Abstract
The cecal ligation perforation (CLP) model of sepsis is known to induce severe diaphragm dysfunction, but the cellular mechanisms by which this occurs remain unknown. We hypothesized that CLP induces diaphragm caspase-3 and calpain activation, and that these two enzymes act at the level of the contractile proteins to reduce muscle force generation. Rats (n = 4/group) were subjected to 1) sham surgery plus saline (intraperitoneal); 2) CLP; 3) CLP plus administration of calpain inhibitor peptide III (12 mg/kg ip); or 4) CLP plus administration of a caspase inhibitor, zVAD-fmk (3 mg/kg). At 24 h, diaphragms were removed, and the following were determined: 1) calpain and caspase-3 activities by fluorogenic assay; 2) caspase-3 and calpain I protein levels; 3) the intact diaphragm force-frequency relationship; and 4) the force generated by contractile proteins of single, permeabilized diaphragm fibers in response to exogenous calcium. CLP significantly increased diaphragm calpain activity (P < 0.02), caspase-3 activity (P < 0.02), active calpain I protein levels (P < 0.02), and active caspase-3 protein (P < 0.02). CLP also reduced the force generated by intact diaphragm muscle (P < 0.001) and the force generated by single-fiber contractile proteins (P < 0.001). Administration of either calpain inhibitor III or zVAD-fmk markedly improved force generation of both intact diaphragm muscle (P < 0.01) and single-fiber contractile proteins (P < 0.001). CLP induces significant reductions in diaphragm contractile protein force-generating capacity. This force reduction is mediated by the combined effects of activated caspase and calpain. Inhibition of these pathways may prevent diaphragm weakness in infected patients.
Keywords: peritonitis, proteolysis
recent work has found that critically ill patients have severe respiratory muscle weakness (12, 30). On average, intensive care unit patients generate transdiaphragmatic pressures that are only 20–25% of those measured in healthy individuals (12, 30). Several factors may contribute to the development of diaphragm weakness in this patient population, including infections, hyperglycemia, neuromuscular blocking agents, steroids, and respiratory muscle disuse due to mechanical ventilation (15, 24, 28). Infections, in particular, appear to markedly reduce respiratory muscle force-generating capacity and can induce 50–60% reductions in diaphragm force-generating capacity in as little as 24 h (24).
Studies using endotoxin administration to induce an animal model of sepsis have suggested that both caspase-3 and calpain are activated in the diaphragm and may contribute to the development of sepsis-induced diaphragm weakness (24, 25). The subcellular site(s) within muscle altered by caspase and calpain activation are not known, however, and it is theoretically possible that these enzymes may alter muscle function by affecting action potential propagation, excitation-contraction coupling, energy metabolism, or contractile protein function. In addition, there are several theoretical mechanisms by which caspase-3 activation can influence calpain activation and, conversely, by which caspase-3 can affect calpain activity. It is not known if the caspase and calpain pathways act independently to alter muscle function in response to sepsis, or if activation of one of these pathways is completely dependent on activation of the other.
The purpose of the present experiment was to address these specific questions and to test the hypotheses that 1) both caspase-3 and calpain affect force generation primarily by altering contractile protein function, i.e., the force generated by the contractile apparatus in response to application of a given level of calcium; and 2) these pathways operate in parallel to alter muscle function, and the blockade of one of these pathways does not completely prevent activation of the other during sepsis. We thought these hypotheses were reasonable, since both caspase and calpain are known to degrade contractile proteins when incubated with these proteins in vitro, and since studies of nonmuscular tissues have shown that there are multiple signaling pathways that can activate caspase and calpain independently of one another (1, 21).
These experiments were carried out in rats that underwent either CLP or sham surgery, with and without administration of caspase and calpain inhibitors. At 24 h after surgery, animals were killed, and diaphragms removed for assessment of casp ase activation, calpain activation, intact muscle bundle specific force generation, and permeabilized single-fiber contractile protein force generation, i.e., the force-pCa relationship.
METHODS
Experimental protocol.
Experiments were performed using adult male rats, 225–300 g in weight. Approval for this work was granted by the Institutional Animal Care and Use Committee. Animals were given food and water ad libitum and housed in university facilities.
Four groups of animals were studied (n = 4/group): 1) control, sham-operated rats given saline (0.3 ml) intravenously via tail vein injection; 2) rats subjected to cecal ligation perforation (CLP) and given saline (0.3) intravenously; 3) rats with CLP given a caspase inhibitor, Z-Val-Ala-Asp(OCH3)-fluoromethylketone (zVAD-fmk), intravenously (3 mg/kg, via tail vein injection); and 4) rats with CLP given calpain inhibitor III, carbobenzoxy-valinyl-phenylalaninal, 12 mg/kg, intravenously. Our selection of zVAD-fmk as a caspase inhibitor is based on the fact that we have previous experience using this agent (24) and have previously defined a systemic dose and route of administration that effectively inhibits diaphragmatic caspase-3. Calpain inhibitor III was chosen based on the knowledge that this agent is fairly specific for calpain and does not inhibit the proteasome or caspase-3. Saline (60 mg·kg−1·day−1) was administered subcutaneously daily to all animals to maintain fluid volume status. Animals were sedated with pentobarbital (50 mg/kg intraperitoneally) 24 h after injections and then euthanized by opening the chest. The diaphragm was removed; a portion of the left costal diaphragm was used for assessment of force generation and stored for single-fiber force-pCa measurements; and the remaining diaphragm was frozen to −80°C and latter assayed for calpain activity, caspase activity, calpain I protein levels, and caspase protein levels.
CLP.
All animals underwent either sham abdominal surgery or cecal ligation puncture. For these procedures, animals were first anesthetized using inhalational halothane (2–4%) delivered via a nose cone attached to a Harvard small-animal ventilator interfaced with an anesthetic gas vaporizer. Once a steady plane of anesthesia was achieved as assessed by no response to tail and toe pinch, the abdomen was prepped with betadine and alcohol, and a small incision was made in the right lower quadrant (∼2 cm in length). The cecum was identified and a portion ligated, keeping the gut lumen open, using sterile suture (2–0 silk). An 18-gauge sterile needle was then used to puncture the ligated cecum. Sterile suture was used to close the abdominal musculature, and the abdominal wall was approximated and closed using surgical staples. For sham surgery, the abdomen was opened and closed without cecal ligation or puncture.
Calpain and caspase activity assays.
For calpain and caspase activity assays, diaphragm muscle homogenate (100 μm of protein) was added to assay buffer, and either a fluorogenic substrate cleaved by calpain [succinyl-Leu-Leu-Val-Tyr-7-amido-4-methyl-coumarin (Suc-LLVY-AMC)] or a caspase-3-specific substrate [N-acetyl-Asp-Glu-Val-Asp-7-amino-4-methylcoumarin (Ac-DEVD-AMC)]. Duplicate determinations were made for each sample using a mixture of diaphragm muscle homogenate, assay buffer, fluorogenic substrate, and either a highly specific calpain inhibitor (0.1 mg/ml calpain inhibitor III, carbobenzoxy-valinyl-phenylalaninal) or a caspase-3 inhibitor [20 nM N-acetyl-Asp-Glu-Val-Asp-aldehyde (DEVD-CHO)]. Immediately after addition of the fluorogenic substrate, a baseline fluorescent measurement of AMC (7-amino-4-methylcoumarin) was performed using a Molecular Devices spectrofluorophotometer (excitation frequency of 360 nm and an emission frequency of 460 nm). This measurement was then repeated after 0.5 h of incubation at 30°C for caspase assays or after 0.5 h of incubation at 25°C for calpain assays. AMC standards were used to create a calibration curve, and activity was quantified as nanomoles of AMC generated per minute per milligram of tissue homogenate protein. The difference between AMC generation from incubation of homogenates with Suc-LLVY-AMC in the presence and absence of calpain inhibitor III was taken as an index of calpain activity; note that calpain inhibitor III blocks calpain activity but does not inhibit the proteasome or other chymotrypsin-like proteases. The difference between AMC generation from incubation of homogenates with Ac-DEVD-AMC in the presence and absence of DEVD-CHO was taken as an index of caspase-3 activity. Note that DEVD-CHO does not inhibit calpain, caspase-8, or caspase-9, but inhibits caspase-3.
Calpain and caspase protein levels.
Western blotting was employed to measure diaphragm levels of caspase-3 and calpain I proteins. We also measured levels of α-tubulin as a loading control. For these determinations, muscle samples were diluted with an equal volume of loading buffer (126 mM Tris·HCl, 20% glycerol, 4% SDS, 1.0% 2-mercaptoethanol, 0.005% bromphenol blue, pH 6.8) and loaded onto Tris glycine polyacrylamide gels, and protein mixtures were separated by electrophoresis (Novex Minicell II, Carlsbad, CA). Proteins were then transferred to polyvinylidene fluoride membranes and incubated over night at 4°C with primary antibodies to targeted proteins (calpain I from Biomol, Plymouth Meeting, PA, and caspase-3 from Cell Signaling, Danvers, MA). Membranes were then incubated with horseradish peroxidase-conjugated secondary antibodies and antibody binding detected on film using enhanced chemiluminescence (Western Lightning, Perkin Elmer, Boston, MA). Densitometry of filmed gels was performed using a Microtek scanner (Carson, CA) and UN-SCAN-IT software (Silk Scientific, Orem, UT). After initial determinations, membranes were stripped and reprobed with primary antibodies to α-tubulin (Santa Cruz Biotechnology, Santa Cruz, CA) to verify equal loading among lanes. We chose α-tubulin for this normalization, because previous experiments indicate this protein is not altered in skeletal muscle by sepsis. Densities of the α-tubulin blots were determined using a Microtek scanner; these values were used to normalize densitometry values for other proteins.
Intact muscle force-frequency determinations.
Diaphragm force generation was assessed as we have previously reported (24). In brief, after diaphragms were excised, two muscle strips were dissected from the left midcostal portion for each animal. Strips were then mounted in water-jacketed organ baths containing Krebs-Henseleit solution (22°C, curare 50 mg/l, pH 7.40, NaCl 135 mM, KCl 5 mM, dextrose 11.1 mM, CaCl2 2.5 mM, MgSO4 1 mM, NaHCO3 14.9 mM, NaHPO4 1 mM, insulin 50 U/l, 95% O2/5% CO2). One end of each strip was tied to the organ bath base, and the other end to a Grass force transducer (West Warwick, RI). Platinum field electrodes were used to deliver supramaximal currents using a current amplifier driven by a Grass S48 stimulator. After a 15-min equilibration, muscle length was adjusted to optimum length, i.e., the length at which strip force generation was maximal. Strips were then sequentially stimulated with trains of 1, 10, 20, 50, and 80 Hz stimuli (train duration 800 ms, 30 s between adjacent trains), and force was recorded. Cross-sectional area was calculated as muscle strip weight divided by muscle density (1.06) and muscle length; specific muscle force was calculated as raw force divided by cross-sectional area.
Contractile protein force-pCa determinations.
For these assessments, single fibers (15/animal) were carefully dissected from muscle bundles under a microscope, as previously described (5), and then permeabilized by incubation in 0.1% Triton X-100 (containing protease inhibitors) for 30 min. Note that this permeabilization process removes sarcolemma, mitochondria, and sarcoplasmic reticulum, allowing examination of the functional properties of the contractile elements per se. Fibers were then mounted between a stationary arm and the adjustable arm of a Harvard force transducer, then adjusted to a sarcomere length of 2.6 μm by visualizing the diffraction pattern of a helium-neon laser aimed through the fiber. Fibers were sequentially exposed to solutions containing different Ca2+ concentrations (pCa 8.5 to pCa 5.0). The composition of these solutions was calculated by using a computer program (Borland International, Scotts Valley, CA) that takes into account stability constants to produce final solutions of the correct ionic strength and pCa (5). The force produced by exposure of the fiber to each of these solutions was recorded and normalized to fiber cross-sectional area by measuring the diameter of the cylindrically shaped fibers with a micrometer attached to one of the eyepieces of the microscope. After completion of this protocol, fibers were removed from the force transducer and placed in SDS-PAGE loading buffer. Individual fibers were subjected to SDS-PAGE and typed based on myosin isoform banding, as described previously (5).
Statistical analysis.
ANOVA was used to compare variables (e.g., force) for data sets that passed normality testing across groups of animals treated with different agents, with post hoc testing (Tukey) to determine differences between groups. For data sets that did not pass normality tests, a nonparametric test (ANOVA on ranks) was employed. A P value of less than 0.05 was taken as indicating statistical significance.
RESULTS
Caspase activation following CLP.
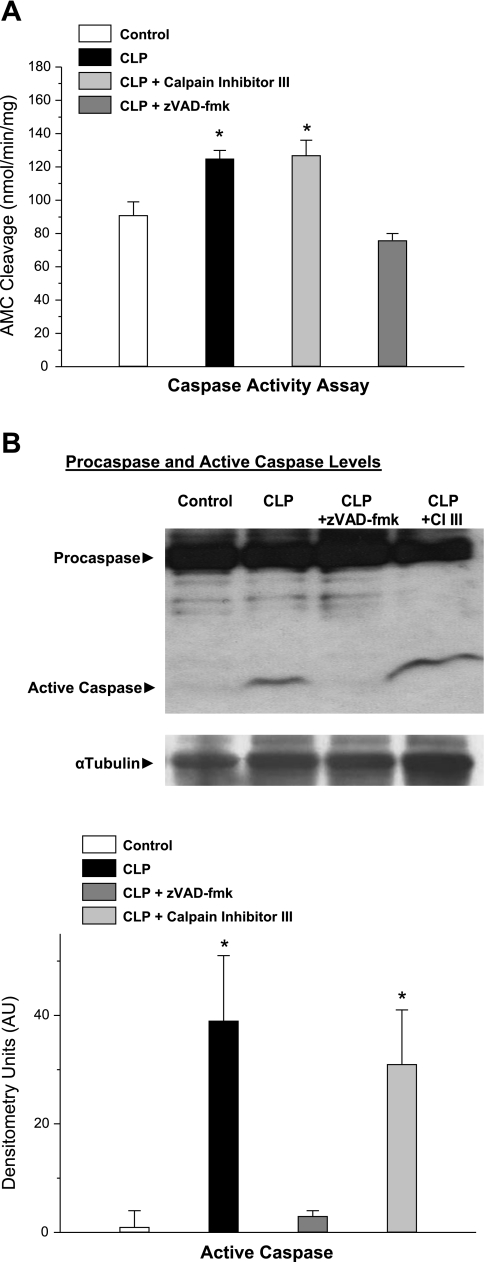
CLP elicted a significant increase in diaphragm caspase activity assessed by measuring the cleavage of a caspase-3-specific substrate by diaphragm homogenates (Fig. 1A, P < 0.02 for comparison of control to the CLP group). Administration of a caspase inhibitor, zVAD-fmk, prevented this increase in diaphragm caspase-3 activity for CLP-treated animals (P < 0.01 for comparison of CLP to CLP plus zVAD-fmk-treated groups). On the other hand, administration of a calpain-specific inhibitor, calpain inhibitor III, did not prevent CLP-induced increases in diaphragm caspase activation. Data obtained measuring another indicator of diaphragm caspase-3 activation, namely diaphragm levels of active caspase-3 protein, showed a similar pattern. Specifically, diaphragm caspase-3 active protein levels increased markedly following CLP (Fig. 1B, P < 0.02 for comparison of control to CLP groups). Administration of zVAD-fmk to CLP-treated animals blocked this increase in diaphragm active caspase-3 protein levels, while administration of calpain inhibitor III had no effect on this index (P < 0.02 for comparison of CLP to CLP plus zVAD-fmk groups).
Fig. 1.
Diaphragm caspase-3 activation. Diaphragm caspase-3 activation was assessed, both by assessing caspase-3 enzymatic activity from the rate of cleavage of a caspase fluorogenic substrate by diaphragm homogenates (A), and by employing Western blotting to determine intact and active cleaved caspase-3 levels for diaphragm homogenates (B) from control, cecal ligation perforation (CLP), CLP plus calpain inhibitor (CI) III, and CLP plus zVAD-fmk groups. A: diaphragm samples from CLP animals (solid bar) had higher caspase activities than samples from control animals (open bar) (P < 0.02). Administration of CI III to CLP animals (light shaded bars) did not reduce caspase activation, but administration of zVAD-fmk, a caspase inhibitor, did reduce caspase activation in CLP animals (dark shaded bars; P < 0.01 for the comparison of CLP to CLP plus zVAD-fmk). B: CLP also induced an increase in active caspase-3 protein (P < 0.02 for comparison to control). Administration of zVAD-fmk to CLP animals reduced caspase-3 protein levels (P < 0.02), whereas administration of CI III did not. *Significant statistical difference compared with the control group, P < 0.05. Error bars for this and all other figures represent ±1 SE. AMC, 7-amino-4-methylcoumarin; AU, arbitrary units.
Calpain activation after CLP.
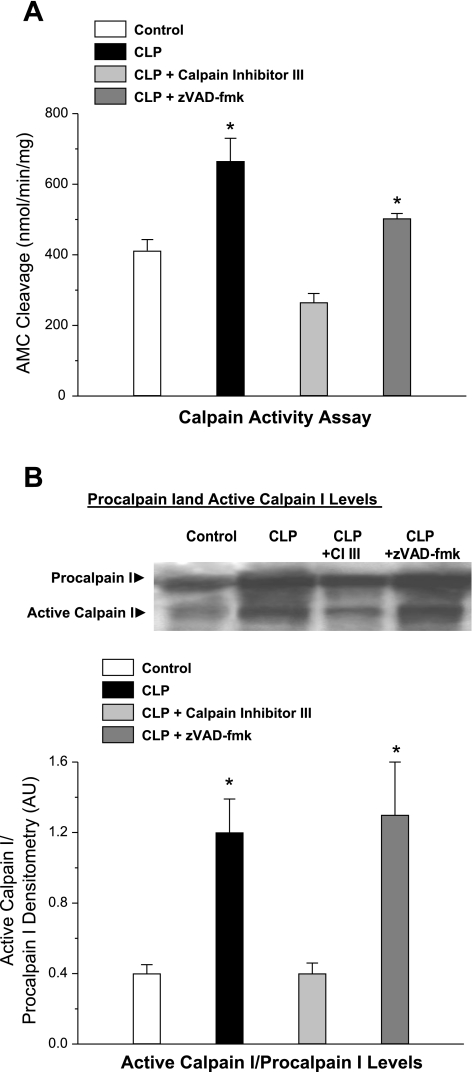
CLP also induced a significant increase in diaphragm calpain activity, as judged by two different indexes, namely, assessment of cleavage of a fluorescent substrate by diaphragm muscle homogenates (an index of calpain catalytic capacity, Fig. 2A, P < 0.02 for comparison of control to CLP groups) and levels of active calpain I protein (an index of in vivo calpain activation, Fig. 2B, P < 0.02 for comparison of control to CLP groups). Administration of zVAD-fmk to CLP animals had no effect on the CLP-induced increase in calpain activity or active calpain I protein levels, but administration of calpain inhibitor III prevented increases in both indexes of calpain activation (P < 0.02 for comparison of CLP to CLP plus calpain inhibitor III groups). These findings suggest that caspase inhibition did not affect calpain activation, arguing that these two particular pathways are not dependent on each other in this animal model of sepsis.
Fig. 2.
Diaphragm calpain activation. Calpain activity was assessed by determining the cleavage of a fluorogenic substrate by diaphragm homogenates (A) and by using Western blotting (B) to determine intact and cleaved calpain I protein levels for diaphragm homogenates from control, CLP, CLP plus CI III, and CLP plus zVAD-fmk groups. A: diaphragm samples from CLP animals (solid bar) had higher calpain activities than samples from control animals (open bar) (P < 0.02). Administration of CI III to CLP animals (light shaded bar) reduced calpain activation (P < 0.01), but administration of zVAD-fmk, a caspase inhibitor, had no effect on calpain activation in CLP animals (dark shaded bar). B: CLP also resulted in an increase in cleaved calpain I protein (P < 0.02 for comparison to control). Administration of CI III to CLP animals reduced cleaved calpain I protein levels (P < 0.02), whereas administration of zVAD-fmk did not. *Significant statistical difference compared with the control group, P < 0.05.
Effect of CLP on force generation by the intact diaphragm.
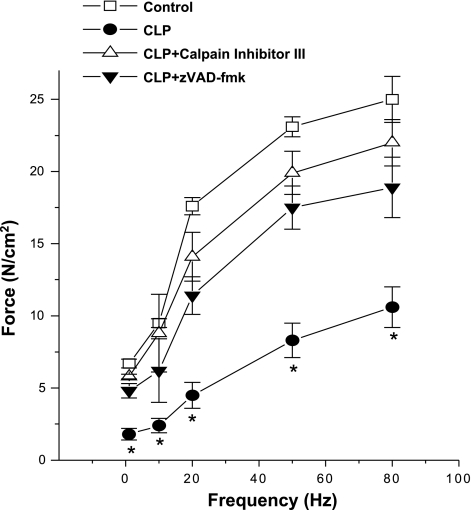
Diaphragm muscle strips taken from CLP-treated animals had lower levels of force generation in response to electrical stimulation in isolated organ baths for stimulation frequencies from 1 to 80 Hz, as shown in Fig. 3 (P < 0.001 for comparison of control to CLP for stimulation frequencies 1–80 Hz; maximal force was 45% lower in the muscles from CLP animals compared with controls). Administration of either zVAD-fmk or calpain inhibitor protein III partially prevented this CLP-induced reduction in whole muscle force generation, with diaphragm force-frequency curves for CLP plus zVAD-fmk and CLP plus calpain inhibitor III treated group lying between the curves for control and CLP-treated animals (Fig. 3, P < 0.01 for comparison of CLP to CLP plus zVAD-fmk or CLP to CLP plus calpain inhibitor III groups for frequencies 1–80 Hz).
Fig. 3.
Intact diaphragm force-frequency curves. Intact muscle force generation was significantly lower at stimulation frequencies from 1 to 80 Hz for diaphragms from CLP-treated animals (●) than for control animals (□) (P < 0.001). Diaphragms from CLP animals given CI III (▵) generated forces significantly higher than diaphragms from CLP animals for frequencies from 1 to 80 Hz (P < 0.01). Force generation for muscles taken from CLP animals given zVAD-fmk (▼) was also greater than for CLP animals (frequencies 1–80 Hz, P < 0.01). *Significant statistical difference between CLP and the other groups, P < 0.05.
Effect of CLP on the single-diaphragm fiber force-pCa relationship.
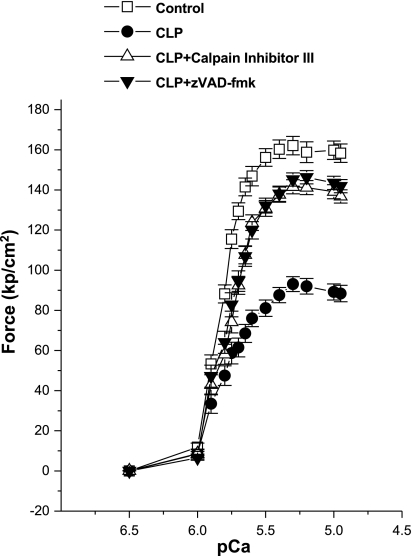
In addition to assessing diaphragm force generation by examining the force produced by intact diaphragm muscle strips, we also directly examined the force-generating capacity of diaphragm contractile elements by measuring the forces generated by single permeabilized muscle fibers to increasing levels of exogenously applied calcium solutions (Figs. 4 and 5). For this analysis, we also performed gel electrophoresis on the individual fibers used for assessment for the four experimental groups of animals. We found that CLP induced a downward shift in the diaphragm single-fiber force-pCa relationship, as shown for group mean data in Fig. 4. Administration of either zVAD-fmk or calpain inhibitor peptide III to CLP-treated animals markedly attenuated this CLP-induced effect, with force-pCa curves for CLP plus zVAD-fmk and CLP plus calpain inhibitor peptide III groups lying between curves for control and CLP groups (Fig. 4).
Fig. 4.
Single-skinned fiber force-pCa curves. Single skinned fiber force generation for diaphragm muscle fibers from CLP animals (●) was significantly reduced compared with the force generated by single-diaphragm fibers from control animals (□) (P < 0.001). Forces generated by single skinned fibers from either CLP plus CI III (△) or CLP plus zVAD-fmk groups (▼) were significantly higher than the force generated by fibers from the CLP group (P < 0.001).
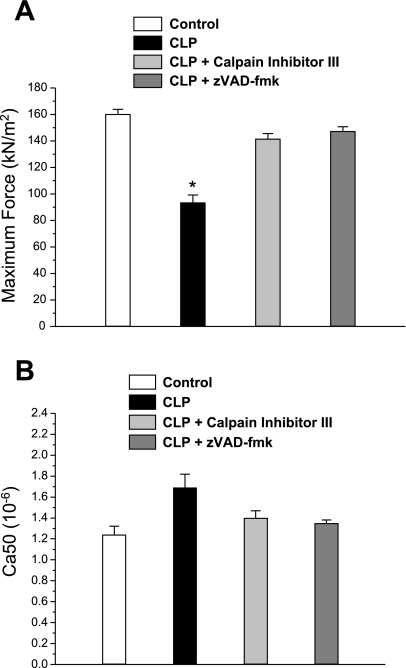
Fig. 5.
Parameters of skinned fiber force generation. A: maximum single-fiber force generation was reduced for fibers from CLP animals (solid bar) compared with force for fibers from control animals (open bar) (P < 0.001). Fibers from CLP animals given either CI III (light shaded bar) or zVAD-fmk (dark shaded bar) generated forces that were significantly higher than that observed for the CLP group (P < 0.001). B: Ca2+ concentration producing half-maximal activation (Ca50) for force-pCa curves of single fibers trended to higher levels for the CLP group (solid bar) compared with the control (open bar), CLP plus CI III (light shaded bar), and CLP plus zVAD-fmk (dark shaded bar) groups, but this difference did not reach statistical significance. *Significant statistical difference between CLP and the other groups, P < 0.05.
We fit the data for force-pCa curves for the various experimental groups to the Hill equation and found that CLP administration reduced the Fmax (maximum force generating capacity of single fibers) compared with controls by 51% (Fig. 5A, P < 0.001 for comparison of controls to CLP). Administration of either zVAD-fmk or calpain inhibitor peptide III prevented this CLP-induced downward shift in Fmax (P < 0.001 for comparison of CLP to either CLP plus zVAD-fmk or CLP plus calpain inhibitor peptide III groups). We also found that CLP had no significant effect on single-fiber calcium sensitivity (i.e., the Ca2+ concentration producing half-maximal activation, as shown in Fig. 5B).
When performing gel electrophoresis fiber typing on each of the individual fibers used for analysis of force-pCa curves, we found that fiber-type distributions examined were similar for the four experimental groups. Specifically, the proportion of type IIX, type IIB, type IIA, and type I fibers for control group experiments were, respectively, 43, 40, 10, and 7%. Similarly, the proportion of these fibers (type IIX, type IIB, type IIA, and type I fibers, respectively) were 45, 39, 11, and 5% for the CLP group, were 45, 42, 10, and 3% for the CLP plus calpain inhibitor III group, and were 50, 41, 6, and 3% for the CLP plus zVAD-fmk group. To further assess our force-pCa data, we compared the mean Fmax for groups of fibers with the same fiber type across the four experimental treatment groups. This analysis found that the average forces for IIX, IIB, and IIA fibers taken from animals receiving CLP were all significantly lower (99 ± 6, 94 ± 5, and 87 ± 13 kPa/cm2, respectively, P < 0.01 for each comparison) than values from control animals (164 ± 2, 161 ± 2, and 165 ± 5 kPa/cm2) or animals from the CLP plus calpain inhibitor III (147 ± 5, 150 ± 5, and 149 ± 6 kPa/cm2) or CLP plus zVAD-fmk (140 ± 5, 146 ± 5, and 133 ± 11 kPa/cm2) groups. The number of slow, type I fibers sampled for the four experimental groups was too small to permit adequate statistical testing, but, again, the trend favored lower forces for fibers from the CLP animals (54 ± 6 kPa/cm2) compared with control, CLP plus calpain inhibitor III, or CLP plus zVAD-fmk groups (132 ± 3, 106 ± 25, and 142 ± 10 kPa/cm2, respectively).
DISCUSSION
Infection-induced diaphragm weakness.
It has been recently discovered that critically ill patients have surprisingly severe respiratory muscle weakness, with the diaphragm strength reduced to only 20–25% of the normal level in these patients (12, 30). While several factors appear to contribute to the development of weakness in this patient population, many of these patients are infected, and increasing evidence indicates that even minor infections can produce profound reductions in diaphragmatic force-generating capacity (6, 7, 13, 17, 24, 27).
One mechanism by which infections induce diaphragmatic weakness is via activation of the caspase and calpain pathways. In two recent studies using the endotoxin model of sepsis, we found that both caspase-3 and caspase-8 are activated in the diaphragm and that selective inhibition of either caspase enzyme reduced the level of endotoxin-induced diaphragmatic weakness (24, 26). In other work, we have shown that calpain I and calpain II are also activated in the diaphragm in endotoxin-induced sepsis and that inhibition of these enzymes with a specific calpain inhibitor also reduced diaphragm weakness (25). The present study extends these previous observations to another, more clinically relevant model of sepsis, the CLP model, demonstrating that both caspase-3 and calpain I are also activated in the diaphragm in CLP and that both enzymes contribute to the development of diaphragmatic weakness following endotoxin administration. Evidence supporting caspase-3 activation include formation of the active cleaved caspase-3 protein in the diaphragm following CLP, as well as increases in diaphragm homogenate caspase-3 activity measured by assessing cleavage of a caspase-3-specific fluorogenic substrate. Evidence that calpain I was activated include formation of a 78-kDa calpain I cleavage product in the diaphragms of animals with CLP-induced peritonitis.
The 78-kDa calpain I cleavage product is known to be formed autocatalytically when calpain I is activated (3). Assessment of calpain I cleavage is a relatively new approach to determining calpain activation in skeletal muscle. Arguably, this may be a superior means of determining whether calpain I is actually active in intact tissues than commonly used traditional indexes. Most traditional activity assays used to assess calpain activity in muscle homogenates are performed using homogenized samples, and homogenization, per se, will release calcium from the sarcoplasmic reticulum, altering calpain activation from that present under in vivo conditions. Some techniques employ initial isolation of calpain I, calpain II, and/or calpastatin from tissues and subsequent assessment of calpain or calpastatin activity in vitro, but these latter approaches assess the capacity of these enzymes or their inhibitor (calpastatin) and do not allow a determination as to the activity of these enzymes under in vivo conditions. In contrast, the presence of these specific cleavage products in diaphragm homogenates can only occur from in vivo calpain I activation in the diaphragm.
Of note, there are several mechanisms by which calpain I and caspase-3 activation can interact with each other (20). For example, under some circumstances, calpain I can directly cleave procaspase-3, leading directly to caspase-3 activation. Caspase-3 can also inactivate calpastatin, the natural inhibitor of calpain, thereby enhancing calpain activity. In addition, cytokines have been reported to induce activation of either calpain I or caspase-3, and both enzymes are influenced by oxidative stress. As a result, these enzymes can sometimes be activated in parallel, downstream of the same upstream trigger (cytokines, reactive oxygen species) or can be activated sequentially. Our hypothesis was that these enzymes were primarily activated in parallel, and the findings of this study are consistent with this possibility. Specifically, we found that calpain inhibitor administration did not block caspase-3 activation, and, conversely, caspase-3 inhibition did not block calpain I activation, arguing that neither of these pathways is activated “downstream” of the other in the diaphragm in response to peritonitis. Instead, our findings would suggest that both are activated downstream of some common upstream inflammatory stimulus (e.g., cytokines) in this particular animal model of disease. These findings also argue that neither calpain inhibitor or caspase inhibitors acted to “nonspecifically” reduce systemic inflammation in the present study, since a nonspecific effect of either of these agents to reduce systemic inflammation would have been expected to also nonspecifically inhibit both caspase-3 and calpain I activation.
It is important to recognize that normal skeletal muscle force generation depends on the coordinated interaction of multiple cellular organelles, requiring propagation of an action potential into the muscle t-tubular apparatus, subsequent sarcoplasmic reticulum calcium release, calcium activation of a normally functioning contractile protein lattice, and sufficient cellular energy metabolism to maintain adequate levels of intracellular high-energy phosphate compounds (ATP, creatine phosphate) (8). In theory, infections could compromise diaphragm force generation by altering any or all of these cellular processes, and previous work examining the effects of bacterial infections (e.g., peritonitis, pneumonia) on diaphragm function did not determine which of these possible sites was critically altered (6, 7, 9, 17). The present study is the first to directly examine the effects of peritonitis on the diaphragm contractile protein force-pCa relationship. We found a profound sepsis-induced reduction in the force-generating capacity of the contractile proteins of permeabilized, single fibers (51%). This was comparable to the observed reduction in maximum force generation in intact muscle strips, which decreased by 45%. If other sites of dysfunction (e.g., reductions in action potential propagation, alterations in sarcoplasmic reticulum calcium handling or alterations in cellular energetics) played a major role in reducing force, then, theoretically, sepsis-induced reductions in force generation by intact muscle should have far exceeded the observed reductions in single permeabilized force generation. The fact that reductions in maximum force in intact muscle and single permeabilized fibers were approximately equal argues, therefore, that a reduction in diaphragm contractile protein function is the major cause of diaphragm weakness produced by peritonitis. Moreover, the fact is that both calpain and caspase inhibitors effectively attenuated CLP-induced reductions in single-fiber maximal force generation in response to exogenous calcium, arguing that the major effect of both calpain I and caspase-3 to reduce muscle force-generating ability is directed at the level of the contractile proteins.
Implications.
These findings are in keeping with a recent theory proposed by Powers et al. (19) regarding the mechanism(s) by which most catabolic processes cause muscle weakness. Specifically, this theory postulates that catabolic processes generally act via a two-step process to alter contractile protein composition. In the first step, the contractile protein lattice is modified and disrupted by proteolytic enzymes, such as calpain and caspase, which cleave a small number of key contractile and cytoskeletal proteins. Once these proteins are cleaved, other myofilament components are released from the lattice and enter the general cytosolic space, where they can be further degraded, as a second step, by components of the proteasomal proteolytic system. This theory is in keeping with several papers that argue the proteasome has a limited role in producing disruption of intact contractile protein lattices and does not degrade myofibrillar component proteins until these are released from the lattice (10, 22). The present findings are consistent with this hypothesis and provide evidence that both caspase-3 and calpain I are likely candidates for initiating diaphragm contractile protein dysfunction in sepsis.
It should also be pointed out that there are extensive data indicating that oxidative stress plays a role in modulating muscle functional alterations during infections. It is possible that oxidative pathways and proteolytic pathways are linked, and that oxidative species (i.e., superoxide and other reactive oxygen species) are involved in triggering cellular signaling events that activate caspase and calpain in muscle. Recent work is consistent with this possibility (16).
The present study is the first to examine the role of caspase-3 activation in producing diaphragm skeletal muscle dysfunction in the CLP animal model of sepsis, but caspase-3 activation has been previously implicated as a potential contributor to dysfunction of a number of other organs (kidney, liver, heart, intestine, thymus, spleen) following CLP (18, 29, 31). Caspase-3 activation has traditionally been linked to apoptotic cell death, but more recent evidence suggests that low-level activation of caspase-3 may cause cell damage rather than cell death (18, 24). This may be particularly true in striated muscles like the heart and skeletal muscle, where activation of this enzyme would be expected to cleave contractile proteins and cause force loss but, because of a redundancy in nuclear content, not necessarily induce cell death. As a result, the present results contribute to a growing literature implicating caspase-3 activation as a potential bodywide modulator of organ dysfunction in sepsis and extends these previous studies by specifically demonstrating that administration of caspase inhibitors has the potential to diminish respiratory muscle dysfunction in sepsis. In view of recent studies demonstrating severe respiratory muscle weakness in critically ill patients, this finding may be of therapeutic importance, and it is conceivable that caspase inhibitors could be used therapeutically to block or reduce respiratory muscle contractile dysfunction in infected, critically ill patients.
The present data also suggest that administration of a relatively selective calpain inhibitor provides a benefit in terms of preventing diaphragm skeletal muscle dysfunction following CLP-induced peritonitis. There are a number of recent reports indicating that it is possible to also block calpain activation with a number of other biopharmaceutical agents that have low levels of toxicity, suggesting that it may be possible to introduce a skeletal muscle protective calpain inhibitor into clinical usage (23).
On the other hand, it is possible that inhibition of caspase-3 and calpain may, under some conditions, alter normal muscle cellular function. For one thing, inhibition of these enzymes may promote retention of abnormal muscle proteins. In addition, it is known that activation of calpain and caspase-3 is required for myocyte differentiation, and it is, therefore, conceivable that prolonged inhibition of caspase and calpain may impair muscle regeneration (2). As a result, it may prove that careful attention to dose and timing of caspase and calpain inhibitors may be required to prevent deleterious side effects on muscle function. A significant amount of additional investigation will, therefore, be needed to determine how inhibitors of caspase or calpain can be safely given to patients to prevent muscle dysfunction during infections.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants 80429, 81525, 63698, 80609, and 69821.
REFERENCES
- 1. Attaix D, Ventadour S, Codran A, Béchet D, Taillandier D, Combaret L. The ubiquitin-proteasome system and skeletal muscle wasting. Essays Biochem 41: 173– 186, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Barnoy S, Kosower NS. Caspase-1-induced calpastatin degradation in myoblast differentiation and fusion: cross-talk between the caspase and calpain systems. FEBS Lett 546: 213– 217, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Barta J, Tóth A, Edes I, Vaszily M, Papp JG, Varró A, Papp Z. Calpain-1-sensitive myofibrillar proteins of the human myocardium. Mol Cell Biochem 278: 1– 8, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Brooks HF, Osabutey CK, Moss RF, Andrews PL, Davies DC. Caecal ligation and puncture in the rat mimics the pathophysiological changes in human sepsis and causes multi-organ dysfunction. Metab Brain Dis 22: 353– 373, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Callahan LA, Nethery D, Stofan D, DiMarco A, Supinski GS. Free radical induced contractile protein dysfunction in endotoxin-induced sepsis. Am J Respir Cell Mol Biol 24: 210– 217, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Divangahi M, Matecki S, Dudley RW, Tuck SA, Bao W, Radzioch D, Comtois AS, Petrof BJ. Preferential diaphragmatic weakness during sustained Pseudomonas aeruginosa lung infection. Am J Respir Crit Care Med 169: 679– 686, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Drew JS, Farkas GA, Pearson RD, Rochester DF. Effects of a chronic wasting infection on skeletal muscle size and contractile properties. J Appl Physiol 64: 460– 465, 1988 [DOI] [PubMed] [Google Scholar]
- 8. Dulhunty AF. Excitation-contraction coupling from the 1950s into the new millennium. Clin Exp Pharmacol Physiol 33: 763– 772, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Fujimura N, Sumita S, Aimono M, Masuda Y, Schichinohe Y, Narimatsu E, Namiki A. Effect of free radical scavengers on diaphragmatic contractility in septic peritonitis. Am J Respir Crit Care Med 162: 2159– 2165, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Goll DE, Neti G, Mares SW, Thompson VF. Myofibrillar protein turnover: the proteasome and the calpains. J Anim Sci 20: 334– 338, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Hubbard WJ, Choudhry M, Schwacha MG, Kerby JD, Rue LW 3rd, Bland KI, Chaudry IH. Cecal ligation and puncture. Shock Suppl 1: 52– 57, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Laghi F, Cattapan SE, Jubran A, Parthasarathy S, Warshawsky P, Choi YS, Tobin MJ. Is weaning failure caused by low-frequency fatigue of the diaphragm? Am J Respir Crit Care Med 167: 120– 127, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Laghi F, Tobin MJ. Disorders of the respiratory muscles. Am J Respir Crit Care Med 168: 10– 48, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Li P, Waters RE, Redfern SI, Zhang M, Mao L, Annex BH, Yan Z. Oxidative phenotype protects myofibers from pathological insults induced by chronic heart failure in mice. Am J Pathol 170: 599– 608, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maes K, Testelmans D, Powers S, Decramer M, Gayan-Ramirez G. Leupeptin inhibits ventilator-induced diaphragm dysfunction in rats. Am J Respir Crit Care Med 175: 1134– 1138, 2007 [DOI] [PubMed] [Google Scholar]
- 16. McClung JM, Judge AR, Talbert EE, Powers SK. Calpain-1 is required for hydrogen peroxide-induced myotube atrophy. Am J Physiol Cell Physiol 296: C363– C371, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mier-Jedrzejowicz M, Brophy C, Green M. Respiratory muscle weakness during upper respiratory tract infections. Am Rev Respir Dis 138: 5– 7, 1988 [DOI] [PubMed] [Google Scholar]
- 18. Neviere R, Fauvel H, Chopin C, Formstecher P, Marchetti P. Caspase inhibition prevents cardiac dysfunction and heart apoptosis in a rat model of sepsis. Am J Respir Crit Care Med 163: 218– 225, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Powers SK, Kavazis AN, DeRuisseau KC. Mechanisms of disuse muscle atrophy: role of oxidative stress. Am J Physiol Regul Integr Comp Physiol 288: R337– R344, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Rami A, Agarwal R, Spahn A. Synergetic effects of caspase 3 and mu-calpain in XIAP-breakdown upon focal cerebral ischemia. Neurochem Res 32: 2072– 2079, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Ray SK. Currently evaluated calpain and caspase inhibitors for neuroprotection in experimental brain ischemia. Curr Med Chem 13: 3425– 3440, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Solomon V, Goldberg AL. Importance of the ATP-ubiquitin-proteasome pathway in degradation of soluble and myofibrillar proteins in rabbit muscle extracts. J Biol Chem 271: 26690– 26697, 1996 [DOI] [PubMed] [Google Scholar]
- 23. Sun M, Xu C. Neuroprotective mechanism of taurine due to up-regulating calpastatin and down-regulating calpain and caspase-3 during focal cerebral ischemia. Cell Mol Neurobiol 28: 593– 611, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Supinski GS, Callahan LA. Caspase activation contributes to endotoxin-induced diaphragm weakness. J Appl Physiol 100: 1770– 1777, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Supinski GS, Callahan LA. Calpain inhibitor III administration attenuates endotoxin-induced diaphragm dysfunction (Abstract). Am J Respir Crit Care Med 175: A502, 2007 [Google Scholar]
- 26. Supinski GS, Ji X, Wang W, Callahan LA. The extrinsic caspase pathway modulates endotoxin-induced diaphragm contractile dysfunction. J Appl Physiol 102: 1649– 1657, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Uzuki M, Yamakage M, Fujimura N, Namiki A. Direct inotropic effect of the beta-2 receptor agonist terbutaline on impaired diaphragmatic contractility in septic rats. Heart Lung 36: 140– 147, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Van den Berghe G, Schoonheydt K, Becx P, Bruyninckx F, Wouters PJ. Insulin therapy protects the central and peripheral nervous system of intensive care patients. Neurology 64: 1348– 1353, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Wan L, Bellomo R, Di Giantomasso D, Ronco C. The pathogenesis of septic acute renal failure. Curr Opin Crit Care 9: 496– 502, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Watson AC, Hughes PD, Louise Harris M, Hart N, Ware RJ, Wenon J, Green M, Moxham J. Measurement of twitch transdiaphragmatic, esophageal, and endotracheal tube pressure with bilateral anterolateral magnetic phrenic nerve stimulation in patients in the intensive care unit. Crit Care Med 29: 1325– 1331, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Wesche-Soldato DE, Chung CS, Gregory SH, Salazar-Mather TP, Ayala CA, Ayala A. CD8+ T cells promote inflammation and apoptosis in the liver after sepsis. Role of Fas-FasL. Am J Pathol 156: 234– 239, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]