Abstract
Objective
To examine the relationships among the variable number of tandem repeats in the monoamine oxidase-A linked polymorphic region allelic variation (MAOA-uVNTR) and the symptoms of depression and sleep quality. The monoamine oxidase-A (MAOA) gene, which plays a vital role in degradation of neurotransmitters such as serotonin, norepinephrine, and dopamine, contains a polymorphism in its promoter region (MAOA-uVNTR) that affects transcriptional efficiency. MAOA-uVNTR genotype has been associated with both psychological and physical measures.
Methods
The sample consisted of 74 males enrolled in a case/control study of caregivers for relatives with dementia. Age- and race-adjusted linear regression models were used to examine the association between low versus high MAOA-uVNTR activity alleles, symptoms of depression (Center for Epidemiological Studies of Depression), and sleep quality ratings (Pittsburgh Sleep Quality Index).
Results
MAOA-uVNTR alleles associated with less transcriptional activity were related to increased symptoms of depression (p < .04; Cohen’s d = 0.52) and poorer sleep quality (p < .04; Cohen’s d = 0.31).
Conclusions
Individuals with less active MAOA-uVNTR alleles may be at increased risk for depressive symptoms and poor sleep.
Keywords: monoamine oxidase-A, MAOA-uVNTR, depression, sleep
INTRODUCTION
Monoamine oxidase-A (MAOA) is a mitochondrial enzyme that degrades the neurotransmitters serotonin, norepinephrine, and dopamine—systems involved with both psychological and physical functioning. The gene that encodes MAOA is found on the X chromosome and contains a polymorphism (MAOA-uVNTR) located 1.2 kb upstream of the MAOA coding sequences (1). In this polymorphism, consisting of a 30-base pair repeated sequence, six allele variants containing either 2-, 3-, 3.5-, 4-, 5-, or 6-repeat copies have been identified (2). Functional studies indicate that certain alleles may confer lower transcriptional efficiency than others. The 3-repeat variant conveys lower efficiency, whereas 3.5- and 4-repeat alleles result in higher efficiency (1,3,4). The 3- and 4-repeat alleles are the most common (2,5), and to date there is less consensus regarding the transcriptional efficiency of the other less commonly occurring alleles (e.g., 2-, 5-, and 6-repeat).
The primary role of MAOA in regulating monoamine turn-over, and hence ultimately influencing levels of norepinephrine, dopamine, and serotonin, indicates that its gene is a highly plausible candidate for affecting individual differences in the manifestation of psychological traits and psychiatric disorders (6). For example, recent evidence indicates that the MAOA gene may be associated with depression (7). However, evidence regarding whether higher or lower MAOA gene transcriptional efficiency is positively associated with psychological pathology has been mixed. The low-activity 3-repeat allele of the MAOA-uVNTR polymorphism has been positively related to symptoms of antisocial personality (8) and cluster B personality disorders (2). Other studies, however, suggest that alleles associated with higher transcriptional efficiency are related to unhealthy psychological characteristics such as trait aggressiveness and impulsivity (9,10). Finally, others have reported finding no significant association between the MAOA-uVNTR polymorphism and negative characteristics such as recurrent major depression and bipolar disorder (11).
Sleep disturbances are also linked to the MAOA gene. Specifically, sleep problems are found in those with Norrie disease, which is caused by X-chromosomal microdeletions that include the MAOA gene. Such sleep disturbances may be partly attributed to deficient MAO activity (12,13). Furthermore, male mice lacking MAOA show, among other behavioral problems, violent motions during sleep (14). Finally, MAOA inhibitors used in the treatment of depression are associated with both sleep disruption and daytime sedation (15,16).
In terms of the pharmacology of these effects, inhibition of the degradation of each of the monoamines, 5-hydroxytryptamine (5HT), dopamine, and norepinephrine, has the potential to contribute to the observed sleep disturbance. Impaired degradation of 5HT would be expected to have effects similar to 5HT reuptake inhibitors, which are associated with sleep disturbance and suppression of rapid eye movement (REM) (14,17). However, adult knockout mice lacking the serotonin transporter have more REM sleep than wild type animals (18). Whether there is a corresponding difference in acute versus lifelong impairment of MAOA function is unclear. If so, it would speak against the importance of decreased 5HT degradation as a mechanism for sleep disturbance in low MAOA activity individuals. The suppression of REM sleep that accompanies medication-related increases in 5HT is mediated by binding to 5HT1A receptors; 5HT2 receptor activation diminishes non-REM sleep (19 –21).
There are several lines of evidence suggesting that MAO-A inhibition may disturb sleep via increasing noradrenergic activity. Chronic inhibition of MAO-A with clorgyline has been demonstrated to significantly enhance noradrenaline release in the central nervous system (22). Implicating this mechanism in sleep disruption is that specific norepinephrine reuptake inhibitors have sleep disturbance as a common side-effect (23,24). An increase in synaptic norepinephrine concentration also plays a role in the wake-promoting effects of amphetamines (25,26). A factor promoting increased extracellular norepinephrine with low MAOA activity is that there is a buildup of normetanephrine (normally metabolized to vannillylmandelic acid by MAOA), which seems to inhibit norepinephrine reuptake (27,28). In addition, mice with mutations of the gene for tyrosine hydroxylase do not synthesize epinephrine and norepinephrine and sleep an average of 2 hours longer than normals (29). The sleep-disturbing effects of increased synaptic levels of norepinephrine are mediated by agonist effects at α1-adrenergic receptors as well as by a down-regulation of presynaptic α2-inhibitory autoreceptors, which occurs with chronic increases in synaptic norepinephrine (30 –32).
Decreased metabolism of dopamine and sleep disturbance are linked by evidence that increasing extracellular dopamine is one of the key wake-promoting mechanisms of the most potent stimulants including methamphetamine, d-amphetamine, cocaine, and methylphenidate (26,33). Further, there is evidence that dopamine transporter knockout mice have reduced non-REM sleep and increased waking (34). The receptors which mediate these dopaminergic effects are unknown.
Recent research (35) indicates that, in studies examining genetic effects, it is important to consider gene by environmental interactions when analyzing the relationship between genetic markers and health. Caspi et al. (36) reported that children with a history of maltreatment, in conjunction with the presence of MAOA-uVNTR alleles that confer low activity, were more likely to develop antisocial problems. Similarly, Foley et al. (37) found that an adverse childhood environment was a significant moderator of MAOA activity such that low activity was associated with higher risk for conduct disorder only in children with a background of maltreatment. Kim-Cohen et al. (38) demonstrated that MAOA-uVNTR polymorphism interacts with early exposure to physical abuse, resulting in poor scores on a composite measure of mental health problems in individuals with the genotype conferring low versus high activity. Such findings suggest that the MAOA gene influences vulnerability to environmental stressors.
Consistent findings demonstrate that the burden associated with providing care for a loved one suffering from dementia is considerable (39). Caregiving has been associated with a number of negative health outcomes (40), including worse ratings of physical health, significant decreases in cellular immunity (41), and higher levels of triglycerides (42). Our own research in caregivers has also shown that they suffer from both increased symptoms of depression and poorer sleep quality (43). Caregiving has also been related to increased rates of early mortality in older caregivers who experience mental and emotional caregiving strain (44), and conclusions from a meta-analysis that combined the results of 23 studies indicate that caregivers are at significantly higher risk for health problems than noncaregivers (45). Thus, in light of the evidence suggesting gene by environment interactions, we hypothesized that the role of caregiving for a loved one with dementia would moderate the effect of MOA-uVNTR allele variation on symptoms of depression and sleep quality.
In sum, MAOA activity, as measured directly or inferred from the MAOA-uVNTR genotype, has been associated with a number of psychological (e.g., cluster B personality disorders, depression) and physical (e.g., disturbed sleep) characteristics. Based on these findings, we examined the relationship between allelic variation (low- versus high-activity alleles) in the MAOA-uVNTR polymorphism and symptoms of depression and sleep quality in a group of 74 males, who were enrolled in a case/control study of caregiving for a relative with dementia. Initially, we examined the main effect of MAOA-vUNTR on each outcome. In secondary analyses, we examined the MAOA-uVNTR gene as a moderator of caregiving stress with respect to each outcome.
METHODS
Patient Population
Participants were recruited to be part of a study designed to examine the underlying biological and behavioral mechanisms whereby stressful social and physical environments might lead to health disparities. Caregivers were recruited using flyers, ads in the local media, and community outreach efforts. Noncaregiver controls were recruited by asking caregivers to nominate two to five friends who live in their neighborhood and are similar with respect to demographic factors (i.e., gender, age, and race). Data collection began in May 2001 and concluded in June 2004. The study was approved by the Duke University Medical Center Institutional Review Board and all subjects gave informed consent before their participation in the study. Individuals who were experiencing an acute major medical or psychiatric disorder that would render them unable to fully participant in the study, or to assume the role of primary caregiver, were excluded from the study. These exclusion criteria resulted in the loss of one individual, who was actively psychotic during the intake interview.
The full study sample consisted of 85 males and 259 females. Because the MAOA gene is located on the X chromosome (Xp11.23), it is difficult to assess high versus low transcription in females. That is, current evidence suggests that it is not clear whether levels of MAOA transcription in females is the product of one or both copies of the gene (46,47). Therefore, as with many studies in this area, only the 85 males were included in the present study. Eleven individuals had incomplete genetic data, leaving the present sample with 74 males (42 caregivers and 32 controls). Individuals with missing data for MAOA did not differ from those with data on any independent or dependent variables.
Measures
Genotyping
Fresh blood samples were obtained and signed into the Center for Human Genetics DNA Bank. Deoxyribonucleic acid (DNA) was extracted and stored according to methods and quality checks as previously reported (48). An aliquot of DNA was used for MAOA genotyping. The MAOA-u VNTR region was amplified with primers: Forward-FAM-CAGCCTGACCGTGGAGAAG and Reverse-GAACGGACGCTCCATTCGGA (1). Polymerase chain reaction products were separated by polyacrylamide gel electrophoresis and visualized using a multi-view scanner (FMBIO IIT, Hitachi, San Francisco, CA). We required that each assay achieve 95% efficiency (i.e., the genotypes of at least 95% of the samples could be called with certainty) to be considered for statistical analysis.
Symptoms of Depression
The Center for Epidemiologic Studies Depression Scale (CES-D) (49) is a widely used 20-item self-report scale designed to measure depressive symptomatology in a general population. Items are scored on a 4-point scale, with the total CES-D score ranging between 0 and 60. Higher scores represent depressive responses, and a score of ≥16 is generally considered suggestive of a depressive disorder. Within the CES-D, there are the following four subscales: depressive affect, well-being, interpersonal, and somatic.
Sleep Quality
The Pittsburgh Sleep Quality Index (PSQI) (50) was used to measure sleep quality. The scale consists of 19 items that assess the following subcomponents of sleep during the past month: overall sleep quality, sleep latency (time to fall asleep on going to bed), sleep duration, sleep efficiency (time asleep/time in bed × 100), sleep disturbances, problems with daytime functioning, and medications taken for sleep. Items are combined to provide a global score (PSQI), with higher scores reflecting poorer sleep quality. A score of ≥5 on the global rating suggests moderate sleep problems in three or more areas, or more severe problems in at least two areas.
Statistical Analysis
General Linear Models were used to assess the relationship among MAOA-uVNTR transcription, symptoms of depression (CES-D), and sleep quality (PSQI). MAOA-uVNTR alleles were categorized according to transcriptional functionality, with the 3-repeat variant defined as low activity (coded 0) and longer alleles (3.5- and 4-repeat) defined as high activity (coded 1). In the present sample, there were no individuals with other MAOAuVNTR alleles, i.e., 2-, 5-, and 6-repeat variants.
The main effect of allelic variation in MAOA-uVNTR was examined as a predictor of CES-D and PSQI scores. These models included MAOA activity (0/1), caregiver status (0/1), age in years, and race (0/1). In secondary analyses, caregiver status was examined as a moderator of the potential association between MAOA-uVNTR and each study outcome. Interaction models included MAOA activity, caregiver status, age in years, race, and MAOA activity × caregiver status. SAS 9.1 (Cary, NC) was used to conduct all analyses.
Along with the potential to influence ratings of depression, antidepressants are known to influence assessments of sleep. Of the 74 participants in the study, nine (12.2%) reported that they were currently taking antidepressant medication (none of which were MAOIs). Analyses were conducted both with and without individuals taking an antidepressant.
RESULTS
Table 1 provides the characteristics of the present sample. On average, the participants were 68 years old, white, and had minor sleep difficulties. Regarding MAOA-uVNTR frequencies, 67.6% of the sample had alleles that indicate higher transcription of MAOA; this distribution is similar to those seen in participants without existing psychiatric disorders from two other US samples (2,51) i.e., approximately 60% and 65%, respectively.
TABLE 1.
Sample Characteristics (n = 74)
| Characteristic | Total n = 74 |
Caregivers n = 42 |
Noncaregivers n = 32 |
|---|---|---|---|
| Age (years), mean ± SD | 68.4 ± 13.9 | 70.1 ± 13.6 | 66.1 ± 14.1 |
| White/African American | 59 (79.7%)/15 (20.3%) | 34 (80.9%)/8 (19.1%) | 25 (78.1%)/7 (21.9%) |
| Symptoms of depression, mean ± SD* | 9.5 ± 8.3 | 11.7 ± 8.8 | 6.5 ± 6.5 |
| Sleep quality, mean ± SD** | 5.8 ± 3.7 | 6.6 ± 3.8 | 5.1 ± 3.6 |
| MAOA-uVNTR frequencies | |||
| 3 Repeat | 24 (32.4%) | 16 (38.1%) | 8 (25.0%) |
| 3.5 Repeat | 2 (2.7%) | 1 (2.4%) | 1 (3.1%) |
| 4 Repeat | 48 (64.9%) | 25 (59.5%) | 23 (71.9%) |
Mean and SD values are unadjusted for age and race.
SD = standard deviation; MAOA-uVNTR = variable number of tandem repeats in the monoamine oxidase-A linked polymorphic region.
p < .05
p < .10 in analyses comparing caregivers versus controls.
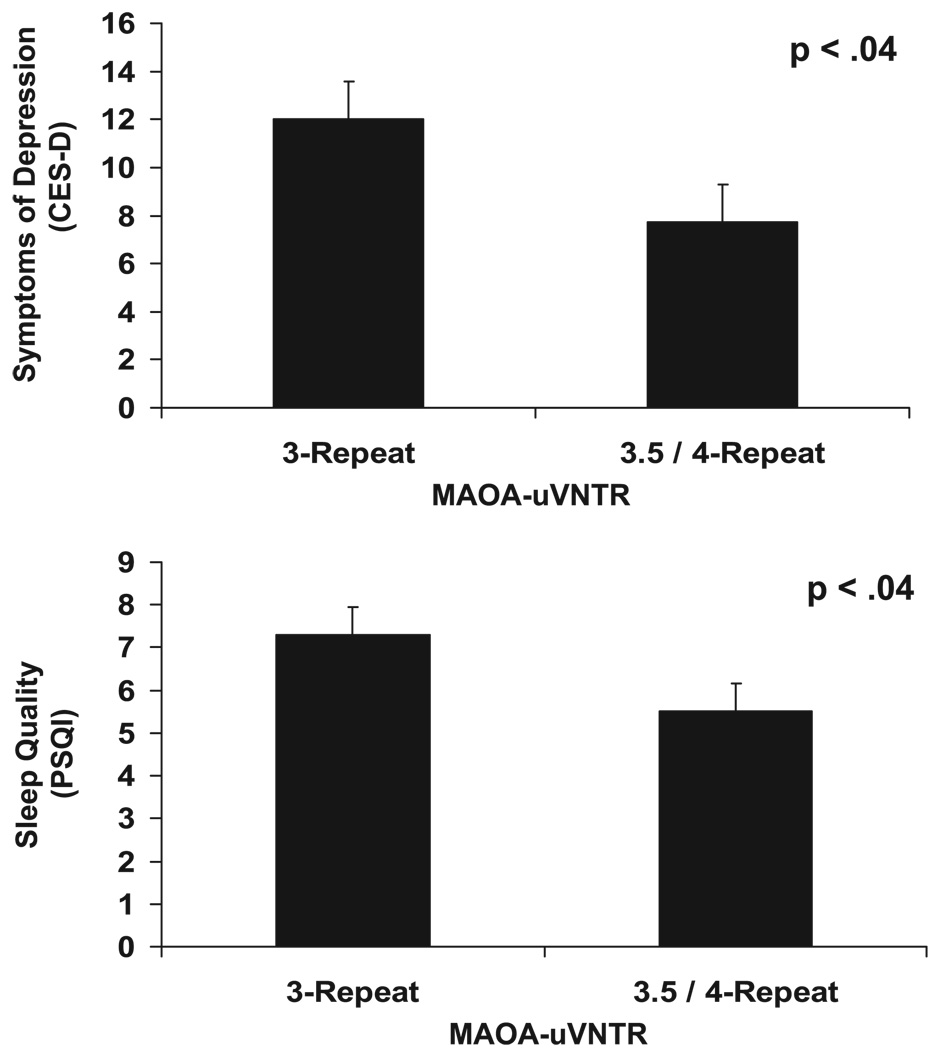
The presence of low-activity alleles (3-repeat), as compared with the presence of higher-activity alleles (3.5- and 4-repeat), was associated with higher ratings on the CES-D (p < .04; Cohen’s d = 0.52) (52). The main effect analysis for MAOA-uVNTR was also significant for PSQI ratings (p < .04; Cohen’s d = 0.31), with MAOA-uVNTR activity accounting for 6% of the model variance. The form of the effect was such that the presence of low-activity alleles was associated with a global rating of poorer sleep quality, as compared with higher-activity alleles. Results are shown in Figure 1. Analyses of both the CES-D and the PSQI remained significant when the nine individuals on antidepressant medication were removed (p < .05 and p < .01, respectively). All product terms modeling the interaction between group (caregiver versus control) and MAOA-uVNTR genotype as a predictor of depressive symptoms and sleep quality were nonsignificant.
Figure 1.
Association of variable number of tandem repeats in the MAOA linked polymorphic region (MAOA-uVNTR) genotypes with symptoms of depression and sleep quality (adjusted means and standard errors). CES-D = Center for Epidemiological Studies of Depression; PSQI = Pittsburgh Sleep Quality Index.
Post Hoc Follow-Up Analyses
To determine which symptoms of depression and aspects of sleep quality were most closely related to MAOA-uVNTR activity, in post hoc analyses, we examined each of the subscales for these measures. For the CES-D, the somatic symptoms subscale was strongly related to MAOA-uVNTR activity (p < .01; Cohen’s d = 0.71), with smaller effects for the Depressive affect (p < .12), Well-Being (p < .62), and Interpersonal (p < .55) subscales. MAOA-vNTR activity explained 16% of the model variance when predicting the CES-D somatic subscale. Regarding the subcomponents of the PSQI, overall sleep quality, sleep latency, and sleep efficiency were worse in the presence of low-activity alleles as compared with higher-activity alleles (p < .10); and the other four subcomponents were less strongly related (p values between .16 and .95).
DISCUSSION
The present results suggest that MAOA-uVNTR genotypes conferring lower rates of transcription exert an influence on symptoms of depression and sleep quality. The form of this association was consistent across both measures. Given the role of the monoamines, 5HT, dopamine, and norepinephrine, in regulating multiple behavioral, and physiological functions, such findings are not surprising.
Follow-up analyses demonstrated that allelic variation in MAOA-uVNTR was not significantly associated with measures of depression reflecting disturbances in affect, well being, or interpersonal associations. This lack of association is consistent with prior findings in this area that have failed to demonstrate an association of MAOA-uVNTR with depression (11) and bipolar disorder (53). In the present data, MAOA alleles conferring lower activity were, however, related to higher levels of somatic symptoms that primarily reflect lethargy (e.g., poor concentration, motivation, sleep, and lack of energy). It is possible that these fatigue-related symptoms are a product of the poorer sleep quality that was demonstrated among participants with lower rates of MAOA transcription in the present study. In addition, one of the items comprising the somatic subscale of the CES-D asks about “restless sleep.” We recalculated the measure of somatic symptoms omitting the sleep item and the association between MAOA-uVNTR remained significant for this abbreviated somatic components subscale. We also tested whether or not the effect of the MAOA polymorphism on somatic symptoms was mediated by the effects on sleep quality by including the PSQI score as an additional covariate to the model containing MAOA-uVNTR as a predictor of somatic symptoms. Results of this analysis provided evidence for partial mediation (Sobel test t = 1.8; p < .07).
The association of low MAOA activity and diminished sleep quality is in agreement with the observed effects of medications, which inhibit MAOA function. These agents, most frequently administered for the treatment of major depression or anxiety disorders, very frequently lead to sleep disturbance (16,54). Although they are also potent suppressors of REM sleep, the clinical significance of this is unknown (15). Disturbed sleep has been the most frequent side-effect of the treatment of patients with major depression, panic disorder, and social phobia with the reversible MAOA inhibitor brofaromine and the irreversible inhibitor tranylcypromine, reported by as many as 53% (16,55,56). In addition, normal controls treated with brofaromine reported shorter sleep duration and lower sleep quality (57). In a polysomnographic study in depressed patients, the irreversible selective MAO-A inhibitor clorgyline nearly totally suppressed REM, significantly decreased total sleep time, and increased wake time during the night (58).
Given findings demonstrating the potential for environmental stressors to moderate the relationship between MAOA-uVNTR and certain outcomes (36 –38), a significant stress (i.e., caregiving) by genotype interaction might have been expected. However, detecting interaction terms in observational studies often requires larger samples (59); it is important to note that this study was underpowered to fully examine the proposed interaction. For 80% power to detect an effect of 0.66 standard deviation would require a sample of three to four times that of the current sample. Thus, the study did not have the ability to provide an adequate test of the gene by environment hypothesis and the null finding should be interpreted in this light.
Certain other limitations should be noted with respect to the present findings. Because the sample consisted of only 74 participants, all results should be interpreted with proper caution and replication in additional samples will be required to determine their validity. We were unable to examine the ethnicity by gene interaction due to insufficient numbers of participants in certain cells (e.g., n = 2 for African Americans classified in the low MAOA activity group). It should also be noted that the present study only involved males and it is possible that gender differences may exist with respect to coping with the stress of caregiving, as well as with differences in depression rates or severity. Thus, the current findings may not generalize to females. There seems to be a consensus regarding which of the more commonly occurring MAOA-uVNTR alleles confer higher or lower transcription, but there remains dispute about the transcriptional activity of the less commonly found alleles (i.e., five copies of VNTR). Fortunately, none of the individuals within the present sample were found to have the less frequently occurring alleles. In the present sample of male caregivers, allelic variation in MAOA-uVNTR is also related to lipid levels and anger expression, but not body mass index (60). Similar to the current results, MAOA-uVNTR alleles associated with decreased activity were positively related to anger expression and lipid levels, suggesting the potential pleiotropic effect of this gene.
In sum, the present findings suggest that decreased transcriptional activity of the MAOA-uVNTR genotype is associated with adverse health outcomes. Specifically, individuals with less active MAOA-uVNTR alleles may be at increased risk for symptoms of depression, in particular, somatic symptoms, and poor sleep quality.
Acknowledgments
This research was supported by Grant R01AG19605 (R.B.W.) from the National Institute on Aging, with co-funding by National Institute of Environmental Health Sciences; Grant M01RR30 from the Clinical Research Unit, Duke University Medical Center; Grant R01MH57663 (R.B.W.) from the National Institutes of Mental Health; and Grant 3P01 HL036587 (R.B.W.) from the National Heart, Lung, and Blood Institutes.
Abbreviations
- CAD
coronary artery disease
- CES-D
Center for Epidemiological Studies of Depression
- MAOA
monoamine oxidase-A
- MAOA-uVNR
variable number of tandem repeats in the MAOA linked polymorphic region
- PSQI
Pittsburgh Sleep Quality Index
REFERENCES
- 1.Sabol SZ, Hu S, Hamer D. A functional polymorphism in the monoamine oxidase A gene promoter. Hum Genet. 1998;103:273–279. doi: 10.1007/s004390050816. [DOI] [PubMed] [Google Scholar]
- 2.Jacob CP, Muller J, Schmidt M, Hohenberger K, Gutknecht L, Reif A, Schmidtke A, Mossner R, Lesch KP. Cluster B personality disorders are associated with allelic variation of monoamine oxidase A activity. Neuropsychopharmacology. 2005;4:1–8. doi: 10.1038/sj.npp.1300737. [DOI] [PubMed] [Google Scholar]
- 3.Deckert J, Catalano M, Syagailo YV, Bosi M, Okladnova O, Di Bella D, Nothen MM, Maffei P, Franke P, Fritze J, Maier W, Propping P, Beckmann H, Bellodi L, Lesch KP. Excess of high activity monoamine oxidase A gene promoter alleles in female patients with panic disorder. Hum Mol Genet. 1999;8:621–624. doi: 10.1093/hmg/8.4.621. [DOI] [PubMed] [Google Scholar]
- 4.Denney RM, Koch H, Craig IW. Association between monoamine oxidase A activity in human male skin fibroblasts and genotype of the MAOA promoter-associated variable number tandem repeat. Hum Genet. 1999;105:542–551. doi: 10.1007/s004399900183. [DOI] [PubMed] [Google Scholar]
- 5.Huang Y, Cate SP, Battistuzzi C, Oquendo MA, Brent D, Mann JJ. An association between a functional polymorphism in the monoamine oxidase A gene promoter, impulsive traits and early abuse experiences. Neuropsychopharmacology. 2004;29:1498–1505. doi: 10.1038/sj.npp.1300455. [DOI] [PubMed] [Google Scholar]
- 6.Shih JC, Chen K, Ridd MJ. Monoamine oxidase: from genes to behavior. Annu Rev Neurosci. 1999;22:197–217. doi: 10.1146/annurev.neuro.22.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du L, Faludi G, Palkovits M, Sotonyi P, Bakish D, Hrdina PD. High activity-related allele of MAO-A gene associated with depressed suicide in males. Neuroreport. 2002;13:1195–1198. doi: 10.1097/00001756-200207020-00025. [DOI] [PubMed] [Google Scholar]
- 8.Contini V, Marques FZC, Garcia CED, Hutz MH, Bau CHD. MAOA-uVNTR polymorphism in a Brazilian sample: further support for the association with impulsive behaviors and alcohol dependence. Am J Med Genet Part B (Neuropsychiatr Genet) 2006;141B:305–308. doi: 10.1002/ajmg.b.30290. [DOI] [PubMed] [Google Scholar]
- 9.Manuck SB, Flory JD, Ferrell RE, Mann JJ, Muldoon MF. A regulatory polymorphism of the monoamine oxidase-A gene may be associated with variability in aggression, impulsivity, and central nervous system serotonergic responsivity. Psychiatry Res. 2000;95:9–23. doi: 10.1016/s0165-1781(00)00162-1. [DOI] [PubMed] [Google Scholar]
- 10.Manor I, Tyano S, Mel E, Eisenberg J, Bachner-Melman R, Kotler M, Ebstein RP. Family-based and association studies of monoamine oxidase A and attention deficity hyperactivity disorder (ADHD): preferential transmission of the long promoter-region repeat and its association with impaired performance on a continuous performance test (TOVA) Mol Psychiatry. 2002;7:626–632. doi: 10.1038/sj.mp.4001037. [DOI] [PubMed] [Google Scholar]
- 11.Syagailo YV, Stober G, Grassle M, Reimer E, Knapp M, Jungkuz G, Okladnova O, Meyer J, Lesch KP. Association analysis of the functional monoamine oxidase A gene promoter polymorphism in psychiatric disorders. Am J Med Genet. 2001;105:168–171. doi: 10.1002/ajmg.1193. [DOI] [PubMed] [Google Scholar]
- 12.Sims KB, de la Chapelle A, Norio R, Sankila EM, Hus YP, Reinehart WB, Corey TJ, Ozelius L, Powell JF, Bruns G, Gusella JF, Murphy DL, Breakefield XO. Monoamine oxidase deficiency in males with an X chromosome deletion. Neuron. 1989;2:1069–1076. doi: 10.1016/0896-6273(89)90231-6. [DOI] [PubMed] [Google Scholar]
- 13.Murphy DL, Sims KB, Karoum F, de la Chapelle A, Norio R, Sankila EM, Breakefield XO. Marked amine and amine metabolite changes in Norrie disease patients with an X-chromosomal deletion affecting monoamine oxidase. J Neurochem. 1990;54:242–247. doi: 10.1111/j.1471-4159.1990.tb13307.x. [DOI] [PubMed] [Google Scholar]
- 14.Cases O, Seif I, Grimsby J, Gaspar P, Chen K, Pournin S, Muller U, Aquet M, Babinet C, Shih JC, de Maeyer E. Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science. 1995;268:1763–1766. doi: 10.1126/science.7792602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Landolt HP, Raimo EB, Schnierow BJ, Kelsoe JR, Rapaport MH, Gillian JC. Sleep and sleep electroencephalogram in depressed patients treated with phenelzine. Arch Gen Psychiatry. 2001;58:268–276. doi: 10.1001/archpsyc.58.3.268. [DOI] [PubMed] [Google Scholar]
- 16.Remick RA, Froese C, Keller FD. Common side effects associated with monoamine oxidase inhibitors. Prog Neuropsychopharmacol Biol Psychiatry. 1989;13:497–504. doi: 10.1016/0278-5846(89)90137-1. [DOI] [PubMed] [Google Scholar]
- 17.Gottesmann C. Brain inhibitory mechanisms involved in basic and higher integrated sleep processes. Brain Res Brain Res Rev. 2004;45:230–249. doi: 10.1016/j.brainresrev.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Alexandre C, Popa D, Fabre V, Bouali S, Venault P, Lesch KP, Hamon M, Adrien J. Early life blockade of 5-hydroxytryptamine 1A receptors normalizes sleep and depression-like behavior in adult knock-out mice lacking the serotonin transporter. J Neurosci. 2006;26:5554–5564. doi: 10.1523/JNEUROSCI.5156-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landolt H, Meier V, Burgess HJ, Finelli LA, Cattelin F, Achermann P, Borbely AA. Serotonin-2 receptors and human sleep: effect of a selective antagonist on EEG power spectra. Neuropsychopharmacology. 1999;21:455–466. doi: 10.1016/S0893-133X(99)00052-4. [DOI] [PubMed] [Google Scholar]
- 20.Adrien JJ. Implication of serotonin in the control of vigilance states as revealed by knockout-mouse studies. Soc Biol. 2004;198:30–36. [PubMed] [Google Scholar]
- 21.Monaca C, Boutrel B, Hen R, Hamon M, Adrien J, Monaca C, Boutrel B, Hen R, Hamon M, Adrien J. 5-HT 1A/1B receptor-mediated effects of the selective serotonin reuptake inhibitor, citalopram, on sleep: studies in 5-HT 1A and 5-HT 1B knockout mice. Neuropsychopharmacology. 2003;28:850–856. doi: 10.1038/sj.npp.1300109. [DOI] [PubMed] [Google Scholar]
- 22.Finberg JP. Pharmacology of reversible and selective inhibitors of monoamine oxidase type A. Acta Psychiatr Scand Suppl. 1995;386:8–13. doi: 10.1111/j.1600-0447.1995.tb05918.x. [DOI] [PubMed] [Google Scholar]
- 23.Hilakivi I, Kovala T, Leppavuori A, Shvaloff A. Effects of serotonin and noradrenaline uptake blockers on wakefulness and sleep in cats. Pharmacol Toxicol. 1987;60:161–166. doi: 10.1111/j.1600-0773.1987.tb01725.x. [DOI] [PubMed] [Google Scholar]
- 24.Simpson D, Plosker GL. Atomoxetine: a review of its use in adults with attention deficit hyperactivity disorder. Drugs. 2004;64:205–222. doi: 10.2165/00003495-200464020-00005. [DOI] [PubMed] [Google Scholar]
- 25.Vanderschuren LJ, Beemster P, Schoffelmeer AN. On the role of noradrenaline in psychostimulant-induced psychomotor activity and sensitization. Psychopharmacology (Berl) 2003;169:176–185. doi: 10.1007/s00213-003-1509-8. [DOI] [PubMed] [Google Scholar]
- 26.Berridge CW, Stalnaker TA. Relationship between low-dose amphet-amine-induced arousal and extracellular norepinephrine and dopamine levels within prefrontal cortex. Synapse. 2002;46:140–149. doi: 10.1002/syn.10131. [DOI] [PubMed] [Google Scholar]
- 27.Schildkraut JJ, Mooney JJ. Toward a rapidly acting antidepressant: the normetanephrine and extraneuronal monoamine transporter (uptake 2) hypothesis. Am J Psychiatry. 2004;161:909–911. doi: 10.1176/appi.ajp.161.5.909. [DOI] [PubMed] [Google Scholar]
- 28.Burgen ASV, Iversen LL. The inhibition of noradrenaline uptake by sympathomimetic amines in the rat isolated heart. Br J Pharmacol. 1965;25:34–49. doi: 10.1111/j.1476-5381.1965.tb01754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ouyang M, Hellman K, Abel T, Thomas SA. Adrenergic signaling plays a critical role in the maintenance of waking and in the regulation of REM sleep. J Neurophysiol. 2004;92:2071–2082. doi: 10.1152/jn.00226.2004. [DOI] [PubMed] [Google Scholar]
- 30.De Sarro GB, Ascioti C, Froio F, Libri V, Nistico G. Evidence that locus coeruleus is the site where clonidine and drugs acting at alpha 1- and alpha 2-adrenoceptors affect sleep and arousal mechanisms. Br J Pharmacol. 1987;90:675–685. doi: 10.1111/j.1476-5381.1987.tb11220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mateo Y, Pineda J, Meana JJ. Somatodendritic 2 adrenoceptors in the locus coeruleus are involved in the in vivo modulation of cortical noradrenaline release by the antidepressant desipramine. J Neurochem. 1998;71:790–798. doi: 10.1046/j.1471-4159.1998.71020790.x. [DOI] [PubMed] [Google Scholar]
- 32.Linnér L, Arborelius L, Nomikos GG, Bertilsson L, Svensson TH. Locus coeruleus neuronal activities and noradrenaline availability in the frontal cortex of rats chronically treated with imipramine: effect of 2-adrenoceptor blockade. Biol Psychiatry. 1999;46:766–774. doi: 10.1016/s0006-3223(99)00126-2. [DOI] [PubMed] [Google Scholar]
- 33.Sandoval V, Riddle EL, Ugarte YV, Hanson GR, Fleckenstein AE. Methamphetamine-induced rapid and reversible changes in dopamine transporter function: an in vitro model. J Neurosci. 2001;21:1413–1419. doi: 10.1523/JNEUROSCI.21-04-01413.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wisor JP, Nishino S, Sora I, Uhl GH, Mignot E, Edgar DM. Dopaminergic role in stimulant-induced wakefulness. J Neurosci. 2001;21:1787–1794. doi: 10.1523/JNEUROSCI.21-05-01787.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moffitt TE, Caspi A, Rutter M. Strategy for investigating interactions between measured genes and measured environments. Arch Gen Psychiatry. 2005;62:473–481. doi: 10.1001/archpsyc.62.5.473. [DOI] [PubMed] [Google Scholar]
- 36.Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- 37.Foley DL, Eaves LJ, Wormley B, Silberg JI, Maes HH, Kuhn J, Riley B. Childhood adversity, monoamine oxidase A genotype, and risk for conduct disorder. Arch Gen Psychiatry. 2004;61:738–744. doi: 10.1001/archpsyc.61.7.738. [DOI] [PubMed] [Google Scholar]
- 38.Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, Moffitt TE. MAOA, maltreatment, and gene-environment interaction predicting children’s mental health: new evidence and a meta-analysis. Mol Psychiatry. 2006;11:1–11. doi: 10.1038/sj.mp.4001851. [DOI] [PubMed] [Google Scholar]
- 39.Schulz R, O’Brien AT, Bookwala J, Fleissner K. Psychiatric and physical morbidity effects of dementia caregiving: prevalence, correlates, and causes. Gerontologist. 1995;35:771–791. doi: 10.1093/geront/35.6.771. [DOI] [PubMed] [Google Scholar]
- 40.Baumgarten M, Battista RN, Infante-Rivard C, Hanley JA, Becker R, Gauthier S. The psychological and physical health of family members caring for an elderly person with dementia. J Clin Epidemiol. 1992;45:61–70. doi: 10.1016/0895-4356(92)90189-t. [DOI] [PubMed] [Google Scholar]
- 41.Kiecolt-Glaser JK, Dura JR, Speicher CE, Trask J, Glaser R. Spousal caregivers of dementia victims: longitudinal changes in immunity and health. Psychosom Med. 1991;53:345–362. doi: 10.1097/00006842-199107000-00001. [DOI] [PubMed] [Google Scholar]
- 42.Vitaliano PP, Russo J, Niaura R. Plasma lipids and their relationships with psychosocial factors in older adults. J Gerontol B Psychol Sci Soc Sci. 1995;50:P18–P24. doi: 10.1093/geronb/50b.1.p18. [DOI] [PubMed] [Google Scholar]
- 43.Brummett BH, Siegler IC, Rohe WM, Barefoot JC, Vitaliano PP, Surwit RS, Feinglos MN, Williams RB. Neighborhood characteristics moderate effects of caregiving on glucose functioning. Psychocom Med. 2005;67:752–758. doi: 10.1097/01.psy.0000174171.24930.11. [DOI] [PubMed] [Google Scholar]
- 44.Schulz R, Beach SR. Caregiving as a risk factor for mortality: the caregiver health effects study. JAMA. 1999;282:2215–2219. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- 45.Vitaliano PP, Jianping Z, Scanlan JM. Is caregiving hazardous to one’s physical health? A meta-analysis. Psychol Bull. 2003;129:946–972. doi: 10.1037/0033-2909.129.6.946. [DOI] [PubMed] [Google Scholar]
- 46.Benjamin D, Van Bakel I, Craig IW. A novel expression based approach for assessing the inactivation status of human X-linked genes. Eur J Hum Genet. 2000;8:103–108. doi: 10.1038/sj.ejhg.5200427. [DOI] [PubMed] [Google Scholar]
- 47.Carrel L, Willard H. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 2005;434:400–404. doi: 10.1038/nature03479. [DOI] [PubMed] [Google Scholar]
- 48.Rimmler J, McDowell JG, Slotterback BD, Haynes CS, Menold MM, Rogala A, Speer MC, Gilbert JR, Hauser ER, Vance JM, Pericak-Vance MA. Development of a data coordinating center (DCC): data quality control for complex disease studies. Am J Hum Genet. 1998;63:240. [Google Scholar]
- 49.Radloff LS. The CES-D scale: A self report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 50.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 51.Williams RB, Marchuk DA, Gadde KM, Barefoot JC, Grichnik K, Helms MJ, Kuhn CM, Lewis JG, Schanberg SM, Stafford-Smith M, Suarez EC, Clary GL, Svenson IK, Siegler IC. Serotonin-related gene polymorphisms and central nervous system serotonin function. Neuropsychopharmacology. 2003;28:533–541. doi: 10.1038/sj.npp.1300054. [DOI] [PubMed] [Google Scholar]
- 52.Cohen J. Statistical power analyses for the behavioral sciences. Hillsdale, NJ: Lawerence Erlbaum Associated; 1988. [Google Scholar]
- 53.Kunugi H, Ishida S, Kato T, Tatsumi M, Saki T, Hattori M, Hirose T, Nanko S. A functional polymorphism in the promoter region of monoamine oxidase-A gene and mood disorders. Mol Psychiatry. 1999;4:393–395. doi: 10.1038/sj.mp.4000558. [DOI] [PubMed] [Google Scholar]
- 54.Schiwy W, Heath WR, Delini-Stula A. Therapeutic and side-effect profile of a selective and reversible MAO-A inhibitor, brofaromine. Results of dose-finding trials in depressed patients. J Neural Transm Suppl. 1989;28:33–44. [PubMed] [Google Scholar]
- 55.Chouinard G, Saxena BM, Nair NP, Kutcher SP, Bakish D, Bradwejn J, Kennedy SH, Sharma V, Remick RA, Kukha-Mohamad SA. A Canadian multicentre placebo-controlled study of a fixed dose of brofaromine, a reversible selective MAO-A inhibitor, in the treatment of major depression. J Affect Disord. 1994;32:105–114. doi: 10.1016/0165-0327(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 56.Fahlen T, Nilsson HL, Borg K, Humble M, Pauli U A double-blind placebo-controlled study. Social phobia: the clinical efficacy and tolerability of the monoamine oxidase-A and serotonin uptake inhibitor brofaromine. Acta Psychiatr Scand. 1995;92:351–358. doi: 10.1111/j.1600-0447.1995.tb09596.x. [DOI] [PubMed] [Google Scholar]
- 57.Ramaekers JG, van Veggel LM, O’Hanlon JF. A cross-study comparison of the effects of moclobemide and brofaromine on actual driving performance and estimated sleep. Clin Neuropharmacol. 1994;1 Suppl 17:S9–S18. doi: 10.1097/00002826-199417001-00003. [DOI] [PubMed] [Google Scholar]
- 58.Cohen RM, Pickar D, Garnett D, Lipper S, Gillin JC, Murphy DL. REM sleep suppression induced by selective monoamine oxidase inhibitors. Psychopharmacology (Berl) 1982;78:137–140. doi: 10.1007/BF00432251. [DOI] [PubMed] [Google Scholar]
- 59.McClelland GH, Judd CM. Statistical difficulties of detecting interactions and moderator effects. Psychol Bull. 1993;114:376–389. doi: 10.1037/0033-2909.114.2.376. [DOI] [PubMed] [Google Scholar]
- 60.Brummett BH, Krystal AD, Siegler IC, Kuhn C, Surwit RS, Zuchner S, Ashley-Koch A, Barefoot JC, Williams RB. Associations of MAOA-uVNTR with symptoms of depression, anger expression, sleep quality, body mass index, and lipids. program and abstracts of the American Psychosomatic Society annual meeting; Budapest, Hungary. 2007. Mar, Abstract. [Google Scholar]