Abstract
The sensory neurons (photoreceptors) in the visual system of Hermissenda are one site of plasticity produced by Pavlovian conditioning. A second site of plasticity produced by conditioning is the type I interneurons in the cerebropleural ganglia. Both photoreceptors and statocyst hair cells of the graviceptive system form monosynaptic connections with identified type I interneurons. Two proposed neurotransmitters in the graviceptive system, serotonin (5-HT) and γ-aminobutyric acid (GABA), have been shown to modify synaptic strength and intrinsic neuronal excitability in identified photoreceptors. However, the potential role of 5-HT and GABA in plasticity of type I interneurons has not been investigated. Here we show that 5-HT increased the peak amplitude of light-evoked complex excitatory postsynaptic potentials (EPSPs), enhanced intrinsic excitability, and increased spike activity of identified type Ie(A) interneurons. In contrast, 5-HT decreased spike activity and intrinsic excitability of type Ie(B) interneurons. The classification of two categories of type Ie interneurons was also supported by the observation that 5-HT produced opposite effects on whole cell steady-state outward currents in type Ie interneurons. Serotonin produced a reduction in the amplitude of light-evoked complex inhibitory PSPs (IPSPs), increased spontaneous spike activity, decreased intrinsic excitability, and depolarized the resting membrane potential of identified type Ii interneurons. In contrast to the effects of 5-HT, GABA produced inhibition in both types of Ie interneurons and type Ii interneurons. These results show that 5-HT and GABA can modulate the intrinsic excitability of type I interneurons independent of the presynaptic effects of the same transmitters on excitability and synaptic efficacy of photoreceptors.
INTRODUCTION
Both γ-aminobutyric acid (GABA) and serotonin (5-HT) are putative neurotransmitters in the graviceptive sensory system of Hermissenda (for reviews, see Blackwell 2006; Crow 2004). The graviceptive sensory organ, the statocyst, contains endogenous GABA, and immunocytochemical procedures have localized GABA in statocyst hair cell axons and axon terminal processes (Alkon et al. 1993). Statocyst hair cells and photoreceptors form reciprocal monosynaptic inhibitory connections where caudal hair cells inhibit photoreceptors and cephalic hair cells are inhibited by type B photoreceptors (Alkon 1973). Stimulation of statocyst hair cells elicits a monosynaptic GABAergic inhibitory postsynaptic potential (IPSP) recorded in type B photoreceptors (Alkon et al. 1993; Blackwell 2002; Rogers et al. 1994; Sakakibara et al. 1993), and enhanced excitability is produced by GABA application paired with depolarization of type B photoreceptors (Matzel and Alkon 1991). With regard to the second putative neurotransmitter of the graviceptive system, the terminal processes of serotonergic interneurons have been shown to form rings of varicosities surrounding photoreceptor axons in the optic nerve before entry into the cerebropleural ganglion (Land and Crow 1985). In addition, immunoreactive processes from 5-HT containing neurons project to a region near the eyes and photoreceptor synapses in the cerebropleural ganglion (Auerbach et al. 1989; Land and Crow 1985; Tian et al. 2006). Both GABA (Schultz and Clark 1997) and 5-HT produce synaptic facilitation of type B to type A photoreceptor monosynaptic IPSPs (Frysztak and Crow 1997; Schuman and Clark 1994) and changes in the intrinsic excitability of identified photoreceptors (Crow and Bridge 1985; Crow and Forrester 1991; Farley and Wu 1989; Matzel and Alkon 1991).
Aggregates of type I interneurons in the cerebropleural ganglion form a second site of synaptic convergence between the graviceptive system and visual system. Photoreceptors and statocyst hair cells form monosynaptic excitatory connections with type Ie interneurons and monosynaptic inhibitory connections with type Ii interneurons (Akaike and Alkon 1980; Crow and Tian 2000). Previous studies have shown that conditioning produces changes in intrinsic excitability in both photoreceptors and type I interneurons (Crow and Alkon 1980; Crow and Tian 2003). Because hair cells form synaptic connections with photoreceptors, type I interneurons, and 5-HT immunoreactive interneurons, the endogenous release of 5-HT and GABA could modulate excitability and synaptic strength in both photoreceptors and type I interneurons. To date, the potential effects of 5-HT and GABA on type Ie and type Ii interneurons have not been investigated. In the present study, we examined the effect of 5-HT and GABA on complex PSPs associated with presynaptic input from photoreceptors and intrinsic excitability in identified type I interneurons.
METHODS
Animals
Adult Hermissenda crassicornis were used in the experiments. The animals were obtained from Sea Life Supply (Sand City, CA) and maintained in closed artificial seawater (ASW) aquaria at 14 ± 1°C on a 12-h light/dark cycle. Electrophysiological data were collected during the light phase of the light/dark cycle.
Intracellular recordings
Circumesophageal nervous systems were isolated in ASW (∼14°C) and desheathed to expose the cell bodies of type I interneurons. The desheathed circumesophageal nervous systems were pinned to a silicone elastomer (Sylgard, Dow Chemical) stage in a recording chamber filled with ASW of the following composition (in mM): 460 NaCl, 10 KCl, 10 CaCl2, and 55 MgCl2, buffered with 10 mM HEPES and brought to pH 7.46 with NaOH solution. Type I interneurons were identified using established anatomical and electrophysiological criteria as described previously (Akaike and Alkon 1980; Crow and Tian 2000, 2002a,b). The ASW in the recording chamber was monitored by a thermistor and held at 14.5 ± 0.5°C. The illumination of the eyes was provided by a tungsten halogen incandescent lamp attached to a fiber optic bundle mounted underneath the recording chamber. Identified type I interneurons were impaled with microelectrodes filled with 4 M potassium acetate and connected to the head stage of an Axoclamp 2A (Axon Instruments, Foster City, CA). Electrode resistances varied between 70 and 130 MΩ. Extrinsic current pulses were applied through a bridge circuit.
Stimulation protocol
After 12 min of dark adaptation, the amplitude of light-evoked complex PSPs was assessed before and after the bath administration of 5-HT or GABA. Complex PSPs were evoked by the presentation of a 10-s period of illumination of the photoreceptors. The peak amplitude of light-evoked complex PSPs was determined by measuring the amplitude of the depolarization during the light step relative to the prelight baseline membrane potential. This measure of complex PSP amplitude is consistent with previous reports when spike activity was blocked by hyperpolarizing type Ie interneurons below threshold for spike generation during the light step (Crow and Tian 2000, 2002a). Spike activity elicited during the 10-s light step was compared with an equivalent period of spontaneous activity before light. Intrinsic excitability was assessed in the dark by presenting 1.8-s depolarizing current pulses (0.1, 0.2, and 0.3 nA) from a holding potential of −60 mV maintained by injection of steady depolarizing or hyperpolarizing current. Current pulses were presented at 1-min intervals before and after the bath application of 5-HT or GABA delivered to the recording chamber by either perfusion or direct injection. Membrane potential was determined by measurement of the potential between spikes during a 10-s pause in extrinsic current injection. The experimental protocol was repeated three times at 6-min intervals before and after the bath application of 5-HT or GABA. The final concentration of 5-HT and GABA in ASW was 10-4 M and 10-3 M, respectively. Digitized electrophysiological data were stored on a computer hard drive and analyzed using Spike 2 software (Cambridge Electronic Design).
Whole cell voltage-clamp recordings
Type Ie interneurons were identified by recording inward current underling complex light-evoked EPSPs. After 12 min of dark adaptation, macroscopic whole cell currents were recorded at 15 ± 0.5°C using an Axopatch 200A amplifier (Axon Instruments). Borosilicate glass pipettes (1.5 mm OD; 1.17 mm ID) were pulled on a horizontal Flaming-Brown microelectrode puller (Model P80/PC, Sutter Instrument, San Rafael, CA) and fire-polished with a micro forge (MF-830, Narishige, Japan) to obtain tip diameters of 1–2 μm. Only cells in experiments with seal resistances >1 GΩ were accepted for analysis. Records were filtered at 5 kHz with a low-pass Bessel filter and digitized at 10 kHz with a Digidata interface controlled by pClamp software, version 10.0.0.61 (Axon Instruments). Data analysis was performed with Clampfit (Axon Instruments) and Origin (Microcal Software, Northampton, MA) software programs. The composition of bath solutions for recording outward currents was as follows (in mM): 450 choline-chloride, 10 KCl, 50 MgCl2, 0.5 CaCl2, and 15 HEPES, the pH value was adjusted with Tris to 7.46 at 20°C. The osmolarity of the bath solution was adjusted to 996–1005 mosM. The composition of the pipette solution used for whole cell recordings was (in mM): 430 KCl, 20 NaCl, 2 MgCl2, 2 EGTA, 50 HEPES, 10 glutathione (reduced), 5 Mg-ATP, and 1 Na2-GTP, the pH value is adjusted with KOH to 7.30 at 15°C. The osmolarity of the internal solution was adjusted to 970 mosM. Whole cell currents presented in I-V plots were adjusted for the correct junction potential (∼7.6 mV). All chemicals were obtained from Sigma.
Statistical analysis
Descriptive statistics are expressed as means ± SE. Overall significant differences involving multiple groups were determined by a repeated-measure ANOVA. Two group inferential statistical comparisons consisted of paired t-tests.
RESULTS
Previous work has shown that 5-HT modulates generator potentials and membrane conductances in type B photoreceptors (Acosta-Urquidi and Crow 1993; Crow and Bridge 1985; Crow and Forrester 1991; Farley and Wu 1989; Rogers and Matzel 1995; Yamoah and Crow 1995, 1996). Spikes in identified type A and B photoreceptors elicit monosynaptic EPSPs in type Ie interneurons and monosynaptic IPSPs in type Ii interneurons (Akaike and Alkon 1980; Crow and Tian 2000). Therefore potential changes in type I interneurons produced by 5-HT and GABA may be induced by both presynaptic and postsynaptic processes.
Excitatory effect of 5-HT on light-evoked complex EPSPs, spike activity, intrinsic excitability, and membrane potential in type Ie(A) interneurons
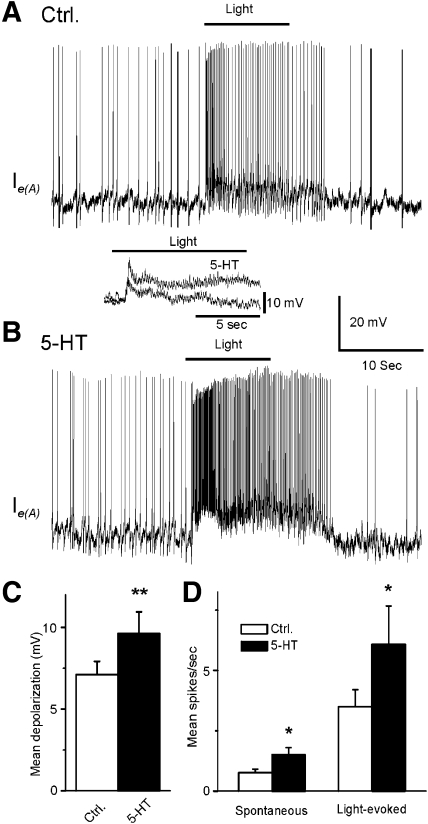
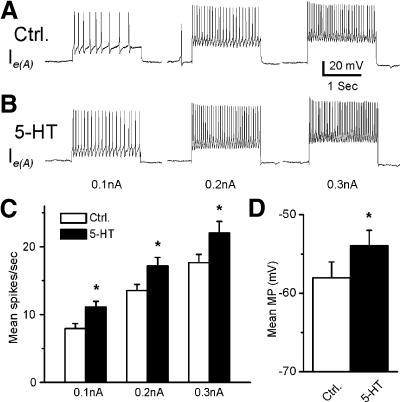
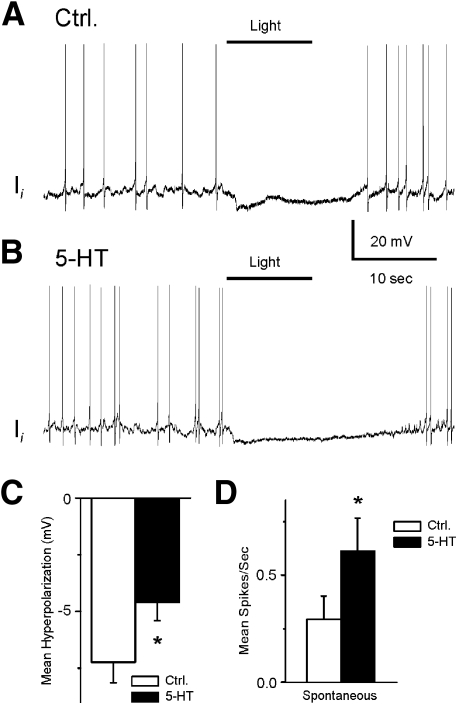
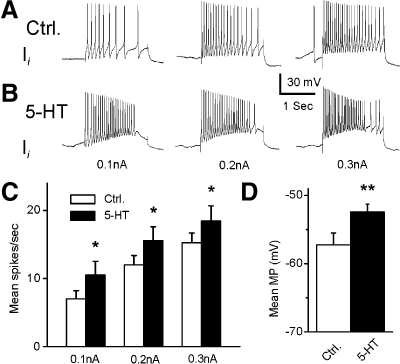
Representative recordings from a type Ie(A) interneuron after 12 min of dark adaptation and after 5-HT application are shown in Fig. 1. The 10-s period of illumination produced a complex EPSP and an increase in spike activity in the type Ie(A) interneuron (Fig. 1A). As shown in Fig. 1B, bath application of 5-HT produced an increase in the amplitude of the light-evoked complex EPSP and an increase in spontaneous and light-evoked spike activity. Figure 1, inset, shows an example of the enhancement of the complex light-evoked EPSP by 5-HT in a preparation without spontaneous spike activity. The analysis of the group summary data (n = 6) shown in Fig. 1, C and D, revealed that 5-HT significantly increased spontaneous (t5 = 2.9; P < 0.05) and the light-evoked spike activity (t5 = 2.8; P < 0.05) and increased the amplitude of the light-evoked complex EPSP (t5 = 4.1; P < 0.01). The excitatory effect of 5-HT may be due to the 5-HT-dependent excitatory effect on photoreceptors (Crow and Bridge 1985; Crow and Forrester 1991; Farley and Wu 1989) or direct effects on type Ie(A) interneurons. This issue was examined by assessing intrinsic excitability of Ie(A) interneurons before and after the bath application of 5-HT. Spike activity evoked by 0.1-, 0.2-, or 0.3-nA current injection was increased in the presence of 5-HT (Fig. 2B). The analysis of the group summary data (n = 6; Fig. 2C) indicated that 5-HT significantly increased intrinsic excitability of type Ie(A) interneurons at all current levels that were applied (0.1 nA, t5 = 7.1; P < 0.01; 0.2 nA, t5 = 5.7; P < 0.01; 0.3 nA, t5 = 4.1; P < 0.01). In addition, 5-HT produced a statistically significant depolarization of the membrane potential of type Ie(A) interneurons (t5 = 7.1; P < 0.01; Fig. 2D).
Fig. 1.
Serotonin (5-HT) enhances the amplitude of light-evoked complex excitatory postsynaptic potentials (EPSPs) and increases spontaneous and light-evoked spike activity in type Ie(A) interneurons. A representative recording from a dark-adapted type Ie(A) interneuron before (A) and after 5-HT application (B). Inset: an example of light-evoked complex EPSPs before and after 5-HT application in a type Ie(A) interneuron that did not exhibit spontaneous spike activity. The bar above the recordings indicates the presentation of the 10-s light. Group summary data of 5-HT-induced increase in the amplitude of light-evoked complex EPSPs (mean depolarization), spontaneous and light-evoked spike activity are shown in C and D, respectively. * P < 0.05, **P < 0.01.
Fig. 2.
Excitatory effect of 5-HT on intrinsic excitability and membrane potential (MP) in type Ie(A) interneurons. Representative examples of spike activity evoked by 0.1-, 0.2-, or 0.3-nA current injection before (Ctrl) (A) and after 5-HT (B). The group summary data for mean spike activity and MP are shown in C and D. *P < 0.01. The 5-HT-dependent increase in excitability and membrane depolarization suggests a direct excitatory effect of 5-HT on type Ie(A) interneurons.
Inhibitory effect of 5-HT on spike activity, intrinsic excitability, and membrane potential of type Ie(B) interneurons
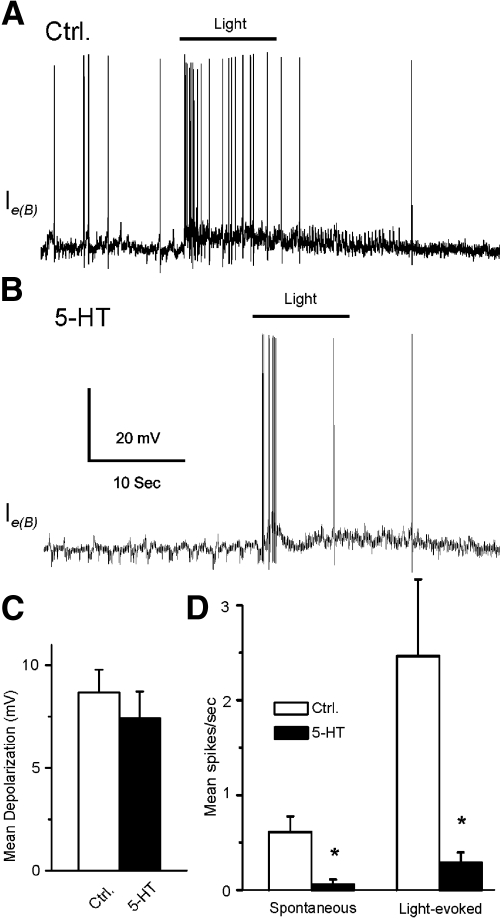
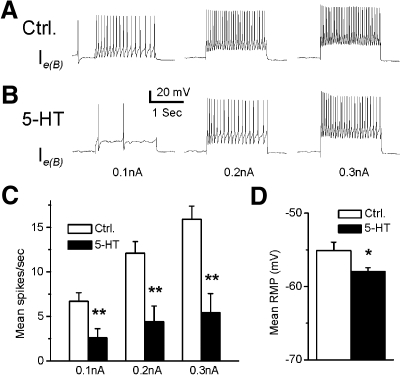
In 7 of 13 type Ie interneurons, the bath application of 5-HT produced inhibition of light-elicited spike activity. These cells were classified as type Ie(B) interneurons. A representative example of spike activity elicited by a 10-s light presentation in ASW and after 5-HT application is shown in Fig. 3. The bath application of 5-HT did not produce a significant change in the amplitude of light-evoked complex EPSPs (n = 7) (t6 = 0.71; NS; Fig. 3C). However, 5-HT significantly decreased the spontaneous and light-evoked spike activity of type Ie(B) interneurons. The analysis of the group summary data shown in Fig. 3D revealed that 5-HT significantly reduced spontaneous (t6 = 3.6; P < 0.05) and light-evoked spike activity (t6 = 2.9; P < 0.05). We examined direct (intrinsic) versus indirect (presynaptic) effects of 5-HT by examining intrinsic excitability of Ie(B) interneurons before and after the bath application of 5-HT. Spike activity evoked by 0.1-, 0.2-, or 0.3-nA current injection was decreased in the presence of 5-HT (n = 7) (Fig. 4, A and B). The analysis of the group data (Fig. 4, C and D) showed that 5-HT significantly decreased intrinsic excitability of type Ie(B) interneurons as measured by current-evoked spike activity (0.1 nA, t6 = 6.2; P < 0.01; 0.2 nA, t6 = 5.7; P < 0.01; 0.3 nA, t6 = 6.7; P < 0.01). In addition, 5-HT application resulted in a significant hyperpolarization of the membrane potential of type Ie(B) interneurons (t6 = 3.3; P < 0.05; Fig. 4D).
Fig. 3.
5-HT decreases the amplitude of light-evoked complex EPSPs and spike activity in type Ie(B) interneurons. A representative recording from a dark-adapted type Ie(B) interneuron before (A) and after 5-HT application (B). The bar above the recordings indicates the presentation of the 10-s light. The group summary data of 5-HT effects on light-evoked complex EPSPs, spontaneous and light-evoked spike activity are shown in C and D. *P < 0.05.
Fig. 4.
5-HT decreases intrinsic excitability and hyperpolarizes the MP in type Ie(B) interneurons. A representative example of spike activity evoked by 0.1-, 0.2-, or 0.3-nA current injections before (A) and after 5-HT (B). The group summary data for 5-HT effects on spike activity and MP are shown in C and D. *P < 0.05, **P < 0.01. The 5-HT-induced decrease in excitability and membrane hyperpolarization suggest a direct inhibitory effect on type Ie(B) interneurons.
Dual effect of 5-HT on whole cell currents in type Ie interneurons
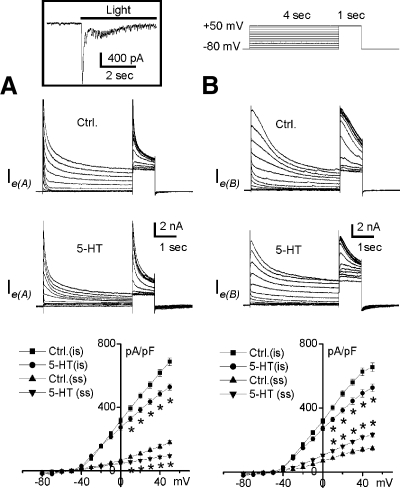
The results obtained from intracellular recordings of type Ie interneurons showed that the bath application of 5-HT produced different effects on excitability, suggesting that there may be two types of Ie interneurons that we have classified as Ie(A) and Ie(B). To further investigate this classification, whole cell currents were examined in a sample of type Ie interneurons (n = 8). To exclude the contamination of Na+ and minimize Ca2+ effects, Na-free and low-Ca2+ (0.5 mM) bath solutions were prepared by ionic substitution of Na+ and Ca2+ with choline. Choline substitution did not result in a change in holding current. Under these experimental conditions, macroscopic outward whole cell currents were recorded from a sample of type Ie interneurons. Figure 5 illustrates a family of outward current traces evoked by voltage-clamp steps from −80 to +50 mV in 10-mV increments from a holding potential of −80 mV. The transient component of the outward current was activated at step voltages positive to −50 mV, followed by a sustained component activated at a voltage step positive to −20 mV. The net outward whole cell currents were reversed at −53.1 ± 3.7 mV. The bath application of 5-HT decreased the peak amplitude of the initial transient currents in all type Ie interneurons examined (n = 8). Overall significant decreases were found in the instantaneous current after 5-HT application for putative type Ie(A) [F(1,3) = 32; P < 0.01] and Ie(B) [F(1,3) = 180; P < 0.001] interneurons. However 5-HT produced opposite effects on the sustained steady-state outward currents in different type Ie interneurons as shown in Fig. 5. The steady-state net outward current was significantly decreased in putative type Ie(A) interneurons [F(1,3) = 106.8; P < 0.002; Fig. 5A] and significantly increased in putative type Ie(B) interneurons [F(1,3) = 62.9; P < 0.004; Fig. 5B]. This suggests that the differential effect of 5-HT on intrinsic excitability of type Ie(A) and Ie(B) interneurons may be the result of the effect of 5-HT on the contribution of the delayed rectifier to the net outward current.
Fig. 5.
Differential effects of 5-HT on whole cell outward currents in identified type Ie interneurons. Inset: type Ie interneurons were identified by detecting an increase in the amplitude of whole cell inward currents and an enhancement in spike activity at the holding potential of −60 mV in response to illumination of the eyes. Representative current traces for control (Ctrl) and after 5-HT application are shown in the top and middle panels in A and B. The outward currents began to active at −50 mV from a holding potential of −80 mV. Averaged instantaneous (is) and steady-state (ss) current density-voltage relationships of the net outward currents at the holding potential of −80 mV are shown in the bottom panels. The initial transient outward current was inhibited by the bath application of 5-HT in all type Ie interneurons examined. However, 5-HT produced a decrease in the steady-state outward current in 50% of type Ie interneurons tested (A) and an increase in the steady-state net outward current in the remaining type Ie interneurons (B), consistent with the type Ie(A) and Ie(B) classification obtained from the intracellular recordings (see Fig. 1–4). *P < 0.01 for all paired comparisons.
Effect of 5-HT on light-evoked complex IPSPs, spike activity, intrinsic excitability, and membrane potential in type Ii interneurons
In all of the type Ii interneurons tested (n = 6), the bath application of 5-HT produced excitation. A representative example of light-evoked spike activity in a type Ii interneuron in ASW and after 5-HT application is shown in Fig. 6. The bath application of 5-HT produced a decrease in the amplitude of light-evoked complex IPSPs and an increase in spontaneous spike activity (Fig. 6, A and B). The analysis of the group summary data shown in Fig. 6, C and D, revealed that 5-HT significantly decreased the amplitude of light-evoked complex IPSPs (t5 = 4.7; P < 0.01) and increased the spontaneous spike activity in type Ii interneurons (t5 = 4.1; P < 0.01). It is likely that the excitatory effect of 5-HT on type Ii interneurons is not due to 5-HT-dependent excitatory effects on photoreceptors as described previously (Crow and Bridge 1985; Crow and Forrester 1991; Farley and Wu 1989). We examined this by assessing intrinsic excitability of Ii interneurons before and after the bath application of 5-HT. Because pronounced spike frequency accommodation occurred during the current step, we examined current elicited spike activity during the initial 1-s depolarization before and after 5-HT application. Spike activity evoked by 0.1-, 0.2-, or 0.3-nA current injection was increased in the presence of 5-HT (see Fig. 7). The analysis of the group data (Fig. 7, C and D) indicated that 5-HT significantly increased intrinsic excitability of type Ii interneurons at all levels of applied current (0.1 nA, t5 = 2.4; P < 0.05; 0.2 nA, t5 = 3.6; P < 0.01; 0.3 nA, t5 = 3.2; P < 0.025). In addition, the membrane potential of type Ii interneurons was significantly depolarized by the 5-HT application (t5 = 5.0; P < 0.01).
Fig. 6.
5-HT decreases the amplitude of light-evoked complex IPSPs and increases spontaneous spike activity of type Ii interneurons. A representative type Ii recording from a dark-adapted type Ii interneuron (A) and after 5-HT application (B). The bar above the recordings indicates the presentation of the 10-s light. The group summary data of 5-HT-dependent effects on light-evoked complex IPSPs and spontaneous spike activity are shown in C and D. *P < 0.01.
Fig. 7.
The effect of 5-HT on intrinsic excitability and MP in the type Ii interneurons. Representative examples of spike activity evoked by 0.1-, 0.2-, or 0.3-nA current injection before (Ctrl, A) and after 5-HT application (B). The group summary data of 5-HT effects on spike activity during the first 1-s depolarizing current injection are shown in C. The group data for 5-HT effects on MP are shown in D. *P < 0.05 and **P < 0.01.
Inhibitory effect of GABA on light-evoked complex EPSPs, spike activity, intrinsic excitability, and membrane potential in type Ie interneurons
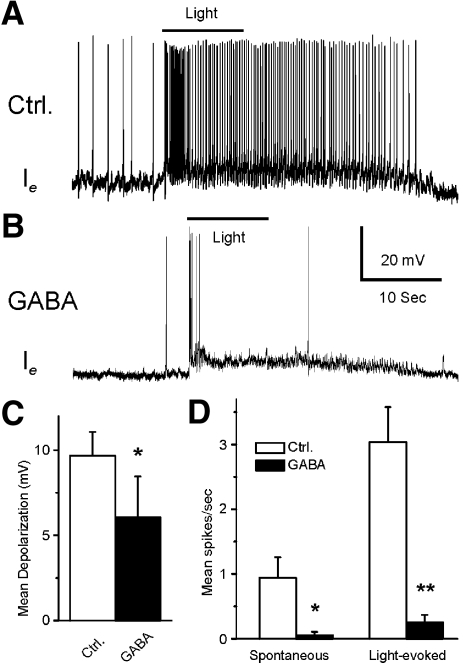
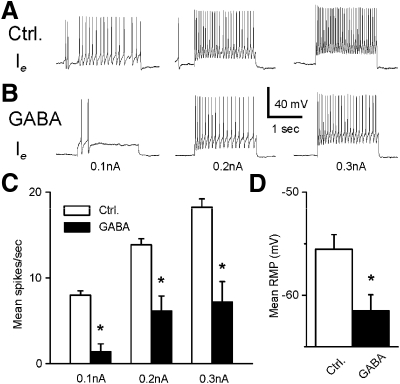
In all type Ie interneurons that were examined (n = 7), GABA produced an inhibitory effect. A representative Ie recording before and after GABA application is shown in Fig. 8. Consistent with other Ie recordings, light evoked a complex EPSP in the control recording (Fig. 8A). As shown in Fig. 8B, bath application of GABA produced a decrease in the amplitude of the light-evoked complex EPSP and a decrease in spontaneous and light-evoked spike activity. The analysis of group summary data shown in Fig. 8, C and D, revealed that GABA significantly decreased the amplitude of the light-evoked complex EPSPs (t6 = 2.9; P < 0.05), spontaneous (t6 = 2.9; P < 0.05), and light-evoked spike activity (t6 = 5.6; P < 0.01). The inhibitory effects of GABA may be due to the GABA-dependent inhibitory effect on photoreceptors (Alkon et al. 1993; Matzel et al. 1995; Rogers et al. 1994) or a direct effect of GABA on type Ie interneurons. This was examined by assessing intrinsic excitability of Ie interneurons before and after the bath application of GABA. Spike activity evoked by 0.1-, 0.2-, or 0.3-nA current injection was decreased in the presence of GABA (see Fig. 9). The analysis of the group data (Fig. 9, C and D) indicated that GABA significantly decreased intrinsic excitability of type Ie interneurons as measured by current evoked spike activity (0.1 nA, t6 = 7.7; P < 0.01; 0.2 nA, t6 = 3.9; P < 0.01; 0.3 nA, t6 = 4.1; P < 0.01). In addition, GABA significantly hyperpolarized type Ie interneurons (t6 = 3.7; P < 0.01).
Fig. 8.
The inhibitory effect of GABA on light-evoked complex EPSPs and spike activity in type Ie interneurons. Representative examples of type Ie light-evoked complex EPSPs after dark adaptation before (A) and after GABA application (B). The bar above the recordings indicates the presentation of the 10-s light. The group summary data of GABA-induced effects on light-evoked complex EPSPs, spontaneous and light-evoked spike activity are shown in C and D. *P < 0.05 and **P < 0.01. The inhibition of the light-evoked activity of type Ie interneurons may be due to a GABA-dependent decrease in excitability of photoreceptors and/or its direct inhibitory effect on type Ie interneurons.
Fig. 9.
The inhibitory effect of GABA on intrinsic excitability and MP of type Ie interneurons. Representative examples of spike activity evoked by 0.1-, 0.2-, or 0.3-nA current injections before (A) and after 5-HT application (B). The group summary data on the effects of GABA on spike activity and MP are shown in C and D. *P < 0.01. The GABA-induced decrease in excitability and hyperpolarization of the MP suggest a direct inhibitory effect of GABA on type Ie interneurons.
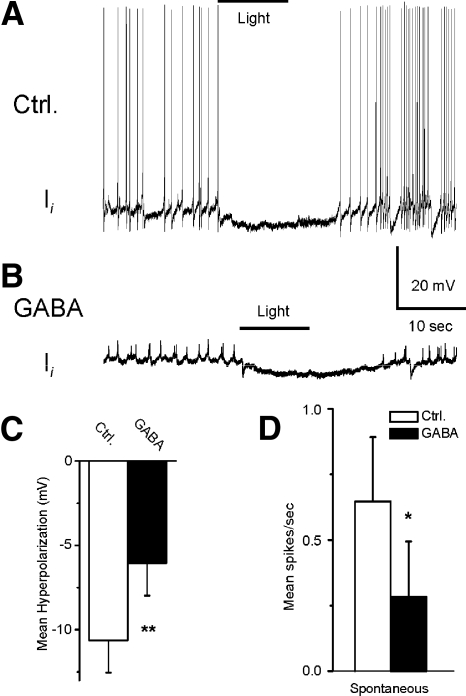
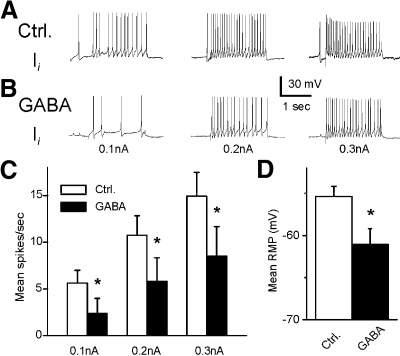
Effect of GABA on light-evoked complex IPSPs, spike activity, intrinsic excitability, and membrane potential of type Ii interneurons
A representative recording from a type Ii interneuron before (control) and after GABA application is shown in Fig. 10, A and B. The bath application of GABA decreased the amplitude of the light-evoked complex IPSP and decreased spontaneous spike activity (Fig. 10B). The analysis of group summary data shown in Fig. 10, C and D, revealed that GABA significantly decreased the amplitude of light-evoked complex IPSPs (t7 = 4.4; P < 0.01) and decreased spontaneous spike activity in type Ii interneurons (t7 = 3.3; P < 0.05). The results of GABA application shown here may be due to the GABA-dependent inhibitory effect on photoreceptors or a direct effect on the type Ii interneurons. This was examined by assessing intrinsic excitability of Ii interneurons before and after the bath application of GABA. Spike activity evoked by 0.1-, 0.2-, or 0.3-nA current injection was decreased after the application of GABA (Fig. 11). The analysis of the group summary data (Fig. 11, C and D) indicated that GABA significantly decreased intrinsic excitability of type Ii interneurons as measured by current evoked spike activity (0.1 nA, t7 = 2.6; P < 0.05; 0.2 nA, t7 = 3.1; P < 0.05; 0.3 nA, t7 = 3.3; P < 0.05). In addition, GABA produced a significant hyperpolarization of type Ii interneurons (t7 = 2.7; P < 0.05).
Fig. 10.
GABA decreases the amplitude of light-evoked complex IPSPs and spontaneous spike activity in the type Ii interneurons. Representative examples of light-evoked complex IPSPs in type Ii recordings after dark adaptation before (A) and after GABA application (B). The bar above the recordings indicates the presentation of the 10-s light. The group summary data of GABA-induced effects on light-evoked complex IPSPs and spontaneous spike activity are shown in C and D. *P < 0.05, **P < 0.01. The inhibition of the light-evoked activity in the type Ii interneurons may be due to a GABA-dependent decrease in excitability of photoreceptors and/or its direct inhibitory effect on type Ii interneurons.
Fig. 11.
Inhibitory effect of GABA on intrinsic excitability and membrane hyperpolarization in type Ii interneurons. Representative examples of spike activity evoked by 0.1-, 0.2-, or 0.3-nA current injections before (A) and after 5-HT application (B). The group summary data for spike activity and membrane hyperpolarization are shown in C and D. *P < 0.05. The GABA-induced decrease in excitability and membrane hyperpolarization suggest a direct inhibitory effect on type Ii interneurons.
DISCUSSION
Serotonin produces two effects on excitability of type Ie interneurons
In ∼50% of the type Ie interneurons examined in this study, 5-HT produced an increase in spontaneous and light-evoked spike activity, an increase in the amplitude of light-evoked complex EPSPs, an enhancement of intrinsic excitability, and a depolarization of the membrane potential. The remaining type Ie interneurons exhibited a decrease in the different measures of excitability as a result of 5-HT application. This suggests that there are two types of Ie interneurons that we have designated as Ie(A) and Ie(B), respectively. Additional support for the classification was obtained from whole cell voltage-clamp experiments. The sustained outward currents were decreased by 5-HT in four of eight type Ie interneurons examined under whole cell voltage clamp. The remaining type Ie interneurons exhibited an increase in sustained outward currents in the presence of 5-HT. In these experiments, most of the delayed rectifier K+ current remained at steady-state conditions because A-type K+ currents are typically inactivated after a 4-s voltage command step. It is well-documented that voltage-gated-delayed rectifier K+ currents play an important role in maintaining membrane potential and regulating electrical excitability in neurons as well as many other kinds of cells (Gutman et al. 2005). Thus the differential effects of 5-HT on the delayed rectifier K+ current may contribute to the differential effect of 5-HT on type Ie interneuron excitability. Similar differential effects of 5-HT were observed in two-electrode voltage-clamp studies of Hermissenda photoreceptors (Acosta-Urquidi and Crow 1993). The question could be raised concerning the coexistence of two different 5-HT receptors. There is evidence to suggest that two pharmacologically and physiologically distinct 5-HT receptors are expressed in the Leech and 5-HT terminals are capable of selectively activating only one of the receptors (Drapeau and Sanchez-Armass 1988). Although it is possible that two distinct 5-HT receptors might be differentially regulated by functionally distinct serotonergic terminals, under the present experimental conditions, the bath administration of 5-HT cannot specifically regulate presynaptic terminals and synaptic receptors. It has been shown that PKA and PKC are differentially recruited depending on the duration of 5-HT application (Braha et al. 1990; Hochner and Kandel 1992; Sugita et al. 1992) and on the state of the synapse (Braha et al. 1990; Ghirardi et al. 1992; Goldsmith and Abrams 1991). For example, facilitation of depressed synapses is blocked by inhibitors of PKC but not PKA, whereas the converse has been shown for nondepressed synapses (Braha et al. 1990; Ghirardi et al. 1992; Goldsmith and Abrams 1991). This state and time dependence of PKA and PKC recruitment by 5-HT remains unexplained (Byrne and Kandel 1996). Alternatively, it is possible that 5-HT activates two different receptor types expressed in the different type Ie(A) and Ie(B) interneurons.
Previous work has shown that photoreceptors form monosynaptic connections with type Ie interneurons (Akaike and Alkon 1980; Crow and Tian 2000). Because 5-HT has been shown to enhance photoreceptor excitability (Crow and Bridge 1985; Farley and Wu 1989), the increase in spontaneous and light-evoked spike activity, and the amplitude of the complex light-evoked EPSPs detected following 5-HT application in type Ie interneurons may be presynaptic. However, the present results also show that 5-HT produces an intrinsic enhancement of excitability in type Ie(A) interneurons.
It is likely that several cellular processes, both pre- and postsynaptic, contribute to the enhancement of activity in type Ie interneurons. The increase in spontaneous spike activity, light-evoked spikes, and light-evoked complex EPSPs may have both pre- and postsynaptic contributions.
It has been shown that 5-HT as a modulatory neurotransmitter is critical for associative learning of Leech shortening (Ehrlich et al. 1992; Sahley 1994) and may contribute to Pavlovian conditioning in Hermissenda (Crow 2004). Potentiation of excitability is an important mechanism for encoding and storing information during learning and memory in both vertebrates and invertebrates (Alkon et al. 1985; Antonov et al. 2001; Burrell et al. 2001; Cleary et al. 1998; Crow and Alkon 1980; Gainutdinov et al. 1998; Moyer et al. 1996, 2000; Oh et al. 2003; Saar et al. 1998; Stackman et al. 2002; Straub and Benjamin 2001; Thompson et al. 1996), and dysfunctions in the modulation of excitability may contribute to age-related deficits in learning and memory (Moyer et al. 2000; Wu et al. 2002).
Effect of 5-HT on type Ii interneurons
In contrast to the differential effect of 5-HT on type Ie(A) and Ie(B) interneurons, 5-HT increased the spontaneous spike activity in type Ii interneurons. Previous work has shown that 5-HT increases intrinsic excitability and potentiates the amplitude of generator potentials in Hermissenda photoreceptors (Crow and Bridge 1985; Crow and Siddiqi 1997; Grover et al. 1989). Therefore the 5-HT-dependent increase in the spontaneous spike activity in type Ii interneurons may be due to a 5-HT-induced increase in the activity of photoreceptors. In addition, 5-HT may also contribute to increased spike activity of the type Ii interneurons by enhanced excitability and membrane potential shifts. This is supported by the observation that 5-HT significantly increases intrinsic excitability. It has been reported that 5-HT2 receptor activation facilitates a persistent Na+ current in spinal motoneurons of rats (Harvey et al. 2006). It is also likely that closure of K+ channels may also contribute to the increase in excitability because 5-HT depolarizes the membrane potential in the type Ii interneurons. Serotonin can also attenuate Ca2+-activated K+ currents (Yamoah and Crow 1995) and delayed rectifier K+ currents as well as A-type K+ currents in Hermissenda photoreceptors (Acosta-Urquidi and Crow 1993). The effect of 5-HT on the reduction of the hyperpolarizing afterpotential is more pronounced in the type Ie(A) interneurons as compared with the type Ii interneurons, suggesting that the mechanism by which 5-HT attenuates accommodation may be different.
Effects of GABA on type Ie and Ii interneurons
As shown in Fig. 8 and 10, GABA produced a hyperpolarization of membrane potential in both type Ie and Ii interneurons. Previous work in Hermissenda photoreceptors reported that baclofen induced an increase in the amplitude of nonvoltage and voltage-dependent conductances, which contribute to the slow hyperpolarization that can be blocked by TEA (Matzel et al. 1995). These conductances may also be activated by GABA and contribute to the GABA-induced hyperpolarizations of both the type Ie and Ii interneurons. The application of GABA reduced the amplitude of the light-evoked complex EPSPs in type Ie interneurons and the light-evoked complex IPSPs in type Ii interneurons. Presynaptic GABA effects from photoreceptors may reduce the spontaneous and light-evoked spike activity while decreased intrinsic excitability of type Ie and type Ii interneurons is most likely postsynaptic. The inhibitory effect of GABA on the type Ie and Ii interneurons may be due to the activation of GABA receptors that results in a hyperpolarization and decreased spike activity of type B photoreceptors (Alkon et al. 1993; Matzel et al. 1995; Rogers et al. 1994) and decreased activity in the type Ie and Ii interneurons. Importantly, some effects of GABA on the type I interneurons may be different from those in B photoreceptors of Hermissenda. For example, GABA paired with intracellular depolarizations can induce enhanced excitability of type B photoreceptors (Matzel and Alkon 1991), a cellular change associated with Pavlovian conditioning (Crow and Alkon 1980; Farley 1987a,b; Farley and Alkon 1982; West et al. 1982). However, in the type Ie interneurons, the complex EPSP evoked by illumination in the presence of GABA induces not an increase, but a decrease in intrinsic excitability. Further study may be needed to unveil the mechanism of the discrepancy.
GRANTS
This work was supported by National Institute of Mental Health Grants MH-40860 and MH-58698 to T. Crow.
REFERENCES
- Acosta-Urquidi and Crow, 1993.Acosta-Urquidi J, Crow T. Differential modulation of voltage-dependent currents in Hermissenda type B photoreceptors by serotonin. J Neurophysiol 70: 541–548, 1993 [DOI] [PubMed] [Google Scholar]
- Akaike and Alkon, 1980.Akaike T, Alkon DL. Sensory convergence on central visual neurons in Hermissenda. J Neurophysiol 44: 501–513, 1980 [DOI] [PubMed] [Google Scholar]
- Alkon, 1973.Alkon DL. Neural organization of a molluscan visual system. J Gen Physiol 61: 444–461, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkon et al., 1993.Alkon DL, Anderson MJ, Kuzirian AJ, Rogers DF, Fass DM, Collin C, Nelson TJ, Kapetanovic IM, Matzel LD. GABA-mediated synaptic interaction between the visual and vestibular pathways of Hermissenda. J Neurochem 61: 556–566, 1993 [DOI] [PubMed] [Google Scholar]
- Alkon et al., 1985.Alkon DL, Sakakibara M, Forman R, Harrigan J, Lederhendler I, Farley J. Reduction of two voltage-dependent K+ currents mediates retention of a learned association. Behav Neural Biol 44: 278–300, 1985 [DOI] [PubMed] [Google Scholar]
- Antonov et al., 2001.Antonov I, Antonova I, Kandel ER, Hawkins RD. The contribution of activity-dependent synaptic plasticity to classical conditioning in Aplysia. J Neurosci 21: 6413–6422, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach et al., 1989.Auerbach SB, Grover LM, Farley J. Neurochemical and immunocytochemical studies of serotonin in the Hermissenda central nervous system. Brain Res Bull 22: 353–361, 1989 [DOI] [PubMed] [Google Scholar]
- Blackwell, 2002.Blackwell KT. Calcium waves and closure of potassium channels in response to GABA stimulation in Hermissenda type B photoreceptors. J Neurophysiol 87: 776–792, 2002 [DOI] [PubMed] [Google Scholar]
- Blackwell, 2006.Blackwell KT. Subcellular, cellular, and circuit mechanisms underlying classical conditioning in Hermissenda crassicornis. Anat Rec B New Anat 289: 25–37, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braha et al., 1990.Braha O, Dale N, Hochner B, Klein M, Abrams TW, Kandel ER. Second messengers involved in the two processes of presynaptic facilitation that contribute to sensitization and dishabituation in Aplysia sensory neurons. Proc Natl Acad Sci USA 87: 2040–2044, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrell et al., 2001.Burrell BD, Sahley CL, Muller KJ. Non-associative learning and serotonin induce similar bi-directional changes in excitability of a neuron critical for learning in the medicinal leech. J Neurosci 21: 1401–1412, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne and Kandel, 1996.Byrne JH, Kandel ER. Presynaptic facilitation revisited: state and time dependence. J Neurosci 16: 425–435, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary et al., 1998.Cleary LJ, Lee WL, Byrne JH. Cellular correlates of long-term sensitization in Aplysia. J Neurosci 18: 5988–5998, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow, 2004.Crow T. Pavlovian conditioning of Hermissenda: current cellular, molecular, and circuit perspectives. Learn Mem 11: 229–238, 2004 [DOI] [PubMed] [Google Scholar]
- Crow and Alkon, 1980.Crow TJ, Alkon DL. Associative behavioral modification in Hermissenda: cellular correlates. Science 209: 412–414, 1980 [DOI] [PubMed] [Google Scholar]
- Crow and Bridge, 1985.Crow T, Bridge MS. Serotonin modulates photoresponses in Hermissenda type-B photoreceptors. Neurosci Lett 60: 83–88, 1985 [DOI] [PubMed] [Google Scholar]
- Crow and Forrester, 1991.Crow T, Forrester J. Light paired with serotonin in vivo produces both short- and long-term enhancement of generator potentials of identified B-photoreceptors in Hermissenda. J Neurosci 11: 608–617, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow and Siddiqi, 1997.Crow T, Siddiqi V. Time-dependent changes in excitability after one-trial conditioning of Hermissenda. J Neurophysiol 78: 3460–3464, 1997 [DOI] [PubMed] [Google Scholar]
- Crow and Tian, 2000.Crow T, Tian LM. Monosynaptic connections between identified A and B photoreceptors and interneurons in Hermissenda: evidence for labeled-lines. J Neurophysiol 84: 367–375, 2000 [DOI] [PubMed] [Google Scholar]
- Crow and Tian, 2002a.Crow T, Tian LM. Facilitation of monosynaptic and complex PSPs in type I interneurons of conditioned Hermissenda. J Neurosci 22: 7818–7824, 2002a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow and Tian, 2002b.Crow T, Tian LM. Morphological characteristics and central projections of two types of interneurons in the visual pathway of Hermissenda. J Neurophysiol 87: 322–332, 2002b [DOI] [PubMed] [Google Scholar]
- Crow and Tian, 2003.Crow T, Tian LM. Neural correlates of Pavlovian conditioning in components of the neural network supporting ciliary locomotion in Hermissenda. Learn Mem 10: 209–216, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapeau and Sanchez-Armass, 1988.Drapeau P, Sanchez-Armass S. Selection of postsynaptic serotonin receptors during reinnervation of an identified leech neuron in culture. J Neurosci 8: 4718–4727, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich et al., 1992.Ehrlich JS, Boulis NM, Karrer T, Sahley CL. Differential effects of serotonin depletion on sensitization and dishabituation in the leech, Hirudo medicinalis. J Neurobiol 23: 270–279, 1992 [DOI] [PubMed] [Google Scholar]
- Farley, 1987a.Farley J. Contingency learning and causal detection in Hermissenda. I. Behavior. Behav Neurosci 101: 13–27, 1987a [DOI] [PubMed] [Google Scholar]
- Farley, 1987b.Farley J. Contingency learning and causal detection in Hermissenda. II. Cellular mechanisms. Behav Neurosci 101: 28–56, 1987b [DOI] [PubMed] [Google Scholar]
- Farley and Alkon, 1982.Farley J, Alkon DL. Associative neural and behavioral change in Hermissenda: consequences of nervous system orientation for light and pairing specificity. J Neurophysiol 48: 785–807, 1982 [DOI] [PubMed] [Google Scholar]
- Farley and Wu, 1989.Farley J, Wu R. Serotonin modulation of Hermissenda type B photoreceptor light responses and ionic currents: implications for mechanisms underlying associative learning. Brain Res Bull 22: 335–351, 1989 [DOI] [PubMed] [Google Scholar]
- Frysztak and Crow, 1997.Frysztak RJ, Crow T. Synaptic enhancement and enhanced excitability in presynaptic and postsynaptic neurons in the conditioned stimulus pathway of Hermissenda. J Neurosci 17: 4426–4433, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainutdinov et al., 1998.Gainutdinov KL, Chekmarev LJ, Gainutdinova TH. Excitability increase in withdrawal interneurons after conditioning in snail. Neuroreport 9: 517–520, 1998 [DOI] [PubMed] [Google Scholar]
- Ghirardi et al., 1992.Ghirardi M, Braha O, Hochner B, Montarolo PG, Kandel ER, Dale N. Roles of PKA and PKC in facilitation of evoked and spontaneous transmitter release at depressed and nondepressed synapses in Aplysia sensory neurons. Neuron 9: 479–489, 1992 [DOI] [PubMed] [Google Scholar]
- Goldsmith and Abrams, 1991.Goldsmith BA, Abrams TW. Reversal of synaptic depression by serotonin at Aplysia sensory neuron synapses involves activation of adenylyl cyclase. Proc Natl Acad Sci USA 88: 9021–9025, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover et al., 1989.Grover LM, Farley J, Auerbach SB. Serotonin involvement during in vitro conditioning of Hermissenda. Brain Res Bull 22: 363–372, 1989 [DOI] [PubMed] [Google Scholar]
- Gutman et al., 2005.Gutman GA, Chandy KG, Grissmer S, Lazdunski M, McKinnon D, Pardo LA, Robertson GA, Rudy B, Sanguinetti MC, Stuhmer W, Wang X. International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacol Rev 57: 473–508, 2005 [DOI] [PubMed] [Google Scholar]
- Harvey et al., 2006.Harvey PJ, Li X, Li Y, Bennett DJ. 5-HT2 receptor activation facilitates a persistent sodium current and repetitive firing in spinal motoneurons of rats with and without chronic spinal cord injury. J Neurophysiol 96: 1158–1170, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochner and Kandel, 1992.Hochner B, Kandel ER. Modulation of a transient K+ current in the pleural sensory neurons of Aplysia by serotonin and cAMP: implications for spike broadening. Proc Natl Acad Sci USA 89: 11476–11480, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land and Crow, 1985.Land PW, Crow T. Serotonin immunoreactivity in the circumesophageal nervous system of Hermissenda crassicornis. Neurosci Lett 62: 199–205, 1985 [DOI] [PubMed] [Google Scholar]
- Matzel and Alkon, 1991.Matzel LD, Alkon DL. GABA-induced potentiation of neuronal excitability occurs during contiguous pairings with intracellular calcium elevation. Brain Res 554: 77–84, 1991 [DOI] [PubMed] [Google Scholar]
- Matzel et al., 1995.Matzel LD, Muzzio IA, Rogers RF. Diverse current and voltage responses to baclofen in an identified molluscan photoreceptor. J Neurophysiol 74: 506–518, 1995 [DOI] [PubMed] [Google Scholar]
- Moyer JR, Jr, Power JM, Thompson LT, Disterhoft JF. Increased excitability of aged rabbit CA1 neurons after trace eyeblink conditioning. J Neurosci 20: 5476–5482, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer JR, Jr, Thompson LT, Disterhoft JF. Trace eyeblink conditioning increases CA1 excitability in a transient and learning-specific manner. J Neurosci 16: 5536–5546, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh et al., 2003.Oh MM, Kuo AG, Wu WW, Sametsky EA, Disterhoft JF. Watermaze learning enhances excitability of CA1 pyramidal neurons. J Neurophysiol 90: 2171–2179, 2003 [DOI] [PubMed] [Google Scholar]
- Rogers et al., 1994.Rogers RF, Fass DM, Matzel LD. Current, voltage and pharmacological substrates of a novel GABA receptor in the visual-vestibular system of Hermissenda. Brain Res 650: 93–106, 1994 [DOI] [PubMed] [Google Scholar]
- Rogers and Matzel, 1995.Rogers RF, Matzel LD. G-protein mediated responses to localized serotonin application in an invertebrate photoreceptor. Neuroreport 6: 2161–2165, 1995 [DOI] [PubMed] [Google Scholar]
- Saar et al., 1998.Saar D, Grossman Y, Barkai E. Reduced after-hyperpolarization in rat piriform cortex pyramidal neurons is associated with increased learning capability during operant conditioning. Eur J Neurosci 10: 1518–1523, 1998 [DOI] [PubMed] [Google Scholar]
- Sahley, 1994.Sahley CL. Serotonin depletion impairs but does not eliminate classical conditioning in the leech Hirudo medicinalis. Behav Neurosci 108: 1043–1052, 1994 [DOI] [PubMed] [Google Scholar]
- Sakakibara et al., 1993.Sakakibara M, Takagi H, Yoshioka T, Alkon DL. Propagated calcium modulates the calcium-dependent potassium current by the activation of GABAB receptor at the axonal branch in the type B photoreceptor of Hermissenda. Ann NY Acad Sci 707: 492–495, 1993 [DOI] [PubMed] [Google Scholar]
- Schultz and Clark, 1997.Schultz LM, Clark GA. GABA-induced synaptic facilitation at type B to A photoreceptor connections in Hermissenda. Brain Res Bull 42: 377–383, 1997 [DOI] [PubMed] [Google Scholar]
- Schuman and Clark, 1994.Schuman EM, Clark GA. Synaptic facilitation at connections of Hermissenda type B photoreceptors. J Neurosci 14: 1613–1622, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackman et al., 2002.Stackman RW, Hammond RS, Linardatos E, Gerlach A, Maylie J, Adelman JP, Tzounopoulos T. Small conductance Ca2+-activated K+ channels modulate synaptic plasticity and memory encoding. J Neurosci 22: 10163–10171, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub and Benjamin, 2001.Straub VA, Benjamin PR. Extrinsic modulation and motor pattern generation in a feeding network: a cellular study. J Neurosci 21: 1767–1778, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita et al., 1992.Sugita S, Goldsmith JR, Baxter DA, Byrne JH. Involvement of protein kinase C in serotonin-induced spike broadening and synaptic facilitation in sensorimotor connections of Aplysia. J Neurophysiol 68: 643–651, 1992 [DOI] [PubMed] [Google Scholar]
- Thompson LT, Moyer JR, Jr, Disterhoft JF. Transient changes in excitability of rabbit CA3 neurons with a time course appropriate to support memory consolidation. J Neurophysiol 76: 1836–1849, 1996 [DOI] [PubMed] [Google Scholar]
- Tian et al., 2006.Tian LM, Kawai R, Crow T. Serotonin-immunoreactive CPT interneurons in Hermissenda: identification of sensory input and motor projections. J Neurophysiol 96: 327–335, 2006 [DOI] [PubMed] [Google Scholar]
- West et al., 1982.West A, Barnes E, Alkon DL. Primary changes of voltage responses during retention of associative learning. J Neurophysiol 48: 1243–1255, 1982 [DOI] [PubMed] [Google Scholar]
- Wu et al., 2002.Wu WW, Oh MM, Disterhoft JF. Age-related biophysical alterations of hippocampal pyramidal neurons: implications for learning and memory. Ageing Res Rev 1: 181–207, 2002 [DOI] [PubMed] [Google Scholar]
- Yamoah and Crow, 1995.Yamoah EN, Crow T. Evidence for a contribution of ICa to serotonergic modulation of IK,Ca in Hermissenda photoreceptors. J Neurophysiol 74: 1349–1354, 1995 [DOI] [PubMed] [Google Scholar]
- Yamoah and Crow, 1996.Yamoah EN, Crow T. Protein kinase and G-protein regulation of Ca2+ currents in Hermissenda photoreceptors by 5-HT and GABA. J Neurosci 16: 4799–4809, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]