Abstract
Many synapses contain both AMPA receptors (AMPAR) and N-methyl-d-aspartate receptors (NMDAR), but their different roles in synaptic computation are not clear. We address this issue at the auditory nerve fiber synapse (called the endbulb of Held), which is formed on bushy cells of the cochlear nucleus. The endbulb refines and relays precise temporal information to nuclei responsible for sound localization. The endbulb has a number of specializations that aid precise timing, including AMPAR-mediated excitatory postsynaptic currents (EPSCs) with fast kinetics. Voltage-clamp experiments in mouse brain slices revealed that slow NMDAR EPSCs are maintained at mature endbulbs, contributing a peak conductance of around 10% of the AMPAR-mediated EPSC. During repetitive synaptic activity, AMPAR EPSCs depressed and NMDAR EPSCs summated, thereby increasing the relative importance of NMDARs. This could impact temporal precision of bushy cells because of the slow kinetics of NMDARs. We tested this by blocking NMDARs and quantifying bushy cell spike timing in current clamp when single endbulbs were activated. These experiments showed that NMDARs contribute to an increased probability of firing, shorter latency, and reduced jitter. Dynamic-clamp experiments confirmed this effect and showed it was dose-dependent. Bushy cells can receive inputs from multiple endbulbs. When we applied multiple synaptic inputs in dynamic clamp, NMDARs had less impact on spike timing. NMDAR conductances much higher than mature levels could disrupt spiking, which may explain its downregulation during development. Thus mature NMDAR expression can support the conveying of precise temporal information at the endbulb, depending on the stimulus conditions.
INTRODUCTION
A critical component of how presynaptic inputs contribute to postsynaptic firing is the diverse set of ionotropic receptors on the postsynaptic membrane. This is particularly important at glutamatergic synapses, which contain both AMPA receptors (AMPARs) and NMDA receptors (NMDARs). These receptors have very different kinetics, which could affect how presynaptic activity influences postsynaptic firing, quite separate from the effects of NMDARs on longer-term plasticity. The specific consequences of this are not well understood.
We address this issue in the endbulb of Held, which is the synapse formed by auditory nerve (AN) fibers onto bushy cells (BC) in the anteroventral cochlear nucleus (AVCN) (Brawer and Morest 1975; Lorente de Nó 1981; Ryugo and Fekete 1982). AN fibers convey timing information about sounds in the precise timing of spikes. The endbulb and BCs have a number of specializations to preserve precise timing, including rapid AMPARs (Gardner et al. 2001; Isaacson and Walmsley 1995b) and low-threshold potassium conductances (Manis and Marx 1991; Oertel 1983; Rothman and Manis 2003). In vivo experiments indicate that BCs have lower variability in spike timing (“jitter”) compared with AN fibers, presumably through convergence of multiple inputs (Joris et al. 1994; Louage et al. 2005; Paolini et al. 2001; Xu-Friedman and Regehr 2005a,b).
BCs express both AMPARs and NMDARs (Isaacson and Walmsley 1995a,b). Unlike AMPARs, NMDARs have slow kinetics, which in other systems is known to reduce the precision of spike timing. For example, in thalamic relay neurons, the AMPAR component of the EPSC is responsible for generating short-latency, temporally precise spikes, while NMDARs trigger longer-latency spikes with low temporal precision (Blitz and Regehr 2003; Miyata and Imoto 2006). Such effects of NMDARs seem at odds with the function of BCs in mature mice.
During development, NMDAR expression may be necessary for processes such as synaptic refinement. Mice begin hearing at P10-12 (Ehret 1976; Kamiya et al. 2001; Romand 2003), and the auditory system undergoes considerable anatomical and physiological changes around this time (Limb and Ryugo 2000; Taschenberger and von Gersdorff 2000). NMDAR expression is effectively eliminated from the developing calyx of Held (in the medial nucleus of the trapezoid body; MNTB) by 2–3 wk postnatally, which is consistent with the refinement of the temporal precision (Futai et al. 2001; Joshi and Wang 2002). Similar downregulation was found electrophysiologically in the rat endbulb by P18 (Bellingham et al. 1998). However, in situ hybridization and immunolabeling have suggested that NMDARs persist in older endbulbs and that NMDAR subunit composition matures by P21 (Joelson and Schwartz 1998; Sato et al. 1998).
To resolve these contradictory observations and to understand how NMDARs contribute to BC firing, we made patch-clamp recordings from brain slices of mice up to P30. Voltage-clamp recordings showed that NMDAR expression reaches a plateau after P21. In current clamp, blocking NMDARs with CPP reduced the probability of BC firing and increased the latency and jitter of firing. We tested these effects further using dynamic clamp (DC). The level of the NMDAR conductance affected probability, latency, and jitter in a dose-dependent way. However, increasing NMDAR conductance much higher than normal resulted in tonic depolarization and disrupted spiking. Thus mature expression levels of NMDARs appear to be optimized to support precise spiking at the endbulb.
METHODS
All animal procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by Institutional Animal Care and Use Committee of the State University of New York at Buffalo. Parasagittal slices of brainstems containing the AVCN were prepared from 14- to 30-day-old (P14-30) CBA/CaJ mice as described previously (Yang and Xu-Friedman 2008). Briefly, slices were cut in ice-cold, high-sucrose solution containing (in mM) 76 NaCl, 25 NaHCO3, 75 sucrose, 25 glucose, 1.25 NaH2PO4, 2.5 KCl, 7 MgCl2, and 0.5 CaCl2. Slices were incubated at 33°C for 20 min and transferred to normal recording solution containing (in mM) 125 NaCl, 26 NaHCO3, 1.25 NaH2PO4, 2.5 KCl, 20 glucose, 1 MgCl2, 1.5 CaCl2, 4 Na-L-lactate, 2 Na-pyruvate, and 0.4 Na-l-ascorbate. Recordings were made at 32–34°C. Strychnine (10 μM) was present during all recordings. In some experiments, 10 μM 2,3-dioxo-6-nitro-1,2,3,4 tetrahydrobenzo[f]quinoxaline-7-sulfonamide (NBQX) or 5 μM (RS)-3-(2-carboxypiperazin-4-yl)-propyl-l-phosphoric acid (CPP) (Harris et al. 1986; Lehmann et al. 1987) were added to block AMPAR or NMDAR currents, respectively. NBQX and CPP were from Tocris Bioscience (Ellisville, MO); all other chemicals were from Sigma (St. Louis, MO). For voltage-clamp recording, pipettes were filled with internal solution (in mM) 35 CsF, 100 CsCl, 10 EGTA, 10 HEPES, and 1 N-(2,6-dimethylphenylcarbamoylmethyl)triethylammonium chloride (QX-314; pH 7.3, osmolarity: 310 mosM). For current clamp, recording pipettes were filled with internal solution (in mM) 130 KMeSO3, 10 NaCl, 2 MgCl2, 0.16 CaCl2, 0.5 EGTA, 10 HEPES, 4 ATP, 0.4 GTP and 14 di(tris) phosphocreatine (pH 7.3, osmolarity: 300 mOsm). Electrode resistances were 1–2 MΩ. In voltage clamp, BCs were held at –70 mV to record AMPAR or at +40 mV for NMDA. Whole cell compensation parameters used were cell capacitance of 22.3 ± 0.9 pF, and series resistance of 4 ± 0.2 MΩ, which was compensated to 70%. In current clamp, holding current was applied to maintain resting membrane potential between trials at –60 mV. For current clamp, pipette capacitance neutralization was within ±1 pF and bridge balance was 4–10 MΩ. The cell input resistance at –60 mV was 37 ± 11 MΩ (n = 11 cells). Single presynaptic AN fibers were stimulated using a micropipette filled with standard external solution and placed in an area 30–50 μm away from the cell being patched. Stimuli of 5–15 μA were delivered through a stimulus isolator (A360, WPI, Saratoga, FL). Recordings were made using the Multiclamp 700B (Molecular Devices, Sunnyvale, CA) and digitized through an ITC-18 (Instrutech, Port Washington, NY) at 50 kHz controlled through custom-written software (mafPC) running in Igor (WaveMetrics, Lake Oswego, OR). Most recordings were made in the anterior part of the AVCN, where BCs receiving low-frequency input reside (Rouiller and Ryugo 1984). BCs were identified in voltage clamp by fast, depressing EPSCs with a large amplitude (2–20 nA) (Isaacson and Walmsley 1995b); In current clamp BCs were identified by their single, undershooting spikes in response to current injection (Oertel 1983). It is not possible to distinguish spherical and globular BCs based on their physiological properties (Ferragamo and Oertel 2001), so all recordings are grouped together.
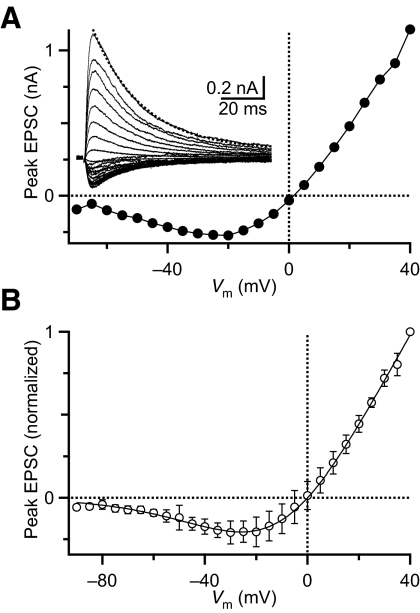
In DC experiments, the AMPAR component was controlled by the ITC-18 DC interface. The nonlinearity of the NMDAR conductance was implemented using a slave computer running a custom-written Igor XOP and a National Instruments interface (PCI-6229). The voltage dependence of NMDAR conductance resulting from block by Mg2+ was measured in voltage-clamp experiments by holding BCs at potentials ranging from –70 to +40 mV and stimulating individual AN inputs in the presence of NBQX (Fig. 1A). NMDAR EPSC peak amplitudes were normalized to the amplitude measured at +40 mV and averaged over five experiments. There were no systematic changes in I–V curves for endbulbs from different aged mice (ages tested ranged from P14 to P26, average 20 ± 3 days). We fit the average data to a modified Woodhull equation, with form I = A [Vm(t) – Vrev]/[1 + [Mg] K0–1exp(–zδFVm/RT)], where K0 is the dissociation constant for Mg2+ with the channel at 0 mV, z is the valence of Mg2+, δ is the fraction of the membrane potential affecting Mg2+ binding, F is Faraday's constant, R is the gas constant, and T is the temperature. Using a least-squares approach, the key free parameters were K0 = 1.9 mM, and δ = 0.73. To simplify the calculation for the dynamic-clamp interface, we also fit the data to a modified Boltzmann equation, with the form I = A [Vm(t) – Vrev]/{1 + exp[(V1/2 – Vm)/Vrate]} using least-squares, yielding values of V1/2 = –1.2 mV and Vrate = 18.1 mV. These values were then used by the XOP on the slave computer to calculate the current through the NMDAR conductance as I(t) = [Vrev – Vm(t)] GNMDA (t)/{1 + exp[(V1/2 – Vm(t))/Vrate]}. The XOP ran at ∼10 kHz. AMPAR and NMDAR currents were summed with a summing amplifier and fed into the command input on the Multiclamp 700B. Threshold was measured in DC for each BC as described previously (Xu-Friedman and Regehr 2005a). Thresholds were 10–35 nS for different cells (average: 20.4 nS ±1.9, n = 22). Endbulb AMPA EPSCs had amplitudes of 2–20 nA in voltage-clamp experiments (average: 6.9 nA ±3.6, n = 89). The relationship between threshold and EPSC amplitude could not be determined for individual cells, so AMPAR conductances in dynamic-clamp experiments were scaled to 10 times threshold. When single inputs were mimicked, the NMDAR conductance waveform was based on NMDAR EPSCs recorded in voltage clamp (Fig. 3E). When multiple inputs were mimicked, a constant NMDAR conductance was applied. The time course of AMPAR conductances was based on single EPSCs recorded in voltage clamp.
Fig. 1.
Voltage dependence of the N-methyl-d-aspartate receptor (NMDAR) conductance at 32–34°C. A: I–V relationship for NMDAR currents for a representative endbulb synapse. Inset: example traces of NMDAR currents following auditory nerve (AN) stimulation with holding potentials ranging from –70 to +40 mV with a 5-mV step. The dotted line is a single-exponential fit to the +40-mV response beginning at 5 ms after the peak and running through the end of the trace, which yielded a τdecay = 23 ms. B: average I–V relationship for NMDAR currents in bushy cells. Curves were normalized to the current at +40 mV and averaged (n = 5). The line is the fit to these data of a modified Boltzmann equation (see methods).
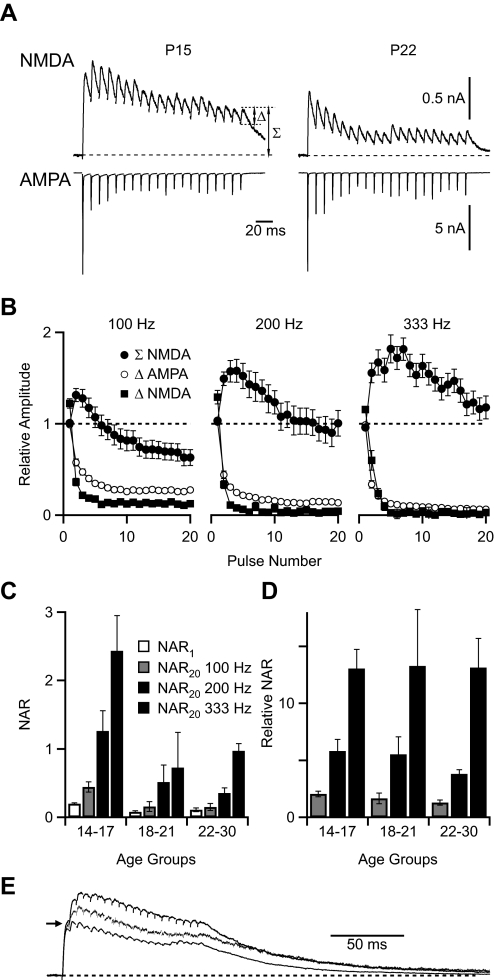
Fig. 3.
Glutamatergic currents in the endbulb following trains of stimulation. A: example traces of NMDAR and AMPAR currents following 100 Hz stimulation in juvenile (P15) and older (P22) mice. Measurement of the relative (Δ) and integrated (Σ) amplitude is shown. Traces are averages of 15–17 trials. Horizontal scale applies to both AMPA and NMDA traces. B: relative amplitudes of NMDAR and AMPAR currents (Δ) and integrated amplitude of NMDAR current (Σ) following stimulation with 100-, 200-, and 333-Hz trains (n = 20 cells). C: NAR at 1st (NAR1) and last (NAR20) pulse of the train in P14–17 (n = 9 cells), P18–21 (n = 6), and P22–30 (n = 5) mice following stimulation at 100, 200 and 333 Hz. Integrated (Σ) values were used for calculations. D: relative NAR for 100-, 200-, and 333-Hz stimulation. Integrated (Σ) values were used for calculations. E: variation in temporal summation of NMDAR currents in different experiments. Traces are average NMDAR currents at 200-Hz stimulation in 3 cells, representing large, average, and small summation. Each response is normalized to the same amplitude for the 1st EPSC peak (arrow). The peak amplitudes were 845, 339, and 1,230 pA for the large, average, and small summation traces, respectively. Recordings from these cells were used to drive the DC experiments in Fig. 5C.
For current- and dynamic-clamp experiments with single inputs, latency was quantified as the duration between the start of the stimulus pulse and the point of maximum slope of the resulting spike (if any). For DC experiments with multiple jittery inputs, latency was quantified as the time difference between the average input time and the resulting spike. Jitter was the SD of the latency. All data are reported as means ± SE.
RESULTS
NMDARs persist at older endbulbs
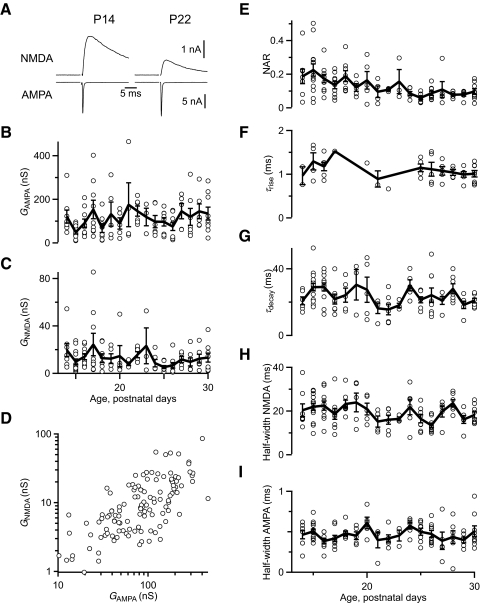
We assessed the functional expression of AMPARs and NMDARs using voltage-clamp recordings in brain slices from CBA/CaJ mice aged P14 to P30. We stimulated individual AN fiber inputs and recorded AMPAR EPSCs at –70 mV. At this holding potential, NMDAR conductance is negligible. NMDAR currents were subsequently measured in the same cells holding at +40 mV. All endbulbs contained both AMPAR and NMDAR currents at all ages studied. The amplitude of the NMDAR current changed with age. Two representative cells are shown in Fig. 2A, that have matched AMPAR current amplitudes. The NMDAR current amplitude in the cell from the younger animal was larger than from the older animal (Fig. 2A, left vs. right). The amplitudes of both AMPAR and NMDAR currents were variable from cell to cell (Fig. 2, B and C), but they seemed to be highly correlated (D). This probably reflects the variability in endbulb size seen in anatomical studies (Limb and Ryugo 2000) and likely results from differing numbers of release sites between different endbulbs. To control for this variability, we used the ratio of the NMDAR conductance at +40 mV to the AMPAR conductance at –70 mV (NMDAR/AMPAR ratio, NAR). NAR decreased significantly with age from 0.19 ± 0.02 for P14–17 (n = 41) to 0.09 ± 0.01 for P22–30 (n = 59; P < 0.001, Student's t-test, Fig. 2E).
Fig. 2.
Development of glutamatergic currents in the endbulb of Held following single stimuli. A: example traces of NMDAR and AMPA receptor (AMPAR) currents in juvenile (P14) and older (P22) mice. AMPAR currents throughout were measured at –70 mV, and NMDAR currents at +40 mV. Some NMDAR currents were recorded in the presence of NBQX. Traces are averages of 5–8 trials. Horizontal scale applies to both AMPA and NMDA traces. B and C: absolute conductance of AMPAR (B) and NMDAR (C) currents over the age studied (n = 122 cells). D: correlation between NMDAR and AMPAR conductances (n = 122 cells). E: NMDAR conductance measured at +40 mV normalized to the AMPAR conductance measured at –70 mV for each cell as a function of age (n = 122). This value is referred to as the NMDAR/AMPAR ratio, or NAR. NAR reaches a plateau after ∼P18. F and G: kinetics of NMDAR currents over the ages studied, using single-exponential fits. For NMDAR currents measured in the presence of NBQX (n = 49 cells), it was possible to measure the rate of rise (F). The rate of decay was measured in 117 cells (G). These measures show no significant changes with age. H and I: AMPAR current half-width (H) and NMDAR current half-width (I) over the ages studied (n = 122 cells). AMPAR and NMDAR half-widths do not change. Bold lines in B–I are the average values for all recordings at each postnatal day.
By contrast, neither AMPAR nor NMDAR currents showed changes in kinetics over this time period. We quantified the rise and decay of NMDAR currents by fitting to a single exponential: y = y0 + A exp[ –(t – t0)/τ]. The rising phase was fit over the period from 10% of peak amplitude through the peak. The decay phase was fit starting 5 ms after the peak of the NMDAR current, through the end of the recording (usually >180 ms). A sample decay fit is shown in Fig. 1A. The time constants (τ) did not change over the ages studied (Fig. 2, F and G) and were similar to those reported before (Bellingham et al. 1998). We also quantified half-width of AMPAR and NMDAR components, and neither showed significant changes over the ages studied (Fig. 2, H and I). Changes in kinetics have been documented in slices from animals younger than P14 (Bellingham et al. 1998). However, these changes appear to be largely concluded in the older slices studied here, suggesting they are mature in this respect.
While NMDAR conductance following isolated stimuli appeared relatively minor compared with AMPAR, during normal activity it could be different. AN fibers can fire at over 300 Hz for extended periods during normal sounds (Joris et al. 1994; Kiang et al. 1965; Sachs and Abbas 1974; Taberner and Liberman 2005); this can have significant effects on EPSC amplitude as a result of short-term synaptic plasticity (Isaacson and Walmsley 1996; Oleskevich et al. 2000; Wang and Manis 2008; Yang and Xu-Friedman 2008). To match these firing rates, we studied NMDAR and AMPAR currents during trains of 20 stimuli at 100, 200, or 333 Hz. As with single stimuli, the amplitude of the NMDAR component during trains was lower in older mice compared with younger mice but still persisted (Fig. 3A). Both AMPAR and NMDAR components showed significant depression during trains. We quantified the peak amplitude for each EPSC in the train as the step increase in current after each stimulation (“Δ” in Fig. 3, A and B). This value represents the additional receptors that are activated after each stimulus. Depression of the NMDAR EPSC was even greater than depression of the AMPAR EPSC at all frequencies used probably because of receptor saturation (Yang and Xu-Friedman 2008).
However, a major effect of multiple stimulation was that NMDAR currents showed considerable temporal summation (TS). AMPAR currents showed no TS even at the highest frequencies because AMPAR currents at the endbulb decay particularly rapidly. We quantified the integrated NMDAR current for each EPSC by measuring the peak current after each stimulation relative to the baseline (Σ in Fig. 3, A and B). TS increased with stimulation frequency even though depression was greater at higher frequencies.
We used the integrated NMDAR current to calculate the NAR during trains. Because of depression of AMPAR EPSCs and TS of NMDAR EPSCs, the NAR changed throughout the train. We assessed this change by comparing the NAR of the first pulse of the train (NAR1) with the final pulse of the train (NAR20; Fig. 3C). As with single stimuli, NAR1 decreased with age from 0.2 ± 0.01 at P14–P17 (n = 9) to 0.1 ± 0.03 at P22–30 (n = 5). NAR20 depended highly on both age and stimulation rate. NAR20 was greater than NAR1, particularly at higher stimulation rates (Fig. 3C). NAR20 was greatest over all stimulation rates in the youngest mice examined (P14–17), reaching 2.4 ± 0.5 at 333 Hz. Even in older animals (P22–30), NAR20 reached parity at 1.0 ± 0.1 at 333 Hz despite the fact that NAR1 was low at these ages. The increases in NAR20 were quite similar across ages when normalized to NAR1 (Fig. 3D); thus each age group showed similar responses to stimulation frequency. This suggests that TS develops at similar rates and follows similar mechanisms across all age groups studied.
There was considerable cell-to-cell variability in the amount of TS. There were differences in the rate of build-up as well as the rate of decay. There were no obvious groupings that might indicate the existence of different classes of synaptic inputs. To illustrate the range of TS, we show NMDAR currents with particularly large, close to average, and particularly small TS in Fig. 3E.
Synaptically activated NMDARs affect BC firing
Because NMDARs are present even into older endbulbs, they may modulate BC firing, such as by affecting the probability or timing of BC spiking. NMDARs activate and inactivate slowly, so one might expect that under conditions in which NMDARs are highly activated, BC spikes may be more likely yet less precise. By contrast, when NMDARs are blocked, BC spikes may be less likely but more precise.
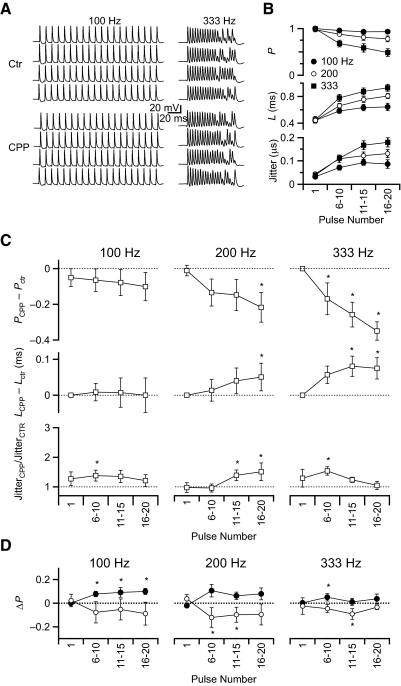
To test this, we made current-clamp recordings from BCs in mice P15 to P20 (average age P17 ± 1), stimulating AN fibers in trains at the same rates as in the voltage-clamp experiments and quantifying characteristics of the BC spikes. In control conditions, the spike firing probability was near 1 at the beginning of the train but decreased later in the train especially at higher stimulation frequencies (Fig. 4, A and B). Both latency and jitter increased over the course of the train (Fig. 4B). This effect is consistent with depression of the AMPAR EPSC during the train (Fig. 3): as the EPSP amplitude decreases, the latency to spiking increases and the likelihood of spiking decreases. The AMPAR component appeared to be essential for firing in the endbulb. NMDARs alone were not able to support BC firing, as application of 10 μM NBQX abolished all BC firing following stimulation with 100-, 200-, or 333-Hz trains (n = 3, data not shown).
Fig. 4.
Effects of changes in NMDAR conductance on bushy cells (BC) spiking in current clamp. A: example traces of 100- and 333-Hz trains before (Ctr) and after application of CPP in a P17 mouse. B: probability, latency, and jitter of control BC firing in regular recording solution and stimulation with 100, 200, and 333 Hz (n = 11). C: probability, latency, and jitter of BC firing in the presence of CPP (n = 9–11). Data were normalized to the control values (- - -). *, significant changes from control (P < 0.05, Student's t-test). D: probability of BC firing at different holding potentials in the presence of CPP (n = 11). Data for VM = –58 mV (closed circles) and VM = –62 mV (open circles) were normalized to the control condition of normal resting potential (–60 mV; - - -). Asterisks indicate significant changes from control (P < 0.05, Student's t-test).
To evaluate the impact of NMDARs on BC firing, we blocked them with 5 μM CPP. CPP application led to a decrease in the probability of firing for all stimulation frequencies (Fig. 4C). In addition, CPP caused a further increase in latency. Both these effects are consistent with the NMDAR component of the EPSC being generally excitatory, increasing the likelihood of spiking and causing BCs to reach threshold sooner and thereby shortening the latency. Thus the effects of CPP suggest that NMDAR currents do have a major impact on BCs firing under physiologically relevant conditions.
An important function of BCs is to relay and refine precise timing information. We quantified the relative change in jitter of BC spikes and found that where there were significant changes, jitter increased on application of CPP (Fig. 4C). Under no conditions did jitter decrease with CPP application. Thus NMDARs appear to support jitter reduction, and when CPP is applied, jitter increases. We had initially expected that the slow NMDAR current would increase jitter, but this was not the case.
The effects found in CPP experiments on probability and latency of BC firing were most prominent during 200- and 333-Hz stimulation. We consider this most likely results from major increases in NAR due to high TS at these frequencies (Fig. 3B). In sum, the effects observed when NMDA current was pharmacologically manipulated suggest that NMDARs play a computational role during normal activity, supporting a higher probability of BC firing, shorter latency, and lower jitter.
NMDARs could affect BC firing by depolarizing the cell. Such depolarization would bring the BC closer to threshold, thereby increasing the probability of response and shortening the latency. In addition, depolarization could activate other voltage-sensitive conductances, such as low-threshold potassium channels (Manis and Marx 1991; Rothman and Manis 2003), which could shorten the time constant of the cell. To test this, we examined BC firing at different membrane potentials in the presence of CPP. The appropriate amount of depolarization to apply was difficult to assess as the hyperpolarization caused by CPP application was not measurable during trains, suggesting that NMDAR activation triggers only slight depolarization. Therefore we tested deviations of ±2 mV from the normal resting potential of –60 mV. Increasing holding potential to –58 mV increased the probability of firing while decreasing it to –62 mV reduced firing probability (Fig. 4D). However, these changes were much smaller than those resulting from NMDA block with CPP (Fig. 4C). This indicates that the depolarization caused by NMDAR activation only partially accounts for the increase in BC spike probability and that it is necessary to take into account the more complex properties of NMDARs, such as their voltage-dependent conductance, which cannot be tested with simple current injection.
Amplitude and kinetics of NMDAR conductances affect BC firing
To test the role of NMDARs more fully, we turned to dynamic clamp (DC) (Robinson and Kawai 1993; Sharp et al. 1993). This technique allows us to mimic AMPAR and NMDAR conductances and to specify the amplitude, timing, and number of synaptic inputs, and then to quantify the effects on BC firing. The details of configuring the DC parameters are described more fully in methods.
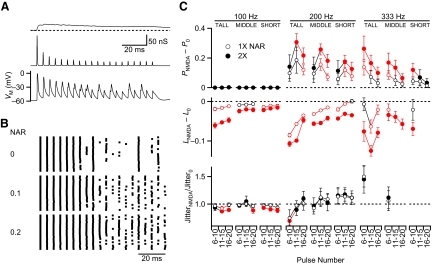
We began by considering single inputs. NMDAR and AMPAR conductances were based on the voltage-clamp recordings of the AMPAR and NMDAR currents (Fig. 5A). In this example, the first NMDAR conductance peak was scaled to 0.1 of the first AMPAR conductance (i.e., NAR = 0.1 or “1×”) to match the average obtained from voltage-clamp recordings. The initial AMPAR conductance was scaled to 10 times the minimum AMPAR conductance that elicited a BC spike (threshold). The DC interface calculated the appropriate AMPAR and NMDAR currents in response to the BC membrane potential, which were then summed and injected into the BC, which then fired a train of action potentials (Fig. 5A). Responses were qualitatively similar to those observed in current clamp. Most conspicuously, firing was less reliable in the second part of the train. This reliability depended on the size of the NMDAR component (Fig. 5B). When it was eliminated (“0” in Fig. 5B), spiking was even less reliable. This situation was similar to what was found when applying CPP to block NMDARs in current clamp. The DC also allowed us to explore a larger NMDAR contribution, NAR = 0.2 (twice normal or “2×”). In this condition, the firing probability was greater by the end of the train (Fig. 5B). To compensate for baseline differences between cells, we normalized all the values within each cell to those for NAR = 0. We tested different stimulation frequencies (100, 200, 333 Hz), NAR (0, 1×, 2× normal), and the position in the train (pulses 6–10, 11–15, and 16–20). In addition, we wanted to test the effect of TS. To do this, we drove the DC using NMDAR EPSCs that were recorded from cells that showed TS that was particularly large (“tall”), close to average (“middle”), or particularly small (“short”) (see Fig. 3E).
Fig. 5.
Using dynamic clamp to study the effects of NMDARs on BC firing. A: examples of injected NMDAR (top) and AMPAR (middle) conductances and the BC response (bottom) for a 200-Hz train with 1× NMDAR/AMPAR ratio (NAR) and “middle” temporal summation. B: spike raster for BC firing at different NAR (n = 20 of each). Increasing the NAR increases the spike probability. C: effects of NMDAR for a single input on spike probability, latency, and jitter in dynamic clamp. Several factors are tested, including input frequency (100, 200, 333 Hz), amount of temporal summation (TS; “tall,” “middle,” “short”; see Fig. 3E), NAR (0, 1×, and 2×), and latency within the train. The responses are quantified relative to NAR = 0. Red symbols indicate significant changes from control (P < 0.05, Student's t-test). Data are averages of 5–10 cells.
Considering firing probability (Fig. 5C, top), for 100-Hz trains, changing the NAR had no effect, because the probability of spiking was close to 100%. For 200- and 333-Hz trains, increasing the NAR to 1× or 2× normal significantly increased the probability of spiking. In addition, larger increases were seen with greater TS (tall > middle > short). Thus NMDAR currents enhance the probability of spiking in a dose-dependent manner, both by changes in NAR and TS.
The effects on latency paralleled the effects on the probability of firing (Fig. 5C, middle). Under conditions with larger NMDAR conductance (greater TS, larger NAR), the latency shift was more negative. These effects are consistent with NMDAR conductance generally contributing greater excitation, which increased the probability of firing and shortened the latency of firing. Also, similar to the results of synaptic stimulation, larger NMDAR conductance either reduced jitter or showed no significant effect, but never significantly increased it (red symbols, Fig. 5C, bottom).
Impact of NMDARs depends on the number of active inputs
The experiments conducted so far suggested that NMDAR activation actually supports transmission of precise temporal information at the endbulb. This raises the obvious question of why NMDARs are downregulated during development. There are several possible explanations for this. One is that deleterious effects of NMDARs on electrical properties of BCs come into play under different conditions from what we examined so far. We tested this possibility using DC.
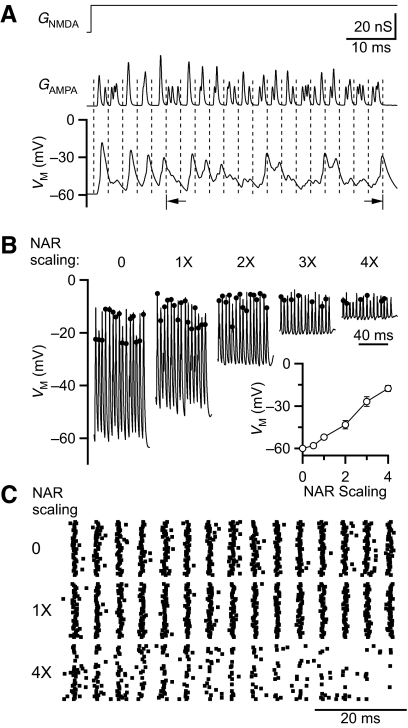
One common set of conditions experienced by BCs is that they receive multiple inputs (Oertel 1985; Xu-Friedman and Regehr 2005a). If those inputs are all active simultaneously, the total conductance would increase, possibly interfering with transmission of precise timing information. To test this possibility, we used DC to mimic multiple inputs. For this, we imagine a stimulus that causes three AN inputs to fire phase-locked spikes with some jitter, triggering synaptic conductances in the postsynaptic cell. We modeled the jitter after the shape of cycle histograms of AN fibers (Johnson 1980), using a Gaussian distribution with SD = 0.5 ms. These times were then convolved with a representative AMPAR conductance (based on voltage-clamp recordings) scaled to the steady-state train amplitudes in Fig. 2B (Fig. 6A, middle). For NMDAR conductances, we could not use the convolution approach because of the complexities introduced by receptor saturation. Instead we assigned each synaptic input a constant NMDAR conductance, the amplitude of which was set by the average integrated NMDAR conductance at the end of a train in Fig. 3B (Fig. 6A, top). We notate this physiologically based NAR as “1×.” To probe the effects of NMDARs, we scaled the NAR from 0 to 4× normal. To avoid changes in firing that result from changes in the activation state of BC channels, we analyzed BC firing after the first five cycles (Fig. 6A, bottom).
Fig. 6.
Using dynamic clamp to study the effects of NMDAR on interactions of multiple inputs. NMDAR conductance is simplified as a constant at the steady-state level. AMPAR peak conductance is the average steady-state level. Three inputs are mimicked at 200 Hz, with Gaussian-distributed jitter (0.5 ms SD). A: examples of applied NMDAR (top) and AMPAR (middle) conductances with the BC responses (bottom). The NAR is scaled to 1× (i.e., normal). Data for subsequent figures are grouped from cycles 6–20 (arrows). B: representative traces for NAR scaling at 0 to 4× normal. Dots mark spike times. At greater depolarization levels some excitatory postsynaptic potentials (EPSPs) do not give rise to spikes. Discrimination of spikes requires using the derivative and comparing against a passive model of the cell (for methodology, see Supplementary Fig. S2). Inset: average effects of NAR scaling on membrane potential during a train. Each point is the average of 4–11 recordings. C: representative spike raster of BC firing in response to NAR scaling 0, 1, and 4× (n = 20 of each).
Changes in the NAR had a striking effect on the baseline membrane potential (Fig. 6B). For example, when 4× normal NAR was applied, BCs depolarized by ∼40 mV (Fig. 6B, inset). We attribute this to the measurable NMDAR conductance at resting membrane potential (Fig. 1B). Despite the strong somatic depolarization, spikes were still elicited, presumably in the axon, and could be detected (Supplemental Figure S11). At the higher NAR scale, there were fewer spikes, particularly by the end of the train (Fig. 6, B and C).
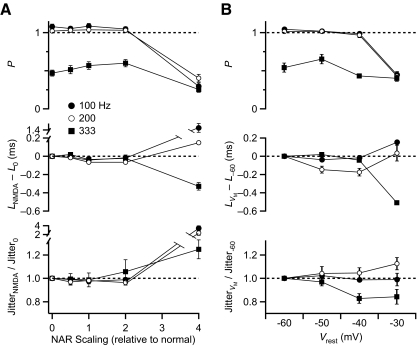
Average results from several experiments indicated that the number of BC spikes per cycle was slightly >1 for inputs firing at 100 and 200 Hz and ∼0.5 at 333 Hz (Fig. 7A, top). This probably happens because inputs at 100 and 200 Hz are above threshold but at 333 Hz are too small to trigger spikes unless multiple inputs occur simultaneously. Probability did not change much for NAR 0–2× normal but decreased at 4× for all frequencies tested (Figs. 6B and 7A, middle). This suggests that for normal NAR, multiple inputs provide sufficient excitation through AMPARs and NMDARs to ensure reliable firing of BCs but that 4× NAR is too much. Latency and jitter showed only small changes for NAR 0–2× normal but were greatly different for 4×.
Fig. 7.
Probability, latency and jitter of BC firing in response to 3 inputs at multiple frequencies. A: influence of NAR on BC firing (n = 7). B: influence of baseline membrane potential on BC firing (n = 5).
To determine if these effects resulted primarily from the tonic depolarization induced by NMDAR activation, we repeated these experiments with no NMDA component but depolarizing the BC to different baseline membrane potentials (−30, −40, or −50 mV, Fig. 7B). Changes in the probability and latency of firing were qualitatively similar to those at differently scaled NAR (Fig. 7A). There were no major changes in firing at lower membrane potentials. However, firing was disrupted at −30 mV with much lower probability of spiking and large shifts in latency.
These data suggest that increasing NAR can have adverse effects on firing resulting from the large shifts in baseline membrane potential. Thus increases in NAR over normal levels can lead to adverse effects on BC spiking when there are multiple converging inputs.
DISCUSSION
In this study, we show that synaptic currents mediated through NMDARs support the relaying of precise timing information in the auditory system. NMDARs are retained at the endbulb of Held late in development, suggesting they play a functional role in mature synapses. In current-clamp recordings, eliminating the NMDAR component pharmacologically reduced the probability of BC firing, increased the latency of firing, and increased jitter. DC experiments showed that these effects were dose-dependent over a physiologically-relevant range. Thus despite slow kinetics of NMDARs, they can play an important computational role in precise timing when single AN inputs are active. However, when multiple inputs are active, the NMDAR component did not play a major role. This indicates that the role of NMDARs in BC firing depends on the stimulus context. Furthermore, NMDARs could disrupt precise spiking, when presented at levels much higher than normal. This suggests there may be constraints on NMDAR expression at the endbulb.
Downregulation of NMDAR conductance during maturation of the endbulb
NMDAR expression persisted in the endbulb in the oldest animals we studied. As endbulbs matured, the amplitude of the NMDAR component decreased until ∼P21, where it stabilized at a plateau. The mouse endbulb continues to show anatomical changes after that age (Limb and Ryugo 2000). However, smaller-scale structures such as vesicular density and mitochondrial volume appear to solidify before that (Ryugo et al. 2006). In addition, subunit composition of the endbulb receptors appears to be relatively stable over the ages studied here: mature subunit composition is achieved for AMPARs by P14 in rats (Koike-Tani et al. 2005) and for NMDARs by P21 in gerbils (Joelson and Schwartz 1998). We saw no systematic changes over development in AMPAR or NMDAR EPSC kinetics in the population of endbulbs studied, suggesting that any late changes in receptor composition do not significantly affect the electrophysiological properties. Therefore we consider the ages studied here in mice to be essentially mature.
This situation differs from the calyx of Held in the MNTB. There AMPAR kinetics show changes until P14–18, probably reflecting changes in subunit composition and synaptic structure (Joshi and Wang 2002; Taschenberger and von Gersdorff 2000; Taschenberger et al. 2002; Youssoufian et al. 2005). Furthermore, NMDARs appear to be greatly reduced or eliminated from the calyx by postnatal week 2–3 (Futai et al. 2001; Joshi and Wang 2002), which is quite different from the endbulb. In addition, the fidelity of firing in the MNTB was unaffected by blocking the NMDAR EPSC with APV in mature synapses (Futai et al. 2001).
Our results emphasize that characterizing single EPSCs underestimates the importance of NMDARs during physiological activity. While AMPAR EPSCs depress (Isaacson and Walmsley 1995b; Oleskevich et al. 2000; Wang and Manis 2005; Xu-Friedman and Regehr 2005b; Yang and Xu-Friedman 2008), NMDAR EPSCs show significant temporal summation. At normal firing rates, NMDAR activation reached conductances equal to or greater than those of AMPARs. The relative build-up of the NMDAR/AMPAR ratio was similar for all ages studied (Fig. 2D). This suggests that the balance of NMDAR/AMPAR mainly depends on activity but not age.
TS of the NMDAR current showed cell-to-cell variability. The amount of TS did not appear to be age-dependent. This variability is likely to reflect differences in NMDAR subunit composition or localization. NMDARs in the mature endbulb contain about equal composition of NR2A-C (Joelson and Schwartz 1998). NR2A-expressing synapses are likely to have faster kinetics than synapses expressing other subunits (Flint et al. 1997). Therefore the subunit expression in each individual cell may explain the scatter we observed in NMDAR EPSC half-width and variability in TS.
Role of NMDAR in BC firing
Because the NMDAR component persists developmentally and becomes increasingly significant during extended activity, it is likely to play a functional role. Indeed pharmacological elimination of NMDAR reduced BC firing probability and increased latency in response to the activation of single AN inputs. These findings are consistent with NMDARs generally increasing excitation. This could happen in at least two ways. First, the small depolarizing conductance of NMDARs at rest may contribute sufficient depolarization to bring BCs closer to threshold. We tested this possibility and found that slight depolarization could only account for part of the change in response probability. Second, NMDARs could effectively amplify depressed AMPAR EPSPs if they are large enough to relieve Mg block. Endbulb NMDARs are insufficient by themselves to trigger firing because synaptic activation could not elicit spikes in the presence of NBQX. In addition, application of tonic NMDAR-like conductances in DC did not produce firing without the further addition of AMPAR-like conductances. NMDAR activation decreased the latency of BC spiking by as much as 0.2 ms. This is striking because this shift is much larger than the precise times that are used as cues for such behaviors as sound localization (e.g., 10 μs) (Klumpp and Eady 1956; Moiseff and Konishi 1981).
While other studies have found that NMDARs support higher response probability, this occurs in part by adding late or temporally imprecise spikes (retinal ganglion cell to lateral geniculate synapses: Augustinaite and Heggelund 2007; Blitz and Regehr 2003; visual cortex: Harsch and Robinson 2000; corticothalamic synapses: Miyata and Imoto 2006). This does not seem consistent with BC function. However, our results indicate that temporal precision at the endbulb is improved, and not degraded, by NMDARs. This is likely to result from increases in membrane conductance, either directly through the NMDARs themselves or indirectly through activation of potassium channels. Depolarization by NMDARs would activate voltage-gated potassium channels, which would decrease the input resistance of the BC and speed the time constant, which may enhance temporal precision. In addition, calcium influx through NMDARs could in principle activate calcium-activated potassium channels, which are present in the cochlear nucleus (Friedland et al. 2007) but have not yet been described in BCs. Another possibility is that the NMDARs amplify the AMPAR EPSPs (as suggested in the preceding text), making each AMPAR EPSP effectively larger and thereby increasing the precision of spike generation.
The effects of NMDAR conductance on spike probability, latency, and jitter were dose-dependent. We tested this in DC using NMDAR conductances with different amplitude or TS. Variability in TS has been shown to play an important role in other systems (Augustinaite and Heggelund 2007; Wu et al. 2004). Our experiments indicate that firing properties of BCs change with different degrees of TS. This raises the possibility that an individual cell could adjust its response by modifying the amplitude or temporal properties of NMDAR conductances, such as by changing receptor density, spatial distribution, or subunit composition. At the calyx of Held, Joshi et al. (Joshi and Wang 2007) found that paired stimulation induced an LTD-like phenomenon for summated NMDAR EPSCs in trains but not in single AMPAR and NMDAR EPSCs, which led to reductions of temporally imprecise spikes. Our results suggest that in the endbulb, such changes could have quite different effects. A reduction in NMDARs would be expected to reduce firing probability and increase latency and jitter. If long-term plasticity of NMDAR expression is also present in BCs, it could provide ways for BCs to optimize the timing properties of their responses, depending on their activity.
NMDARs and multiple inputs
We also investigated how NMDARs could influence BC firing when multiple inputs are active. BCs in mice appear to receive one to five inputs (Limb and Ryugo 2000; Oertel 1985; Xu-Friedman and Regehr 2005a). During normal sounds, the number of inputs that are active from moment to moment is likely to vary depending on the thresholds of different AN inputs and the stochastic nature of their firing. In addition, individual firing histories may affect the contribution of each AN by short-term synaptic plasticity (Yang and Xu-Friedman 2008). We investigated a simplified case using DC for three identical inputs with NAR ranging from 0 to 4 times normal. Under these conditions, the NMDAR component made only a small contribution to the probability, latency, and jitter of BC firing over the physiological range of NAR. Based on this, it appears that the NMDAR component has greater importance under stimulus conditions when there is only one active input but would have less impact as more inputs are recruited, such as with louder sounds.
With very large NAR (4×), BCs showed highly depolarized baseline membrane potentials and disrupted firing. This suggests that under some conditions, NMDARs could negatively impact firing. However, we did not observe such extreme levels of NMDAR conductance even at the earliest ages examined, so our data do not explain the 50% downregulation from P14 to P21. It may be that other naturalistic conditions that we did not test can lead to extreme NMDAR activation, such as with yet larger numbers of active inputs. Alternatively it may be other aspects of NMDAR physiology that are related to their downregulation, such as their role in intracellular signaling pathways for long-term changes in synaptic strength.
Relevance to other time-coding systems
Our findings may have implications for other systems where precise timing is important. Precise timing has been best explored in systems near the sensory or motor periphery. In the electrosensory system, precise spike timing is important for the jamming-avoidance response as well as species recognition (Carr 1993; Carr and Friedman 1999; Heiligenberg 1991; Hopkins and Bass 1981; Kawasaki and Guo 1996). Precise spike timing also seems to be important in the visual system (Dan et al. 1998) and motor system (Hahnloser et al. 2002; Yu and Margoliash 1996). In some systems far from the periphery, such as in cortex, neurons do appear to have adaptations for generating precisely timed spikes (Mainen and Sejnowski 1995), although the behavioral significance is not yet clear. Experiments in visual thalamus have suggested that NMDARs increase the likelihood of spiking, but the additional spikes were not as precisely timed (Blitz and Regehr 2003). Our data indicate that in the AVCN, NMDARs can contribute to precise spike timing. It will be interesting to ascertain how NMDARs contribute to spiking in other systems and what other features are important in determining whether the spikes are more or less precisely timed.
GRANTS
This study was supported by National Institute of Deafness and Other Communication Disorders Grant R01 DC-008125 to M. A. Xu-Friedman.
Supplementary Material
ACKNOWLEDGMENTS
We thank S. Chanda, K.M. Luce, S. R. Oh, and W.C. Hsu for help during the project and for comments on the manuscript.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Augustinaite S, Heggelund P. Changes in firing pattern of lateral geniculate neurons caused by membrane potential dependent modulation of retinal input through NMDA receptors. J Physiol 582: 297–315, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellingham MC, Lim R, Walmsley B. Developmental changes in EPSC quantal size and quantal content at a central glutamatergic synapse in rat. J Physiol 511: 861–869, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz DM, Regehr WG. Retinogeniculate synaptic properties controlling spike number and timing in relay neurons. J Neurophysiol 90: 2438–2450, 2003 [DOI] [PubMed] [Google Scholar]
- Brawer JR, Morest DK. Relations between auditory nerve endings and cell types in the cat's anteroventral cochlear nucleus seen with the Golgi method and Nomarski optics. J Comp Neurol 160: 491–506, 1975 [DOI] [PubMed] [Google Scholar]
- Carr CE. Processing of temporal information in the brain. Annu Rev Neurosci 16: 223–243, 1993 [DOI] [PubMed] [Google Scholar]
- Carr CE, Friedman MA. Evolution of time coding systems. Neural Comput 11: 1–20, 1999 [DOI] [PubMed] [Google Scholar]
- Dan Y, Alonso JM, Usrey WM, Reid RC. Coding of visual information by precisely correlated spikes in the lateral geniculate nucleus. Nat Neurosci 1: 501–507, 1998 [DOI] [PubMed] [Google Scholar]
- Ehret G. Development of absolute auditory thresholds in the house mouse (Mus musculus). J Am Audiol Soc 1: 179–184, 1976 [PubMed] [Google Scholar]
- Ferragamo MJ, Oertel D. Functional circuitry of the cochlear nucleus: in vitro studies in slices. In: Handbook of Mouse Auditory Research: From Behavior to Molecular Biology, edited by Willott JF. Boca Raton, FL: CRC, 2001, p. 297–315 [Google Scholar]
- Flint AC, Maisch US, Weishaupt JH, Kriegstein AR, Monyer H. NR2A subunit expression shortens NMDA receptor synaptic currents in developing neocortex. J Neurosci 17: 2469–2476, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedland DR, Eernisse R, Popper P. Potassium channel gene expression in the rat cochlear nucleus. Hear Res 228: 31–43, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futai K, Okada M, Matsuyama K, Takahashi T. High-fidelity transmission acquired via a developmental decrease in NMDA receptor expression at an auditory synapse. J Neurosci 21: 3342–3349, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner SM, Trussell LO, Oertel D. Correlation of AMPA receptor subunit composition with synaptic input in the mammalian cochlear nuclei. J Neurosci 21: 7428–7437, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahnloser RH, Kozhevnikov AA, Fee MS. An ultra-sparse code underlies the generation of neural sequences in a songbird. Nature 419: 65–70, 2002 [DOI] [PubMed] [Google Scholar]
- Harris EW, Ganong AH, Monaghan DT, Watkins JC, Cotman CW. Action of 3-((+/−)-2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid (CPP): a new and highly potent antagonist of N-methyl-d-aspartate receptors in the hippocampus. Brain Res 382: 174–177, 1986 [DOI] [PubMed] [Google Scholar]
- Harsch A, Robinson HP. Postsynaptic variability of firing in rat cortical neurons: the roles of input synchronization and synaptic NMDA receptor conductance. J Neurosci 20: 6181–6192, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiligenberg Neural Nets in Electric Fish Cambridge, MA: Bradford Book, 1991 [Google Scholar]
- Hopkins CD, Bass AH. Temporal coding of species recognition signals in an electric fish. Science 212: 85–87, 1981 [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Walmsley B. Counting quanta: direct measurements of transmitter release at a central synapse. Neuron 15: 875–884, 1995a [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Walmsley B. Receptors underlying excitatory synaptic transmission in slices of the rat anteroventral cochlear nucleus. J Neurophysiol 73: 964–973, 1995b [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Walmsley B. Amplitude and time course of spontaneous and evoked excitatory postsynaptic currents in bushy cells of the anteroventral cochlear nucleus. J Neurophysiol 76: 1566–1571, 1996 [DOI] [PubMed] [Google Scholar]
- Joelson D, Schwartz IR. Development of N-methyl-d-aspartate receptor subunit immunoreactivity in the neonatal gerbil cochlear nucleus. Microsc Res Tech 41: 246–262, 1998 [DOI] [PubMed] [Google Scholar]
- Johnson DH. The relationship between spike rate and synchrony in responses of auditory-nerve fibers to single tones. J Acoust Soc Am 68: 1115–1122, 1980 [DOI] [PubMed] [Google Scholar]
- Joris PX, Smith PH, Yin TC. Enhancement of neural synchronization in the anteroventral cochlear nucleus. II. Responses in the tuning curve tail. J Neurophysiol 71: 1037–1051, 1994 [DOI] [PubMed] [Google Scholar]
- Joshi I, Wang LY. Developmental profiles of glutamate receptors and synaptic transmission at a single synapse in the mouse auditory brain stem. J Physiol 540: 861–873, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya K, Takahashi K, Kitamura K, Momoi T, Yoshikawa Y. Mitosis and apoptosis in postnatal auditory system of the C3H/He strain. Brain Res 901: 296–302, 2001 [DOI] [PubMed] [Google Scholar]
- Kawasaki M, Guo YX. Neuronal circuitry for comparison of timing in the electrosensory lateral line lobe of the African wave-type electric fish Gymnarchus niloticus. J Neurosci 16: 380–391, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiang NY, Pfeiffer RR, Warr WB, Backus AS. Stimulus coding in the cochlear nucleus. Ann Otol Rhinol Laryngol 74: 463–485, 1965 [DOI] [PubMed] [Google Scholar]
- Klumpp RG, Eady HR. Some measurements of interaural time difference thresholds. J Acoust Soc Am 28: 859–860, 1956 [Google Scholar]
- Koike-Tani M, Saitoh N, Takahashi T. Mechanisms underlying developmental speeding in AMPA-EPSC decay time at the calyx of Held. J Neurosci 25: 199–207, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J, Schneider J, McPherson S, Murphy DE, Bernard P, Tsai C, Bennett DA, Pastor G, Steel DJ, Boehm C, Cheney DL, Liebman JM, Williams M, Woud PL. CPP, a selective N-methyl-d-aspartate (NMDA)-type receptor antagonist: characterization in vitro and in vivo. J Pharmacol Exp Ther 240: 737–746, 1987 [PubMed] [Google Scholar]
- Limb CJ, Ryugo DK. Development of primary axosomatic endings in the anteroventral cochlear nucleus of mice. J Assoc Res Otolaryngol 1: 103–119, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorente de Nó R. The Primary Acoustic Nuclei New York: Raven, 1981 [Google Scholar]
- Louage DH, van der Heijden M, Joris PX. Enhanced temporal response properties of anteroventral cochlear nucleus neurons to broadband noise. J Neurosci 25: 1560–1570, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainen ZF, Sejnowski TJ. Reliability of spike timing in neocortical neurons. Science 268: 1503–1506, 1995 [DOI] [PubMed] [Google Scholar]
- Manis PB, Marx SO. Outward currents in isolated ventral cochlear nucleus neurons. J Neurosci 11: 2865–2880, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata M, Imoto K. Different composition of glutamate receptors in corticothalamic and lemniscal synaptic responses and their roles in the firing responses of ventrobasal thalamic neurons in juvenile mice. J Physiol 575: 161–174, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moiseff A, Konishi M. Neuronal and behavioral sensitivity to binaural time differences in the owl. J Neurosci 1: 40–48, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel D. Synaptic responses and electrical properties of cells in brain slices of the mouse anteroventral cochlear nucleus. J Neurosci 3: 2043–2053, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel D. Use of brain slices in the study of the auditory system: spatial and temporal summation of synaptic inputs in cells in the anteroventral cochlear nucleus of the mouse. J Acoust Soc Am 78: 328–333, 1985 [DOI] [PubMed] [Google Scholar]
- Oleskevich S, Clements J, Walmsley B. Release probability modulates short-term plasticity at a rat giant terminal. J Physiol 524: 513–523, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolini AG, FitzGerald JV, Burkitt AN, Clark GM. Temporal processing from the auditory nerve to the medial nucleus of the trapezoid body in the rat. Hear Res 159: 101–116, 2001 [DOI] [PubMed] [Google Scholar]
- Robinson HP, Kawai N. Injection of digitally synthesized synaptic conductance transients to measure the integrative properties of neurons. J Neurosci Methods 49: 157–165, 1993 [DOI] [PubMed] [Google Scholar]
- Romand R. The roles of retinoic acid during inner ear development. Curr Top Dev Biol 57: 261–291, 2003 [DOI] [PubMed] [Google Scholar]
- Rothman JS, Manis PB. The roles potassium currents play in regulating the electrical activity of ventral cochlear nucleus neurons. J Neurophysiol 89: 3097–3113, 2003 [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Ryugo DK. Intracellular marking of physiologically characterized cells in the ventral cochlear nucleus of the cat. J Comp Neurol 225: 167–186, 1984 [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Fekete DM. Morphology of primary axosomatic endings in the anteroventral cochlear nucleus of the cat: a study of the endbulbs of Held. J Comp Neurol 210: 239–257, 1982 [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Montey KL, Wright AL, Bennett ML, Pongstaporn T. Postnatal development of a large auditory nerve terminal: the endbulb of Held in cats. Hear Res 216– 217: 100–115, 2006 [DOI] [PubMed] [Google Scholar]
- Sachs MB, Abbas PJ. Rate versus level functions for auditory-nerve fibers in cats: tone-burst stimuli. J Acoust Soc Am 56: 1835–1847, 1974 [DOI] [PubMed] [Google Scholar]
- Sato K, Kuriyama H, Altschuler RA. Differential distribution of NMDA receptor subunit mRNA in the rat cochlear nucleus. Microsc Res Tech 41: 217–223, 1998 [DOI] [PubMed] [Google Scholar]
- Sharp AA, O'Neil MB, Abbott LF, Marder E. The dynamic clamp: artificial conductances in biological neurons. Trends Neurosci 16: 389–394, 1993 [DOI] [PubMed] [Google Scholar]
- Taberner AM, Liberman MC. Response properties of single auditory nerve fibers in the mouse. J Neurophysiol 93: 557–569, 2005 [DOI] [PubMed] [Google Scholar]
- Taschenberger H, Leao RM, Rowland KC, Spirou GA, von Gersdorff H. Optimizing synaptic architecture and efficiency for high-frequency transmission. Neuron 36: 1127–1143, 2002 [DOI] [PubMed] [Google Scholar]
- Taschenberger H, von Gersdorff H. Fine-tuning an auditory synapse for speed and fidelity: developmental changes in presynaptic waveform, EPSC kinetics, and synaptic plasticity. J Neurosci 20: 9162–9173, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Manis PB. Synaptic transmission at the cochlear nucleus endbulb synapse during age-related hearing loss in mice. J Neurophysiol 94: 1814–1824, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Manis PB. Short-term synaptic depression and recovery at the mature mammalian endbulb of Held synapse in mice. J Neurophysiol 100: 1255–1264, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SH, Ma CL, Kelly JB. Contribution of AMPA, NMDA, and GABA(A) receptors to temporal pattern of postsynaptic responses in the inferior colliculus of the rat. J Neurosci 24: 4625–4634, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu-Friedman MA, Regehr WG. Dynamic-clamp analysis of the effects of convergence on spike timing. I. Many synaptic inputs. J Neurophysiol 94: 2512–2525, 2005a [DOI] [PubMed] [Google Scholar]
- Xu-Friedman MA, Regehr WG. Dynamic-clamp analysis of the effects of convergence on spike timing. II. Few synaptic inputs. J Neurophysiol 94: 2526–2534, 2005b [DOI] [PubMed] [Google Scholar]
- Yang H, Xu-Friedman MA. Relative roles of different mechanisms of depression at the mouse endbulb of held. J Neurophysiol 99: 2510–2521, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssoufian M, Oleskevich S, Walmsley B. Development of a robust central auditory synapse in congenital deafness. J Neurophysiol 94: 3168–3180, 2005 [DOI] [PubMed] [Google Scholar]
- Yu AC, Margoliash D. Temporal hierarchical control of singing in birds. Science 273: 1871–1875, 1996 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.