Abstract
Studies were designed to determine the effects of increases of renal perfusion pressure (RPP) upon the production of H2O2 and NO-2+NO-3 (NOx) within the renal outer medulla. Sprague-Dawley rats were studied with either the renal capsule intact or removed to ascertain the contribution of changes of medullary blood flow (MBF) and renal interstitial hydrostatic pressure (RIHP) upon H2O2 and NOx production. Responses to three 30 minute step changes of renal perfusion pressure (RPP; from ∼85 to ∼115 to ∼145 mmHg) were studied using adjustable aortic occluders proximal and distal to the left renal artery. Medullary interstitial H2O2 determined by microdialysis increased at each level of RPP from 640 to 874 to 1593 nmol/L as did H2O2 urinary excretion rates and these responses were significantly attenuated by decapsulation. Medullary interstitial NOx increased from 9.2 to 13.8 to 16.1 μmole/L with parallel changes in urine NOx, but decapsulation did not significantly blunt these responses. Over the range of RPP, MBF (laser-Doppler flowmetry) rose ∼30% and RIHP from 7.8 to 19.7 cmH2O. RIHP and the natriuretic and diuretic responses were significantly attenuated with decapsulation but MBF was not affected. The data indicate that pressure-induced increases of H2O2 emanated largely from increased tubular flow rates to mTAL and NO largely from increased MBF to the vasa recta. The parallel pressure-induced increases of H2O2 and NO indicate a participation in shaping the “normal” pressure-natriuresis relationship and explain why an imbalance in either would affect the blood pressure salt-sensitivity.
Keywords: renal medullary oxidative stress, hydrogen peroxide, nitrate and nitrite, nitric oxide, pressure-natriuresis, renal medullary blood flow
Renal oxidative stress is enhanced in many animal models of hypertension and renal disease and is associated with renal fibrosis, vasoconstriction, apoptosis, and a reduction of urinary excretion of sodium (UNaV)1. It has been demonstrated that either a reduction of NO production or an increase in renal oxidative stress within the renal medulla can produce hypertension and renal injury1,2,3,4,5,6,7. The mechanisms leading to excess production of reactive oxygen species within the kidney are beginning to be understood. It is evident, for example, that both elevations of hormones such as angiotensin II (Ang II) and increased tubular or extracellular sodium concentrations can stimulate the production of superoxide (O2-) within the medullary thick ascending limbs of Henle (mTAL) and contributes to hypertension and renal injury8,9,10.
The elevation of renal perfusion pressure (RPP) with hypertension can contribute importantly to the progressive renal injury generally observed in hypertension11 as demonstrated in two rat models of hypertension; infusion of low pressor angiotensin II (AngII) + high salt diet8, and the Dahl salt-sensitive rat strain12 in which RPP to one kidney was chronically protected from elevated pressures using a computerized servo-controlled balloon occluder implanted between the distal left and proximal right renal arteries8. In the kidneys exposed to high RPP, molecules related to pathways of oxidative stress such as TGF-β and NF-κB, exhibited enhanced expression compared to the pressure-protected kidney. Kidneys exposed to the higher perfusion pressure also exhibited greater glomerular and medullary tubular sclerosis and interstitial fibrosis with an exaggerated expression of genes related to pathways of oxidative stress and apoptosis12 when compared to the pressure-protected kidney. These results indicate that RPP in some manner stimulated oxidative stress and contributed to the progression of hypertension and renal injury.
Since it was found that increased delivery of NaCl to the mTAL by microperfusion results in increased production of superoxide in this tubular segment of the outer medulla13, we hypothesized that elevations of RPP with a resulting increased delivery of NaCl would produce oxidative stress in the this region of the kidney. Given that elevations of RPP are known to increase medullary blood flow (MBF) and renal interstitial hydrostatic pressure (RIHP), these parameters were also determined in order to indirectly assess the mechanisms responsible for observed changes of H2O2 in the outer medulla. The study therefore determined if kidneys of normal Sprague Dawley rats when subjected to acute increases of RPP (∼85 to ∼115 to ∼145 mmHg) responded with increased levels of H2O2 within the outer medulla and whether these responses were driven by increases of MBF and RIHP by comparing responses in kidneys with intact renal capsules and to responses in decapsulated kidneys. Also, changes in NO production in response to elevations of RPP were also studied to determine how these events may be interrelated.
Methods
Experimental Animals
Male Sprague Dawley (SD) rats were used in all protocols (Protocol 1 and 2 used Harlan SD, Madison, WI.; Protocol 3 used SLC SD rats, Shizuoka, Japan) aged 10-11 weeks. Rats were anesthetized with ketamine (20mg/kg, i.m.) and inactin (50mg/kg, i.p), and placed on a temperature-controlled surgical table to maintain body temperature at 37°C. All procedures were approved by the Institutional Animal Care and Use Committee.
Surgical Preparation
In studies to determine the relationship between three levels of RPP (80-85, 110-115, and 140-145 mmHg) and H2O2 or NOx production in a single kidney, the left kidney was isolated, the renal artery denervated, and an adjustable micro-Blalock clamp placed around the aorta proximal and distal to the renal arteries to raise or lower RPP. Superior mesenteric and celiac arteries were tied off to achieve the highest increase of RPP to 140-145 mmHg. The left ureter was catheterized for collection of urine and the femoral artery and vein catheterized for measurement of aortic renal perfusion pressure (RPP) and for infusion of solutions respectively. An intravenous infusion of 3% bovine serum albumin in saline was given at a rate that maintained blood volume constant as estimated by hematocrit. Following one hour of equilibration, RPP was measured for two 30-min. baseline periods to ensure the stability of the preparation before RPP was changed and adjusted for measurements at the three levels of RPP described above. Urine was collected for 30-minute corresponding to the duration of each step change in RPP for determination of urine sodium and volume as well as either H2O2 or NOx. Three distinct protocols were carried out:
Protocol 1. Effects of step increases in RPP upon production of renal medullary H2O2 or NOx in rats with intact kidney capsule and in rats with the capsule removed
In these studies, a linear microdialysis fiber (320 μm OD, 5mm membrane window, LM-5, BAS Inc., West Lafeyette, IN) was inserted longitudinally using a 30-gauge needle from the lower to the upper pole of the kidney to pass through the outer medulla and anchored in place on the kidney surface with cyanoacrylate adhesive. This dialysis fiber was perfused with 0.9% NaCl at a rate of 2μl/min throughout the study. Dialysate was collected continuously throughout each 30 min. period for determination of either H2O2 or NOx. The placement of the fiber in the outer medulla was confirmed at the end of the experiment by careful visual examination, and rats with incorrectly placed fibers were discarded from the study. In one group of rats, the renal capsule remained intact while in a separate group of rats, the capsule was removed as previously described14. Two separate groups of rats were surgically prepared in the identical manner but RPP was maintained constant at control levels throughout the entire study. H2O2 was determined in one time control group and NOx in the second time control group (“time control”; Table S1A and B; please see http://hyper.ahajournals.org).
Protocol 2. Effect of step increases of RPP on medullary renal interstitial hydrostatic pressure (RIHP) or medullary blood flow (MBF) in rats with intact kidney capsule and in rats with the capsule removed
Since simultaneous implantation into the same kidney of the microdialysis fiber, the implanted polyethylene catheter for RIHP measurement, and the optical fiber for measurement of MBF was too disruptive to normal function, separate groups of rats were studied. For the determination of RIHP, rats were prepared as described above except rather than implanting a microdialysis fiber, a polyethylene catheter (PE50) with a polyethylene matrix in the tip was implanted into the outer medulla of the kidney as previously described14. For rats in which MBF was determined, an optical fiber was inserted into the outer medulla as previously described15. RIHP or MBF was measured continuously during the step changes in RPP. These measurements were made in groups of rats with the kidney capsule either intact or removed.
Protocol 3. Urinary H2O2 responses of the left and right kidney within the same rat
In another group of rats, the capsule of the left kidney remained intact while that of the right kidney was removed. RPP was then increased from 119 to 145 mmHg to both kidneys by tying off the celiac and superior mesenteric arteries and adjusting the final elevation of RPP with a ligature placed around the aorta distal to both renal arteries. Urine was collected bilaterally from catheters inserted into both ureters for measurement of changes of urinary H2O2 excretion rates associated with the increase of RPP.
Protocol 4. Effect of step increases of RPP on glomerular filtration rate (GFR)
GFR was determined by inulin clearance of FITC-inulin16. FITC-inulin (5 mg/ml; Sigma) dissolved in BSA and saline was infused at 3 ml/hr for 60 min. prior to the collection of the first urine sample. Blood samples were taken at the midpoint of each urine collection period. Collected samples were diluted with phosphate buffered saline (pH 7.4) and the fluorescence measured with a microplate reader.
Biochemical Measurements
H2O2 concentration was determined in interstitial fluid collected by microdialysis and urine using a fluorescence spectrometric assay (Amplex Red Hydrogen Peroxide Assay kit, Molecular Probes, Eugene, OR) as previously described3,5,17. NOx was determined in interstitial fluid collected by microdialysis and urine with absorbance spectrophotometer using the Greiss reaction (Nitrate/Nitrite Colorimetric Assay Kit, Cayman Chemical Company, MI) as previously described18. Since all of the nitrite in the sample is converted by the reaction to nitrate, the final determination is the sum of the converted nitrite plus the nitrate already in the sample. This sum is designated as NOx in this manuscript. Urine volume for calculation of urine flow rate (UV) was determined gravimetrically and urine sodium for calculation of urinary excretion of sodium (UNaV) measured by flame photometry.
Statistical analysis
Data are presented as mean ± SE. For statistical comparisons, 2-way ANOVA with repeated measures was used followed by a Duncan’s post hoc test. All statistical analyses were performed on the raw data. P<0.05 was considered to be statistically significant.
Results
Relationship of RPP to sodium and water excretion, renal medullary interstitial concentrations and urinary excretion of H2O2 and NOx in rats with intact renal capsule and in rats with the renal capsule removed
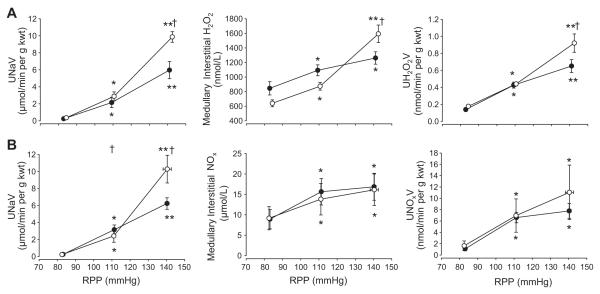
Figure 1 (Panel A) summarizes changes of urine sodium excretion (UNaV) and medullary interstitial and urine H2O2 in response to RPP adjusted to three levels in one group rats (n=7) with an intact renal capsule compared to a group with the renal capsule removed (n=8). In rats with the renal capsule intact, RPP was adjusted downward from resting control levels of 112±2.0 to 84±0.8 mmHg then to 111±0.3 mmHg (not different from pressure setting of time control group) and finally to 143±0.3mmHg. Associated with these step increases of RPP, UNaV rose significantly from 0.36±0.1 to 2.88±0.5 to 9.86±0.6 μmol/min per gram kidney weight (g kwt) and urine volume from 4.3±0.5 to 18.3±3.1 to 54.4±4.9 μL/min per g kwt (data not shown). Elevation of RPP significantly increased medullary interstitial H2O2 at each pressure step (640±47, 874±53 and 1593±120 nmol/L; p<0.05). Parallel increases of urinary H2O2 excretion were observed in these rats increasing significantly from 0.18±0.01, 0.44±0.02 to 0.92±0.11 nmol/min per g kwt with the increases of RPP. In a separate group of rats (n=8), GRF was determined and found to autoregulate in a manner similar to that reported by others19. With RPP fixed at 85+0.5 mmHg, GFR averaged 0.5+0.05 ml/min/g kwt in the left kidney. When RPP was increased to 109+1.1mmHg, GFR rose significantly to 0.69+0.05 ml/min/g kwt. GFR was not further increased (0.78 ml/min/g) at the highest RPP fixed at 139+2.1mmHg. RPP in the time control group (n=7) was adjusted to remain constant at 110±4.9, 111±5 and 110±4.8 mmHg, respectively for the three collection periods. No significant changes in any measured variable were observed in the time control group (Table S1A; please see http://hyper.ahajournals.org).
Figure 1.
Responses to changes in renal perfusion pressure (RPP) are shown in kidneys with intact capsules (open circles) and following decapsulation (closed circles). In Panel A, the changes in excretion of urinary sodium (UNaV; top figure), medullary interstitial H2O2 (middle figure), and urinary excretion of H2O2 (UH2O2V; bottom figure) following a change in RPP are shown. Panel B shows the change in excretion of urinary sodium (UNaV; top figure), medullary interstitial NOx (middle figure), and urinary excretion of NOx (UNOxV; bottom figure) following a change in RPP. Values are means ± SE. * indicates significant change from the lowest pressure (p<0.05); ** indicates significant change from both the lowest and the intermediate pressure (p<0.05); † indicates significant difference from the decapsulated group at the same pressure step (p<0.05).
Similar step increases of RPP were evaluated in the decapsulated group (83±0.6 to 109±1.0 and then 141±0.8 mmHg; (n=8; Figure 1A). Elevation of RPP from 83 to 109 mmHg increased renal interstitial H2O2 in the decapsulated kidney significantly and to nearly the same amount seen in the group with the intact capsule. However, when RPP was elevated from 109 to 141 mmHg, medullary interstitial H2O2 was significantly attenuated in this group increasing only 15% compared to the 82% increase observed in the group with the intact capsule. Similarly urinary excretion of H2O2 increased with each pressure step with the excretion in the intact kidney being significantly greater than the increase measured in the decapsulated kidney at the highest pressure step.
Figure 1B summarizes changes of medullary and urine NOx in response to similar changes of RPP in renal intact (n=7) and decapsulated (n=6) rats. With the renal capsule intact, RPP was adjusted downward from resting control levels of 116±3.2 to 83±0.5mmHg, then to 111±0.6 mmHg and finally to 140±2.3 mmHg (n=7). Associated with the step changes of RPP, rising from 83 to 111 to 140 mmHg, UNaV increased significantly from 0.22±0.1 to 2.42±0.7 to 10.28±1.6 μmol/min per g kwt and UV from 3.4±0.5 to 20.4±3.5 to 53.2±5.3 μL/min per g kwt (data not shown). Medullary interstitial NOx increased significantly when RPP was increased from 83 to 111 mmHg (9.2+2.8 to 13.8+3.9; P<0.05). As RPP was increased further to 140 mmHg, medullary interstitial NOx rose to 16.1+3.9 μmol/L. Similarly, urinary NOx excretion increased from 1.6+0.9 to 7.0+2.9 to 11.1+4.8 nmol/min per g kwt at the three respective pressure steps. In decapsulated kidneys, the pressure-natriuresis response was significantly reduced compared to the intact group (UNaV 0.25±0.1 to 3.15±0.6 to 6.24±0.9 μmol/min per g kwt; urine volume 3.1+0.7 to 18.1+3.7 to 31.4+5.2 μL/min per g kwt-data not graphed). In contrast, to the H2O2 responses, renal decapsulation did not significantly attenuate the relationship between RPP and medullary interstitial NOx (8.9±2.4; 15.9±3.2; and 16.9±3.3 μmol/L), or urinary excretion of NOx (1.1±0.3 to 6.6±0.9 to 7.8±1.3 nmol/min per g kwt. No significant changes in any measured variable were observed in the time control group (Table S1B; please see http://hyper.ahajournals.org).
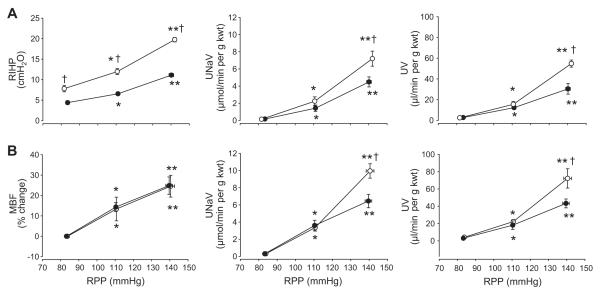
Effect of step increases of RPP on medullary renal interstitial hydrostatic pressure (RIHP) and medullary blood flow (MBF) responses to decapsulation
In rats with intact renal capsules and implanted catheter for determination of RIHP (n=7), as RPP was adjusted from 82±0.6 to 111±0.8 to 142±0.6 mmHg, RIHP increased significantly from 7.8±0.8, 12±0.7 to 19.7±0.5 cmH2O (Figure 2A). UNaV increased from 0.1±0.04 to 2.2±0.5 to 7.2±0.9 μmol/min per g kwt while UV increased from 2.5±0.4, 15.5±2.7 to 54.8±3.4 μL/min per g kwt with the three pressure steps. With decapsulation (n=7), increase of RIHP was significantly attenuated rising from 4.4±0.5 to 6.5±0.4 to 11.1±0.4 cmH2O for the same pressure steps. The increase in UNaV was significantly blunted in the kidneys that were decapsulated compared to the kidneys with intact renal capsule (4.5±0.6 vs 7.2±0.9 μmol/min per g kwt; p<0.05). Similarly, UV was also attenuated by decapsulation averaging 2.7±0.4 to 12.3±1.9 to 30.4±5.1 μL/min per g kwt.
Figure 2.
Changes in renal interstitial hydrostatic pressure (RIHP; Panel A) or medullary blood flow (MBF; Panel B) as well as urinary excretion of sodium (UNaV) and volume (UV) in response to changes in renal perfusion pressure (RPP) are shown in groups of rats with either intact (open circles) or decapsulated kidneys (closed circles). Values are means ± SE. * indicates significant change from the lowest pressure (p<0.05); ** indicates significant change from both the lowest and the intermediate pressure (p<0.05); † indicates significant difference from the decapsulated group at the same pressure step (p<0.05).
In rats with intact renal capsules and optical fibers implanted for the measurement of MBF (n=5), as RPP was adjusted from 84±0.5 to 111±0.9 to 141±2.1 mmHg, MBF increased 13±6% above the lowest pressure at the intermediate step and then 25±5% at the highest pressure step (Figure 2B). UNaV increased from 0.3±0.1 to 3.4±0.4 to 10.0±0.8 μmol/min per g kwt while UV increased from 4.2±0.6 to 22.2±2.5 to 72.2±11.2 μL/min per g kwt with the three pressure steps. In contrast to the RIHP, MBF was not significantly attenuated by decapsulation (n=6). However, since the rise of RIHP was clearly blunted by decapsulation, increases in UNaV and urine volume were also blunted in this group of rats.
Changes in urinary oxidative stress with differing RPP between two kidneys
The relationship between RPP, urinary H2O2 excretion, and the influence of renal interstitial hydrostatic pressure (RIHP) were further confirmed in another group of rats (N=7) by comparing responses within the same rat. The renal capsule was removed from the left kidney while the capsule of the right kidney remained intact. In these rats, the aortic occluder was placed below both of the renal arteries so RPP increased from 119±3.6 to 145±5.3 mmHg to both kidneys as shown in Figure 3. Urinary H2O2 excretion of the intact right kidney increased from 0.12±0.02 to 0.48±0.09 nmol/min per g kwt (p<0.05), an increase significantly greater than the response of the decapsulated left kidney in which H2O2 excretion was increased slightly with the pressure step and did not reach significance. These data support the results shown above and emphasize the importance of changes of RIHP upon the production of renal H2O2.
Figure 3.
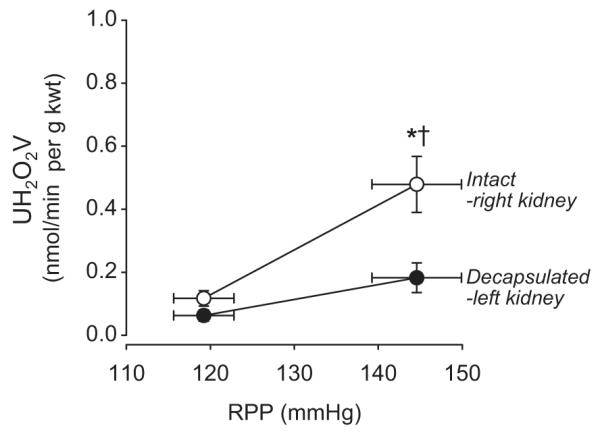
Urinary oxidative stress determined as urinary excretion of H2O2 (UH2O2V) increases with an increase in renal perfusion pressure (RPP) in the intact right kidney (open circles) but the decapsulated left kidney (closed circles) of the same rat showed significantly less excretion with the same change of pressure. Values are means ± SE. * significantly different from the baseline pressure in the same kidney (p<0.05). † significantly different from the decapsulated left kidney (p<0.05).
Discussion
Renal medullary oxidative stress as driven by acute increases of renal perfusion pressure
The concept of pressure-natriuresis and the relationships between RPP, medullary blood flow (MBF), renal interstitial hydrostatic pressure (RIHP), and UNaV has been well established20,21 . It was the goal in the present study to establish how these factors were involved in the observed increases of H2O2 and NO in response to elevations of RPP. The present study provides the first evidence that acute increases of RPP elevate the production of H2O2 in the outer medulla and that this response is triggered by an increase of RIHP.
Since blood flow to the renal medulla of the rat is poorly autoregulated, increases of RPP are transmitted to the vasa recta circulation21,22 and as RPP and vasa recta flow increase, vasa recta capillary hydrostatic pressure increases producing parallel increases in RIHP due to a net filtration of fluid into the renal interstitial space14,23. Pressure-natriuresis results from reduction of Na+ reabsorption both in the proximal tubules24,25 and in the deep medullary nephrons. An increase of RPP with an increase of RIHP signals the release of 20-HETE within the proximal tubules24 which inhibits Na+-K+-ATPase activity and results in the internalization of the sodium/hydrogen exchanger from the brush border and a reduction in Na+ reabsorption. This results in a greater delivery of NaCl to the distal tubules24,25 and natriuresis which is further enhanced by washout of the medullary solute gradient that affects the passive reabsorption of Na+ in deep medullary loops of Henle1,21. It is the increased delivery of NaCl to the mTAL that we hypothesized would stimulate the enhanced production of O2- and H2O2 in this tubular segment when RPP is increased. Since removal of the renal capsule significantly blunted the rise of RIHP, pressure-natriuresis, and the rise of H2O2 within the outer medulla, (all by approximately 40%), we conclude that NaCl delivery to the mTAL was critical for this response. It is possible that changes of RIHP could in some way have directly stimulated H2O2 production in various tubular segments, however, such responses have not yet been explored._ Although the precise origin of H2O2 production was not determined in the present study, it is recognized that the mTAL can be a major source of oxygen free radical (O2-) production4,9,10,13 and increased luminal flow and NaCl delivery to the mTAL can stimulate O2- production13,26. It is of interest that changes of urinary H2O2 excretion in the present study were remarkably parallel to those seen in the microdialysis samples of the outer medullary interstitial fluid. This suggests that urinary H2O2 excretion could serve as a good marker of renal medullary oxidative stress since H2O2 is relatively stable in aqueous solutions and a more specific assay than other currently used markers of renal oxidative stress such as 8-isoprotanes determined by enzyme immunoassay, and products of lipid peroxidation as determined by a colorimetric assay for thiobarbituric acid reactive substances.
Finally, it should also be noted that although we have previously used microdialysis techniques to measure nitric oxide27, NOx18, superoxide18,28 and H2O23,5 in the renal medulla, these earlier studies utilized a needle type microdialysis probe (BR-2, BAS, Inc.) that provided a 2 mm length of membrane for dialysis. The linear microdialysis fiber (with nearly a 5 mm length of membrane) used in the current study provided a greater membrane surface area and enhanced the dialysis efficiency from 32% seen with the needle probe to 52%. The linear fiber maintained a more stable position in the face of increases of RPP. Since fluorescence units were not corrected for the efficiency of the membranes, the absolute values are greater than previously reported from our laboratory.
Effects of increases of RPP upon renal medullary NO production
Medullary interstitial NOx concentrations and urinary NOx excretion levels also increased as RPP was increased, confirming observations of others29. This, however, was mostly associated with the increases of RPP from the lowest (85 mmHg) to the intermediate pressure step (110 mmHg). Changes in MBF following renal decapsulation have not been previously reported and a novel finding of the present study is that in contrast to the changes observed with RIHP, changes of MBF with RPP were unaffected by decapsulation. Microperfusion studies by Pallone et al.30 found flowdependent increases of NO production in isolated perfused vasa recta of the outer medulla of the rat. A rise of RPP with an increase of MBF would therefore be expected to produce an increased endothelial release of NO and not be affected by renal decapsulation. Although we have reported that increased tubular flow and delivery of Na+ to mTAL reduces NO production within isolated perfused mTAL13 , since medullary interstitial NOx was increased in the present study, it appears that these levels were dominated by NO produced by the vasa recta vessels. These relationships are clearly complex and since medullary interstitial NO did not rise substantially between RPP of 110 to 140 mmHg even though MBF did rise significantly over this range RPP, it is evident that something else is going on at these pressure levels. It is possible that as RPP and MBF are increased to these higher levels, further increases of interstitial NO concentrations may be attenuated by medullary washout.
We conclude that elevations of RPP stimulate the release of both NO and H2O2 within the renal outer medulla. The mechanisms responsible for the parallel release of these important vasoactive molecules differ whereby NO appears to be driven by increases of MBF while increases of H2O2 results from increased mTAL delivery of NaCl. The balance of H2O2 and NO production in the outer medulla are therefore important considerations in understanding the interrelationships between renal perfusion pressure, sodium excretion, and the long-term control of arterial blood pressure. The parallel increases of H2O2 and NO produced by elevations of RPP appear to be important determinants of the “normal” pressure-natriuresis relationship.
Perspectives
Pressure-natriuresis appears to come with the price of also producing oxidative stress within the outer medulla of the kidney unless offset by a parallel production of NO. This would appear to explain why excess levels of medullary H2O2 can lead to a salt-sensitive form of hypertensive31 while greater levels of medullary NO can reduce salt-sensitivity and lower arterial pressure32. One can also speculate that in the chronic state, if both the production of superoxide and NO occur with elevations of RPP, greater amounts of peroxynitrite would also be produced in renal outer medulla that may be associated with greater tissue fibrosis and injury. The present data suggest that the development of antioxidant agents that could effectively reduce medullary O2- and H2O2 production may be of greater clinical benefit than those that focus upon producing greater production of NO, such as L-arginine.
Supplementary Material
Acknowledgments
The authors thank Jenifer Goepfert for measurement of urine sodium; Terry Kurth and Yoshimi Yoneki for their excellent technical assistance.
Sources of Funding This work was supported by National Heart Lung and Blood Institute grant HL-29587 and Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (No. 17390245 and No. 17590181).
Footnotes
Disclosures None
References
- 1.Cowley AW., Jr. Renal medullary oxidative stress, pressure-natriuresis and hypertension. Hypertension. 2008;52:777–786. doi: 10.1161/HYPERTENSIONAHA.107.092858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Makino A, Skelton MM, Zou AP, Roman RJ, Cowley AW., Jr. Increased renal medullary oxidative stress produces hypertension. Hypertension. 2002;39:667–672. doi: 10.1161/hy0202.103469. [DOI] [PubMed] [Google Scholar]
- 3.Taylor NE, Cowley AW., Jr. Effect of renal medullary H2O2 on salt-induced hypertension and renal injury. Am J Physiol. 2005;289:R1573–1579. doi: 10.1152/ajpregu.00525.2005. [DOI] [PubMed] [Google Scholar]
- 4.Zou AP, Li N, Cowley AW., Jr. Production and actions of superoxide in the renal medulla. Hypertension. 2001;37:547–553. doi: 10.1161/01.hyp.37.2.547. [DOI] [PubMed] [Google Scholar]
- 5.Makino A, Skelton MM, Zou AP, Cowley AW., Jr. Increased renal medullary H2O2 leads to hypertension. Hypertension. 2003;42:25–30. doi: 10.1161/01.HYP.0000074903.96928.91. [DOI] [PubMed] [Google Scholar]
- 6.Taylor NE, Cowley AW., Jr. Effect of renal medullary H2O2 salt-induced hypertension and renal injury. Am J Physiol. 2005;289:R1573–R1579. doi: 10.1152/ajpregu.00525.2005. [DOI] [PubMed] [Google Scholar]
- 7.Guarasci G, Kline RL. Pressure-natriuresis following acute and chronic inhibition of nitric oxide synthase in rats. Am J Physiol. 1996;270:R469–R478. doi: 10.1152/ajpregu.1996.270.2.R469. [DOI] [PubMed] [Google Scholar]
- 8.Mori T, Cowley AW., Jr. Role of pressure in angiotensin II-induced renal injury: Chronic servo-control of renal perfusion pressure in rats. Hypertension. 2004;43:752–759. doi: 10.1161/01.HYP.0000120971.49659.6a. [DOI] [PubMed] [Google Scholar]
- 9.Mori T, Cowley AW., Jr. Renal oxidative stress in medullary thick ascending limbs produced by elevated NaCl and glucose. Hypertension. 2004;43:341–346. doi: 10.1161/01.HYP.0000113295.31481.36. [DOI] [PubMed] [Google Scholar]
- 10.Mori T, O’Connor PM, Abe M, Cowley AW., Jr. Enhanced superoxide production in renal outer medulla of Dahl salt-sensitive rats reduces nitric oxide tubular-vascular cross-talk. Hypertension. 2007;49:1336–1341. doi: 10.1161/HYPERTENSIONAHA.106.085811. [DOI] [PubMed] [Google Scholar]
- 11.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 12.Mori T, Polichnowski A, Glocka P, Kaldunski M, Ohsaki Y, Liang M, Cowley AW., Jr. High perfusion pressure accelerates renal injury in salt-sensitive hypertension. J Am Soc Nephrol. 2008;19:1472–1482. doi: 10.1681/ASN.2007121271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abe M, O’Connor P, Kaldunski M, Liang M, Roman RJ, Cowley AW., Jr. Effect of sodium delivery on superoxide and nitric oxide in the medullary thick ascending limb. Am J Physiol. 2006;291:F350–F357. doi: 10.1152/ajprenal.00407.2005. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Estan J, Roman RJ. Role of renal interstitial hydrostatic pressure in the pressure diuresis response. Am J Physiol. 1989;256:F63–70. doi: 10.1152/ajprenal.1989.256.1.F63. [DOI] [PubMed] [Google Scholar]
- 15.Nakanishi k, Mattson DL, Gross V, Roman RJ, Cowley AW., Jr. Control of renal medullary blood flow by vasopressin V1 and V2 receptors. Am J Physiol. 1995;879:R193–R200. doi: 10.1152/ajpregu.1995.269.1.R193. [DOI] [PubMed] [Google Scholar]
- 16.Patrick KK, Yang LE, Holstein-Tathlou N-H, McDonough AA. Angiotensin II clamp prevents the second step in renal apical NHE3 internalization during acute hypertension. Am J Physiol. 2002;283:F1142–F1150. doi: 10.1152/ajprenal.00178.2002. [DOI] [PubMed] [Google Scholar]
- 17.Chen YF, Cowley AW, Jr, Zou AP. Increased H2O2 counteracts the vasodilator and natriuretic effects of superoxide dismutation by tempol in renal medulla. Am J Physiol. 2003;285:R827–33. doi: 10.1152/ajpregu.00636.2002. [DOI] [PubMed] [Google Scholar]
- 18.Taylor NE, Glocka P, Liang M, Cowley AW., Jr. NADPH oxidase in the renal medulla causes oxidative stress and contributes to salt-sensitive hypertension in Dahl S rats. Hypertension. 2006;47:692–698. doi: 10.1161/01.HYP.0000203161.02046.8d. [DOI] [PubMed] [Google Scholar]
- 19.Wang C-T, Chin SY, Navar LG. Impairment of pressure-natriureesis and renal autoregulation in Ang II-infused hypertensive rats. Am J Physiol. 2000;279:F319–F325. doi: 10.1152/ajprenal.2000.279.2.F319. [DOI] [PubMed] [Google Scholar]
- 20.Guyton AC, Coleman TG, Cowley AW, Jr, Scheel KW, Manning RD, Jr, Norman RA., Jr. Arterial pressure regulation. Overriding dominance of the kidneys in long-term regulation and in hypertension. Am J Med. 1972;52:584–594. doi: 10.1016/0002-9343(72)90050-2. [DOI] [PubMed] [Google Scholar]
- 21.Cowley AW., Jr. Long-term control of arterial blood pressure. Physiol Rev. 1992;72:231–300. doi: 10.1152/physrev.1992.72.1.231. [DOI] [PubMed] [Google Scholar]
- 22.Cowley AW, Jr, Roman RJ. The role of the kidney in hypertension. JAMA. 1996;275:1581–1589. [PubMed] [Google Scholar]
- 23.Granger JP. Pressure natriuresis. Role of renal interstitial hydrostatic pressure. Hypertension. 1992;19:I9–I17. doi: 10.1161/01.hyp.19.1_suppl.i9. [DOI] [PubMed] [Google Scholar]
- 24.Williams JM, Sarkis A, Lopez B, Ryan RP, Flasch AK, Roman RJ. Elevations in renal interstitial hydrostatic pressure and 20-hydroxyeicosatetraenoci acid contribute to pressure natriuresis. Hypertension. 2007;49:687–694. doi: 10.1161/01.HYP.0000255753.89363.47. [DOI] [PubMed] [Google Scholar]
- 25.Zhang YB, Magyar CE, Holstein-Rathlou NH, McDonough AA. The cytochrome P-450 inhibitor cobalt chloride prevents inhibition of renal Na, K-ATPase and redistribution of apical NHE-3 during acute hypertension. J Am Soc Nephrol. 1998;9:531–537. doi: 10.1681/ASN.V94531. [DOI] [PubMed] [Google Scholar]
- 26.Garvin JL, Hong NJ. Cellular stretch increases superoxide production in the thick ascending limb. Hypertension. 2008;51:488–493. doi: 10.1161/HYPERTENSIONAHA.107.102228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zou AP, Cowley AW., Jr. Nitric oxide in renal cortex and medulla: an in vivo microdialysis study. Hypertension. 1997;29:194–198. doi: 10.1161/01.hyp.29.1.194. [DOI] [PubMed] [Google Scholar]
- 28.Taylor NE, Maier KG, Roman RJ, Cowley AW., Jr. NO synthase uncoupling in the kidney of Dahl S rats: Role of dihydrobiopterin. Hypertension. 2006;48:1066–1071. doi: 10.1161/01.HYP.0000248751.11383.7c. [DOI] [PubMed] [Google Scholar]
- 29.Majid DS, Godfrey M, Grisham MB, Navar LG. Relation between pressure natriuresis and urinary excretion of nitrate/nitrite in anesthetized dogs. Hypertension. 1995;25:860–865. doi: 10.1161/01.hyp.25.4.860. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Z, Pallone TL. Response of descending vasa recta to luminal pressure. Am J Physiol. 2004;287:F535–F542. doi: 10.1152/ajprenal.00394.2003. [DOI] [PubMed] [Google Scholar]
- 31.Chen YF, Cowley AW, Jr, Zou AP. Increased H2O2 counteracts the vasodilator and natriuretic effects of superoxide dismutation by tempol in renal medulla. Am J Physiol. 2003;285:R827–R833. doi: 10.1152/ajpregu.00636.2002. [DOI] [PubMed] [Google Scholar]
- 32.Miyata N, Cowley AW., Jr. Renal medullary interstitial infusion of L-arginine prevents reduction of medullary blood flow and hypertension in Dahl salt-sensitive rats. Hypertension. 1999;33:446–450. doi: 10.1161/01.hyp.33.1.446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.