Abstract
The oncogene EVI1 has been implicated in the etiology of AML and MDS. Although AML cells are characterized by accelerated proliferation and differentiation arrest, MDS cells hyperproliferate when immature but fail to differentiate later and die instead. In agreement with its roles in AML and in immature MDS cells, EVI1 was found to stimulate cell proliferation and inhibit differentiation in several experimental systems. In contrast, the variant protein MDS1/EVI1 caused the opposite effect in some of these assays. In the present study, we expressed EVI1 and MDS1/EVI1 in a tetracycline-regulable manner in the human myeloid cell line U937. Induction of either of these proteins caused cells to accumulate in the G0/G1-phase of the cell cycle and moderately increased the rate of spontaneous apoptosis. However, when EVI1- or MDS1/EVI1-expressing cells were induced to differentiate, they massively succumbed to apoptosis, as reflected by the accumulation of phosphatidylserine in the outer leaflet of the plasma membrane and increased rates of DNA fragmentation. In summary, these data show that inducible expression of EVI1 in U937 cells causes phenotypes that may be relevant for its role in MDS and provides a basis for further investigation of its contribution to this fatal disease.
Keywords: ecotropic viral integration site 1, apoptosis, cell proliferation
Introduction
MDS are a group of clonal malignant hematopoietic diseases with an annual incidence of two to 12 per 100,000 and an almost invariably fatal outcome [1]. Approximately one-third of the patients die, as the disease progresses into therapy-resistant sAML, and the remaining two-thirds succumb to the complications of peripheral blood pancytopenia. Reduced blood cell counts with concomitant hyper- or normoplasia and dysplasia of bone marrow are the principal biological characteristics of MDS [1, 2]. These apparently contradictory features are attributed to the increased proliferation of immature cells, accompanied by high rates of apoptosis in maturing cells. The latter phenomenon has been termed differentiation failure and contributes markedly to, but probably does not fully account for, the peripheral blood hypocellularity in MDS [1]. The molecular and genetic basis of MDS is poorly understood, and no MDS-specific genetic alterations have been identified thus far [2, 3]. Point mutations in the gene for the hematopoietic transcription factor AML1, which are rare but recurrent in AML, have been found in >20% of patients with advanced MDS and constitute the most frequent, currently known molecular alteration in this disease entity [4]. Mutations in the N-RAS, FLT3, and MLL genes have also been observed in MDS but significantly less often than in AML [5]. Approximately one-half of patients with MDS bears cytogenetic abnormalities such as deletions in or losses of chromosomes 5, 7, 17, or 20, trisomy 8, or structural aberrations affecting the MLL and EVI1 genes in chromosome bands 11q23 and 3q26, respectively [1, 2].
Rearrangements of chromosome band 3q26, the majority of which leads to overexpression of the EVI1 gene, are found in AML, chronic myeloid leukemia, and MDS [1, 2, 6, 7]. In addition, EVI1 is expressed aberrantly in some cases of these disease entities as a result of yet-unknown reasons [7,8,9,10,11]. It codes for a zinc finger transcription factor [12, 13] and through alternative promoter use and alternative splicing, gives rise to several mRNA variants [7, 14,15,16,17]. These are translated into two major protein forms: the 145-kD EVI1 protein and the 170-kD MDS1/EVI1 protein [12, 15, 18]. MDS1/EVI1, which differs from EVI1 by the presence of an N-terminal PR domain, exhibited biological activity contrary to or different from that of EVI1 in some experimental systems [18,19,20,21].
Research about EVI1 has focused largely on its role in AML, as its overexpression in this disease is associated with a poor prognosis [10, 11, 22]. In agreement with a contribution to the pathogenesis of AML, the overexpression of EVI1 promoted cellular proliferation and inhibited differentiation and apoptosis in certain experimental systems [13, 19, 23,24,25]. However, EVI1 levels appear to be increased even more frequently in MDS and sAML than in de novo AML [8,9,10,11]. Furthermore, mice transplanted with bone marrow cells ectopically expressing EVI1 developed a fatal disorder resembling human MDS but without progression to AML [23, 25]. The latter disease emerged only when mice were transplanted with bone marrow cells coexpressing EVI1 with the leukemogenic oncogenes HOXA9 and MEIS1 [25] or with a mutant AML1 gene [26]. These observations suggest that in vivo, EVI1 promoted the proliferation of immature cells but exerted antiproliferative and/or proapoptotic effects that counteracted further cellular expansion in more differentiated cells. Full-blown leukemia developed only when this property of EVI1 was overcome by additional genetic events.
In the present study, we found that inducible expression of EVI1 or MDS1/EVI1 in the human myelomonocytic cell line U937T led to cell-cycle arrest and massive apoptosis upon exposure to differentiation stimuli, thereby recapitulating salient aspects of the biology of MDS in vitro.
MATERIALS AND METHODS
Plasmids, cell culture, and transfections
The plasmids EVI1-HA/pUHD10S and MDS1/EVI1-HA/pUHD10S harbor the human EVI1 and MDS1/EVI1 cDNAs, respectively, both with HA epitope tags at their 3′-ends in the pUHD10S vector backbone. The epitope tags did not affect the activities of EVI1 and MDS1/EVI1 in reporter gene assays. In addition, preliminary data suggested that transient transfection of a plasmid harboring an untagged EVI1 cDNA into U937 cells led to a growth phenotype similar to that observed upon induction of EVI-HA in stably transfected U937 cells (T. A. Konrad, unpublished results). pUHD10S contains seven copies of the tetracycline operator, facilitating tetracycline-regulable expression of its cDNA inserts [27].
U937T cells had been derived from U937 human histiocytic lymphoma cells by stable transfection with a construct driving tetracycline-regulable expression of the tetVP16 fusion protein [28]. They were cultured in a humidified incubator at 37°C and 5% CO2 in RPMI-1640 medium (Invitrogen, Carlsbad, CA, USA) containing 10% FBS (Invitrogen), 0.5 μg/ml puromycin (Sigma Chemical Co., St. Louis, MO, USA), and 1 μg/ml tetracycline (Serva Electrophoresis, Germany). To obtain U937T_EVI1-HA and U937T_MDS1/EVI1-HA cells, the plasmids EVI1-HA/pUHD10S and MDS1/EVI1-HA/pUHD10S were electroporated (0.17 kV, 950 μF) into U937T cells. Transfectants were selected, cloned, and propagated in RPMI-1640 medium supplemented with 10% FBS, 0.5 μg/ml puromycin, 1 μg/ml tetracycline, and 500 μg/ml hygromycin (PAA, Austria). To induce the expression of EVI1-HA or MDS1/EVI1-HA, exponentially growing cells were washed three times with PBS and resuspended in growth media without tetracycline. Control cultures were washed in the same manner but resuspended in media with tetracycline. To induce differentiation, TPA, at a final concentration of 50 ng/ml, was added to cells that had been transferred to media with or without tetracycline and adjusted to a density of 60 cells/μl on the previous day (Day 0 of the experiment). Control cultures were treated with an equivalent amount of solvent (ethanol).
HNT-34 [29] and MPD cells [30] were maintained at 37°C and 5% CO2 in RPMI-1640 medium supplemented with 10% FBS.
Immunoblot and immunofluorescence analyses
U937T cells and their derivatives were transferred to media containing the specified concentrations of tetracycline. After the indicated periods of time, protein extracts were prepared by subjecting cells to repeated freeze-thawing in a buffer consisting of 20 mM Tris, pH 8.0, 100 mM NaCl, 1 mM EDTA, 0.5% Nonidet P-40, 0.5 mM PMSF, and protease inhibitor cocktail (Roche, Nutley, NJ, USA). Polyacrylamide gel electrophoresis, tank-blotting onto Bio Trace NT nitrocellulose membranes (Pall Corp., East Hills, NY, USA), and antibody hybridizations were performed using standard procedures. Primary antibodies directed against the HA-tag (mouse anti-HA.11 clone 16B12, Covance, Princeton, NJ, USA), EVI1 (rabbit anti-EVI1 C50E12, Cell Signaling Technology, Beverly, MA, USA), or β-tubulin (mouse anti-β-tubulin clone TUB 2.1, Sigma Chemical Co.) were used at dilutions of 1:10,000, 1:1000, and 1:2500, respectively. HRP-conjugated goat anti-mouse and goat anti-rabbit secondary antibodies were used at dilutions of 1:50,000–1:100,000 and detected by the Super Signal West Femto kit (Pierce, Rockford, IL, USA).
For immunofluorescence analysis, U937T cells and their derivatives were transferred to media with or without tetracycline and spun onto coverslips 48 h later. Cells were fixed with 100% methanol, permeabilized with 1% Triton X-100, and blocked with 5% goat serum (PAA). Cells were incubated with the HA antibody at a dilution of 1:200, followed by a fluorescein (FITC)-conjugated goat anti-mouse secondary antibody [FITC-conjugated AffiniPure goat anti-mouse IgG (H+L), Jackson ImmunoResearch, West Grove, PA, USA; 1:200]. Counterstaining of DNA with DAPI and mounting of coverslips were performed in one step using Vectashield Mounting Medium with DAPI (Vector Laboratories, Burlingame, CA, USA). Images were obtained with an Axiovert microscope (Zeiss, Thornwood, NY, USA).
Analyses of cellular growth and cell-cycle distribution
To monitor cellular proliferation, exponentially growing U937T cells and their derivatives were transferred to media containing the indicated concentrations of tetracycline. During this process or 2 days later, all cultures were adjusted to the same cell density. On the indicated days after density adjustment, Trypan blue-excluding viable cells were counted in a hematocytometer.
For cell-cycle analyses, cells were transferred to media with or without tetracycline and adjusted to the same density. After 4 days, they were washed with PBS and incubated for 5 min in ice-cold 0.5 M citrate/0.5% Tween-20. Cell membranes were disrupted mechanically before the nuclei were pelleted and resuspended in PBS containing 100 μg/ml RNase A and 50 μg/ml propidium iodide. The nuclear DNA content was analyzed on a FACSCalibur (Becton Dickinson, San Diego, CA, USA) using CellQuest Pro and ModFit software for data acquisition and analysis, respectively.
Differentiation and apoptosis assays
Spontaneous apoptosis was determined 5 days after exponentially growing cells had been transferred to media with or without tetracycline and adjusted to a density of 50 cells/μl. Accumulation of outer membrane leaflet phosphatidylserine and the loss of membrane integrity were assessed using the Annexin V-FITC/7-AAD kit from Beckman Coulter (Fullerton, CA, USA), according to the manufacturer’s instructions. Flow cytometric analyses were performed on a FACSCalibur using CellQuest Pro software. Annexin V-negative 7-AAD-negative cells were considered viable; Annexin V-positive 7-AAD-negative cells were deemed early-apoptotic; and double-positive cells were considered late-apoptotic/necrotic.
To investigate the effects of EVI1 and MDS1/EVI1 on cellular differentiation, cells were transferred to media with or without tetracycline and adjusted to a density of 60 cells/μl. On the following day (Day 1 of the experiment), TPA at a final concentration of 50 ng/ml was added to one-half of each culture and an equivalent amount of solvent (ethanol) to the other half. Cellular differentiation was monitored on Days 4 and 6 of the experiment by staining for the monocyte/macrophage-specific cell-surface marker CD11b. Cells were incubated in a 1:48 dilution of a PE-conjugated CD11b antibody (Becton Dickinson) in PBS for 15–30 min at room temperature in the dark. Flow cytometric analyses were performed on a FACSCalibur. The percentage of CD11b-positive cells was used as a measure of differentiation. A second aliquot of each culture was subjected to Annexin V-FITC/7-AAD staining as described above.
For DNA fragmentation assays, cells were cultured in the presence or absence of tetracycline and TPA as described above. On Day 5 of the experiment, cells were subjected to TUNEL assays using the APO-DIRECT™ kit (Becton Dickinson) according to the manufacturer’s instructions. Flow cytometric analyses were performed on a FACSAria (Becton Dickinson) using FACSDiva™ software.
Statistical analyses
Statistical analyses were performed using the two-tailed t-test and the software package R 2.6.0. (http://www.r-project.org/). Data derived from several independent experiments are presented as mean ± standard error of the mean. P values <0.05 were considered significant.
RESULTS
Establishment of human myeloid cell lines expressing EVI1 or MDS1/EVI1 in an inducible manner
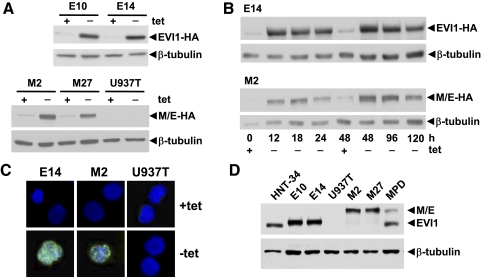
U937 histiocytic lymphoma cells were chosen as a model to study the functions of EVI1 and MDS1/EVI1 in myeloid malignancies, as they are of human origin, can be stimulated to differentiate along the myelomonocytic lineage, do not express endogenous EVI1 (T. A. Konrad, unpublished observations), and have been reported to be responsive to exogenously expressed EVI1 [31]. The U937-derivative cell line U937T expresses the tetVP16 fusion protein in a tetracycline-regulated manner [28]. It was transfected with constructs containing the human EVI1 and MDS1/EVI1 cDNAs with HA epitope tags at their 3′-ends under the control of a tetVP16-responsive promoter. After hygromycin selection, U937T_EVI1-HA clones E10 and E14 and U937T_MDS1/EVI1-HA clones M2 and M27, which upon removal of tetracycline strongly induced EVI1-HA or MDS1/EVI1-HA, respectively (Fig. 1A), were selected for further investigation. Time-course experiments showed that the exogenously expressed proteins were highly abundant as early as 12 h after tetracycline withdrawal and continued to be elevated for at least 120 h (Fig. 1B). Immunofluorescence analysis confirmed their location in the nucleus (Fig. 1C). During these investigations, a purchasable antibody capable of detecting endogenous EVI1 became available and was used to show that exogenous protein levels in U937T_EVI1-HA and U937T_MDS1/EVI1-HA cells 48 h after removal of tetracycline were comparable with endogenous EVI1 and MDS1/EVI1 protein levels in the human myeloid cell lines HNT-34 and MPD [29, 30] (Fig. 1D).
Figure 1.
Expression of EVI1 and MDS1/EVI1 in U937T transfectants. (A) Immunoblot analysis of U937T_EVI1-HA E10 and E14, U937T_MDS1/EVI1-HA M2 and M27, and U937T cells grown in media with or without tetracycline (tet) for 48 h. Experimentally expressed proteins were detected using an antibody recognizing the HA epitope tag (upper panels); hybridization with an antibody against β-tubulin was used as loading control (lower panels). M/E, MDS1/EVI1. (B) Time-course analysis. Proteins were extracted from exponentially growing E14 or M2 cells maintained in media with or without tetracycline for the indicated times and subjected to immunoblot analysis as described above. (C) Immunofluorescence analysis demonstrating nuclear location of experimentally expressed EVI1 and MDS1/EVI1 proteins. E14, M2, and U937T cells were maintained in the presence or absence of tetracycline for 48 h, fixed, permeabilized, and probed with the HA antibody and a FITC-conjugated secondary antibody. DNA was counterstained with DAPI. Similar results were obtained for clones E10 and M27. (D) Protein extracts prepared from E10, E14, M2, M27, and U937T cells maintained in the absence of tetracycline for 48 h and from the human myeloid leukemia cell lines HNT-34 and MPD were subjected to immunoblot analysis with an antibody recognizing human EVI1 or with a β-tubulin antibody as a loading control. Small size differences between endogenously and exogenously expressed proteins are a result of the presence of the HA epitope tag in the latter. All experiments were repeated several times and yielded comparable results.
Induction of EVI1 or MDS1/EVI1 reduces the proliferation of U937T cells
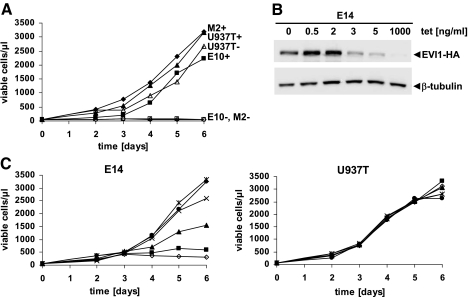
To investigate the effects of experimental EVI1 and MDS1/EVI1 expression on cell proliferation, U937T_EVI1-HA, U937T_MDS1/EVI1-HA, and U937T cells were transferred to media with or without tetracycline. Two days later, they were adjusted to the same starting cell density. Viable cells were then counted daily over a period of 6 days. Unexpectedly, induction of EVI1 or MDS1/EVI1 strongly inhibited cell proliferation, while control U937T cells grew at comparable rates in the presence or absence of tetracycline (Fig. 2A). Similar results were obtained when cells were cultured in media containing 1% rather than 10% FBS (data not shown).
Figure 2.
EVI1 and MDS1/EVI1 inhibit proliferation of U937T cells. (A) U937T_EVI1-HA E10 (squares), U937T_MDS1/EVI1-HA M2 (diamonds), and U937T cells (triangles) were transferred to media containing 1000 (closed symbols) or 0 (open symbols) ng/ml tetracycline. Two days later, their density was adjusted to 50 cells/μl (Day 0). On the indicated days, viable cells were identified by Trypan blue exclusion and counted in a hematocytometer. Comparable results were obtained for E14 and M27 cells. (B) Immunoblot analysis of E14 cells maintained in media containing the indicated concentrations of tetracycline for 3 days. (Upper panel) Staining with an HA antibody; (lower panel) staining with a β-tubulin antibody as a loading control. (C) E14 and U937T cells were transferred to media containing 1000 (circles), 5 (asterisks), 3 (crosses), 2 (triangles), 0.5 (squares), or 0 (diamonds) ng/ml tetracycline and adjusted to the same starting density. On the indicated days thereafter, viable cells were identified by Trypan blue exclusion and counted in a hematocytometer. All experiments were repeated several times and yielded comparable results.
To determine whether EVI1 inhibited cellular growth in a dose-dependent manner, E14 cells were transferred to media containing 1000, 5, 3, 2, 0.5, or 0 ng/ml tetracycline. Immunoblot analysis was performed on protein extracts derived 3 days after transfer to these media and showed that EVI1 protein levels increased with decreasing tetracycline concentrations (Fig. 2B). Accordingly, cell growth was maximal or near-maximal in the presence of 1000, 5, and 3 ng/ml tetracycline, intermediate at 2, and strongly reduced at 0.5 or 0 ng/ml tetracycline (Fig. 2C).
The reduced proliferation after exogenous expression of EVI1 or MDS1/EVI1 could be a result of slowed cell-cycle progression, increased apoptosis, or a combination of both. To address the first possibility, the nuclei of cells that had been maintained in the presence or absence of tetracycline for 4 days were stained with propidium iodide and analyzed by flow cytometry. In the presence of tetracycline, ∼40% of the cells were in S-phase, consistent with the rapid growth observed under these conditions. When EVI1 was induced through removal of tetracycline, the percentage of cells in S-phase dropped to 4%, with a concomitant increase of the G0/G1 population (Fig. 3, A and B). A similar but slightly milder effect was observed for U937T_MDS1/EVI1-HA cells (Fig. 3, A and B). As reported previously [28], the percentage of cells in S-phase was also somewhat reduced upon removal of tetracycline from U937T cells (Fig. 3, A and B), but this nonspecific effect was significantly milder than the specific effect observed as a result of the induction of EVI1 or MDS1/EVI1.
Figure 3.
EVI1 and MDS1/EVI1 cause G0/G1 arrest in U937T cells. (A) U937T_EVI1-HA E14, U937T_MDS1/EVI1-HA M2, and U937T cells were maintained in the presence or absence of tetracycline for 4 days. Nuclear DNA content was analyzed by flow cytometry after staining with propidium iodide. Similar data were obtained with E10 and M27 cells. (B) Quantitative evaluation of cell-cycle experiments. The percentage of cells in each phase of the cell cycle is indicated. E, M, U, Results obtained for U937T_EVI1-HA (n=5), U937T_MDS1/EVI1-HA (n=4), and U937T cells (n=3), respectively. Data have been derived from several independent experiments. Values for U937T_EVI1-HA clones E10 and E14 were pooled, as were values for U937T_MDS1/EVI1-HA clones M2 and M27. Open and solid bars, Cells grown in the presence or absence of tetracycline, respectively. Asterisks above bars indicate the level of significance between cells grown in the presence or absence of tetracycline. Asterisks above brackets indicate the level of significance as compared with U937T cells under the same conditions. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
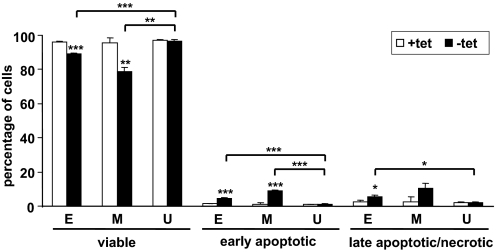
To determine whether expression of EVI1 or MDS1/EVI1 caused cells to undergo apoptosis, U937T_EVI1-HA, U937T_MDS1/EVI1-HA, and U937T cells were maintained in the presence or absence of tetracycline for 5 days, stained with FITC-conjugated Annexin V and 7-AAD, and analyzed by flow cytometry. Tetracycline removal was found to cause a small but significant proportion of U937T_EVI1-HA cells to undergo apoptosis. This effect was more pronounced in U937T_MDS1/EVI1-HA cells but absent in parental U937T cells (Fig. 4).
Figure 4.
Induction of EVI1 or MDS1/EVI1 in U937T cells increases the rate of spontaneous apoptosis. U937T_EVI1-HA, U937T_MDS1/EVI1-HA, and U937T cells were maintained in the presence (open bars) or absence (solid bars) of tetracycline for 5 days, stained with Annexin V-FITC and 7-AAD, and analyzed by flow cytometry. The percentages of viable (double-negative), early-apoptotic (Annexin V-positive 7-AAD-negative), and late-apoptotic/necrotic (double-positive) cells are indicated. E, M, U: results obtained for U937T_EVI1-HA (n=6), U937T_MDS1/EVI1-HA (n=4), and U937T cells (n=4), respectively. Data have been derived from several independent experiments. Values for U937T_EVI1-HA clones E10 and E14 were pooled, as were values for U937T_MDS1/EVI1-HA clones M2 and M27. Asterisks above bars indicate the level of significance between cells grown in the presence or absence of tetracycline. Asterisks above brackets indicate the level of significance as compared with U937T cells under the same conditions. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
In summary, EVI1 and MDS1/EVI1 inhibited U937T cell proliferation, primarily by causing cell-cycle arrest and to a minor extent by increasing the rate of spontaneous apoptosis.
U937T cells expressing EVI1 or MDS1/EVI1 undergo massive apoptosis when stimulated to differentiate
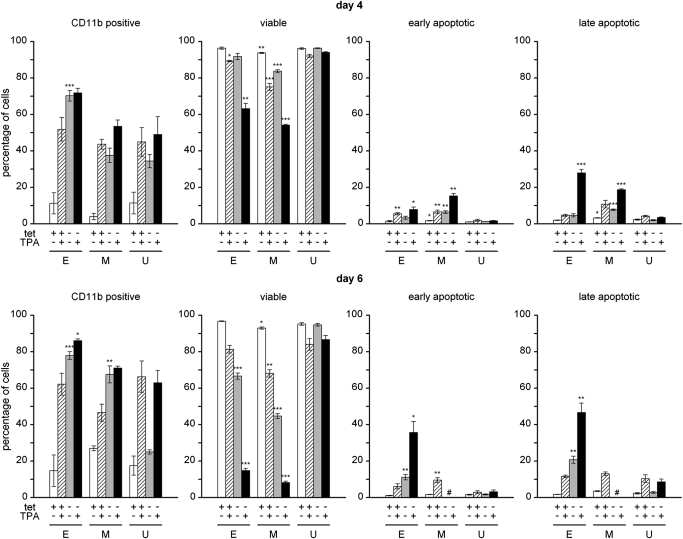
To investigate the effects of EVI1 and MDS1/EVI1 on cellular differentiation, U937T_EVI1-HA, U937T_MDS1/EVI1-HA, and control U937T cells were transferred to media with or without tetracycline, and the differentiation-inducing agent TPA was added to one-half of each culture on the following day (Day 1 of the experiment). On Days 4 and 6, cellular differentiation was monitored by flow cytometric analysis for the monocyte/macrophage-specific cell-surface marker CD11b. As expected, TPA caused a time-dependent increase in the percentage of CD11b-positive cells in all three investigated cell types (Fig. 5). Similarly, removal of tetracycline led to enhanced CD11b expression in U937T_EVI1-HA, U937T_MDS1/EVI1-HA, and U937T cells (Fig. 5). This effect was strongest in U937T_EVI1-HA cells and least pronounced in U937T cells. In the latter cell line, it also declined over time (Fig. 5), probably as a result of overgrowth of differentiated cells by cycling cells. Addition of TPA after tetracycline withdrawal caused an only moderately stronger effect on cell-surface CD11b levels than either treatment alone (Fig. 5). However, a markedly increased rate of cell death was noted in U937T_EVI1-HA and U937T_MDS1/EVI1-HA cells under these conditions. To investigate this phenomenon further, aliquots of the cultures used for CD11b staining were labeled with Annexin V-FITC and 7-AAD and subjected to flow cytometry. Removal of tetracycline led to a moderate increase in apoptosis in U937T_EVI1-HA and U937T_MDS1/EVI1-HA cells but not parental U937T cells on Day 6 of the experiment (Fig. 5). Addition of TPA to tetracycline-containing cultures caused the death of a small proportion of cells of all three types. Notably, a marked and synergistic increase in the numbers of dying and dead cells was observed when U937T_EVI1-HA and U937T_MDS1/EVI1-HA cells were exposed to TPA after transfer to tetracycline-free media (Fig. 5). This effect was absent in parental U937T cells (Fig. 5), demonstrating that it was a specific consequence of the expression of EVI1 or MDS1/EVI1.
Figure 5.
Expression of EVI1 or MDS1/EVI1 causes massive apoptosis in U937T cells exposed to a differentiation stimulus. U937T_EVI1-HA, U937T_MDS1/EVI1-HA, and parental U937T cells were transferred to media with or without tetracycline. On the next day (Day 1 of the experiment), 50 ng/ml TPA was added to one-half of each culture. On Days 4 (upper panel) and 6 (lower panel), one aliquot of each culture was stained with a PE-conjugated CD11b antibody and another aliquot with Annexin V-FITC and 7-AAD, and cells were analyzed by flow cytometry. The percentages of CD11b-positive cells as well as the percentages of viable (Annexin V-negative 7-AAD-negative), early-apoptotic (Annexin V-positive 7-AAD-negative), and late-apoptotic/necrotic (Annexin V-positive 7-AAD-positive) cells are shown in separate panels as indicated. E, M, U: results obtained with U937T_EVI1-HA (n=4), U937T_MDS1/EVI1-HA (n=4), and U937T cells (n=4), respectively. Data have been derived from several independent experiments. Values for U937T_EVI1-HA clones E10 and E14 were pooled, as were values for U937T_MDS1/EVI1-HA clones M2 and M27. Open bars, Cells maintained in the presence of tetracycline and the absence of TPA; hatched bars, cells maintained in the presence of tetracycline and TPA; shaded bars, cells maintained in the absence of tetracycline and TPA; solid bars, cells maintained in the absence of tetracycline and the presence of TPA. Asterisks above bars indicate the level of significance as compared with U937T cells under the same conditions. *, P < 0.05; **, P < 0.01; ***, P < 0.001. #, On Day 6 of the experiment, U937T_MDS1/EVI1-HA cells without tetracycline had lost viability to an extent that did not allow a reliable distinction between early- and late-apoptotic cells. Therefore, only the number of viable cells is indicated.
To corroborate these results, U937T_EVI1-HA, U937T_MDS1/EVI1-HA, and U937T cells were cultured as described above and subjected to TUNEL assays on Day 5 of the experiment. Again, a limited proportion of U937T_EVI1-HA cells underwent programmed death upon exposure to TPA or removal of tetracycline, and the combination of these treatments caused a more-than-additive proportion of cells to die (Fig. 6, A and B). Similar effects were seen in U937T_MDS1/EVI1-HA cells but not in parental U937T cells (Fig. 6, A and B). In summary, these data show that the expression of EVI1 or MDS1/EVI1 causes massive apoptosis in cells exposed to a differentiation stimulus.
Figure 6.
TUNEL assays confirm massive apoptosis in EVI1- or MDS1/EVI1-expressing U937T cells exposed to a differentiation stimulus. (A) U937T_EVI1-HA E14, U937T_MDS1/EVI1-HA M27, and parental U937T cells were transferred to media with or without tetracycline. On the next day (Day 1 of the experiment), 50 ng/ml TPA was added to one-half of each culture. On Day 5, DNA breaks were labeled using terminal deoxynucleotidyl transferase and FITC-labeled dUTP and quantified by flow cytometry. Similar results were obtained with E10 and M2 cells. (B) Quantitative evaluation of TUNEL experiments. The percentage of TUNEL-positive (dead) cells is indicated for each culture condition. E, M, U: data obtained with U937T_EVI1-HA (n=3), U937T_MDS1/EVI1-HA (n=4), and U937T cells (n=3), respectively. Data have been derived from several independent experiments. Values for U937T_EVI1-HA clones E10 and E14 were pooled, as were values for U937T_MDS1/EVI1-HA clones M2 and M27. Open bars, Cells maintained in the presence of tetracycline and the absence of TPA; hatched bars, cells maintained in the presence of tetracycline and TPA; shaded bars, cells maintained in the absence of tetracycline and TPA; solid bars, cells maintained in the absence of tetracycline and the presence of TPA. Asterisks above bars indicate the level of significance as compared with U937T cells under the same conditions. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
DISCUSSION
Overexpression of the EVI1 gene is presumed to contribute to the pathogenesis of AML and MDS [6,7,8,9,10,11]. In agreement with its role in the former disease, experimental expression of Evi1 enhanced the proliferation of immature hematopoietic cells [19, 23,24,25, 32, 33], and targeted disruption of its gene locus impaired proliferation and enhanced spontaneous apoptosis of murine hematopoietic stem and progenitor cells [13, 34]. EVI1 inhibited programmed cell death in response to a variety of apoptotic stimuli [24, 31, 35], and its ectopic expression interfered with erythroid and granulocytic differentiation [23, 36]. Of these phenotypes, EVI1-mediated augmentation of proliferation and survival is not only likely to be relevant for the role of EVI1 in AML but may also contribute to the bone marrow hypercellularity observed frequently in MDS. However, more mature MDS cells are impaired in their ability to grow and survive. Indeed, EVI1 also evoked phenotypes that may be related to this aspect of the disease. Thus, ectopic expression of Evi1 caused an MDS-like disease in a mouse bone marrow transduction/transplantation system [23, 25]. Compared with controls, bone marrow cells from diseased mice yielded reduced numbers of colonies in soft agar containing differentiation-promoting cytokines [23, 25], most likely reflecting impaired growth and/or enhanced rates of cell death. In ovarian epithelial cells, only short-term expression of EVI1 increased proliferation, whereas long-term expression strongly reduced growth and/or survival [37]. EVI1 overexpression also delayed proliferation of human myeloid UT-7/GM cells and of erythroid cells from various sources [23, 36, 38, 39]. Furthermore, when Evi1-transduced murine 32Dcl3 or 32DEpo cells were induced to differentiate, they lost their viability in a manner not characterized further [20, 38, 40], possibly reflecting the phenotype of differentiation failure. In the present investigation, it was found that inducible expression of EVI1 in the human myeloid cell line U937T caused cells to cease proliferation, accumulate in the G0/G1-phase of the cell cycle, increase cell-surface CD11b expression, undergo a mild degree of spontaneous apoptosis, and massively succumb to programmed cell death when induced to differentiate.
Notably, tetracycline withdrawal caused a shift from the S- to the G0/G1-phase of the cell cycle, not only in U937T_EVI1-HA cells but, as reported previously [28], in parental U937T cells as well. However, the alterations in cell-cycle distribution were significantly more pronounced in U937T_EVI1-HA cells than in the parental line. Therefore, they appear to constitute a genuine and specific consequence of EVI1 expression. Similarly, removal of tetracycline led to increased cell-surface CD11b expression in U937T_EVI1-HA and in U937T cells. Again, this effect has been reported previously for U937T cells [41] but was less pronounced than in U937T_EVI1-HA cells in our experiments. Although we cannot rule out the possibility of differentiation being induced as a specific consequence of EVI1 expression in U937T cells, we believe it is more likely a secondary effect of cell-cycle arrest. This assumption is supported by the following observations: First, the extent of the G0/G1 arrest correlated with that of CD11b induction in the cell lines used in this study. Second, a large proportion of U937T cells remained in a cycling state and outgrew the CD11b-positive cells over time, whereas in the U937T_EVI1-HA clones, of which virtually all cells arrested in G0/G1, cell-surface CD11b expression increased with time. Third, it has been shown previously that cell-cycle arrest caused by experimental expression of the cyclin-dependent kinase inhibitors p21 and p27 or by glutamine depletion was sufficient to induce differentiation of U937 cells [42, 43], suggesting that cell-cycle arrest per se is sufficient to promote differentiation in this cell line.
The most striking phenotype observed after induction of EVI1, namely, the occurrence of massive apoptosis after exposure to a differentiation signal, was entirely absent in parental cells, showing that it was a genuine and specific effect of EVI1. Interestingly, TPA caused a higher rate of cell death in U937T_EVI1-HA than in U937T cells even in the presence of tetracycline. This may be a result of basal EVI1 expression in U937T_EVI1-HA cells in the presence of the antibiotic (Fig. 1A) and suggests that the ability of EVI1 to cause apoptosis in cells exposed to differentiation signals is dose-dependent. For the following reasons, we believe it unlikely that the massive apoptosis observed in EVI1-expressing, TPA-treated cells was merely a consequence of excessive differentiation signals: i) the rate of apoptosis in EVI1-expressing, TPA-treated cells greatly exceeded that observed under any other conditions, including some that led to comparable degrees of CD11b positivity, and ii) the combined effects of EVI1 induction and TPA treatment on CD11b expression were only moderately greater than their individual effects, but their combined effects on apoptosis were synergistic. Therefore, our results suggest that expression of EVI1 in U937 cells causes differentiation failure, which is a key feature of MDS.
Our data appear to contradict those of Kurokawa et al. [31], who reported that constitutive EVI1 overexpression inhibited TNF-α-induced apoptosis in U937 cells. A possible explanation for this discrepancy is that constitutive expression of EVI1 may select for transfectants with genetic or epigenetic alterations that cooperate with EVI1 to promote cellular growth and survival. Alternatively, U937 cells maintained in different laboratories, or U937 and U937T cells, may exhibit certain genetic and/or gene-expression differences a priori. For example, our batch of U937 cells transcribed HOXA9, a gene highly expressed in, and important for the function of, hematopoietic stem cells [44], while U937T cells, for unknown reasons, did not (T. A. Konrad, unpublished results).
A further, unexpected finding was that EVI1 and MDS1/EVI1 evoked qualitatively identical and quantitatively similar cellular phenotypes in our experimental system. Like a number of other related protein pairs, EVI1 and MDS1/EVI1 differ from each other by the absence or presence of an N-terminal PR domain, respectively. In many such protein pairs, the variant lacking the PR domain has oncogenic and the PR-containing variant tumor suppressive properties [45]. Likewise, Evi1 and Mds1/Evi1 exhibited opposing biological activities in some previous experiments [18,19,20, 37]. However, in other assay systems, Evi1 elicited specific effects, whereas Mds1/Evi1 was inactive [19,20,21], and in yet others, the expression of the two protein variants had the same biological consequences [21, 37, 46, 47]. Thus, it appears that EVI1 and MDS1/EVI1 may act in a similar, different, or opposite manner depending on the biological effect and the experimental system investigated. Notably, however, two recent studies reported that overexpression of EVI1 and MDS1/EVI1 had contrary effects on prognosis in ovarian cancer [37] and in AML [11]. Comparable data for MDS are not available at the present time. Although our results show that both EVI1 and MDS1/EVI1 bring about phenotypes that may be relevant for peripheral blood cytopenia in MDS, their effects on immature MDS cells and/or the prognostic significance of their overexpression in MDS may differ.
In summary, our study confirms and extends previous results, demonstrating that the effects of EVI1 on the proliferation, differentiation, and death of hematopoietic cells strongly depend on cell lineage and the stage of maturation. Such seemingly contradictory phenotypes have also been observed after experimental expression of an activated N-RAS gene [48,49,50,51,52] and probably reflect the multifaceted roles of these genes in the complex pathologies of MDS and AML. In the present study, we established a cell-culture model that recapitulates cellular phenotypes which may be relevant to the pathology of MDS in response to the inducible expression of EVI1. Based on our own data and those of others, we propose the following working model for the role of EVI1 in normal and malignant hematopoiesis: In the former context, EVI1 promotes the proliferation of immature cells and is down-regulated during differentiation [7, 53,54,55]. In MDS, pathologically activated EVI1 expression augments proliferation and/or survival of immature malignant cells, but the inability to down-regulate this gene properly causes differentiation failure and consequent peripheral pancytopenia. In AML, additional genetic and/or epigenetic alterations keep cells in a state sufficiently immature to benefit from the growth-promoting properties of EVI1, and/or they overcome the failure of more mature, EVI1-expressing cells to differentiate.
Acknowledgments
This work was supported by grants P17896-B14, P19795-B12, and P20920-B12 (all to R. W.) from the Austrian Science Foundation (FWF). We gratefully acknowledge Dr. Gerard Grosveld of the St. Jude Children’s Research Hospital (Memphis, TN, USA) for providing U937T cells and the pUHD10S vector, Dr. Hiroyuki Hamaguchi, Musashino Red Cross Hospital (Tokyo, Japan), for his gift of HNT-34 cells, and Dr. Michael Baumann, Wright State University (Dayton, OH, USA), for supplying MPD cells. We thank Dr. Andreas Spittler, Dr. Herbert Strobl, and Dr. Peter Valent of the Medical University of Vienna (Austria) and Dr. Regina Grillari of the University of Natural Resources and Applied Life Sciences (Vienna, Austria) for discussion and valuable advice. Dr. Nina Brandl at the Medical University of Vienna helped with immunofluorescence microscopy, and Dr. Lukas Mach at the University of Natural Resources and Applied Life Sciences provided an aliquot of the goat anti-mouse antibody. Apoptosis experiments were analyzed at the Cell Sorting Core Facility of the Medical University of Vienna.
Footnotes
Abbreviations: 7-AAD=7-amino-actinomycin D, AML=acute myeloid leukemia, DAPI=4′,6′-diamidino-2-phenylindole dihydrochloride, EVI1=ecotropic viral integration site 1, HA=hemagglutinin, HOXA9=homeobox A9, MDS=myelodysplastic syndrome, MLL=myeloid/lymphoid or mixed lineage leukemia, N-RAS=neuroblastoma RAS, PR=PRDI-BF1/Blimp-1 and RIZ1 related, sAML=secondary AML, TPA=12-O-tetradecanoylphorbol 13-acetate
References
- Corey S J, Minden M, Barber D, Kantarjian H, Wang J, Schimmer A. Myelodysplastic syndromes: the complexity of stem-cell diseases. Nat Rev Cancer. 2007;7:118–129. doi: 10.1038/nrc2047. [DOI] [PubMed] [Google Scholar]
- Nimer S D. Myelodysplastic syndromes. Blood. 2008;111:4841–4851. doi: 10.1182/blood-2007-08-078139. [DOI] [PubMed] [Google Scholar]
- Steensma D P. The spectrum of molecular aberrations in myelodysplastic syndromes: in the shadow of acute myeloid leukemia. Haematologica. 2007;92:723–727. doi: 10.3324/haematol.11471. [DOI] [PubMed] [Google Scholar]
- Harada H, Harada Y, Niimi H, Kyo T, Kimura A, Inaba T. High incidence of somatic mutations in the AML1/RUNX1 gene in myelodysplastic syndrome and low blast percentage myeloid leukemia with myelodysplasia. Blood. 2004;103:2316–2324. doi: 10.1182/blood-2003-09-3074. [DOI] [PubMed] [Google Scholar]
- Bacher U, Haferlach T, Kern W, Haferlach C, Schnittger S. A comparative study of molecular mutations in 381 patients with myelodysplastic syndrome and in 4130 patients with acute myeloid leukemia. Haematologica. 2007;92:744–752. doi: 10.3324/haematol.10869. [DOI] [PubMed] [Google Scholar]
- Poppe B, Dastugue N, Vandesompele J, Cauwelier B, De Smet B, Yigit N, De Paepe A, Cervera J, Recher C, De Mas V, Hagemeijer A, Speleman F. EVI1 is consistently expressed as principal transcript in common and rare recurrent 3q26 rearrangements. Genes Chromosomes Cancer. 2006;45:349–356. doi: 10.1002/gcc.20295. [DOI] [PubMed] [Google Scholar]
- Vinatzer U, Mannhalter C, Mitterbauer M, Gruener H, Greinix H, Schmidt H, Fonatsch C, Wieser R. Quantitative comparison of the expression of EVI1 and its presumptive antagonist, MDS1/EVI1, in patients with myeloid leukemia. Genes Chromosomes Cancer. 2003;36:80–89. doi: 10.1002/gcc.10144. [DOI] [PubMed] [Google Scholar]
- Russell M, List A, Greenberg P, Woodward S, Glinsmann B, Parganas E, Ihle J, Taetle R. Expression of EVI1 in myelodysplastic syndromes and other hematologic malignancies without 3q26 translocations. Blood. 1994;84:1243–1248. [PubMed] [Google Scholar]
- Dreyfus F, Bouscary D, Melle J, Ribrag V, Guesnu M, Varet B. Expression of the Evi-1 gene in myelodysplastic syndromes. Leukemia. 1995;9:203–205. [PubMed] [Google Scholar]
- Haas K, Kundi M, Sperr W, Esterbauer H, Ludwig W, Ratei R, Koller E, Gruener H, Sauerland C, Fonatsch C, Valent P, Wieser R. Expression and prognostic significance of different mRNA 5′-end variants of the oncogene EVI1 in 266 patients with de novo AML: EVI1 and MDS1/EVI1 overexpression both predict short remission duration. Genes Chromosomes Cancer. 2008;47:288–298. doi: 10.1002/gcc.20532. [DOI] [PubMed] [Google Scholar]
- Lugthart S, van Drunen E, van Norden Y, van Hoven A, Erpelinck C, Valk P, Beverloo H, Lowenberg B, Delwel R. High EVI1 levels predict adverse outcome in acute myeloid leukemia: prevalence of EVI1 overexpression and chromosome 3q26 abnormalities underestimated. Blood. 2008;111:4329–4337. doi: 10.1182/blood-2007-10-119230. [DOI] [PubMed] [Google Scholar]
- Matsugi T, Morishita K, Ihle J. Identification, nuclear localization, and DNA-binding activity of the zinc finger protein encoded by the Evi-1 myeloid transforming gene. Mol Cell Biol. 1990;10:1259–1264. doi: 10.1128/mcb.10.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuasa H, Oike Y, Iwama A, Nishikata I, Sugiyama D, Perkins A, Mucenski M, Suda T, Morishita K. Oncogenic transcription factor Evi1 regulates hematopoietic stem cell proliferation through GATA-2 expression. EMBO J. 2005;24:1976–1987. doi: 10.1038/sj.emboj.7600679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aytekin M, Vinatzer U, Musteanu M, Raynaud S, Wieser R. Regulation of the expression of the oncogene EVI1 through the use of alternative mRNA 5′-ends. Gene. 2005;356:160–168. doi: 10.1016/j.gene.2005.04.032. [DOI] [PubMed] [Google Scholar]
- Fears S, Mathieu C, Zeleznik Le N, Huang S, Rowley J, Nucifora G. Intergenic splicing of MDS1 and EVI1 occurs in normal tissues as well as in myeloid leukemia and produces a new member of the PR domain family. Proc Natl Acad Sci USA. 1996;93:1642–1647. doi: 10.1073/pnas.93.4.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer K, Vinatzer U, Zwirn P, Fonatsch C, Wieser R. Comparative expression analysis of the antagonistic transcription factors EVI1 and MDS1-EVI1 in murine tissues and during in vitro hematopoietic differentiation. Biochem Biophys Res Commun. 1998;252:691–696. doi: 10.1006/bbrc.1998.9588. [DOI] [PubMed] [Google Scholar]
- Bordereaux D, Fichelson S, Tambourin P, Gisselbrecht S. Alternative splicing of the Evi-1 zinc finger gene generates mRNAs which differ by the number of zinc finger motifs. Oncogene. 1990;5:925–927. [PubMed] [Google Scholar]
- Soderholm J, Kobayashi H, Mathieu C, Rowley J, Nucifora G. The leukemia-associated gene MDS1/EVI1 is a new type of GATA-binding transactivator. Leukemia. 1997;11:352–358. doi: 10.1038/sj.leu.2400584. [DOI] [PubMed] [Google Scholar]
- Sitailo S, Sood R, Barton K, Nucifora C. Forced expression of the leukemia-associated gene EVI1 in ES cells: a model for myeloid leukemia with 3q26 rearrangements. Leukemia. 1999;13:1639–1645. doi: 10.1038/sj.leu.2401585. [DOI] [PubMed] [Google Scholar]
- Sood R, Talwar Trikha A, Chakrabarti S, Nucifora G. MDS1/EVI1 enhances TGF-β 1 signaling and strengthens its growth-inhibitory effect, but the leukemia-associated fusion protein AML1/MDS1/EVI1, product of the t(3;21), abrogates growth-inhibition in response to TGF-β 1. Leukemia. 1999;13:348–357. doi: 10.1038/sj.leu.2401360. [DOI] [PubMed] [Google Scholar]
- Cuenco G, Ren R. Both AML1 and EVI1 oncogenic components are required for the cooperation of AML1/MDS1/EVI1 with BCR/ABL in the induction of acute myelogenous leukemia in mice. Oncogene. 2004;23:569–579. doi: 10.1038/sj.onc.1207143. [DOI] [PubMed] [Google Scholar]
- Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G, Rees J, Hann I, Stevens R, Burnett A, Goldstone A. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children’s Leukaemia Working Parties. Blood. 1998;92:2322–2333. [PubMed] [Google Scholar]
- Buonamici S, Li D, Chi Y, Zhao R, Wang X, Brace L, Ni H, Saunthararajah Y, Nucifora G. EVI1 induces myelodysplastic syndrome in mice. J Clin Invest. 2004;114:713–719. doi: 10.1172/JCI21716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonamici S, Li D, Mikhail F, Sassano A, Platanias L, Colamonici O, Anastasi J, Nucifora G. EVI1 abrogates interferon-α response by selectively blocking PML induction. J Biol Chem. 2005;280:428–436. doi: 10.1074/jbc.M410836200. [DOI] [PubMed] [Google Scholar]
- Jin G, Yamazaki Y, Takuwa M, Takahara T, Kaneko K, Kuwata T, Miyata S, Nakamura T. Trib1 and Evi1 cooperate with Hoxa and Meis1 in myeloid leukemogenesis. Blood. 2007;109:3998–4005. doi: 10.1182/blood-2006-08-041202. [DOI] [PubMed] [Google Scholar]
- Watanabe-Okochi N, Kitaura J, Ono R, Harada H, Harada Y, Komeno Y, Nakajima H, Nosaka T, Inaba T, Kitamura T. AML1 mutations induced MDS and MDS/AML in a mouse BMT model. Blood. 2008;111:4297–4308. doi: 10.1182/blood-2007-01-068346. [DOI] [PubMed] [Google Scholar]
- Fornerod M, Boer J, van Baal S, Jaegle M, von Lindern M, Murti K, Davis D, Bonten J, Buijs A, Grosveld G. Relocation of the carboxyterminal part of CAN from the nuclear envelope to the nucleus as a result of leukemia-specific chromosome rearrangements. Oncogene. 1995;10:1739–1748. [PubMed] [Google Scholar]
- Boer J, Bonten-Surtel J, Grosveld G. Overexpression of the nucleoporin CAN/NUP214 induces growth arrest, nucleocytoplasmic transport defects, and apoptosis. Mol Cell Biol. 1998;18:1236–1247. doi: 10.1128/mcb.18.3.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi H, Suzukawa K, Nagata K, Yamamoto K, Yagasaki F, Morishita K. Establishment of a novel human myeloid leukemia cell line (HNT-34) with t(3;3)(q21;q26), t(9;22)(q34;q11) and the expression of EVI1 gene, p210 and p190 BCR/ABL chimeric transcripts from a patient with AML after MDS with 3q21q26 syndrome. Br J Haematol. 1997;98:399–407. doi: 10.1046/j.1365-2141.1997.2143029.x. [DOI] [PubMed] [Google Scholar]
- Paul C, Aly E, Lehman J, Page S, Gomez-Cambronero J, Ackerman S, Baumann M. Human cell line that differentiates to all myeloid lineages and expresses neutrophil secondary granule genes. Exp Hematol. 2000;28:1373–1380. doi: 10.1016/s0301-472x(00)00552-x. [DOI] [PubMed] [Google Scholar]
- Kurokawa M, Mitani K, Yamagata T, Takahashi T, Izutsu K, Ogawa S, Moriguchi T, Nishida E, Yazaki Y, Hirai H. The evi-1 oncoprotein inhibits c-Jun N-terminal kinase and prevents stress-induced cell death. EMBO J. 2000;19:2958–2968. doi: 10.1093/emboj/19.12.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi Y, Senyuk V, Chakraborty S, Nucifora G. EVI1 promotes cell proliferation by interacting with BRG1 and blocking the repression of BRG1 on E2F1 activity. J Biol Chem. 2003;278:49806–49811. doi: 10.1074/jbc.M309645200. [DOI] [PubMed] [Google Scholar]
- Chakraborty S, Senyuk V, Sitailo S, Chi Y, Nucifora G. Interaction of EVI1 with CBP and P/CAF results in reversible acetylation of EVI1 and in colocalization in nuclear speckles. J Biol Chem. 2001;276:44936–44943. doi: 10.1074/jbc.M106733200. [DOI] [PubMed] [Google Scholar]
- Goyama S, Yamamoto G, Shimabe M, Sato T, Ichikawa M, Ogawa S, Chiba S, Kurokawa M. Evi-1 is a critical regulator for hematopoietic stem cells and transformed leukemic cells. Cell Stem Cell. 2008;3:207–220. doi: 10.1016/j.stem.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Liu Y, Chen L, Ko T, Fields A, Thompson E. Evi1 is a survival factor which conveys resistance to both TGFβ- and taxol-mediated cell death via PI3K/AKT. Oncogene. 2006;25:3565–3575. doi: 10.1038/sj.onc.1209403. [DOI] [PubMed] [Google Scholar]
- Laricchia-Robbio L, Fazzina R, Li D, Rinaldi C, Sinha K, Chakraborty S, Nucifora G. Point mutations in two EVI1 Zn fingers abolish EVI1-GATA1 interaction and allow erythroid differentiation of murine bone marrow cells. Mol Cell Biol. 2006;26:7658–7666. doi: 10.1128/MCB.00363-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanjundan M, Nakayama Y, Cheng K, Lahad J, Liu J, Lu K, Kuo W, Smith-McCune K, Fishman D, Gray J, Mills G. Amplification of MDS1/EVI1 and EVI1, located in the 3q26.2 amplicon, is associated with favorable patient prognosis in ovarian cancer. Cancer Res. 2007;67:3074–3084. doi: 10.1158/0008-5472.CAN-06-2366. [DOI] [PubMed] [Google Scholar]
- Kreider B, Orkin S, Ihle J. Loss of erythropoietin responsiveness in erythroid progenitors due to expression of the Evi-1 myeloid-transforming gene. Proc Natl Acad Sci USA. 1993;90:6454–6458. doi: 10.1073/pnas.90.14.6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louz D, van den Broek M, Verbakel S, Vankan Y, van Lom K, Joosten M, Meijer D, Lowenberg B, Delwel R. Erythroid defects and increased retrovirally-induced tumor formation in Evi1 transgenic mice. Leukemia. 2000;14:1876–1884. doi: 10.1038/sj.leu.2401887. [DOI] [PubMed] [Google Scholar]
- Morishita K, Parganas E, Matsugi T, Ihle J. Expression of the Evi-1 zinc finger gene in 32Dc13 myeloid cells blocks granulocytic differentiation in response to granulocyte colony-stimulating factor. Mol Cell Biol. 1992;12:183–189. doi: 10.1128/mcb.12.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromleigh V, Freedman L. p21 is a transcriptional target of HOXA10 in differentiating myelomonocytic cells. Genes Dev. 2000;14:2581–2586. doi: 10.1101/gad.817100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Lee M, Cohen M, Bommakanti M, Freedman L. Transcriptional activation of the Cdk inhibitor p21 by vitamin D3 leads to the induced differentiation of the myelomonocytic cell line U937. Genes Dev. 1996;10:142–153. doi: 10.1101/gad.10.2.142. [DOI] [PubMed] [Google Scholar]
- Spittler A, Oehler R, Goetzinger P, Holzer S, Reissner C, Leutmezer F, Rath V, Wrba F, Fuegger R, Boltz-Nitulescu G, Roth E. Low glutamine concentrations induce phenotypical and functional differentiation of U937 myelomonocytic cells. J Nutr. 1997;127:2151–2157. doi: 10.1093/jn/127.11.2151. [DOI] [PubMed] [Google Scholar]
- Argiropoulos B, Humphries R. Hox genes in hematopoiesis and leukemogenesis. Oncogene. 2007;26:6766–6776. doi: 10.1038/sj.onc.1210760. [DOI] [PubMed] [Google Scholar]
- Jiang G, Huang S. The yin-yang of PR-domain family genes in tumorigenesis. Histol Histopathol. 2000;15:109–117. doi: 10.14670/HH-15.109. [DOI] [PubMed] [Google Scholar]
- Vinatzer U, Taplick J, Seiser C, Fonatsch C, Wieser R. The leukaemia-associated transcription factors EVI-1 and MDS1/EVI1 repress transcription and interact with histone deacetylase. Br J Haematol. 2001;114:566–573. doi: 10.1046/j.1365-2141.2001.02987.x. [DOI] [PubMed] [Google Scholar]
- Nitta E, Izutsu K, Yamaguchi Y, Imai Y, Ogawa S, Chiba S, Kurokawa M, Hirai H. Oligomerization of Evi-1 regulated by the PR domain contributes to recruitment of corepressor CtBP. Oncogene. 2005;24:6165–6173. doi: 10.1038/sj.onc.1208754. [DOI] [PubMed] [Google Scholar]
- Darley R, Hoy T, Baines P, Padua R, Burnett A. Mutant 1N-RAS induces erythroid lineage dysplasia in human CD34+ cells. J Exp Med. 1997;185:1337–1347. doi: 10.1084/jem.185.7.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M, Vaque J, Arozarena I, Lopez-Ilasaca M, Martinez C, Crespo P, Leon J. H-, K- and N-Ras inhibit myeloid leukemia cell proliferation by a p21WAF1-dependent mechanism. Oncogene. 2000;19:783–790. doi: 10.1038/sj.onc.1203384. [DOI] [PubMed] [Google Scholar]
- Shen S, Dolnikov A, Passioura T, Millington M, Wotherspoon S, Rice A, MacKenzie K, Symonds G. Mutant N-ras preferentially drives human CD34+ hematopoietic progenitor cells into myeloid differentiation and proliferation both in vitro and in the NOD/SCID mouse. Exp Hematol. 2004;32:852–860. doi: 10.1016/j.exphem.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Passioura T, Dolnikov A, Shen S, Symonds G. N-ras-induced growth suppression of myeloid cells is mediated by IRF-1. Cancer Res. 2005;65:797–804. [PubMed] [Google Scholar]
- Shen S, Passioura T, Symonds G, Dolnikov A. N-ras oncogene-induced gene expression in human hematopoietic progenitor cells: upregulation of p16INK4a and p21CIP1/WAF1 correlates with myeloid differentiation. Exp Hematol. 2007;35:908–919. doi: 10.1016/j.exphem.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Privitera E, Longoni D, Brambillasca F, Biondi A. EVI-1 gene expression in myeloid clonogenic cells from juvenile myelomonocytic leukemia (JMML) Leukemia. 1997;11:2045–2048. doi: 10.1038/sj.leu.2400865. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Nagasawa T, Katoh O, Komatsu N, Yokota J, Morishita K. EVI1 is expressed in megakaryocyte cell lineage and enforced expression of EVI1 in UT-7/GM cells induces megakaryocyte differentiation. Biochem Biophys Res Commun. 2002;292:609–616. doi: 10.1006/bbrc.2002.6693. [DOI] [PubMed] [Google Scholar]
- Scicchitano M S, McFarland D, Tierney L, Narayanan P, Schwartz L. In vitro expansion of human cord blood CD36(+) erythroid progenitors: temporal changes in gene and protein expression. Exp Hematol. 2003;31:760–769. doi: 10.1016/s0301-472x(03)00185-1. [DOI] [PubMed] [Google Scholar]