Abstract
Regeneration of muscle fibers, lost during pathological muscle degeneration or after injuries, is mediated by the production of new myofibres. This process, sustained by the resident stem cells of the muscle, the satellite cells, is finely regulated by local cues, in particular by cytokines and growth factors. Evidence in the literature suggests that nerve growth factor (NGF) is involved in muscle fiber regeneration; however, its role and mechanism of action were unclear. We have investigated this issue in in vivo mouse models of muscle regeneration and in primary myogenic cells. Our results demonstrate that NGF acts through its low-affinity receptor p75NTR in a developmentally regulated signaling pathway necessary to myogenic differentiation and muscle repair in vivo. We also demonstrate that this action of NGF is mediated by the down-regulation of RhoA-GTP signaling in myogenic cells.
INTRODUCTION
Muscle growth and regeneration after damage recapitulates to some extent embryonic myogenesis and is mediated by the satellite cells, resident myogenic stem cells situated within a niche between the cell membrane and the basal lamina of muscle fibers (Tajbakhsh, 2003); muscle injury activates satellite cells, which start proliferating, and after several rounds of proliferation most of them differentiate and fuse to form new fibers or to repair the damaged ones; a minority of satellite cells however return to quiescence to maintain a pool of progenitors (Schmalbruch and Lewis, 2000). Many positive and negative signals responsible for the regulation of myogenesis have been identified, which include transcription factors such as MyoD and related family members, MEF2 and Twist, as well as extracellular agonists, such as members of the insulin-like growth factor (IGF) and transforming growth factor (TGF)-β families, and fibroblast and hepatocyte growth factors (FGFs and HGF; Balemans and Van Hul, 2002; Parker et al., 2003).
Although the role of many of these signaling molecules has been clarified, still unclear is the precise contribution to myogenesis of nerve growth factor (NGF), despite many studies having suggested a crucial role for the neurotrophic factor in this process.
The expression of NGF has been studied both in human and animal models of myogenesis: NGF is expressed in developing muscle (Ernfors et al., 1991; Wheeler and Bothwell, 1992; Ip et al., 2001) and is down-regulated after birth (Ernfors et al., 1991; Ip et al., 2001). It appears to be reexpressed in adult muscle in the course of pathological conditions such as muscular dystrophy (Toti et al., 2003) and amyotrophic lateral sclerosis (Kust et al., 2002): de novo expression of NGF occurs in regenerating fibers and connective tissue myofibroblasts of the damaged muscles (Toti et al., 2003; Chevrel et al., 2006), suggesting that it may have a proregenerative role (Menetrey et al., 2000). Indeed, mice with functional knockdown of NGF signaling display signs of muscle dystrophy (Ruberti et al., 2000). Interestingly, the de novo expression of NGF correlates with the reexpression of the low-affinity receptor for neurotrophins p75NTR (Baron et al., 1994). In addition, p75NTR is expressed in Pax7+ muscle satellite cells (Mousavi and Jasmin, 2006) and human primary myoblasts and myotubes (Baron et al., 1994), and its expression appears to be mediated by MyoD, a muscle-specific bHLH transcription factor, which is able to activate the p75NTR gene promoter (Erck et al., 1998). This receptor binds dimeric NGF and in the nervous system is implicated in key processes such as cell death, survival, and axonal elongation (Bibel and Barde, 2000; Hempstead, 2002; Roux and Barker, 2002; Schor, 2005). Taken together these observations and the fact that the expression of both p75NTR and NGF is developmentally coordinated in muscle raises the possibility that the NGF to p75NTR signaling plays a role also in myogenesis (Rende et al., 2000; Reddypalli et al., 2005). What is the precise function of NGF/p75NTR signaling pathway in myogenesis and muscle repair and the definition of the cellular and molecular mechanisms activated by this pathway and involved in myogenesis, however, is still lacking.
Some studies were indeed performed but only in skeletal muscle-derived cell line models, the C2C12 and L6, which mimic to a limited extent the physiology and behavior of primary myogenic cells in vivo. In addition, these studies yielded inconclusive results that variously suggest a role of NGF and p75NTR in proliferation and fusion and opposite effects on differentiation, without identifying the mechanisms of NGF action (Erck et al., 1998; Seidl et al., 1998; Rende et al., 2000; Chevrel et al., 2006).
We have now investigated the role of the NGF/p75NTR pathway in myogenesis in vivo and dissected its biology in vitro using satellite cell–derived myogenic precursors that are a physiological model faithfully recapitulating the myogenic program in vitro. Our results show that a NGF/p75NTR-activated signaling pathway is necessary in vivo to myogenic differentiation and muscle repair, through specific actions on the cytoskeletal architecture and fusogenic properties of myotubes. We also demonstrate that this pathway operates through RhoA inhibition and is tightly regulated via a controlled expression of NGF and p75NTR during myogenesis.
MATERIALS AND METHODS
The following reagents were purchased as indicated: anti-p75 pAb from Chemicon International (Temecula, CA); anti-sarcomeric myosin MF20 mAb, anti-myogenin mAb from Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA); anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mAb from Biogenesis (Poole, England); anti-proliferating cell nuclear antigen (PCNA) mAb from Santa Cruz Biotechnology (Santa Cruz, CA); anti-α-sarcomeric actinin mAb from Sigma (Saint Louis, MO); anti-paxillin mAb from BD Biosciences (San Jose, CA); anti-focal adhesion kinase (Fak) pAb from Santa Cruz Biotechnology; anti-developmental myosin heavy chain (NCL-MyHCd) from Novocastra (Newcastle upon Tyne, United Kingdom); anti-laminin from Sigma; anti-GFP was from Invitrogen (Carlsbad, CA); anti-RhoA mAb from Santa Cruz Biotechnology. The coating substrates were the following: low factor matrigel and fibronectin were from BD Biosciences (San Jose, CA), laminin was from Invitrogen, type I collagen was from Sigma. Reagents for RNA extraction and RT-PCR were from Promega (Madison, WI); iQ SYBR green supermix for the real-time PCR was from Bio-Rad (Hercules, CA). In immunofluorescence analysis primary Abs were detected by appropriate Alexa-conjugated secondary Abs (Molecular Probes, Invitrogen, Eugene, OR). In immunoblot analysis primary Abs were detected by chemiluminescence with appropriate horseradish peroxidase–conjugated secondary Abs, all purchased from Bio-Rad. Cell culture media and sera were from Cambrex (Walkersville, MD). The transfection reagents LTX and PLUS were from Invitrogen. The plasmid pEGFP-RHOV14 was a kind gift from Dr. Germana Falcone (Italian National Research Council, Naples, Italy). The NGF was from Alomone Labs (Jerusalem, Israel). The sequence of cyclic decapeptide antagonist of NGF binding to p75NTR was previously described (Botchkarev et al., 2003); for animal injections, cyclic decapeptides were modified by N-terminal conjugation to an undecapeptide (11-mer) viral TAT4 permeabilization sequence, which facilitates tissue penetration, and TAT4 alone was synthesized as the control peptide (Turner et al., 2004): all these peptides were synthesized by R.L.. The Hoechst dye cardiotoxin (CTX) and l-α-lysophosphatidic acid (LPA) were from Sigma; cell-permeable C3 transferase (recombinant protein) was from Cytoskeleton (Denver, CO).
Satellite Cell–derived Myogenic Precursors Culture, Infection, and Transfection
Satellite cell–derived myogenic precursors were isolated from newborn wild-type (wt) mice of the CD1 strain as previously described (Cossu et al., 1980) and plated on a coating of low factor matrigel. Cells were grown in proliferation medium (DMEM supplemented with 20% fetal bovine serum, 3% chick embryo extract, 100 U/ml penicillin, 100 μg/ml streptomycin, and 50 μg/ml gentamicin), and after 3 d of culture they were switched to the differentiating medium (DMEM supplemented with 2% horse serum, 100 U/ml penicillin, and 100 μg/ml streptomycin).
Satellite cell–derived myogenic precursors were treated with NGF (50 ng/ml), the blocking antibody anti-p75 pAb (1:100) and the blocking peptide (50 mM), either constantly in proliferation medium or for 12 h in differentiating medium for the postfusion experiments.
Satellite cell–derived myogenic precursors to be injected into mice (see Regeneration Assay below) were plated at high density and infected with the lentiviral vector pRRLCMVLacZ (Galvez et al., 2008; MOI = 10) 24 h after isolation; the day after they were transfected with pRHOV14 plasmids or the empty vector (Vartiainen et al., 2000; Castellani et al., 2006) by using LTX and PLUS reagents; after 8 h they were injected in the crushed tibialis anterior muscle (TA) and part of them were induced to differentiate in vitro in order to test the effects of RHOV14 protein on myoblasts fusion after 12 h of differentiation.
Quantification of NGF Protein by ELISA
NGF was quantified in the supernatant of satellite cell–derived myogenic precursors, both during proliferation and at different time points of differentiation. A commercially available NGF-specific, highly-sensitive, two-site ELISA kit was used following the manufacturer's instructions (Promega). The optical density was measured in duplicate at 450 nm. The detection range was 7.8–500 pg/ml.
RT-PCR and Real-Time PCR
RNA (1 μg) was collected from primary satellite cell–derived myogenic precursors using an SV total RNA isolation system (Promega) and subsequently converted into double-stranded cDNA using the cDNA synthesis kit ImProm II Reverse Transcription System (Promega), according to the manufacturer's instructions. The primers used for NGF, p75NTR TrkA, and Mlc1F amplification were previously described (Syroid et al., 2000; Deponti et al., 2007). The primers used for the amplification of p75NTR and the ribosomal subunit 36BA in real-time PCR were the following: P75NTR Fw: 5′-cattgtggagagcctgtg-3′ and Rev 5′-ctcggttctgactgttgg-3′; and 36BA Fw: 5′-aggatatgggattcggtctcttc-3′ and Rev: 5′-tcatcctgcttaagtgaacaaact-3′.
Immunofluorescence
Immunofluorescence on the various cell cultures was performed using antibodies specific for sarcomeric myosin MF20, green fluorescent protein (GFP), paxillin, Fak, and α-actinin as described (Pisconti et al., 2006).
The fusion index of differentiating satellite cell–derived myogenic precursors was measured as the number of nuclei in sarcomeric myosin-expressing cells with more than two nuclei versus the total number of nuclei (Pisconti et al., 2006).
Protein Extraction and Immunoblot Analysis
Satellite cell–derived myogenic precursors were homogenized in a buffer containing 50 mM Tris-HCl, 150 mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate, 1% NP40, pH 7.4, supplemented with protease inhibitor cocktail (Roche Diagnostics, Alameda, CA) and centrifuged at 14,000 rpm at 4°C for 10 min to discard cellular debris. Protein concentration in the extracts was determined using BCA protein assay from Perbio (Bezons, France). Sample preparation and Western blot analyses were carried out as previously described (Pisconti et al., 2006). After electrophoresis polypeptides were electrophoretically transferred to nitrocellulose filters (Schleicher & Schuell, Dassel, Germany) that were blocked in 5% fat-free milk for 1 h at room temperature and then incubated with the respective primary antibody for 2 h at room temperature.
RhoA-GTP Pulldown Assay
Satellite cell–derived myogenic precursors were switched into the differentiating medium, and after 24 h of differentiation the medium was removed and replaced with fresh serum-free medium in order to eliminate endogenous NGF; myotubes were treated with NGF (50 ng/ml), the competing peptide (50 mM), and the blocking antibody (1:200) in three separate experiments: cells were collected at time 0 and after 10 and 20 min of treatment and processed for RhoA-GTP pulldown, carried out using the Rhotekin binding domain affinity precipitation assay according to the manufacturer's protocol (Cytoskeleton). The reversion of RhoA activation induced by NGF antagonist was performed by pretreating myotubes with the cell-permeable form of C3 transferase (1.5 μg/ml for 4 h in serum-free medium; myotubes were also treated with LPA (1 μg/ml) for 10 min as positive control for RhoA activation.
Quantification of Rho-GTP levels was performed also in the regenerating TA according to the same protocol.
Regeneration Assay
To evaluate the ability of the satellite cell–derived myogenic precursors to participate to the regeneration process in vivo, injury was performed on the TA of 3-mo-old CD1 mice by injecting 25 μl of 10 μM of CTX. Two types of experiments were carried out: in the first, mice were injected in the damaged TA with 1 mg/kg the tissue-permeable form of the p75NTR-competing peptide p75NTRTAT4 in one leg and the TAT4 control peptide in the other leg. Injections were performed every day for 16 d after CTX injection. Mice (n = 4) were killed at 6, 10, and 16 d after the CTX injection: histological analysis was performed at all time points, whereas Rho-GTP levels were measured only at day 6. In the second type of experiments mice were injected in the TA with β-galactosidase (β-Gal)-overexpressing satellite cell–derived myogenic precursors prepared as described above: the injection was performed 48 h after CTX treatment, and muscles were collected after 3 additional days and stained for β-Gal activity (n = 6).
Histology and Immunostaining
TA were dissected from CTX-injected mice and frozen in liquid N2-cooled isopentane. Serial muscle sections (8 μm) were stained for Azan Mallory and in hematoxylin-eosin (H&E) as previously described (Sciorati et al., 2006).
Sections were also stained for laminin and embryonal myosin for the regeneration experiments, whereas staining for β-Gal activity was performed on sections of TA injected with β-Gal–overexpressing satellite cell–derived myogenic precursors, according to a previously described protocol (Pisconti et al., 2006).
RESULTS
Primary Satellite Cell–derived Myogenic Precursors Up-Regulate Expression of NGF and p75NTR during Their Differentiation into Myotubes
We investigated p75NTR, TrkA, and NGF expression, as well as NGF secretion in primary myogenic precursors isolated from newborn mice and induced to differentiate on a matrigel coating. The extent of myogenic differentiation was assessed by measuring the expression of myogenin and myosin light and heavy chains (Figure 1, A and B).
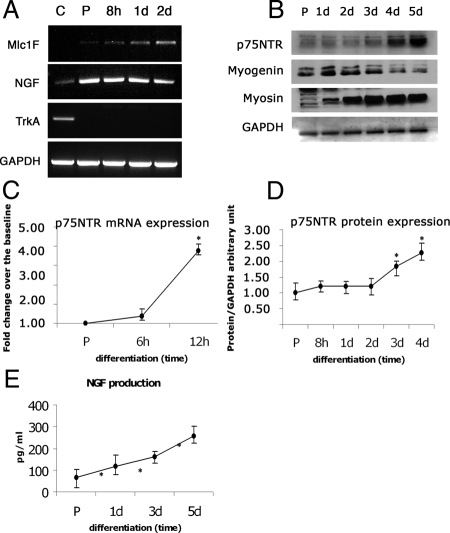
Figure 1.
The expression of NGF and p75NTR is regulated during primary satellite cell–derived myogenic precursors proliferation and differentiation. (A) RNA was collected from satellite cell–derived myogenic precursors during proliferation and at different stages of differentiation (c = PC12 cells, p75-positive control; p = proliferating; 8 h, 1 d, 2 d = hours or days of differentiation); RT-PCR was performed with specific primers for skeletal muscle specific Myosin light chain (Mlc1F), NGF, TrkA, and GAPDH. (B) Primary satellite cell–derived myogenic precursors were collected and lysed at different time points, both in proliferating and differentiating condition; the expression of p75NTR, sarcomeric myosin (myosin), myogenin, and GAPDH were evaluated by Western blot using specific antibodies. (C) Quantitative analysis of p75NTR mRNA levels during the first phases of differentiation. (D) Quantitative analysis of p75NTR protein levels during early and late differentiation. (E) Conditioned medium from proliferating and differentiating satellite cell–derived myogenic precursors was collected at different time points (P = proliferating; 1 d, 3 d, and 5 d = days of differentiation), and the NGF produced and released by the cells was quantified by ELISA: increasing amounts of NGF were found in the medium from a minimum of 60 ± 25 pg/ml in proliferating condition to a maximum of 250 ± 35 pg/ml at the latest stage of differentiation (5 d). Error bars, SEM. *p < 0.05 versus proliferating satellite cell–derived myogenic precursors; n = 4.
Proliferating satellite cell–derived myogenic precursors expressed p75NTR (both mRNA and protein), and this expression increased during differentiation (Figure 1, B–D). In parallel, we observed high levels of NGF transcripts throughout differentiation (Figure 1A) and increased NGF secretion by primary satellite cell–derived myogenic precursors during differentiation (from 60 to 250 pg/ml; Figure 1E).
These results indicate that both NGF and p75NTR are expressed by primary myoblasts and that their expression increases during differentiation. By contrast, TrkA was found to be expressed neither by proliferating myoblasts nor by differentiating myotubes at any stage (Figure 1A).
NGF Regulates Myoblasts Fusion and Myotube Cytoskeletal Organization
To investigate the role of NGF/p75NTR signaling in myogenic differentiation, satellite cell–derived myogenic precursors were differentiated in the absence or presence of NGF (50 ng/ml), a peptide that competes with NGF binding to receptors, thus inhibiting its activity (peptide; 50 mM; Botchkarev et al., 2003; Turner et al., 2004) or of a p75NTR-blocking Ab (anti-p75; 1:100; Pehar et al., 2004). After 12 h the expression of the myogenic markers sarcomeric myosin (MF20) and myogenin were assessed, and the myogenic fusion index was calculated (Figure 2, A–C).
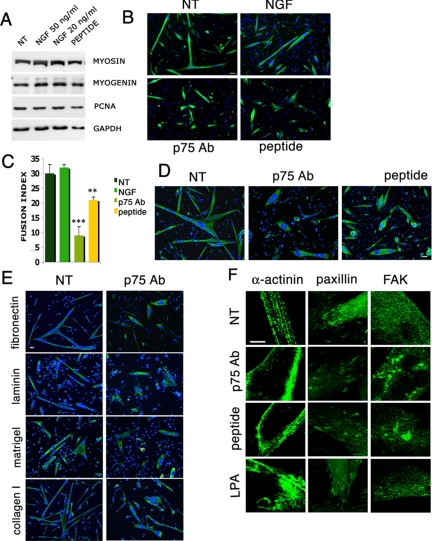
Figure 2.
NGF signaling is required for satellite cell–derived myogenic precursors fusion but not for differentiation. (A) Satellite cell-derived myogenic precursors were grown on matrigel and treated with NGF, 20 and 50 ng/ml, and with the blocking peptide, 50 mM (peptide); after 3 d in proliferation medium they were switched into the differentiating medium for 12 h and then lysed in order to test the expression of proteins involved in proliferation (PCNA) or differentiation (myogenin and sarcomeric myosin) of myoblasts, by Western blot. (B) Satellite cell–derived myogenic precursors were grown on matrigel and constantly treated with NGF, p75NTR-blocking antibody 1:100 (p75 Ab), and with the blocking peptide 50 mM (peptide); after 3 d in proliferation medium they were switched to the differentiating medium for 12 h, and then they were fixed and immunostained for sarcomeric myosin (MF 20). Scale bar, 100 μm. (C) The fusion index of the experiments in B was calculated as the number of nuclei in myotubes with more than two nuclei per myotube derived from myoblasts treated with the blocking antibody, the blocking peptide, or NGF. (D) Primary satellite cell–derived myogenic precursors were allowed to fuse for 12 h in differentiating medium, and then they were treated with the blocking antibody (p75 Ab) and the blocking peptide (peptide) for the next 12 h; they were fixed and immunostained for sarcomeric myosin (MF 20). Scale bar, 100 μm. (E) Primary satellite cell–derived myogenic precursors were grown on different extracellular matrix components: fibronectin, laminin, collagen I, and matrigel; they were allowed to fuse for 12 h in differentiating medium, and then they were treated or not with the blocking antibody. Scale bar, 100 μm. (F) Primary satellite cell–derived myogenic precursors were allowed to fuse for 12 h in differentiating medium and then treated with the blocking antibody, the blocking peptide, and LPA for the next 12 h; myotubes were fixed and immunostained for α-actinin, paxillin, and Fak. The pictures are magnifications of the myotubes edges represented in Supplementary Figures S2–S4. Scale bar, 50 μm. Error bars, SEM.**p < 0.05 and ***p < 0.001, respectively, versus untreated control; n = 4.
Exposure to NGF, its blocking peptide, or the p75NTR-blocking Ab did not change significantly the expression of the myogenic markers investigated, as revealed by both immunofluorescence and Western blotting analyses (Figure 2, A and B). By contrast, inhibition of NGF/p75NTR signaling with either the peptide or the antibody significantly reduced the fusion index (Figure 2, B and C). Exogenously added NGF did not significantly increase fusion, suggesting that the effects mediated by the endogenous NGF secreted during differentiation (Figure 1, A and E) was already maximal. These results indicate that NGF signaling controls a specific event of myogenesis, i.e., fusion of myogenic cells during myoblasts differentiation.
Because NGF and p75NTR are strongly expressed in the late phases of myogenic differentiation, we investigated whether their signaling is required also for postfusion events. Primary satellite cell–derived myogenic precursors were allowed to differentiate into myotubes on matrigel coating for 12 h and then were treated with NGF, its competing peptide, or the p75NTR-blocking Ab. Both blockers of NGF signaling induced significant changes in the morphology of the myotubes, which became rounded in shape with centrally located nuclei (Figure 2D), suggesting a role for NGF as a regulator of the adhesion properties of the myotube and/or its cytoskeletal architecture.
We investigated this possibility in detail by analyzing adhesion of myotubes on different coating substrates, namely laminin, collagen I, and fibronectin. The change in morphology after NGF signaling blockade was evident on all coating substrates (Figure 2E), suggesting that such an effect is not restricted to specific adhesion molecules.
We next examined whether blockade of the NGF/p75NTR signaling affected cytoskeletal organization, by assessing the intramyotube distribution of α-actinin, paxillin, and Fak: paxillin and Fak are components of focal adhesion sites and contribute to the formation of costameres (Quach and Rando, 2006), whereas α-actinin is a component of myofibrils and its distribution is representative of sarcomeric organization. In myotubes treated with the blocking antibody and peptide the distribution of α-actinin was affected, and the sarcomeres appeared less organized, whereas paxillin and Fak accumulated in clusters, particularly at the terminal extremities of the myotubes (Figure 2F, Supplemental Figures S2–S4).
A significant role in cytoskeletal organization, in particular that of focal adhesions, is played by small RhoGTPases (,Ridley and Hall 1992). We found that the effects of NGF/p75NR blockade on cytoskeletal organization were similar to those observed with LPA, a known trigger of RhoGTPase activity (Moolenaar, 1995; Figure 2F; Supplemental Figures S2–S4). Among RhoGTPases, RhoA plays prominent roles in regulating actin organization and stress fiber formation in fibroblasts (Ridley and Hall, 1992) and smooth muscle cells (Worth et al., 2004; Pacaud et al., 2005); furthermore, it regulates myoblast fusion by modulating M-cadherin expression (Charrasse et al., 2006; Fortier et al., 2008) and may affect myotube morphology (Castellani et al., 2006).
RhoA Mediates NGF/p75NTR Signaling during Muscle Differentiation
We investigated whether NGF/p75NTR acted through RhoA. Myotubes were treated with NGF (50 ng/ml), the blocking antibody (1:200), or the NGF-competing peptide (50 mM) for 10 and 20 min, and RhoA activation was assessed by measuring by the levels of active RhoA-GTP in pulldown experiments. NGF decreased RhoA-GTP levels, whereas the blocking antibody and the competing peptide increased them (Figure 3, A and B). These increases were reversed by the treatment with the RhoA inhibitor exoenzyme C3 (Figure 3, C and D; Aktories et al., 2000). The treatment with exoenzyme C3 also reversed the reduction in the fusion index (Supplemental Figure 5, A and B) and in the changes in the shape of myotubes (Supplemental Figure 5C) observed when NGF/p75NTR signaling was inhibited.
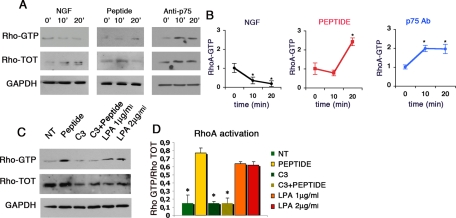
Figure 3.
The binding of NGF to p75NTR receptor modulates RhoA activation in myotubes. (A and B) Primary satellite cell–derived myogenic precursors were grown on matrigel for 3 d in proliferation medium; after 24 h in differentiation medium myotubes were starved in serum-free medium for 3 h and then treated for 10 and 20 min with NGF, the blocking antibody, and the competing peptide. (A) Pulldown assays for RhoA-GTP were performed on myoblasts lysates. (B) Quantification of RhoA-GTP levels normalized on total Rho and they are expressed as fold increase versus basal RhoA-GTP (time 0). (C and D) Primary satellite cell–derived myogenic precursors were grown on matrigel for 3 d in proliferation medium; after 24 h in differentiation medium myotubes were starved in serum-free medium for 3 h and then treated for 10 and 20 min with NGF and the blocking peptide, which followed a pretreatment of myotubes with exoenzyme C3, a RhoA inhibitor. (C) Pulldown experiments for RhoA-GTP were performed on myoblasts lysates (NT, not treated). (D) Quantification of the levels of RhoA-GTP levels normalized on the total amount of RhoA. Values of RhoA activation obtained using LPA in the same experimental conditions are used as comparison. Error bar, SEM. *p <0.05 versus time 0 (B) or untreated myoblasts (D); n = 4.
Taken together, these results indicate that the binding of NGF to p75NTR inhibits RhoA-GTP levels and that this inhibition mediates the effects of the growth factor on myoblast fusion and myotube structural organization.
P75NTR to RhoA Signaling Sustains Skeletal Muscle Regeneration In Vivo
We investigated whether the effects of NGF/p75NTR signaling observed in vitro had a biological role in vivo.
We investigated the de novo myogenesis that occurs during skeletal muscle regeneration; to stimulate regeneration, CTX (10 μM) was injected in the TA, thus inducing reversible muscle damage; a tissue-permeable form of the NGF-competing peptide (p75NTRTAT4) or the control peptide TAT4 were injected in the right and left TA, respectively, each day starting 48 h after damage (n = 4). Mice were killed 6, 10, and 16 d after CTX injection, the TA were collected, and regeneration was assessed by staining with H&E, Azan-Mallory, and after immunostaining for embryonic MHC (hallmark of early regeneration) and laminin. Six days after CTX injection regenerating centro-nucleated fibers are present in the control, whereas no regeneration is apparent in the muscles treated with the blocking peptide (Figure 4A); at 10 d regeneration appeared also in the p75NTRTAT4-treated animals, however, it was significantly less than in controls (Figure 4, A and B) with a persistence of embryonic MHC (Figure 4C). After 16 d the muscle fiber organization was completely restored in the control muscle, whereas in the p75NTRTAT4-treated ones early regenerating fibers could still be detected (Figure 4A). Moreover, increased levels of RhoA-GTP were found in p75NTRTAT4-treated TA, 6 d after CTX damage (Figure 4D): these results suggest that the binding of NGF to p75NTR modulate muscle regeneration by regulating RhoA activity in vivo as well as in vitro.
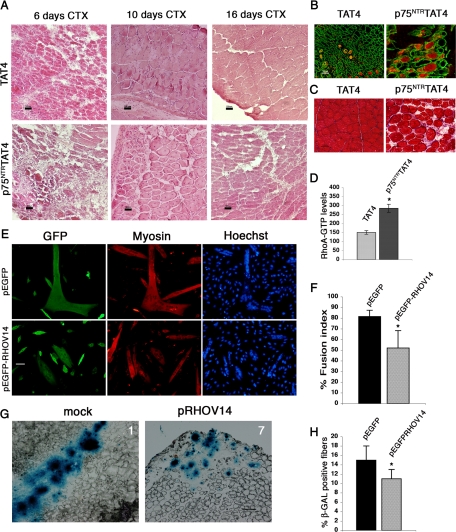
Figure 4.
NGF signaling is necessary for efficient skeletal muscle regeneration. (A) TA muscle of CD1 mice were injured by injecting 50 μl of CTX (10 μM). After 48 h they were treated (by intramuscular injection) with the permeable form of p75NTR-blocking peptide (i.e., conjugated to the TAT4) sequence or with the TAT4 peptide as control. At each time point (6, 10, and 16 d after CTX injection, n = 4) the TA were collected, and sections were stained with H&E. Scale bar, 100 μm. (B and C) Section of TA muscle collected 10 d after CTX injection were stained for embryonic myosin (red) and laminin (green) (B) and for Azan-Mallory (C). (D) A pulldown assay was performed on lysates from total TA after 6 d from CTX injection, and RhoA-GTP levels were measured and normalized on total RhoA. (E–H) Satellite cells derived-myoblasts were infected with β-gal–overexpressing lentivirus and then transfected with pEGFP-RHOV14 plasmid or the empty vector (mock). (E) Transfected myoblasts were induced to differentiate in vitro and immunostained for sarcomeric myosin (myosin) and GFP, and the fusion index was calculated as number of nuclei in myotubes with more than two nuclei per myotube (F). (G) Transfected myoblasts were injected in TA of CD1 mice (n = 6) 48 h after the CTX injection. Muscles were collected after 3 d and stained with X-Gal in order to show the fusion efficiency of the injected cells, and the panels show representative images of muscle sections of animals 1 (mock transfected) and 7 (pEGFP-RHOV14 transfected). (H) The fusion extent of the different cell populations was measured by calculating the ratio between X-Gal–positive areas and the overall section area; the results are expressed as percentage. Error bars, SEM. *p < 0.05 versus control muscles; n = 6.
We investigated the cell-autonomous role of RhoA in the proregenerative action of NGF. Primary satellite cell–derived myogenic precursors, infected with a lentiviral vector expressing β-Gal, were transfected with pRHOV14, a constitutive active isoform of RhoA (Castellani et al., 2006), or its control vector. We tested the myogenic potential and the fusion abilities of these myoblasts in vitro by inducing differentiation and measuring the fusion index: the expression of RHOV14 decreased the fusion index (Figure 4, E and F) without affecting proliferation (data not shown) indicating a cell-autonomous effect of RhoA. Cells were then injected in the TA of host mice 48 h after CTX treatment, and muscles were collected after 3 additional days. Satellite cell–derived myogenic precursors expressing the control vector fused to the host muscle fibers with a significantly enhanced efficiency compared with myogenic precursors overexpressing constitutively active RhoA (Figure 4, G and H, and Supplemental Figure S6). These results indicate that constitutive RhoA activation inhibits muscle regeneration in vivo by acting on myoblasts fusion and that NGF signaling contributes to muscle regeneration in vivo by modulating this small GTPase.
DISCUSSION
In this study we addressed a long-standing but still open question in the biology of skeletal muscle, that is, the role played by NGF in regulating muscle differentiation and repair. Such a role is suggested by strong morphological evidence of NGF and p75NTR expression during developmental myogenesis and their reexpression in adult regenerating muscle fibers of patients with muscular dystrophy and inclusion-body myositis (Baron et al., 1994; Toti et al., 2003; Chevrel et al., 2006; Lavasani et al., 2006). Morphological observations, however, were not supported so far by conclusive functional studies, because the three studies addressing this issue (Erck et al., 1998; Rende et al., 1999, 2000) yielded inconclusive and conflicting results and did not define the molecular correlates to the phenomenological observations. Moreover, they were carried out using myoblast-derived cell lines that mimic only in part the actual behavior of myogenic cells in vivo. In addition, two of these studies used L6-derived myoblasts, where dissection of NGF-dependent effects was hampered by coexpression of both the high- and low-affinity neurotrophin receptors (Rende et al., 1999, 2000). We decided to use a completely different approach in which we used primary mouse satellite cell–derived myogenic precursors and confirmed the biological relevance of the evidence obtained in vitro using an in vivo model of regeneration. Such a strategy allowed us to unambiguously demonstrate that NGF plays a specific role in increasing the ability of myogenic precursors to repair damaged muscle, by regulating their cytoskeletal architecture and enhancing their fusogenic potential. Furthermore, the fact that mouse myogenic precursor cells do not express the high-affinity trk receptors allowed for a clear-cut definition of the role and mechanism of action of p75NTR in mediating NGF muscular actions. Thus we established that these actions of NGF are mediated through a p75NTR-dependent inhibition of RhoA signaling in vivo.
These observations shed light on several important aspects of NGF function in skeletal muscle. The first is that the NGF/p75NTR-signaling pathway appears to be regulated in a coordinated way during differentiation, because myogenic precursors were found to express increasing amounts of both NGF and p75NTR during the process: interestingly, the NGF generated was released in the medium to act in an autocrine way on its receptor to stimulate fusion. The effect of NGF was selective because it did not affect the expression of proteins involved in differentiation such as myogenin and myosin. In addition, the up-regulation of this pathway appears to have long-lasting effects even after the establishment of myotubes. In particular, we found that inhibition of NGF signaling after completion of the fusion process led to the formation of abnormally shaped myotubes, characterized by an aberrant cytoskeletal organization, specifically at the level of the sarcomere and costamere organization, which were assessed, by evaluating α-actinin expression and paxillin and Fak expression, respectively (Quach and Rando, 2006).
Another important aspect emerging from our studies is the identification of RhoA as the key intermediate transducer regulated by NGF. Although RhoA had been previously associated with NGF signaling through p75NTR in neurons (Yamashita et al., 1999), this is the first time that such a link was established in muscle. Our results clearly show that both the NGF-stimulated fusion and the alterations of cytoskeleton with formation of myosac-like myotubes required RhoA inhibition. A role of RhoA in modulating myoblasts fusion was recently reported, in particular that constitutive RhoA activation inhibited fusion and caused the formation of myosac-like myotubes (Castellani et al., 2006; Charrasse et al., 2006; Fortier et al., 2008). Our results now identify the signaling events upstream RhoA and show how this GTPase is regulated by autocrine signals.
The evidence from our in vivo experiments shed further light on the biological function of NGF in muscle homeostasis. The fact that NGF and p75NTR are reexpressed in dystrophic and inclusion body myositis muscle when regeneration occurs suggested the idea that this signaling is crucial whenever enhanced myoblasts function is required to repair damaged fibers. Our results in the CTX model of damage now demonstrate that this is the case since the tissue-permeable form of NGF antagonist delayed muscle regeneration, with persistence of embryonic myosin and a decreased number of regenerating fibers.
The delay in regeneration we observed was due to inhibition of myoblasts fusion and hyper-activation of RhoA, as confirmed by the injection of TA with primary myoblasts transfected with a constitutive active form of RhoA, which mimics the activation of RhoA induced by the block of NGF signaling. In these experiments, the use of β-Gal–expressing exogenous myoblasts allowed to estimate their integration into the regenerating fibers by assessing their β-Gal positivity. Myoblasts in which RhoA is hyperactivated, showed a decreased fusion index in culture and a reduced integration into damaged myofibers.
In conclusion, our experiments demonstrate that the signaling of NGF through its low-affinity p75NTR receptor mediated by RhoA in muscle cells and is required for physiological myoblast fusion and to maintain a functional cytoskeletal organization of myotubes; these effects have a particular relevance in vivo during damage where myogenic precursors need to get activated and to fuse to existing myofibres in order to contribute to the formation of fibers with a functional contractile apparatus and allow an efficient repair of damaged areas of skeletal muscle. These observations therefore add novel and important information on the biological function of a signaling pathway, which is present during developmental myogenesis and is turned on in adult life every time skeletal muscle needs to be remodeled.
Supplementary Material
ACKNOWLEDGMENTS
We thank Giuliano Della Valle for advice. This work was supported by grants from Telethon (GGP07013 to S.B. and GGP07006 to E.C.), Fondazione Cariplo (E.C. and S.B.), Fondazione Invernizzi (E.C.) and the Italian Ministry of Health (E.C., M.T., and S.B., ex.PE56).
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-01-0012) on June 24, 2009.
REFERENCES
- Aktories K., Schmidt G., Just I. Rho GTPases as targets of bacterial protein toxins. Biol. Chem. 2000;381:421–426. doi: 10.1515/BC.2000.054. [DOI] [PubMed] [Google Scholar]
- Balemans W., Van Hul W. Extracellular regulation of BMP signaling in vertebrates: a cocktail of modulators. Dev. Biol. 2002;250:231–250. [PubMed] [Google Scholar]
- Baron P., Scarpini E., Meola G., Santilli I., Conti G., Pleasure D., Scarlato G. Expression of the low-affinity NGF receptor during human muscle development, regeneration, and in tissue culture. Muscle Nerve. 1994;17:276–284. doi: 10.1002/mus.880170304. [DOI] [PubMed] [Google Scholar]
- Bibel M., Barde Y. A. Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 2000;14:2919–2937. doi: 10.1101/gad.841400. [DOI] [PubMed] [Google Scholar]
- Botchkarev V. A., Yaar M., Gilchrest B. A., Paus R. p75 Neurotrophin receptor antagonist retards apoptosis-driven hair follicle involution (catagen) J. Invest. Dermatol. 2003;120:168–169. doi: 10.1046/j.1523-1747.2003.12003.x. [DOI] [PubMed] [Google Scholar]
- Castellani L., Salvati E., Alema S., Falcone G. Fine regulation of RhoA and Rock is required for skeletal muscle differentiation. J. Biol. Chem. 2006;281:15249–15257. doi: 10.1074/jbc.M601390200. [DOI] [PubMed] [Google Scholar]
- Charrasse S., Comunale F., Grumbach Y., Poulat F., Blangy A., Gauthier-Rouviere C. RhoA GTPase regulates M-cadherin activity and myoblast fusion. Mol. Biol. Cell. 2006;17:749–759. doi: 10.1091/mbc.E05-04-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrel G., Hohlfeld R., Sendtner M. The role of neurotrophins in muscle under physiological and pathological conditions. Muscle Nerve. 2006;33:462–476. doi: 10.1002/mus.20444. [DOI] [PubMed] [Google Scholar]
- Cossu G., Zani B., Coletta M., Bouchè M., Molinaro M. In vitro differentiation of satellite cells isolated from normal and dystrophic mammalian muscles. A comparison with embryonic myogenic cells. Cell Differ. 1980;9:357–368. doi: 10.1016/0045-6039(80)90035-4. [DOI] [PubMed] [Google Scholar]
- Deponti D., et al. Necdin mediates skeletal muscle regeneration by promoting myoblast survival and differentiation. J. Cell Biol. 2007;179:305–319. doi: 10.1083/jcb.200701027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erck C., Meisinger C., Grothe C., Seidl K. Regulation of nerve growth factor and its low-affinity receptor (p75NTR) during myogenic differentiation. J. Cell Physiol. 1998;176:22–31. doi: 10.1002/(SICI)1097-4652(199807)176:1<22::AID-JCP3>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Ernfors P., Wetmore C., Eriksdotter-Nilsson M., Bygdeman M., Stromberg I., Olson L., Persson H. The nerve growth factor receptor gene is expressed in both neuronal and non-neuronal tissues in the human fetus. Int. J. Dev. Neurosci. 1991;9:57–66. doi: 10.1016/0736-5748(91)90073-u. [DOI] [PubMed] [Google Scholar]
- Fortier M., Comunale F., Kucharczak J., Blangy A., Charrasse S., Gauthier-Rouviere C. RhoE controls myoblast alignment prior fusion through RhoA and ROCK. Cell Death Differ. 2008;15:1221–1231. doi: 10.1038/cdd.2008.34. [DOI] [PubMed] [Google Scholar]
- Galvez B. G., et al. Cardiac mesoangioblasts are committed, self-renewable progenitors, associated with small vessels of juvenile mouse ventricle. Cell Death Differ. 2008;15:1417–1428. doi: 10.1038/cdd.2008.75. [DOI] [PubMed] [Google Scholar]
- Hempstead B. L. The many faces of p75NTR. Curr. Opin. Neurobiol. 2002;12:260–267. doi: 10.1016/s0959-4388(02)00321-5. [DOI] [PubMed] [Google Scholar]
- Ip F. C., Cheung J., Ip N. Y. The expression profiles of neurotrophins and their receptors in rat and chicken tissues during development. Neurosci. Lett. 2001;301:107–110. doi: 10.1016/s0304-3940(01)01603-2. [DOI] [PubMed] [Google Scholar]
- Kust B. M., Copray J. C., Brouwer N., Troost D., Boddeke H. W. Elevated levels of neurotrophins in human biceps brachii tissue of amyotrophic lateral sclerosis. Exp. Neurol. 2002;177:419–427. doi: 10.1006/exnr.2002.8011. [DOI] [PubMed] [Google Scholar]
- Lavasani M., Lu A., Peng H., Cummins J., Huard J. Nerve growth factor improves the muscle regeneration capacity of muscle stem cells in dystrophic muscle. Hum. Gene Ther. 2006;17:180–192. doi: 10.1089/hum.2006.17.180. [DOI] [PubMed] [Google Scholar]
- Menetrey J., Kasemkijwattana C., Day C. S., Bosch P., Vogt M., Fu F. H., Moreland M. S., Huard J. Growth factors improve muscle healing in vivo. J. Bone Joint Surg. 2000;82:131–137. doi: 10.1302/0301-620x.82b1.8954. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H. Lysophosphatidic acid signalling. Curr. Opin. Cell Biol. 1995;7:203–210. doi: 10.1016/0955-0674(95)80029-8. [DOI] [PubMed] [Google Scholar]
- Mousavi K., Jasmin B. J. BDNF is expressed in skeletal muscle satellite cells and inhibits myogenic differentiation. J. Neurosci. 2006;26:5739–5749. doi: 10.1523/JNEUROSCI.5398-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacaud P., Sauzeau V., Loirand G. Rho proteins and vascular diseases. Arch. Mal. Coeur Vaiss. 2005;98:249–254. [PubMed] [Google Scholar]
- Parker M. H., Seale P., Rudnicki M. A. Looking back to the embryo: defining transcriptional networks in adult myogenesis. Nat. Rev. Genet. 2003;4:497–507. doi: 10.1038/nrg1109. [DOI] [PubMed] [Google Scholar]
- Pehar M., Cassina P., Vargas M. R., Castellanos R., Viera L., Beckman J. S., Estevez A. G., Barbeito L. Astrocytic production of nerve growth factor in motor neuron apoptosis: implications for amyotrophic lateral sclerosis. J. Neurochem. 2004;89:464–473. doi: 10.1111/j.1471-4159.2004.02357.x. [DOI] [PubMed] [Google Scholar]
- Pisconti A., Brunelli S., Di Padova M., De Palma C., Deponti D., Baesso S., Sartorelli V., Cossu G., Clementi E. Follistatin induction by nitric oxide through cyclic GMP: a tightly regulated signaling pathway that controls myoblast fusion. J. Cell Biol. 2006;172:233–244. doi: 10.1083/jcb.200507083. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Quach N. L., Rando T. A. Focal adhesion kinase is essential for costamerogenesis in cultured skeletal muscle cells. Dev. Biol. 2006;293:38–52. doi: 10.1016/j.ydbio.2005.12.040. [DOI] [PubMed] [Google Scholar]
- Reddypalli S., Roll K., Lee H. K., Lundell M., Barea-Rodriguez E., Wheeler E. F. p75NTR-mediated signaling promotes the survival of myoblasts and influences muscle strength. J. Cell Physiol. 2005;204:819–829. doi: 10.1002/jcp.20330. [DOI] [PubMed] [Google Scholar]
- Rende M., Brizi E., Conner J., Treves S., Censier K., Provenzano C., Taglialatela G., Sanna P. P., Donato R. Nerve growth factor (NGF) influences differentiation and proliferation of myogenic cells in vitro via TrKA. Int. J. Dev. Neurosci. 2000;18:869–885. doi: 10.1016/s0736-5748(00)00041-1. [DOI] [PubMed] [Google Scholar]
- Rende M., Brizi E., Sorci G., Bianchi R., Provenzano C., Bruno R., Donato R. Regulation of the p75 neurotrophin receptor in a rat myogenic cell line (L6) Histochem. J. 1999;31:589–601. doi: 10.1023/a:1003851024732. [DOI] [PubMed] [Google Scholar]
- Ridley A. J., Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Roux P. P., Barker P. A. Neurotrophin signaling through the p75 neurotrophin receptor. Prog. Neurobiol. 2002;67:203–233. doi: 10.1016/s0301-0082(02)00016-3. [DOI] [PubMed] [Google Scholar]
- Ruberti F., Capsoni S., Comparini A., Di Daniel E., Franzot J., Gonfloni S., Rossi G., Berardi N., Cattaneo A. Phenotypic knockout of nerve growth factor in adult transgenic mice reveals severe deficits in basal forebrain cholinergic neurons, cell death in the spleen, and skeletal muscle dystrophy. J. Neurosci. 2000;20:2589–2601. doi: 10.1523/JNEUROSCI.20-07-02589.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalbruch H., Lewis D. M. Dynamics of nuclei of muscle fibers and connective tissue cells in normal and denervated rat muscles. Muscle Nerve. 2000;23:617–626. doi: 10.1002/(sici)1097-4598(200004)23:4<617::aid-mus22>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Schor N. F. The p75 neurotrophin receptor in human development and disease. Prog. Neurobiol. 2005;77:201–214. doi: 10.1016/j.pneurobio.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Sciorati C., Galvez B. G., Brunelli S., Tagliafico E., Ferrari S., Cossu G., Clementi E. Ex vivo treatment with nitric oxide increases mesoangioblast therapeutic efficacy in muscular dystrophy. J. Cell Sci. 2006;119:5114–5123. doi: 10.1242/jcs.03300. [DOI] [PubMed] [Google Scholar]
- Seidl K., Erck C., Buchberger A. Evidence for the participation of nerve growth factor and its low-affinity receptor (p75NTR) in the regulation of the myogenic program. J. Cell Physiol. 1998;176:10–21. doi: 10.1002/(SICI)1097-4652(199807)176:1<10::AID-JCP2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Syroid D. E., et al. Induction of postnatal Schwann cell death by the low-affinity neurotrophin receptor in vitro and after axotomy. J. Neurosci. 2000;20:5741–5747. doi: 10.1523/JNEUROSCI.20-15-05741.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajbakhsh S. Stem cells to tissue: molecular, cellular and anatomical heterogeneity in skeletal muscle. Curr. Opin. Genet. Dev. 2003;13:413–422. doi: 10.1016/s0959-437x(03)00090-x. [DOI] [PubMed] [Google Scholar]
- Toti P., Villanova M., Vatti R., Schuerfeld K., Stumpo M., Barbagli L., Malandrini A., Costantini M. Nerve growth factor expression in human dystrophic muscles. Muscle Nerve. 2003;27:370–373. doi: 10.1002/mus.10332. [DOI] [PubMed] [Google Scholar]
- Turner B. J., Murray S. S., Piccenna L. G., Lopes E. C., Kilpatrick T. J., Cheema S. S. Effect of p75 neurotrophin receptor antagonist on disease progression in transgenic amyotrophic lateral sclerosis mice. J. Neurosci. Res. 2004;78:193–199. doi: 10.1002/jnr.20256. [DOI] [PubMed] [Google Scholar]
- Vartiainen M., Ojala P. J., Auvinen P., Peranen J., Lappalainen P. Mouse A6/twinfilin is an actin monomer-binding protein that localizes to the regions of rapid actin dynamics. Mol. Cell Biol. 2000;20:1772–1783. doi: 10.1128/mcb.20.5.1772-1783.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler E. F., Bothwell M. Spatiotemporal patterns of expression of NGF and the low-affinity NGF receptor in rat embryos suggest functional roles in tissue morphogenesis and myogenesis. J. Neurosci. 1992;12:930–945. doi: 10.1523/JNEUROSCI.12-03-00930.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worth N. F., Campbell G. R., Campbell J. H., Rolfe B. E. Rho expression and activation in vascular smooth muscle cells. Cell Motil. Cytoskelet. 2004;59:189–200. doi: 10.1002/cm.20036. [DOI] [PubMed] [Google Scholar]
- Yamashita T., Tucker K. L., Barde Y. A. Neurotrophin binding to the p75 receptor modulates Rho activity and axonal outgrowth. Neuron. 1999;24:585–593. doi: 10.1016/s0896-6273(00)81114-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.