Abstract
The generation of prolonged neuronal activity depends on the maintenance of synaptic neurotransmitter pools. The astrocytic glutamate-glutamine cycle is a major mechanism for recycling the neurotransmitters GABA and glutamate. Here we tested the effect of disrupting the glutamate-glutamine cycle on two types of neuronal activity patterns in the thalamus: sleep-related spindles and epileptiform oscillations. In recording conditions believed to induce glutamine scarcity, epileptiform oscillations showed a progressive reduction in duration that was partially reversible by the application of exogenous glutamine (300 μM). Blocking uptake of glutamine into neurons with α-(methylamino) isobutyric acid (5 mM) caused a similar reduction in oscillation duration, as did blocking neuronal GABA synthesis with 3-mercaptoproprionic acid (10 μM). However, comparable manipulations did not affect sleep spindles. Together, these results support a crucial role for the glutamate-glutamine cycle in providing the neurotransmitters necessary for the generation of epileptiform activity and suggest potential therapeutic approaches that selectively reduce seizure activity but maintain normal neuronal activity.
INTRODUCTION
Reliable synaptic transmission is supported by neuronal pools of neurotransmitter. The possibility of transmission failure resulting from depletion of neurotransmitter pools is reduced by mechanisms that replenish neurotransmitters, including de novo synthesis and recycling of previously released transmitters (Hertz 2006). The most direct neurotransmitter recycling mechanism relies on presynaptic neurons to internalize released neurotransmitters from the extracellular space (Krantz et al. 1999).
Indirect mechanisms that recycle the neurotransmitters glutamate and GABA also exist. Specifically, high-affinity transporters take up GABA and glutamate into astrocytes (Chaudhry et al. 2002; Hamberger et al. 1979; Laake et al. 1995; Schousboe 2000), where they are ultimately converted via GABA decarboxylase and glutamine synthetase into glutamine (Bak et al. 2006). Astrocytic system-N transporters efflux glutamine to the extracellular space, where it is taken up into neurons by system-A transporters (Chaudhry et al. 2002; Fricke et al. 2007). Neuronal glutamine is converted into glutamate by phosphate-activated glutaminase (Bak et al. 2006; Kvamme 1998). In inhibitory neurons, glutamate is further converted into GABA by glutamate decarboxylase (Liang et al. 2006) before being packaged into vesicles.
Evidence is emerging that the glutamate-glutamine cycle is crucial for the proper maintenance of synaptic activity. Pharmacological disruption of the glutamate-glutamine cycle in the hippocampus and cortex reduces vesicular glutamate and GABA levels (Laake et al. 1995; Liang et al. 2006; Rae et al. 2003) and blocks epileptiform activity (Bacci et al. 2002; Tani et al. 2007). However, previous studies have also shown that glutamate-glutamine cycle disruption does not affect all types of neuronal activity generated in the hippocampus (Kam and Nicoll 2007; Liang et al. 2006). These contradictory results suggest that the importance of the glutamate-glutamine cycle as a neurotransmitter recycling mechanism is activity and structure dependent.
One structure where the maintenance of synaptic transmission is likely to depend on astrocytic neurotransmitter recycling is the thalamus, a subcortical sensory processing structure. Previous studies have shown that plasmalemmal GABA and glutamate transporters are localized exclusively on astrocytes in the thalamus (Danbolt 2001; De Biasi et al. 1998; Vitellaro-Zuccarello et al. 2008), precluding significant neurotransmitter reuptake by presynaptic neurons. This anatomical specificity suggests that astrocytes play a dominant role in recycling thalamic neurotransmitters and that perturbation of the glutamate-glutamine cycle would greatly affect thalamic activity.
The thalamus is involved in generating both sleep-related and epileptic activity (Huguenard and Prince 1994; McCormick and Bal 1997; Steriade and Llinás 1988; Steriade et al. 1993). Under normal conditions, the thalamus can generate 6- to 14-Hz spindles—oscillations that are expressed predominantly during sleep. These oscillations are generated by networks of reciprocally connected glutamatergic thalamocortical (TC) neurons and GABA-ergic reticular (RT) neurons. During some forms of epilepsy, these thalamocortical networks become dysfunctional and are involved in generating the oscillatory spike-wave discharges associated with absence seizures (Huguenard and Prince 1994; McCormick and Bal 1997). Activity patterns resembling both sleep spindles and spike-wave oscillations can be generated in vitro (Jacobsen et al. 2001; McCormick 2002; von Krosigk et al. 1993). Specifically, spindle-like activity in ferret and rat thalamic brain slices can be transformed into highly synchronous, 2- to 4-Hz epileptiform oscillations by bath application of bicuculline methiodide (BMI), a GABAA receptor antagonist that increases thalamic dependence on GABAB-mediated currents (Huguenard and Prince 1994; Kleinman-Weiner et al. 2009; McCormick 2002; von Krosigk et al. 1993).
Here, we determine the dependence of spindle-like and epileptiform activity on the glutamate-glutamine cycle. We take advantage of a previously reported time-dependent depletion of glutamine that occurs during in vitro experiments. Specifically, glutamine, and to a lesser extent glutamate and GABA, is gradually depleted from brain slices because of diffusion from the tissue into the bathing medium (Kapetanovic et al. 1993). Here we assess the functional relevance of such depletion. We report that under conditions matching those previously reported (Kapetanovic et al. 1993), epileptiform oscillations, but not nonpathological spindle-like activity, exhibit a progressive, activity-dependent rundown that is rescued by exogenous application of glutamine. Furthermore, this glutamine-induced recovery is reversed after pharmacological blockade of either system-A transporters or GABA synthesis. Together, these results show a dependence of thalamic epileptiform oscillations on the neurotransmitter recycling sustained by the astrocytic glutamate-glutamine cycle.
METHODS
Slice preparation
Experiments involving the use of animals were conducted in compliance with the rules set forth by the Stanford Institutional Animal Care and Use Committee. Sprauge-Dawley rat pups of both sexes, 11–15 days of age, were anesthetized (50 mg/kg sodium pentobarbital, ip) and decapitated. Brains were removed and transferred to chilled (4°C), oxygenated slicing solution (in mM): 234 sucrose, 11 glucose, 26 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 10 MgSO4, and 0.5 CaCl2 (310 mOsm). Horizontal slices (400 μM) containing somatosensory thalamus were made as previously described (Huguenard and Prince 1994) using a Leica VT1200 microtome. Slices were incubated in warm (32–33°C) artificial cerebrospinal fluid (ACSF; in mM): 10 glucose, 26 NaHCO3, 2.5 KCl, 1.25 NaHPO4, 1 MgSO4, 2 CaCl2, and 120 NaCl (298 mOsm) for 1 h and subsequently incubated at room temperature (23°C).
Slice recordings
Slices were placed in an interface chamber for recording and perfused with warm, oxygenated ACSF at a rate of 2 ml/min. Extracellular multiunit field recordings were made using monopolar tungsten microelectrodes (50–100 kΩ, FHC, Bowdoin, ME) placed in the thalamic reticular nucleus (containing RT neurons) and the ventrobasal somatosensory relay nucleus (VB, containing TC neurons). Signals were amplified 10,000 times and band-pass filtered between 100 Hz and 3 kHz. Electrical stimuli were delivered to the internal capsule with a pair of tungsten microelectrodes (50–100 kΩ, FHC). The stimuli were 100 μs in duration, 50 V in amplitude, and delivered once every 30 s.
Pharmacology
Epileptiform oscillations were induced by adding bicuculline methiodide (BMI; 10 μm, Tocris Bioscience, Ellisville, MO) to the ACSF solution (Huguenard and Prince 1994; Kleinman-Weiner et al. 2009; McCormick 2002; von Krosigk et al. 1993). Unless otherwise noted, slices were exposed to drugs for ∼30 min. l-glutamine (hereafter referred to as glutamine; 300 μM, Sigma, St. Louis, MO) (Hagenfeldt et al. 1984; Lerma et al. 1986) was added to ACSF after 30 min of stimulation. For some experiments, a higher concentration of glutamine (600 μM, Sigma) was applied 30 min after application of 300 μM glutamine. d-glutamine (300 μM, Sigma), a stereoisomer of glutamine, was used during control experiments. For experiments testing the activity dependence of rundown, slices were stimulated for 2.5 min, left unstimulated for 25 min, and stimulated for an additional 2.5 min. α-(methylamino) isobutyric acid (MeAIB; 5 mM, Sigma) (Bröer and Brookes 2001; Liang et al. 2006) was used to block system-A transporters. d-Mannitol was added to control solutions for MeAIB experiments to equalize changes in osmolarity (final osmolarity, 305 ± 1 mOsm). 3-Mercaptopropionic acid (MPA; Sigma) (Engel et al. 2001; Lindgren 1983; Murphy et al. 1998; Netopilová et al. 1997; Yamauchi et al. 1989) was used to block glutamate decarboxylase (GAD) at 10 μM, a concentration twofold higher than the Ki (Netopilová et al. 1995). For experiments testing the effect of GAD blockage on epileptiform activity in the absence of glutamine, MPA was applied after ∼22 min. During experiments testing the effect of GAD blockage on epileptiform activity in the presence of glutamine, glutamine was added to the ACSF after 7–13 min, followed 18–22 min later by application of MPA.
Data analysis
Data were collected with an Axon Instruments DigiData 1200 and pClamp 8 software. Data were analyzed with Matlab scripts (MathWorks, Natick, MA) designed to quantify burst activity during thalamic oscillations. Briefly, voltage deflections with a slope 3 times greater than background noise were defined as action potentials. Bursts of action potentials were defined as a minimum number of spikes separated from the previous spike by a maximum time interval. Oscillations were defined as groups of bursts containing a minimum number of bursts with a maximum allowable time between bursts. Because the properties of spindle and BMI-induced activity are different, the parameters used to quantify the two types of oscillations were distinct. For spindle activity, a burst was defined as at least four spikes with interspike intervals of <5 ms, with an oscillation defined as at least four bursts with a maximum interburst interval of 400 ms. For BMI-induced activity, a burst was defined as at least four spikes with interspike intervals of <15 ms. BMI-induced oscillations were defined as containing at least four bursts, with a maximum interburst interval of 1 s. The data we report reflect the average of five sweeps during a given condition. RM ANOVA refers to the repeated measures ANOVA on ranks test. Linear fits and correlation values (Pearson product-moment correlation coefficients) were generated in Origin 7 (OriginLab, Northhampton, MA).
RESULTS
Evoked spindle oscillations are not affected by glutamine-scarce conditions
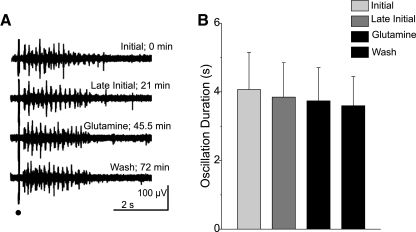
Kapetanovic et al. (1993) showed a significant, time-dependent loss of glutamine content from brain slice preparations incubated in the absence of exogenous glutamine. We tested whether such a loss of endogenous glutamine could provide a novel, nonpharmacological model of glutamate-glutamine cycle disruption in the thalamus. In horizontal rat thalamic slices, electrical stimulation of the internal capsule evokes spindle-like oscillations evident in RT and VB nuclei (Jacobsen et al. 2001). These oscillations consist of 6- to 14-Hz waxing and waning activity and are similar to spindle oscillations recorded in vivo (Steriade et al. 1993). In the absence of exogenous glutamine, spindle activity evoked at regular intervals (30 s) showed no significant change in duration [initial: 4.1 ± 2.9 s; late initial (after 30 min): 3.8 ± 2.7 s; n = 7; P > 0.05; RM ANOVA; Fig. 1]. Furthermore, subsequent exposure of the slice to physiological amounts of glutamine (300 μM) (Hagenfeldt et al. 1984; Lerma et al. 1986) failed to alter evoked spindle activity (glutamine: 3.7 ± 2.6 s; wash: 3.6 ± 2.3 s; n = 7; P > 0.05, RM ANOVA; Fig. 1). Presuming our experiments replicate the glutamine depletion shown by Kapetanovic et al. (1993), these results show that physiological, spindle-like activity is not susceptible to either rundown by glutamine depletion or to changes induced by glutamine restoration, suggesting that the glutamate-glutamine cycle is not necessary for the maintenance of such activity.
Fig. 1.
Spindle activity is not affected by rundown. A: sample traces show spindles evoked under different experimental conditions at the times indicated. Stimulus is indicated by black dot. B: population data from 7 slices depicting mean ± SE duration of spindle responses in the conditions noted in A. No time- or glutamine-dependent changes were observed (P > 0.05, RM ANOVA).
BMI-evoked oscillations show rundown of activity
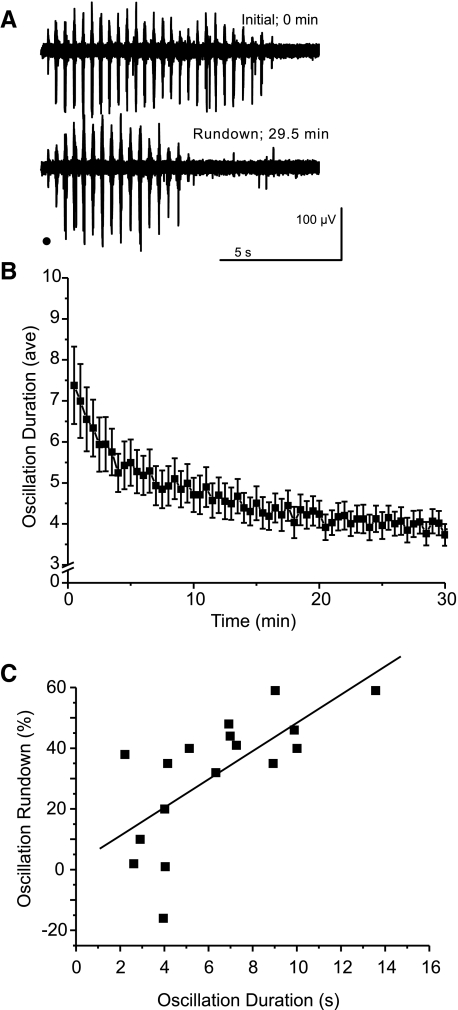
After application of BMI to a thalamic slice, 6- to 14-Hz spindle oscillations are converted into highly synchronous 2- to 4-Hz epileptiform oscillations (Jacobsen et al. 2001; Kleinman-Weiner et al. 2009; von Krosigk et al. 1993). Unlike spindle activity, we observed a progressive time-dependent decrease in the duration of epileptiform oscillations. After evoking epileptiform responses every 30 s for 30 min, the duration of the evoked oscillations decreased by an average of 31% [initial: 6.4 ± 3.2 s; late initial (after 30 min): 3.9 ± 1.3 s; n = 17 slices; P < 0.0001, RM ANOVA; Figs. 2A and 4C]. We refer to this decrease in duration as oscillation “rundown.” Rundown was usually characterized by two phases: an early rapid decrease over the first minute or so, followed by a more gradual depression over 30 min. Because oscillation frequency was unchanged (P > 0.05, RM ANOVA), the oscillation rundown was primarily caused by a decrease in the number of bursts evoked per stimulus (initial: 18.3 ± 8.7 bursts; rundown: 12.0 ± 3.6 bursts; P < 0.005, RM ANOVA). The decrease in duration was positively correlated with the initial oscillation duration (R = 0.69; n = 17; P < 0.005, Pearson correlation; Fig. 2C), indicating that slices with stronger initial activity exhibit greater rundown.
Fig. 2.
Epileptiform oscillations show rundown of their duration. A: sample traces of initial oscillations and oscillations evoked after ∼30 min. Stimulus is indicated by black dot. B: the time course of evoked oscillation duration shows activity-dependent rundown toward a plateau. Plot shows average response duration at 0.5-min intervals (n = 17 slices, error bars indicate SE). C: the degree of rundown is correlated with the original oscillation duration (R = 0.69; n = 17; P < 0.005, Pearson correlation, line shows linear fit).
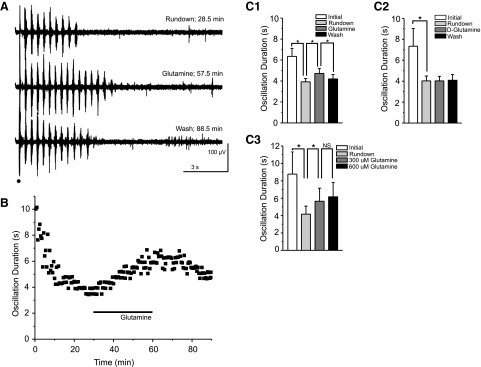
Fig. 4.
Exogenous glutamine (300 μM) partially and reversibly rescues evoked oscillations. A and B: sample traces (A) and time course of a typical experiment (B) show rundown of oscillation duration, the rescue effect of exogenous glutamine, and the secondary rundown that occurs after wash of glutamine. Stimulus is indicated by black dot and glutamine application by black bar. C: averaged durations at initial (00:00–02:30), rundown (27:30–30:00), glutamine (57:30–60:00), and wash (87:30–90:00) time bins show rundown and the reversible effect of glutamine (C1; 300 μM; *P < 0.0001, RM ANOVA; error bars indicate SE), but not d-glutamine (C2; 300 μM; *P > 0.05, RM ANOVA; error bars indicate SE) on oscillation duration. C3: averaged durations at initial (0–2.5 min), rundown (27.5–30 min), 300 μM glutamine (57.5–60 min), and 600 μM glutamine (87.5–90 min) time bins show no additional rescue of oscillation duration with an increased glutamine concentration (n = 5; P > 0.05, RM ANOVA).
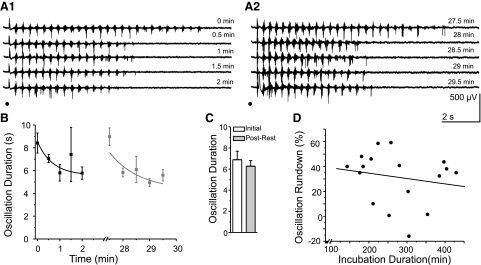
The positive correlation between initial duration and percent rundown was likely not skewed by changes in slice glutamine concentration associated with prolonged incubation time between slice preparation and recording. First, the initial oscillation duration observed in a slice was not correlated with time elapsed between the preparation of the slice and the start of the experiment (i.e., incubation duration, R = −0.192; n = 17; P > 0.05, Pearson correlation). Moreover, the decrease in duration observed in a slice was not correlated with incubation duration (R = −0.185; n = 17; P > 0.05, Pearson correlation; Fig. 3D).
Fig. 3.
Oscillation rundown is activity dependent. A1 and A2: sample traces of initial oscillations and oscillations evoked after a 25-min stimulation-free rest period. Stimulus indicated by black dot. B: the time course of evoked oscillation duration shows no rundown after a 25-min rest period. Plot shows average response duration at 0.5-min intervals (n = 5, error bars indicate SE). C: averaged durations at initial (0–2.5 min) and post-rest (27.5–30 min) time bins show no rundown of activity when stimulation is halted for 25 min between stimulation periods. D: the degree of rundown is not correlated with the incubation duration (R = −0.185, n = 17, P > 0.05, Pearson correlation, line shows linear fit).
To directly test the activity dependence of rundown, we evoked epileptiform responses every 30 s for 2.5 min, halted stimulation for 25 min, and resumed stimulation for an additional 2.5 min. Both the initial oscillations (i.e., those in the 1st 2.5 min; Fig. 3, A1 and B, black symbols) and those recorded after 25 min without stimulation (Fig. 3, A2 and B, gray symbols) showed an early decrease in duration over the first minute or so, followed by relatively stable oscillations at a slightly decreased level. The activity-free period was not associated with changes in either the first oscillation in each series or the later oscillations (initial duration, 5-sweep average: 6.9 ± 1.8 s; post-rest duration, 5-sweep average: 6.3 ± 1.2 s; n = 5; P > 0.05, Signed rank test; Fig. 3C).
Glutamine partially restores oscillation activity lost during rundown
If the observed rundown of oscillatory activity was caused by the loss of glutamine in brain slices as described by Kapetanovic et al. 1993, supplementation of rundown slices with glutamine should reverse the loss and restore activity. Application of 300 μM glutamine partially restored evoked oscillation duration to 81% of pre-rundown oscillation duration (initial: 6.4 ± 3.2 s; rundown: 3.9 ± 1.3 s; glutamine: 4.7 ± 1.9 s; n = 17; P < 0.0001, RM ANOVA; Fig. 4, A, B, and C1). As was the case during oscillation rundown, the glutamine-dependent rescue of oscillation duration was primarily caused by a change in the number of bursts per oscillation (rundown: 12.0 ± 3.6 bursts; glutamine: 14.7 ± 5.3 bursts; P < 0.005, RM ANOVA). Subsequent washout of exogenous glutamine yielded a decrease in oscillation duration to levels approximating the initial rundown (wash: 4.2 ± 1.8 s; 106% of rundown oscillation duration; P < 0.0001, RM ANOVA; Fig. 4, A, B, and C1). Oscillation frequency did not significantly change during any of the three experimental conditions (P > 0.05, RM ANOVA). The partial rescue of oscillation duration by 300 μM glutamine was not further enhanced by the application of a higher concentration of glutamine (600 μM; initial: 8.8 ± 2.6 s; rundown: 4.2 ± 0.9 s; 300 μM glutamine: 5.7 ± 1.5 s; 600 μM glutamine: 6.2 ± 1.7 s; n = 5; P > 0.05; 300 vs. 600 μM glutamine; P < 0.005 all other conditions, RM ANOVA; Fig. 4C3).
d-Glutamine does not rescue rundown epileptiform oscillation activity
To control for nonspecific effects of glutamine, we exposed thalamic slices to an inert stereoisomer of glutamine, d-glutamine, which is transported into neurons (Pow and Crook 1996) but accumulates in presynaptic neurons because it is not converted into excitatory glutamate by phosphate-activated glutaminase (Brown et al. 2008). To test the effect of d-glutamine on rundown, we stimulated slices in glutamine-free conditions to promote rundown and exposed slices to d-glutamine. Unlike l-glutamine, d-glutamine had no effect on rundown oscillations (300 μM, initial: 7.3 ± 4.5 s; rundown: 4.0 ± 1.2 s; d-glutamine: 4.0 ± 1.1 s; wash: 4.1 ± 1.5 s; n = 6; P > 0.05, RM ANOVA; Fig. 4C2).
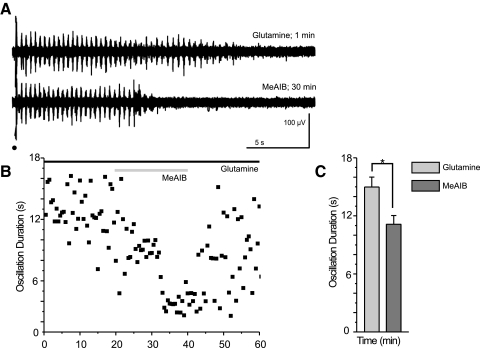
MeAIB reverses the rescue of epileptiform oscillations
Current models of the astrocytic glutamate-glutamine cycle ascribe glutamine uptake into neurons to system-A transporter activity (Chaudhry et al. 2002; Fricke et al. 2007). Blockade of these transporters reduces the amount of recycled neurotransmitter (Armano et al. 2002). MeAIB is a competitive inhibitor of system-A transporters (Bröer and Brookes 2001). To isolate the effects of MeAIB, we included 300 μM glutamine in the ACSF to prevent rundown. Subsequent application of MeAIB onto glutamine-supplemented slices reduced the duration of the evoked oscillations by 24% (glutamine: 15.0 ± 2.5 s; MeAIB: 11.1 ± 2.2 s; n = 6; P < 0.05, signed rank test; Fig. 5).
Fig. 5.
Blocking uptake of glutamine into neurons causes oscillation run down. A and B: sample traces (A) and the time course of a typical experiment (B) show a reduction in oscillation duration after application of the system-A transporter antagonist, α-(methylamino) isobutyric acid (MeAIB), in the presence of glutamine. Stimulation is indicated by a black dot, glutamine application by a black bar, and MeAIB application by a gray bar. C: averaged durations, at times of equilibrium glutamine (0–2.5 min) and MeAIB (17.5–20.83 min after MeAIB application) effect, show that MeAIB causes a reduction in oscillation duration even in the presence of exogenous glutamine (*P < 0.05, signed rank test, error bars indicate SE).
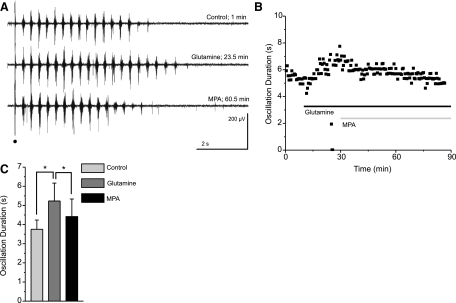
MPA reverses the rescue of epileptiform oscillations
The glutamine supplied by the astrocytic glutamate-glutamine cycle is used to replenish two distinct neurotransmitters: glutamate and GABA. Individually blocking the recycling of these neurotransmitters could show distinct roles of glutamate and GABA in maintaining epileptiform activity. In GABAergic neurons, GABA is converted from glutamate by the enzyme glutamate decarboxylase (Martin and Tobin 2000), which can be blocked by 3-mercaptopropionic acid (MPA) (Engel et al. 2001; Murphy et al. 1998; Netopilová et al. 1995). Before block of GABA synthesis, rundown of evoked oscillations was induced in thalamic slices and rescued through the application of 300 μM glutamine (Fig. 6). The subsequent application of 10 μM MPA onto these glutamine-supplemented slices reversed glutamine-rescue of evoked oscillatory activity (17% reduction in oscillation duration by MPA; control 3.8 ± 1.2 s; glutamine: 5.2 ± 2.3 s; MPA: 4.4 ± 2.2 s; n = 6; P < 0.01, RM ANOVA; Fig. 6). In the absence of glutamine, MPA application would be expected to induce negligible effects. Indeed, MPA does not significantly reduce the duration of rundown oscillations in slices that had not been supplemented with glutamine (rundown: 8.8 ± 2.4 s; MPA 8.3 ± 2.6 s; n = 8; P > 0.05, RM ANOVA).
Fig. 6.
Blocking the conversion of glutamate into GABA reverses the glutamine-dependent rescue of evoked oscillations. A and B: representative traces (A) and the time course of a typical experiment (B) show glutamine-dependent rescue of rundown followed by a secondary run down in the presence of 3-mercaptoproprionic acid (MPA), a glutamate decarboxylase inhibitor. Stimulation is indicated by a black dot, glutamine application by a black bar, and MPA application by a gray bar. C: averaged durations at times of equilibrium control (0–2.5 min), glutamine (22.5–25 min), and MPA (60–62.5 min) effect show the rescue effect of glutamine and the reversal of that effect by application of MPA (*P < 0.01, RM ANOVA, error bars indicate SE).
DISCUSSION
The functional relevance of the glutamate-glutamine cycle in recycling neurotransmitter and supporting neuronal activity has been studied in multiple neuronal systems, including hippocampus and neocortex (Bacci et al. 2002; Kam and Nicoll 2007; Laake et al. 1995; Liang et al. 2006; Rae et al. 2003; Tani et al. 2007). Here we assess glutamate-glutamine cycle dependence in the thalamus, a brain structure that generates two types of network oscillations: sleep-related spindles and seizure-related spike-wave discharges. We showed that thalamic epileptiform oscillations, but not spindle activity, are dependent on the glutamate-glutamine cycle.
Glutamine is a major precursor of glutamate and GABA (Lebon et al. 2002; Patel et al. 2001; Paulsen et al. 1988; Peng et al. 1993; Tapia and González 1978). A crucial step in the glutamate-glutamine cycle is the astrocytic release of glutamine into the extracellular space (Chaudhry et al. 2002). We developed a model of glutamate-glutamine cycle disruption based on depletion of extracellular glutamine. In vivo, the extracellular space contains 200–500 μM glutamine (Hagenfeldt et al. 1984; Lerma et al. 1986). Perfusing brain slices with glutamine-free ACSF causes diffusion of extracellular glutamine out of the tissue and into the bathing medium (Kapetanovic et al. 1993). Over time, this diffusion results in a pronounced decrease in glutamine tissue concentrations (90% lost after 3 h) and, to a lesser extent, neurotransmitters such as glutamate and GABA (45 and 25%, respectively, lost after 3 h) (Kapetanovic et al. 1993). Although we did not directly measure glutamine tissue concentrations, our recording conditions were comparable to those used by Kapetanovic et al. (1993) and are likely to induce comparable decreases in tissue concentrations of glutamine, glutamate, and GABA. Glutamine depletion is expected to act as a potent disruptor of the glutamate-glutamine cycle and to yield functionally relevant changes in neuronal activity.
Thalamic dependence on the glutamate-glutamine cycle is activity dependent
Despite the proposed disruption of the glutamate-glutamine cycle, the duration and frequency of thalamic spindle activity was not affected by prolonged exposure to glutamine-free ACSF. Spindle activity is hypothesized to represent sparse network oscillations, with only a small percentage of thalamic neurons participating during any given cycle of the oscillation (Huguenard and McCormick 2007; Steriade and Llinás 1988; von Krosigk et al. 1993). Specifically, each spindle oscillation is thought to be generated by a dynamically changing subpopulation of thalamic neurons (Steriade and Llinás 1988; von Krosigk et al. 1993), an effect mediated by the inhibitory connections between RT neurons that tend to decrease network synchronization (for review, Huguenard and McCormick 2007). If true, such sparseness provides individual neurons with periods of inactivity during which they may replenish neurotransmitter stores. Indeed, previous reports in the hippocampus have shown that alternate sources of glutamate and GABA may be sufficient to support low levels of synaptic activity (Liang et al. 2006; Mathews and Diamond 2003). Alternatively, spindles may be maintained by the glutamine and neurotransmitter concentrations that survive diffusion (Kapetanovic et al. 1993).
In contrast to spindles, epileptiform activity in the thalamus seems highly dependent on the glutamate-glutamine cycle. BMI disrupts thalamocortical networks, wherein reduced intra-RT inhibition and enhanced RT excitability transform sparse spindle activity into robust epileptiform oscillations (Huguenard and Prince 1994; Kleinman-Weiner et al. 2009; McCormick and Bal 1997; von Krosigk et al. 1993). We hypothesized that the enhanced activity of epileptiform oscillations might unmask glutamate-glutamine cycle dependence. Indeed, BMI-treated slices showed progressive rundown of epileptiform oscillations evoked in glutamine-free ACSF. Rundown was associated with a decrease in the number of action potential bursts evoked by a stimulus, rather than with changes in oscillation frequency. This distinction indicates that, during rundown, the thalamic network is activated for shorter periods of time but is not intrinsically modified. Oscillation rundown was partially reversed by adding glutamine to the ACSF, a manipulation previously shown to restore tissue glutamine and neurotransmitter levels (Kapetanovic et al. 1993). This rescue of epileptiform activity was maintained only if exogenous glutamine was constantly supplied, suggesting that glutamine diffusion out of brain slices is a continuous process.
Our results suggest that functionally relevant neurotransmitter losses are activity dependent. First we observed that oscillation rundown was greatest in the most active slices. Consistent with this observation, rundown could be prevented by halting stimulation for 25 min. During incubation, glutamine rapidly diffuses from the extracellular space into the bathing medium (Kapetanovic et al. 1993). Because we did not observe any correlation between duration of slice incubation and percent rundown, our results suggest that extracellular glutamine concentration changes that occur after the required 1-h slice incubation are not functionally relevant. However, the correlation between initial oscillation duration and the percent decrease in oscillation duration suggests an activity-dependent depletion of neurotransmitter stores separate from the extracellular contents lost into the bathing medium during incubation. A likely candidate is intracellular neurotransmitter stores, which may be maintained if synaptic activity remains low during the incubation period. If so, repeated stimulation of activity during experiments would likely result in the release of neurotransmitter stores into the extracellular space followed by astrocytic conversion to glutamine, which may diffuse out of the slice. Such diffusion would deprive neurons of a source of neurotransmitter replenishment and thus deplete neurotransmitter stores, reducing the ability of the slice to generate prolonged oscillations. More active slices would release more neurotransmitter, yielding greater glutamine loss and greater rundown.
Previous reports support the hypothesis that the functional significance of the glutamate-glutamine cycle is dependent on the level of activity. In hippocampal CA1 pyramidal neurons, exogenous glutamine application does not affect the amplitude or frequency of spontaneous miniature inhibitory postsynaptic currents (mIPSCs) or miniature excitatory post synaptic currents (mEPSCs) (Kam and Nicoll 2007). Similarly, glutamate-glutamine cycle disruption does not affect the amplitudes of evoked inhibitory postsynaptic potentials (IPSPs) in the CA1 pyramidal neurons of relatively inactive slices (Liang et al. 2006). However, GABAergic IPSP amplitudes are reduced when glutamate-glutamine cycle disruption is paired with burst stimulation at physiologically relevant rates (Liang et al. 2006). Furthermore, glutamate-glutamine cycle blockade reduces the frequency of spontaneous epileptiform activity in the hippocampus (Bacci et al. 2002) and reduces the likelihood of evoking epileptiform activity in the neocortex (Tani et al. 2007). Together, these results support the hypothesis that higher activity rates show greater dependence on the glutamate-glutamine cycle—a hypothesis that our study further supports.
Roles for GABA and glutamate recycling in maintenance of epileptiform oscillations
In the thalamus, both glutamate and GABA recycling seem to depend on the exogenous glutamine supplied by the astrocytic glutamate-glutamine cycle (Danbolt 2001; De Biasi et al. 1998; Vitellaro-Zuccarello et al. 2003). Therefore disruptions to the glutamate-glutamine cycle should affect both neurotransmitter concentrations. MeAIB, a competitive inhibitor of system-A transporter-mediated neuronal glutamine uptake (Bröer and Brookes 2001), progressively shortened oscillations by an average of 24% in slices that were provided with exogenous glutamine. This finding supports the hypothesis that extracellular glutamine is a crucial substrate for glutamate and/or GABA and that blocking uptake of exogenous glutamine into neurons diminishes the thalamic circuit's ability to maintain prolonged epileptiform oscillations.
However, glutamatergic and GABA-ergic synapses have distinct vesicle refilling and release properties (Li et al. 2005; Moulder et al. 2007), suggesting that glutamate-glutamine cycle disruptions may not equally affect glutamate and GABA synaptic function. To address this issue, we aimed to selectively block transmitter production. Selectively blocking glutamate synthesis poses a technical challenge because glutamate is a GABA precursor (Liang et al. 2006; Martin and Tobin 2000), and therefore manipulations that target glutamate stores indirectly affect GABA stores.
The functional relevance of GABA can be examined by selectively blocking the synthesis of GABA from glutamate using MPA (Engel et al. 2001; Murphy et al. 1998; Netopilová et al. 1995), which reduced oscillation duration by an average of 17% in the presence of glutamine. The significant reduction of oscillations that occurs in MPA indicates that selective disruption of GABA production in RT neurons is sufficient to affect epileptiform activity.
Might decreases in glutamate levels after glutamine depletion also contribute to the rundown of epileptiform activity? The slightly greater rundown caused by MeAIB compared with MPA could reflect the fact that maintenance of epileptiform activity requires the recycling of both GABA and glutamate via the glutamate-glutamine cycle. The lack of an MPA effect in the absence of glutamine suggests that glutamine depletion results in decreased glutamate levels, especially those relevant for synaptic GABA recycling. Whether similar depletion of glutamate is relevant for synaptic pools of glutamate itself remains to be determined, but seems likely.
However, the smaller rundown caused by MPA compared with MeAIB could result from incomplete block of GABA synthesis. At high concentrations (>50 μM) MPA is reported to have nonspecific effects, including inhibition of the catabolic enzyme GABA transaminase (Lamar 1970; Loscher and Vetter 1985). Therefore in our experiments, we used 10 μM MPA, a relatively low concentration that is nevertheless double the reported Ki for GAD (Netopilová et al. 1995), and thus likely to produce robust inhibition.
Survival of thalamic activity despite glutamate-glutamine cycle disruption
Interestingly, we observed two forms of activity that persist despite presumed glutamine depletion: 1) spindle-like activity and 2) post-rundown epileptiform activity. The maintenance of these two activity types suggests that weaker/shorter patterns of activity are resistant to glutamine depletion. Contributory mechanisms might include alternative pathways such as non–system-A glutamine uptake, synthesis of glutamate from glucose (Hertz 2006), and/or synthesis of GABA from glutamate sources not associated with astrocytic metabolism (Mathews and Diamond 2003), whose contributions might be sufficient to maintain neurotransmitter at concentrations that can support low levels of activity. Furthermore, the possibility exists that previously unidentified neurotransmitter transporters on thalamic neurons might allow maintenance of some activity.
Targeting the glutamate-glutamine cycle for selective seizure relief
Previous studies have shown that the role of the astrocytic glutamate-glutamine cycle in supporting neuronal activity is highly structure and activity dependent (Bacci et al. 2002; Kam and Nicoll 2007; Laake et al. 1995; Liang et al. 2006; Rae et al. 2003; Tani et al. 2007). Here, we showed that, in the thalamus, the functional significance of the astrocytic glutamate-glutamine cycle is also highly activity dependent. We showed that glutamine depletion or pharmacological disruption of the astrocytic glutamate-glutamine cycle restricts the ability of the thalamic circuit to maintain intense, long-lasting 2- to 4-Hz epileptiform activity. Although it is unknown whether the intact brain can experience local glutamine depletion, our study nonetheless identifies disruption of the glutamate-glutamine cycle as a potential in vivo therapeutic tool. Indeed, considering the maintenance of normal neuronal activity during disruption of the glutamate-glutamine cycle, it may be possible to provide therapeutic approaches that target the cycle to selectively reduce epileptiform activity.
GRANTS
M. P. Beenhakker was funded through an Epilepsy Foundation Fellowship. This research was supported by National Institute of Neurological Disorders and Stroke Grants NS-12151 and NS-034774.
ACKNOWLEDGMENTS
We thank M. Kleiman-Weiner, H. Tani, and all the members of the Huguenard Laboratory for technical guidance and discussions.
REFERENCES
- Armano S, Coco S, Bacci A, Pravettoni E, Schenk U, Verderio C, Varoqui H, Erickson DJ, Matteoli M. Localization and functional relevance of system A neutral amino acid transporters in cultured hippocampal neurons. J Biol Chem 277: 10467–10473, 2002 [DOI] [PubMed] [Google Scholar]
- Bacci A, Sancini G, Verderio C, Armano S, Pravettoni E, Fesce R, Franceschetti S, Matteoli M. Block of glutamate-glutamine cycle between astrocytes and neurons inhibits epileptiform activity in hippocampus. J Neurophysiol 88: 2302–2310, 2002 [DOI] [PubMed] [Google Scholar]
- Bak LK, Schousboe A, Waagepetersen HS. The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. J Neurochem 98: 641–653, 2006 [DOI] [PubMed] [Google Scholar]
- Bradford HF, Ward HK, Foley P. Glutaminase inhibition and the release of neurotransmitter glutamate from synaptosomes. Brain Res 476: 29–34, 1989 [DOI] [PubMed] [Google Scholar]
- Bröer S, Brookes N. Transfer of glutamine between astrocytes and neurons. J Neurochem 77: 705–719, 2001 [DOI] [PubMed] [Google Scholar]
- Brown G, Singer A, Proudfoot M, Skarina T, Kim Y, Chang C, Dementieva I, Kuznetsova E, Gonzalez CF, Joachimiak A, Savchenko A, Yakunin AF. Functional and structural characterization of four glutaminases from Escherichia coli and Bacillus subtilis. Biochem 47: 5724–5735, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry FA, Reimer RJ, Edwards RH. The glutamine commute: take the N line and transfer to the A. J Cell Biol 157: 349–355, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol 65: 1–105, 2001 [DOI] [PubMed] [Google Scholar]
- De Biasi S, Vitellaro-Zuccarello L, Brecha NC. Immunoreactivity for the GABA transporter-1 and GABA transporter-3 is restricted to astrocytes in the rat thalamus. A light and electron-microscopic immunolocalization. Neuroscience 83: 815–828, 1998 [DOI] [PubMed] [Google Scholar]
- Dericioglu N, Garganta CL, Petroff OA, Mendelsohn D, Williamson A. Blockade of GABA synthesis only affects neural excitability under activated conditions in rat hippocampal slices. Neurochem Int 53: 22–32, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel D, Pahner I, Schulze K, Frahm C, Jarry H, Ahnert-Hilger G, Draguhn A. Plasticity of rat central inhibitory synapses through GABA metabolism. J Physiol 535: 473–482, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD, Ellisman MH. Synaptic morphology and differences in sensitivity. Science 228: 197–199, 1985 [DOI] [PubMed] [Google Scholar]
- Fricke MN, Jones-Davis DM, Mathews GC. Glutamine uptake by system A transporters maintains neurotransmitter GABA synthesis and inhibitory synaptic transmission. J Neurochem 102: 1895–1904, 2007 [DOI] [PubMed] [Google Scholar]
- Hagenfeldt L, Bjerkenstedt L, Edman G, Sedvall G, Wiesel FA. Amino acids in plasma and CSF and monoamine metabolites in CSF: interrelationship in healthy subjects. J Neurochem 42: 833–837, 1984 [DOI] [PubMed] [Google Scholar]
- Hamberger AC, Chiang GH, Nylén ES, Scheff SW, Cotman CW. Glutamate as a CNS transmitter. I. Evaluation of glucose and glutamine as precursors for the synthesis of preferentially released glutamate. Brain Res 168: 513–530, 1979 [DOI] [PubMed] [Google Scholar]
- Hertz L. Glutamate, a neurotransmitter – and so much more. A synopsis of Wierzba III. Neurochem Int 48: 416–425, 2006 [DOI] [PubMed] [Google Scholar]
- Huguenard JR, McCormick DA. Thalamic synchrony and dynamic regulation of global forebrain oscillations. Trends Neurosci 30: 350–356, 2007 [DOI] [PubMed] [Google Scholar]
- Huguenard JR, Prince DA. Thalamic rhythmicity studied in vitro: nominal t-current modulation causes robust antioscillatory effects. J Neurosci 14: 5485–5502, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen RB, Ulrich D, Huguenard JR. GABAB and NMDA receptors contribute to spindle-like oscillations in rat thalamus in vitro. J Neurophysiol 86: 1365–1375, 2001 [DOI] [PubMed] [Google Scholar]
- Kam K, Nicoll R. Excitatory synaptic transmission persists independently of the glutatmate-glutamine cycle. J Neurosci 27: 9192–9200, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapetanovic IM, Yonekawa WD, Kupferbery HJ. Time-related loss of glutamine from hippocampal slices and concomitant changes in neurotransmitter amino acids. J Neurochem 61: 865–872, 1993 [DOI] [PubMed] [Google Scholar]
- Kleinman-Weiner M, Beenhakker MP, Segal WA, Huguenard JR. Synergistic roles of GABAA receptors and SK channels in regulating thalamocortical oscillations. J Neurophysiol 102: 203–213, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krantz DE, Chaudry FA, Edwards RH. Neurotransmitter transporters. In: Neurotransmitter Release, edited by Bellen H. Oxford, UK: Oxford Press, 1999, p. 145–200 [Google Scholar]
- Kvamme E. Synthesis of glutamate and its regulation. Prog Brain Res 116: 73–85, 1998 [DOI] [PubMed] [Google Scholar]
- Laake JH, Slyngstad TA, Haug F-MS, Ottersen OP. Glutamine from glial cells is essential for the maintenance of the nerve terminal pool of glutamate: immunogold evidence from hippocampal slice cultures. J Neurochem 65: 871–881, 1995 [DOI] [PubMed] [Google Scholar]
- Lamar C., Jr Mercaptopropionic acid: a convulsant that inhibits glutamate decarboxylase. J. Neurochem 17: 165–170, 1970 [DOI] [PubMed] [Google Scholar]
- Lebon V, Petersen KF, Cline GW, Shen J, Mason GF, Dufour S, Behar KL, Shulman GI, Rothman DL. Astroglial contribution to brain energy metabolism in humans revealed by 13C nuclear magnetic resonance spectroscopy: elucidation of the dominant pathway for neurotransmitter glutamate repletion and measurement of astrocytic oxidative metabolism. J Neurosci 22: 1523–1531, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma J, Herranz AS, Herreras O, Abraira V, Martín del Río R. In vivo determination of extracellular concentration of amino acids in the rat hippocampus. A method based on brain dialysis and computerized analysis. Brain Res 384: 145–155, 1986 [DOI] [PubMed] [Google Scholar]
- Li Z, Burrone J, Tyler WJ, Hartman KN, Albeanu DF, Murthy VN. Synaptic vesicle recycling studied in transgenic mice expressing synaptopHluorin. Proc Natl Acad Sci USA 102: 6131–6136, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S, Carlson GC, Coulter DA. Dynamic regulation of synaptic GABA release by the glutamate-glutamine cycle in hippocampal area CA1. J Neurosci 26: 8537–8548, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren S. Effects of the glutamic acid decarboxylase inhibitor 3-mercaptopropionic acid on the synthesis of brain GABA in vivo and postmortally. J Neural Transm 58: 75–82, 1983 [DOI] [PubMed] [Google Scholar]
- Loscher W, Vetter M. In vivo effects of aminoxyacetic acid and valproic acid on nerve terminal (synaptosomal) GABA levels in discrete brain areas of the rat. Correlation to pharmacological activities. Biochem Pharmacol 34: 1747–1756, 1985 [DOI] [PubMed] [Google Scholar]
- Martin DL, Tobin AJ. Mechanisms controlling GABA synthesis and degradation in the brain. In: GABA in the Nervous System, edited by Martin DL, Olsen RW. Philadelphia, PA: Lippincott Williams and Wilkins, 2000, p. 25–41 [Google Scholar]
- Mathews GC, Diamond JS. Neuronal glutamate uptake contributes to GABA synthesis and inhibitory synaptic strength. J Neurosci 23: 2040–2048, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA. Cortical and subcortical generators of normal and abnormal rhythmicity. Int Rev Neurobiol 49: 99–114, 2002 [DOI] [PubMed] [Google Scholar]
- McCormick DA, Bal T. Sleep and arousal: thalamocortical mechanisms. Annu Rev Neurosci 20: 185–215, 1997 [DOI] [PubMed] [Google Scholar]
- Moulder KL, Jiang X, Taylor AA, Shin W, Gillis KD, Mennerick S. Vesicle pool heterogeneity at hippocampal glutamate and GABA synapses. J Neurosci 27: 9846–9854, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DD, Cole NB, Greenberger V, Segal M. Estradiol increases dendritic spine density by reducing GABA neurotransmission in hippocampal neurons. J Neurosci 18: 2550–2559, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netopilová M, Dršata J, Haugvicová R, Kubová H, Mareš P. Inhibition of glutamate decarboxylase activity by 3-mercaptopropionic acid has different time course in the immature and adult rat brains. Neurosci Lett 266: 68–70, 1997 [DOI] [PubMed] [Google Scholar]
- Netopilová M, Dršata J, Kubová H, Mareš P. Differences between immature and adult rats in brain glutamate decarboxylase inhibition by 3-mercaptoproprionic acid. Epilepsy Res 20: 179–184, 1995 [DOI] [PubMed] [Google Scholar]
- Patel AB, Rothman DL, Cline GW, Behar KL. Glutamine is the major precursor for GABA synthesis in rat neocortex in vivo following acute GABA-transaminase inhibition. Brain Res 919: 207–220, 2001 [DOI] [PubMed] [Google Scholar]
- Paulsen RE, Odden E, Fonnum F. Importance of glutamine for gamma-aminobutyric acid synthesis in rat neostriatum in vivo. J Neurochem 13: 637–641, 1988 [DOI] [PubMed] [Google Scholar]
- Peng L, Hertz L, Huang R, Sonnewald U, Petersen SB, Westergaard N, Larsson O, Schousboe A. Utilization of glutamine and of TCA cycle constituents as precursors for transmitter glutamate and GABA. Dev Neurosci 15: 367–377, 1993 [DOI] [PubMed] [Google Scholar]
- Pow DV, Crook DK. Direct immunocytochemical evidence for the transfer of glutamine from glial cells to neurons: use of specific antibodies directed against the d-stereoisomers of glutamate and glutamine. Neurosci 70: 295–302, 1996 [DOI] [PubMed] [Google Scholar]
- Rae C, Hare N, Bubb WA, McEwan SR, Bröer A, McQuillian JA, Balcar VJ, Conigrave AD, Bröer S. Inhibition of glutamine transport depletes glutamate and GABA neurotransmitter pools: further evidence for metabolic compartmentation. J Neurochem 85: 503–514, 2003 [DOI] [PubMed] [Google Scholar]
- Rizzoli SO, Betz WJ. Synaptic vesicle pools. Nat Rev Neurosci 6: 57–69, 2005 [DOI] [PubMed] [Google Scholar]
- Schousboe A. Pharmacological and functional characterization of astrocytic GABA transport: a short review. Neurochem Rev 25: 1241–1244, 2000 [DOI] [PubMed] [Google Scholar]
- Steriade M, Llinás RR. The functional states of the thalamus and the associated neuronal interplay. Physiol Rev 68: 649–742, 1988 [DOI] [PubMed] [Google Scholar]
- Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science 262: 679–685, 1993 [DOI] [PubMed] [Google Scholar]
- Tani H, Bandrowski AE, Parada I, Wynn M, Huguenard JR, Prince DA, Reimer RJ. Modulation of epileptiform activity by glutamine and system A transport in a model of post-traumatic epilepsy. Neurobiol Dis 25: 230–238, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia R, González RM. Glutamine and glutamate as precursors of the releasable pool of GABA in brain cortex slices. Neurosci Lett 10: 165–169, 1978 [DOI] [PubMed] [Google Scholar]
- Ventura R, Harris KM. Three-dimensional relationships between hippocampal sysnapses and astrocytes. J Neurosci 19: 6897–6906, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitellaro-Zuccarello L, Calvaresi N, De Biasi S. Expression of GABA transporters GAT-1 and GAT-3, in the cerebral cortex and thalamus of the rat during postnatal development. Cell Tissue Res 313: 245–257, 2003 [DOI] [PubMed] [Google Scholar]
- von Krosigk M, Bal T, McCormick DA. Cellular mechanisms of a synchronized oscillation in the thalamus. Science 261: 361–364, 1993 [DOI] [PubMed] [Google Scholar]
- Yamauchi R, Amatsu M, Okada Y. Effect of GABA (γ-aminobutyric acid) on neurotransmission in inferior colliculus slices from the guinea pig. Neurosci Res 6: 446–455, 1989 [DOI] [PubMed] [Google Scholar]