Abstract
Learning is known to cause alterations in intrinsic cellular excitability but, to date, these changes have been seen only after multiple training trials. A powerful learning task that can be quickly acquired and extinguished with a single trial is fear conditioning. Rats were trained and extinguished on a hippocampus-dependent form of fear conditioning to determine whether learning-related changes in intrinsic excitability could be observed after a few training trials and a single extinction trial. Following fear training, hippocampal slices were made and intrinsic excitability was assayed via whole cell recordings from CA1 neurons. Alterations in intrinsic excitability, assayed by the postburst afterhyperpolarization and firing frequency accommodation, were observed after only three trials of contextual or trace-cued fear conditioning. Animals that had been trained in contextual and trace-cued fear were then extinguished. Context fear-conditioned animals extinguished in a single trial and the changes in intrinsic excitability were reversed. Trace-cue conditioned animals only partially extinguished in a single trial and reductions in excitability remained. Thus a single learning experience is sufficient to alter intrinsic excitability. This dramatically extends observations of learning-specific changes in intrinsic neuronal excitability previously observed in paradigms requiring many training trials, suggesting the excitability changes have a basic role in acquiring new information.
INTRODUCTION
An important regulator of intrinsic excitability is the postburst afterhyperpolarization (AHP), a calcium-dependent potassium current that hyperpolarizes the membrane following a burst of action potentials (APs) (Alger and Nicoll 1980; Hotson and Prince 1980; Schwartzkroin and Stafstrom 1980). Learning-related increases in cellular excitability have been demonstrated across a variety of species, tasks, and brain regions (for review, see Disterhoft and Oh 2006). In these studies, an animal is trained in a task and cellular excitability is assessed by measuring the AHP and accommodation. Learning-related increases in excitability have been demonstrated following trace eyeblink conditioning in hippocampus CA1 (Moyer et al. 1996) and CA3 (Thompson et al. 1996) in rabbits and CA1 of rats (Kuo et al. 2008). Spatial learning is correlated with reductions in the AHP, as measured in rats following water maze learning (Oh et al. 2003; Tombaugh et al. 2005). Increases in excitability are also observed in piriform cortex following odor-discrimination learning in rats (Saar et al. 1998). The above-cited tasks all require numerous repeated trials before intrinsic plasticity is seen, although rapid changes in intrinsic excitability can be elicited using in vitro preparations (Kaczorowski et al. 2007; Zhang and Linden 2003). Changes in intrinsic excitability have not yet been demonstrated after a single learning experience.
Fear conditioning takes advantage of an animal's natural fear response by pairing a neutral stimulus, such as an environment or a tone, with an aversive stimulus, such as a shock. Although the amygdala is critical for learning this task (Blanchard and Blanchard 1972; LeDoux 2000), certain forms also require the hippocampus. The hippocampus is required for learning contextual (Kim and Fanselow 1992) and trace-cued (McEchron et al. 1998) fear conditioning and is important in the extinction of contextual fear conditioning (Corcoran et al. 2005). In neurons of the infralimbic prefrontal cortex, the AHP and accommodation are increased after learning (Santini et al. 2008). A learning-related decrease in excitability in these cells is consistent with the region's potential role in providing a tonic brake on fear expression in the amygdala (Corcoran and Maren 2001; Maren and Holt 2000). Extinction reversed the increase in the AHP (Santini et al. 2008). Middle-aged mice that learn contextual fear conditioning have reduced AHPs in CA1 pyramidal neurons compared with controls that failed to learn (Kaczorowski and Disterhoft 2009).
We sought to determine whether learning hippocampus-dependent forms of fear conditioning using very few training trials is accompanied by altered excitability of CA1 pyramidal neurons. We found that the AHP was reduced after both trace-cued and contextual fear conditioning compared with control conditions. In addition, the learning-related increase in excitability was reset to naïve levels after a single extinction trial, at a time point when freezing to the learned context was abolished. These data are a compelling demonstration that intrinsic excitability, as assayed by the AHP and firing frequency accommodation, can be modulated by a single experience.
METHODS
Animals
Two- to 3-mo-old males of the F1 generation of Fisher 344 X Brown Norway rats (Harlan, Indianapolis, IN) were used. Fifty-nine animals were trained in the study, divided between three cohorts, plus 6 naïve animals. Rats were housed in small groups with unrestricted access to food and water on a 14/10-h light/dark cycle. Rats were handled and housed in accordance with the standards established by the Institutional Animal Care and Use Committee of Northwestern University and National Institutes of Health.
Behavior
Rats were divided into three cohorts; each cohort was subdivided between the behavioral paradigms. The Trained cohort was trained in the fear task for three trials spaced over 2 days, then killed on day 3 with no additional testing. The Tested cohort was trained in the fear task for three trials over 2 days, given a single testing trial for both tone and context on the second day, then killed on day 3. The Extinguished cohort were also trained in the fear task for three trials over 2 days and were tested/extinguished for both the tone and context three times over days 2 and 3. Hippocampal slices were made from the Extinguished cohort 18–24 h after the final testing trial.
Within the cohorts, animals were randomly assigned to tone alone (Tone), shock alone (Shock), or paired (Paired) training paradigms; the Extinguished cohort did not include a tone alone group. In each training trial, Tone animals were presented with one tone, Shock animals were presented with one shock, and Paired animals received a tone and shock separated by a 30-s “trace” stimulus-free interval. Naïve animals were neither handled nor exposed to the training context.
Training took place in a 40 × 40 × 40-cm clear plastic box with a grid floor and an open top in a curtained 3 × 3-m room with movable cues. The stimuli presented consisted of a 15-s, 4-kHz, 75-dB tone and a 1-s, 0.8-mA shock delivered through the floor, as appropriate. All trials lasted 226 s, with the tone (if used) presented 90 s after the rat was placed in the chamber and the shock (if used) presented 136 s after the rat was placed in the chamber. Testing was divided into two parts, each also lasting 226 s. First, animals were tested for contextual learning, during which time they were placed in the original context, no stimuli were presented, and freezing was measured. To test cue learning, animals were placed in a clear plastic chamber in which a new floor had been placed (wood shavings on a plastic floor), the distal cues were changed, a novel scent (banana extract) was added, and the lighting was altered. The tone stimulus was presented in this novel context and freezing was measured.
Freezing behavior was video recorded for the duration of training and testing sessions and analyzed using FreezeFrame software (Actimetrics, Wilmette, IL). FreezeFrame uses a pixel-based algorithm to detect motion. The minimum freezing bout length was set at 1 s; the movement threshold was determined based on a histogram analysis of total movement during the entire 226-s trial. This allowed each animal's freezing threshold to be standardized to its overall activity level. Freezing behavior during training and the context test was averaged over the 135 s preceding the shock onset. Freezing behavior during the tone test was averaged over the 135 s following the tone presentation.
Electrophysiological recordings
Eighteen to 24 h after training and testing were complete, rats were anesthetized by isoflurane and decapitated. The brains were quickly removed and immersed in ice-cold artificial cerebrospinal fluid (aCSF), consisting of (in mM) 124 NaCl, 1.25 NaH2PO4, 2.5 KCl, 26 NaHCO3, 25 glucose, 2.4 CaCl2, and 2.0 MgSO4. These solutions were saturated with 95% O2-5% CO2 to maintain a pH of 7.4 and to oxygenate slices. The dorsal half of the hippocampus was dissected out and sliced into 300-μm-thick sections using a Leica vibratome. Slices were held at 34°C for 30 min and then allowed to sit at room temperature (∼22°C) until biophysical recordings were made (≥1 h after slicing). The experimenter was blind to the training status of the animal during recording and analysis.
CA1 pyramidal neurons were visually identified for whole cell current-clamp recordings. Recordings were made at 32°C. Patch electrodes contained (in mM): 120 KMeSO4, 10 KCl, 10 HEPES, 10 phosphocreatine sodium salt, 4 ATP magnesium salt, 0.4 GTP sodium salt, and 0.5% neurobiotin, with pH corrected to 7.4 with KOH and osmolarity of 285 ± 5 mOsm. Neurons were included if they had a resting membrane potential of less than −58 mV, an input resistance >25 MΩ, AP amplitude of >80 mV from rest, and stable series resistance of <20 MΩ. Electrode capacitance and series resistance were monitored and compensated throughout recording; cells were held at or near −65 mV with injected current. Data were collected using a Dagan BVC-700 amplifier and pClamp 9.2 (Axon) and digitized using a Digidata 1322A A-to-D converter. Data were analyzed using pClamp 9.2 (Axon).
The AHP was elicited by injecting 2-ms current pulses of 1.8 nA, 15 times, at 50 Hz to elicit 15 APs. The amplitude of the AHP was measured relative to baseline at its peak (peak AHP) and 1 s following the end of the final AP (slow AHP). The integrated area of the AHP was measured from the end of the last AP in the train until the membrane potential returned to baseline levels. Accommodation was measured by giving a 1,000-ms current step of sufficient strength to elicit five APs in the first 100 ms. The total number of APs in the entire step was counted. A single AP was elicited to measure the spike threshold, rheobase, and amplitude. Current–voltage (I–V) relations were studied using an 800-ms current injection (−0.3 to +0.1 nA). Sag was measured as the difference between the peak and steady-state hyperpolarization in response to an 800-ms, −300-pA current step. The input resistance was calculated as the slope of the line of the current plotted against the voltage at the last 100 ms of the current step.
Statistics
Statistics were performed using Microsoft Excel and StatView. Differences were evaluated using t-test, one-way ANOVA, repeated-measures ANOVA, and Fisher's protected least significant difference post hoc tests where appropriate. All data are reported as means ± SE.
RESULTS
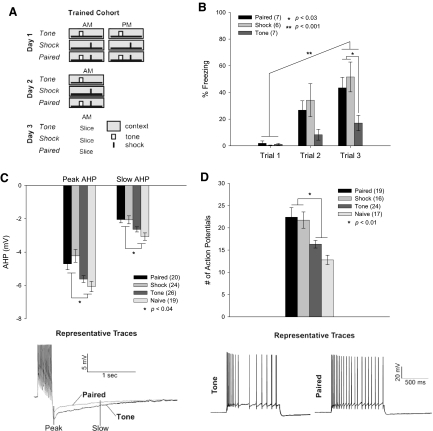
Young adult male rats were divided into Trained, Tested, and Extinguished cohorts. Within cohorts, groups were Naïve, tone-contextual control (Tone), contextually conditioned (Shock), or trace-cue conditioned (Paired). In each group, rats were placed in the training apparatus for three 226-s trials spaced over 2 days. A trial consisted of a 90-s baseline, a 15-s tone, 30-s trace interval, 1-s shock, and a 90-s postshock period, as appropriate (Figs. 1A, 2, A and D). Fear was measured by freezing. In the Trained cohort, Shock and Paired groups showed contextual fear conditioning as measured during each training session in the baseline period prior to the shock (Fig. 1B) [repeated-measures ANOVA, Paired: F(2,10) = 16.744, P = 0.0006; Shock: F(2,10) = 10.496, P = 0.004]. Rats from the Tested cohort also developed contextual fear conditioning during training (Fig. 2B) [repeated-measures ANOVA, Paired: F(2,14) = 20.504, P < 0.0001; Shock: F(2,10) = 6.551, P = 0.02], as did rats from the Extinguished cohort (Fig. 2E) [repeated-measures ANOVA, Paired: F(2,18) = 13.955, P = 0.0002; Shock: F(2,16) = 6.412, P = 0.009]. Rats from the Tested and Trained cohorts also showed contextual learning compared with the tone-alone group during the final trial [ANOVA, Trained: F(2,17) = 4.775, P = 0.023; Tested: F(2,17) = 3.793, P = 0.04]. Detailed freezing averages are listed in Supplemental Table S1.1
Fig. 1.
Learning trace and contextual fear increases intrinsic excitability. A: training paradigm for Trained cohort. In each training session, Tone animals were presented with one tone (small white box), Shock animals were presented with one shock (small black dash), and Paired animals received a tone and shock separated by a 30-s “trace” stimulus-free interval. Naïve animals were not handled or exposed to the training context. B: Paired and Shock groups froze more across the training sessions and also froze significantly more than Tone animals in the last training session, indicating robust contextual learning. All bar graphs show means ± SE. C: cells from Paired and Shock animals had increased intrinsic excitability compared with Tone or Naïve animals as measured by the peak and 1-s amplitude of the afterhyperpolarization (AHP). Example traces from Paired and Tone animals are shown (peak AHP: Paired −4.70 ± 0.36 mV, Shock −4.23 ± 0.38 mV, Tone −5.62 ± 0.21 mV, Naïve −6.07 ± 0.31 mV; slow AHP: Paired −2.05 ± 0.19 mV, Shock −2.06 ± 0.24 mV, Tone −2.64 ± 0.15 mV, Naïve −3.07 ± 0.2 mV). D: cells from Shock and Paired animals showed decreased accommodation by firing more action potentials (APs) during a 1-s current step sufficient to produce 5 APs in the first 100 ms, with no difference in the current step (P = 0.23). Example traces from Paired and Tone animals are shown (Paired 22.4 ± 2.1 APs, Shock 21.7 ± 1.8 APs, Tone 16.3 ± 0.9 APs, Naïve 12.8 ± 1.1 APs).
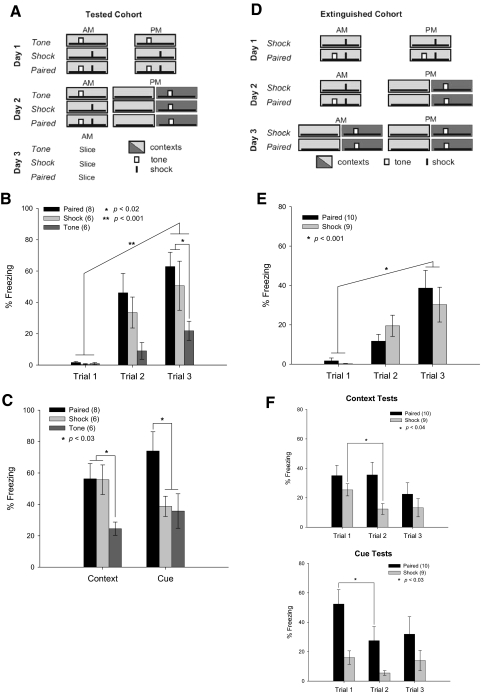
Fig. 2.
A single testing session confirms learning, but also extinguishes contextual freezing. A: training paradigm for the Tested cohort. Testing sessions consisted of 226 s in the original context (light gray), with no other stimuli, followed 15 min later by exposure to the tone in a novel context (dark gray). B: Paired and Shock groups froze more across the training sessions and also froze significantly more than Tone animals in the last training session, indicating robust contextual learning. C: all animals from the Tested cohort were tested for contextual and cue learning. Freezing during the testing session of the Tested cohort revealed robust contextual learning in Shock and Paired groups compared with the Tone group. Only Paired animals exhibited freezing behavior in response to the cue. D: training paradigm for the Extinguished cohort. E: Paired and Shock groups froze more across the training sessions, indicating robust contextual learning. F: during the first testing session, freezing was the same as that for the Tested cohort—i.e., Shock and Paired groups learned the context, whereas only Paired learned the cue. In the second testing session, Paired animals continued to freeze to the context, but showed extinction to the cue. Shock animals exhibited extinction of contextual learning after only a single testing session; this was the time point at which physiologic recordings were made in the Tested cohort. Although it appears that the Shock animals froze less to the cue in the second testing session, this was not significant [repeated-measures ANOVA, F(8,2) = 1.305, P = 0.29]. By the third testing session, extinction was complete for both groups.
Intrinsic excitability was measured 24 h after the last training trial from animals in the Trained cohort. The AHP was elicited by 15 APs and was measured at its peak and 1 s later, reflective of the slow AHP. For both the peak and slow AHP, CA1 pyramidal neurons from Shock and Paired animals were significantly more excitable than those from Naïve and Tone animals [ANOVA, peak AHP: F(3,85) = 6.917, P = 0.0003; slow AHP: F(3,85) = 5.039, P = 0.0019] (Fig. 1C). The integrated area of the AHP was also significantly smaller in cells from Paired and Shock animals than that in Tone and Naïve animals [data not shown; F(3,85) = 3.600, P = 0.017, Paired −7.64 ± 0.65 mV·s, Shock −8.06 ± 0.79 mV·s, Tone −9.52 ± 0.54 mV·s, Naïve −10.67 ± 0.82 mV·s]. Distributions of these data can be seen in Supplemental Fig. S1.
To measure accommodation, cells were depolarized for 1,000 ms with a current sufficient to elicit five APs in the first 100 ms. Cells from Paired and Shock animals fired significantly more APs in response to this step than cells from Tone or Naïve controls [ANOVA: F(3,72) = 8.698, P < 0.0001] (Fig. 1D). There was no significant difference between the groups in the amount of current needed to elicit the first five APs [F(3,55) = 1.47, P = 0.23]. Further, no differences between all cells recorded were observed in the resting membrane potential, the threshold for AP firing, sag, or the input resistance (see Table 1). The AHP and accommodation measures confirm that intrinsic excitability is increased in those animals that learn either contextual or trace-cued fear.
Table 1.
Intrinsic properties of cells included in this study
| Cohort/Group | Number of Cells, n | Vrest, mV | Vthresh, mV | Sag, mV | Rinput, MΩ |
|---|---|---|---|---|---|
| Trained | |||||
| Paired | 20 | −65.2 ± 0.8 | −42.9 ± 2.6 | −3.7 ± 0.2 | 72.1 ± 4.5 |
| Shock | 19 | −65.2 ± 1.0 | −39.4 ± 2.7 | −3.3 ± 0.3 | 88.6 ± 3.9* |
| Tone | 26 | −66.8 ± 0.7 | −43.7 ± 2.4 | −4.1 ± 0.3 | 78.0 ± 4.9 |
| Naïve | 19 | −67.0 ± 0.6 | −45.6 ± 1.5 | −3.8 ± 0.2 | 70.9 ± 4.8 |
| Extinguished | 20 | −64.3 ± 0.8 | −35.1 ± 4.4** | −3.8 ± 0.3 | 80.7 ± 5.6 |
| Tested | |||||
| Paired | 22 | −64.3 ± 0.7 | −45.8 ± 1.4 | −3.5 ± 0.3 | 71.0 ± 5.0 |
| Shock | 22 | −65.0 ± 0.5 | −42.6 ± 2.0 | −3.5 ± 0.3 | 67.8 ± 5.0 |
| Tone | 19 | −65.2 ± 0.9 | −41.3 ± 1.5 | −4.3 ± 0.3 | 78.3 ± 3.3 |
Values are means ± SE. Resting potential (Vrest), action potential threshold (Vthresh), and input resistance (Rinput) were measured for every cell. Vrest was calculated as the potential with 0-pA current current injection; Vthresh was calculated as the voltage where the first derivative of the action potential equaled 20 mV/ms; Rinput was calculated as the slope of the I–V curve generated with an 800-ms current injection from −0.3 to +0.1 nA; Sag was measured as the difference between the peak and steady-state hyperpolarization in response to an 800-ms, −300-PA current step.
= F(7,159) = 1.98, P = 0.062;
= F(7,159) = 1.85, P = 0.081.
Learning can be more thoroughly measured by placing the animal in the original context, to assess contextual conditioning, and in a novel context with the cue, to assess cued learning (Fig. 2A). The Tested cohort was given these testing trials before hippocampal recordings were made and both Paired and Shock rats demonstrated more freezing in the original context than that in Tone animals (Fig. 2C) [ANOVA, F(2,17) = 5.205, P = 0.033]. In the novel context, the Paired group froze more in response to the tone than did the Shock and Tone groups [ANOVA, F(2,17) = 4.159, P = 0.034].
Because the testing session presents the training cues in the absence of the shock, it also acts as an extinction trial. We hypothesized that the single testing session was acting to extinguish fear learning in the Shock group. To test this idea, the Extinguished cohort was given repeated testing sessions until freezing, to both the context and the cue, was extinguished in all animals (Fig. 2D). During the second test, the Shock animals had significantly reduced freezing to the context, compared with their first testing trial [ANOVA, F(1,8) = 5.89, P = 0.04], whereas the Paired animals still showed fear [ANOVA, F(1,9) = 0.01, P = 0.97]. Importantly, the Paired group continued to show freezing behavior in the original context after the first extinction trial, although they extinguished to the tone cue after only a single exposure in a new context [ANOVA, F(1,9) = 6.19, P = 0.03]. By the third trial, both groups had extinguished to both the cue and the original context (Fig. 2F).
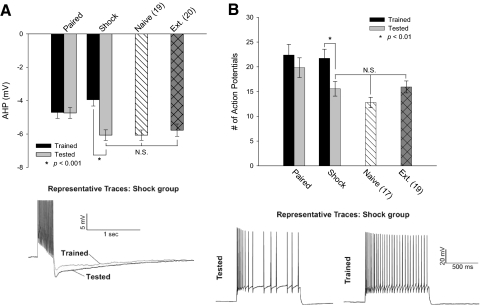
Intrinsic excitability measures were made 24 h following the last testing period from animals that were extinguished for one or three sessions (Tested and Extinguished cohorts). To determine the effect of a single extinction trial, comparisons were made between the Tested and Trained cohorts. For the Paired animals, testing had no effect on intrinsic excitability, as measured by comparing the AHP and accommodation of the Tested and Trained cohorts (Fig. 3, A and B) [ANOVA, peak: F(1,37) = 0.03, P = 0.86; accommodation: F(1,34) = 1.95, P = 0.17]. However, neurons from Shock animals from the Tested cohort had significantly larger AHPs and exhibited increased accommodation than those in the Trained cohort [ANOVA, peak: F(1,29) = 6.73, P = 0.015; accommodation: F(1,26) = 5.44, P = 0.03]. After a single testing trial, the excitability of neurons from Shock animals was no different from that from Naïve animals [ANOVA, peak: F(1,32) = 0.11, P = 0.741; accommodation: F(1,30) = 1.142, P = 0.294]. The reversal of the excitability increase in neurons from the Shock animals is not due to the exposure to the novel context in the testing session because there was no difference in the size of the AHP from Tone animals that were and were not tested [data not shown; Tone: F(1,31) = 2.11, P = 0.16]. The idea that complete extinction was responsible for the difference between the Shock animals versus the Paired animals was expanded by measuring the excitability from the Extinguished cohort. There was no significant difference in the freezing behavior after extinction between the Paired and Shock groups, nor in either the AHP or the accommodation measures. Consequently, the electrophysiological data for these groups were pooled. Cells from Extinguished animals had AHPs like those of Naïve animals (Fig. 3A). Indeed, there was no difference in the size of the AHP between Naïve, Extinguished, and Shock animals from the Tested cohort [ANOVA, F(2,56) = 0.267, P = 0.77]. There was also no difference in accommodation measures (Fig. 3B) [ANOVA, F(2,53) = 1.709, P = 0.19].
Fig. 3.
Behavioral extinction in single or multiple trials is sufficient to reverse the learning-related increases in excitability. A: cells from Paired animals did not show any effect of a single testing trial and their excitability changes remained (peak: Trained −4.70 ± 0.36 mV; Tested −4.68 ± 0.34 mV). In Shock animals, a single testing trial reversed the reduction of the AHP seen after learning but before testing (peak: Trained −4.23 ± 0.38 mV; Tested −6.06 ± 0.32 mV). The AHP from these cells, after a single testing trial, was no different from that from Naïve cells (peak: −6.06 ± 0.31 mV) or from that of animals from the Extinguished cohort who showed complete extinction (peak: −5.77 ± 0.37 mV). B: after a single testing trial, the learning-related reduction of firing frequency accommodation was also reversed in the Shock group, as measured by firing fewer APs during a 1-s current injection sufficient to produce 5 APs in the first 100 ms (Trained: 21.7 ± 1.8 APs; Tested: 15.6 ± 1.5 APs). After a single testing session, firing frequency accommodation in the Shock group was no different from that of Naïve animals (12.8 ± 1.1 APs) or from that of animals from the Extinguished cohort (15.9 ± 1.2 APs). The Paired group showed no effect of a single testing session on firing frequency accommodation (Trained: 22.4 ± 2.1 APs; Tested: 19.8 ± 2.0 APs).
Together, these data suggest that learning cue or contextual fear conditioning increases excitability. Furthermore, at a time point when freezing behavior and thus learning were extinguished, the learning-related increase in excitability is rapidly reversed in as little as a single trial.
DISCUSSION
We have shown here that learning either contextual or trace-cued fear increases intrinsic excitability in only three trials and those changes can be reversed by a single experience sufficient to extinguish the learned behavior. The Paired animals learned both contextual and trace-cued fear conditioning, as evidenced by their freezing to both the context and the cue during the testing trial. Shock animals learned only the context, as was evident by their lack of freezing in the cue-testing trial.
Since hippocampal CA1 neurons in both Paired and Shock alone animals showed AHPs reduced to similar levels, the conclusion can be drawn that learning contextual fear conditioning is sufficient to reduce the AHP. However, this does not mean that learning trace-cued fear conditioning does not alter intrinsic excitability (recall that the Paired animals learned to fear both the original context and the tone in a new context). The degree of AHP reduction we observe here, roughly 30%, is similar to values from previous studies in rats after learning trace eyeblink or Morris water maze (Matthews et al. 2008; Oh et al. 2003). This level of AHP reduction may be optimal to support learning and further learning may not have an additive effect, possibly to protect the hippocampus from excessive excitation. The fact that excitability is reduced the same degree by learning one association (contextual fear conditioning) or two associations (trace and contextual fear conditioning) suggests that AHP modulation is not additive. However, a reduction in the AHP caused by learning one task could aid in learning a second one. In fact, learning both Morris water maze and trace eyeblink conditioning reduce the AHP, and simultaneous training in the two tasks facilitates learning trace eyeblink conditioning (Kuo et al. 2006). Olfactory discrimination also increases intrinsic excitability in CA1 pyramidal neurons during and on the day of learning, but not after the animal has learned the rule. Learning the Morris water maze is enhanced if it is started immediately following olfactory discrimination rule learning but not a few days later, and the facilitation is correlated with time periods in which olfactory discrimination reduces the AHP (Zelcer et al. 2006). Thus learning one task and its subsequent increases in intrinsic excitability appears to facilitate the learning of other hippocampus-dependent tasks. The learning-related increase in excitability seen with fear conditioning may facilitate the learning of other hippocampus-dependent tasks.
A testing trial provided the opportunity to more fully assess the degree of learning. However, the act of testing the animals exposed them to an extinction trial since the tone and context cues were present but the shock was not. To determine whether the animals from the Tested cohort did indeed show behavioral extinction after a single trial, the Extinguished cohort was given repeated testing trials until freezing returned to baseline levels. Indeed, by the second extinction trial, at which point AHP recordings were made for the Tested cohort, the Extinguished cohort demonstrated significantly less freezing than that during the previous testing trial, but with a differentiation between contextual and cued learning. The Paired animals showed significantly reduced freezing to the cue (while retaining contextual freezing levels) and the Shock animals had significantly reduced freezing to the context, compared with each group's performance in the first testing trial (Fig. 2F). The electrophysiological measures from the Tested cohort showed that a single extinction trial reset the size of the AHP in neurons from Shock animals to that of Naïve controls. However, this was not the case for the Paired animals, which had reduced AHPs in both the Tested and Trained cohorts (Fig. 3A). Note that in the Trained cohort, both the excitability changes and the freezing behavior to the context were similar in the Paired and Shock groups, so the level of intrinsic excitability after learning was correlated with the behavioral index of learning. The demonstration that cells from animals from the Extinguished cohort had large AHPs further supports this idea. Furthermore, the AHP size after a single extinction trial was correlated with behavioral contextual freezing demonstrated by the Paired and Shock groups.
The slower extinction of freezing to the context in the Paired group than that in the Shock group may have occurred because the Paired group made a compound association during training (context + cue) and therefore the testing sessions exposed the Paired animals to two separate partial extinction sessions (context only or cue only), whereas the Shock group received a complete extinction session with each test (Rescorla and Wagner 1972). Another hypothesis is that the slower rate of contextual extinction for the Paired group may be indicative of the richer informational content created by the addition of a cue to the training paradigm—i.e., the Paired animals learned a more complex stimulus and thus extinguished more slowly. Regardless of why the extinction rates differed, the AHP was predictive of the freezing behavior of the animals in the context.
The difference between the reversal in AHP reduction between the Paired and the Shock animals with extinction may also be due to the involvement of different neuronal circuits for the two tasks. The extinction of contextual fear conditioning is hippocampus dependent (Corcoran et al. 2005), but the involvement of the hippocampus in extinction of trace-cued fear is unknown. Other brain regions are required for cued fear conditioning and extinction. For example, the prefrontal cortex is important for extinction of cued fear, but not of contextual fear conditioning (Morgan and LeDoux 1995, 1999; Quirk et al. 2006). The reversal of the AHP reduction observed in the Shock animals after a single extinction trial is qualitatively similar to that observed in the infralimbic prefrontal cortex, which inhibits fear expression. Learning cued fear conditioning is correlated with a reduction in intrinsic excitability in this region, whereas its extinction increases it (Santini et al. 2008). Thus although the hippocampus is involved in extinction of contextual conditioning, the involvement of other brain regions may mean that other sites of plasticity are involved in extinguishing cued fear.
The central observation in this study is that learning-related changes in intrinsic excitability were evident after just three training trials or only a single extinction trial. Use of this training paradigm in future experiments could help to elucidate the not yet fully understood mechanism(s) of the learning-related reduction in the postburst AHP. It has been shown that protein kinase A (PKA) mediates this reduction (Oh et al. 2009), but the time course of the changes relative to the training time course is unknown. Previous demonstrations of learning-related alterations in the AHP used learning paradigms that required many training trials over several days. The data reported here, however, indicate that the cellular changes underlying excitability modulation can be rapidly induced. It is well documented that a single experience, requiring learning or merely novel, is sufficient to induce changes in gene expression of proteins known to be important for learning and memory (Sweatt 2004; Tzingounis and Nicoll 2006). For example, mice that were exposed to a novel context for 3 min with or without a shock and a tone show high levels of FOS production in the hippocampus (Radulovic et al. 1998). A single lap around a rectangular track is sufficient to trigger immediate early gene Arc transcription in both CA3 and CA1 (Miyashita et al. 2009). Further, a single contextual fear conditioning trial with a single shock results in up-regulation of Erk1/2 and Elk1 signaling in CA3 and the dentate gyrus (Sananbenesi et al. 2002). Erk activation is often preceded or paralleled by PKA activation (Ferguson and Storm 2004; Winder and Sweatt 2001), providing a possible mechanism for the learning-related changes in excitability reported here. Also, Erk signaling can lead to cAMP response element binding (CREB) activation, which has also been shown to correlate with AHP modulation (Lopez de Armentia et al. 2007). These changes in gene expression after a single trial could activate signaling cascades that result in the alterations in the AHP observed after three training trials or a single extinction trial.
The finding that intrinsic excitability is increased after learning contextual or trace-cued fear and is rapidly decreased after a single extinction event sufficient to eliminate the learned behavior underscores the importance of intrinsic plasticity as a cellular substrate for learning. A more complete understanding of the mechanisms by which excitability is modulated and interacts with synaptic properties that also change during learning should augment treatments to restore the impaired cognitive ability attributed to aging or injury.
GRANTS
This work was supported by National Institutes of Health Grants R37 AG-08796 to J. F. Disterhoft, F31 NS-053434 to E. A. Matthews, and T32 AG-20506 to B. M. McKay.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. J. Radulovic for helpful discussion of the behavioral experiments. Behavioral training was done in the Behavioral Phenotyping Core at Northwestern University.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Alger and Nicoll, 1980.Alger BE, Nicoll RA. Epileptiform burst afterhyperolarization: calcium-dependent potassium potential in hippocampal CA1 pyramidal cells. Science 210: 1122–1124, 1980 [DOI] [PubMed] [Google Scholar]
- Blanchard and Blanchard, 1972.Blanchard DC, Blanchard RJ. Innate and conditioned reactions to threat in rats with amygdaloid lesions. J Comp Physiol Psychol 81: 281–290, 1972 [DOI] [PubMed] [Google Scholar]
- Corcoran et al., 2005.Corcoran KA, Desmond TJ, Frey KA, Maren S. Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. J Neurosci 25: 8978–8987, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disterhoft and Oh, 2006.Disterhoft JF, Oh MM. Learning, aging and intrinsic neuronal plasticity. Trends Neurosci 29: 587–599, 2006 [DOI] [PubMed] [Google Scholar]
- Ferguson and Storm, 2004.Ferguson GD, Storm DR. Why calcium-stimulated adenylyl cyclases? Physiology (Bethesda) 19: 271–276, 2004 [DOI] [PubMed] [Google Scholar]
- Hotson and Prince, 1980.Hotson JR, Prince DA. A calcium-activated hyperpolarization follows repetitive firing in hippocampal neurons. J Neurophysiol 43: 409–419, 1980 [DOI] [PubMed] [Google Scholar]
- Kaczorowski et al., 2007.Kaczorowski CC, Disterhoft J, Spruston N. Stability and plasticity of intrinsic membrane properties in hippocampal CA1 pyramidal neurons: effects of internal anions. J Physiol 578: 799–818, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczorowski and Disterhoft, 2009.Kaczorowski CC, Disterhoft JF. Memory deficits are associated with impaired ability to modulate neuronal excitability in middle-aged mice. Learn Mem 16: 362–366, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim and Fanselow, 1992.Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science 256: 675–677, 1992 [DOI] [PubMed] [Google Scholar]
- Kuo et al., 2006.Kuo AG, Lee G, Disterhoft JF. Simultaneous training on two hippocampus-dependent tasks facilitates acquisition of trace eyeblink conditioning. Learn Mem 13: 201–207, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo et al., 2008.Kuo AG, Lee G, McKay BM, Disterhoft JF. Enhanced neuronal excitability in rat CA1 pyramidal neurons following trace eyeblink conditioning acquisition is not due to alterations in IM. Neurobiol Learn Mem 89: 125–133, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux, 2000.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci 23: 155–184, 2000 [DOI] [PubMed] [Google Scholar]
- Lopez de Armentia et al., 2007.Lopez de Armentia M, Jancic D, Olivares R, Alarcon JM, Kandel ER, Barco A. cAMP response element-binding protein-mediated gene expression increases the intrinsic excitability of CA1 pyramidal neurons. J Neurosci 27: 13909–13918, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews et al., 2008.Matthews EA, Weible AP, Shah S, Disterhoft JF. The BK-mediated fAHP is modulated by learning a hippocampus-dependent task. Proc Natl Acad Sci USA 105: 15154–15159, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEchron et al., 1998.McEchron MD, Bouwmeester H, Tseng W, Weiss C, Disterhoft JF. Hippocampectomy disrupts auditory trace fear conditioning and contextual fear conditioning in the rat. Hippocampus 8: 638–646, 1998 [DOI] [PubMed] [Google Scholar]
- Miyashita et al., 2009.Miyashita T, Kubik S, Haghighi N, Steward O, Guzowski JF. Rapid activation of plasticity-associated gene transcription in hippocampal neurons provides a mechanism for encoding of one-trial experience. J Neurosci 29: 898–906, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan and LeDoux, 1995.Morgan MA, LeDoux JE. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav Neurosci 109: 681–688, 1995 [DOI] [PubMed] [Google Scholar]
- Morgan and LeDoux, 1999.Morgan MA, LeDoux JE. Contribution of ventrolateral prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Neurobiol Learn Mem 72: 244–251, 1999 [DOI] [PubMed] [Google Scholar]
- Moyer JR, Jr, Thompson LT, Disterhoft JF. Trace eyeblink conditioning increases CA1 excitability in a transient and learning-specific manner. J Neurosci 16: 5536–5546, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh et al., 2003.Oh MM, Kuo AG, Wu WW, Sametsky EA, Disterhoft JF. Watermaze learning enhances excitability of CA1 pyramidal neurons. J Neurophysiol 90: 2171–2179, 2003 [DOI] [PubMed] [Google Scholar]
- Oh et al., 2009.Oh MM, McKay BM, Power JM, Disterhoft JF. Learning-related postburst afterhyperpolarization reduction in CA1 pyramidal neurons is mediated by protein kinase A. Proc Natl Acad Sci USA 106: 1620–1625, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk et al., 2006.Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry 60: 337–343, 2006 [DOI] [PubMed] [Google Scholar]
- Radulovic et al., 1998.Radulovic J, Kammermeier J, Spiess J. Relationship between fos production and classical fear conditioning: effects of novelty, latent inhibition, and unconditioned stimulus preexposure. J Neurosci 18: 7452–7461, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla and Wagner, 1972.Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: variations in the effectiveness of reinforcement and nonreinforcement. In: Classical Conditioning II: Current Research and Theory, edited by Black AH, Prokasy WF. New York: Appleton–Century–Crofts, 1972, p. 64–99 [Google Scholar]
- Saar et al., 1998.Saar D, Grossman Y, Barkai E. Reduced after-hyperpolarization in rat piriform cortex pyramidal neurons is associated with increased learning capability during operant conditioning. Eur J Neurosci 10: 1518–1523, 1998 [DOI] [PubMed] [Google Scholar]
- Sananbenesi et al., 2002.Sananbenesi F, Fischer A, Schrick C, Spiess J, Radulovic J. Phosphorylation of hippocampal Erk-1/2, Elk-1, and p90-Rsk-1 during contextual fear conditioning: interactions between Erk-1/2 and Elk-1. Mol Cell Neurosci 21: 463–476, 2002 [DOI] [PubMed] [Google Scholar]
- Santini et al., 2008.Santini E, Quirk GJ, Porter JT. Fear conditioning and extinction differentially modify the intrinsic excitability of infralimbic neurons. J Neurosci 28: 4028–4036, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzkroin and Stafstrom, 1980.Schwartzkroin PA, Stafstrom CE. Effects of EGTA on the calcium-activated afterhyperpolarization in hippocampal CA3 pyramidal cells. Science 210: 1125–1126, 1980 [DOI] [PubMed] [Google Scholar]
- Sweatt, 2004.Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol 14: 311–317, 2004 [DOI] [PubMed] [Google Scholar]
- Thompson LT, Moyer JR, Jr, Disterhoft JF. Transient changes in excitability of rabbit CA3 neurons with a time course appropriate to support memory consolidation. J Neurophysiol 76: 1836–1849, 1996 [DOI] [PubMed] [Google Scholar]
- Tombaugh et al., 2005.Tombaugh GC, Rowe WB, Rose GM. The slow afterhyperpolarization in hippocampal CA1 neurons covaries with spatial learning ability in aged Fisher 344 rats. J Neurosci 25: 2609–2616, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzingounis and Nicoll, 2006.Tzingounis AV, Nicoll RA. Arc/Arg3.1: linking gene expression to synaptic plasticity and memory. Neuron 52: 403–407, 2006 [DOI] [PubMed] [Google Scholar]
- Winder and Sweatt, 2001.Winder DG, Sweatt JD. Roles of serine/threonine phosphatases in hippocampal synaptic plasticity. Nat Rev 2: 461–474, 2001 [DOI] [PubMed] [Google Scholar]
- Zelcer et al., 2006.Zelcer I, Cohen H, Richter-Levin G, Lebiosn T, Grossberger T, Barkai E. A cellular correlate of learning-induced metaplasticity in the hippocampus. Cereb Cortex 16: 460–468, 2006 [DOI] [PubMed] [Google Scholar]
- Zhang and Linden, 2003.Zhang W, Linden DJ. The other side of the engram: experience-driven changes in neuronal intrinsic excitability. Nat Rev Neurosci 4: 885–900, 2003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.