Abstract
Although rare, interneurons are pivotal in governing striatal output by extensive axonal arborizations synapsing on medium spiny neurons. Using a genetically modified mouse strain in which a green fluorescent protein (GFP) is driven to be expressed under control of the neuropeptide Y (NPY) promoter, we identified NPY interneurons and compared them with striatal principal neurons. We found that the bacteria artificial chromosome (BAC)-npy mouse expresses GFP with high fidelity in the striatum to the endogenous expression of NPY. Patch-clamp analysis from NPY neurons showed a heterogeneous population of striatal interneurons. In the majority of cells, we observed spontaneous firing of action potentials in extracellular recordings. On membrane rupture, most NPY interneurons could be classified as low-threshold spiking interneurons and had high-input resistance. Voltage-clamp recordings showed that both GABA and glutamate gated ion channels mediate synaptic inputs onto these striatal interneurons. AMPA receptor–mediated spontaneous excitatory postsynaptic currents (sEPSCs) were small in amplitude and infrequent in NPY neurons. Evoked EPSCs did not show short-term plasticity but some rectification. Evoked N-methyl-d-aspartate (NMDA) EPSCs had fast decay kinetics and were poorly sensitive to an NR2B subunit containing NMDA receptor blocker. Spontaneous inhibitory postsynaptic currents (sIPSCs) were mediated by GABAA receptors and were quite similar among all striatal neurons studied. On the contrary, evoked IPSCs decayed faster in NPY neurons than in other striatal neurons. These data report for the first time specific properties of synaptic transmission to NPY striatal interneurons.
INTRODUCTION
The striatum is a key brain center for coordinating the proper selection of motor behaviors (Graybiel 2008). It is a heterogeneous nucleus containing a majority of medium spiny projection neurons (MSNs) and a minority of spiny and aspiny or sparsely spiny interneurons (Tepper and Bolam 2004). Among striatal interneurons, at least four classes have been identified in rodent brain. Choline acetyl-transferase, parvalbumin, calretinin, and neuropeptide Y (NPY) immunoreactive neurons have been differentiated anatomically. Many striatal interneurons have also been described electrophysiologically with the exception of calretinin positive neurons (Bennett et al. 2000; Kawaguchi 1993; Koos and Tepper 1999).
The expression pattern of NPY protein in the CNS is generally associated with inhibitory interneurons of several different classes (Allen et al. 1983; Aoki and Pickel 1989; Karagiannis et al. 2009). Within the rodent striatum, most NPY positive interneurons co-express somatostatin, NO synthase, and NADPH diaphorase (Figueredo-Cardenas et al. 1996). These neurons form a distinct class of striatal GABAergic interneurons from those that express parvalbumin and calretinin. Together, the complement of striatal GABAergic interneurons makes ∼2–3% of striatal neurons. Parvalbumin neurons are referred to as fast-spiking (FS) based on their electrophysiological properties. NPY neurons are likely associated with cells that fire low threshold spikes (LTSs), sometimes associated with a persistent plateau and that show an aspiny phenotype (Kawaguchi 1993, 1997; Koos and Tepper 1999). In target MSNs, most striatal GABAergic interneurons form extensive functional inhibitory synapses. These produce large inhibitory postsynaptic currents (IPSCs) making interneurons very important players in striatal function (Koos and Tepper 1999; Koos et al. 2004; Tepper and Bolam 2004).
NPY is a major neuropeptide transmitter in the CNS and peripheral nervous system. This neurotransmitter has pleiotropic activities in vivo ranging from stimulating appetite (Chee and Colmers 2008) to promoting stress-induced obesity (Kuo et al. 2007). NPY also participates in the regulation of vasoconstriction (Ruohonen et al. 2008), vascular remodeling (Abe et al. 2007), and seizure susceptibility (Thorsell and Heilig 2002). Elevated levels of NPY in the striatum have been associated with behavioral social isolation (Thorsell et al. 2006) and alcohol preference in rats (Kimpel et al. 2007). Gaining a better understanding of striatal NPY interneurons will be an important step in clarifying the contribution of these cell types to basal ganglia output. In this study, we took advantage of genetic manipulations that allow identification of NPY expressing neurons. Because they are quite rare, very little is known about the synaptic afferent properties of these neurons.
Transgenic mice in which the NPY promoter drives the expression of a green fluorescent protein (GFP) have been previously used to characterize NPY interneurons in the hippocampus (Fu and van den Pol 2007). We took advantage of a similar mouse model, selecting GFP expressing cells (NPY+) to study and compare membrane properties, action potential firing, and synaptic physiology with nonfluorescent striatal cells (NPY−). We specifically studied AMPA, N-methyl-d-aspartate (NMDA), and GABAA receptor–mediated synaptic responses. We found that NPY+ neurons had similarities and differences in their synaptic responses in comparison to NPY− cells. These findings extend our knowledge on how specific types of neurons in the striatum process information to incoming excitatory and inhibitory inputs.
METHODS
Slice preparation
Bacteria artificial chromosome (BAC) mice aged 2–3 wk, in which the NPY (BAC-npy) promoter was attached to a humanized Renilla GFP (Stock 006417, Jackson Laboratory, Bar Harbor, ME) (van den Pol et al. 2009) were killed by decapitation in agreement with the Georgetown University Animal Care and Use Committee. In a subset of experiments to confirm NPY− characteristics, we examined BAC-drd2-EGFP animals (Gong et al. 2003). Brains were rapidly removed and placed in an ice-cold slicing solution containing (in mM) 75 sucrose, 85 NaCl, 3 KCl, 1 CaCl2, 4 MgCl2, 1 NaH2PO4, 25 NaHCO3, and 25 glucose. Coronal slices (250 μm) containing the striatum were prepared using a Vibratome 3000 Plus Sectioning System (Vibratome, St. Louis, MO). Sections recovered in slicing solution at 32°C for 30 min were transferred to artificial cerebrospinal fluid (ACSF) containing (in mM) 124 NaCl, 4.5 KCl, 1.2 Na2HPO4, 26.0 NaHCO3, 2.0 CaCl2, 1.0 MgCl2, and 10.0 dextrose at 32°C for 30 min. During experiments, slices were completely submerged and continuously perfused (2–3 ml/min) with ACSF at room temperature (23–26°C). All solutions were maintained at pH 7.4 by continuous bubbling with 95% O2-5% CO2.
Electrophysiological recordings
Hemislices containing the cortex and the anterior striatum were completely submerged in a perfusion chamber. Slices were visualized under an upright microscope equipped with Nomarski optics and an electrically insulated ×60 water immersion objective with a long working distance (2 mm) and high numerical aperture (1.0). Identification of GFP-expressing neurons was performed by epifluorescent excitation of the tissue with a mercury-based lamp and standard GFP filter sets. Recording electrodes were pulled on a vertical pipette puller from borosilicate glass capillaries (Wiretrol II, Drummond, Broomall, PA). For examination of glutamate receptor responses, ruptured-patch whole cell voltage-clamp recordings were obtained using pipettes (4- to 6-MΩ tip resistance) filled with an internal solution containing (in mM) 145 potassium gluconate, 10 HEPES, 10 BAPTA, 0.2 Na-GTP, and 5 Mg-ATP, and supplemented with 5 lidocaine N-ethyl bromide (QX-314), with pH adjusted to 7.4 with KOH. When examining synaptic NMDA responses, extracellular magnesium was eliminated, whereas 10 μM d-serine (Sigma-Aldrich) was included in the recording media. GABAA responses were examined similarly except patch pipettes were filled with KCl containing (in mM) 145 KCl 145, 10 HEPES, 5 Mg-ATP, 0.2 Na-GTP, and 10 EGTA, adjusted to pH 7.2 with KOH. AMPA receptor–mediated current-voltage relationships were measured using pipettes filled with a solution that consisted of (in mM) 120 CsMeSO3, 5 NaCl, 10 TEA-Cl, 10 HEPES, 5 QX-314, 1.1 EGTA, 4 Mg-ATP, and 0.3 Na-GTP, with and without the addition of 0.1 spermine. Voltage-clamp recordings were performed using the whole cell configuration of the patch-clamp technique at a holding potential of −70 (glutamate transmission) or −60 mV (GABA transmission) using either a Multiclamp 700B or an Axopatch 200B amplifier (Molecular Device, Sunnyvale CA). Input and access resistances were monitored by 5-mV hyperpolarization steps during recordings, and experiments with >5% change were discarded.
For anatomical reconstruction, biocytin was added at a final concentration of 1% to the intracellular solution. On achieving whole cell recordings, biocytin was allowed to diffuse through the cell for ∼3 min. After whole cell recording, the pipette was gently removed to form an outside-out patch, and the slices were fixed in 4% paraformaldehyde for 30 min. After fixation, slices were washed in PBS and stained with Texas red–conjugated avidin-D dye (Invitrogen, Carlsbad, CA) at 2.5 μl/ml for 2 h. Slices were mounted on microscope slides and processed for confocal imaging as described below.
Stock solutions of bicuculline methobromide (BMR), TTX, 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulfonamide disodium salt hydrate (NBQX), (±)-3-(2-carboxypiperazin-4-yl)propyl-1-phosphonic acid (CPP), spermine (all from Sigma), and (2S*,3R*)-1-(phenanthrene-2-carbonyl)piperazine-2,3-dicarboxylic acid (PPDA; Tocris Bioscience, Ellisville, MO) were prepared in water. (1S,2S)-1-(4-hydroxyphenyl)-2-(4-hydroxy-4-phenylpiperidino)-1-propanol (CP101606), a gift from Dr. Richard Woodward (Acea Pharmaceutical, Irvine, CA), was dissolved in dimethylsulfoxide (<0.1% final concentration). Drug-containing stock solutions were diluted to the desired concentration in ACSF and applied locally through the “Y tube” method (Murase et al. 1989). Currents were low-pass filtered at 2 kHz and digitized at 5–10 kHz using a Dell computer equipped with Digidata 1322A data acquisition board and pCLAMP9 software (Molecular Devices). Off-line data analysis, curve fitting, and figure preparation were performed with Clampfit 9 or Igor 6 software (Wavemetrics, Lake Oswego, OR).
Immunolocalization
Brain sections were prepared from mice aged ∼3 wk postnatally. After isoflurane anesthesia, mice were perfused transcardially with 5% glutaraldehyde, and whole brains were dissected and stored in 30% sucrose solution overnight at 4°C. Coronal sections (100 μm) were cut with a Lancer Vibrotome (Series 1000, Sherwood Medical, St. Louis, MO). Fixed, free-floating tissue sections were blocked with 4% normal donkey serum in PBS for 1 h at room temperature and washed three times for 10 min in PBS/0.1% Triton-X100 (Tx). The following 1° antibodies were diluted in PBS/Tx/1% BSA: rabbit α-NPY (ab10980; 1:800; Abcam, Cambridge, MA), mouse α-parvalbumin (P3088 1:100; Sigma), and goat α-ChAT (ab144p; 1:100; Millipore, Bellerica, MA). The 1° antibody incubation step was 12–18 h in duration at 4°C, and slices were washed three times for 10 min in PBS/Tx. 2° antibodies, conjugated to Alexa-fluor 555 (A-21428, A-21422, and A-21432, Invitrogen, Carlsbad, CA), specific for the 1° antibody species of origin were diluted 1:500 in PBS/Tx/BSA and exposed to tissue for 2–4 h at room temperature. The 2° antibody was washed three times for 10 min with PBS/Tx, and sections were placed on microscope slides and sealed under glass coverslips with VectaShield H-1000 mounting media (Vector Labs, Burlingame, CA). For confocal imaging, an Olympus Fluoview-FV300 laser scanning confocal microscope was used for excitation of endogenous GFP or Alexa-555 fluorophores. z-stack images (1 μm thick) were processed with Metamorph software (Molecular Devices). Images presented in Fig. 1,C–F, represent a composite image of 14–16 z-stacks. Colocalization of immunostaining overlap was performed by manually tracing regions of interest corresponding to the cell bodies of GFP + neurons or Alexa-555 + neurons.
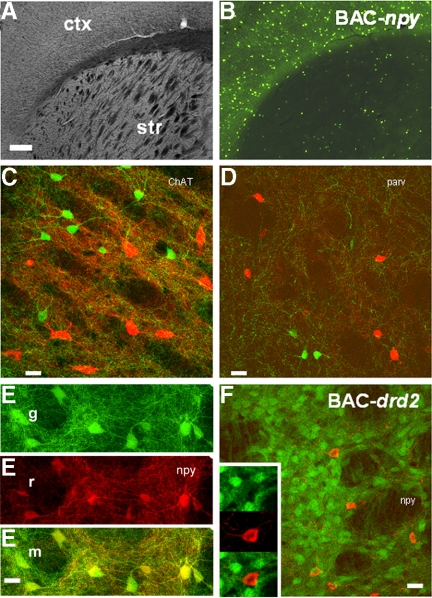
Fig. 1.
Distribution of green fluorescent protein (GFP) in the bacteria artificial chromosome (BAC)-npy mouse strain. Phase contrast (A) and fluorescent (B) photomicrographs of a coronal section of mouse brain including cortex (ctx) and striatum (str). Note the sparse distribution of GFP-expressing neurons in striatum compared with cortex. Images shown in A and B show the same field of view. Scale bar, 250 μm. Immunohistochemical detection of choline acetyltransferase (ChAT, C), parvalbumin (parv, D) and neuropeptide Y (NPY; npy, E and F) in confocal z-stack projections of striatum. Antigen detection visualized with species selective 2°antibody conjugated to Alexa Fluor 555 (in red). Eg and Er images correspond to fluorescence detection on green and red channels, respectively. Em image is merged image of these 2 channels. F: NPY immunoreactivity (in red) in the BAC-drd2 mouse strain showing the lack of expression overlap. Insets: 1.5× zoom for more detail. Scale bars in C–F, 25 μm.
Data analysis
To sample a representative population, each variable examined in this study was derived from at least three different animals from at least “two” or more breeding pairs. Spontaneous and miniature postsynaptic currents (PSCs), both excitatory and inhibitory, were identified using semiautomated threshold-based mini-detection software (Mini Analysis, Synaptosoft, Fort Lee, NJ) and were visually confirmed. Event detection threshold was set at 5 times the RMS level of baseline noise. PSC average values were based on >50 events in each cell studied, and the decay kinetics were determined using double exponential curve fittings and reported as weighted time constants (Tw) (Logan et al. 2007). NBQX was not included in sIPSC measurements to not perturb the network activity. AMPA-mediated spontaneous EPSCs could easily be identified by the rapid decay kinetics (<2 ms) (Ortinski et al. 2004) and were excluded from the analysis. mIPSCs were isolated by application of TTX (0.5 μM). All detected events were used for event frequency analysis; however, superimposing events were eliminated for the amplitude, rise time, and decay kinetic analysis. Box and whisker plots were used to show the distribution of data points in some data sets. The whiskers correspond to data set percentiles of 10 and 90. The box portion of these plots corresponds to percentiles of 25 and 75, with the median shown by a central bar. Statistical significance was determined using the unpaired two-tailed Student's t-test. All data values are expressed as means ± SE.
RESULTS
We used epifluorescent microscopy to ascertain the expression pattern of fluorescent protein in the BAC-npy strain. Shown in Fig. 1A is a brightfield image of a 100-μm coronal brain slice obtained from a BAC-npy mouse showing striatum, the overlying corpus callosum, and cortex. In Fig. 1B, the same field was viewed with a GFP excitation/emission filter to show GFP-positive cells. The relative abundance of green cells in the cortex in comparison to the striatum is consistent with NPY expression in the rat and mouse nervous system (Allen et al. 1983; Aoki and Pickel 1989). Next we used selective antibodies to known striatal interneuron antigens, in combination with confocal microscopy, to verify that the BAC-npy mouse was suitable to study a specific striatal interneuron subclass. In Fig. 1C, choline acetyltransferase antibodies were used to identify the cholinergic class of striatal interneurons. Secondary antibodies tagged with a red fluorophore were subsequently used to identify these large aspiny neurons. As shown in Fig. 1C, there is no overlap of red signal with endogenous green fluorescent signal. We conducted similar experiments in Fig. 1, D–F. In Fig. 1D, we used an antibody selective for parvalbumin, and in Fig. 1E, we used an antibody selective for NPY. There was no overlap in signal with the parvalbumin antibody, whereas there was strong overlap of both red and green signals with the NPY antibody. Analysis of >300 neurons from 10 images and three BAC-npy mice showed that 9% of GFP+ cells did not stain with the NPY antibody and 1% of antibody staining did not co-localize with endogenous GFP expression. Additionally, we performed NPY immunostaining in a separate mouse strain, the BAC-drd2, used to differentiate striato-pallidal output neurons and observed no overlap of red and green signals in this mouse strain. Together, these data show that the mouse BAC-npy strain is an excellent model in which to selectively study NPY-expressing neurons within the striatum.
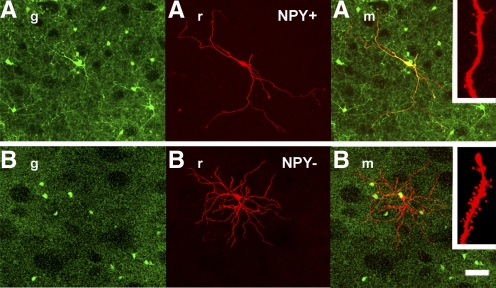
We next injected biocytin to better characterize morphological aspects of NPY+ and NPY− neurons. As shown in the example in Fig. 2, NPY+ cells differ considerably from NPY− cells. NPY+ cells showed infrequent, longitudinal, and sparsely spiny dendrites. Conversely, NPY− cells had characteristics typical of medium spiny neurons including frequent, tortuous branching and spiny dendrites.
Fig. 2.
Morphology of BAC-npy striatal neurons. Confocal z-stack images showing images of biocytin-filled NPY+ (A) and NPY− (B) cells. g and r images correspond to fluorescence detection on green and red channels, respectively. m image is the merged images of these 2 channels. Insets: magnification of a dendrite to show sparsely spiny (NPY+) or spiny (NPY−) dendritic segments. Note that the soma of the filled neuron in B is in proximity of an GFP+ neuron, but the fluorescence does not overlap. Calibration bar, 50 and 5 μm for insets.
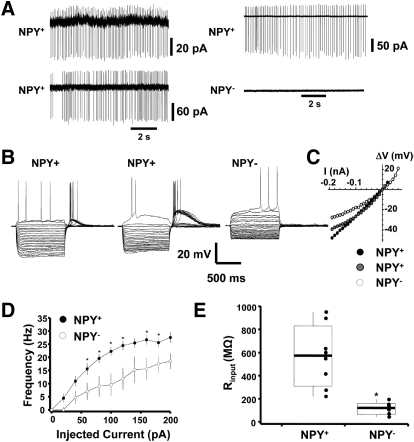
We next determined the membrane properties of NPY+ cells within the striatum. Initially, we conducted extracellular recordings with cell-attached or loose-patch configurations from neighboring NPY+ and NPY− cells located in the visual field (∼150 μm). Figure 3 shows simultaneous dual recordings from two NPY+ neurons (Fig. 3A, left) and one NPY+ cell and one NPY− neuron (Fig. 3A, right). We observed spontaneous firing of action potentials in most NPY+ neurons in this configuration (16 of 19 cells, 84%). In six NPY+ neurons, the average firing rate was 4.5 ± 1.1 Hz at room temperature when ACSF was in the internal pipette. Spontaneous firing frequency did not change at 32°C (n = 4, P > 0.05) or when K-gluconate–based solution (n = 6, P > 0.05) was in the recording pipette. Simultaneous loose-patch recordings from six NPY+ pairs showed that the spontaneous firing was not synchronized between cells.
Fig. 3.
Passive and active membrane properties of BAC-npy striatal neurons. A: simultaneous loose patch recordings from 2 NPY+ neurons (left) and 1 NPY+ and 1 NPY− neuron (right). Spontaneous action potentials were detected in NPY+ neurons only. B: superimposed representative current-clamp records to hyperpolarizing and depolarizing current injections in distinct examples of NPY+ cells (left, center) and an NPY− cell (right). C: voltage changes (ΔV) after current injections (10-pA steps) from a set membrane potential. D: spike frequency plots of NPY+ and NPY− neurons as a function of depolarizing injection current amplitude. Data derived from 9 NPY+ and 6 NPY− neurons held at −70 mV. E: box and whisker plot range of input resistance (Rinput) values.
We also used whole cell patch-clamp experiments in voltage- and current-clamp modes to determine electrophysiological parameters of these cell types. We found that, in current clamp with KCl-based internal solution, many NPY+ neurons still fired spontaneously, confounding a reliable measure of resting membrane potential. The firing frequency in this condition was significantly (P < 0.05) higher and averaged 12.3 ± 1.8 Hz (n = 10). We did not observe any spontaneous firing in NPY− cells in either recording condition. To compare membrane properties, we injected steady currents (ranging from >10 to 100 pA) to drive membrane potentials to near −70 mV for NPY− and −60 mV for NPY+ neurons. Under these conditions, we subjected neurons to 1,000-ms hyperpolarizing and depolarizing current injections, as in Fig. 3B. We studied 20 NPY+ neurons with this protocol. In 15 of these neurons, we observed repetitive firing to depolarizing current injections and rebound action potentials after hyperpolarizing current pulses, as shown in the example in Fig. 3B (left). Some of these properties have been reported as hallmark features of LTS striatal interneurons (Dehorter et al. 2009; Tepper and Bolam 2004). In the remaining five NPY+ neurons, we observed the presence of a persistent depolarization as shown in Fig. 3B (center), similar to persistent, low threshold spike (PLTS) neurons previously described (Kawaguchi 1993). As shown in this example, strong hyperpolarization showed the presence of an anomalous rectifying voltage sag. In addition, the afterhyperpolarization after each spike was remarkably large. However, the occurrence of voltage sags or persistent depolarizations was often observed in many NPY+ cells, making it difficult to classify these neurons unequivocally as LTS or PLTS types. We did not observe this firing pattern in NPY− neurons as shown in Fig. 3B (right). Current-voltage relationships shown in Fig. 3C illustrate the differences between these types of striatal cells. The relationship between injection current amplitude and spike frequency were compared between NPY+ and NPY− neurons in current clamp. The NPY+ cells began to fire action potentials with smaller current injections than the NPY− cells (NPY+, 28 ± 4 pA, n = 8; NPY−, 73 ± 24 pA, n = 6; P < 0.05). The NPY+ cells had a significantly faster firing frequency at several values of injected current than the NPY− cells (Fig. 3D). Input resistance was also compared between these neurons from the steady-state portion of the voltage response to current injections. As shown in the box and whisker plot in Fig. 3E, NPY+ neurons had significantly higher input resistance than NPY− neurons (P < 0.01), another characteristic of striatal LTS or PLTS neurons.
We characterized pharmacologically isolated spontaneous and evoked excitatory glutamatergic EPSCs. As shown in Fig. 4, sEPSCs were recorded in both NPY+ (n = 5) and NPY− (n = 7) neurons in the presence of 25 μM BMR and 10 μM CPP in voltage clamp at −70 mV. In NPY+ interneurons, sEPSCs were significantly less frequent and smaller in amplitude than sEPSCs in NPY− cells from BAC-npy mice or BAC-drd2 MSNs (Fig. 4B). We also characterized EPSCs evoked when stimulating local afferents within the striatum in the presence of 25 μM BMR and 10 μM CPP (Fig. 4, C and D). Under these conditions, EPSCs did not differ between NPY+ and NPY− neurons and were completely abolished by 5 μM NBQX (data not shown). EPSC size depended on stimulation intensity in both cell types, and the decay time was 9.9 ± 1.6 and 9.5 ± 2.2 ms for five NPY+ and eight NPY− neurons, respectively. To ascertain short-term plasticity, we performed paired pulse stimulation using a 50-ms interval as shown in Fig. 4C. The paired pulse ratios were near 1.0 with this protocol in both NPY+ and NPY− cells, consistent with previous reports in MSNs of mice at matching ages (Tang et al. 2001). We studied current to voltage relationships using an intracellular recording solution with cesium methane sulfonate with and without 100 μM spermine (see methods). In 11 NPY+ interneurons, the EPSC reversed close to 0 mV (Fig. 4, E and F). Current size was smaller at positive potentials when spermine was included, suggesting that EPSCs are mediated in part by AMPA receptors lacking GluR2 subunits, in constrast to what has been reported in MSNs (Kreitzer and Malenka 2007).
Fig. 4.
AMPA receptor–mediated synaptic transmission in BAC-npy striatal neurons. Representative spontaneous excitatory postsynaptic current (sEPSC) traces from an NPY+ (left) and an NPY− cell (right). B: summary histograms of the mean sEPSC frequency (left) and amplitude (right) observed in 5 NPY+ (filled bar), 7 NPY− (open bar), and 5 drd2+ medium spiny neurons (MSNs; gray bar). Error bars represent the SE in each data set. *P < 0.05 comparing data from the pool of NPY− and drd2+ MSNs. (C) Averaged traces of evoked AMPA receptor–mediated currents to a paired stimulation protocol from an NPY+ (left) and an NPY− (right) cell. D: summary plot of the AMPA receptor–mediated paired pulse ratio (PPR) from 5 NPY+ (filled bar) and 7 NPY− (open bar) neurons. Each recording was performed in the presence of 25 μM bicuculline methobromide (BMR) and 10 μM (±)-3-(carboxypiperazin-4-yl)propyl-1-phosphonic acid (CPP) to block GABAA and N-methyl-d-aspartate (NMDA) receptor responses, respectively. E: superimposed traces of evoked AMPA EPSCs recorded at distinct holding potentials in an NPY+ neuron with intracellular spermine. F: summary plot of the current-voltage relationship (circles with connecting line) showing the voltage dependence of the percent of AMPA EPSCs recorded at −60 mV in 6 NPY+ neurons in the absence of intracellular spermine (○) and 11 NPY+ neurons in the presence of spermine (●). Error bars represent SE.
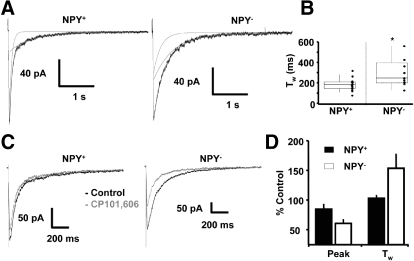
In separate experiments, we characterized NMDA receptor–mediated EPSCs on extensive perfusion of the striatal slice with a solution lacking Mg2+ while in the presence of GABAA and AMPA receptor antagonists BMR and NBQX (Fig. 5). Double exponential fitting of the NMDA EPSCs decay showed significantly faster currents in NPY+ neurons as summarized in Fig. 5B. Stimulation intensity studies showed NMDA EPSCs of increasing size with stimulus strength in NPY+ neurons (data not shown). The fold change in peak amplitude averaged 3.8 ± 1.0 under these conditions. The decay of the NMDA EPSCs slightly increased with stimulus strength from that measured with smallest stimulus intensity in nine cells studied (fold change, 1.1 ± 0.1). In some neurons, there was a more substantial change in Tw on increasing stimulus intensity. We also compared the sensitivity of the NMDA EPSCs to the NR1/NR2B receptor subtype–specific antagonist CP101606 (10 μM). As shown in Fig. 5, C and D, a small decrease of current amplitude (15 ± 9%) in the presence of this drug was not paralleled by changes in the NMDA EPSC decay in six NPY+ neurons. However, in five NPY− neurons, CP101606 decreased the peak NMDA EPSC amplitude by 39 ± 7%. In contrast to NPY+ neurons, CP101606 increased the Tw by 53 ± 24%. We also attempted to pharmacologically dissect NMDA EPSCs in striatal neurons with the putative NMDA receptor submit 2D. (NR2D) antagonist PPDA (500 nM) (Feng et al. 2004). At this concentration, this agent blocked NMDA EPSCs similarly in four NPY+ neurons (32 ± 13%) and five NPY− neurons (27 ± 10%).
Fig. 5.
NMDA receptor–mediated transmission in BAC-npy striatal neurons. A: averaged (n = 10–12) evoked NMDA EPSCs from an NPY+ (left) and NPY− (right) neuron. Superimposed on each trace is the double exponential curve used to fit the decay phase together with the 2 exponential components shown individually. Weighted decay time constant values were 176 and 377 ms, respectively. B: summary box and whisker plot of the distribution of weighted time constants (Tw) for NMDA-mediated current decay from 16 NPY+ and 9 NPY− neurons; *P < 0.05, Student's t-test. C: superimposed NMDA EPSCs averaged from 10–12 individual evoked currents obtained in absence (black trace) and presense (gray trace) of 10 μM CP101606 from an NPY+ cell (left). Same experiment performed in an NPY− cell (right). D: summary of the effect of CP101606 on the peak NMDA EPSC amplitude and Tw expressed as percent control in 6 NPY+ neurons (filled bars) and 5 NPY− neurons (open bars).
We recorded GABAergic synaptic activity with KCl-based internal solutions at holding potentials of −60 mV. Figure 6A shows examples of sIPSCs recorded from an NPY+ and an NPY− neuron. We did not observe significant differences in sIPSC and mIPSC decay time, amplitude, or frequency (Fig. 6B), suggesting a similar population of GABAA receptors at inhibitory synapses on both NPY+ and NPY− neurons. We studied evoked GABA responses in NPY+ and NPY− cells while stimulating local afferent fibers within the striatum. Figure 6C shows examples of evoked IPSCs in the presence of glutamate receptor blockers from NPY+ and NPY− cells in the presence and the absence of BMR. As shown in Fig. 6D, in both cell types, the decay of evoked IPSCs was significantly longer than sIPSC decay (NPY+: 188 ± 35% of sIPSC Tw, n = 8, P < 0.001; NPY−: 398 ± 56% of sIPSC Tw, n = 7, P < 0.01). Furthermore, NPY+ eIPSC decay times (60 ± 6 ms, n = 8) were significantly faster than those in NPY− cells (159 ± 33 ms, n = 7; P < 0.005).
Fig. 6.
GABAergic synaptic transmission in BAC-npy striatal neurons. A: representative spontaneous inhibitory postsynaptic current (sIPSC) traces from an NPY+ (left) and an NPY− (right) neuron. B: box and whisker plots for both sIPSC and mIPSC Twvalues (left), amplitudes (middle), and event frequencies (left) in NPY+ (gray boxes) and NPY− (open boxes) cells. C: representative traces of NPY+ (left) and NPY− (right) evoked IPSCs with intrastriatal stimulation. NBQX (5 μM) and CPP (10 μM) application (black) and BMR (25 μM) application (gray) showed pure GABAA-evoked current. D: sIPSC and evoked (eIPSC) Tw values for NPY+ (black) and NPY− (gray) cells are plotted as open circles with connecting lines. Each circle represents a single cell's Tw value, and the line connecting each circle shows the change in Tw when comparing sIPSC to eIPSCs in the same cell. Data derived from 8 NPY+ and 7 NPY− cells.
DISCUSSION
Electrophysiological recordings from genetically identified neurons in the CNS allow important advancement to our understanding of brain physiology (Lichtman et al. 2008). Expression of one or more fluorescent proteins has previously been used to study specific neurons and their synaptic relationships. To our knowledge, these findings represent an initial systematic analysis of excitatory and inhibitory synaptic connectivity in NPY containing interneurons in mouse striatum. The types of neurons we examined in this study are partly consistent with previous characterizations of firing patterns of these neurons in rat (Kawaguchi 1993; Koos and Tepper 1999) and extend our understanding of the functional synaptic inputs to these interneurons.
The distribution of GFP-positive neurons agrees with previous immunocytochemical studies that showed a sparse distribution of NPY+ interneurons in the rodent striatum, whereas in cortex, they are more abundant (Allen et al. 1983; Aoki and Pickel 1989). The comparison of GFP and NPY labeling in our study showed that most NPY immunopositive cells also expressed GFP. Thus we believe that this mouse model provides a significant advancement in our ability to identify and characterize these rare GABAergic interneurons. The results of our comparative immunocytochemical analysis with several interneuron markers showed that this mouse strain differentiates the NPY+ interneurons from several others. In support of this finding, we never observed electrophysiological characteristics of other interneurons such as cholinergic or FS type of interneurons (Kawaguchi 1993).
Our anatomical and electrophysiological findings are fairly consistent with previous studies. The NPY+ neurons studied here were found to have dendritic ramifications and fusiform cell bodies with sparsely spiny dendrites similar to those characterized in rats (Kawaguchi 1993). These neurons also showed LTS patterns and high-input resistance values. Previous reports have made distinctions between LTS and PLTS based on the occurrence of persistent depolarization and Ih sag (Koos and Tepper 1999; Tepper et al. 2008). Our results showed the occurrence of these characteristics in a subset of NPY+ cells. However, species and developmental stages may contribute to a less well-defined classification of electrophysiological properties for neuronal subtypes.
Most NPY+ neurons showed spontaneous action potential firing. This characteristic was observed with noninvasive loose patch recordings and whole cell current-clamp recordings. Recently, mouse striatal LTS neurons were reported to fire spontaneous action potentials in this configuration, supporting our observations. Firing patterns of these LTS neurons became oscillatory under chronic dopamine deprivation, influencing medium spiny neuron GABAergic input (Dehorter et al. 2009). Simultaneous recordings from NPY+ neuron pairs showed that the spontaneous firing is not synchronized between cells in our experimental conditions, suggesting a lack of electrical coupling. This has importance because it has been shown that networks of “LTS-like” interneurons can be entrained to fire synchronously in the rodent cortex (Fanselow et al. 2008; Gibson et al. 1999). In our study, we also report electrophysiological characteristics of NPY− neurons. Because they are most consistent with those seen in studies of MSNs, throughout the remaining discussion, we will refer to NPY− neurons as MSNs, with the limitation that a small percent of these neurons may not be MSNs.
The aim of this study was the identification and characterization of synaptic connectivity on striatal NPY/LTS/PLTS neurons. Electrophysiological recordings examined the influence of glutamate and GABAA ionotropic receptors at these synapses. We examined basic excitatory synaptic transmission through AMPA receptor–mediated sEPSCs. These pharmacologically isolated events were of much lower amplitude and frequency in comparison to MSNs. Evoked EPSCs in a paired pulse protocol suggest that there is no overt difference in glutamate release probability at these synapses. In combination, these data support the hypothesis that NPY+ neurons may have more sparse glutamatergic innervation, at least in well-clamped regions near the soma and proximal dendrites. Because the presence of glutamate receptors lacking GluR2 subunit in GABAergic interneurons can induce “anti-Hebbian long-term potentiation” (Kullmann and Lamsa 2008), it was important to study the presence of these AMPA receptor subtypes in NPY+ interneurons. The distinct current-voltage relationship from that reported for MSNs (Kreitzer and Malenka 2007) suggests a significant reduction in abundance of GluR2 subunits in NPY+ interneurons.
We also characterized the NMDA receptor–mediated component of synaptic transmission onto NPY+ striatal neurons. The faster decay of evoked NMDA-EPSCs in NPY+ neurons, compared with MSNs, may be related to the presence of specific and distinct NMDA receptor subtypes (Chapman et al. 2003; Ding et al. 2008; Logan et al. 2007). This is further supported by the strong trend for a reduced sensitivity to the NR2B specific blocker in NPY+ neurons. However, the pharmacological results are of limited interpretation because of the possible presence of triheterotrimeric NMDA receptors (Neyton and Paoletti 2006). Fast NMDA EPSCs in NPY+ neurons may be important because they decrease the time window for coincidence detection for NMDA-mediated plastic changes. It can also affect the charge contribution of NMDA receptors to basal synaptic activation, particularly with short high-frequency presynaptic trains. Among the possible reasons underlying the fast NMDA EPSCs in NPY+ neurons are the abundance of NR2A subunit and the presence of the exon 5–containing splice variants of the NR1 subunit (Cull-Candy and Leszkiewicz 2004; Rumbaugh et al. 2000). However, the physical location of glutamatergic synapses in relation to the soma and the lack of spines in NPY+ neurons could contribute to these observed differences. A better space clamp control caused by the high-input resistance in NPY+ neurons may also produce faster EPSCs than MSNs (Williams and Mitchell 2008).
In addition to glutamatergic innervation, GABAergic input onto NPY+ interneurons in the striatum, to the best of our knowledge, has not been characterized. What remains unclear is if these interneurons receive GABAergic projections from other interneurons or MSN collaterals. Although our study does not address the source of GABAergic innervation to NPY+ neurons, we provide the first characterization of GABAA receptors at these synapses. Our results showed that NPY+ neurons receive inhibitory input. Activation of these impinging GABAergic projections to NPY+ neurons dampen feed-forward inhibition onto MSNs and may play important roles in the striatal network (Tepper et al. 2008). Initially, we compared TTX-sensitive and insensitive spontaneous GABA release in NPY+ neurons and MSNs. Our results showed a similar decay of sIPSCs and mIPSCs, suggesting little or no differences in the composition of NPY and MSN GABAA receptors. In addition, the similar amplitude of these currents in both cell types implies that the number of postsynaptic GABAA receptors per synapse is not different. Also, NPY+ interneurons and MSNs have a similar number of active GABAergic synaptic connections because mIPSC frequency does not differ between cell types. However, MSNs tended to have a higher frequency of sIPSCs events than NPY+ neurons, suggesting that MSNs may receive more inputs from spontaneously active interneurons.
Although evoked GABAergic IPSCs have been studied in MSNs, they have not been characterized in NPY interneurons. Evoked IPSCs could be elicited by stimulation in both NPY neurons and MSNs and were antagonized by a GABAA receptor blocker. Evoked IPSCs were slower in MSNs than in NPY neurons. Furthermore, although evoked IPSCs were slower than sIPSCs in both cell types, the difference was strikingly larger in MSNs. One explanation of this finding is that MSNs receive less synchronized synaptic GABAergic input on intrastriatal stimulation. Second, the slower decay in MSNs may be caused by greater input from GABAergic interneurons predisposed to high-frequency, repetitive firing. A third possibility could be a delayed activation of distinct components of the inhibitory striatal network by excitatory afferents. Thus, this reasoning further supports the possibility that NPY+ interneurons receive greater input from MSNs than other GABAergic interneurons. However, one has to keep in mind that axon collaterals between MSNs make weaker synapses than those made by interneuron afferents (Koos et al. 2004; Kubota and Kawaguchi 2000), whereas this is not known for NPY+ interneurons.
Our data specify properties of synaptic connections onto NPY+ interneurons and′ elucidate the integration of these neurons in striatal microcircuits. Because the axonal fields of NPY+ interneurons are extensive (Tepper and Bolam 2004), these interneurons have wide ranging influence on information processing and selection within the striatal network. As different GABAergic interneuron subtypes are involved in setting network oscillatory patterns relevant for a variety of behaviors in other areas of the CNS (Engel et al. 2001), NPY interneurons may be crucial in the striatal network for movement control and its dysfunction occurring in several neurological disorders.
ACKNOWLEDGMENTS
We thank L. E. Dettin and E.-J. Li for technical assistance, D. Pak for providing antibodies, and D. Lovinger at the National Institute on Alcoholism and Alcohol Abuse for providing the BAC-drd2 EGFP mice.
GRANTS
This work was supported by National Institutes of Health Grants NS-047700 and T32-DA-007291.
REFERENCES
- Abe K, Tilan JU, Zukowska Z. NPY and NPY receptors in vascular remodeling. Curr Top Med Chem 7: 1704–1709, 2007 [DOI] [PubMed] [Google Scholar]
- Allen YS, Adrian TE, Allen JM, Tatemoto K, Crow TJ, Bloom SR, Polak JM. Neuropeptide Y distribution in rat brain. Science 221: 877–879, 1983 [DOI] [PubMed] [Google Scholar]
- Aoki C, Pickel VM. Neuropeptide Y in the cerebral cortex and the caudate-putamen nuclei: ultrastructural basis for interactions with GABAergic and non-GABAergic neurons. J Neurosci 9: 4333–4354, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett BD, Callaway JC, Wilson CJ. Intrinsic membrane properties underlying spontaneous tonic firing in neostriatal cholinergic interneurons. J Neurosci 20: 8493–8503, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman DE, Keefe KA, Wilcox KS. Evidence for functionally distinct synaptic NMDA receptors in ventromedial versus dorsolateral striatum. J Neurophysiol 89: 69–80, 2003 [DOI] [PubMed] [Google Scholar]
- Chee MJ, Colmers WF. Y eat? Nutrition 9: 869–877, 2008 [DOI] [PubMed] [Google Scholar]
- Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Sci STKE 2004: re16, 2004 [DOI] [PubMed] [Google Scholar]
- Dehorter N, Guigoni C, Lopez C, Hirsch J, Eusebio A, Ben-Ari Y, Hammond C. Dopamine-deprived striatal GABAergic interneurons burst and generate repetitive gigantic IPSCs in medium spiny neurons. J Neurosci 29: 7776–7787, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Peterson JD, Surmeier DJ. Corticostriatal and thalamostriatal synapses have distinctive properties. J Neurosci 28: 6483–6492, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AK, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci 2: 704–716, 2001 [DOI] [PubMed] [Google Scholar]
- Fanselow EE, Richardson KA, Connors BW. Selective, state-dependent activation of somatostatin-expressing inhibitory interneurons in mouse neocortex. J Neurophysiol 100: 2640–2652, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B, Tse HW, Skifter DA, Morley R, Jane DE, Monaghan DT. Structure-activity analysis of a novel NR2C/NR2D-preferring NMDA receptor antagonist: 1-(phenanthrene-2-carbonyl) piperazine-2,3-dicarboxylic acid. Br J Pharmacol 141: 508–516, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueredo-Cardenas G, Morello M, Sancesario G, Bernardi G, Reiner A. Colocalization of somatostatin, neuropeptide Y, neuronal nitric oxide synthase and NADPH-diaphorase in striatal interneurons in rats. Brain Res 735: 317–324, 1996 [DOI] [PubMed] [Google Scholar]
- Fu LY, van den Pol AN. GABA excitation in mouse hilar neuropeptide Y neurons. J Physiol 579: 445–464, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature 402: 75–79, 1999 [DOI] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature 425: 917–925, 2003 [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Habits, rituals, and the evaluative brain. Annu Rev Neurosci 31: 359–387, 2008 [DOI] [PubMed] [Google Scholar]
- Karagiannis A, Gallopin T, Dávid C, Battaglia D, Geoffroy H, Rossier J, Hillman EM, Staiger JF, Cauli B. Classification of NPY-expressing neocortical interneurons. J Neurosci 29: 3642–3659, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y. Physiologicial, morphological, and histochemical characterization of three classes of interneurons in rat neostriatum. J Neurosci 13: 4908–4923, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y. Neostriatal cell subtypes and their functional roles. Neurosci Res 27: 1–8, 1997 [DOI] [PubMed] [Google Scholar]
- Kimpel MW, Strother WN, McClintick JN, Carr LG, Liang T, Edenberg HJ, McBride WJ. Functional gene expression differences between inbred alcohol-preferring and -non-preferring rats in five brain regions. Alcohol 41: 95–132, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koos T, Tepper JM. Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nat Neurosci 5: 467–472, 1999 [DOI] [PubMed] [Google Scholar]
- Koos T, Tepper JM, Wilson CJ. Comparison of IPSCs evoked by spiny and fast-spiking neurons in the neostriatum. J Neurosci 24: 7916–7922, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson's disease models. Nature 445: 643–647, 2007 [DOI] [PubMed] [Google Scholar]
- Kubota Y, Kawaguchi Y. Dependence of GABAergic synaptic areas on the interneuron type and target size. J Neurosci 20: 375–386, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann DM, Lamsa K. Roles of distinct glutamate receptors in induction of anti-Hebbian long-term potentiation. J Physiol 586: 1481–1486, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo LE, Kitlinska JB, Tilan JU, Li L, Baker SB, Johnson MD, Lee EW, Burnett MS, Fricke ST, Kvetnansky R, Herzog H, Zukowska Z. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat Med 13: 803–811, 2007 [DOI] [PubMed] [Google Scholar]
- Lichtman JW, Livet J, Sanes JR. A technicolour approach to the connectome. Nat Rev Neurosci 9: 417–422, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan SM, Partridge JG, Matta JA, Buonanno A, Vicini S. Long-lasting NMDA receptor-mediated EPSCs in mouse striatal medium spiny neurons. J Neurophysiol 98: 2693–2704, 2007 [DOI] [PubMed] [Google Scholar]
- Murase K, Ryu PD, Randic M. Excitatory and inhibitory amino acids and peptide-induced responses in acutely isolated rat spinal dorsal horn neurons. Neurosci Lett 103: 56–63, 1989 [DOI] [PubMed] [Google Scholar]
- Neyton J, Paoletti P. Relating NMDA receptor function to receptor subunit composition: limitations of the pharmacological approach. J Neurosci 26: 1331–1333, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortinski PI, Lu C, Takagaki K, Fu Z, Vicini S. Expression of distinct alpha subunits of GABAA receptor regulates inhibitory synaptic strength. J Neurophysiol 92: 1718–1727, 2004 [DOI] [PubMed] [Google Scholar]
- Rumbaugh G, Prybylowski K, Wang JF, Vicini S. Exon 5 and spermine regulate deactivation of NMDA receptor subtypes. J Neurophysiol 83: 1300–1306, 2000 [DOI] [PubMed] [Google Scholar]
- Ruohonen ST, Savontaus E, Rinne P, Rosmaninho-Salgado J, Cavadas C, Ruskoaho H, Koulu M, Pesonen U. Stress-induced hypertension and increased sympathetic activity in mice over expressing neuropeptide Y in noradrenergic neurons. Neuroendocrinology 89: 351–360, 2008 [DOI] [PubMed] [Google Scholar]
- Tang K, Low MJ, Grandy DK, Lovinger DM. Dopamine-dependent synaptic plasticity in striatum during in vivo development. Proc Natl Acad Sci USA 98: 1255–1260, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Bolam JP. Functional diversity and specificity of neostriatal interneurons. Curr Opin Neurobiol 14: 685–692, 2004 [DOI] [PubMed] [Google Scholar]
- Tepper JM, Wilson CJ, Koós T. Feedforward and feedback inhibition in neostriatal GABAergic spiny neurons. Brain Res Rev 58: 272–281, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsell A, Heilig M. Diverse functions of neuropeptide Y revealed using genetically modified animals. Neuropeptides 36: 182–193, 2002 [DOI] [PubMed] [Google Scholar]
- Thorsell A, Slawecki CJ, El Khoury A, Mathe AA, Ehlers CL. The effects of social isolation on neuropeptide Y levels, exploratory and anxiety-related behaviors in rats. Pharmacol Biochem Behav 83: 28–34, 2006 [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Yao Y, Fu L, Foo K, Huang H, Coppari R, Lowell BB, Broberger C. Neuromedin B and gastrin-releasing peptide excite arcuate nucleus neuropeptide Y neurons in a novel transgenic mouse expressing strong Renilla green fluorescent protein in NPY Neurons. J Neurosci 29: 4622–4639, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SR, Mitchell SJ. Direct measurement of somatic voltage clamp errors in central neurons. Nat Neurosci 11: 790–798, 2008 [DOI] [PubMed] [Google Scholar]