Abstract
Numerous studies have shown that older adults exhibit deficits in motor sequence learning, but the mechanisms underlying this effect remain unclear. Our recent work has shown that visuospatial working-memory capacity predicts the rate of motor sequence learning and the length of motor chunks formed during explicit sequence learning in young adults. In the current study, we evaluate whether age-related deficits in working memory explain the reduced rate of motor sequence learning in older adults. We found that older adults exhibited a correlation between visuospatial working-memory capacity and motor sequence chunk length, as we observed previously in young adults. In addition, older adults exhibited an overall reduction in both working-memory capacity and motor chunk length compared with that of young adults. However, individual variations in visuospatial working-memory capacity did not correlate with the rate of learning in older adults. These results indicate that working memory declines with age at least partially explain age-related differences in explicit motor sequence learning.
INTRODUCTION
The ability to acquire new action sequences is critical to functional independence with advancing age. Although normal aging does not appear to affect the acquisition of relatively simple motor sequences (e.g., Frensch and Miner 1994; Howard and Howard 1989; Seidler 2006), numerous studies have reported a decline in the ability of older adults to learn action sequences with complex structure under both implicit and explicit learning conditions (e.g., Curran 1997; Howard et al. 2004; Shea et al. 2006). For example, Howard et al. (2004) found that older adults could not learn new sequences in which the fixed and random elements were intermixed (alternating serial reaction time task). Curran et al. (1997) reported that learning-related improvements in the serial reaction time (SRT) task (Nissen and Bullemer 1987) are less in older than in young adults (YA). The underlying mechanisms of such age-related declines in motor sequence learning remain unclear.
It may be that cognitive declines associated with aging underlie deficits in motor sequence learning. Aging has a detrimental impact on many cognitive functions, including working memory (e.g., Reuter-Lorenz et al. 2000). It has been suggested that declines in working-memory capacity may mediate age-related changes in many complex tasks, including sequential behavior (e.g., Cornoldi et al. 2007; Gomez-Perez and Ostrosky-Solis 2006; Mayr and Kliegl 1993). In addition, studies have shown that individual differences in working-memory capacity play a significant role in explicit motor sequence learning (e.g., Bo and Seidler 2009; Unsworth and Engle 2005). It has been shown that older adults rely more on cognitive resources for the performance of simple motor tasks than young adults, such as balance (Huxhold et al. 2006) and walking (Lindenberger et al. 2000). It is unclear how and whether older adults rely on their relatively limited cognitive resources (e.g., Craik and Byrd 1982) when performing complex motor tasks. Therefore the current study examined age-related changes in cognitive functions, including working-memory capacity and temporal control, and determined whether they are correlated with motor sequence learning deficits in older adults.
To explain the process of sequence learning, Verwey (1996, 2001) proposed a model that includes components of “buffer loading” and “dual-processing,” in which sequences are executed by a cognitive and a motor processor. Participants have to rely on the cognitive processor to select individual sequence elements one by one when learning a new sequence. However, once a sequence is learned, the cognitive processor selects a single representation (i.e., a “chunk” of the sequence), whereas a dedicated motor processor is running in parallel to execute the sequence. Buffer loading may be the engagement of a kind of short-term motor memory (Henry and Rogers 1960; Sternberg et al. 1978) that allows each chunk to be programmed in advance of execution. Thus the motor chunks develop as a result of repeatedly filling the motor buffer through the development of interelement associations (Verwey 1996). Here, we propose that the buffer is not specific to the motor domain and the size of each chunk is generally determined by short-term memory capacity.
Recently, Shea et al. (2006) reported that older adults could not develop a clear chunking pattern during sequence learning whereas young adults did, even though older adults were able to execute the sequence more quickly with practice. However, our recent study in young adults (Bo and Seidler 2009) as well as other studies on motor sequence chunking (e.g., Kennerley et al. 2004; Verwey and Eikelboom 2003) show substantial individual differences in chunk development (e.g., the location of chunks varies across participants). Thus age group comparisons of motor sequence chunking patterns may conceal individual differences.
It has been shown that chunking patterns can be complex and exhibit little consistency across participants (e.g., Kennerley et al. 2004; Sakai et al. 2003; Verwey 2003; Verwey and Eikelboom 2003; Verwey et al. 2009). It is typical to quantify individual differences in chunking patterns via visual detection of relatively longer interelement durations, followed by statistical analysis across a group of participants (Kennerley et al. 2004; Sakai et al. 2003). Here, we used a similar technique that defines chunks with at least two elements. Although it is theoretically possible to have a one-element chunk, it is hard to justify whether no statistical differences among neighbors were actually 12 one-element chunks (12-element sequence in the current experiment); one 12-element chunk; or no chunks. It is also mathematically possible to have a 10-element chunk (maximum possibility) in a 12-element sequence. Although a 10-element chunk seems quite long based on the literature (e.g., Verwey and Eikelboom 2003), we decided not to put a constraint on the analysis from the outset. Thus we assume that a chunk is a group of 2–10 elements in the current study.
In addition to working memory, temporal control processes may also contribute to the development of chunking patterns when older adults learn new motor sequences. A “central timing mechanism” has been proposed to explain correlations among various tasks that exhibit temporal structures (e.g., Ivry and Hazeltine 1995). General age-related slowing may affect the pace of the central timing mechanism and thereby limit temporal resolution. In tasks that assess “central timing,” older adults move more slowly and have larger temporal variability than that of young adults (e.g., Rakitin and Malapani 2008; Rakitin et al. 2005; Vanneste et al. 2001). Although producing a sequence is not necessarily rhythmic, it is a task that is mainly measured by reaction time. Low temporal resolution and slow speed in older adults can result in slow buffer loading during motor sequence learning. In other words, slow processes for a previous chunk can affect the processes for the next chunk. In addition, multiple brain imaging studies have demonstrated a general timing network including the dorsolateral prefrontal cortex (DLPFC), presupplementary motor area (pre-SMA), supplementary motor area (SMA), and cerebellum (Maquet et al. 1996; Smith et al. 2003). These areas are also engaged during motor sequence learning (Boyer et al. 2005; Doyon et al. 2002; Kennerley et al. 2004). Thus it is possible that older adults who have impaired temporal control cannot develop consistent motor chunking patterns. The current experiment investigated whether age-related impairments in motor sequence learning might be partially due to declines in temporal control with age as well.
Therefore the current study examined whether age-related declines in explicit motor sequence learning and the development of motor sequence chunks are related to cognitive declines, particularly in terms of visuospatial working memory and temporal control. To measure the development of motor sequence chunks, we asked young and older adult participants to perform an explicit motor sequence learning task through four learning phases, progressing from a serial reaction time type task to a discrete sequence production task. The last block of training was used to define the final chunking pattern for each participant. To measure working-memory capacity, we used the visuospatial working-memory task introduced by Luck and Vogel (1997; experiment 1). Last, we used the continuous tapping task (Wing and Kristofferson 1973) to evaluate individuals' temporal control ability. The temporal variability (i.e., coefficient of variation [CV]) was calculated using the SD of the absolute tapping interval divided by the mean, then multiplied by 100.
We predicted that older adults would exhibit shorter sequence chunk lengths, compared with young adults, and that they would acquire motor sequences more slowly. Moreover, we expected that individual differences in working-memory capacity and temporal control would be positively correlated with the length of motor chunks formed and the rate of sequence learning in older adults. We also expected that older adults would have lower scores on all measures relative to young adults. Finally, because chunking patterns are thought to be represented in an abstract fashion that is not tied to the effector used during training (e.g., Keele et al. 1995; Sternberg et al. 1990; Verwey and Clegg 2005; Young and Schmidt 1991; but also see Verwey et al. 2009), we predicted that acquired chunk patterns would be maintained when participants performed the same sequence with either hand. We previously found that young adults showed transfer patterns if they developed chunks in the earlier learning phases. In this experiment, we explored whether older adults who developed consistent chunks would show similar patterns when they changed their response effectors.
METHODS
Participants
Thirty-two older adults [OA: age range = 65.0–78.7 yr, 12 males and 20 females; age = 70.6 ± 4.5 (mean ± SD)] and 27 younger adults (YA: age range = 18.8–28.8 yr, 12 males and 15 females; age = 20.9 ± 2.1) participated in this study. All individuals were right-handed (determined by self-report and the Edinburgh handedness inventory; Oldfield 1971) with normal or corrected vision. They provided their consent before the experiment and were paid $15 per hour for their participation. The experimental procedures were approved by the Institutional Review Board of the University of Michigan.
Procedure
Participants first completed general health history questionnaires (Table 1). Older adults with a history of stroke, diabetes, alcoholism, arthritis, or neurological disease were excluded from the study. The Mattis Dementia Rating Scale (Mattis 1988) and the Mini-Mental State Examination (MMSE; Folstein et al. 1975) were administered to screen out older adults with dementia. The CHAMPS physical activity questionnaire for older adults (Stewart et al. 2001) was used to assess how active each participant was during his/her daily life.
Table 1.
Questionnaires and screening tests for young and older adults
| Young Adults |
Older Adults |
|||
|---|---|---|---|---|
| Questionnaire/Screening Test | Mean | SD | Mean | SD |
| General information | ||||
| Age | 20.93 | 2.13 | 70.58 | 4.52 |
| Gender | 12M/15F | — | 12M/20F | — |
| Year of high education** | 2.88 | 1.33 | 5.04 | 2.19 |
| Number of medications** | 0.20 | 0.58 | 3.60 | 2.00 |
| Screening tests | ||||
| Mattis Dementia | 143.80 | 0.58 | 143.24 | 0.93 |
| MMSE | 29.72 | 0.74 | 29.44 | 0.58 |
| CHAMPS | ||||
| kcal/wk exercise-related activities | 5,538.09 | 4,305.95 | 3,504.17 | 2,865.15 |
| kcal/wk moderate exercise-related | 4,072.55 | 3,566.01 | 2,714.36 | 2,550.98 |
| Frequency/wk exercise-related activities* | 21.68 | 6.61 | 12.24 | 5.39 |
| Frequency/wk exercise-related | 13.68 | 8.07 | 8.76 | 4.37 |
P < 0.05,
P < 0.01.
Participants were then asked to perform 1) an explicit motor sequence learning task, 2) a visuospatial working-memory task, and 3) a continuous tapping timing task. All stimuli were controlled by a PC using custom software written in E-Prime version 1.0 (Psychology Software Tools, Pittsburgh, PA).
EXPLICIT MOTOR SEQUENCE LEARNING TASK.
Participants were instructed to learn a color-cued 12-element sequence (purple, yellow, blue, purple, red, blue, red, yellow, blue, red, yellow, purple) of finger movements. The colors “red,” “yellow,” “blue,” and “purple” were mapped onto the middle and index fingers of the left hand and the index and middle fingers of the right hand to the four adjacent buttons on the keyboard (c, v, b, n above the spacebar), respectively. We selected this sequence because 1) the probability of each element within the sequence was equally distributed; 2) this sequence did not have a fixed grouping pattern (i.e., no regularity; e.g., ABCD); 3) the sequence did not have runs of three (i.e., triplets) or trills (e.g., ABAB), even when the stimuli were presented continuously in phases 1 and 2 (i.e., no breaks between sequences). To display the sequence while in the learning phase, four visual stimulus boxes were presented side by side on a computer screen. Each square was assigned one of four colors (“red,” “yellow,” “blue,” and “purple” from the most left to the most right), which remained “fixed” to that spatial position for the remainder of the experiment. There were four learning phases and two transfer phases in the task.
Phase 1.
The sequence was presented element by element every 1,000 ms (900-ms stimulus duration, 100-ms interstimulus delay) and the participants were instructed to press the corresponding buttons as fast as possible after seeing each stimulus. There were no breaks between completions of a sequence and participants were not told where a sequence starts. We used a paced 1,000-ms interstimulus delay because one of our behavioral studies (Bo and Seidler, unpublished data) showed that older adults were able to show equivalent learning as young adults at this rate and were comfortable with the task. If participants made a response >1,000 ms, we took that element out of the analysis. A complete sequence (12 elements) defined one trial and 10 trials constituted one block (12 elements × 10 trials). Phase 1 contained 3 blocks (12 element × 10 trials × 3 blocks) of training.
Phase 2.
The task was the same as that in phase 1. However, participants had to reach 80% accuracy (to accommodate a potentially large performance range for older participants) on 10 consecutive trials for the last block of phase 2 to move onto phase 3. If participants' accuracy was <80%, they were asked to repeat phase 2.
Phase 3.
For this phase, participants were asked to refrain from responding until all 12 elements of the sequence were shown (one element every 500 ms). After presentation of the last element of the sequence in each trial, an instruction screen appeared directing the participant to begin reproducing the entire sequence from memory (i.e., discrete sequence production task). A trial in phase 3 consisted of the visually presented sequence and the subsequent sequence reproduction generated by the participants. Each trial concluded after the participant had input 12 responses. There were three blocks in phase 3 and accuracy feedback was given at the end of each block. If accuracy was <80% at the last block of this phase, participants had to repeat phase 3.
Phase 4.
During this phase, participants performed the sequence solely from memory, without visual cues (i.e., they were not shown the sequence) at the beginning of each trial. If they could respond with ≥80% accuracy for three continuous blocks (10 trials each), the sequence was considered learned. The last block of this phase was used to define the final chunking pattern for each participant (see following text).
Transfer phases (5 and 6).
As they had done in phase 4, participants were again asked to generate the acquired sequence purely from memory, but they were asked to use the fingers of only one hand for these trials. Two blocks of responses contained 10 trials each of the sequence, in which responding involved the index, middle, ring, and little fingers of the right hand in phase 5 and the little, ring, middle, and index fingers of the left hand for phase 6. Accuracy feedback was reported at the end of each trial. All participants were asked to perform the two transfer conditions in a counterbalanced fashion (i.e., half of the participants performed the phase 5 first, whereas the other half performed the phase 6 before phase 5).
VISUOSPATIAL WORKING-MEMORY TASK.
We slightly modified the visuospatial memory task published by Luck and Vogel (1997; experiment 1) (i.e., we reduced the number of array sizes from 12 to 10 and omitted the array size of one to shorten the testing time). Participants viewed a sample array (within a 9 × 9-in. region) of colored squares (1 × 1-in.) followed by a test array. Then, they had to press the “s” key if the two arrays were the same or the “d” key if the two arrays were different. The arrays consisted of 2–10 (array size) colored squares (drawn randomly from seven colors: red, blue, violet, green, yellow, black, and white). Each color appeared no more than twice for the 8–10 squares conditions. The sample array was presented for 100 ms, followed by a 900-ms blank screen delay, and then a 2,000-ms presentation of the test array. The test array was either the same as the sample array or different in the color of one of the squares. In other words, only one of the colors, not the locations, was changed in the different scenario. The ratio of same to different arrays was 1:1. For each trial, only one of the colors was changed for the test array. Thus it is possible that the test array contains a color that had not occurred in the sample array on that trial. Therefore this task relied on detection of a change in color and/or location. We used nine different arrays as the stimulus set, each having between 2 and 10 squares. Each array appeared five times in random order.
CONTINUOUS TAPPING TASK.
Participants wore a pair of over-the-ear headphones and sat comfortably in front of a computer. They were instructed to tap the spacebar using their index finger to coincide with given tones in three interval conditions—500, 1,000, and 1,500 ms—while looking at a fixation cross in the center of the screen. After 15 (audibly paced) tapping responses were recorded, the audible tones ceased and the participants were to continue tapping as consistently as possible for another 30 intervals (unpaced) at the respective interval. Three blocks of five trials were tested for each interval condition. The blocks were presented in a random order.
Analysis
EXPLICIT MOTOR SEQUENCE LEARNING.
All error trials were removed for reaction time analyses.
Early learning phases: phases 1 and 2.
Data from phases 1 and 2 were treated as the early phase of learning. The reaction time was the time from the appearance of stimulus to the onset of the response. The mean reaction time for every trial was computed. In our previous study in young adults, we used a power-fitting function to evaluate the rate of learning. However, a power function did not provide a good fit to the data for one third of older participants nor did other learning-curve functions such as linear or exponential functions (i.e., 8, 12, and 9 older adults' data sets showed R2 values <0.10 using linear, exponential, and power functions, respectively). Therefore we computed a reaction time change score for each phase as the change in reaction time from the first block (10 trials) to the last block (10 trials) to represent the rate of learning for old and young adults. We did not use accuracy data to calculate the rate of learning because most of the participants attained a high accuracy level after just one or two trials of practice (>90% correct).
Late learning phases: phases 3 and 4.
We were interested in observing the motor chunking pattern when participants had to reproduce all the elements of the sequence at once without visual cues in phases 3 and 4. The response time for the first movement in the sequence was defined from the appearance of the go signal to the onset of the first response. Response times for the later sequential elements were calculated between two consecutive responses. All the error trials were removed for further analyses.
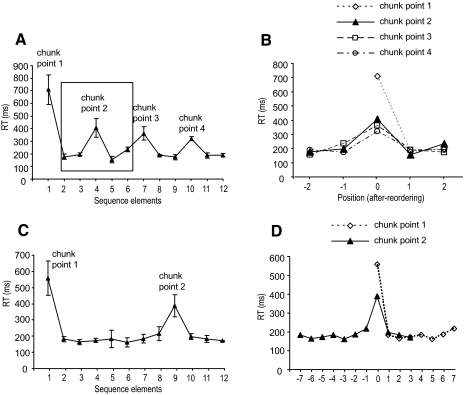
First, we defined the “preliminary” chunk points. The last block of phase 4 was used to predefine these chunking points for each participant, assuming that by this phase the sequence had already been learned and the execution was fluid. A chunk was determined by the number of elements grouped together according to duration in neighboring response times (Kennerley et al. 2004). Longer interresponse times between elements represent the divisions of chunks, whereas shorter interresponse times refer to a strong association within each chunk. We used one-tail paired t-test, from the 3rd to 11th elements of the sequence, to evaluate whether each element was significantly longer than the elements that came before and after it (alpha value was preset at 0.2 level). We adjusted the alpha value based on our young adults' data (Bo and Seidler 2009) and the fact that it was applied for a within-subjects analysis across only 10 trials. A chunk is a group of at least two elements. So naturally, the 1st and 2nd elements were omitted from the analyses because the 1st element is always the start of a chunk and the 2nd element must fall into the first chunk. Similarly, the 12th element must fall into the last chunk and was therefore also excluded from the analysis.
Once these predefined chunking points were established, we confirmed individual chunk points at the group level using the reordering procedure based on that used by Kennerley et al. (2004; see also Bo and Seidler 2009). The individual data were replotted according to the longest response time for each chunk. All the chunks were treated equally during the analysis. That is, regardless of the length of the chunks, we aligned all the chunk elements to the longest response time at the beginning of each chunk. Figure 1 illustrates this aligning procedure. The initial analysis identified four chunk points (Fig. 1A). Taking the second chunk point as an example, the 4th element of the sequence was labeled as position 0 because the longest response time for that chunk preceded this element. The 3rd and 2nd elements were labeled as −1, −2, whereas the 5th and 6th elements were labeled as 1 and 2 for that particular chunk. The same procedure was used for the rest of the chunks for every participant (Fig. 1B). Figure 1C illustrates the same procedure when the lengths of the chunks were different. These two chunks were weighted equally when all the elements were aligned (Fig. 1D). After the chunk points were reorganized, we performed a one-way ANOVA on response time (individual participant data) to examine whether the response time at position 0 was significantly longer than the response times at any other positions. Only when the response time at position 0 was found to be significantly higher than the response times at any other positions did we accept the preliminary chunks as the final chunk points. The mean chunk length was calculated using 12 (elements) divided by the number of chunks.
Fig. 1.
Illustration of the chunking realignment procedure. A: 4 initial chunking points identified for one representative participant. B: the plots realigned with respect to each chunk point in the sequence. C: 2 initial chunking points with different chunk lengths for another participant. D: the plots realigned to each chunk point for the example in C.
Then, we defined how quickly chunks formed during training. The mean response time for each element of the sequence was computed for every block in phases 3 and 4. Then, the sequence elements were reordered from the longest to the shortest response time for each block. If any earlier block showed the same reordered pattern as the last block of phase 4, we marked that block as the beginning of the developed chunks.
Transfer conditions: phases 5 and 6.
To evaluate the transfer, we first calculated the mean response times for the first block in phases 5 and 6. Then we used the same reordering and matching procedures as described earlier. If either of the phases showed the same pattern as that in phase 4, the transfer was deemed successful for that participant. Then, we performed a chi-square test to examine whether transfer was significant for older adults. Finally, a nonparametric independent sample test (Mann–Whitney test) was used to compare transfer effects between young and older adults.
VISUOSPATIAL WORKING-MEMORY TASK.
The memory capacity (K) = S(H − F), where S is the size of the array, H is the observed hit rate, and F is the false alarm rate (Vogel and Machizawa 2004). We computed the K value for each array size and took the average K across all array sizes to represent the visuospatial memory capacity for each participant.
CONTINUOUS-TAPPING TASK.
The mean and SD of the unpaced intertap intervals were calculated. Any tapping intervals that were shorter or longer than 2SDs of the mean were excluded from the analysis. The coefficient of variation was calculated using the SD of the absolute tapping interval divided by the mean, then multiplied by 100.
RESULTS
All older participants reached a minimum Mini-Mental State Examination (MMSE; Folstein et al. 1975) score of 27 and a Mattis Dementia Rating Scale (Mattis 1988) score of 123. Table 1 lists the results of questionnaires and screening tests for older participants.
Explicit motor sequence learning
Results for the young adults have been previously presented in detail (Bo and Seidler 2009) and are included here for a comparison with the older adult data. Seven of 32 older adults and 2 of 27 young adults were not able either to remember the sequence (i.e., explicitly expressed that they could not continue the task at any learning phases) or to develop chunking patterns (i.e., our statistical procedure failed to identify clear chunking points). We believe that this is one index of poorer sequence learning in older adults. However, given the goals of the current study, these data sets were excluded from further analysis.
EARLY LEARNING PHASES: PHASES 1 AND 2.
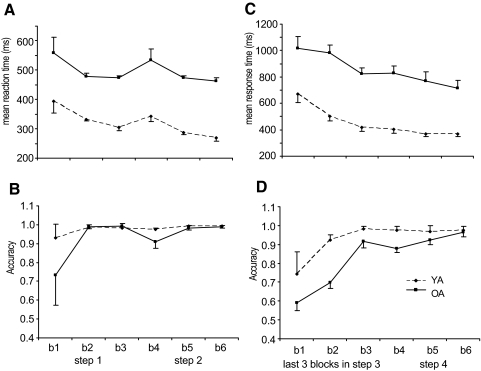
Because all the included 25 older adults were able to reach the accuracy cutoff without repetitions in phase 2, we labeled the first block of phase 1 as block 1, the second block of phase 1 as block 2, the third block of phase 1 as block 3, the first block of phase 2 as block 4, the second block of phase 2 as block 5, and the third block of phase 2 as block 6. Figure 2, A and B illustrates the overall performance for 60 trials from blocks 1 to 6 in phases 1 and 2. As expected, a mixed ANOVA with block as the within-subjects factor and group (young, older) as the between-subjects factor revealed that older adults had longer reaction times [F(1,48) = 133.86, P < 0.01] and lower accuracy [F(1,48) = 19.82, P < 0.01] than those of young adults. Both young and older adults responded faster [F(5,240) = 61.42, P < 0.01] and more accurately [F(5,240) = 133.86, P < 0.01] as they practiced across blocks. A group × block interaction [F(5,240) = 19.72, P < 0.01] on accuracy revealed that older adults had significantly lower accuracy in blocks 1 and 4 (Bonferroni-corrected post hoc procedure, all P < 0.05) than that of young adults.
Fig. 2.
A: the mean reaction times for each block in phases 1 and 2. B: the mean accuracy for each block in phases 1 and 2. C: the mean response times for each block in the last 3 blocks of phases 3 and 4. D: the mean accuracy for each block in the last 3 blocks of phases 3 and 4.
Late learning phases and transfer: phases 3–6.
late learning phases: phases 3 and 4. Two of 25 older adults had to repeat phase 3 once due to the accuracy cutoff requirements. Figure 2, C and D illustrates the mean response time and accuracy in the last three blocks of phases 3 and 4. A mixed ANOVA revealed that older adults had longer response times [F(1,48) = 55.28, P < 0.01] and lower accuracy [F(1,48) = 24.87, P < 0.01] than those of young adults. Both young and older adults responded faster [F(5,240) = 28.97, P < 0.01] and more accurately [F(5,240) = 64.02, P < 0.01] as they practiced across blocks. A group × block interaction on accuracy [F(5,240) = 6.97, P < 0.01] revealed that older adults had significantly lower accuracy in all blocks of phase 3 and the first block of phase 4 (Bonferroni-corrected, all P < 0.05) than that of young adults.
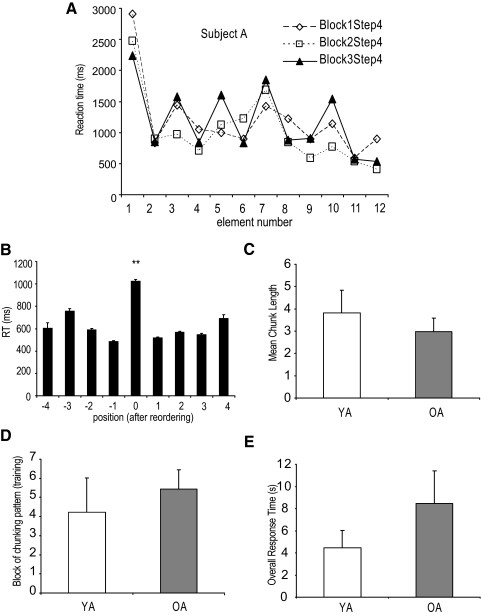
Figure 3A shows the performance of one representative older adult during the last three blocks of phase 4. Participant A has divided the whole sequence into five chunks: at the first, third, fifth, seventh, and tenth elements for the last block in phase 4. Interestingly, the chunking pattern was not the same in the first or second block in phase 4, suggesting that the final chunking pattern was not developed until after the second block of phase 4.
Fig. 3.
A: the response time from the 3 blocks of phase 4 of sequence training are depicted for one representative older participant. B: group mean response time data (last block of phase 4), after replotting with respect to each older adult's initially determined chunk points. C: mean chunk length for young and older adults. D: block at which participants formed their final chunking pattern during training in young and older adults. E: overall response time for completing a learned sequence in young and older adults.
Using the methods outlined earlier in Analysis, we first identified the beginning of each chunk based on multiple paired t-tests for every participant. Then, we confirmed these points at the group level using a procedure modified from Kennerley et al. (2004) (see also Bo and Seidler 2009). A mixed-model ANOVA was performed where the position from the predefined chunks was the within-subjects factor and the age group was the between-subjects factor. A significant position effect was found [F(8,171) = 10.66 and 8.73, both P < 0.01] for both young and older adults. Post hoc tests (Bonferroni-corrected) showed that the chunk point response time at position 0 was significantly longer than any of the adjacent response times (all P < 0.01, Fig. 3B), confirming these predefined chunk points.
Then, we observed whether the locations of those chunking points were similar across participants. Substantial individual differences were found, suggesting that the property of the selected sequence did not evoke particular chunking locations (also see Verwey and Eikelboom 2003).
After all the chunk points were confirmed, a correlation analysis between the chunk length and the response time ratio between and within chunks for the last block of phase 4 was performed to examine whether longer chunks required a longer initial reaction time. Although young adults had a significant correlation between the chunk length and response time ratio (R = 0.48, P < 0.05), no significant correlation was found in older adults (R = 0.10, P > 0.10). However, when we compared the correlations between young and older adults (Fisher r-to-z transformation to test the significance of difference between two correlation coefficients), no group difference was found either (z = 1.40, P = 0.16).
Tables 2 and 3, respectively, list the training block number at which the final chunking pattern started to form for both older and young adults. Eighteen of 25 older participants did not show their final chunking patterns until the last block of learning. Two of these 18 participants had to repeat phase 3, which means that they had nine blocks of training in phases 3 and 4. Four participants developed their stable chunks at the second block of phase 4. Two participants showed chunks at the first block of phase 4. There was only one older adult who had his final chunks at the second block of phase 3.
Table 2.
How quickly the final chunk pattern formed and transfer of the chunking pattern for older adults
| Participant | Repetition of Phase 3 | Block That Showed Final Defined Chunk Points | Number of Blocks of Training Before Forming the Final Chunks | Transfer Left | Transfer Right |
|---|---|---|---|---|---|
| A. Full transfer | |||||
| 11 | No | 2nd block, phase 3 | 2 | Yes | Yes |
| 6 | No | 2nd block, phase 4 | 5 | Yes | Yes |
| 5 | Yes | 3rd block, phase 4 | 9 | Yes | Yes |
| B. Partial transfer | |||||
| 14 | No | 3rd block, phase 4 | 6 | No | Yes |
| 18 | No | 3rd block, phase 4 | 6 | No | Yes |
| C. No transfer | |||||
| 2 | No | 1st block, phase 4 | 4 | No | No |
| 19 | No | 1st block, phase 4 | 4 | No | No |
| 12 | No | 2nd block, phase 4 | 5 | No | No |
| 15 | No | 2nd block, phase 4 | 5 | No | No |
| 24 | No | 2nd block, phase 4 | 5 | No | No |
| 1 | No | 3rd block, phase 4 | 6 | No | No |
| 3 | No | 3rd block, phase 4 | 6 | No | No |
| 4 | No | 3rd block, phase 4 | 6 | No | No |
| 7 | No | 3rd block, phase 4 | 6 | No | No |
| 8 | No | 3rd block, phase 4 | 6 | No | No |
| 9 | No | 3rd block, phase 4 | 6 | No | No |
| 10 | No | 3rd block, phase 4 | 6 | No | No |
| 13 | No | 3rd block, phase 4 | 6 | No | No |
| 16 | No | 3rd block, phase 4 | 6 | No | No |
| 17 | No | 3rd block, phase 4 | 6 | No | No |
| 20 | No | 3rd block, phase 4 | 6 | No | No |
| 21 | No | 3rd block, phase 4 | 6 | No | No |
| 22 | No | 3rd block, phase 4 | 6 | No | No |
| 23 | No | 3rd block, phase 4 | 6 | No | No |
| 25 | Yes | 3rd block, phase 4 | 9 | No | No |
Table 3.
How quickly the final chunk pattern formed and transfer of the chunking pattern for young adults
| Participant | Repetition of Phase 3 | Block That Showed Final Defined Chunk Points | Number of Blocks of Training Before Forming the Final Chunks | Transfer Left | Transfer Right |
|---|---|---|---|---|---|
| A. Full transfer | |||||
| 1 | No | 1st block, phase 3 | 1 | Yes | Yes |
| 6 | No | 1st block, phase 3 | 1 | Yes | Yes |
| 14 | No | 1st block, phase 3 | 1 | Yes | Yes |
| 2 | No | 1st block, phase 4 | 4 | Yes | Yes |
| 5 | No | 1st block, phase 4 | 4 | Yes | Yes |
| 22 | No | 1st block, phase 4 | 4 | Yes | Yes |
| 24 | No | 1st block, phase 4 | 4 | Yes | Yes |
| B. Partial transfer | |||||
| 19 | No | 1st block, phase 3 | 1 | Yes | No |
| 25 | No | 3rd block, phase 3 | 3 | Yes | No |
| 9 | No | 3rd block, phase 4 | 6 | Yes | No |
| 10 | No | 3rd block, phase 4 | 6 | No | Yes |
| 11 | No | 3rd block, phase 4 | 6 | No | Yes |
| C. No transfer | |||||
| 17 | No | 3rd block, phase 3 | 3 | No | No |
| 13 | No | 1st block, phase 4 | 4 | No | No |
| 15 | No | 1st block, phase 4 | 4 | No | No |
| 3 | No | 2nd block, phase 4 | 5 | No | No |
| 8 | No | 2nd block, phase 4 | 5 | No | No |
| 21 | No | 2nd block, phase 4 | 5 | No | No |
| 4 | No | 3rd block, phase 4 | 6 | No | No |
| 7 | No | 3rd block, phase 4 | 6 | No | No |
| 12 | No | 3rd block, phase 4 | 6 | No | No |
| 16 | No | 3rd block, phase 4 | 6 | No | No |
| 18 | No | 3rd block, phase 4 | 6 | No | No |
| 20 | No | 3rd block, phase 4 | 6 | No | No |
| 23 | No | 3rd block, phase 3 | 6 | No | No |
Transfer conditions: phases 5 and 6.
Using the methods outlined earlier in Analysis, the results for the transfer conditions with “Y” representing positive transfer and “N” representing no transfer are listed in Tables 2 and 3. Twenty of 25 older adults did not keep the same pattern of chunking when they changed the response effector. Three older adults appeared to have a consistent chunking pattern regardless of the response effector (full transfer: transfer to both left and right fingers) and the remaining two showed partial transfer (i.e., transfer to either left or right fingers). A chi-square test revealed that the older group did not show transfer (χ2 = 14.44, P < 0.01). When we compared the young and older groups, a nonparametric independent sample test (Mann–Whitney test) showed that older adults had significantly less transfer than that of young adults (Z = −2.75, P < 0.01). Overall, older adults formed their chunking patterns later than young adults [t(48) = 3.01, P < 0.05] and were less successful at transfer.
Comparing between young and older groups, significant differences were found in the mean chunk length [12 elements divided by number of chunks, t(48) = 3.59, P < 0.01; Fig. 3C], overall response time at the last block of phase 4 [t(48) = −6.04, P < 0.01; Fig. 3E], and the number of the training block that first started to show the final chunk pattern [t(48) = −2.93, P < 0.01; Fig. 3D]. In brief, older adults had shorter chunk lengths, required a longer time to reproduce the sequence, and formed their final chunking pattern later compared with young adults during the sequence learning.
Visuospatial working memory
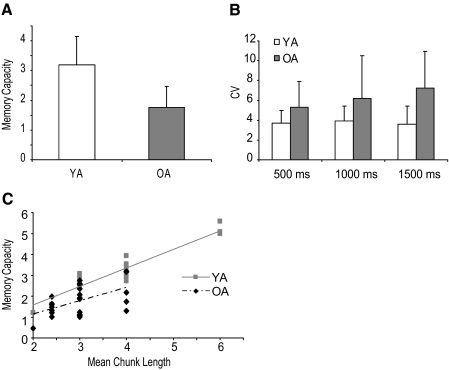
The overall performance for the visuospatial working-memory task showed a similar pattern between young and older adults: the accuracy for arrays of size two to four items was relatively higher than that for sizes >four. Comparing between young and older adults, older adults had significantly lower visuospatial working-memory capacity than that of the young adults [t(48) = 6.00, P < 0.01; Fig. 4A].
Fig. 4.
A: mean working-memory capacity for young and older adults. B: mean CV in 500, 1,000, and 1,500 ms between young and older adults. C: correlation between working-memory capacity (K) and mean chunk length for young and older adults.
Continuous tapping task
The average CVs were 3.7, 3.9, and 3.6 for young adults and 5.3, 5.5, and 6.8 for older adults during the 500-, 1,000-, and 1,500-ms conditions, respectively. A mixed ANOVA revealed a significant condition × group interaction [F(2,144) = 4.28, P < 0.01] and group main effects [F(1,144) = 55.35, P < 0.01], whereas the condition effect approached significance [F(2,144) = 2.85, P = 0.06]. Post hoc analysis suggested that although young adults did not show differences across the three intervals (suggesting a general timing ability), older adults increased temporal variability as the interval increased from 500 to 1,500 ms (Fig. 4B). In addition, we ran correlations among CV500, CV1,000, and CV1,500 to examine whether older adults who had a higher CV in one interval would also show a higher CV in other intervals. Similar to young adults, significant correlations on the CV among these three intervals were found in older adults (R = 0.70, P < 0.01; R = 0.57, P < 0.01; R = 0.81, P < 0.01 for CV500 and CV,1000; CV500 and CV,1500; and CV1,000 and CV1,500, respectively).
Relationships among working memory, temporal consistency, and sequence learning measures
In our previous work in young adults, we found a correlation between working-memory capacity and rate of sequence learning, as defined with power function coefficients (Bo and Seidler 2009). Since a power function did not provide a good fit for each individual older adult's data, we computed the reaction time change scores to represent the rate of learning. No correlation between the rate of learning and working-memory capacity was found. Analysis of the young adults' data in the same way showed trends for a correlation between reaction time change scores within phase 1 and mean chunk length (R = 0.36, P = 0.08) and a correlation between reaction time change scores within phase 2 and working-memory capacity (R = 0.38, P = 0.06). In addition, no correlations were found between any of the timing measures and the rate of learning for all sequence learning phases in both young and older adults.
Figure 4C depicts the scatterplot for individuals' memory capacity and mean chunk length for both young and older adults. Significant positive correlations between working-memory capacity and mean chunk length were found in both groups (R = 0.78, 0.54 for young and older adults, respectively, both P < 0.01), suggesting that the number of items participants could accurately retain in working memory predicted the temporal pattern of the acquired sequence regardless of age. That is, participants with low working-memory capacity formed shorter chunks, whereas high-capacity participants had relatively longer chunks. In a comparison of the correlations between working memory and chunk length, we found no difference between young and older adults (z = 1.46, P = 0.14).
Similar to young adults, no other correlations were found in any combinations between the temporal measures, memory capacity, chunk length, and overall response time (all P > 0.05) in older adults.
DISCUSSION
In our previous study, we found a positive correlation between visuospatial working-memory capacity and both the rate of early motor sequence learning and the length of motor chunks that young adult participants formed (Bo and Seidler 2009). In the current study we report that older adults exhibited an overall reduction in both working-memory capacity and motor chunk length compared with those of young adults. Moreover, there was a positive correlation between older adults' visuospatial working-memory capacity and the length of motor chunks. However, in contrast to young adults, older adults did not show a correlation between working-memory capacity and the rate of learning. Thus it seems that older adults may rely on visuospatial working memory during early sequence learning in a manner similar to that of young adults, although other factors also influence the overall rate of learning.
It is important to note that 7 of 32 older participants were not able to remember the sequence (n = 4) or to develop a consistent chunking pattern (i.e., the statistical procedure used in the current study failed to identify any chunk points, n = 3). When we compared the visuospatial working-memory capacity for these 7 older adults with the remaining 25 successful older adults, we found that 6 of these 7 were below the mean capacity. These results provide further evidence for some motor sequence learning deficits in older adults and highlight the substantial individual differences that exist. In addition, these excluded cases suggest that not all the participants had successful explicit learning.
Twenty-five of 32 older adults were able to develop clear chunking patterns, suggesting that this ability is primarily not impaired by normal aging. However, using the 25 data sets from the older participants who did develop a consistent chunking pattern, we found that the length of motor chunks was significantly shorter than that seen in young adults. This result is different from that of Shea et al. (2006), who reported that older adults could not develop a consistent movement sequence structure. We believe that this discrepancy arose due to several experimental differences between the two studies. First, we used only the successful participants' data when measuring chunks, whereas Shea et al. (2006) compared group performance between all their older and young adults. Second, we used a key-press version of the discrete sequence production task, whereas Shea et al. (2006) used an arm-aiming version of the task where participants had to move a lever to different target locations in a sequential order. Our task required finger-press movements, whereas their task involved more complex multijoint movements. Third, we explicitly instructed the participants to remember the sequence, whereas Shea et al. (2006) did not, although all participants developed explicit knowledge of the sequence by the end of the experiment. Thus our study forced participants to use an explicit memory strategy to remember a sequence of items throughout the whole learning process, whereas participants in the Shea et al. (2006) study gradually developed explicit awareness from the early to the late learning phase. It is possible that the older adults in the Shea et al. (2006) study would have gone on to develop consistent chunks as participants did in our study if they were given more practice. In addition, the older adult participants in Shea et al. (2006) were younger than ours. It is not clear how this could have led to the differences, however, given that presumably these younger individuals would have less working-memory impairment. Last, we focused on individual differences, whereas they performed only a group comparison. The current study revealed that most older adults could develop a consistent chunking pattern during motor sequence learning, although they needed additional practice to remember a new sequence and had shorter chunk lengths when producing a learned sequence.
Since most of the older participants developed consistent chunks at the last block of the training phase, one may question the stability of these patterns. Although it is possible that the chunking pattern may have changed with more practice, our statistical procedure at least ensures that the pattern is consistent across the last block. Previous studies have judged chunk boundaries based on visual inspection of reaction times (e.g., Kennerley et al. 2004; Shea et al. 2006). In other words, as long as one interval was relatively longer than the neighboring intervals, that interval was defined as the boundary of a chunk. This “relatively longer interval” could be easily biased by one or two trials, however (i.e., a lack of consistency). To address this, we developed and used a statistical procedure (Bo and Seidler 2009) to test whether one “relatively longer interval” was significantly longer than that of its two neighbors at the 0.2 alpha level. Because of this statistical cutoff, we rejected the data from 3 of the 32 older adults, even though they exhibited some patterns of “relatively longer” and “relatively shorter” intervals. Thus we ensured that at least most of the trials within the last training block were exhibiting the same chunking pattern.
We previously found that the pattern of motor chunks was not always transferable (Bo and Seidler 2009). Young participants who formed their chunking patterns earlier in the experiment exhibited better transfer of the pattern to new response effectors. Comparatively, it seems older adults had a similar pattern in which they had less successful transfer and later development of consistent chunking patterns. These results suggest that chunking patterns are initially effector dependent and then become more abstractly represented with additional practice.
It is perhaps not surprising that older adults showed lower visuospatial working-memory capacity and poorer temporal control compared with those of young adults, given that several other studies have reported these effects as well (e.g., Baudouin et al. 2004; Gunstad et al. 2006; Jonides et al. 2000; Nordahl et al. 2006; Reuter-Lorenz et al. 2000; Stebbins et al. 2002). However, it was an open question whether older adults would still engage these limited cognitive resources to perform explicit motor sequence learning. The positive correlation between visuospatial working-memory capacity and mean motor chunk length that we observed suggests that older adults were able to use their working memory during learning. Awh et al. (2007) argued that mean working-memory capacity represents a fixed number of items that people can hold in short-term working memory regardless of object complexity. This claim is quite consistent with our previous finding in young adults: the ratio between mean motor chunk length and working-memory capacity was very close to 1 (3.81/3.18). It is surprising that the ratio between these two variables was even higher for older adults (2.97/1.76). It seems that older adults may have been using other memory strategies. In addition, older adults might also have relied on other memory domains (i.e., auditory, verbal, etc.). In the current study, however, we did not measure other domains of working memory. Thus it remains an open question as to whether and how they would relate to motor sequence learning. An alternative explanation for the result that the chunk length was longer than what was predicted by working-memory capacity in older adults might relate to the additional involvement of motor components at the later learning phases. Hikosaka et al. (1999) argued that both spatial and motor components are involved in sequence learning and that the motor component develops more slowly than the spatial one. This implies that there might be a decreasing correlation between working memory and sequencing with practice.
One may question our interpretation of working-memory contributions to motor sequence learning in older adults because we did not find a significant correlation between working-memory capacity and the rate of sequence learning. In young adults, the power-fitting method reasonably represented rate of change for each individual. In contrast, learning patterns were so variable for older adults that we could not select a single fitting method that would provide a reasonable fit to the data of each participant (8, 12, and 9 older adults' data sets showed R2 values <0.10 using linear, exponential, and power functions, respectively). Such results do not indicate that older adults were not learning the sequence, however. Indeed, our results showed that 25 of 32 older participants had not only learned the sequence but also developed clear chunking patterns. Rather, the older adults were highly variable in the shape of their learning curves across participants. It may be that individuals engaged different learning strategies during the early learning phase. In addition, the lack of correlation between the rate of learning and working-memory capacity suggests that older adults were engaging resources other than visuospatial working memory to perform the task. In a study of implicit motor sequence learning, Frensch and Miner (1994) found that digit-span performance correlated with the magnitude of implicit learning that occurred, for both young and older adults. Although it is hard to extrapolate from implicit to explicit learning conditions, it may be that older adults in the current study were also engaging some (potentially less effective) verbal strategies.
We also predicted that age-related declines in temporal control ability (e.g., Rakitin and Malapani 2008) would interrupt the development of motor sequence chunking patterns in older adults. In contrast to this hypothesis, we did not find a correlation between individual differences in temporal control and the motor sequence chunking patterns. In combination with our previous findings in young adults (Bo and Seidler 2009), these data suggest that a “common timing” mechanism does not play a significant role in the temporal structure of acquired motor sequences. In addition, there was no evidence to suggest that older adults engaged temporal control resources to compensate for declines in visuospatial working memory to optimize learning.
One may question the sensitivity of the current study to detect correlations, given declines in working memory and temporal control abilities in older adults. However, the fact that we found strong correlations between working-memory capacity and mean chunk length—as well as positive correlations among the three temporal conditions—argues against this criticism. Another potential limitation of the current study is whether the correlation between working memory and chunk length was skewed by one or two extreme points due to the limited range of chunk length data, particularly for the older participants. In our older adult data set, one older participant had a mean chunk length of 2, 5 had a mean chunk length of 4, 8 had a mean chunk length of 2.4 (i.e., 12 elements/5 chunks), and the remaining 11 had a mean chunk length of 3. After removing the one participant with a chunk length of 2, a significant correlation was still observed (R = 0.47, P < 0.05), supporting the robustness of our findings.
In summary, we found that older adults exhibited an overall reduction in both working-memory capacity and motor chunk length compared with that of young adults. Furthermore, we found a positive correlation between visuospatial working-memory capacity and mean motor chunk length, suggesting that older adults relied on working-memory resources to maximize motor sequence learning.
GRANTS
This work was supported by National Institute on Aging Grants R01-AG-024106 to R. D. Seidler and AG-08808 (University of Michigan Claude D. Pepper Geriatrics Center—Human Subjects Core).
ACKNOWLEDGMENTS
We thank all the research assistants who helped with data collection and the participants who willingly gave time and effort during the study.
REFERENCES
- Awh et al., 2007.Awh E, Barton B, Vogel EK. Visual working memory represents a fixed number of items regardless of complexity. Psychol Sci 18: 622–628, 2007 [DOI] [PubMed] [Google Scholar]
- Baudouin et al., 2004.Baudouin A, Vanneste S, Isingrini M. Age-related cognitive slowing: the role of spontaneous tempo and processing speed. Exp Aging Res 30: 225–239, 2004 [DOI] [PubMed] [Google Scholar]
- Bo and Seidler, 2009.Bo J, Seidler RD. Visuospatial working memory capacity predicts the organization of acquired explicit motor sequences. J Neurophysiol 101: 3116–3125, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornoldi et al., 2007.Cornoldi C, Bassani C, Berto R, Mammarella N. Aging and the intrusion superiority effect in visuo-spatial working memory. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 14: 1–21, 2007 [DOI] [PubMed] [Google Scholar]
- Craik and Byrd, 1982.Craik FIM, Byrd M. Aging and cognitive deficits: the role of attentional resources. In: Aging and Cognitive Processes, edited by Craik FIM, Trehub S. New York: Plenum, 1982, p. 191–211 [Google Scholar]
- Curran, 1997.Curran T. Effects of aging on implicit sequence learning: accounting for sequence structure and explicit knowledge. Psychol Res 60: 24–41, 1997 [DOI] [PubMed] [Google Scholar]
- Folstein et al., 1975.Folstein MF, Folstein SE, McHugh PR. Mini-mental state: practical method for grading cognitive state of patients for clinician. J Psychiatr Res 12: 189–198, 1975 [DOI] [PubMed] [Google Scholar]
- Frensch and Miner, 1994.Frensch PA, Miner CS. Effects of presentation rate and individual differences in short-term memory capacity on an indirect measure of serial learning. Mem Cogn 22: 95–110, 1994 [DOI] [PubMed] [Google Scholar]
- Gomez-Perez and Ostrosky-Solis, 2006.Gomez-Perez E, Ostrosky-Solis F. Attention and memory evaluation across the life span: heterogeneous effects of age and education. J Clin Exp Neuropsychol 28: 477–494, 2006 [DOI] [PubMed] [Google Scholar]
- Gunstad et al., 2006.Gunstad J, Cohen RA, Paul RH, Luyster FS, Gordon E. Age effects in time estimation: relationship to frontal brain morphometry. J Integr Neurosci 5: 75–87, 2006 [DOI] [PubMed] [Google Scholar]
- Henry and Rogers, 1960.Henry FM, Rogers DE. Increased response latency for complicated movements and a “memory drum” theory of neuromotor reaction. Res Q 31: 448–458, 1960 [Google Scholar]
- Hikosaka et al., 1999.Hikosaka O, Nakahara H, Rand MK, Sakai K, Lu X, Nakamura K, Miyachi S, Doya K. Parallel neural networks for learning sequential procedures. Trends Neurosci 22: 464–471, 1999 [DOI] [PubMed] [Google Scholar]
- Howard DV, Howard JH., Jr Age differences in learning serial patterns: direct versus indirect measures. Psychol Aging 4: 357–364, 1989 [DOI] [PubMed] [Google Scholar]
- Howard DV, Howard JH, Jr, Japikse K, DiYanni C, Thompson A, Somberg R. Implicit sequence learning: effects of level of structure, adult age, and extended practice. Psychol Aging 19: 79–92, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxhold et al., 2006.Huxhold O, Li SC, Schmiedek F, Lindenberger U. Dual-tasking postural control: aging and the effects of cognitive demand in conjunction with focus of attention. Brain Res Bull 69: 294–305, 2006 [DOI] [PubMed] [Google Scholar]
- Ivry and Hazeltine, 1995.Ivry RB, Hazeltine RE. Perception and production of temporal intervals across a range of durations: evidence for a common timing mechanism. J Exp Psychol Hum Percept Perform 21: 3–18, 1995 [DOI] [PubMed] [Google Scholar]
- Jonides et al., 2000.Jonides J, Marshuetz C, Smith EE, Reuter-Lorenz PA, Koeppe RA, Hartley A. Age differences in behavior and PET activation reveal differences in interference resolution in verbal working memory. J Cogn Neurosci 12: 188–196, 2000 [DOI] [PubMed] [Google Scholar]
- Keele et al., 1995.Keele SW, Jennings P, Jones S, Caulton D, Cohen A. On the modularity of sequence representation. J Mot Behav 27: 17–30, 1995 [Google Scholar]
- Kennerley et al., 2004.Kennerley SW, Sakai K, Rushworth MF. Organization of action sequences and the role of the pre-SMA. J Neurophysiol 91: 978–993, 2004 [DOI] [PubMed] [Google Scholar]
- Lindenberger et al., 2000.Lindenberger U, Marsiske M, Baltes PB. Memorizing while walking: increase in dual-task costs from young adulthood to old age. Psychol Aging 15: 417–436, 2000 [DOI] [PubMed] [Google Scholar]
- Luck and Vogel, 1997.Luck SJ, Vogel EK. The capacity of visual working memory for features and conjunctions. Nature 390: 279–281, 1997 [DOI] [PubMed] [Google Scholar]
- Mattis, 1988.Mattis S. Dementia Rating Scale: Professional Manual Odessa, FL: Psychological Assessment Resources, 1988 [Google Scholar]
- Mayr and Kliegl, 1993.Mayr U, Kliegl R. Sequential and coordinative complexity: age-based processing limitations in figural transformations. J Exp Psychol Learn Mem Cogn 19: 1297–1320, 1993 [DOI] [PubMed] [Google Scholar]
- Nissen and Bullemer, 1987.Nissen MJ, Bullemer P. Attentional requirements of learning: evidence from performance measures. Cogn Psychol 19: 1–32, 1987 [Google Scholar]
- Nordahl et al., 2006.Nordahl CW, Ranganath C, Yonelinas AP, Decarli C, Fletcher E, Jagust WJ. White matter changes compromise prefrontal cortex function in healthy elderly individuals. J Cogn Neurosci 18: 418–429, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield, 1971.Oldfield RC. The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia 9: 97–113, 1971 [DOI] [PubMed] [Google Scholar]
- Rakitin and Malapani, 2008.Rakitin BC, Malapani C. Effects of feedback on time production errors in aging participants. Brain Res Bull 75: 23–33, 2008 [DOI] [PubMed] [Google Scholar]
- Rakitin et al., 2005.Rakitin BC, Stern Y, Malapani C. The effects of aging on time reproduction in delayed free-recall. Brain Cogn 58: 17–34, 2005 [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz et al., 2000.Reuter-Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz C, Koeppe RA. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. J Cogn Neurosci 12: 174–187, 2000 [DOI] [PubMed] [Google Scholar]
- Seidler, 2006.Seidler RD. Differential effects of age on sequence learning and sensorimotor adaptation. Brain Res Bull 70: 337–346, 2006 [DOI] [PubMed] [Google Scholar]
- Shea et al., 2006.Shea CH, Park JH, Braden HW. Age-related effects in sequential motor learning. Phys Ther 86: 478–488, 2006 [PubMed] [Google Scholar]
- Stebbins et al., 2002.Stebbins GT, Carrillo MC, Dorfman J, Dirksen C, Desmond JE, Turner DA, Bennett DA, Wilson RS, Glover G, Gabrieli JDE. Aging effects on memory encoding in the frontal lobes. Psychol Aging 17: 44–55, 2002 [DOI] [PubMed] [Google Scholar]
- Sternberg et al., 1990.Sternberg S, Knool RL, Turock KD. Hierarchical control in the execution of action sequences: tests of two invariance properties. In: Attention and Performance XIII, edited by Jeannerod M. Hillsdale, NJ: Erlbaum, 1990, p. 3–55 [Google Scholar]
- Sternberg et al., 1978.Sternberg S, Monsell S, Knoll RL, Wright CE. The latency and duration of rapid movement sequence: comparison of speech and typewriting. In: Information Processing in Motor Control and Learning, edited by Stelmach GE. New York: Academic Press, 1978, p. 117–152 [Google Scholar]
- Stewart et al., 2001.Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc 33: 1126–1141, 2001 [DOI] [PubMed] [Google Scholar]
- Unsworth and Engle, 2005.Unsworth N, Engle RW. Individual differences in working memory capacity and learning: evidence from the serial reaction time task. Mem Cogn 33: 213–220, 2005 [DOI] [PubMed] [Google Scholar]
- Vanneste et al., 2001.Vanneste S, Pouthas V, Wearden JH. Temporal control of rhythmic performance: a comparison between young and old adults. Exp Aging Res 27: 83–102, 2001 [DOI] [PubMed] [Google Scholar]
- Verwey, 1996.Verwey WB. Buffer loading and chunking in sequential keypressing. J Exp Psychol Hum Percept Perform 22: 544–562, 1996 [Google Scholar]
- Verwey, 2001.Verwey WB. Concatenating familiar movement sequences: the versatile cognitive processor. Acta Psychol (Amst) 106: 69–95, 2001 [DOI] [PubMed] [Google Scholar]
- Verwey et al., 2009.Verwey WB, Abrahamse EL, Jimenez L. Segmentation of short keying sequences does not spontaneously transfer to other sequences. Hum Mov Sci 28: 348–361, 2009 [DOI] [PubMed] [Google Scholar]
- Verwey and Clegg, 2005.Verwey WB, Clegg BA. Effector dependent sequence learning in the serial RT task. Psychol Res 69: 242–251, 2005 [DOI] [PubMed] [Google Scholar]
- Verwey and Eikelboom, 2003.Verwey WB, Eikelboom T. Evidence for lasting sequence segmentation in the discrete sequence-production task. J Mot Behav 35: 171–181, 2003 [DOI] [PubMed] [Google Scholar]
- Vogel and Machizawa, 2004.Vogel EK, Machizawa MG. Neural activity predicts individual differences in visual working memory capacity. Nature 428: 748–751, 2004 [DOI] [PubMed] [Google Scholar]
- Wing and Kristofferson, 1973.Wing A, Kristofferson A. Response delays and the timing of discrete motor response. Percept Psychophysiol 14: 5–12, 1973 [Google Scholar]
- Young and Schmidt, 1991.Young DE, Schmidt RA. Motor programs as units of movement control. In: Making Them Move: Mechanics, Controls, and Animation of Articulated Figures, edited by Badler NI, Barsky BA, Zeltser D. San Mateo, CA: Morgan Kaufmann, 1991, p. 129–155 [Google Scholar]