Abstract
It is unclear whether intrinsic excitabilities of specific interneurons are modulated by sensory experiences. Here, I examined the intrinsic excitabilities of interneurons in “sensory-spared” and “sensory-deprived” cortices of GAD67-GFP mice. The results showed that whisker trimming, begun at postnatal day 7 for 3 wk, induced significant changes in intrinsic and firing properties of fast-spiking (FS) but not regular spiking nonpyramidal (RSNP) cells. Firing threshold, spike frequency, spike adaptation index, and input resistance of FS cells were significantly altered by sensory deprivation such that FS cells became less excitable. An up-regulation of IA currents in FS cells appeared to be responsible. Along with changes in the intrinsic properties of FS cells, whisker trimming also induced a robust reduction in the number of vesicular glutamate transporter 2 positive varicosities and parvalbumin expression and the strength of thalamocortical (TC) excitatory postsynaptic currents in FS cells in the “sensory-deprived barrels.” The probability of spike induction by TC stimulus was reduced by 30% and the spike jitter was increased in sensory-deprived FS cells. These results suggest that the FS networks are selectively inhibited by sensory deprivation. The concurrent changes of intrinsic properties and expression of parvalbumin in FS but not RSNP cells with TC synapses support a contribution from the TC pathway and glutamate to sensory-induced activity-dependent intrinsic plasticity of inhibitory networks in barrel cortex.
INTRODUCTION
Sensory experience drives the refinement of sensory maps in developing and adult sensory cortices (Crair et al. 1998; Feldman and Brecht 2005; Stryker 1978; Wiesel and Hubel 1974). Tremendous progress has been made toward understanding the maturation of excitatory networks (Feldman and Brecht 2005). However, the process of experience-dependent plasticity of inhibitory networks and its underlying mechanisms remain elusive (reviewed by Hensch 2005). A thorough understanding of the roles of activity and γ-aminobutyric acid (GABA) in sculpting the development of cortical systems will reveal how activity is translated in mature, functional systems. Within the sensory system (Chattopadhyaya et al. 2004; Morales et al. 2002), neocortical inhibitory networks exhibit experience-dependent maturation (Hensch 2005; Huang et al. 2007; Jiang et al. 2005; Sun 2007). How specific interneurons respond to sensory experiences is unclear. In principal neurons, both the strength of inhibitory synapses (Jiao et al. 2006; Maffei and Turrigiano 2004; Maffei et al. 2006) and the firing properties of principal neurons (Maffei and Turrigiano 2008; Maffei et al. 2004, 2006; Xu et al. 2007) are regulated by sensory-induced activities. So far, there is no evidence regarding sensory-dependent intrinsic plasticity in interneurons. To date, circuit-wide changes induced by homeostatic mechanisms have been described in the visual cortex. Maffei et al. (2004) showed that short deprivation early in life had distinct effects on regular-spiking nonpyramidal (RSNP) and fast-spiking (FS) cells. Maffei and Turrigiano suggest that the timing, length, and mode of deprivation, as well as the layers under study, can affect the outcome of plasticity (Maffei and Turrigiano 2008; Maffei et al. 2004). It is unclear whether this interneuron-specific effect can be induced in other sensory cortices and whether activity-dependent mechanisms are involved. Within layer IV barrel cortex, we have reported changes of perisomatic inhibitory synaptic strength (Jiao et al. 2006). In this study, I primarily focused on regulation of intrinsic excitability by whisker trimming. To answer the question regarding whether there is any cell-type-specific intrinsic plasticity in GABA-releasing interneurons, I took advantage of two functionally and structurally distinct types of interneurons (i.e., RSNP and FS cells) in the barrel cortex and examined the effect of whisker trimming on their intrinsic firing properties.
The next question to be addressed is how whisker trimming induces intrinsic plasticity. Although the idea of regulation of intrinsic plasticity has been described in sensory neural circuits (Maffei and Turrigiano 2008; Maffei et al. 2004; Xu et al. 2007), little is known about the underlying induction and expression mechanisms (Nelson and Turrigiano 2008). Here, I focused on answering the following two questions. 1) Are the changes of intrinsic properties correlated with glutamatergic synaptic changes? 2) Which ion conductance contributes to the intrinsic plasticity? I simultaneously measured the strength of glutamatergic synapses, intrinsic electrical properties of interneurons, and isolated IA-type voltage-gated potassium currents. Results reported here highlight a cell-type-specific effect of sensory deprivation and support the contribution of glutamate and activity-dependent mechanisms to sensory-deprivation induced intrinsic plasticity of FS cells.
METHODS
Animals and treatment groups
I used glutamate decarboxylase (GAD) 67-green fluorescent protein (GFP) (Δneo) mice, in which GFP is selectively expressed under the control of the endogenous GAD67 gene promoter (Tamamaki et al. 2003). In this study, these transgenic mice are called GAD67-GFP mice for simplicity. The GAD67-GFP transgenic mouse line was used to study the effects of whisker removal on the maturation of inhibitory networks in sensory “spared” and “deprived” cortices in the adolescent barrel cortex (Jiao et al. 2006). All mice used in this study were GFP-positive mice (i.e., expression GAD67-GFP). Newborn mice were examined under a homemade epifluorescent dissecting microscope or simply using Uvex safety eyeglasses with filtering properties appropriate for GFP emissions. Non-GFP mice were excluded from experiments. Mice were divided into two groups: one group of mice had row D whiskers (in the left mystacial pad) trimmed daily and another group were sham-treated normal mice (i.e., Control group; Woolsey and Van der Loos 1970). In the whisker-trimmed group, interneurons were further separated into “sensory-deprived group” if they were located in row D and “sensory-spared group” if they were located in other rows (A–C and E). Within barrel cortex layer IV of the D-row trimmed group, depending on the location of the interneurons, animals in the experimental groups began to have whiskers (only whiskers of the left mystacial pad) removed at neonatal day 7 and this process lasted until the mice were 30 days old (Jiao et al. 2006). All immunohistochemistry and electrophysiology experiments were performed at this age (postnatal day 30 [P30]). Slices were processed for cytochrome oxidase (CO) histochemistry for validation of recording locations with respect to barrels. These mice were then used in either electrophysiological or immunohistochemistry experiments. For immunohistochemistry experiments, at P30, mice were given a lethal injection of nembutal and perfused intracardially with 0.1 M sodium phosphate buffer, pH 7.4, followed by 4% paraformaldehyde. The brain was then removed and the whole cortex was dissected. Thalamocortical (TC) sections (30 μm in thickness) were prepared based on methods described by Agmon and Connors (1991). Alternative sections from the barrel cortex were processed for CO and PV (or VGlut2) staining (Jiao et al. 2006).
Immunohistochemistry
CYTOCHROME OXIDASE (CO) HISTOCHEMISTRY.
Methods for CO staining were modified from the method published by Wong-Riley (1979). Sections were incubated in 3,3′-diaminobenzidine tetrahydrochloride (DAB, Sigma), cytochrome c (type III, Sigma), and sucrose in 0.1 M PB (pH 7.4) for 3 h, then TBS washed, mounted, and coverslipped. Reconstructed contours of barrel structures and slice outlines were quantitatively analyzed with NeuroExplorer (MicroBrightField).
FLUORESCENT LABELING.
Sections were incubated in 0.6% H2O2 for 30 min, PBS washed, switched to 50% alcohol for 10 min, PBS washed, then incubated in TBS with 0.5% Triton X-100, 2% BSA, and 10% normal goat serum for 2 h, and incubated in primary antibodies directed against: PV (polyclonal anti-PV: 1:500, Calbiochem, cat. #PC255L) or vesicular glutamate transporter 2 (VGlut2, 1:300, monoclonal, Chemicon/Millipore, cat. #MAB5504) overnight. The next day, after PBS rinsing, sections were incubated in Alexa Fluor 594, goat anti-rabbit IgG (H + L) for PV for 3 h, then rinsed, mounted, and coverslipped. The immunofluorescent specimens were examined using an epifluorescence microscope (Carl Zeiss, Thornwood, NY) equipped with an AxioCam digital color camera. Double immunofluorescent images were analyzed using an AxioVision LE imaging suite (Carl Zeiss).
Identification of barrels was achieved by aligning imaging data with underlying CO traced maps. The present study used a computer-aided image analysis system to determine the changes in PV-positive and GFP-positive neuronal density in the barrel cortex after whisker trimming. The image of the CO-stained cortical sections (30–40 μm in thickness) was used as a reference and imported into NeuroExplorer to trace barrel-related columns throughout layer IV of the barrel cortex. Photographs of the DAB-stained and/or double-labeled immunofluorescent stained cortical sections (30–40 μm in thickness) were digitized directly from the microscope images using a CCD camera (Carl Zeiss) and imported into Photoshop (version 7.0; Adobe Systems, San Jose, CA) for each brain section. Then, the images of DAB or double immunofluorescent-stained cortical sections (30–40 μm in thickness) were superimposed over the appropriate trace of CO-stained barrels using an AxioVision LE imaging suite (Carl Zeiss) and Corel Draw (Corel, Fremont, CA). With this method, the location of the immunohistochemically stained neurons, with regard to the outline of barrels, could be accurately determined. Finally, within the outlined barrel columns of layer IV barrel cortex, the densities of specific marked cells and boutons were analyzed.
Brain slice preparations, electrophysiological recordings, and cell tracing
GAD67-GFP mice were deeply anesthetized with pentobarbital sodium (55 mg/kg) and decapitated. The brains were quickly removed and placed into cold (∼4°C) oxygenated slicing medium containing (in mM): 2.5 KCl, 1.25 NaH2PO4, 10.0 MgCl2, 0.5 CaCl2, 26.0 NaHCO3, 11.0 glucose, and 234.0 sucrose. TC slices were prepared according to methods described by Agmon and Connors (1991). Tissue slices (300–400 μm) were cut using a vibratome (TPI, St. Louis, MO), transferred to a holding chamber, and incubated (35°C) for ≥1 h. Individual slices were then transferred to a recording chamber, fixed to a modified microscope stage, and allowed to equilibrate for ≥30 min before recording. Slices were minimally submerged and continuously superfused with oxygenated physiological saline at the rate of 4.0 ml/min. The physiological perfusion solution contained (in mM): 126.0 NaCl, 2.5 KCl, 1.25 NaH2PO4, 1.0 MgCl2, 2.0 CaCl2, 26.0 NaHCO3, and 10.0 glucose. Solutions were gassed with 95% O2-5% CO2 to a final pH of 7.4 at a temperature of 35 ± 1°C. The method for identification of the barrel subfield in living TC slices was described in earlier studies (Sun et al. 2006). A low-power objective (×2.5) was used to identify barrels and thalamic nuclei and a high-power water-immersion objective (×60) with Nomarski optics and infrared video was used to visualize individual neurons. Recording pipettes were pulled from capillary glass obtained from World Precision Instruments (M1B150F-4), using a Sutter Instrument P80 puller, and had tip resistances of 2–5 MΩ when filled with the intracellular solutions (see the following text). A MultiClamp 700B amplifier (Axon Instruments, Foster City, CA) was used for voltage-clamp and current-clamp recordings. Patch pipette saline, modified according to Brecht and Sakmann (2002), was composed of (in mM): 100 K-gluconate, 10.0 phosphocreatine-Tris, 3.0 MgCl2, 0.07 CaCl2, 4 EGTA, 10.0 HEPES, 4.0 Na2-ATP, and 1.0 Na-GTP, pH adjusted to 7.4 and osmolarity adjusted to 280 mOsm. Neurobiotin (0.5%; Vector Labs) was regularly added to the patch pipette solution. Data were accepted for analysis when access resistance in whole cell recordings ranged from 15 to 35 MΩ, and was stable (<25% change) during the recording. The resting membrane potential and the resting input resistance of the cell were also monitored to ensure a stable baseline recording. Current- and voltage-clamp protocols were generated using pCLAMP9.2 software (Axon Instruments). A sharpened bipolar tungsten electrode, placed about 200 μm away from recorded cells in the cortical layer IV, was used to activate intracortical fibers. TC–excitatory postsynaptic currents (EPSCs) were evoked based on methods decribed by Agmon and Connors (1991). Monosynaptic evoked excitatory postsynaptic currents (eEPSCs) were evoked in GAD67-GFP–positive interneurons with the stimuli and were recorded at a holding potential of −70 mV. eEPSCs were evoked in the presence of a cocktail artificial cerebrospinal fluid (ACSF) solution containing GABAA antagonist picrotoxin (50 μM) and a low concentration of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate receptor antagonist 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX; 0.05 μM) to reduce excitation and prevent hyperexcitability (Kumar and Huguenard 2003). Evoked EPSCs events were detected using a Clampfit event-detection function with either the “threshold detection” or the “template detection” method. Spontaneous excitatory postsynaptic currents (sEPSCs) were also recorded using the cocktail ACSF solution. In addition, in some experiments sEPSCs were recorded in the presence of picrotoxin (50 μM) only (Table 4).
Table 4.
Properties of eEPSCs and sEPSCs in RSNP cells of control, sensory-spared, and sensory-deprived areas
| Property | RSNP-Spared (n = 10) | RSNP-Deprived (n = 10) | RSNP-Control (n = 10) |
|---|---|---|---|
| 1. eEPSC amplitude, pA | −30.0 ± 3.0 | −36.0 ± 3.0 | −35.6 ± 4.0 |
| 2. CV (eEPSCs) | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 |
| 3. Paired-pulse ratio | 0.9 ± 0.0 | 0.9 ± 0.1 | 0.9 ± 0.1 |
| 4. Half-width, ms | 7.6 ± 0.5 | 9.4 ± 0.6 | 8.1 ± 0.7 |
| 5. Rise τ, ms | 3.6 ± 0.3 | 5.3 ± 0.5 | 3.7 ± 0.3 |
| 6. Decay τ, ms | 9.6 ± 1.0 | 7.4 ± 0.3 | 7.5 ± 0.4 |
| 7. Max rise slope, mV/ms | −22.0 ± 2.0 | −32.0 ± 2.0 | −30.0 ± 2.0 |
| 8. Max decay slope, mV/ms | 6.5 ± 0.6 | 10.0 ± 1.0* | 8.0 ± 1.0 |
| 9. Area, mV·ms | −513.0 ± 43.0 | −879.0 ± 116.0 | −600.0 ± 39.0 |
| 10. Frequency of sEPSCs, Hz | 3.5 ± 0.5 | 3.1 ± 0.3 | 3.0 ± 0.4 |
| 11. sEPSC amplitude | −6.5 ± 0.5 | −5.6 ± 0.3 | −6.0 ± 0.4 |
| 12. CV (sEPSCs) | 0.4 ± 0.1 | 0.4 ± 0.0 | 0.5 ± 0.1 |
Values are means±SD. 1. 1–12: data obtained from recordings made from cocktail solution (see methods). Paired (or unpaired) t-test and/or one-way ANOVA analysis were used with P < 0.05 considered significant.
P < 0.05.
CHEMICALS.
The following were used in this study: AMPA antagonist GYKI 52466 hydrochloride [1-(4-aminophenyl)-4-methyl-7,8-methylenedioxy-5H-2,3-benzodiazepine] (Sigma–Aldrich, St. Louis, MO); NBQX (Tocris, Ellisville, MO 63021), d-AP5 (Tocris), picrotoxin (Tocris), 4-aminopyridine (4-AP, Tocris), and tetrodotoxin (TTX, Sigma).
ACTION POTENTIAL PARAMETERS.
The following operational definitions were used.
-
Fast afterhyperpolarization (fAHP, mV): the difference between threshold and the most negative membrane potential immediately after the spike, measured on the first evoked action potential (AP).
Spike frequency: the reciprocal of the first interspike interval (ISI).
Frequency (initial, Hz): the reciprocal of the first ISI, measured at the smallest threshold current step that induced spikes.
Frequency (300 pA, Hz): the reciprocal of the first ISI, measured at the 300-pA current step.
Rin (MΩ): input resistance, the slope of linear fit to membrane voltage responses to small current steps resulting in deflections of ±10 mV on resting potentials.
Max rise slope (mV/ms): maximal voltage slope (dV/dt) during AP upswing.
Max decay slope (mV/ms): maximal voltage slope (dV/dt) during AP downswing.
Peak Amp (mV): the difference between threshold and voltage at the most positive value.
Half-width (ms): the period between the AP's crossing the half-amplitude point in its rising and decaying stages. The crossover point nearest the peak is used in each case. Times at which the half-amplitude threshold is crossed may be calculated as falling between data points.
90% width of AP: the period between the AP's crossing the 10% point in its rising and decaying stages.
τ (ms): membrane time constant, determined from the single-exponential curve best fitting the rising phase of the membrane response to a 5-mV hyperpolarizing current step.
AP-threshold (mV): the membrane potential value at which the interpolated rate of rise (dV/dt) of the AP equaled 5 V/s. Before I determine the AP threshold, I make the dV/dt plot of the entire AP waveform. Based on the dV/dt plot of five FS and five RSNP cells, I determine the AP threshold. I found that on average, the rate of voltage change at below threshold range between 1 and 3 V/S, range between 4 and 6 V/S near threshold and range between 6 and 60 V/S above threshold. Medium rates of 5 V/S at near threshold are used for defining AP threshold.
Resting membrane potential (RM, mV): the stable membrane potential reached 3 min after breaking the seal, with no current applied.
Spike adaptation index (SAI): the reciprocal of the first ISI divided by the reciprocal of the last ISI during a 1-s, 300-pA current injection.
Stuttering frequency (Hz): events of stuttering occurred during a 1-s repetitive firing induced by near threshold (1.5-fold) current injection. Stuttering was defined as a self-evident drop in the reciprocal ISI value compared with the average of the last three ISI values.
EPSC PARAMETERS.
These are similar to those defined in APs. In addition, several parameters were unique to EPSCs.
-
Paired-pulse ratio: amplitude of second evoked EPSC divided by the amplitude of the first evoked EPSC.
CV: coefficient of variance of evoked or spontaneous EPSCs.
Area: the area bound by the data trace (EPSC) and the baseline, during the event.
Rise τ (ms): time constant for the rising section of EPSC, obtained by a single-exponential curve best fitting the rising phase of the EPSC.
Decay τ: time constant for the decaying section of EPSC, obtained by a single-exponential curve best fitting the decaying phase of the EPSC.
K+ CURRENTS (INCLUDING IA) RECORDING AND ISOLATIONS.
Methods for isolating A-type currents (IA) were adopted from the Stern lab (Sonner and Stern 2007). Briefly, slices were bathed in a solution with nominal Ca2+ (0 mM) containing (in mM): 105 NaCl, 3 MgSO4, 2.5 KCl, 1.25 NaH2PO4, 26 NaHCO3, 20 d-glucose, 0.2 ascorbic acid, 2 pyruvic acid, 3 EGTA, 200 μM CdCl2, 30 TEA, and 0.2 μM TTX (pH 7.4; 290–300 mOsm). Series resistance was electronically compensated for throughout the recordings. Voltage protocols used to isolate IA currents are plotted in Fig. 4A.
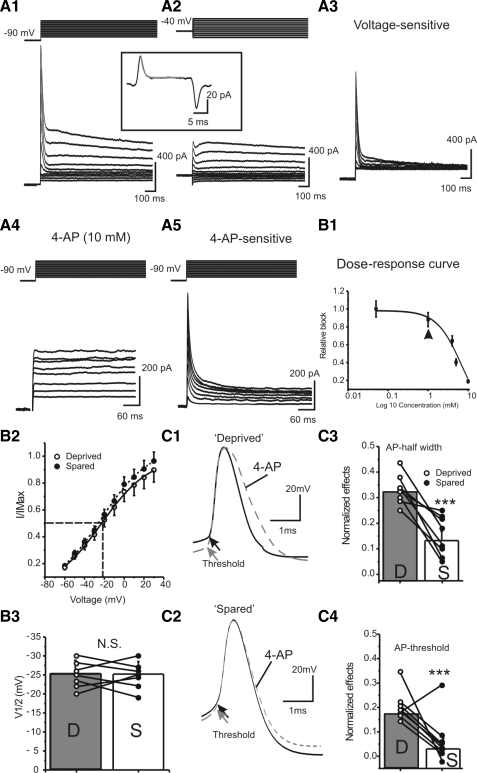
Fig. 4.
4-Aminopyridine (4-AP) blocks a transient voltage-gated potassium current in FS neurons. A: characterization of 4-AP and voltage-sensitive currents in FS cells. Current series in A1 and A4 were obtained with the same voltage-clamp protocol, in which the prepulse voltage was −90 mV. Prepulse voltage in A2 was −40 mV. A3 was the result of subtraction of A2 from A1; A5 was the result of subtraction of A4 from control (not shown). Inset: whole cell transient elicited by a voltage step. Solid ray line: exponential fitting curve. The estimated capacitance for this cell was 44 pF. B1: dose–response curve of 4-AP-induced currents in FS cells. Solid line: data were fitted with a Hill equation: base + {(max − base)/[(1 + Xhalf/X)rate]}, where Xhalf = 6.4 mM and rate = 1.5. Arrowhead indicates the effects of 4-AP at a concentration of 1 mM. B2: normalized IA currents, in cells located in the sensory-deprived (open circles and solid line) and spared areas (filled circles and dotted line), as a function of voltage fitted with a Boltzmann relationship, I/Imax = {1 + exp[(V + V1/2)/K]} under control conditions, where V1/2 = −25 ± 3 mV and K = 13 ± 1 mV. B3: V1/2 obtained from the Boltzmann relationship in sensory-deprived (D) and spared (S) regions; N.S., no statistical significance, n = 7 in each group. C: effects of 4-AP (1 mM) on single AP in sensory-deprived (C1) and sensory-spared areas (C2). Grouped data showing effects of 4-AP on AP half-width (C3) and threshold (C4) in cells located in the sensory-deprived (open circles) and sensory-spared areas (filled circles). **P < 0.01, n = 7 in each group.
NEUROBIOTIN HISTOLOGY AND CELL TRACING.
Patch recordings were performed for ≥30 min before slices were fixed in 4% paraformaldehyde. Fixed slices were processed for neurobiotin-3,3′-diaminobenzidine tetrahydrochloride (DAB, D5905, Sigma) histochemistry. GFP-expressing neurons were first identified under an epifluorescent microscope, which was switched to infrared differential interference contrast microscopy for visualized patch-clamp recording. Three-dimensional neuron models were reconstructed using the Neurolucida system (MicroBrightField, Williston, VT) and a brightfield light microscope (Carl Zeiss MicroImaging, Thornwood, NY). Shrinkage was not corrected in this study. Neurons with poor or unclear labeling were discarded. Reconstructed neurons were quantitatively analyzed with NeuroExplorer (MicroBrightField).
STATISTICS.
Paired (or unpaired) t-test and/or one-way ANOVA analysis were used with P < 0.05 considered significant.
Simulations
All the simulations were carried out with the NEURON simulation program, version 5.9 (Hines and Carnevale 1997). The simulation consists of two FS cells connected via GABAA synapses, two spiny stellate cells connected via glutamatergic synapses, and two separate sets of TC inputs onto the FS and spiny stellate cells via AMPA- and N-methyl-d-aspartate (NMDA)-mediated synapses. Both FS and spiny stellate cells consist of an active somatic compartment and passive dendritic compartments. Each cell was implemented with an axon, soma, and a number of dendrites. The geometries of soma and dendrites of FS and SS cells were constructed using neurolucida data and based on realistic neurobiotin-labeled basket cells and spiny stellate cells. Membrane biophysics of the FS cells is based on data reported by Beierlein et al. (2003) and Sun et al. (2006). For each cell type (FS) the resting potential, input resistance, membrane time constant, AP half-width, amplitude of afterhyperpolarization potentials (AHPs), and firing rate at threshold were closely matched to those published by Beierlein et al. (2003) and patch-clamp recordings from mouse FS cells. In addition to passive (i.e., leak) conductance, the following conductances were included in the model: a voltage-gated calcium conductance (gcabar); a voltage-gated Na+ conductance (gnabar); a calcium-activated potassium conductance (gkcbar); and a transient potassium conductance (IA, gabar). The conductances of FS cells were specified as follows: gcabar_spike = 0.0015 nS/cm2, gkbar_spike = 0.018 nS/cm2, gabar_spike = 0.054 nS/cm2, gkcbar_spike = 0.000065 nS/cm2, gnabar_spike = 0.10 nS/cm2. In the dendritic compartments, only leak conductance was present, with gleak = 0.00045 nS/cm2. It should be noted that several additional mechanisms were not included in my model, for example, persistent Na+ conductance, other voltage-gated or inwardly rectifying K+ conductances, and voltage-gated Ca2+ conductance. The reason that I did not include these conductances was that these conductances may modulate spike synchrony. The calcium-dependent potassium conductance was determined by intracellular calcium concentration, which was dynamically regulated by voltage-gated calcium conductance. Briefly, the biophysical properties of the FS cells are: resting membrane potential: −66 mV; input resistance: 220 MΩ (sensory-spared cells) or 148 MΩ (sensory-deprived cells); membrane time constant: 5 ms; AP half-width: 0.4 ms; steady-state firing rate at threshold: 60 Hz; spike frequency adaptation index: 0.7. Synaptic connection characteristics were based on data provided by Sun et al. (2006), Bacci et al. (2002), and Xiang et al. (1998). Briefly, TC–EPSCs in FS cell: amplitude: 2.2–5.0 mV; rise time: 0.4 ms; decay time: 1.5 ms; CV: 0.2. Recurrent GABAA-mediated inhibition in FS cell: amplitude: 1.5–2.5 mV; rise time: 2.0 ms; decay time: 37 ms; CV: 0.4. The geometry of FS and SS cells, constructed using Neurolucida, was based on realistic neurobiotin labeled from basket cells and SS cells. Additional information regarding the simulation is available in the Supplemental materials.1
RESULTS
Effects of whisker trimming on intrinsic firing properties of FS interneurons
We have shown that whisker trimming induces down-regulation in PV expression and perisomatic inhibitions in spiny neurons (Jiao et al. 2006). To continue examining mechanisms of sensory deprivation, I examined the effects of whisker trimming on intrinsic properties in specific FS interneurons. Because the intrinsic firing properties are determined by both the expression of voltage-gated ion channels and the geometry of dendritic trees (Mainen and Sejnowski 1996), I first examined the effects of whisker trimming on dendritic geometry in specific FS interneurons. FS cells encompass structurally and biochemically distinct subgroups in the barrel cortex (Ma et al. 2006). Among all reconstructed FS cells, six neurons (three pairs: three in spared and three in deprived areas) belonged to type A FS cells that were characterized by a crescent (or oval) shaped cell body with form factor value of 0.6 ± 0.1 (form factor for a perfect circle is 1.0) and roundness value of 0.45 ± 0.1 (e.g., Fig. 1A, white arrowheads). Type A FS cells typically had two or three large-diameter dendrites emerging from the two ends of the cell body, which were then extended toward the opposite directions (e.g., Fig. 1A). Eight neurons (four pairs: four in spared and four in deprived) belonged to type B FS cells, which were characterized by a roundish shaped cell body with a form factor value of 0.85 ± 0.1 and roundness value of 0.65 ± 0.1 (e.g., Fig. 1B, white arrowheads). There were 6 ± 1 main dendrites emerged on all directions from the cell body. These dendrites branched much less frequently than type A cells (e.g., Fig. 1, B vs. A). Overall, type B cell dendrites covered a smaller volume than type A cells (545 ± 25 vs. 1,364 ± 165 μm3; Fig. 1C3).
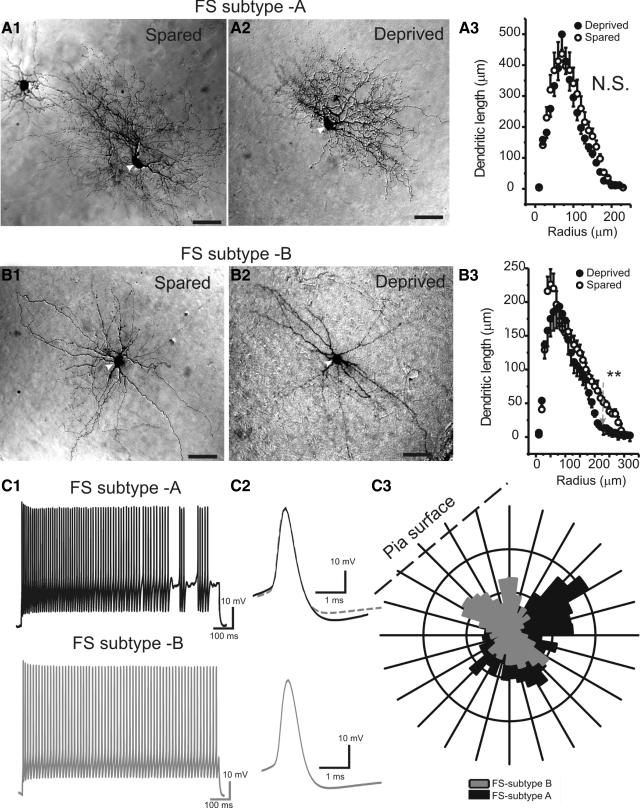
Fig. 1.
Whisker trimming had only a slight effect on the dendritic structure of fast-spiking (FS) interneurons. A1 and A2: photomicrographs of 2 type A FS cells in the spared (A1) and deprived (A2) regions, respectively. A3: Sholl analysis of the dendritic arbors of type A FS cells in deprived (filled circles) and spared regions (open circles), respectively (n = 3 in each group, P > 0.5). B1 and B2: photomicrographs of 2 type B FS cells in the spared (B1) and deprived (B2) regions, respectively. B3: Sholl analysis of the dendritic arbors of type B FS cells in deprived (filled circles) and spared regions (open circles), respectively (n = 4 in each group, P < 0.01 at values larger than >220 μm). C: firing pattern and dendritic arborizations in type A and type B cells. C1: action potentials (APs) elicited by long depolarizing currents (1 s, 210 pA). Top: type A cell; bottom: type B cell. C2: single APs. Top: type A cell; bottom: type B cell; gray dashed trace on top overlay the 3 traces. C3: polar histogram showing the distribution of dendrites of a type A (black) and type B (gray) cells, respectively.
Despite the marked differences in their dendritic morphology, type A and type B cells shared the same FS firing properties with or without stuttering (e.g., Fig. 1, C1 and C2). The single AP properties of type A and type B cells were identical (e.g., Fig. 1C2, n = 6 and 8 in each group, P > 0.5). I performed Sholl analysis and compared the effects of whisker trimming on the distribution of dendrites in the two cell types. I found that whisker trimming had no significant effect on the dendrites of type A cells (Fig. 1A) and had only negligible effects on type B cells: i.e., affecting the distal dendrites located at >200 μm away from the cell body (Fig. 1B, gray arrow, P < 0.01 at 220–300 μm). Thus whisker trimming had a very small effect on the dendritic distal structure in a subgroup of FS cells. I next assessed the effects of persistent whisker trimming on the physiological firing properties of FS and RSNP cells. Both the ability to fire repetitive APs in response to prolonged depolarizing current injections and the properties of single AP waveforms were assessed using parameters explained in methods. d-AP-5 (30 μM), NBQX (10 μM), and picrotoxin (300 μM) were present during this experiment to block modulation by synaptic conductance. RSNP and FS cells differ in their firing properties and can be readily distinguished based on 1) maximum firing frequency, 2) 90% width of AP, and 3) cell time constant (Sun et al. 2009). RSNP interneurons were defined by having a firing frequency (induced at currents of ≥200 pA) of <120 Hz, a 90% AP width of >1 ms, and a cell membrane time constant >7 ms (Beierlein et al. 2003; Ma et al. 2006; Sun et al. 2009). Next I compared the properties of excitability in cells recorded in sensory-spared versus sensory-deprived barrels as well as cells in naïve untreated barrels (control, Table 1). Two cells were usually recorded from the same brain slice located in spared and deprived barrels, respectively, in a random order.
Table 1.
Properties of action potentials in FS cells of control, sensory-spared, and sensory-deprived areas
| Property | FS-Spared (n = 16) | FS-Deprived (n = 16) | FS-Control (n = 16) |
|---|---|---|---|
| 1. AP-threshold | −37.0 ± 1.0 | −29.0 ± 1.0*** | −36.0 ± 2.0 |
| 2. Frequency, Hz | 172.0 ± 10.0 | 106.0 ± 7.0*** | 195.0 ± 14.0 |
| 3. Frequency, initial Hz | 61.0 ± 3.0 | 29.0 ± 5.0*** | 58.0 ± 4.0 |
| 4. SAI | 0.7 ± 0.03 | 0.9 ± 0.03** | 0.7 ± 0.1 |
| 5. Stuttering frequency, Hz | 1.9 ± 0.6 | 0.6 ± 1.2* | 1.4 ± 0.7 |
| 6. RM, mV | −63.0 ± 2.0 | −60.0 ± 1.0 | −64.0 ± 3.0 |
| 7. Peak Amp, mV | 68.0 ± 1.0 | 67.0 ± 2.0 | 70.0 ± 3.0 |
| 8. Half-width, ms | 0.4 ± 0.04 | 0.5 ± 0.1 | 0.4 ± 0.1 |
| 9. Max rise slope, mV/ms | 107.0 ± 7.0 | 122.0 ± 10.0 | 115.0 ± 9.0 |
| 10. Max decay slope, mV/ms | −68.0 ± 4.0 | −81.0 ± 7.0 | −78.0 ± 7.0 |
| 11. Rin, MΩ | 221.0 ± 10.0 | 148.0 ± 8.0*** | 207.0 ± 13.0 |
| 12. τ, ms | 4.4 ± 0.3 | 6.2 ± 0.5** | 4.7 ± 0.4 |
| 13. fAHP, mV | −16.0 ± 1.0 | −14.0 ± 2.0 | −18.0 ± 2.0 |
Values are means±SD. 1. AP-threshold: action potential threshold; 3. Frequency (initial, Hz): firing frequency induced by threshold current depolarization; 4. SAI: spike-adaptation index; 6. RM: resting membrane potential (mV); 7. Peak Amp: peak amplitude of action potential (mV); 11. Rin: input resistance (MΩ); 12. τ: membrane time constant (ms); 13. fAHP: fast afterhyperpolarization potential (mV). Paired (or unpaired) t-test and/or one-way ANOVA analysis were used with P < 0.05 considered significant.
P < 0.05;
P < 0.01;
P < 0.001.
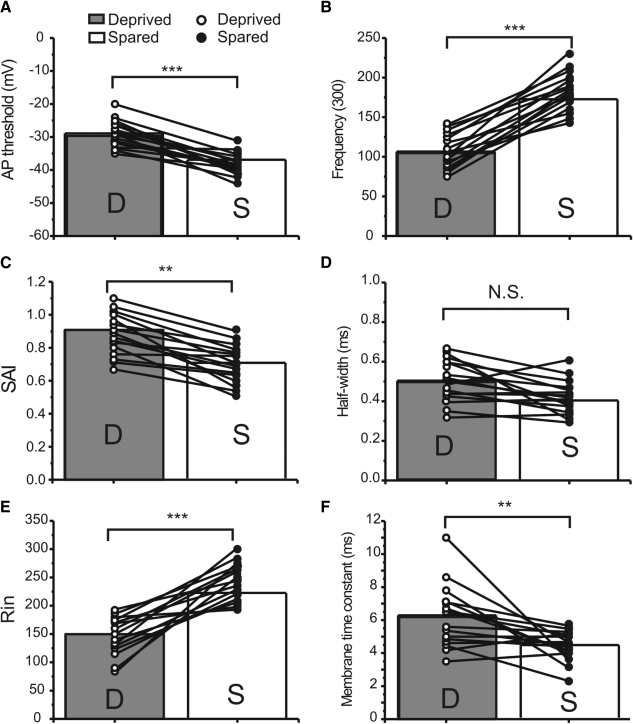
As shown in Table 1 and Fig. 2, whisker trimming had several significant effects on the firing and intrinsic properties of FS cells. First, in FS cells located in the “sensory-deprived” barrels, the firing frequency (initial and 300 pA) became significantly slower than cells in sensory-spared and control groups (e.g., Fig. 2, A2 vs. A1, B2 vs. B1; see Table 1 and Fig. 3B for group data, P < 0.001) and the firing threshold was significantly more positive (e.g., Fig. 2, C2 vs. C1; see Table 1 and Fig. 3A for group data, P < 0.001). However, there were no significant differences in single AP width between spared and deprived cells (e.g., Fig. 2C; see Table 1 and Fig. 3D for group data, P > 0.1). There was a rightward shift of the frequency–current (F–I) curve in sensory-deprived cells as well as a change in the slope of the linear regression fit of the F–I curve (Fig. 2D1). Second, the stuttering frequency was significantly lower (0.6 ± 2.3 Hz in sensory-deprived vs. 1.9 ± 0.6 in sensory-spared and 1.4 ± 0.7 in control, Table 1; P < 0.05) and SAI significantly decreased from 0.9 ± 0.0 in sensory-deprived to 0.7 ± 0.0 in sensory-spared cells (Table 1 and Fig. 3C; P < 0.01). Third, passive membrane properties such as Rin and time constant (τ), but not resting membrane potentials, also showed significant differences between “sensory-deprived” and control or “spared” cortices (Table 1 and Fig. 3, E and F). However, I did not find any significant differences in peak amplitude, max rise slope, and max decay slope for single APs between the FS spared and FS deprived groups (Table 1). There were no significant differences in any AP parameters between spared and control groups in FS cells (see Table 1). In contrast, in RSNP cells, whisker trimming had no significant effect on repetitive firing parameters, single AP waveforms (AP-width, amplitudes, max rise slope), or intrinsic membrane properties (τ, Rin, RM, Table 1). All AP parameters were similar between sensory-spared, sensory-deprived, and control groups (Supplemental Fig. S1 and Table 2).
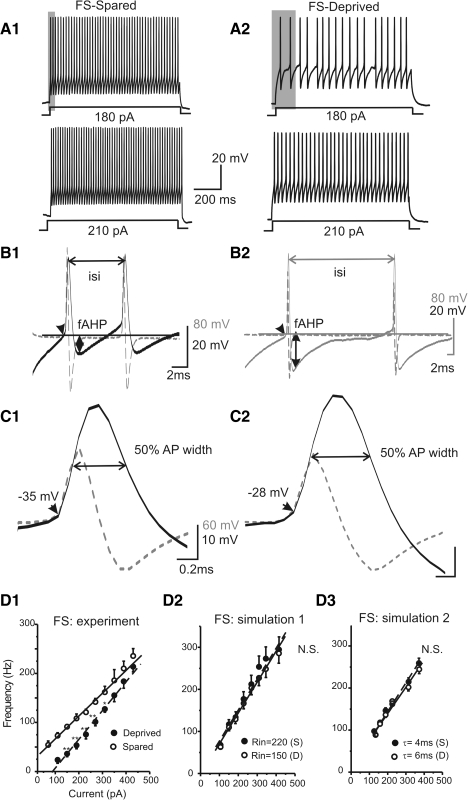
Fig. 2.
Effects of whisker trimming on firing pattern of FS interneuron. A: APs elicited by long depolarizing currents (1 s, 180 and 210 pA, respectively) in FS cells located in sensory-spared (A1, left) or sensory-deprived (A2, right) cortices. B: doublets AP waveform (solid line) and its differentiated waveform (dotted line) in sensory-spared (B1) vs. sensory-deprived (B2) cortices. ISI, interspike interval; fAHP, fast afterhyperpolarization potential. Scale bar indicates the voltage and timescale value for the AP (20 mV/2 ms) and its dV/dt (80 mV/2 ms), respectively. C: single AP waveform (solid line) and its differentiated waveform (dotted line) in sensory-spared (C1) vs. sensory-deprived (C2) cortices. D: frequency–current (F–I) plot in FS cells. Scale bar indicates the voltage and timescale value for the AP (10 mV/0.2 ms) and its dV/dt (60 mV/0.2 ms), respectively. D1: F–I plot in FS cells of sensory-spared (open circles) and sensory-deprived areas (filled circles). n = 16 in sensory-deprived region; n = 10 in sensory-spared region. **P < 0.01; *P < 0.05. Solid line: linear regression fit for “spared” (Y = A + BX), where B = 0.46, R = 0.99, P < 0.0001. Dashed line: linear regression fit for “sensory-deprived” (Y = A + BX), where B = 0.58, R = 0.99, P < 0.0001. D2: F–I plot in 2 computational simulations, in which input resistance was altered from 210 MΩ (filled circles) to 150 MΩ (open circles, n = 5 trials, P > 0.5). D3: F–I plot in 2 computational simulations, in which membrane time constant was altered from 6 ms (filled circles, “deprived”) to 4 ms (open circles, “spared,” n = 5 trials, P > 0.5).
Fig. 3.
Properties of APs in FS cells of sensory-spared and sensory-deprived areas. Parameters for single and repetitive APs in cells located in deprived (open circles) and spared areas (filled circles). Solid lines link cells located in the same brain slice. A: AP-threshold, action potential threshold. B: frequency (300 pA, Hz), firing frequency induced by 300-pA depolarizing current. C: SAI, spike-adaptation index. D: half-width of single AP. E: Rin, input resistance (MΩ). F: membrane time constant (ms). Paired t-test and one-way ANOVA analysis were used, with P < 0.05 considered significant. ***P < 0.001; **P < 0.01; N.S., no statistical significance.
Table 2.
Properties of action potentials in RSNP cells of control, sensory-spared, and sensory-deprived areas
| Property | RSNP-Spared (n = 10) | RSNP-Deprived (n = 10) | RSNP-Control (n = 12) |
|---|---|---|---|
| 1. AP-threshold | −32.0 ± 3.0 | −30.0 ± 3.0 | −33.0 ± 3.0 |
| 2. Frequency, initial Hz | 10.0 ± 2.0 | 10.0 ± 1.0 | 14.0 ± 4.0 |
| 3. SAI | 0.7 ± 0.1 | 0.8 ± 0.1 | 0.6 ± 0.3 |
| 4. Stuttering frequency, Hz | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 5. RM, mV | −62.0 ± 2.0 | −60.0 ± 3.0 | −63.0 ± 3.0 |
| 6. Peak Amp, mV | 78.0 ± 5.0 | 78.0 ± 5.0 | 74.0 ± 6.0 |
| 7. Half-width, ms | 1.4 ± 0.2 | 1.4 ± 0.4 | 1.4 ± 0.3 |
| 8. Max rise slope, mV/ms | 88.0 ± 10.0 | 84.0 ± 7.0 | 89.0 ± 13.0 |
| 9. Max decay slope, mV/ms | −25.0 ± 3.0 | −30.0 ± 5.0 | −33.0 ± 6.0 |
| 10. Rin, MΩ | 222.0 ± 38.0 | 271.0 ± 40.0 | 256.0 ± 43.0 |
| 11. τ, ms | 11.0 ± 1.0 | 12.0 ± 2.0 | 10.0 ± 3.0 |
| 12. fAHP, mV | −13.0 ± 2.0 | −12.0 ± 2.0 | −12.0 ± 3.0 |
Values are means±SD. 1. AP-threshold: action potential threshold; 2. Frequency (initial, Hz): firing frequency induced by threshold current depolarization; 3. SAI: spike-adaptation index; 5. RM: resting membrane potential (mV); 6. Peak Amp: peak amplitude of action potential (mV); 10. Rin: input resistance (MΩ); 11. τ: membrane time constant (ms); 12. fAHP: fast afterhyperpolarization potential (mV). Paired (or unpaired) t-test and/or one-way ANOVA analysis were used with P < 0.05 considered significant.
These data suggested that whisker trimming did have significant effects on the firing and intrinsic properties of FS but not RSNP interneurons. Thus although both FS cells and RSNP cells were located in the same barrels, the electrical properties of only FS cells were modulated, suggesting cell-type-specific effects of sensory deprivation. To evaluate whether changes in input resistance or membrane time-constant alone (see Table 1) could be responsible for changes in the observed firing properties (F–I curve) in FS cells, I performed computational simulation experiments, in which model FS cells were created based on realistic FS conductance and dendritic data (see methods and Supplemental materials). In simulation experiments, input resistance was changed to mimic the changes induced by whisker trimming. As shown in Fig. 2D2, there was little effect of Rin on the F–I curves (n = 5 simulations in each condition, P > 0.1). I also modeled whether changes in membrane time constant (τ) could explain the changes in the F–I curve. The F–I curves were almost identical when I changed the membrane τ from 4 to 6 ms (Fig. 2D3, n = 5 simulations in each condition, P > 0.1). These results suggest that the change in F–I was not the result of altered Rin or membrane time constant, but was likely the result of altered expression of voltage-gated conductances.
Ionic mechanisms underlying the effects of whisker experiences on intrinsic firing properties of FS cells
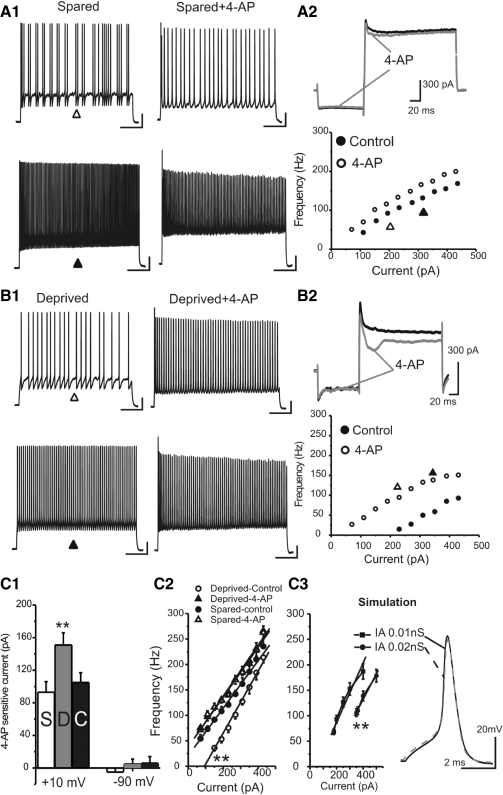
I next examined which ionic conductances are responsible for the decrease in firing rate in FS cells. Because there was little effect of whisker trimming on the properties of single action potentials (APs, with the exception of AP threshold), I examined conductances whose roles are to modulate the properties of spike trains (such as spike adaptation, etc.). Using a voltage protocol selectively for IA current activation (Jackson and Bean 2007; Song et al. 1998; Sonner and Stern 2007), in all GAD67-GFP cells tested (n = 20 cells), I isolated a transient outward current whose voltage dependence was similar to that of the IA currents (e.g., Fig. 4A3). IA currents can be selectively blocked by potassium channel blocker 4-AP (Bourdeau et al. 2007; Jackson and Bean 2007; Song et al. 1998; Sonner and Stern 2007). Indeed, in the same neurons, the transient IA currents isolated by voltage protocol were similar to the 4-AP-sensitive currents (e.g., Fig. 4, A5 vs. A3 and A6; n = 20 cells). I then examined the dose–response properties of the 4-AP-sensitive currents, which was also consistent with previous studies (Jackson and Bean 2007). Thus IA currents were present in all GAD67-GFP neurons. I next assessed the effects of whisker trimming on IA activation. As shown in Fig. 4B, there were no significant differences in slope or the half-activation voltages (n = 7 pairs, P > 0.5). I then examined the effects of low concentration of 4-AP (1 mM) on single APs. As shown in Fig. 4B1, at 1 mM, 4-AP blocks <10% of total K+ currents. In this concentration, 4-AP (1 mM) had a modest effect (30 ± 4%) on AP half-width in deprived cells but not in spared cells (e.g., Fig. 4, C1 vs. C2, P < 0.01 spared vs. deprived). Similar effects were found on the spike threshold, i.e., greater effects on deprived cells than those on spared cells (Fig. 4C4, P < 0.01, n = 7 pairs). As expected, low doses of 4-AP (1 mM) also increased the firing frequency in sensory-spared FS cells and induced a modest leftward shift of the F–I curve (e.g., Fig. 5A2). In the sensory-deprived FS cells, 4-AP (1 mM) produced a greater effect on spike frequencies and a larger leftward shift in the F–I curve (e.g., Fig. 5, B vs. A). In cells in which spikes were induced with a repeatable fixed current (300 pA, 500 ms), 1 mM 4-AP increased the firing frequency by 153 ± 28% (n = 7 cells) in deprived cells and by only 42 ± 9% in spared cells (n = 7, P < 0.01 vs. deprived).
Fig. 5.
Low concentration of 4-AP abolished differences in firing frequencies between sensory-deprived and sensory-spared neurons. A1: APs elicited by long depolarizing currents (1 s, 200 and 260 pA, respectively, in the top and bottom panels) in FS cells located in sensory-spared cortices before (A1, left) and after 1 mM 4-AP (A1, right) application. A2, top: effect of 4-AP on currents induced by the voltage protocol depicted here. Note that the voltage protocol was not shown in the same timescale. Bottom: effects of 4-AP on the F–I curve in the same cell shown in A1. B1: APs elicited by long depolarizing currents (1 s, 200 and 260 pA, respectively, in the top and bottom panels) in FS cells located in sensory-deprived cortices before (B1, left) and after 1 mM 4-AP (B1, right) application. B2, top: effects of 4-AP on currents induced by voltage protocols. Bottom: effects of 4-AP on the F–I curve in the same cell shown in B1. C1: effects of 1 mM 4-AP on currents elicited at +10 mV (left columns) and −90 mV (right columns) in sensory-spared (S, n = 12) and sensory-deprived (D, n = 10) cortices and in control (C, n = 8). **P < 0.01 vs. S and C, respectively. C2: F–I plot in FS cells located in sensory-deprived and sensory-spared cortices in the absence and presence of 1 mM 4-AP. Solid and dashed lines: linear fit of the F–I data. C3, left: F–I plot in simulated FS cells with different IA (control, gIA = 0.01 nS; additional IA, gIA = 0.02 nS). Solid and dashed lines: linear fit of the F–I data. Right: single AP waveforms in simulated FS cells with different IAs (solid black trace, gIA = 0.01 nS; dashed gray trace, gIA = 0.02 nS).
This result suggests that a larger 4-AP-sensitive current plays a role in regulating the intrinsic firing properties in FS cells of sensory-deprived cortices. I next examined the relative expression of the IA currents in FS cells by examining the amount of 4-AP-sensitive currents. A low concentration of 4-AP (1 mM) was used to compare its effects on firing. The 4-AP (1 mM) sensitive currents were significantly up-regulated in the sensory-deprived area (n = 10 cells) compared with sensory-spared (n = 12 cells) or control groups (n = 8; e.g., Fig. 5, B2 vs. A2; see group data in Fig. 5C1, P < 0.01). In contrast, there was very little effect of 4-AP (1 mM) on leak currents and no significant differences in leak currents in sensory-deprived versus spared or control group (Fig. 5C1, −90 mV). This result suggests that in sensory-deprived cortices, the 4-AP-sensitive currents were up-regulated, compared with the sensory-spared or control groups. If the up-regulation of the IA currents were responsible for the slowing of spike trains, the effects should be abolished by a low concentration of 4-AP. Indeed, in the presence of this very low concentration of 4-AP (1 mM), the difference in firing frequency between sensory-deprived and sensory-spared groups was totally abolished (Fig. 5C2). These results suggest that the up-regulation of IA currents is likely responsible for the slowing of firing frequencies in sensory-deprived FS cells. To further evaluate whether up-regulation of IA alone mimics the effects of sensory deprivation, I performed computational simulation experiments. As shown in Fig. 5C3, increasing IA currents by onefold (from 0.01 to 0.02 nS) caused a rightward shift of the F–I curve (five trials, P < 0.05). Other changes in intrinsic properties in sensory-deprived FS cells, such as an increase in AP threshold (Fig. 5C3) and SAI (not shown), but not the AP half-width (e.g., Fig. 5C3, right, n = 5, P > 0.5), were also observed. This result suggests that up-regulation of IA alone can explain most of the observed changes in the intrinsic properties of FS cells. The lack of differences in AP half-width can also be explained by the simulation results as well (e.g., Fig. 5C3, right).
Effects of whisker trimming on properties of excitatory synaptic currents in FS cells
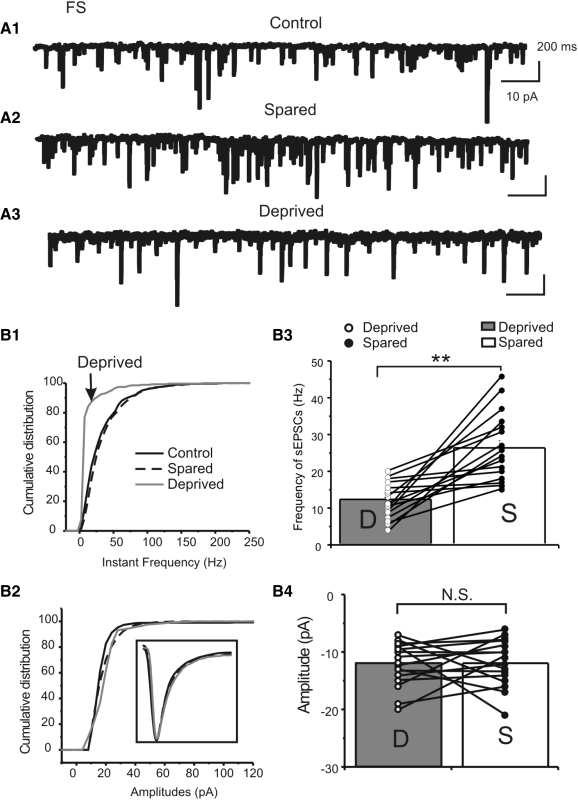
I next examined the effects of whisker trimming on the spontaneously occurring EPSCs and EPSCs evoked by stimulation of TC fibers. Putative AMPA-receptor mediated sEPSCs and eEPSCs were recorded at holding potential of −80 mV in the presence of a cocktail ACSF solution containing GABAA antagonist picrotoxin (50 μM) and a very low concentration of AMPA/kainate receptor antagonist NBQX (0.05 μM) to reduce excitation and prevent hyperexcitability (Kumar and Huguenard 2003). Under these conditions, the sEPSCs and eEPSCs were entirely blocked by selective AMPA receptor antagonist GYKI52466 hydrochloride. In FS cells located in the sensory-deprived barrels, the sEPSCs frequency (12 ± 7 Hz) was significantly slower than that recorded in sensory-spared (26 ± 2 Hz, P < 0.01) or control barrels (20 ± 3 Hz, P < 0.05, e.g., Fig. 6, A vs. B1; see group data in Fig. 6B3 and Table 3, P < 0.01, n = 16 in each group). However, there were no significant effects on the amplitudes of sEPSCs (e.g., Fig. 6B2; see group data in Table 3 and Fig. 6B4, P > 0.5, n = 16 in each group), suggesting that the effects of whisker trimming are predominantly presynaptic. In contrast, there were no statistical differences in sEPSC parameters (frequency, amplitudes, decay time constant) recorded in RSNP interneurons located in the sensory-deprived, spared barrels, or control groups (Supplemental Fig. S2 and Table 4). To further evaluate pathway-specific EPSCs, I applied minimal stimulus to the TC fiber by placing the stimulating electrode in the internal capsule or striatum (Sun et al. 2006). The monosynaptic nature of the TC-induced EPSCs was determined by consistent latency, consistent rise time (e.g., Fig. 7,A1 and A2, and additional characterization in Supplemental Figs. S3 and S4), and the ability to follow a high-frequency stimulus (not shown; Sun et al. 2005, 2006). A minimal stimulus protocol was used to evoke all or none TC–EPSCs (Fig. 7A1). As shown in Fig. 7A1, the minimally induced TC–EPSCs in FS cells had mean amplitudes of −111 ± 13 pA (n = 16), a value that was more than twofold larger than the value of EPSCs induced in RSNP cells (−30 ± 3, n = 10, Fig. 7B3 and Table 4).
Fig. 6.
Effects of whisker trimming on spontaneous excitatory postsynaptic currents (sEPSCs). A: examples of representative sEPSCs recorded in FS interneurons of control (A1), sensory-spared (A2), or sensory-deprived (A3) traces. B: cumulative distribution of frequency (B1) and amplitudes (B2) of sEPSCs in FS cells. Inset in B3: example of averaged and normalized sEPSCs in control (black solid), sensory-spared (black dotted), and sensory-deprived (gray solid) traces. Group data showing frequency (B3) and amplitudes (B4) of EPSCs in cells located in deprived (open circles) and spared areas (filled circles), respectively. Solid lines link cells recorded from the same brain slice. **P < 0.01; N.S.: no statistical significance, n = 16 in each group.
Table 3.
Properties of eEPSCs and sEPSCs in FS cells of control, sensory-spared, and sensory-deprived areas
| Property | FS-Spared (n = 16) | FS-Deprived (n = 16) | FS-Control (n = 16) |
|---|---|---|---|
| 1. eEPSC amplitude, pA | −111.0 ± 13.0 | −43.0 ± 3.0* | −120.0 ± 21.0 |
| 1′. eEPSC amplitude, pA | −119.0 ± 11.0 | −51.0 ± 4.0* | N.A. |
| (n = 6) | (n = 6) | ||
| 2. CV (eEPSCs) | 0.3 ± 0.0 | 0.4 ± 0.0* | 0.3 ± 0.0 |
| 2′. CV (eEPSCs) | 0.3 ± 0.0 | 0.5 ± 0.0** | N.A. |
| 3. Paired-pulse ratio | 0.6 ± 0.0 | 0.6 ± 0.1 | 0.6 ± 0.1 |
| 4. Half-width, ms | 2.6 ± 0.2 | 2.6 ± 0.1 | 2.6 ± 0.1 |
| 5. Rise τ, ms | 1.6 ± 0.2 | 2.1 ± 0.4 | 1.5 ± 0.4 |
| 6. Decay τ, ms | 3.4 ± 0.4 | 3.6 ± 0.4 | 3.5 ± 0.4 |
| 7. Max rise slope, mV/ms | −150.0 ± 18.0 | −55.0 ± 5.0* | −165.0 ± 8.0 |
| 8. Max decay slope, mV/ms | 67.0 ± 8.0 | 22.0 ± 2.0* | 62.0 ± 7.0 |
| 9. Area, mV·ms | −469.0 ± 32.0 | −285.0 ± 50.0* | −500.0 ± 43.0 |
| 9′. Area, mV·ms | −511.0 ± 25.0 | −315.0 ± 54.0* | N.A. |
| 10. Frequency of sEPSCs, Hz | 26.0 ± 2.0 | −12.0 ± 1.0**/* | 20.0 ± 3.0 |
| 10′. Frequency, Hz | 25.0 ± 3.0 | −17.0 ± 4.0* | N.A. |
| (n = 6) | (n = 6) | ||
| 11. sEPSC amplitude | −12.0 ± 1.0 | −12.0 ± 1.0 | −12.0 ± 1.0 |
| 11′. sEPSC amplitude | −14.0 ± 1.0 | −12.0 ± 1.0 | N.A. |
| 12. CV (sEPSCs) | 0.6 ± 0.1 | 1.0 ± 0.2* | 0.5 ± 0.2 |
| 12′. CV (sEPSCs) | 0.7 ± 0.2 | 1.1 ± 0.2* | N.A. |
Values are means±SD. 1. 1–12: data obtained from recordings made from cocktail solution (see methods); 1′, 2′, 9′, 10′, 11′, and 12′: data obtained from recordings made from ACSF solution containing picrotoxin (n = 6 in sensory-spared and sensory-deprived, respectively); N.A.: data not available. Paired (or unpaired) t-test and/or one-way ANOVA analysis were used with P < 0.05 considered significant.
P < 0.05;
P < 0.01;
***P < 0.001.
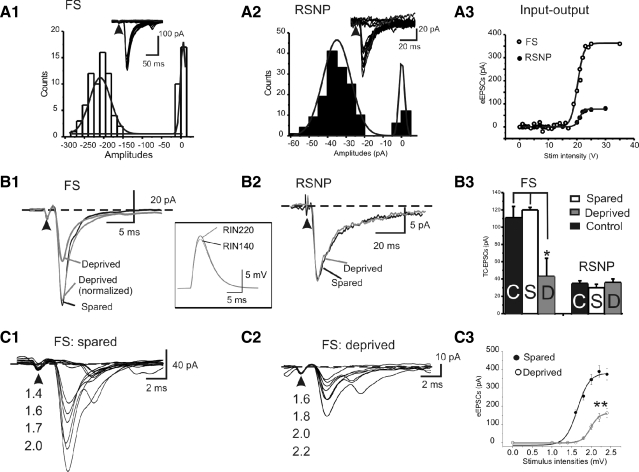
Fig. 7.
Effects of whisker trimming on evoked (e)EPSCs. A: amplitudes of EPSCs evoked by minimal stimulus, where the same stimulus induced all-or-none responses in the FS (A1, open bars) or regular spiking nonpyramidal (RSNP, A2, black bars) interneurons. Insets: example of 20 EPSCs evoked by the minimal stimulus. Arrowheads: stimulus artifact indicates the time of stimulus. A3: amplitude of eEPSCs vs. stimulus intensity in a representative RSNP (filled circles) vs. FS (open circles) cells. B1 and B2: example of representative EPSCs evoked the minimal stimulus recorded in FS (B1) or RSNP (B2) interneurons of sensory-spared (black) or sensory-deprived (gray) traces. Inset: computational simulations of TC-induced EPSPs in a model FS cell with 2 different input resistances (140 and 220 MΩ, respectively). B3: mean amplitudes of EPSCs induced by the minimal stimulus in control (open bar), sensory-spared (gray bar), or sensory-deprived (black bar) conditions in FS (left) or RSNP (right) interneurons. *P < 0.05 control and sensory-spared vs. sensory-deprived, n = 16 for each FS groups. C1 and C2: example of representative EPSCs evoked by varying stimulus intensities in FS in sensory-spared (C1) or sensory-deprived (C2) areas. C3) Input (i.e., stimulation intensities) and output (i.e., amplitudes of eEPSCs) in spared and deprived cells (n = 12 cells in each group, **P < 0.01). Solid line is the best fit of Boltzmann relation, y = {1 + exp[(a + a1/2)/k]−1}−1, where a1/2 = 1.98 ± 0.06 mV and k = 0.11 ± 0.03 (n = 12) for the deprived group and a1/2 = 1.64 ± 0.03 mV and k = 0.15 ± 0.04 (n = 12) for the spared group.
I next examined whether TC–EPSCs in FS and RSNP cells show any differences in sensory-spared versus sensory-deprived conditions. In FS cells, the amplitudes and CVs, but not other kinetic properties (half-width, rise and decay τs, rise and decay slopes, Table 3), were significantly affected by whisker-trimming treatment. In the sensory-deprived area, the amplitudes of eEPSCs (e.g., Fig. 7A3) were significantly smaller (−43 ± 3 pA in sensory-deprived vs. −111 ± 13 pA in sensory-spared and 120 ± 21 pA in control, P < 0.05; Table 3). As expected from a presynaptic effect, the CV value of TC–EPSCs was significantly larger in FS cells recorded from the sensory-deprived area (Table 3). If the TC projections to the deprived area are down-regulated, then the recruitment of TC axons by increased stimulation intensities should be impaired.
I next examined the classical input–output relation (i.e., stimulus intensity–EPSC amplitudes relation) of TC-induced EPSCs in FS cells. As shown in Fig. 7C, the input–output curves were significantly smaller at peak amplitude (n = 12 cells in deprived vs. spared, respectively, P < 0.01); the half-activation intensities shifted rightward, indicating an impaired recruitment of TC axons in the deprived area. In contrast, in the RSNP cells, similar to the effects of whisker trimming on APs in RSNP cells, there was also no statistical difference in evoked EPSCs in RSNP neurons (Table 4). Together, these results suggest that whisker trimming induced selective presynaptic effects on glutamatergic transmission in FS cells, but not in RSNP cells. To examine whether the reduced TC transmission could be induced by simply changing the input resistance (see Rin values in Table 1), I performed computational simulation studies (see methods). The simulation results showed that changes of input resistance alone (from 220 to 150 MΩ) had negligible effects on the amplitude of TC–EPSPs (<5 ± 1%, n = 10 trials; P > 0.1, e.g., Fig. 7B2, inset), which cannot account for the large differences seen in the recordings (61% decrease in the cocktail recordings and 57% decrease in ACSF alone).
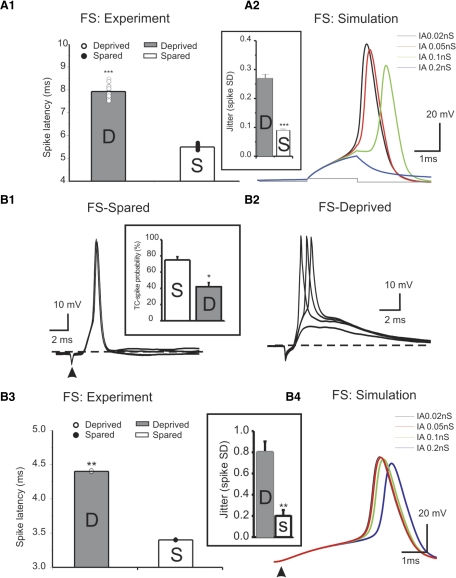
The timing of spikes from interneurons controls the cortical response (Gabernet et al. 2005). The latency to spike time is key in determining the role of interneurons in the cortical circuit. I next examined whether the current- induced spike latency in FS cells was altered. As shown in Fig. 8A1, there was a significant increase in the spike latency in deprived FS cells (n = 7 cells in deprived and spared, respectively, P < 0.001). The increase in spike latency was mimicked by increasing IA currents in simulated FS cells (Fig. 8A2). I next examined whether the TC- induced synchronization of spiking in FS cells was altered. In current-clamp mode, TC induced highly synchronized spikes in FS cells under control conditions (Beierlein et al. 2003; Cruikshank et al. 2007; Sun et al. 2006), as well as in sensory-spared cortices. In the sensory-deprived area, the TC–EPSCs were smaller (Fig. 7A2) and the probability of inducing spikes was significantly reduced (Fig. 8B1, inset). In addition, the suprathreshold spikes in sensory-deprived cortices showed greater jitter in spike time (Fig. 8, B2 vs. B1 and B3, inset). The SD of spike time was significantly larger than that recorded in sensory-spared areas (Fig. 8B3, inset, n = 5 in each groups, P < 0.01). In addition, there was significant increase in the latency of TC-induced spike as well (Fig. 8B3). This effect was mimicked by increasing IA currents in simulation experiments (Fig. 8B4). These results suggest that the effect of sensory deprivation on the strength of the TC synapse is dependent on the postsynaptic target—i.e., in FS cells the TC synapses exhibited robust down-regulation, whereas in RSNP cells it remained unchanged.
Fig. 8.
Effects of whisker trimming on spike induction in FS cells. A: whisker trimming prolongs spike latency in real FS cells (A1, n = 7 cells in each group) and increases spike jitter (A2 inset). In simulated FS cells, increase of IA stepwise gradually increases latency of spikes induced by direct depolarizing current (A2). B: example of thalamocortical (TC)-induced spikes in a representative spared (B1) and deprived cell (B2), respectively. Inset: TC-spike probability in spared (n = 5) vs. deprived (n = 5) cells. B3 and inset: the spike latency and spike jitter (inset) in deprived (n = 5) vs. spared cells (n = 5; **P < 0.01. B4: the effect of stepwise increasing IA on latency of spikes induced via TC inputs.
I performed additional experiments to record monosynaptic eEPSCs and sEPSCs in slices perfused with picrotoxin only. I have found that similar differences in the frequency of sEPSCs were still present (Table 3). The magnitude of sEPSCs did not exhibit significant differences. In addition, in the absence of CNQX, it is still possible to induce eEPSCs and measure the amplitude of eEPSCs, although polysynaptic excitatory events often followed the monosynaptic events (Supplemental Fig. S4). There were also significant changes in the amplitudes and CV of evoked eEPSCs (Table 3). Together with previous data, these results support a presynaptic hypothesis of the induced effects.
Concomitant reduction of VGlut2- and PV-immunoreactivity in sensory-deprived barrels
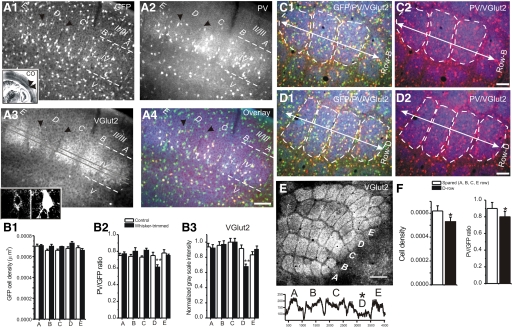
We have shown that whisker trimming induces down-regulation in PV expression and perisomatic inhibition (Jiao et al. 2006). In this study, electrophysiological recordings demonstrated reduced TC–excitatory synaptic currents in FS cells. To compare whether there were any correlations in structural and functional changes of glutamatergic terminals in FS (i.e., PV-positive) interneurons I took advantage of the selective labeling of TC nerve terminals by VGlut2 (Graziano et al. 2008) and examined whether the number of TC synapses was down-regulated in sensory-deprived row(s). Localization of barrels was described in an earlier publication (Jiao et al. 2006). Briefly, the CO staining method was used to identify barrels in an adjacent TC section (Fig. 8A1, inset). The darkness of the CO-positive barrels was examined to assist in the identification of sensory-deprived barrels. As shown in Fig. 9, there was a significant reduction of VGlut2-immunoreactivity in the sensory-deprived row (e.g., Fig. 9, A3, B2, and E). Concomitantly, the PV-expression levels, measured by the PV/GFP ratio as well as the density of PV-positive cells, were significantly reduced in the VGlut2- and CO-stained barrels (e.g., Fig. 9A2; see group data in Fig. 8B2, P < 0.01, n = 15 sections in five brains). In addition to total loss of PV-positive cells, the remainder of the PV-positive cells appeared to have reduced PV immunoreactivity, suggesting that similar to changes in firing properties, the down-regulation of FS cells was a common phenomenon among all PV-positive cells (see Jiao et al. 2006). To further validate this effect, I examined PV expression in flattened barrel cortex. In the right cerebral hemisphere, the entire barrel field can be visualized and the reduction of VGlut2 immunoreactivity in row D was apparent (Fig. 9E). In the sensory-deprived row (D), both the down-regulation of PV and the reduction in total PV-positive cells were clearly shown (Fig. 9, D vs. C). It appeared that there were more green cells (GFP-positive but PV-negative), due to reduced expression of PV. Confocal images of double immunohistochemical staining of PV and VGlut2 showed colocalization of VGlut2-positive puncta near the cell body region of the PV-positive cells (e.g., Fig. 9A3, inset). The reduction of PV but not GFP is similar to results reported earlier (Jiao et al. 2006). These results suggest that there is a concomitant reduction in the number of VGlut2-positive terminals (i.e., presumably the number of TC synapses) and PV expression in sensory-deprived barrels. Because PV expression is regulated by sensory activity (Jiao et al. 2006) and the main physiological role of PV in FS cells is to decrease network synchrony and oscillations (Schwaller et al. 2004), I suspect that the down-regulation of PV and the reduced excitability of FS cells were related to a common up-stream mechanism.
Fig. 9.
Immunohistochemical examination of the whisker-trimming-induced effects. A1: photomicrograph of glutamate decarboxylase 67–green fluorescent protein (GAD67-GFP) in a TC section (30 μm in thickness) from a row D whisker-trimmed GAD67-GFP mouse (A1). Rows A–E, barrel areas of layer IV barrel cortex, are shown. White dashed lines demarcate layer IV of the barrel cortex. Black arrowheads mark row D of barrel field. Inset: image of an entire adjacent TC section stained for cytochrome oxidase (CO). Note the reduction in CO staining in row D (black arrowheads). Scale bar = 150 μm (for A1–A4). A2–A4: the same brain section (40 μm in thickness) as that in A1. The sections were stained for parvalbumin (PV, A2) and vesicular glutamate transporter 2 (VGlut2, A3), respectively. A4 shows superimposed image of A1, A2, and A3. Inset in A3: confocal image of a VGlut2-IR (left) in a PV-positive cell (right); note that the VGlut2-IR is clustered around the cell body region. Small red arrowheads in A2 indicate weakly stained PV-IR positive neurons. B1: cell densities of GAD67-GFP positive cells in control (open bars) and row D whisker-trimmed (black bars) mice. There were no significant differences in different rows (n = 12 sections in 3 mice for each group) under any conditions. B2: statistical comparison of PV/GFP ratio in rows A–E barrels of whisker-trimming (black bars) and normal mice (white bars). Only neurons located within the boundary of the barrel were counted. B3: comparison of grayscale line intensity profile of the VGlut2 in rows A–E barrels of whisker-trimming (black bars) and normal mice (white bars). **P < 0.01; n = 5 brains in whisker-trimming and controls, respectively. C and D: photomicrograph of GFP/VGlut2/PV-IR (C1) or PV/VGlut2-IR (C2) in row B (C, spared) or D (D, deprived) of a flattened tangential section (30 μm in thickness) from the right cerebral hemisphere in a row D whisker-trimmed GAD67-GFP mouse. White dashed lines demarcate barrels in the layer IV of the barrel cortex. White arrow indicates the orientation of the row. Scale bar = 100 μm (for C and D). E, top: photomicrograph of VGlut2-IR showing rows A–E barrel areas of layer IV barrel cortex in the same mouse as that shown in C and D. Scale bar = 250 μm. Note that the VGlut2-IRs in row D are weaker. Bottom: grayscale line intensity profile of the VGlut2 across rows A–E showing changes in VGlut2-IR in row D. F: statistical comparison of PV density and PV/GFP ratio in row D vs. other rows.
DISCUSSION
Experience-dependent plasticity of intrinsic properties of FS neurons
My first significant finding is that sensory deprivation induces intrinsic plasticity in GABAergic interneurons in the primary sensory cortex. The next significant finding was that the effect was cell-type specific: i.e., although both RSNP and FS cells were located in the same deprived area, only intrinsic properties of FS cells were affected by sensory deprivation. So far, the mechanism underlying sensory-dependent intrinsic plasticity is unclear (Nelson and Turrigiano 2008). In this study, I investigated circuit level and ionic mechanisms. My other significant findings were: 1) changes in the intrinsic properties of FS cells were accompanied by robust remodeling of TC synapses and PV expression and 2) changes in the intrinsic properties of FS cells were occluded by a very low concentration of the IA channel antagonist 4-AP and mimicked by increasing IA in computational simulations. These results support a contribution from the TC pathway and glutamate to sensory-induced activity-dependent intrinsic plasticity of inhibitory networks in the barrel cortex.
Previous studies demonstrated that changes in the expression level of distinct Kv3 channels contributed to postnatal maturation of the electrical properties of FS cells (Lau et al. 2000). So far, it remains unclear what the contributions of whisker inputs to the maturation of the intrinsic properties of interneurons in the somatosensory cortex are. Several indirect pieces of evidence indicate that sensory experience may play such a role. Simons and colleagues showed that the firing rates of FS cells are negatively modified by sensory deprivation in vivo (Lee et al. 2007). They pointed out that it is unknown whether the effect is due to changes in intrinsic firing properties, such as increased firing threshold (Barth et al. 2004; Lee et al. 2007), or to synaptic changes, such as reduced intracortical inhibition (Jiao et al. 2006). Results shown here suggest that whisker experiences can directly modulate the intrinsic properties of FS cells.
Interestingly, the rapid depolarization characteristics of FS phenotypes, which are one of the most important parameters distinguishing FS from RSNP cells, were not altered by sensory experiences. Despite the fact that RS and FS cells both emerge from immature multiple-spiking (IMS) neurons during the first 2 postnatal weeks (Massengill et al. 1997), there were no effects of whisker trimming on RSNP interneurons (Table 2 and Supplemental data). This suggests that different mechanisms (i.e., different molecular and input-dependent mechanisms such as intracortical vs. TC) may contribute to the maturation of FS and RSNP firing phenotypes. With respect to layer IV inhibitory circuits, we have thoroughly examined the developmental periods for inducing plasticity of inhibitory circuits (Jiao et al. 2006). We have found that a prolonged whisker-trimming duration was required (i.e., from P7 to P20) to induce plasticity of the inhibitory network. In this study, we continued with this protocol. The protocol of prolonged whisker trimming is widely used by a number of groups (Drew and Feldman 2008; Lee et al. 2007). In summary, results shown here clearly demonstrate that maturation of intrinsic properties in FS (but not RSNP interneurons) of cortical layer IV requires intact whisker inputs. In small subgroup of FS cells (i.e., FS cells with diffuse-type multipolar dendrites), there were significant decreases in dendritic arbors (Fig. 1B3, n = 3 in each group). However, in another FS subtype (cells with bipolar-like dendrites, e.g., Fig. 1A, n = 3 in each group), there were no differences in dendritic morphologies. In both groups, the firing pattern and intrinsic pattern were significantly affected by whisker trimming in a similar manner (e.g., Fig. 2). Thus it appears that changes in dendritic length appear to be independent of changes in intrinsic and firing properties. In addition, because the only differences group B FS cells in dendritic arbors occurred at >200 μm away from the soma, it is unlikely that this change alone had significant effects on somatic firing properties. This idea is supported by the fact that there were no differences in single AP properties between deprived and spared groups. However, whether active dendritic properties are changed at distal dendrites is an open question suitable for future investigations.
Intrinsic plasticity induced by activity-dependent versus homeostatic mechanisms
The next question to be resolved by this study is whether the intrinsic plasticity of FS cells is induced by an activity-dependent or homeostatic mechanism. A homeostatic form of plasticity can be thought of as one that acts to stabilize neuronal activity in the face of perturbations (reviewed by Nelson and Turrigiano 2008). Circuit-wide rapid homeostatic plasticity was first described in the visual cortex, where brief visual deprivation alters the excitation and inhibition balance in visual cortex layer IV (Maffei et al. 2004). In the visual cortex layer 2/3, one visual deprivation paradigm (i.e., lid suture) but not another (i.e., intraocular TTX) raised the intrinsic excitability of pyramidal neurons by reducing the threshold of APs and increasing input-spike rates (Maffei and Turrigiano 2008). In these studies, the enhancement of intrinsic excitability is mediated by homeostatic mechanisms in which the overall excitability of a local network is raised to compensate for the lack of exogenous inputs (Maffei et al. 2004; Turrigiano and Nelson 2004). These results suggest that within cortical circuits, the forms of plasticity (e.g., intrinsic vs. synaptic, excitation vs. inhibition, etc.) are determined by the sensory deprivation paradigm as well as cortical layers. Similar homeostatic intrinsic plasticity results have been reported in the auditory system, where hearing loss raised intrinsic biophysical excitabilities (Kotak et al. 2005; Xu et al. 2007).
The intrinsic plasticity of FS interneurons in barrel cortex appears to be different from that reported in principal neurons of visual and auditory cortices. Changes in homeostatic mechanisms have been closely linked with removal of glutamatergic inputs (Maffei et al. 2004; Turrigiano and Nelson 2004) or decreased firing rate in pyramidal neurons (Ibata et al. 2008). In my case, whisker trimming reduced only the amount of TC inputs (Fig. 7 and Table 3). The probability of spike induction was modestly reduced in FS cells by about 30% (Fig. 7B1; see similar results by Celikel et al. 2004). Because the excitability of inhibitory cells was reduced, this could lead to a disinhibitory effect in the deprived barrels (cf. Jiao et al. 2006). In fact, a compensatory increase in the circuit excitability in the deprived area has been documented in vivo (Lee et al. 2007). This situation is clearly opposite from what occurred in the visual and auditory cortices described earlier. Celikel et al. (2004) showed that D-row whisker trimming caused a reversal of firing order for layer 4 and layer 2/3 neurons and substantial decorrelation of spike trains. In contrast, the spike rate changed only modestly.
This study supports a conclusion drawn by Celikel et al. (2004) who suggested that whisker deprivation is likely to drive map plasticity by spike-timing-dependent mechanisms. In another very recent study, using intrinsic signal imaging and in vivo electrophysiological recordings, Drew and Feldman (2008) concluded that D-row whisker deprivation selectively engages the depression component of map plasticity primarily via an activity-dependent and intracolumnar mechanism. My data here clearly support this idea. I therefore conclude that the intrinsic plasticity of FS cells occurred in an activity-dependent manner and that selective D-row whisker trimming reprograms the activity-dependent intrinsic plasticity of FS cells. In an earlier study, we investigated the time period required to produce robust plasticity in FS cells (Jiao et al. 2006). We found that prolonged whisker deprivation is required (>2 wk) to produce robust and persistent FS cell plasticities. In a pilot study (data not shown), I also examined whether such a long deprivation period is required to produce intrinsic plasticity. I did not find significant differences in intrinsic properties between spared and deprived FS cells at the third postnatal week (with whisker trimming starting at the second postnatal week). Unfortunately, because the maturation of intrinsic properties FS cells also occurs between the second postnatal week through the fourth postnatal week, it is still possible that a much larger n number will be required to reach statistical differences, if any. It appears that the effects on intrinsic firing are more related to promoting rearrangement of the size of the barrel (or functional receptive field of D-row), to facilitate expansion of adjacent barrels. Both our earlier anatomical results (Jiao et al. 2006) and in vivo physiological recordings support this idea (Drew and Feldman 2008; Shoykhet et al. 2005).
Roles of glutamate in the activity-dependent plasticity of inhibitory circuits
Neonatal sensory deprivation induced by whisker trimming significantly affects the functional organization of receptive fields in the adult barrel cortex (reviewed by Feldman and Brecht 2005; Lee et al. 2007). Sadaka and colleagues (2003) reported a 43% reduction in the numerical density of TC synapses after deprivation. In addition, whisker trimming appears to affect TC synapses equally in excitatory and inhibitory neurons. My results regarding the strength of TC–FS synapses are consistent with this idea. In addition, the magnitude of reduction in amplitude of TC–EPSCs in FS cells is 61% (Fig. 7 and Table 3), which is larger than the reduction of numerical density of TC synapses reported in rats (Graziano et al. 2008). The changes in the strength of TC–EPSCs and sEPSCs in FS cells correspond well with previously described activity-dependent changes that rely on basket cells, including the level of PV expression (e.g., Fig. 8 and Jiao et al. 2006), and the number and the strength of perisomatic GABAergic synapses (Jiao et al. 2006). Although changes in the strength of glutamatergic synapses alone do not provide direct evidence with regard to the mechanisms underlying the sensory-dependent plasticity of FS networks, these results are consistent with the idea that glutamate may contribute to experience-dependent plasticity of cortical networks, which has been demonstrated in visual (Bender et al. 2006; Kirkwood et al. 1996) and barrel cortical principal neurons (Bender et al. 2006; Clem et al. 2008). Similarly, the double-negative effects in RSNP cells (i.e., no effects of sensory deprivation on the TC–RSNP synapses and firing properties) also support this idea. In addition, it indicates the target-specific effects in whisker-induced plasticities. Possible mechanisms may involve differential expression of specific glutamate receptors (i.e., AMPA, NMDA, mGluRs) and their downstream effects on specific inhibitory cells (cf. Clem et al. 2008; Kumar and Huguenard 2003).
Ionic mechanisms underlying the effects of whisker experiences on intrinsic firing properties of FS cells
Among all parameters, firing frequencies (including a rightward shift of the F–I curve), AP threshold, and SAI have the greatest differences among the sensory-spared and sensory-deprived FS cells (Table 1 and Fig. 3). The parameters related to the properties of a single AP waveform (half-width, rise and decay slopes, and amplitudes) showed no significant differences between the two groups. These results suggest that ionic conductances important for the modulation of spike train properties may be regulated by sensory experience. Several voltage-gated potassium channels are known to be important in regulating AP waveforms. Among these channels, Kv4.2 and Kv4.3 channels are expressed at a modest level in neocortex layer IV (Burkhalter et al. 2006; Serodio and Rudy 1998). These channels are found predominantly in the somatodendritic site, where they operate at subthreshold potentials and contribute to the IA currents in inhibitory interneurons (Bourdeau et al. 2007; Burkhalter et al. 2006; Song et al. 1998). At very low does (1 mM), 4-AP enhanced the spike frequencies and abolished the differences in firing frequencies between sensory-spared and sensory-deprived cells. In addition, there was a significant up-regulation of 4-AP-sensitive currents in the sensory-deprived FS cells, suggesting that 4-AP-sensitive channels, with biophysical properties consistent with IA channels, were up-regulated in the sensory-deprived area. In computational simulation experiments, increased IA mimicked the effects of F–I and increased firing threshold (Fig. 5). This evidence suggests that IA currents underlie the effects of sensory deprivation on repetitive firing. In simulation studies, an increase in IA expression by onefold (from 0.01 to 0.02 nS) caused significant changes in the F–I curve and AP threshold, although it had very little effect on AP half-width (Fig. 5C3). This result suggests that up-regulation of IA alone can explain virtually all of the observed changes in the intrinsic properties of FS cells. This result also suggests that a small amount of IA, such as that in the spared cells, is sufficient to maintain the half-width value. Up-regulation of IA may cause a small down-regulation of other voltage-gated channels, which can explain why 4-AP had greater effects on the AP width in sensory-deprived cells.
To date, intrinsic plasticities have been described primarily in principal neurons in the visual (Cudmore and Turrigiano 2004; Maffei and Turrigiano 2008; Maffei et al. 2004), auditory (Xu et al. 2007), and somatosensory cortices (Barth et al. 2004). In these studies, the intrinsic plasticities exhibited either enhanced or reduced excitabilities and are induced via different physiological mechanisms (e.g., sensory deprivation or acute long-term pairing), respectively. To my knowledge, the ionic mechanisms underlying intrinsic plasticity in the aforementioned studies are unclear. Changes in firing latency and spike adaptation in principal interneurons have been reported in principal neurons (Cudmore and Turrigiano 2004; Xu et al. 2007). These changes are similar (although maybe in the opposite direction) to changes reported here; therefore it is possible that IA currents may be involved as well. A broad review of the literature to include intrinsic plasticities that occurred in other CNS regions reveals that many different ion channels have been implicated [i.e., delayed outward rectifier K+ currents, sIAHP, IK(Ca) BK type, Na+ channels, Ca2+ channels, and Ih channels; Zhang and Linden 2003]; interestingly, IA currents have not been generally implicated in intrinsic plasticities.
GRANTS
This research was supported by National Institute of Neurological Disorders and Stroke Grant 5R01-NS-057415.
Supplementary Material
ACKNOWLEDGMENTS
We thank M. Sikora for assistance with the computational modeling; Dr. Yuchio Yanagawa (Department of Genetic and Behavioral Neuroscience, Gunma University Graduate School of Medicine) for the generous gift of GAD67-GFP mice; C. Zhang and Y. Jiao for excellent assistance with immunohistochemical experiments; and A. Young for providing editorial assistance.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Agmon A, Connors BW. Thalamocortical responses of mouse somatosensory (barrel) cortex in vitro. Neuroscience 41: 365–379, 1991 [DOI] [PubMed] [Google Scholar]
- Bacci A, Huguenard JR, Prince DA. Differential modulation of synaptic transmission by neuropeptide Y in rat neocortical neurons. Proc Natl Acad Sci USA 99: 17125–17130, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth AL, Gerkin RC, Dean KL. Alteration of neuronal firing properties after in vivo experience in a FosGFP transgenic mouse. J Neurosci 24: 6466–6475, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beierlein M, Gibson JR, Connors BW. Two dynamically distinct inhibitory networks in layer 4 of the neocortex. J Neurophysiol 90: 2987–3000, 2003 [DOI] [PubMed] [Google Scholar]
- Bender KJ, Allen CB, Bender VA, Feldman DE. Synaptic basis for whisker deprivation-induced synaptic depression in rat somatosensory cortex. J Neurosci 26: 4155–4165, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdeau ML, Morin F, Laurent CE, Azzi M, Lacaille JC. Kv4.3-mediated A-type K+ currents underlie rhythmic activity in hippocampal interneurons. J Neurosci 27: 1942–1953, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecht M, Sakmann B. Dynamic representation of whisker deflection by synaptic potentials in spiny stellate and pyramidal cells in the barrels and septa of layer 4 rat somatosensory cortex. J Physiol 543: 49–70, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhalter A, Gonchar Y, Mellor RL, Nerbonne JM. Differential expression of I(A) channel subunits Kv4.2 and Kv4.3 in mouse visual cortical neurons and synapses. J Neurosci 26: 12274–12282, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celikel T, Szostak VA, Feldman DE. Modulation of spike timing by sensory deprivation during induction of cortical map plasticity. Nat Neurosci 7: 534–541, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Higashiyama H, Knott GW, Kuhlman SJ, Welker E, Huang ZJ. Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. J Neurosci 24: 9598–9611, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clem RL, Celikel T, Barth AL. Ongoing in vivo experience triggers synaptic metaplasticity in the neocortex. Science 319: 101–104, 2008 [DOI] [PubMed] [Google Scholar]
- Crair MC, Gillespie DC, Stryker MP. The role of visual experience in the development of columns in cat visual cortex. Science 279: 566–570, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudmore RH, Turrigiano GG. Long-term potentiation of intrinsic excitability in LV visual cortical neurons. J Neurophysiol 92: 341–348, 2004 [DOI] [PubMed] [Google Scholar]
- Drew PJ, Feldman DE. Intrinsic signal imaging of deprivation-induced contraction of whisker representations in rat somatosensory cortex. Cereb Cortex 19: 331–348, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman DE, Brecht M. Map plasticity in somatosensory cortex. Science 310: 810–815, 2005 [DOI] [PubMed] [Google Scholar]
- Gabernet L, Jadhav SP, Feldman DE, Carandini M, Scanziani M. Somatosensory integration controlled by dynamic thalamocortical feed-forward inhibition. Neuron 48: 315–327, 2005 [DOI] [PubMed] [Google Scholar]
- Graziano A, Liu XB, Murray KD, Jones EG. Vesicular glutamate transporters define two sets of glutamatergic afferents to the somatosensory thalamus and two thalamocortical projections in the mouse. J Comp Neurol 507: 1258–1276, 2008 [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period mechanisms in developing visual cortex. Curr Top Dev Biol 69: 215–237, 2005 [DOI] [PubMed] [Google Scholar]
- Huang ZJ, Di Cristo G, Ango F. Development of GABA innervation in the cerebral and cerebellar cortices. Nat Rev Neurosci 8: 673–686, 2007 [DOI] [PubMed] [Google Scholar]
- Jackson AC, Bean BP. State-dependent enhancement of subthreshold A-type potassium current by 4-aminopyridine in tuberomammillary nucleus neurons. J Neurosci 27: 10785–10796, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Huang ZJ, Morales B, Kirkwood A. Maturation of GABAergic transmission and the timing of plasticity in visual cortex. Brain Res Brain Res Rev 50: 126–133, 2005 [DOI] [PubMed] [Google Scholar]
- Jiao Y, Zhang C, Yanagawa Y, Sun Q-Q. Major effects of sensory experiences on the neocortical inhibitory circuits. J Neurosci 26: 8691–8701, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood A, Rioult MC, Bear MF. Experience-dependent modification of synaptic plasticity in visual cortex. Nature 381: 526–528, 1996 [DOI] [PubMed] [Google Scholar]
- Kotak VC, Fujisawa S, Lee FA, Karthikeyan O, Aoki C, Sanes DH. Hearing loss raises excitability in the auditory cortex. J Neurosci 25: 3908–3918, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar SS, Huguenard JR. Pathway-specific differences in subunit composition of synaptic NMDA receptors on pyramidal neurons in neocortex. J Neurosci 23: 10074–10083, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau D, Vega-Saenz de Miera EC, Contreras D, Ozaita A, Harvey M, Chow A, Noebels JL, Paylor R, Morgan JI, Leonard CS, Rudy B. Impaired fast-spiking, suppressed cortical inhibition, and increased susceptibility to seizures in mice lacking Kv3.2 K+ channel proteins. J Neurosci 20: 9071–9085, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Land PW, Simons DJ. Layer- and cell-type-specific effects of neonatal whisker-trimming in adult rat barrel cortex. J Neurophysiol 97: 4380–4385, 2007 [DOI] [PubMed] [Google Scholar]
- Ma Y, Hu H, Berrebi AS, Mathers PH, Agmon A. Distinct subtypes of somatostatin-containing neocortical interneurons revealed in transgenic mice. J Neurosci 26: 5069–5082, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei A, Nataraj K, Nelson SB, Turrigiano GG. Potentiation of cortical inhibition by visual deprivation. Nature 443: 81–84, 2006 [DOI] [PubMed] [Google Scholar]
- Maffei A, Nelson SB, Turrigiano GG. Selective reconfiguration of layer 4 visual cortical circuitry by visual deprivation. Nat Neurosci 7: 1353–1359, 2004 [DOI] [PubMed] [Google Scholar]
- Maffei A, Turrigiano GG. Multiple modes of network homeostasis in visual cortical layer 2/3. J Neurosci 28: 4377–4384, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainen ZF, Sejnowski TJ. Influence of dendritic structure on firing pattern in model neocortical neurons. Nature 382: 363–366, 1996 [DOI] [PubMed] [Google Scholar]
- Massengill JL, Smith MA, Son DI, O'Dowd DK. Differential expression of K4-AP currents and Kv3.1 potassium channel transcripts in cortical neurons that develop distinct firing phenotypes. J Neurosci 17: 3136–3147, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales B, Choi SY, Kirkwood A. Dark rearing alters the development of GABAergic transmission in visual cortex. J Neurosci 22: 8084–8090, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SB, Turrigiano GG. Strength through diversity. Neuron 60: 477–482, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaka Y, Weinfeld E, Lev DL, White EL. Changes in mouse barrel synapses consequent to sensory deprivation from birth. J Comp Neurol 457: 75–86, 2003 [DOI] [PubMed] [Google Scholar]
- Schwaller B, Tetko IV, Tandon P, Silveira DC, Vreugdenhil M, Henzi T, Potier MC, Celio MR, Villa AE. Parvalbumin deficiency affects network properties resulting in increased susceptibility to epileptic seizures. Mol Cell Neurosci 25: 650–663, 2004 [DOI] [PubMed] [Google Scholar]
- Serodio P, Rudy B. Differential expression of Kv4 K+ channel subunits mediating subthreshold transient K+ (A-type) currents in rat brain. J Neurophysiol 79: 1081–1091, 1998 [DOI] [PubMed] [Google Scholar]
- Shoykhet M, Land PW, Simons DJ. Whisker trimming begun at birth or on postnatal day 12 affects excitatory and inhibitory receptive fields of layer IV barrel neurons. J Neurophysiol 94: 3987–3995, 2005 [DOI] [PubMed] [Google Scholar]
- Song WJ, Tkatch T, Baranauskas G, Ichinohe N, Kitai ST, Surmeier DJ. Somatodendritic depolarization-activated potassium currents in rat neostriatal cholinergic interneurons are predominantly of the A type and attributable to coexpression of Kv4.2 and Kv4.1 subunits. J Neurosci 18: 3124–3137, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonner PM, Stern JE. Functional role of A-type potassium currents in rat presympathetic PVN neurones. J Physiol 582: 1219–1238, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryker MP. Postnatal development of ocular dominance columns in layer IV of the cat's visual cortex and the effects of monocular deprivation. Arch Ital Biol 116: 420–426, 1978 [PubMed] [Google Scholar]
- Sun Q-Q. The missing piece in the “use it or lose it” puzzle: is inhibition regulated by activity or does it act on its own accord? Rev Neurosci 18: 295–310, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q-Q, Huguenard JR, Prince DA. Reorganization of barrel circuits leads to thalamically-evoked cortical epileptiform activity. Thalamus Relat Syst 3: 261–273, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q-Q, Huguenard JR, Prince DA. Barrel cortex microcircuits: thalamocortical feedforward inhibition in spiny stellate cells is mediated by a small number of fast-spiking interneurons. J Neurosci 26: 1219–1230, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q-Q, Zhang Z, Jiao Y, Zhang C, Szabo G, Erdelyi F. Differential metabotropic glutamate receptor expression and modulation in two neocortical inhibitory networks. J Neurophysiol 101: 2679–2692, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci 5: 97–107, 2004 [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Ordered arrangement of orientation columns in monkeys lacking visual experience. J Comp Neurol 158: 307–318, 1974 [DOI] [PubMed] [Google Scholar]
- Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res 17: 205–242, 1970 [DOI] [PubMed] [Google Scholar]
- Xiang Z, Huguenard JR, Prince DA. GABAA receptor-mediated currents in interneurons and pyramidal cells of rat visual cortex. J Physiol 506: 715–730, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Kotak VC, Sanes DH. Conductive hearing loss disrupts synaptic and spike adaptation in developing auditory cortex. J Neurosci 27: 9417–9426, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Linden DJ. The other side of the engram: experience-driven changes in neuronal intrinsic excitability. Nat Rev Neurosci 4: 885–900, 2003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.