester determined the participants' eccentric and concentric maximum torque as the highest produced torque. The maximum torque was determined to assess whether the participants were generating maximal effort during each set of the acute training bout. The strength testing was only performed once due to a recent investigation that reported repeat isokinetic strength testing is not warranted (37). Participants filled out and orally confirmed a modified Borg soreness scale (6) 2–3 days after the strength testing.
Eccentric resistance exercise bout.
The participants completed one bout of maximal single-leg eccentric knee extension exercise on the dominant leg using the Cybex Norm dynamometer at 60°/s 1 wk after strength testing. The Cybex was calibrated immediately before the exercise bout. Participants were positioned on the Cybex using the settings recorded from the strength testing visit. The exercise protocol consisted of 10 sets of 10 maximal eccentric repetitions with the participants performing the eccentric component while the investigator assisted the participants during the concentric component by moving the limb back to the starting position (15° before full extension). Each repetition was separated by the time it took the testing investigator to manually return the lever arm back to 75° of knee flexion (i.e., starting position). Each of the 10 sets was separated by 20 s. Biofeedback was provided on the computer screen, and verbal encouragement was provided by the tester during each repetition. The training bout occurred ∼2 wk after strength testing and 1 wk after the baseline muscle biopsy to allow recovery from soreness. Participants filled out and orally confirmed a modified Borg soreness scale (6) 2–3 days after the exercise bout.
Muscle biopsies.
Percutaneous muscle biopsies (150–200 mg) were obtained from the vastus lateralis of the exercised leg, 1 wk before the acute exercise bout and 4 h postexercise. Four hours after exercise is an optimal time point to assess changes in mRNA expression stimulated by exercise (22, 44). Biopsy specimens were collected using sterile conditions and local anesthesia (1% lidocaine) with a 5-mm Bergstrom muscle biopsy needle (Micrins Surgical, Lake Forest, IL) from the midportion of the vastus lateralis muscle. The postexercise biopsy was performed at a distance of 2–4 cm proximal to the first site. Muscle tissue samples were immediately flash-frozen in liquid nitrogen and stored at −80°C until processed for analysis.
RNA extraction and cDNA synthesis.
Total RNA was isolated after homogenization (Kinematica Polytron PT1200C) of 30–40 mg of muscle tissue with a monophasic solution containing guanidine-isothiocyanate lysis buffer and β-mercaptoethanol. The concentration and purity of the RNA were determined using a ultraviolet spectrophotometer (NanoDrop ND-1000, Thermo Scientific, Waltham, MA) by measuring absorbance at 260 and 280 nm; 500 ng of total skeletal muscle RNA were reverse transcribed to synthesize cDNA using Taqman reverse transcription reagents, according to the manufacturer's instructions (Applied Biosystems, Branchburg, NJ). RNA was reverse transcribed into cDNA with the following temperature/time protocol: 25°C for 10 min, 48°C for 30 min, 95°C for 5 min, and 4°C infinite (Mycycler; Bio-Rad, Hercules, CA).
Oligonucleotide primers for PCR.
Oligonucleotide primers were used to amplify the mRNA expression of follistatin, FOXO3A, MAFbx, MuRF-1, MyoD, myogenin, myostatin, Myf5, and MRF4. Primer sequences were designed using Primer3 program (32). The primer sequences for the specific target mRNAs and melt curve temperatures are shown in Table 1. Melt curves were determined for each primer set to ensure amplification of pure PCR products. GAPDH was used as an internal control for detecting changes in mRNA expression using quantitative real-time PCR (qRT-PCR), which has previously been used as an internal control to examine myogenic mRNA expression following resistance exercise (4, 13, 18, 24).
Table 1.
Primer sequences used for quantitative real-time PCR
| PCR Primer Sequence 5′ → 3′ | Melt Curve Temperature | |
|---|---|---|
| Follistatin | F: AAGACCGAACTGAGCAAGGA | 84°C |
| R: TTTTTCCCAGGTCCACAGTA | ||
| FOXO3A | F: GAACGTGGGAACTTCACTGGTGCTA | 84°C |
| R: GGTCTGCTTTGCCCACTTCCCCTT | ||
| MAFbx | F: CATCCTTATGTACACTGGTCCAAAGA | 82°C |
| R: TCCGATGATACACCCACATGTTAATG | ||
| MRF4 | F: CCCCTTCAGCTACAGACCCAAACAAGAA | 79°C |
| R: CCCCCTGGAATGATCGGAAACAC | ||
| MURF-1 | F: CTCAGTGTCCATGTCTGGAGGCCGTT | 79°C |
| R: GGCCGACTGGAGCACTCCTGTTTGTA | ||
| Myf5 | F: ATGGACGTGATGGATGGCTGCCAGTT | 83°C |
| R: GCGGCACAAACTCGTCCCCAAATT | ||
| MyoD | F: CACTACAGCGGCGACTCC | 79°C |
| R: TAGGCGCCTTCGTAGCAG | ||
| Myogenin | F: GGTGCCCAGCGAATGC | 84°C |
| R: TGATGCTGTCCACGATGGA | ||
| Myostatin | F: CTGTAACCTTCCCAGGACCA | 82°C |
| R: CCCATCCAAAAGCTCAAAA | ||
| GAPDH | F: AGCCACATCGCTCAGACA | 79°C |
| R: GCCCAATACGACCAAATCC |
F, forward; R, reverse; FOXO3A, forkhead box 3A; MAFbx, muscle atrophy F-box; MuRF-1, muscle ring finger-1; MyoD, myogenic differentiation factor; Myf5, myogenic factor 5; MRF4, muscle regulatory factor 4.
qRT-PCR.
A qRT-PCR method was applied to determine relative expression levels of mRNAs for follistatin, FOXO3A, MAFbx, MuRF-1, MyoD, myogenin, myostatin, Myf5, and MRF4. A total of 10 ng of cDNA were added to each of the 20-μl PCR reaction for follistatin, FOXO3A, MAFbx, MuRF-1, MyoD, myogenin, myostatin, Myf5, MRF4, and GAPDH. Specifically, each PCR reaction contained the following mixture: 10 μl of 2.5× iQSYBRgreen Supermix (Bio-Rad); 7 μl of RNase free water, 10 ng cDNA, and 10 pmol of each primer (forward and reverse) of interest. All samples were run in quadruplicate. Each PCR reaction was amplified using Bio-Rad iCycler iQ thermal cycler. Thermal cycling conditions were as specified by the manufacturer: the amplification profile involved the initial denaturation step at 95°C for 3 min, followed by 60 cycles consisting of denaturation at 95°C for 15 s, and primer annealing and extension at 56°C for 1 min.
mRNA quantification.
All data were determined by normalizing the cDNA measured in eight replicates (4 replicates repeated) for each participant sample to GAPDH (internal control) and then averaging the data to account for the change in mRNA expression as a result of the exercise stimulus. The data were then normalized to biopsy 1 (preexercise) for each participant to determine the change (in fold) of mRNA expression after exercising. All participant samples were then averaged to determine the average mRNA expression before and after exercise for each group. mRNA expression was evaluated by a relative quantification method (27). To compare the relative mRNA expression between the control and HRT groups, the 2^-ΔCT method (34) was used. This method calculates a value in arbitrary units (AU) of the gene of interest (GOI) expression normalized to the internal control gene (ICG) expression (ΔCT = CT GOI − CT ICG). The 2^-ΔΔCT method (21) was used to calculate the changes (in fold) in mRNA expression as a result of the eccentric exercise bout. In this method, GOI expression was normalized to ICG expression and normalized to preexercise value [ΔΔCT = (CT GOI postexercise − CT ICG postexercise) − (CT GOI preexercise − CT ICG preexercise)] within each group.
Statistical methods.
Statistical analyses were performed using SPSS, version 16.0 (SPSS, Chicago, IL). Descriptive statistics and Pearson correlations were used to analyze the control and HRT groups. An independent t-test was used to compare participant characteristics. Data were normally distributed, and parametric analyses were used to compare mRNA expression. For each gene, the changes (in fold) in mRNA expression, calculated by 2^-ΔΔCT, were compared initially using independent t-tests without covariates and additionally using a 2 × 2 analysis of covariance with factors of time (pre- to postexercise) and group (control and HRT). Weight and lean mass served as covariates. For analysis of mRNA baseline differences, calculated by 2^-ΔCT, independent t-tests were performed. A probability level of P ≤ 0.05 was used to determine statistical significance for all analyses.
RESULTS
Fourteen healthy postmenopausal women 55–65 years of age volunteered to participate in the study. Baseline characteristics of the study participants are displayed in Table 2. Weight and lean body mass were significantly greater in the HRT group than in the control group (P < 0.05). There were no statistically significant differences between groups with dietary intake and muscle strength. Peak torque generated during strength testing and the training bout were not significantly different between groups. Although there were group differences in weight and lean mass, the results did not differ when these variables were used as covariates. Therefore, the data presented reflect results from independent t-tests.
Table 2.
Participant characteristics
| Control | HRT | |
|---|---|---|
| (n = 6) | (n = 8) | |
| Age, years | 59.2±4.2 | 58.5±3.7 |
| Height, cm | 160.4±9.0 | 163.8±3.0 |
| Weight, kg | 63.1±17.4 | 89.5±23.7* |
| BMI, kg/cm2 | 35.4±11.5 | 47.2±11.9 |
| Percent fat, % | 34.1±13.6 | 45.1±7.9 |
| LBM, kg | 41.6±4.3 | 49.1±5.0* |
| Estrogen, pg/ml | 54.7±5.3 | 392.6±81.0* |
| Caloric intake, kcal† | 1652±387 | 1765±442 |
| Fat intake, g† | 67.7±39.4 | 82.1±37.7 |
| Protein intake, g† | 62.0±11.0 | 81.8±36.3 |
| Carbohydrate intake, g† | 239.5±59.6 | 197.9±47.1 |
| 1-RM eccentric, Nm | 108.7±52.9 | 132.7±58.6 |
| 1-RM concentric, Nm | 75.3±19.9 | 84.4±28.7 |
Values are means ± SD. HRT, hormone replacement therapy. BMI, body-mass index; LBM, lean body mass; 1-RM, one-repetition maximum.
Significantly different from control group, P < 0.05.
Expressed as daily averages. BMI, Body Mass Index; LBM, Lean Body Mass; kcal, kilocalories; g, grams; Nm, Newton meters.
The HRT group consisted of four estrogen-alone users and four estrogen-progesterone users. Our inclusion criteria specified forms of HRT, which included oral and transdermal types of low doses of estrogen and progesterone. All participants in the HRT group were using some form of oral HRT containing estrogen (Premarin, n = 2; Menest, n = 2) or a combination of estrogens and progesterone (Prempro, n = 2; FemHRT, n = 2). Therefore, whether the HRT use was estrogen alone or estrogen-progesterone, our results did not vary within the HRT group for all gene expression measures. Despite group differences in body composition with the HRT group and significantly heavier weight, group differences in myogenic mRNA expression were not affected by lean body mass or weight as covariates.
Dietary intake (3-day diet analysis) and physical activity levels were assessed to further control for potential group differences. A lack of significant group differences in caloric consumption and nutrient intake presented consistency among the population. Results from physical activity questionnaires did not present significant group differences. All participants had low activity levels with an average of 2.5 ±0.5 h of low-intensity exercise per week, consisting of predominantly household chores and leisure walking. Although dietary intake was not measured over the course of the study and the groups were not matched for body mass, we believe that changes in myogenic mRNA expression were a direct result of the exercise stimulus and HRT. There were no significant group differences in perceived muscle soreness or 1-repetition maximum between groups (Table 2). Average ratings of perceived muscle soreness for both groups was 5.0 ± 0.5 (strong perceived soreness) out of 10 for the 1-repetition maximum testing and 8.0 ± 0.5 (very strong perceived soreness) out of 10 for the acute exercise bout (P < 0.05).
Baseline gene expression.
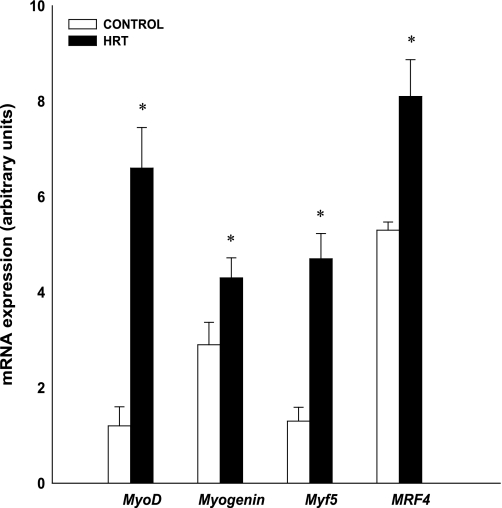
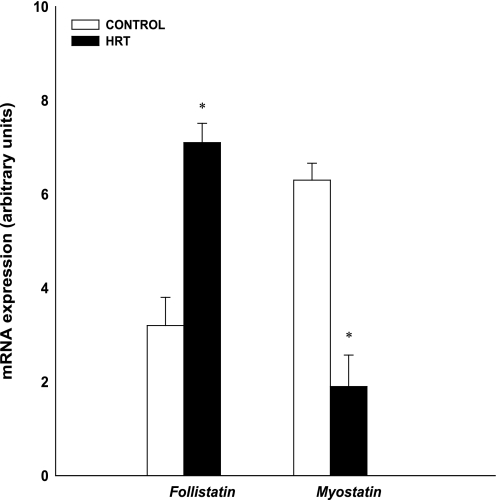
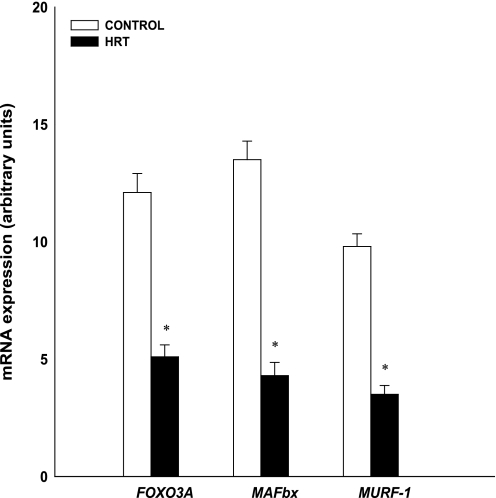
At rest (preexercise), gene expression was higher in the HRT group than in the controls for MyoD (HRT: 6.7 AU; control 1.6 AU), myogenin (HRT: 64.3 AU; control 2.9 AU), Myf5 (HRT: 4.7 AU; control 1.6 AU), MRF4 (HRT: 8.4 AU; control 5.3 AU), and follistatin (HRT: 7.1 AU; control 3.6 AU; Figs. 1 and 3; P < 0.05). Gene expression was higher in the control group than in the HRT group for FOXO3A (control 12.1 AU; HRT 5.1 AU), MAFbx (control 13.5 AU; HRT 4.3 AU), MURF-1 (control 9.8 AU; HRT 3.5 AU), and myostatin (control 6.3 AU; HRT 2.1 AU; Figs. 2 and 3 P < 0.05).
Fig. 1.
Comparison of baseline muscle regulatory factor (MRF) mRNA from skeletal muscle of control and hormone replacement therapy (HRT) groups. Expression of mRNA for each gene of interest was normalized to GAPDH. Values are means ± SE. *Significantly different from control group (P < 0.05). MyoD, myogenic differentiation factor; Myf5, myogenic factor 5; MRF4, muscle regulatory factor 4.
Fig. 3.
Comparison of baseline muscle growth regulator mRNA from skeletal muscle of control and HRT groups. Expression of mRNA for each gene of interest was normalized to GAPDH. Values are means ± SE. *Significantly different from control group (P < 0.05).
Fig. 2.
Comparison of baseline proteolytic mRNA from skeletal muscle of control and HRT groups. Expression of mRNA for each gene of interest was normalized to GAPDH. Values are means ± SE. *Significantly different from control group (P < 0.05). FOXO3A, forkhead box 3A; MAFbx, muscle atrophy F-box; MURF-1, muscle ring finger-1.
Myogenic regulatory factors.
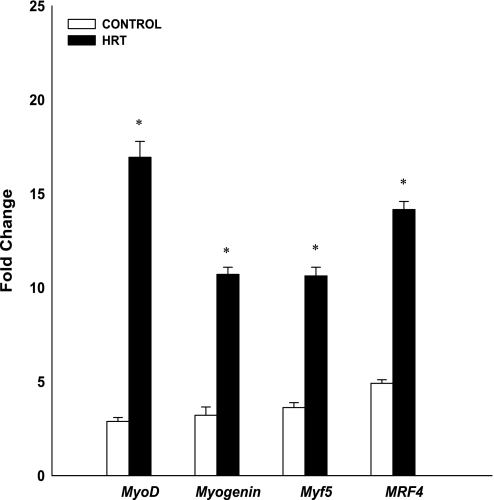
MRF gene expression of MyoD, myogenin, Myf5, and MRF4 increased postexercise for both groups (P < 0.01; Fig. 4). In addition, MRF gene expression was significantly greater for the HRT group than for controls (P < 0.01). MyoD mRNA expression increased 2.8- and 16.9-fold for the control and HRT groups, respectively. Myogenin gene expression increased 3.2- and 10.7-fold for the control and HRT groups, respectively. Myf5 gene expression increased 3.6- and 10.6-fold for the control and HRT groups, respectively. MRF4 gene expression increased 4.9- and 14.2-fold for the control and HRT groups, respectively.
Fig. 4.
Changes (in fold) in MRF mRNA expression after a bout of maximal eccentric resistance exercise normalized to GAPDH and relative to preexercise levels. Values are means ± SE. *Significantly different from control group (P < 0.01).
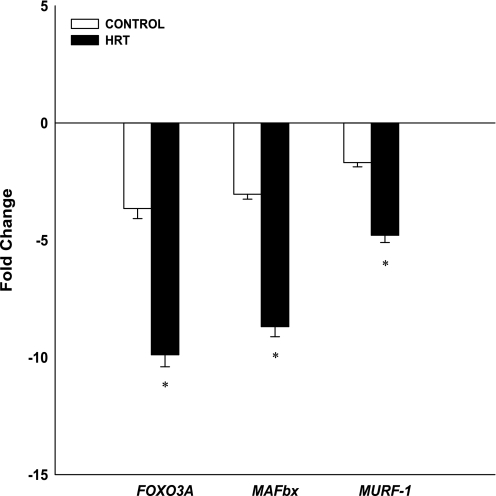
Proteolytic.
Proteolytic gene expression levels of FOXO3A, MAFbx, and MuRF-1 are shown in Fig. 5. FOXO3A, MAFbx, and MuRF-1 gene expression decreased postexercise for both groups (P < 0.01). The decrease in FOXO3A gene expression was significantly greater in the HRT group (−9.9-fold) than in the controls (−3.6-fold; P < 0.05). The decrease in MAFbx gene expression was significantly greater in the HRT group (−8.7-fold) than in the controls (−3.0-fold; P < 0.05). The decrease in MuRF-1 gene expression was significantly greater in the HRT group (−4.8-fold) than in the controls (−1.7-fold; P < 0.05).
Fig. 5.
Changes (in fold) in proteolytic mRNA expression following an acute bout of resistance exercise normalized to GAPDH and relative to preexercise levels. Values are means ± SE. *Significantly different from control group (P < 0.05).
Skeletal muscle growth regulators.
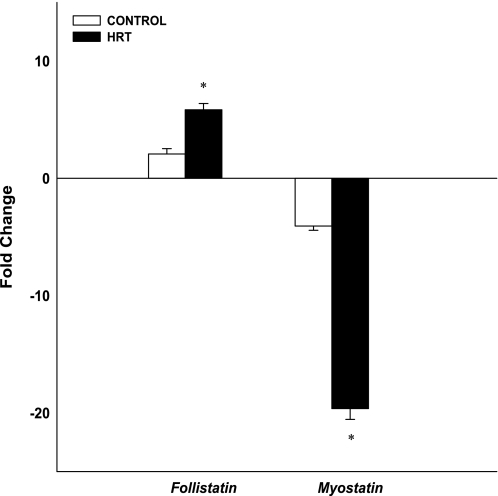
Follistatin and myostatin gene expression are shown in Fig. 6. Follistatin gene expression increased and myostatin gene expression decreased postexercise in both groups (P < 0.01). Follistatin gene expression was significantly greater in the HRT group (5.8-fold) than in controls (2.1-fold; P < 0.05). Decreased myostatin gene expression was significantly greater in the HRT group (−19.6-fold) than in the controls (−4.1-fold; P < 0.05).
Fig. 6.
Changes (in fold) in skeletal muscle growth regulator mRNA expression after an acute bout of resistance exercise normalized to GAPDH and relative to preexercise levels. Values are means ± SE. *Significantly different from control group (P < 0.05).
DISCUSSION
This is a novel study investigating changes in myogenic gene expression at rest and after maximal eccentric resistance exercise in postmenopausal women using HRT. Two important findings from our investigation include 1) at rest, postmenopausal women using HRT expressed higher levels of MRF and follistatin gene expression; and 2) after eccentric exercise, postmenopausal women using HRT experienced a greater mRNA response of all the genes of interest, including follistatin, FOXO3A, MAFbx, MuRF-1, MyoD, myogenin, myostatin, Myf5, and MRF4. These primary findings indicate that HRT use combined with maximal eccentric resistance exercise elicits a greater response in myogenic factors involved in muscle differentiation, growth, and hypertrophy.
Baseline mRNA expression.
Skeletal muscle gene expression profiling from microarray gene chip analysis has demonstrated gene expression differences between young and old adults (10, 31, 41, 42). Our investigation focused on strictly older women and compared myogenic gene expression of HRT users to non-HRT users. Our data support previous findings that suggest estrogen and synthetic HRT act as anabolic agents (30, 35) based on higher gene expression levels of the myogenic regulatory factors and follistatin in the HRT users. Although additional supportive literature is lacking, the role of estradiol in skeletal muscle has been investigated in vitro. Estradiol may play a role in skeletal myoblast growth as indicated by an increase in muscle fibers positively stained for myogenic markers (e.g., MyoD) in estrogen-treated myoblasts (8). Estrogen was reported to influence markers of satellite cell activation and have shown increases in MyoD with estrogen treatment in rat skeletal muscle cells (8, 9, 15). Thus a plausible explanation for our findings may involve satellite cell activation from consistent oral use of synthetic estradiol or estradiol + progestin. Recently, our laboratory demonstrated increased gene expression of MyoD with estradiol-treated human skeletal muscle cells (7), yet the mechanism of the influence of estradiol on MyoD remains unknown. Future investigations should consider the need to explore skeletal muscle gene expression differences with hormone therapy interventions in older women.
MRF mRNA response to exercise.
MRF gene expression of MyoD, myogenin, Myf5, and MRF4 increased postexercise. Our study is in accordance with findings from Raue et al. (27), who reported increases in MyoD, myogenin, and MRF4 after resistance exercise in older women (27). However, our study findings suggest an elevated response with HRT use in postmenopausal women. We report higher levels of MRF gene expression in the postmenopausal women using HRT than in postmenopausal women not using HRT. These results may indicate a potential protective effect of estrogen and or estrogen-progesterone therapy against age-related muscle loss (sarcopenia). MyoD and Myf5 stimulate the early onset of differentiation of myoblasts, whereas MRF4 and myogenin stimulate terminal differentiation of myoblasts (26), and these actions appear to be enhanced with HRT use when combined with maximal eccentric exercise. Therefore, stimulation or activation of these MRFs should contribute to an anabolic environment that would help maintain or augment muscle mass.
Proteolytic mRNA response to exercise.
Proteolytic genes involved in the muscle cell UPP include MuRF-1, MAFbx, and FOXO3A. The UPP is often associated with muscle atrophy (33); therefore, the proteolytic genes are indicative of protein degradation in the UPP. Raue et al. (28) investigated proteolytic gene expression in young and older women after resistance exercise and reported increased gene expression of FOXO3A, MAFbx, and MuRF-1 in both groups (28). Our results differ in that FOXO3A, MAFbx, and MuRF-1 mRNA expression decreased after exercise. Although in both studies muscle biopsies were performed 4 h postexercise, the exercise protocols differed. Raue et al. (28) employed an exercise protocol consisting of 3 sets of 10 repetitions of knee extension at 70% of the 1-RM, whereas our study utilized 10 sets of 10 maximal repetitions of eccentric knee extension. In addition, our study involved postmenopausal women with an average age of 58.5 years, whereas Raue et al. (28) studied older women (average 85 years of age).
Our study indicates that HRT suppresses the UPP and its associated genes. MAFbx and MuRF-1 are associated with skeletal muscle atrophy (5) where FOXO3A acts as a common transcription factor for MAFbx and MuRF-1 (33). Therefore, HRT combined with maximal eccentric resistance exercise may prevent age-related muscle loss by mediating the UPP. However, further investigations are warranted to determine specific mechanisms involved in the interaction between HRT and ubiquitin proteasome-related genes.
Follistatin and myostatin mRNA response to exercise.
Myostatin, a negative regulator of myogenesis, controls the proliferation of muscle precursor cells (39). Myostatin inhibits expression of the myogenic regulatory factors (Myf5 and MyoD), thus inhibiting differentiation of skeletal muscle myoblasts (2, 39). Our study supports these data in that we report increased levels of Myf5 and MyoD with decreased levels of myostatin. Conversely, lower concentrations of myostatin may allow greater activation of myogenic regulatory factors and satellite cells responsible for muscle hypertrophy (25). Our study demonstrated decreases in myostatin levels in both groups, which have been previously shown in older (27) and young (22) women after an acute resistance exercise bout. A novel finding in our study is the greater suppression of the myostatin response in the HRT group, which may prevent or attenuate age-related muscle loss.
Follistatin is a recognized inhibitor of myostatin and allows for greater muscle hypertrophy (2). Follistatin gene expression is not often measured in skeletal muscle tissue via muscle biopsies. We previously reported increases in follistatin gene expression in young and older men after eccentric resistance exercise (14). Other studies have not measured changes in follistatin gene expression in women; however, our study is in agreement with our previous findings in men, indicating increases in follistatin levels in both groups. A unique finding in our study is the elevated follistatin response in the HRT group. HRT combined with eccentric resistance exercise may elicit a greater activation of follistatin to prevent age-related muscle loss. However, exercise intervention studies are necessary to examine the long-term benefits of HRT on follistatin and myostatin in older women.
The role of estrogen has been studied previously in human and animal models with exercise. Estrogen influences MyoD postexercise in animal models (8, 9) and further attenuates leukocyte infiltration (12, 36, 40) and indirect indexes of muscle damage (17). Our data indicate an increase in mRNA expression of MyoD in combination with other myogenic factors when postmenopausal women are taking HRT and performing maximal eccentric exercise. The presence of estrogen receptors have been identified in skeletal muscle in both men and women (11, 20, 43); however, the role of the estrogen receptors in skeletal muscle has not been determined. The increased myogenic gene expression in our study may directly relate to activation of the estrogen receptors; nevertheless, the mechanisms of this relationship have yet to be determined. Of importance is that skeletal muscle mRNA, as measured in the present study, does not always result in similar changes in protein synthesis. Further experiments are needed to verify these findings at the translational level.
In summary, the findings of the present study demonstrate that, at rest, postmenopausal women using HRT expressed greater levels of myogenic genes responsible for muscle growth and differentiation. Furthermore, a maximal eccentric exercise bout combined with HRT in postmenopausal women induces significant changes in gene expression of follistatin, FOXO3A, MAFbx, MURF-1, MyoD, myogenin, myostatin, Myf5, and MRF4 compared with postmenopausal women not taking HRT. This is the first study to indicate that HRT may play a role in skeletal muscle differentiation and growth, but further investigation will be necessary to learn whether this constellation of findings may lead to the attenuation or prevention of sarcopenia in older women.
GRANTS
This study was supported by the Clinical Exercise Research Center at the University of Southern California and local National Center for Research Resources General Clinical Research Center Grant M0I RR-000043.
ACKNOWLEDGMENTS
We thank the nurses and personnel of the general clinical research center of the University of Southern California for help with the clinical portion of this study.
REFERENCES
- 1. Aadahl M, Jorgensen T. Validation of a new self-report instrument for measuring physical activity. Med Sci Sports Exerc 35: 1196– 1202, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Amthor H, Nicholas G, McKinnell I, Kemp CF, Sharma M, Kambadur R, Patel K. Follistatin complexes myostatin and antagonises myostatin-mediated inhibition of myogenesis. Dev Biol 270: 19– 30, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Aubertin-Leheudre M, Audet M, Goulet ED, Dionne IJ. HRT provides no additional beneficial effect on sarcopenia in physically active postmenopausal women: a cross-sectional, observational study. Maturitas 51: 140– 145, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Barber RD, Harmer DW, Coleman RA, Clark BJ. GAPDH as a housekeeping gene: analysis of GAPDH mRNA expression in a panel of 72 human tissues. Physiol Genomics 21: 389– 395, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294: 1704– 1708, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Borg G. Ratings of perceived exertion and heart rates during short-term cycle exercise and their use in a new cycling strength test. Int J Sports Med 3: 153– 158, 1982 [DOI] [PubMed] [Google Scholar]
- 7. Dieli-Conwright CM, Spektor TM, Rice JC, Schroeder ET. Oestradiol and SERM treatments influence oestrogen receptor coregulator gene expression in human skeletal muscle cells. Acta Physiol (Oxf) In press. [DOI] [PubMed] [Google Scholar]
- 8. Enns DL, Iqbal S, Tiidus PM. Oestrogen receptors mediate oestrogen-induced increases in post-exercise rat skeletal muscle satellite cells. Acta Physiol (Oxf) 194: 81– 93, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Enns DL, Tiidus PM. Estrogen influences satellite cell activation and proliferation following downhill running in rats. J Appl Physiol 104: 347– 353, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Giresi PG, Stevenson EJ, Theilhaber J, Koncarevic A, Parkington J, Fielding RA, Kandarian SC. Identification of a molecular signature of sarcopenia. Physiol Genomics 21: 253– 263, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Glenmark B, Nilsson M, Gao H, Gustafsson JA, Dahlman-Wright K, Westerblad H. Difference in skeletal muscle function in males vs. females: role of estrogen receptor-β. Am J Physiol Endocrinol Metab 287: E1125– E1131, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Iqbal S, Thomas A, Bunyan K, Tiidus PM. Progesterone and estrogen influence postexercise leukocyte infiltration in ovariectomized female rats. Appl Physiol Nutr Metab 33: 1207– 1212, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Jemiolo B, Trappe S. Single muscle fiber gene expression in human skeletal muscle: validation of internal control with exercise. Biochem Biophys Res Commun 320: 1043– 1050, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Jensky NE, Sims JK, Rice JC, Dreyer HC, Schroeder ET. The influence of eccentric exercise on mRNA expression of skeletal muscle regulators. Eur J Appl Physiol 101: 473– 480, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Kahlert S, Grohe C, Karas RH, Lobbert K, Neyses L, Vetter H. Effects of estrogen on skeletal myoblast growth. Biochem Biophys Res Commun 232: 373– 378, 1997 [DOI] [PubMed] [Google Scholar]
- 16. Kenny AM, Dawson L, Kleppinger A, Iannuzzi-Sucich M, Judge JO. Prevalence of sarcopenia and predictors of skeletal muscle mass in nonobese women who are long-term users of estrogen-replacement therapy. J Gerontol A Biol Sci Med Sci 58: M436– 440, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Komulainen J, Koskinen SO, Kalliokoski R, Takala TE, Vihko V. Gender differences in skeletal muscle fibre damage after eccentrically biased downhill running in rats. Acta Physiol Scand 165: 57– 63, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Kubica N, Kimball SR, Jefferson LS, Farrell PA. Alterations in the expression of mRNAs and proteins that code for species relevant to eIF2B activity after an acute bout of resistance exercise. J Appl Physiol 96: 679– 687, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Lee SJ, McPherron AC. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci USA 98: 9306– 9311, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lemoine S, Granier P, Tiffoche C, Rannou-Bekono F, Thieulant ML, Delamarche P. Estrogen receptor alpha mRNA in human skeletal muscles. Med Sci Sports Exerc 35: 439– 443, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[−ΔΔC(T)] method. Methods 25: 402– 408, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Louis E, Raue U, Yang Y, Jemiolo B, Trappe S. Time course of proteolytic, cytokine, and myostatin gene expression after acute exercise in human skeletal muscle. J Appl Physiol 103: 1744– 1751, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Maddalozzo GF, Cardinal BJ, Li F, Snow CM. The association between hormone therapy use and changes in strength and body composition in early postmenopausal women. Menopause 11: 438– 446, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Mahoney DJ, Carey K, Fu MH, Snow R, Cameron-Smith D, Parise G, Tarnopolsky MA. Real-time RT-PCR analysis of housekeeping genes in human skeletal muscle following acute exercise. Physiol Genomics 18: 226– 231, 2004 [DOI] [PubMed] [Google Scholar]
- 25. McCroskery S, Thomas M, Maxwell L, Sharma M, Kambadur R. Myostatin negatively regulates satellite cell activation and self-renewal. J Cell Biol 162: 1135– 1147, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Perry RL, Rudnick MA. Molecular mechanisms regulating myogenic determination and differentiation. Front Biosci 5: D750– 767, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Raue U, Slivka D, Jemiolo B, Hollon C, Trappe S. Myogenic gene expression at rest and after a bout of resistance exercise in young (18–30 yr) and old (80–89 yr) women. J Appl Physiol 101: 53– 59, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Raue U, Slivka D, Jemiolo B, Hollon C, Trappe S. Proteolytic gene expression differs at rest and after resistance exercise between young and old women. J Gerontol A Biol Sci Med Sci 62: 1407– 1412, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Ribom EL, Piehl-Aulin K, Ljunghall S, Ljunggren O, Naessen T. Six months of hormone replacement therapy does not influence muscle strength in postmenopausal women. Maturitas 42: 225– 231, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Ronkainen PH, Kovanen V, Alen M, Pollanen E, Palonen EM, Ankarberg-Lindgren C, Hamalainen E, Turpeinen U, Kujala UM, Puolakka J, Kaprio J, Sipila S. Postmenopausal hormone replacement therapy modifies skeletal muscle composition and function: a study with monozygotic twin pairs. J Appl Physiol 107: 25– 33, 2009 [DOI] [PubMed] [Google Scholar]
- 31. Roth SM, Ferrell RE, Peters DG, Metter EJ, Hurley BF, Rogers MA. Influence of age, sex, and strength training on human muscle gene expression determined by microarray. Physiol Genomics 10: 181– 190, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132: 365– 386, 2000 [DOI] [PubMed] [Google Scholar]
- 33. Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117: 399– 412, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schmittgen TD, Zakrajsek BA. Effect of experimental treatment on housekeeping gene expression: validation by real-time, quantitative RT-PCR. J Biochem Biophys Methods 46: 69– 81, 2000 [DOI] [PubMed] [Google Scholar]
- 35. Sorensen MB, Rosenfalck AM, Hojgaard L, Ottesen B. Obesity and sarcopenia after menopause are reversed by sex hormone replacement therapy. Obes Res 9: 622– 626, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Stupka N, Tiidus PM. Effects of ovariectomy and estrogen on ischemia-reperfusion injury in hindlimbs of female rats. J Appl Physiol 91: 1828– 1835, 2001 [DOI] [PubMed] [Google Scholar]
- 37. Symons TB, Vandervoort AA, Rice CL, Overend TJ, Marsh GD. Effects of maximal isometric and isokinetic resistance training on strength and functional mobility in older adults. J Gerontol A Biol Sci Med Sci 60: 777– 781, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Taaffe DR, Sipila S, Cheng S, Puolakka J, Toivanen J, Suominen H. The effect of hormone replacement therapy and/or exercise on skeletal muscle attenuation in postmenopausal women: a yearlong intervention. Clin Physiol Funct Imaging 25: 297– 304, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Thomas M, Langley B, Berry C, Sharma M, Kirk S, Bass J, Kambadur R. Myostatin, a negative regulator of muscle growth, functions by inhibiting myoblast proliferation. J Biol Chem 275: 40235– 40243, 2000 [DOI] [PubMed] [Google Scholar]
- 40. Tiidus PM, Bombardier E. Oestrogen attenuates post-exercise myeloperoxidase activity in skeletal muscle of male rats. Acta Physiol Scand 166: 85– 90, 1999 [DOI] [PubMed] [Google Scholar]
- 41. Welle S, Brooks AI, Delehanty JM, Needler N, Bhatt K, Shah B, Thornton CA. Skeletal muscle gene expression profiles in 20–29 year old and 65–71 year old women. Exp Gerontol 39: 369– 377, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Welle S, Brooks AI, Delehanty JM, Needler N, Thornton CA. Gene expression profile of aging in human muscle. Physiol Genomics 14: 149– 159, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Wiik A, Glenmark B, Ekman M, Esbjornsson-Liljedahl M, Johansson O, Bodin K, Enmark E, Jansson E. Oestrogen receptor beta is expressed in adult human skeletal muscle both at the mRNA and protein level. Acta Physiol Scand 179: 381– 387, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Yang Y, Creer A, Jemiolo B, Trappe S. Time course of myogenic and metabolic gene expression in response to acute exercise in human skeletal muscle. J Appl Physiol 98: 1745– 1752, 2005 [DOI] [PubMed] [Google Scholar]