Response Inhibition and Atomoxetine
Response inhibition is a central element of executive control that is critical for adaptive behavior in a dynamic and unpredictable environment. The inability to inhibit inappropriate responses has been linked to several prevalent disorders, including compulsions, autism, and attention-deficit/hyperactivity disorder (ADHD). Therefore, the ability to develop more effective treatments for these disorders is likely to depend, at least in part, on understanding the brain mechanisms that control response inhibition, including the underlying neurotransmitter systems.
Stimulants have long been the treatment of choice for ADHD, suggesting that the underlying neural dysfunctions involve monoamine neurotransmitter systems. Genetic analyses in the last several years have given more support to this idea, indicating not only that ADHD is highly heritable but also that it is associated with alterations in several monoamine genes, including those for the D4 and D5 dopamine (DA) receptors and the DA transporter (1). These results and others linking the DA system with locomotor activation led to the hypothesis that ADHD reflects primarily deficits in DA systems (2).
Recent evidence indicates that the brain norepinephrine (NE) system also plays an important role in ADHD. Genetic studies find that ADHD is associated with alterations in the genes responsible for making NE from DA (dopamine β hydroxylase) and for the NE transporter (1). Other evidence includes findings from animal studies that α-2 NE receptors in cortex might play a role in locomotor activation and attention (3) as well as unit recordings showing that locus coeruleus (LC) NE neurons respond in relation to attentional performance (4) (discussed in the following text). Finally, the effectiveness of the selective NE uptake blocker atomoxetine in treating ADHD also supports this view (5).
An accompanying article in this issue of Biological Psychiatry by Chamberlain et al. (pg.*) finds that atomoxetine increases inhibitory control in normal humans as measured with a well-established procedure, the stop-signal reaction time task (Figure 1). For this task, subjects perform a simple action like a button press or eye movement immediately in response to a visual cue. On a small subset of trials, a second cue is presented after the “go” cue, indicating that the action should be withheld. The timing of this “stop signal” is critical. Present it immediately after the “go” cue, and the task is easy. Present it too late, and it is ineffective. Chamberlain et al. adjusted the timing between “go” and “stop” cues until subjects appropriately withheld responses approximately one-half the time, allowing them to infer the “stop signal reaction time” (SSRT) that quantifies the speed of response inhibition. They found that atomoxetine decreased SSR (i.e., facilitated the ability to inhibit responses).
Figure 1.
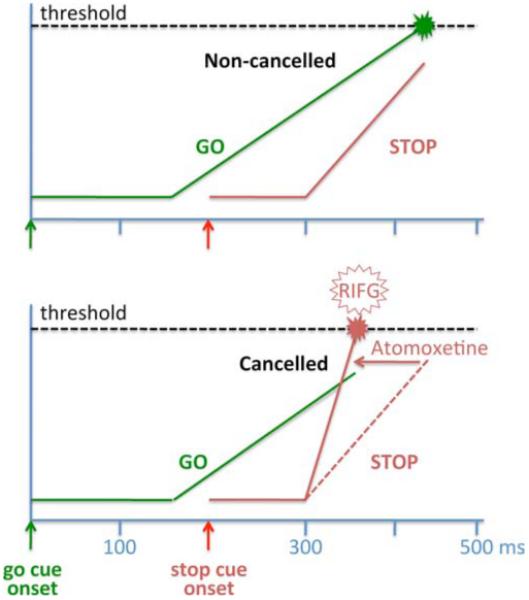
Illustration of the race model for the stop-signal task and the effect of atomoxetine proposed in Chamberlain et al. The race model includes a GO process (green) and a STOP process (red) that are racing independently toward a common threshold. The GO stimulus onset is at time 0 (green arrow), triggering a rise in the GO process (green) after a delay. The stop stimulus onset is at 200 msec (red arrow), triggering a rise in the STOP process (red) after a delay. If the GO process reaches threshold first (upper panel), then a response is generated (non-cancelled trials). Conversely, if the STOP process finishes before the GO process (lower panel), then the response is cancelled. Functional magnetic resonance imaging studies show relatively higher activation of the right inferior frontal gyrus (RIFG) on cancelled trials. Atomoxetine decreases stop-signal reaction times and increases RIFG activation on cancelled trials, possibly by increasing the strength (rate of rise) of the Stop process. Modified with permission from Hanes et al. (6).
Previous work has identified several neural systems involved with inhibitory control on the stop-signal task. The “go” signal is thought to trigger a dynamic process in the brain that builds up to a critical level of activation that, when reached, triggers the action (6). Neural recordings in monkeys performing the task have identified correlates of this process in several brain regions, including the frontal eye field (FEF) for a task using eye-movement responses (6). Response inhibition is thought to involve a race between these go processes and similar, dynamic stop processes (6). In monkeys, neurons in the FEF and other regions of the prefrontal cortex that are related to maintaining fixation are active on successfully inhibited trials in the eye-movement stop-signal task (6). Functional magnetic resonance imaging (fMRI) studies in human subjects suggest that these inhibitory signals originate in a set of circuits that includes the right inferior frontal gyrus (RIFG), possibly acting via the subthalamic nucleus to suppress thalamocortical output that would otherwise trigger response execution (7).
The authors use the stop-signal task combined with fMRI to investigate neural substrates for the improvement in inhibitory control that occurs with atomoxetine. Results show that: 1) the stop-signal task activates distributed neural systems, including the RIFG as previously shown (8); 2) atomoxetine at a dose similar to that used to treat adult ADHD (40 mg) increases brain activation selectively in the RIFG and adjacent areas of the inferior temporal cortex and insula on stop-signal trials, whether or not they were successful; and 3) there is a positive correlation between plasma atomoxetine levels and fMRI activation in the RIFG area on successful but not on failed inhibition trials. Perhaps most exciting in this study is the selectivity of the effect of atomoxetine for the RIFG and adjacent brain regions. This result supports previous studies of RIFG’s role in inhibitory control (8) and extends them by showing that the ADHD medication atomoxetine might facilitate inhibitory control by potentiating activity in this area (Figure 1). This result also extends our knowledge of how NE systems might be involved in ADHD, by linking NE modulation specifically to a cortical region previously shown to be important in ADHD and response inhibition.
Given the fact that atomoxetine is an indirect agonist of NE, and NE systems are highly distributed throughout the brain, this anatomical selectivity for atomoxetine effects is somewhat unexpected. However, this finding might not be surprising when considered in light of other neurobiological features of the brain’s NE system.
NE-LC Role in Decision Execution and Performance
Recent results show that LC neurons in monkeys exhibit phasic activations just preceding behavioral responses, possibly reflecting signals that a decision has been reached (9). The ensuing NE release is thought to transiently increase neuronal gain (synaptic responsiveness) in the widespread LC targets, serving to facilitate processes and behaviors engaged by the decision and its execution (4). Therefore, because the RIFG seems to be substantially involved in response inhibition, it follows that this area might be particularly impacted by phasic NE release if LC neurons are activated by the decision to inhibit responding after the stop-signal. This as-yet unconfirmed hypothesis is under active investigation in our labs.
In contrast, poor attentional performance with high distractibility is proposed to be facilitated by elevated tonic (baseline) LC activity when phasic responses are absent (4). Although such distractibility is adaptive in many contexts by promoting flexible behavior, it can also be maladaptive when carried to an extreme, as in disorders of attention. This idea suggests that ADHD might be associated with overly tonic activity of LC neurons and an absence of decision-related phasic LC activation.
The utility of stimulants or atomoxetine in treating ADHD would seem to conflict with the idea that this disorder is associated with elevated tonic LC activity because these agents are thought to act by blocking reuptake and thereby increasing catecholamine availability. However, such drug actions do not take into account effects of these drugs on the impulse activity of NE source neurons in the LC. A previous study reported that the stimulant and popular ADHD medication methylphenidate (Ritalin) decreases both tonic and phasic LC activity in anesthetized rats, presumably by increasing autoreceptor stimulation (10). The effect of NE transporter blockade on LC impulse activity might be different in waking subjects and depend upon the level of task performance and pattern of LC activity. For example, the autoreceptor agonist clonidine has been found to decrease tonic LC activity but promote phasic task-related LC activity and task performance in otherwise poorly performing monkeys (4). Similarly, a recent study in humans reported that modafinil (a compound that in part acts by decreasing NE reuptake) decreased task-independent tonic LC activity, increased task-associated LC and cortical activation, and potentiated trial-wise LC-cortical coupling (11). If such an effect on LC activity occurs for atomoxetine, the ensuing phasic NE potentiation in target areas such as RIFG could help inhibit inappropriate responses and refocus behavior on more adaptive goals.
The report by Chamberlain et al. is significant for showing a specific target region where NE actions might help to facilitate response inhibition. The finding seems to have important implications for not only normal behavior but also behavioral dysfunctions such as ADHD. Further insight into the brain mechanisms involved in inhibitory control, particularly with regard to brain NE systems, will be found by studying how LC neurons respond in animals performing response inhibition tasks and how their performance is impacted by stimulant or atomoxetine administration.
Acknowledgments
This work was supported by National Institutes of Health Grant P50-MH62196. The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, et al. Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 2.Casey BJ, Nigg JT, Durston S. New potential leads in the biology and treatment of attention deficit-hyperactivity disorder. Curr Opin Neurol. 2007;20:119–124. doi: 10.1097/WCO.0b013e3280a02f78. [DOI] [PubMed] [Google Scholar]
- 3.Ramos BP, Arnsten AF. Adrenergic pharmacology and cognition: Focus on the prefrontal cortex. Pharmacol Ther. 2007;113:523–536. doi: 10.1016/j.pharmthera.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- 5.Prince J. Catecholamine dysfunction in attention-deficit/hyperactivity disorder: An update. J Clin Psychopharmacol. 2008;28:S39–S45. doi: 10.1097/JCP.0b013e318174f92a. [DOI] [PubMed] [Google Scholar]
- 6.Hanes DP, Patterson WF, Schall JD. Role of frontal eye fields in countermanding saccades: Visual, movement, and fixation activity. J Neurophysiol. 1998;79:817–834. doi: 10.1152/jn.1998.79.2.817. [DOI] [PubMed] [Google Scholar]
- 7.Aron AR, Poldrack Cortical and subcortical contributions to stop signal response inhibition: Role of the subthalamic nucleus. J Neurosci. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aron AR, Poldrack The cognitive neuroscience of response inhibition: Relevance for genetic research in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1285–1292. doi: 10.1016/j.biopsych.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 9.Clayton EC, Rajkowski J, Cohen JD, Aston-Jones G. Phasic activation of monkey locus ceruleus neurons by simple decisions in a forcedchoice task. J Neurosci. 2004;24:9914–9920. doi: 10.1523/JNEUROSCI.2446-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devilbiss DM, Berridge CW. Low-dose methylphenidate actions on tonic and phasic locus coeruleus discharge. J Pharmacol Exp Ther. 2006;319:1327–1335. doi: 10.1124/jpet.106.110015. [DOI] [PubMed] [Google Scholar]
- 11.Minzenberg MJ, Watrous AJ, Yoon JH, Ursu S, Carter CS. Modafinil shifts human locus coeruleus to low-tonic, high-phasic activity during functional MRI. Science. 2008;322:1700–1702. doi: 10.1126/science.1164908. [DOI] [PubMed] [Google Scholar]


