Abstract
The short (s) variant of the serotonin transporter (5-HTT) gene linked functional polymorphic region (5-HTTLPR) is associated with depression. Stressful life events, gender, and race have been shown to moderate this association. We examined the relationship between 5-HTTLPR genotype and symptoms of depression in two samples. Study 1 = 288 participants from a study of caregiver stress; and Study 2 = 142 participants from a study examining psychosocial stressors, genetics, and health. Main effects of 5-HTTLPR on symptoms of depression were examined, along with moderation by stress (care-giving status or low childhood socioeconomic status (SES), gender, and race. The 5-HTTLPR × stress group × gender interaction was significant in both samples (P < 0.003, and P < 0.008, respectively). For females, the s allele, combined with caregiving stress (Study 1) or low childhood SES (Study 2), was associated with higher depression scores as compared to participants in the non-stressor group and those with the long (l) allele; whereas, in males, the l allele, combined with a stressor, was associated with higher depression scores as compared to those in the non-stressor group and those with the s allele. Findings from two independent samples suggest that the association of 5-HTTLPR with depression varies according to gender and stressful life events.
Keywords: 5-HTTLPR, Depressive Symptoms, Caregiving, Socioeconomic Status, Race, Gender difference
Introduction
Serotonergic neurotransmission is a substantial modulator of emotional behavior (Whitaker-Azmitia and Peroutka 1990); thus genes involved in serotonergic transmission have received considerable attention with respect to their relation to personality traits and affective disturbances. During the past decade a growing number of studies have examined the association between allelic variation in the serotonin transporter (5-HTT) gene linked functional polymorphic region (5-HTTLPR) and personality traits related to depression, anxiety, and hostility. The results of these studies have been mixed, with some studies demonstrating an association between allelic variation and negative traits e.g., (Gelertner et al. 1998; Greenberg et al. 2000; Hamer et al. 1999; Lesch et al. 1996), and others reporting no association e.g., (Gustavsson et al. 1999; Jorm et al. 1998).
One noted reason for varied findings concerns the stress-diathesis model. This theory suggests that an underlying predisposition exists for certain disorders/diseases, but the presence of an environmental stressor may be necessary in order for the disorder to manifest itself. Accordingly, failure to consider gene by environment interactions may lead to the erroneous conclusion that an effect is not present. The work of Caspi and Moffitt (Caspi et al. 2003; Moffitt et al. 2005) has illustrated this point with findings indicating that allelic variation in the functional polymorphism 5-HTTLPR interacts with stressful life events to predict the occurrence of depression. Specifically, carriers of the less transcriptionally efficient 5-HTTLPR short (s) allele had increased rates of major depression as a function of increased numbers of varied past life stressors. Others have replicated this interaction by examination of environmental stressors such as unemployment (Grabe et al. 2005) and low socio-economic status (SES) (Manuck et al. 2004).
Gender should also be considered when examining the genetic effects of 5-HTTLPR on psychological outcomes (Du et al. 2000). At present it is difficult to draw conclusions about the effects of gender with respect to associations among personality traits, affective disorders, and 5-HTTLPR because the consideration of gender has varied markedly across studies. Some have failed to specifically address gender differences—e.g., gender was modeled as a covariate, the participants were predominately or exclusively male or female, or there was no consideration of potential gender effects (Deary et al. 1999; Gonda et al. 2005; Hamer et al. 1999; Jacobs et al. 2006; Lesch et al. 1996; Nakamura et al. 1997). The results have been inconsistent among studies that have examined potential gender differences. In three instances (Ball et al. 1997; Gelertner et al. 1998; Greenberg et al. 2000) no significant effects for gender were found when examining the association between NEO-PI scores and the 5-HTTLPR genotype; however, one study did report that higher Harm Avoidance was associated with the 5-HTTLPR s allele in males, whereas, the s allele was associated with lower scores for females. Du et al. (2000) reported a positive association between neuroticism and the presence of short (s) alleles, but only for males. Conversely, in two different adult samples, anxiety was significantly lower among males with the s variant of 5-HTTLPR, whereas, no such association was demonstrated in females (Flory et al. 1999). Furthermore, other findings indicate that the 5-HTTLPR genotype may interact with both gender and environmental stress with respect to psychological out-comes (Grabe et al. 2005, Sjoberg et al. 2006).
Race is yet another characteristic that must be taken into account when examining the effects of genes on personality traits and psychiatric disorders (Gelernter et al. 1999). Gelertner et al. (1998) reported that Caucasians with the 5-HTTLPR s allele had higher scores on neuroticism compared to those with the l/l genotype, whereas the opposite pattern of findings was reported for African Americans. Results from samples of Polish (Samochowiec et al. 2001) and Japanese individuals (Katsuragi et al. 1999) suggest that the 5-HTTLPR s allele is associated with higher scores on harm avoidance. Conversely, no association was found in Finnish (Mazzanti et al. 1998), Israeli (Ebstein et al. 1997), and Japanese (Nakamura et al. 1997) samples. Finally, increased anxiety has been significantly related to the 5-HTTLPR s allele in a Swedish study (Melke et al. 2001), while results from an Australian study (Jorm et al. 1998) did not show this association.
Related work from our lab has examined whether the functional polymorphism 5-HTTLPR is associated with CNS serotonin turnover—as indexed by cerebrospinal fluid levels of 5-hydroxyindoleacetic acid (5-HIAA) (Williams et al. 2003). A significant race by genotype interaction was found, indicating homozygosity for the 5-HTTLPR s allele is associated with higher CSF 5-HIAA levels in African Americans, but with lower levels in Caucasians. In addition, a significant gender by genotype interaction indicated that the 5-HTTLPR s/s genotype is associated with higher 5-HIAA levels in women, but with lower levels in men. These findings suggest that effects of the 5-HTTLPR polymorphism on CNS serotonergic function varies as a function of race and gender, further indicating that, as highlighted above, race and gender should be taken into account in research evaluating effects of 5-HTTLPR on behavioral functions that are regulated by CNS serotonergic function. We are aware of only one study that has included gender as a potential moderator of the interaction between 5-HTTLPR and stress as predictors of depressive symptoms (Sjoberg et al. 2006). Findings from their sample of boys and girls aged 16–19, indicated that females homozygous for the 5-HTTLPR s allele, in conjunction with childhood stress, were more likely to develop depression as compared to girls with no such history, or the presence of the long (l) allele. Moreover, their results also suggested that males with the s allele and traumatic backgrounds are protected from depression.
In the present paper, we examined the association of 5-HTTLPR genotype with depressive symptoms in the following two independent samples: (1) Study 1: participants who were caregivers of a relative with Alzheimer’s Disease or other major dementia and non-caregiver controls, and (2) Study 2: participants of varying childhood SES as indexed by father’s educational background. Consistent findings demonstrate that the burden associated with providing care for a loved one suffering from dementia is considerable (Schulz et al. 1995), and has been associated with a number of negative health outcomes (Baumgarten et al. 1992), including worse ratings of physical health, significant decreases in cellular immunity (Kiecolt-Glaser et al. 1991), higher levels of triglycerides (Vitaliano et al. 1995), and increased rates of early mortality, (Schulz and Beach 1999). We have also previously shown, in participants from Study 1, that caregiving is associated with increased symptoms of depression, poorer sleep quality, and increased glucose, as compared to non-caregivers (Brummett et al. 2006, 2005).
The stressor examined in Study 2 pertains to the negative impact of adversity during childhood. Because the risk of poor health is cumulative, starting during childhood and ultimately resulting in disease during adulthood (Power et al. 1999), consideration of factors that occur throughout the lifecourse is important. Low SES during childhood is a stressor that has been associated with poor health, e.g., depression, substance abuse, and early mortality (Bergstrom et al. 1996; Garner 1997; Shilling et al. 2007). Moreover, there is evidence to suggest that father’s educational level is an index of childhood SES that predicts cardiovascular mortality and ischemic heart disease, independently of adult SES (Strand and Kunst 2006).
Thus, based on the research summarized above we examined two different major chronic environmental stressors—caregiving and low childhood SES—along with gender and race, as potentially important moderators of the association between 5-HTTLPR and depressive symptoms. We hypothesized that depressive symptoms would be associated with 5-HTTLPR genotype, with effects magnified by the stress of caregiving and by low childhood SES as represented by father’s education level.
Methods
Sample
Study 1
Individuals were recruited to be part of a study designed to examine the underlying genetic, biological, and behavioral mechanisms whereby stressful social and physical environments lead to health outcomes. Persons who were the primary caregiver for a relative with Alzheimer’s Disease or other major dementia were included as participants who were living in a stressful life situation. Controls were identified by asking each caregiver to nominate two to five friends who live in their neighborhood and are like them with respect to key demographic factors (e.g., race and gender). The study was conducted at Duke University Medical Center, and all subjects gave informed consent prior to their participation in the study using a form approved by the Duke University Medical Center Institutional Review Board. Subjects enrolled in the study received $250 for their participation. Individuals who were experiencing any acute major medical/psychiatric disorder were excluded, resulting in the exclusion of one individual who experienced a severe psychiatric problem. A questionnaire battery was given to participants during a home visit by a study nurse. The questionnaire battery was returned during the same week upon their visit to the General Clinical Research Center at Duke University Medical Center, during which they received a physical examination and blood was drawn for assessment of biological parameters. The full study sample consisted of 344 participants. A total of 17 individuals did not have valid data for 5-HTTLPR. In addition, 39 individuals who reported taking an antidepressant were excluded from analyses, resulting in 288 (142 caregivers and 146 non-caregiving controls) participants who had complete data for each of the primary constructs (genotype, depressive symptoms, gender, and race). The final sample consisted of 215 (74.7%) females, 203 (70.5%) Caucasians, and the mean (SD) age in years was 58.3 (14.7).
Study 2
Participants were recruited to be part of a study designed to examine the moderating effects of genetic, behavioral, and environmental mechanisms on health disparities. The study was conducted at Duke University Medical Center, and all subjects gave informed consent prior to their participation in the study using a form approved by the Duke University Medical Center Institutional Review Board. Participants enrolled in the study received $500 for their participation. This protocol required that participants be in good current health because of the study procedures, see (Williams et al. 2001, 2003), therefore all participants underwent a comprehensive psychological examination, as well as medical history, physical exam, electrocardiogram, chest radiograph, hemoglobin, hematocrit, white cell count, and blood chemistries to rule out current psychiatric and medical disorders. Use of any prescription drugs as well as use of illegal drugs (as detected by a urine screen prior to entry into study) were grounds for exclusion. On day one of the study a questionnaire battery was given to participants and blood was drawn for assessment of biological parameters. The full study sample consisted of 165 participants. A total of 20 individuals did not have valid data for father’s educational background and 3 individuals did not have valid data for 5-HTTLPR, resulting in 142 participants who had complete data for each of the primary constructs (genotype, depressive symptoms, race, and gender). The sample was comprised of 64 (45.1%) females, 67 (47.2%) Caucasians, and the mean (SD) age in years was 34.0 (8.8).
In summary, both samples consisted of community volunteers who were free of acute medical and psychiatric disorders. In addition, race and gender were not significantly related to levels of depression in either study. However, as expected, there was a significant effect of stress group (caregiving and father’s education level) with respect to depression scores such that the caregivers in study 1, and those with lower levels of father’s education in study 2, had higher levels of depressive symptoms (P’s < 0.01).
Measures
Demographic
Gender and race (African American or Caucasian, based on self-report) were each coded as dichotomous variables. In Study 1, the stressor of caregiving status was coded as a dichotomous variable. In Study 2 childhood SES was determined by the participant’s father’s level of education coded in years (mean = 12.9, SD 4.0, range 2–21).
Genotyping
To determine HTTLPR genotypes, DNA was extracted from whole blood according to established protocols (Vance 1998). Polymerase chain reaction amplification to generate a 484- or 528-base pair fragment corresponding to the s and l 5-HTTLPR alleles, respectively, was performed using forward primer GCGTTGCCGCTCTGAATGC and reverse primer 6FAM-GAGGGACTGAGCTGGACAACCAC. Reaction conditions were 1X PCR buffer; 1X Q-solution (Qiagen), 0.2 mM dNTPs, 2 mM MgCl2 (total concentration including from buffer); 20 ng/µl of each primer and a total of 10 ng of DNA in a 10 µl reaction. Cycling conditions were 94°C for 3 min; 5 cycles of 94°C for 30 s, 70°C for 30 s, and 72°C for 1 min; and 30 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 1 min. The labeled products were separated on poly-acrylamide gels, which were scanned to detect the fluorescent products. Data was analyzed using Bioimage software (Genomic Solutions). Pooled samples, ladders, duplicates and CEPH DNA were all used to ensure quality genotyping.
Table 1 provides the distribution of 5-HTTLPR allele frequency by gender and by race for both studies. Studies 1 and 2 were examined for Hardy Weinberg Equlibrium (HWE). Both study samples did deviate somewhat from HWE (Study 1, P < 0.01; Study 2, P < 0.03). However, the frequencies of allele distributions in African Americans as compared to Caucasians likely accounts for the deviation. Confirming this notion is the fact that when we examined HWE within groups of African Americans and Caucasians the results were consistent with HWE. Due to an expected relation between depression and 5-HTTLPR allele frequency, a deviation of HWE in cases might be predicted. In the present samples HWE was tested in cases and controls combined and because HWE was demonstrated when races where analyzed independently this issue was not examined further.
Table 1.
5-HTTLPR allele frequency distributions by gender and race for studies 1 & 2
| Characteristic n, (%)* | s/s | s/l | l/l |
|---|---|---|---|
| Study 1: n = 288 | |||
| Male n = 73 (25.3%) | 21 (28.8%) | 25 (34.2%) | 27 (37.0%) |
| Female n = 215 (74.7%) | 41 (19.1%) | 98 (45.6%) | 76 (35.3%) |
| Caucasian n = 203 (70.5%) | 48 (23.7%) | 90 (44.3%) | 65 (32.0%) |
| African American n = 85 (29.5%) | 14 (16.5%) | 33 (38.8%) | 38 (44.7%) |
| Study 2: n = 142 | |||
| Male n = 78 (54.9%) | 8 (10.3%) | 31 (39.7%) | 39 (50.0%) |
| Female n = 64 (41.5%) | 13 (20.3%) | 17 (26.6%) | 34 (53.1%) |
| Caucasian n = 67 (47.2%) | 14 (20.9%) | 27 (40.3%) | 26 (38.8%) |
| African American n = 75 (52.8%) | 7 (9.3%) | 21 (28.0%) | 47 (62.7%) |
Percentages columns 2–3 represent those for each row total
Note: Within group analyses of African Americans and Caucasians were consistent with HWE
Symptoms of depression
Study 1
The Center for Epidemiologic Studies Depression Scale (CES-D) (Radloff 1977) is a widely used 20-item self-report scale designed to measure depressive symptom-atology (i.e., depressive affect, well-being, somatic complaints, and interpersonal concerns) in a general population. Items are scored on a 4-point scale, with the total score ranging between 0 and 60. Higher scores represent depressive responses, and a score of 16 or greater is generally considered suggestive of a depressive disorder.
Study 2
The 40-item Obvious Depression scale (OBD) (Wiener 1948) from the Minnesota Multiphasic Personality Inventory was used to assess symptoms of depression (MMPI) (Dahlstrom et al. 1960). The OBD is a measure of depressive symptoms (e.g., I am blue most of the time) that is more appropriate for non-clinical samples than the widely known D scale.
Analytic plan
Multiple linear regression analyses were used to examine the hypotheses that allelic variation in 5-HTTLPR is related to symptoms of depression, and that the effect will be moderated by the stressor of caregiving (caregiver versus control) or low father’s education (years of education). Initially for each study, 3-way interaction terms (i.e., gender × stressor status × allele status; and race × stressor status × allele status), along with all appropriate univariate and 2-way product terms, were examined as predictors of symptoms of depression (CES-D or OBD). Next, models were trimmed by removing any non-significant 3-way, and associated non-significant 2-way interaction terms. Age and race were controlled in all models.
Results
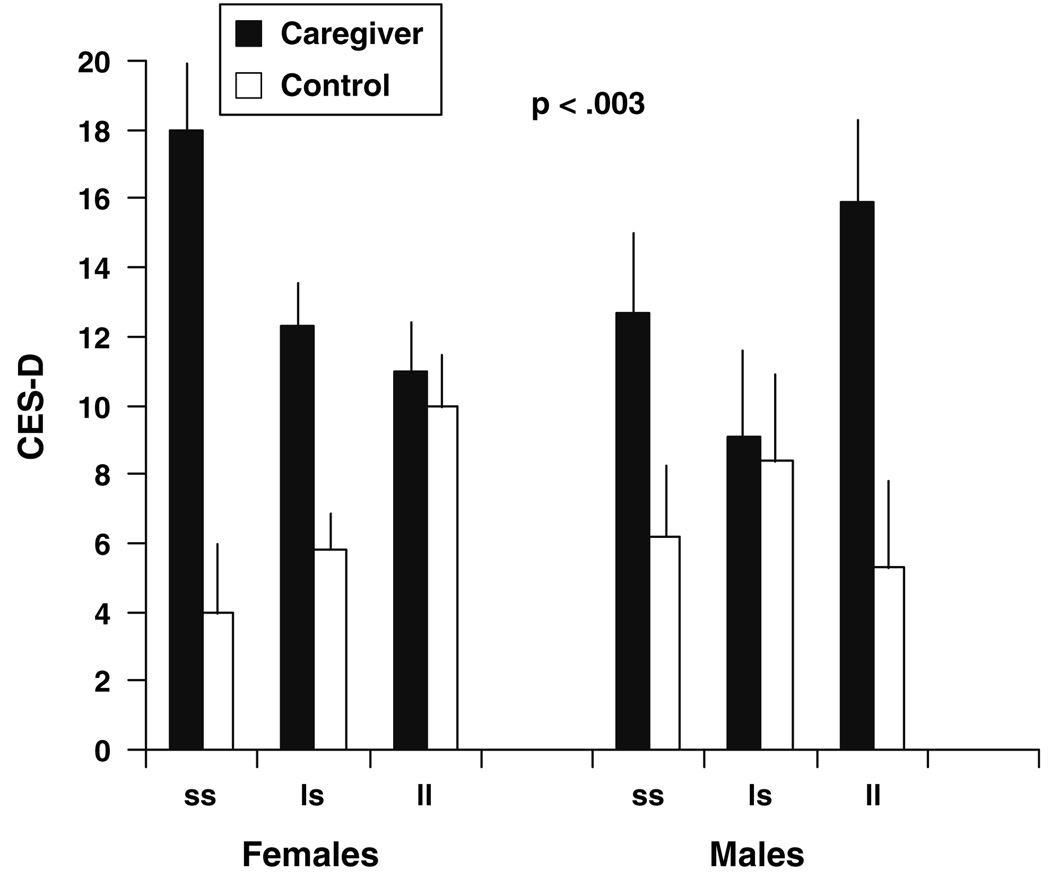
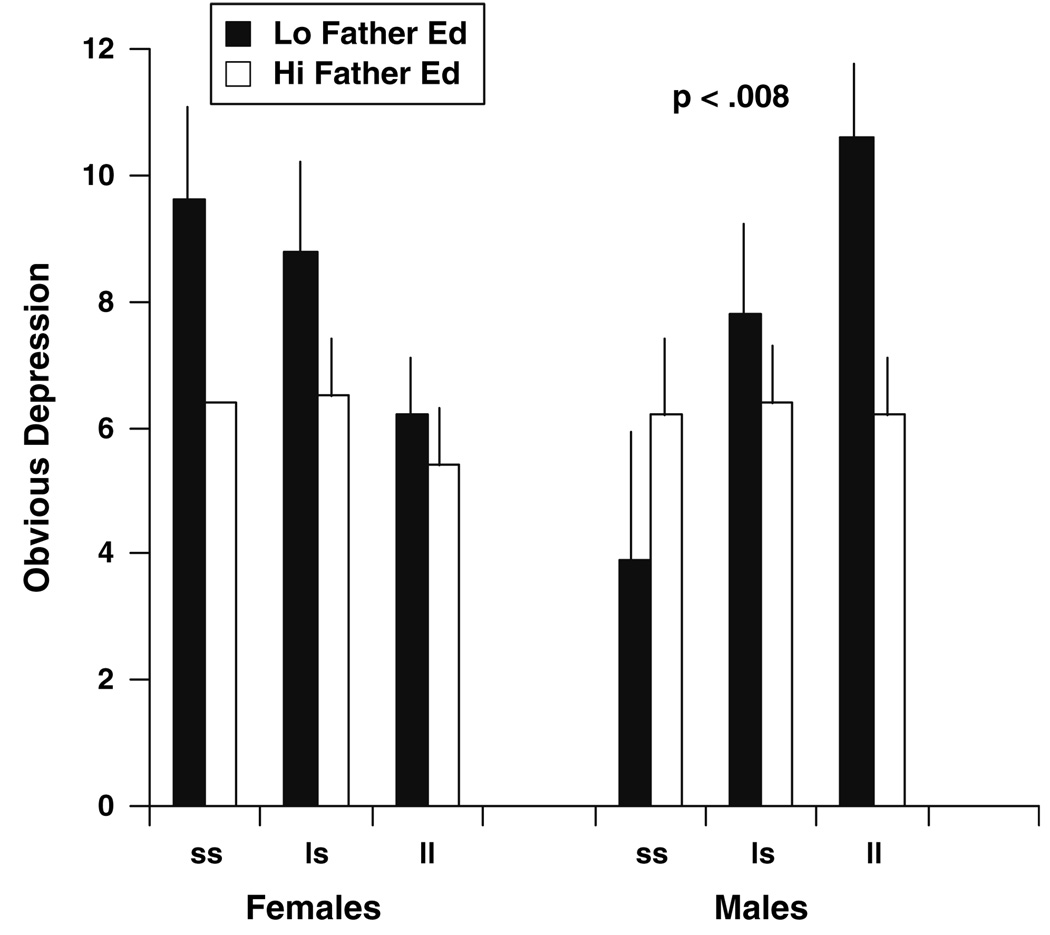
The race × stressor (caregiver status or father’s education) × 5-HTTLPR interaction term was non-significant in both studies. However, the gender × stressor status × 5-HTTLPR interaction was significant in both Study 1 and in Study 2 (P < 0.003, and P < 0.008, respectively). Figure 1 and Figure 2 depict the form of the interaction effects. In Study 1 and Study 2, among females the s allele was associated with increased symptoms of depression for those who were in the higher stress condition, as compared to those who possessed the l allele or were in the low stress condition. In contrast, for males in both studies, presence of the l allele was associated with increased symptoms of depression for those who were in the higher stress condition, as compared to those who possessed the s allele or were in the low stress condition. In marked contrast to those exposed to high stress conditions, whether currently or during childhood, there was little impact of 5-HTTLPR genotype on depressive symptoms among both males and females with low stress exposure levels.
Fig. 1.
Study 1: environmental stressor (caregivers vs. non-caregivers) × 5-HTTLPR × gender: symptoms of depression (CES-D; mean ± S.E.); n = 288
Fig. 2.
Study 2: environmental stressor (childhood SES graphically depicted as father’s education <12 years vs. ≥12 years) × 5-HTTLPR × gender: symptoms of depression (obvious depression; mean ± S.E.); n = 142
In Study 1, the mean score for female caregivers homozygous for the s allele was in the clinical range for depressive symptoms (i.e., 17.8); and was almost 1 standard deviation higher than the mean score for all other females in the study combined; Cohen’s (Cohen 1988) d = 0.78. Similarly, male caregivers homozygous for the l allele had CES-D ratings that were just below the clinical range (i.e., 15.9), and the mean CES-D score for this group was greater than 1 standard deviation higher than all other males in the study (Cohen’s d = 0.94). For female caregivers, genotype accounted for 4% of the variance in CES-D scores; and for male caregivers, genotype accounted for 13.2% of the variance in CES-D scores.
Likewise, in Study 2, homozygous s/s females whose father’s education level was below 12 years had scores on the obvious depression scale that were almost 1 standard deviation higher than that of all other females in the study (Cohen’s d = 0.84). This same pattern held for homozygous l/l males who reported a low level of father’s education (Cohen’s d = 0.94). Within females whose father’s education level was below 12 years, genotype accounted for 7.0% of the variance in OBD scores; and for males whose father’s education was low, genotype accounted for 26.3% of the variance in OBD scores.
Discussion
The current findings from two independent samples suggest that gender has a strong influence on the association among 5-HTTLPR frequency, chronic environmental stress and depressive symptoms in non-patient populations. Specifically, our study suggests that in females the 5-HTTLPR genotype associated with less transcriptional activity (s/s) may increase an individual’s susceptibility to depression under stressful life conditions, whereas the genotype associated with increased activity (l/l) may do so in males. While the interaction between 5-HTTLPR genotype and stress to predict depression has been documented previously e.g., (Caspi et al. 2003; Grabe et al. 2005; Manuck et al. 2004; Moffitt et al. 2005), the role of gender has received scant attention. Effects of stress to increase the impact of 5-HTTLPR genotype on depressive symptoms are strong in both studies—effects sizes (d), 0.78–0.94—whether the exposure to stressful life circumstances is current (being a caregiver in Study 1) or in the past (low father’s education in Study 2).
This study expands upon a growing literature showing that 5-HTTLPR genotype can have an opposite effect on behavioral characteristics in men and women. In a sample of 128 females, participants carrying the s allele had significantly higher ratings on a measure of subthreshold depression, as compared with those who carried the ll genotype (Gonda et al. 2005). Although not present in males, Eley et al. (2004) found a significant 5-HTTLPR × environmental stress interaction in females such that the proportion of depressed subjects who were carrying the s allele, coupled with high environmental stress, was higher than the proportion of non-s allele carriers and those with low environmental stress. Flory et al. have shown that anxiety was significantly lower among males with the short variant of 5-HTTLPR, whereas, no association was present in females (Flory et al. 1999). Likewise, in prior work on another sample we found that men, but not women, with the s variant of the 5-HTTLPR reported lower Anxiety (Brummett et al. 2003). In another study of 374 females, stressful life events interacted with the 5-HTTLPR genotype to predict symptoms of depression such that those with the s allele had significantly higher depression ratings, as compared to those with the l allele (Jacobs et al. 2006). Similarly, in a predominately female sample, lower expressing 5-HTTLPR alleles were associated with increased severity of major depression in those with moderate to severely stressful life events (Zalsman et al. 2006b). Additionally, results from a general population study indicate that for females, but not males, the s allele was associated with perceived mental and physical distress in unemployed participants as compared to those who were employed (Grabe et al. 2005). Finally, in a sample of 81 boys and 119 girls, Sjoberg et al. (2006) found results strikingly similar to those of the present study. Specifically, their findings suggest that females homozygous for the 5-HTTLPR s allele, in conjunction with the stressor of family conflict, are more likely to develop depression than girls with no family conflict, or the presence of the l allele; whereas, for males the s allele in conjunction with a traumatic background serves to protect an individual from depression. Thus, a similar pattern of gender X 5-HTTLPR X environmental stress effects on depression, and/or perceived stress, has now been demonstrated in 4 independent samples. The present results do, however, contrast with those of Caspi et al. (2003) who have shown in a sample of both males and females, that the s allele interacts with environmental stress to increase probability of depression later in life.
Females have higher rates of depression than males (Piccinelli and Wilkinson 2000) and a number of findings suggest that sex differences in serotonergic function may contribute to the enhanced risk in women. Selective serotonin re-uptake inhibitors (SSRI) efficacy is higher in females than in males (Kornstein et al. 2000), and conversely, the emergence of depressive symptoms after tryptophan depletion is significantly greater in women (Booji et al. 2002; Moreno et al. 2006). One meta analysis has even suggested that tryptophan depletion causes depressive symptoms only in women (Jans et al. 2007). The specific sex differences in serotonergic function which contribute to vulnerability to depressive symptoms are not clear, although there are some plausible candidates. Rates of central nervous system (CNS) serotonin synthesis are approximately 50% higher in males than in females (Nishizawa et al. 1997). Our studies and others (Jonsson et al. 2000; Williams et al. 2003) have shown that CSF 5 HIAA is higher in women than in men, although this could reflect either enhanced serotonin neuron firing or enhanced metabolism. Both 5-HT1A and 5-HT2 receptor density is lower in the brains of women than men (Biver et al. 1996; Costes et al. 2005), although such a difference could reflect either lower receptor expression or lower serotonin release to compete with radioligand for binding. How these characteristics combine to produce serotonergic “vulnerability” that leads to depression is not clear, but the consistency of the sex differences is suggestive.
The initial findings of Lesch et al. (1996), that polymorphisms in the serotonin transporter may be associated with normal variation in personality traits, has prompted an exciting area of research. One ongoing controversy about the mechanism by which the 5-HTTLPR influences behavior is the relative importance of neurodevelopmental effects that influence the development of serotonergic circuitry for current transporter expression. Reports vary from the s/s genotype exhibiting more (van Dyck et al. 2004), less (Heinz et al. 2000) or no difference (Shioe et al. 2003; Willeit et al. 2001) from s/l or l/l genotypes in serotonin transporter binding. A pair of exciting recent studies from one research group have particular relevance for the present study. In the first study, they confirmed their previous finding that overall 5-HTTLPR genotype is not associated with serotonin transporter expression in human brain, even when considering the recent triallelic categorization of genotypes (Parsey et al. 2006). However, this same laboratory reported a strong association between the low-expressing 5-HTTLPR genotype and increased vulnerability to depression in persons experiencing high levels of stressful life events. (Zalsman et al. 2006a). Although they did not report a sex difference in this association, 71% of their depressed subjects were female and this association was detected with patients describing either early life and current stress, as in the present study. It is tempting to speculate the neurodevelopmental events as well as transporter expression may play a critical role in the association we report here among sex, stressful life events and depression vulnerability.
Studies of ovarian steroid modulation of serotonergic function in non-human primate brain further support the existence of functionally important sex differences in females that could influence the emergence of depressive symptoms. In female Macaques, estrogen administration has been shown to decrease mRNA levels of the 5-HT1A autoreceptor, monoamine oxidase A and the 5-HTT and increase mRNA of tryptophan hydroxylase in dorsal raphe and hypothalamic nuclei (Bethea et al. 2002; Gundlah et al. 2002; Pecins-Thompson and Bethea 1999) Using protein levels to index expression, estrogen treatment was found to increase tryptophan hydroxylase 2 and 5-HTT expression and decrease 5-HT1A and 5-HT2 receptor expression (Lu and Bethea 2002; Smith et al. 2004). While more research will be required to fully understand effects of ovarian steroids on serotonergic functions, there are clearly effects on serotonin synthesis, its removal from the synapse, its metabolism and also its stimulation of pre and postsynaptic receptor populations that strongly support a role for ovarian steroids in regulating almost every gene, its mRNA and protein that controls extracellular serotonin.
Research in rodent models also has documented sex differences in the effects of variation in serotonin transporter function on serotonergic function that could influence the emergence of depressive symptoms. In serotonin transporter knockout mice, the 5-HT1A autoreceptor is desensitized more extensively in females than males (Bouali et al. 2003; Li et al. 2000). Similarly, in 5-HT1B knockout mice, females exhibit greater disinhibition of serotonin release which manifests as less depressive behavior than 5-HT1B knockout males. These findings suggest that the presynaptic 5-HT1A and 5-HT1B autorecpetors contribute differentially more to regulation of extracellular serotonin in intact females than in intact males.
Finally it is important to consider the possibility that sex differences in effects of 5-HTTLPR interactions with stress on depression could reflect sex differences in stress reactivity that affect serotonergic function. In humans, an extensive literature supports the contribution of early life stressors to the development of depression, an association that is particularly strong in women who have been sexually or physically abused (Heim et al. 2004). Similarly, female macaques who carry the 5-HTTLPR s allele and who have been exposed to early adversity (peer rearing) exhibit lower cortisol responses to stress, a pattern that has been associated with certain stress-related neuropsychiatric disorders (Barr et al. 2004). More research will be required to determine how these mechanisms might account for the opposite effects of the 5-HTTLPR s allele on depressive symptoms that we observe in men vs. women exposed to stressful life circumstances.
Several factors should be noted with respect to the present findings. In addition to moderation by environmental stress, we hypothesized a main effect for 5-HTTLPR with respect to depressive symptoms that was not supported in either study. This highlights the importance of continued examination of potential moderators of genetic effects. Although a significant 3-way interaction was observed with respect to gender × genotype × stressor, this finding resulted under conditions of limited power. Importantly, our inability to detect a similar 3-way interaction with respect to race may have been due to the fact that the present samples were underpowered with regard to detection of potential race effects. Finally, it should be noted that the allele frequencies differed somewhat between the two present studies, a factor that was likely influenced by the differences in racial composition. Thus, these results are to be interpreted with caution prior to replication in additional samples.
Recent research (Hu et al. 2006) has revealed the existence of a common single base substitution (A → G) within the 5-HTTLPR L allele, with the rarer (10–15% in Caucasians, 24% in African Americans) LG allele showing reduced transcriptional efficiency, comparable to that of the S allele, while the LA allele is about twice as transcriptionally efficient as the S or LG alleles. The presence of the less functional LG allele within the LL or LS subjects of our study would dilute the effects of the more active LA allele, making it harder to find an association between 5-HTTLPR genotype and depressive symptoms. It is likely, therefore, that our findings represent a conservative estimate of the impact of 5-HTTLPR genotype on depressive symptoms in persons exposed to stressful life circumstances.
The present study contributes to an emerging literature showing that the 5-HTTLPR genotype and stress interact to affect depression vulnerability by demonstrating that this association is moderated by gender. Our findings, in two independent samples, combine with those of Sjoberg et al. (2006) to suggest that—whether the stressor is early in life or in adulthood—in women, the 5-HTTLPR s/s genotype confers increased risk of depression following exposure to stressful life circumstances, while in men the l/l genotype confers increased risk. It will be important to pursue this finding by exploring the relative importance of current versus developmental contributions of 5-HTTLPR genotype.
The present findings may inform efforts to develop more effective approaches to prevention and treatment. Kaufman et al. (2004) reported, for example, that the presence of positive social support reduces the risk of depression in maltreated children with the 5-HTTLPR s/s genotype. The current findings suggest that, to maximize the chances for success, a clinical trial to test the efficacy of an intervention that increases social support in preventing depression in persons exposed to stress should target women with the s/s genotype and men with the l/l genotype.
Acknowledgements
This research was financially supported by the National Institute on Aging, with co-funding by National Institute of Environmental Health Sciences and National Institute of Mental Health grant R01AG19605; by the Clinical Research Unit grant M01RR30; by the National Heart Lung and Blood Institute grant 5P01 HL036587; and by the Duke Behavioral Medicine Research Center.
Contributor Information
Beverly H. Brummett, Department of Psychiatry and Behavioral Medicine, Duke University Medical Center, Box 2969, Durham, NC 27710, USA, brummett@acpub.duke.edu
Stephen H. Boyle, Department of Psychiatry and Behavioral Medicine, Duke University Medical Center, Box 2969, Durham, NC 27710, USA
Ilene C. Siegler, Department of Psychiatry and Behavioral Medicine, Duke University Medical Center, Box 2969, Durham, NC 27710, USA
Cynthia M. Kuhn, Department of Pharmacology and Cancer Biology, Duke University Medical Center, Durham, NC, USA
Allison Ashley-Koch, Center for Human Genetics, Duke University Medical Center, Durham, NC, USA.
Charles R. Jonassaint, Department of Psychology, Duke University, Durham, USA
Stephan Züchner, Miami Institute of Human Genomics, University of Miami Miller School of Medicine, Miami, Fl, USA.
Ann Collins, Center for Human Genetics, Duke University Medical Center, Durham, NC, USA.
Redford B. Williams, Department of Psychiatry and Behavioral Medicine, Duke University Medical Center, Box 2969, Durham, NC 27710, USA
References
- Ball D, Hill L, Freeman B, et al. The serotonin transporter gene and peer-rated neuroticism. Neuroreport. 1997;8:1301–1304. doi: 10.1097/00001756-199703240-00048. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Schwandt M, Shannon C, Dvoskin RL, Lindell SG, Taubman J, Thompson B, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD. Sexual dichotomy of an interaction between early adversity and the serotonin transporter gene promoter variant in rhesus macaques. PANAS. 2004;101:12358–12363. doi: 10.1073/pnas.0403763101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgarten M, Battista RN, Infante-Rivard C, Hanley JA, Becker R, Gauthier S. The psychological and physical health of family members caring for an elderly person with dementia. J Clin Epidemiol. 1992;45:61–70. doi: 10.1016/0895-4356(92)90189-t. [DOI] [PubMed] [Google Scholar]
- Bergstrom E, Hernell O, Persson LA. Cardiovascular risk indicators cluster in girls from families of low socio-economic status. Acta Paediatr. 1996;85:1083–1090. doi: 10.1111/j.1651-2227.1996.tb14222.x. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Mirkes SJ, Su A, Michelson D. Effects of oral estrogen, raloxifene and arzoxifene on gene expression in serotonin neurons of macaques. Psychoneuroendocrinology. 2002;27:431–445. doi: 10.1016/s0306-4530(01)00054-3. [DOI] [PubMed] [Google Scholar]
- Biver F, Lotstra F, Monclus M, Wikler D, Damhaut P, Mendlewicz J, Goldman S. Sex difference in 5HT2 receptor in the living human brain. Neurosci Letts. 1996;204:25–28. doi: 10.1016/0304-3940(96)12307-7. [DOI] [PubMed] [Google Scholar]
- Booji L, Van der Does W, Benkelfat C, Bremner JD, Cowen PJ, Fava M, Gillin G, Leyton M, Moore PM, Smith KA, Van der Kloot WA. Predictors of mood response to acute tryptophan depletion: a renalaysis. Neuropsychopharmacology. 2002;27:852–861. doi: 10.1016/S0893-133X(02)00361-5. [DOI] [PubMed] [Google Scholar]
- Bouali S, Evrard A, Chastanet M, Lesch K-P, Hamon M, Adrien J. Sex hormone-dependent desensitization of 5-HT1a auto-receptors in knockout mice deficient inn the 5-HT transporter. Eur J Neurosci. 2003;18:2203–2212. doi: 10.1046/j.1460-9568.2003.02960.x. [DOI] [PubMed] [Google Scholar]
- Brummett BH, Babyak MA, Siegler IC, Vitaliano PP, Ballard EL, Gwyther LP, Williams RB. Associations among perceptions of social support, negative affect, and quality of sleep in caregivers and non-caregivers. Health Psychol. 2006;25:220–225. doi: 10.1037/0278-6133.25.2.220. [DOI] [PubMed] [Google Scholar]
- Brummett BH, Siegler IC, McQuoid DR, Svenson IK, Marchuk DA, Steffens DC. Associations among the NEO Personality Inventory, Revised and the serotonin transporter gene-linked polymorphic region in elders: effects of depression and gender. Psychiatr Genet. 2003;13:13–18. doi: 10.1097/00041444-200303000-00002. [DOI] [PubMed] [Google Scholar]
- Brummett BH, Siegler IC, Rohe WM, Barefoot JC, Vitaliano PP, Surwit RS, Feinglos MN, Williams RB. Neighborhood characteristics moderate effects of caregiving on glucose functioning. Psychosom Med. 2005;67:752–758. doi: 10.1097/01.psy.0000174171.24930.11. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analyses for the behavioral sciences. 2nd edn. Hillsdale: Lawerence Erlbaum Associates; 1988. [Google Scholar]
- Costes N, Merlet I, Ostrowsky K, Faillenot I, Lavenne F, Zimmer L, Ryvlin P, Le Bars D. An 18F-MPPF Pet normative database of 5-HT1a receptor binding in men and women over agin. J Nucl Med. 2005;46:1980–1989. [PubMed] [Google Scholar]
- Dahlstrom WG, Welsh GS, Dahlstrom LE. University of Minnesota Press; 1960. An MMPI Handbook Volume II Research Applications. [Google Scholar]
- Deary IJ, Battersby S, Whiteman MC, Connor JM, Fowkes FG, Harmar A. Neuroticism and polymorphisms in the serotonin transporter gene. Psychol Med. 1999;29:735–739. doi: 10.1017/s0033291798007557. [DOI] [PubMed] [Google Scholar]
- Du l, Bakish D, Hrdina PD. Gender differences in association between serotonin transporter gene polymorphism and personality traits. Psychiatr Genet. 2000;10(4):159–164. doi: 10.1097/00041444-200010040-00002. [DOI] [PubMed] [Google Scholar]
- Ebstein RP, Gritsenko I, Nemanov L, Frisch A, Osher Y, Belmaker RH. No association between the serotonin transporter gene regulatory region polymorphism and the Tridimensional Personality Questionnaire (TPQ) temperament of harm avoidance. Mol Psychiatry. 1997;2:224–226. doi: 10.1038/sj.mp.4000275. [DOI] [PubMed] [Google Scholar]
- Eley TC, Sugden K, Corsico A, Gregory AM, Sham P, McGuffin P, Plomin R, Craig IW. Gene-environment interaction analysis of serotonin system markers with adolescent depression. Mol Psychiatry. 2004;9:908–915. doi: 10.1038/sj.mp.4001546. [DOI] [PubMed] [Google Scholar]
- Flory JD, Manuck SB, Ferrell RE, Dent KM, Peters DG, Muldoon MF. Neuroticism is not associated with the serotonin transporter (5-HTTLPR) polymorphism. Mol Psychiatry. 1999;4:93–96. doi: 10.1038/sj.mp.4000466. [DOI] [PubMed] [Google Scholar]
- Garner DM. The body image survey results. Psychol Today. 1997;30:30–47. [Google Scholar]
- Gelernter J, Cubells JF, Kidd JR, Pakstis AJ, Kidd KK. Population studies of polymorphisms of the serotonin transporter protein gene. Am J Med Genet B Neuropsychiat Genet. 1999;88:61–66. [PubMed] [Google Scholar]
- Gelertner J, Kranzler H, Coccaro EF, Siever LJ, New AS. Serotonin transporter protein gene polymorphism and personality measures in African American and European American subjects. Am J Psychiatry. 1998;155:1332–1338. doi: 10.1176/ajp.155.10.1332. [DOI] [PubMed] [Google Scholar]
- Gonda X, Juhasz G, Laszik A, Rihmer Z, Bagdy G. Subthreshold depression is linked to the functional polymorphism of the 5HT transporter gene. J Affect Disord. 2005;87:291–297. doi: 10.1016/j.jad.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Grabe HJ, Lange M, Wolff B, Volzke H, Lucht M, Freyberger HJ, John U, Cascorbi I. Mental and physical distress is modulated by a polymorphism in the 5-HT transporter gene interacting with social stressors and chronic disease burden. Mol Psychiatry. 2005;10:220–224. doi: 10.1038/sj.mp.4001555. [DOI] [PubMed] [Google Scholar]
- Greenberg BD, Li Q, Lucas FR, Hu S, Sirota LA, Benjamin J, Lesch KP, Hamer D, Murphy DL. Association between the serotonin transporter promoter polymorphism and personality traits in a primarily female population sample. Am J Med Genet. 2000;96:202–216. doi: 10.1002/(sici)1096-8628(20000403)96:2<202::aid-ajmg16>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Gundlah C, Lu NZ, Betha CL. Ovarian steroid regulation of monoamine oxidase-A and B mRNAs in the macaque dorsal raphe and hypothalamic nuclei. Psychopharmacology. 2002;160:271–282. doi: 10.1007/s00213-001-0959-0. [DOI] [PubMed] [Google Scholar]
- Gustavsson JP, Nothen MM, Jonsson EG. No association between serotonin transporter gene polymorphisms and personality traits. Am J Hum Genet. 1999;88:430–436. [PubMed] [Google Scholar]
- Hamer DH, Greenberg BD, Sabol SZ, Murphy DL. Role of the serotonin transporter gene in temperament and character. J Person Disord. 1999;13:312–327. doi: 10.1521/pedi.1999.13.4.312. [DOI] [PubMed] [Google Scholar]
- Heim C, Plotsky PM, Nemeroff CB. Importance of studying the contributions of early adverse experience to neurobiological findings in depression. Neuropsychopharmacology. 2004;29:641–648. doi: 10.1038/sj.npp.1300397. [DOI] [PubMed] [Google Scholar]
- Heinz A, Jones DW, Mazzanti C, Goldman D, Ragan P, Hommer D, Linnoila M, Weinberger DR. A relationship between serotonin transporter genotype and in vivo protein expression and alcohol neurotoxicity. Bio Psychiatry. 2000;47:643–649. doi: 10.1016/s0006-3223(99)00171-7. [DOI] [PubMed] [Google Scholar]
- Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Traubman J, Greenberg BD, Xu K, Arnold PD, Richter MA, Kennedy JL, Murphy DL, Goldman D. Serotonin transporter promoter gain-of-function genotypes are linked to obessive-compulsive disorder. Am J Hum Genet. 2006;78:815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs N, GKenis G, Peeters F, Derom C, Vlietinck R, van Os J. Stress-related negative affectivity and genetically altered serotonin transporter function. Arch Gen Psychiatry. 2006;63:989–996. doi: 10.1001/archpsyc.63.9.989. [DOI] [PubMed] [Google Scholar]
- Jans LA, Riedel WJ, Markus CR, Blokland A. Serotonergic vulnerability and depression: assumptions, experimental evidence and implications. Mol Psychiatry. 2007;12:522–543. doi: 10.1038/sj.mp.4001920. [DOI] [PubMed] [Google Scholar]
- Jonsson EG, Norton N, Gustavsson P, Oreland L, Owen MJ, Sedvall GC. A promotoer polymorphism in the monoamine oxidase A gene and its relationships to monoamine metabolite concentrations in CSF of healthy volunteers. J Psychiatric Res. 2000;34:239–244. doi: 10.1016/s0022-3956(00)00013-3. [DOI] [PubMed] [Google Scholar]
- Jorm AF, Henderson AS, Jacomb PA, Christensen H, Korten AE, Rodgers B, Tan X, Easteal S. An association study of a functional polymorphism of the serotonin transporter gene with personality and psychiatric symptoms. Mol Psychiatry. 1998;3:449–451. doi: 10.1038/sj.mp.4000424. [DOI] [PubMed] [Google Scholar]
- Katsuragi S, Kunugi H, Sano A, Tsutsumi T, Isogawa K, Nanko S, Akiyoshi J. Association between serotonin transporter gene polymorphism and anxiety-related traits. Bio Psychiatry. 1999;45:368–370. doi: 10.1016/s0006-3223(98)00090-0. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Yang BZ, Douglas-Palumberi H, Houshyar S, Lipschitz D, Krystal JH, Gelernter J. Social supports and serotonin transporter gene moderate depression in maltreated children. PANAS. 2004;101:17316–17321. doi: 10.1073/pnas.0404376101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Dura JR, Speicher CE, Trask J, Glaser R. Spousal caregivers of dementia victims: Longitudinal changes in immunity and health. Psychosom Med. 1991;53:345–362. doi: 10.1097/00006842-199107000-00001. [DOI] [PubMed] [Google Scholar]
- Kornstein SG, Schatzberg AF, Thase ME, Yonkers KA, McCullough JP, Keitner GI, Gelenberg AJ, Davis SM, Harrison WM, Keller MB. Gender differences in treatment response to sertraline versus imipramine in chronic depression. Am J Psychiatry. 2000;157:1445–1452. doi: 10.1176/appi.ajp.157.9.1445. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg B, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Li Q, Wichems C, Heils A, Lesch K-P, Murphy DL. Reduction in the density and expression, but not G-Protein coupling, of serotonin receptors (5-HT1a) in 5-HT transporter knock-out mice: Gender and brain region differences. J Neurosci. 2000;20:7888–7895. doi: 10.1523/JNEUROSCI.20-21-07888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu NZ, Bethea CL. Ovarian steroid regulation of 5-HT1a receptor binding and G protein activation in female onkeys. Neuropsychopharmacol. 2002;27:21–24. doi: 10.1016/S0893-133X(01)00423-7. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Flory JD, Ferrell RE, Muldoon MF. Socio-economic status covaries with central nervous system serotonergic responsivity as a function of allelic variation in the serotonin transporter gene-linked polymorphic region. Psychoneuroendocrinology. 2004;29:651–668. doi: 10.1016/S0306-4530(03)00094-5. [DOI] [PubMed] [Google Scholar]
- Mazzanti CM, Lappalainen J, Long JC, Bengle D, Naukkarinen H, Eggert M, Virkkunen M, Linnoila M, Goldman D. Role of the serotonin transporter promoter polymorphism in anxiety-related traits. Arch Gen Psychiatry. 1998;55:936–940. doi: 10.1001/archpsyc.55.10.936. [DOI] [PubMed] [Google Scholar]
- Melke J, Landen M, Baghei F, Rosmond R, Holm G, Bjorntorp P, Westberg L, Hellstrand M, Eriksson E. Serotonin transporter gene polymorphisms are associated with anxiety-related personality traits in women. Am J Hum Genet. 2001;105:458–463. doi: 10.1002/ajmg.1434. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Rutter M. Strategy for investigating interactions between measured genes and measured environments. Arch Gen Psychiatry. 2005;62:473–481. doi: 10.1001/archpsyc.62.5.473. [DOI] [PubMed] [Google Scholar]
- Moreno FA, McGahuey CA, Freeman MP, Delgado PL. Sex difference in depressive response during monoamine depletions in remitted depressive subjects. J Clin Psychiatry. 2006;67:1618–1623. doi: 10.4088/jcp.v67n1019. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Muramatsu T, Y O. Serotonin transporter gene regulatory region polymorphism and anxiety-related traits in the Japanese. Am J Med Genet. 1997;74:544–545. doi: 10.1002/(sici)1096-8628(19970919)74:5<544::aid-ajmg18>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Nishizawa S, Benkelfat C, Young SN, Leyton M, Mzengeza S, de Montigny C, Blier P, Diksic M. Differences between males and females in rates of serotonin synthesis in human brain. PANAS. 1997;94:4823–4824. doi: 10.1073/pnas.94.10.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsey RV, Hastings RS, Oquendo MA, Hu XZ, Goldman D, Huang YY, Simpson N, Arcement J, Huang Y, Ogden RT, Van Heertum RL, Arango V, Mann JJ. Effect of triallelic functional polymorphism of the serotonin transporter linked promoter region on expression of serotonin transporter in the human brain. Am J Psychiatry. 2006;163:48–51. doi: 10.1176/appi.ajp.163.1.48. [DOI] [PubMed] [Google Scholar]
- Pecins-Thompson M, Bethea CL. Ovarian steroid regulation of serotonin-1A autoreceptor messenger rna expression in the dorsal raphe of rhesus macaques. Neuroscience. 1999;89:267–277. doi: 10.1016/s0306-4522(98)00326-1. [DOI] [PubMed] [Google Scholar]
- Piccinelli M, Wilkinson G. Gender differences in depression. Critical Review. Br J Psychiatry. 2000;177:486–492. doi: 10.1192/bjp.177.6.486. [DOI] [PubMed] [Google Scholar]
- Power C, Manor O, Matthews S. The duration and timing of exposure: effects of socioeconomic environment on adult health. Am J Public Health. 1999;89:1059–1065. doi: 10.2105/ajph.89.7.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: a self report depression scale for research in the general population. Appl Psych Meas. 1977;1(3):385–401. [Google Scholar]
- Samochowiec J, Rybakowsk iF, Czerski P, Zakrzewska M, Stepien G, Pelka-Wysiecka J, Horodnicki J, Rybakowski JK, Hauser J. Polymorphisms in the dopamine, serotonin, and norepinephrine transporter genes and their relationship to temperamental dimensions measured by the Temperament and Character Inventory in healthy volunteers. Neuropsychobiology. 2001;43:248–253. doi: 10.1159/000054898. [DOI] [PubMed] [Google Scholar]
- Schulz R, Beach SR. Caregiving as a risk factor for mortality: The Caregiver Health Effects Study. JAMA. 1999;282:2215–2219. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- Schulz R, O’Brien AT, Bookwala J, Fleissner K. Psychiatric and physical morbidity effects of dementia caregiving: prevalence, correlates, and causes. Gerontologist. 1995;35:771–791. doi: 10.1093/geront/35.6.771. [DOI] [PubMed] [Google Scholar]
- Shilling AE, Aseltine RJ, Gore S. Adverse childhood experiences and mental health in young adults: a longitudinal survey. BMC Public Health. 2007;7:30. doi: 10.1186/1471-2458-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioe K, Ichimiya T, Suhara T, Takano A, Sudo Y, Yasuno F, Hirano M, Shinohara M, Kagami M, Okubo Y, Nankai M, Kanba S. No association between genotype of the promoter region of serotonin transporter gene and serotonin transporter binding in human brain measured by PET. Synapse. 2003;48:184–188. doi: 10.1002/syn.10204. [DOI] [PubMed] [Google Scholar]
- Sjoberg RL, Nilsson KW, Nordquist N, Ohrvik J, Leppert J, Lindstrom L, Oreland L. Development of depression: sex and the interaction between environment and a promoter polymorphism of the serotonin transporter gene. Int J Neuropsychopharmacol. 2006;9:443–449. doi: 10.1017/S1461145705005936. [DOI] [PubMed] [Google Scholar]
- Smith LJ, Henderson JA, Abell CW, Bethea CL. Effects of ovarian steroids and raloxifene on proteins that synthesize, transport and degrade serotonin in the raphe region of macaques. Neuropsychopharmacol. 2004;29:2035–2045. doi: 10.1038/sj.npp.1300510. [DOI] [PubMed] [Google Scholar]
- Strand BH, Kunst H. Childhood socioeconomic position and cause-specific mortality in early adulthood. Am J Epidemiol. 2006;165:85–93. doi: 10.1093/aje/kwj352. [DOI] [PubMed] [Google Scholar]
- van Dyck CH, Malison RT, Staley JK, Jacobsen LK, Seibyl JP, Laruelle M, Baldwin RM, Innis RB, Gelernter J. Central serotonin transporter availability measured with [123I ]beta-CIT SPECT in relation to serotonin transporter genotype. Am J Psychiatry. 2004;161:525–531. doi: 10.1176/appi.ajp.161.3.525. [DOI] [PubMed] [Google Scholar]
- Vance JM. The collection of biological samples for DNA analysis. In: Haines JL, Pericak-Vance MA, editors. Approaches to gene mapping in complex human diseases. New York: Wiley-Liss; 1998. pp. 201–211. [Google Scholar]
- Vitaliano PP, Russo J, Niaura R. Plasma lipids and their relationships with psychosocial factors in older adults. J Gerontol: Psycholog Sci. 1995;50B:18–24. doi: 10.1093/geronb/50b.1.p18. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM, Peroutka SJ. The neuropharmacology of serotonin. Ann N Y Acad Sci. 1990;600:1–718. [Google Scholar]
- Wiener DN. Subtle and obvious keys for the MMP. J Consult Clin Psychol. 1948;12:164–170. doi: 10.1037/h0055594. [DOI] [PubMed] [Google Scholar]
- Willeit M, Stastny J, Pirker W, Praschak-Rieder N, Neumeister A, Asenbaum S, Tauschera J, Fuchsc K, Sieghartc W, Hornike K, Aschauera HN, Brücked T, Kaspera S. No evidence for in vivo regulation of midbrain serotonin transporter availability by serotonin transporter promoter gene polymorphism. Bio Psychiatry. 2001;50:8–12. doi: 10.1016/s0006-3223(00)01123-9. [DOI] [PubMed] [Google Scholar]
- Williams RB, Marchuk DA, Gadde KM, Barefoot JC, Grichnik K, Helms MJ, Kuhn CM, Lewis JG, Schanberg SM, Stafford-Smith M, Suarez EC, Clary GL, Svenson IK, Siegler IC. Central nervous system serotonin function and cardiovascular responses to stress. Psychosom Med. 2001;63:300–305. doi: 10.1097/00006842-200103000-00016. [DOI] [PubMed] [Google Scholar]
- Williams RB, Marchuk DA, Gadde KM, Barefoot JC, Grichnik K, Helms MJ, Kuhn CM, Lewis JG, Schanberg SM, Stafford-Smith M, Suarez EC, Clary GL, Svenson IK, Siegler IC. Serotonin-related gene polymorphisms and central nervous system serotonin function. Neuropsychopharmacology. 2003;28:533–541. doi: 10.1038/sj.npp.1300054. [DOI] [PubMed] [Google Scholar]
- Zalsman G, Huang Y, Oquendo MA, Burke AK, Hu XZ, Brent DA, Ellis SP, Goldman D, Mann JJ. Association of the triallelic serotonin transporter gene promoter region (5-HTTLPR) polymorphism with stressful life events and severity of depression. Am J Psychiatry. 2006a;163:1588–1593. doi: 10.1176/ajp.2006.163.9.1588. [DOI] [PubMed] [Google Scholar]
- Zalsman G, Huang Y, Oquendo MA, Burke AK, Hu Z, Brent DA, Ellis SP, Goldman D, Mann JJ. Association of a triallelic serotonin transporter gene promoter region (5-HTTLPR) polymorphism with stressful life events and severity of depression. Am J Psychiatry. 2006b;163:1558–1593. doi: 10.1176/ajp.2006.163.9.1588. [DOI] [PubMed] [Google Scholar]