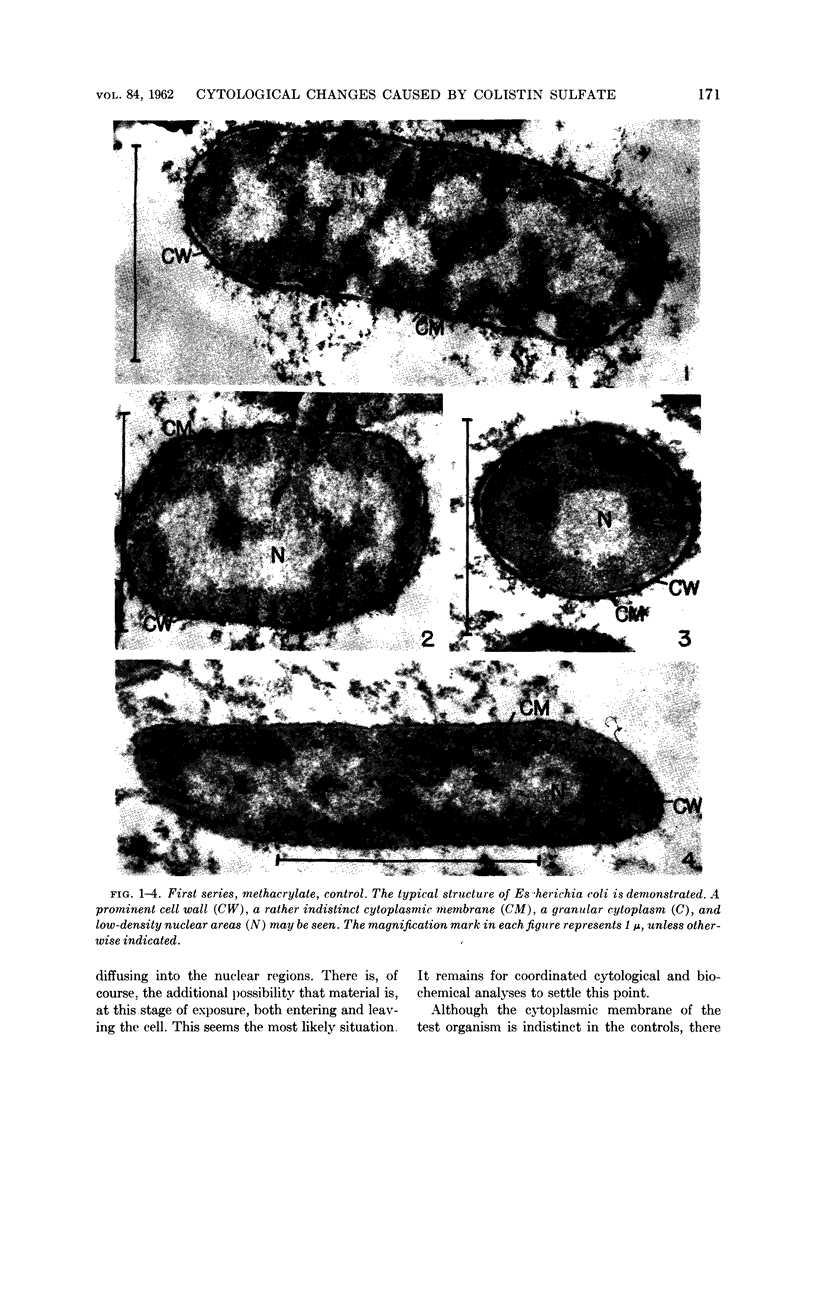
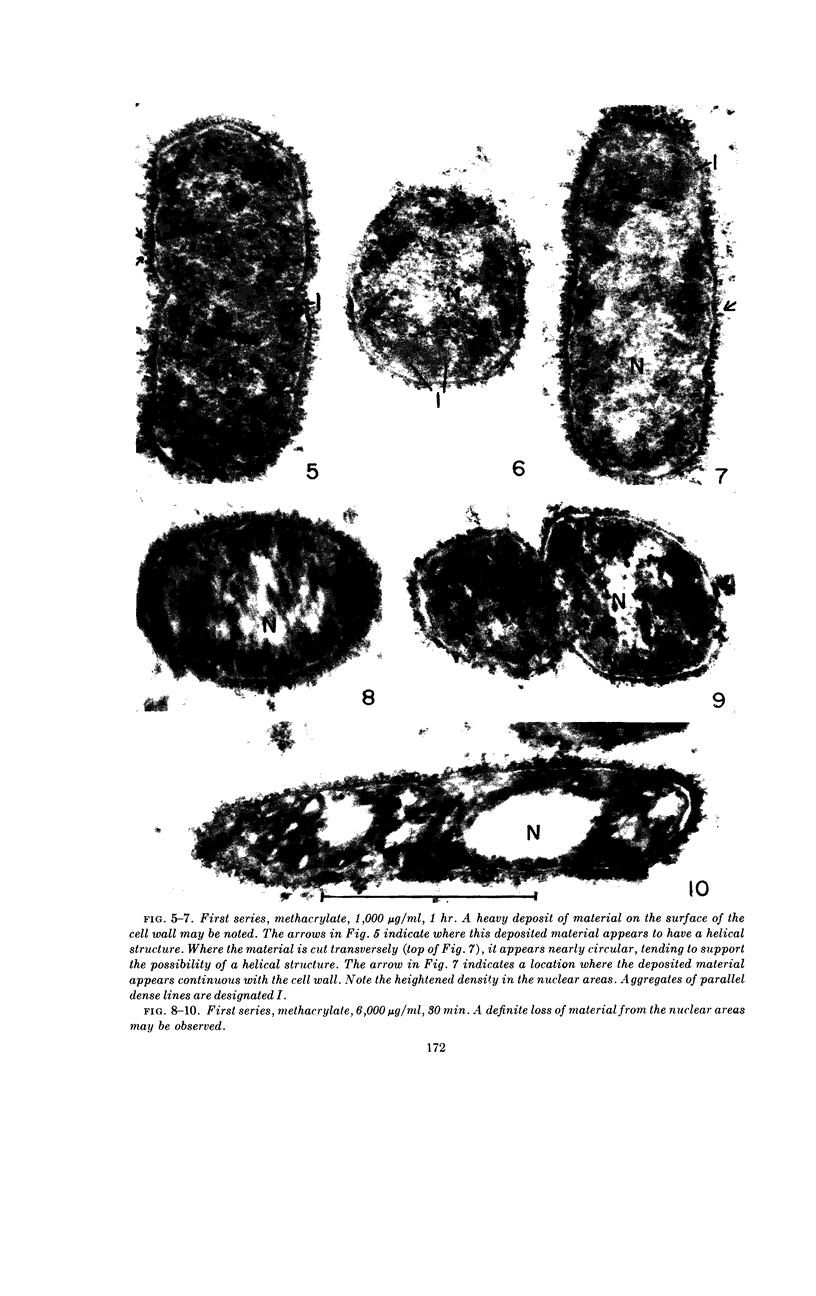
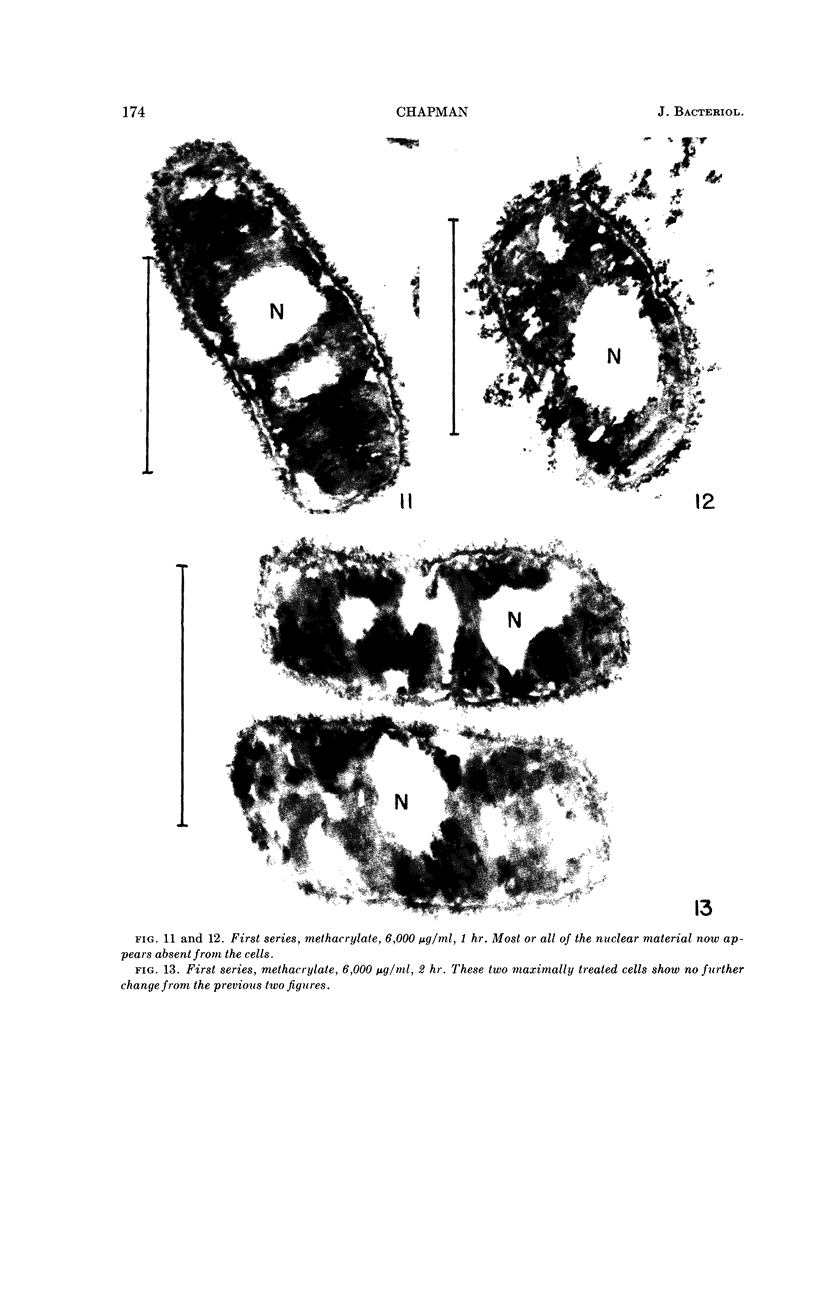
Abstract
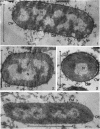
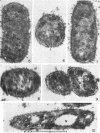
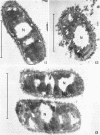
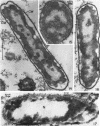
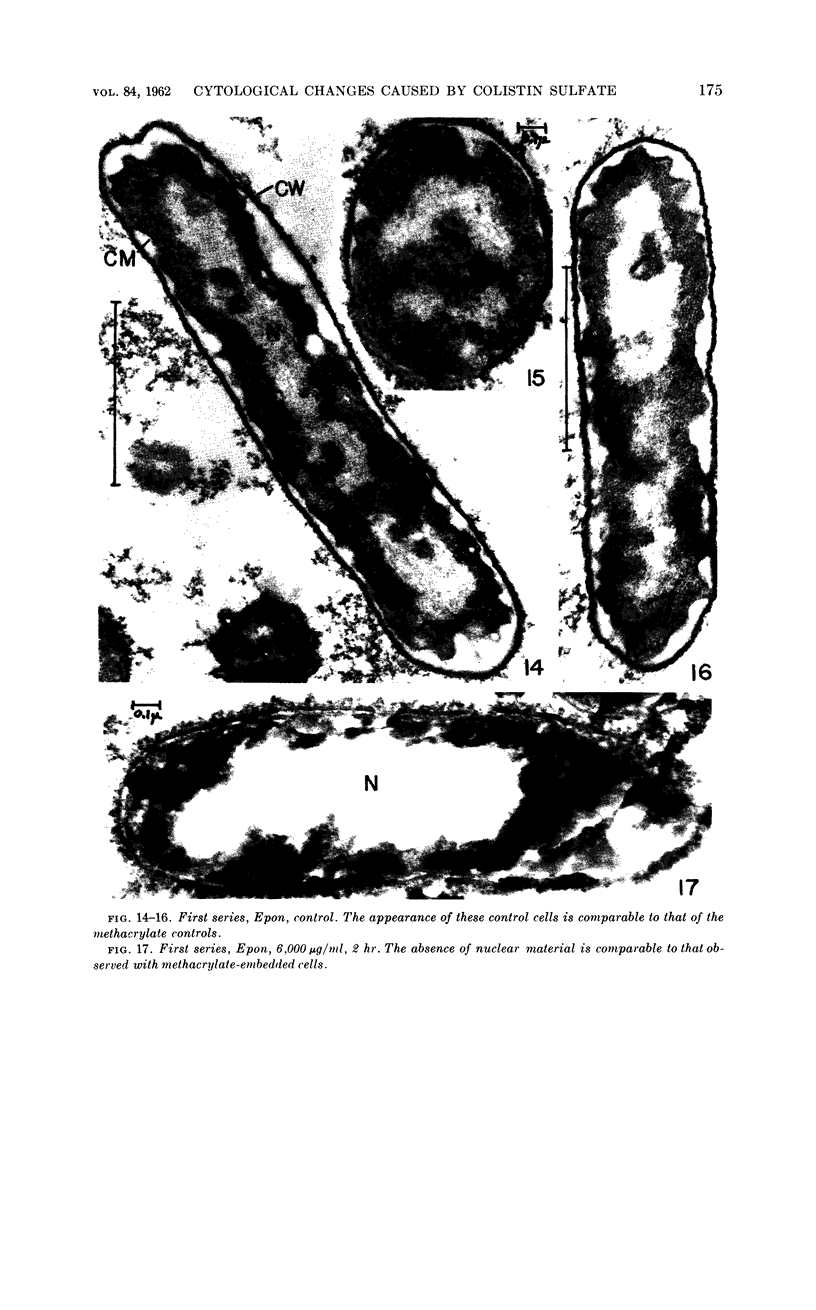
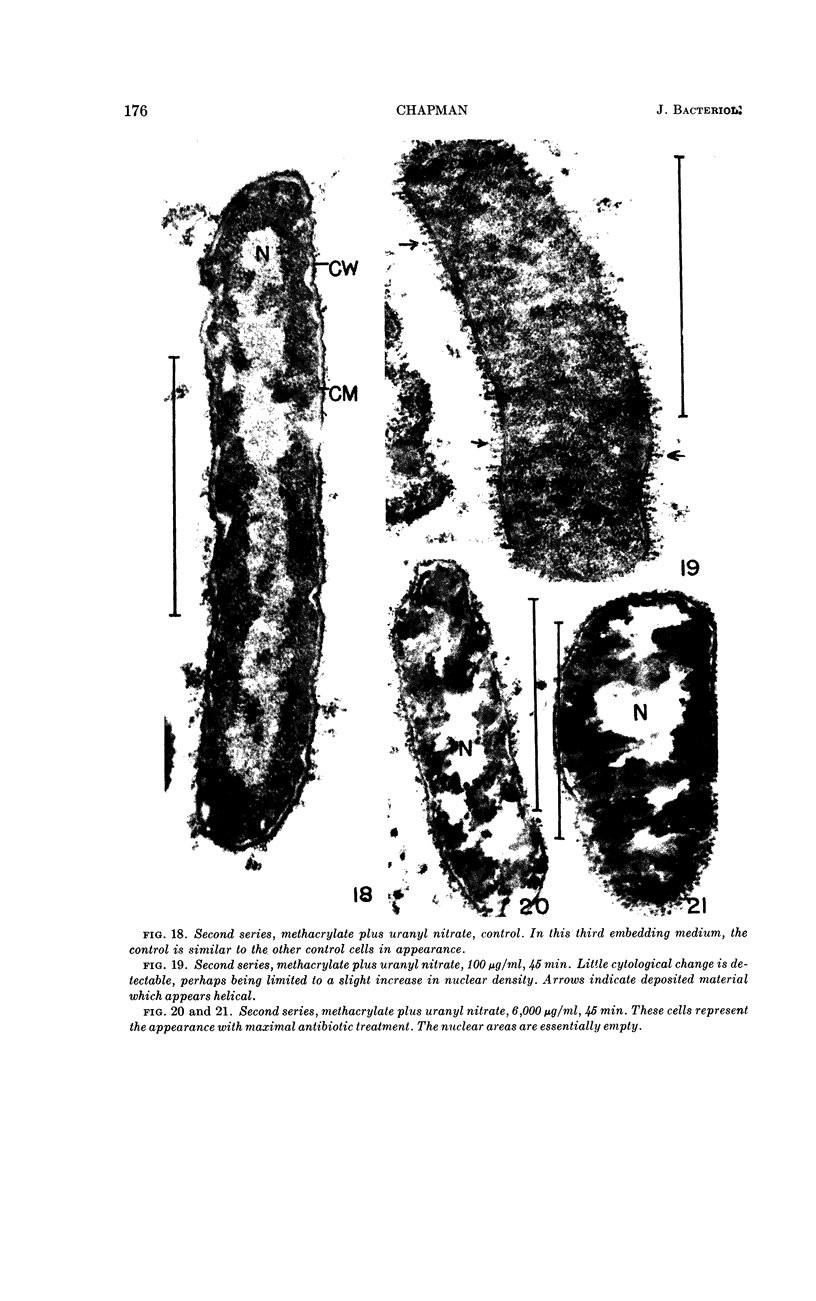
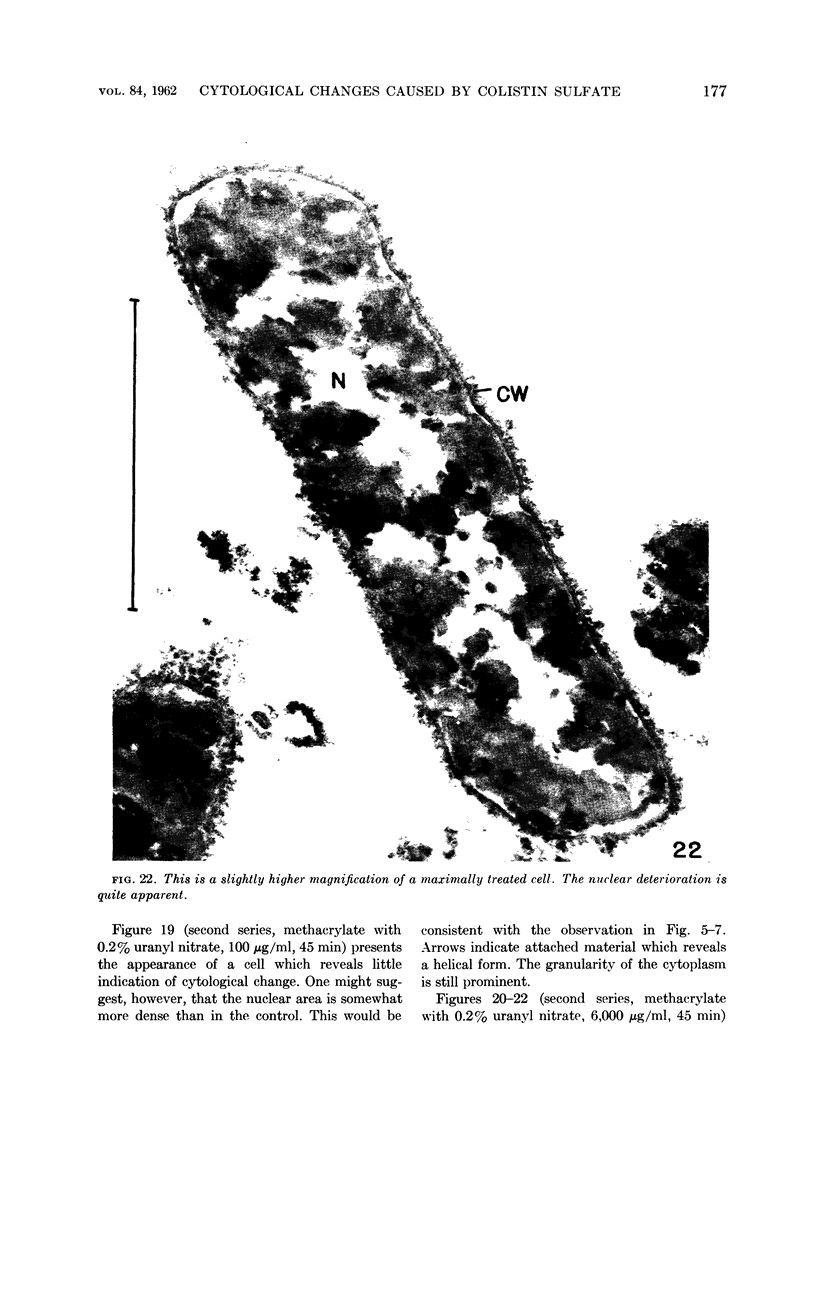
Chapman, George B. (Cornell University Medical College, New York, N.Y.). Cytological aspects of antimicrobial antibiosis. I. Cytological changes associated with the exposure of Escherichia coli to colistin sulfate. J. Bacteriol. 84:169–179. 1962—Broth cultures of Escherichia coli were exposed to different concentrations of the antibiotic colistin sulfate for various lengths of time. Control (untreated) and treated cells were fixed, dehydrated, and embedded in methacrylate or Epon. Ultrathin sections were examined in an RCA EMU2-D electron microscope. Two conspicuous cytological changes were noted. First, the nuclear material disappeared from its normal sites and was no longer demonstrable. Second, the cytoplasm lost its granularity and became homogeneous. Cells which showed these changes were nonviable.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHAPMAN G. B. Cytological aspects of antimicrobial antibiosis. II. Cytological changes associated with the exposure of Pseudomonas aeruginosa and Bacillus megaterium to colistin sulfate. J Bacteriol. 1962 Jul;84:180–185. doi: 10.1128/jb.84.1.180-185.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZ-JAMES P. C. Participation of the cytoplasmic membrane in the growth and spore fromation of bacilli. J Biophys Biochem Cytol. 1960 Oct;8:507–528. doi: 10.1083/jcb.8.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLAUERT A. M., BRIEGER E. M., ALLEN J. M. The fine structure of vegetative cells of Bacillus subtilis. Exp Cell Res. 1961 Jan;22:73–85. doi: 10.1016/0014-4827(61)90087-8. [DOI] [PubMed] [Google Scholar]
- GLAUERT A. M., HOPWOOD D. A. A membranous component of the cytoplasm in Streptomyces coelicolor. J Biophys Biochem Cytol. 1959 Dec;6:515–516. doi: 10.1083/jcb.6.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLAUERT A. M., HOPWOOD D. A. The fine structure of Streptomyces coelicolor. I. The cytoplasmic membrane system. J Biophys Biochem Cytol. 1960 Jun;7:479–488. doi: 10.1083/jcb.7.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOPWOOD D. A., GLAUERT A. M. The fine structure of Streptomyces coelicolor. II. The nuclear material. J Biophys Biochem Cytol. 1960 Sep;8:267–278. doi: 10.1083/jcb.8.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A. Contribution à l'étude du noyau bactérien. Schweiz Z Pathol Bakteriol. 1955;18(5):1122–1137. [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A. Fixation et inclusion du matériel nucléaire de Escherichia coli. Experientia. 1956 Nov 15;12(11):420–421. doi: 10.1007/BF02157362. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederberg J. BACTERIAL PROTOPLASTS INDUCED BY PENICILLIN. Proc Natl Acad Sci U S A. 1956 Sep;42(9):574–577. doi: 10.1073/pnas.42.9.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORE R. T., CHAPMAN G. B. Observations of the fine structure and modes of growth of a streptomycete. J Bacteriol. 1959 Dec;78:878–885. doi: 10.1128/jb.78.6.878-885.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUDD S., HILLIER J., BEUTNER E. H., HARTMAN P. E. Light and electron microscopic studies of Escherichia coli-coliphage interactions. II. The electron microscopic cytology of the E. coli B-T2 system. Biochim Biophys Acta. 1953 Jan;10(1):153–179. doi: 10.1016/0006-3002(53)90224-8. [DOI] [PubMed] [Google Scholar]
- PRIMOSIGH J., PELZER H., MAASS D., WEIDEL W. Chemical characterization of mucopeptides released from the E. coli B cell wall by enzymic action. Biochim Biophys Acta. 1961 Jan 1;46:68–80. doi: 10.1016/0006-3002(61)90647-3. [DOI] [PubMed] [Google Scholar]
- ROBINOW C. F. The chromatin bodies of bacteria. Bacteriol Rev. 1956 Dec;20(4):207–242. doi: 10.1128/br.20.4.207-242.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYTER A. [Electron microscopic study of the nuclear transformations 05 E. coli K12S and K12S (lambda 26) after irradiation with ultraviolet rays and x-rays]. J Biophys Biochem Cytol. 1960 Oct;8:399–412. [PMC free article] [PubMed] [Google Scholar]
- SCHWARTZ B. S., WARREN M. R., BARKLEY F. A., LANDIS L. Microbiological and pharmacological studies of colistin sulfate and sodium colistinmethanesulfonate. Antibiot Annu. 1959;7:41–60. [PubMed] [Google Scholar]
- VAN ITERSON W. Some features of a remarkable organelle in Bacillus subtilis. J Biophys Biochem Cytol. 1961 Jan;9:183–192. doi: 10.1083/jcb.9.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARBASSE W. W., JOHNSON F. H. The influence of penicillin on large body production by luminous bacteria. J Bacteriol. 1950 Sep;60(3):279–282. doi: 10.1128/jb.60.3.279-282.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. J Biophys Biochem Cytol. 1958 Jul 25;4(4):475–478. doi: 10.1083/jcb.4.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIBULL C. Bacterial protoplasts. Annu Rev Microbiol. 1958;12:1–26. doi: 10.1146/annurev.mi.12.100158.000245. [DOI] [PubMed] [Google Scholar]