Abstract
ASK1 cysteine oxidation allows JNK activation upon oxidative stress. Trx1 negatively regulates this pathway by reducing the oxidized cysteines of ASK1. However, precisely how oxidized ASK1 is involved in JNK activation and how Trx1 regulates ASK1 oxidoreduction remains elusive. Here, we describe two different thiol reductase activities of Trx1 on ASK1. First, in H2O2-treated cells, Trx1 reduces the various disulfide bonds generated between cysteines of ASK1 by a rapid and transient action. Second, in untreated cells, Trx1 shows a more stable thiol reductase activity on cysteine 250 (Cys250) of ASK1. After H2O2 treatment, Trx1 dissociates from Cys250, which is not sufficient to activate the ASK1-JNK pathway. Indeed, in untreated cells, a Cys250 to alanine mutant of ASK1 (C250A), which cannot bind Trx1, does not constitutively activate JNK. On the other hand, in H2O2-treated cells, this mutant (C250A) fails to activate JNK and does not induce apoptosis, although it remains fully phosphorylated on Threonine 838 (Thr838) in its activation loop. Overall, our data show that Cys250 is essential for H2O2-dependent signaling downstream from ASK1 but at a step subsequent to the phosphorylation of ASK1 Thr838. They also clarify the thiol reductase function of Trx1 on ASK1 activity.
INTRODUCTION
Hydrogen peroxide (H2O2) has long been considered as an unwanted by-product of cell respiration. However, the capacity of the cell to produce H2O2 in response to stimuli such as ligand-receptor binding and microorganism infection as well as the recognized role of H2O2 in normal cellular processes like proliferation, differentiation, migration and programmed cell death, have changed our vision of this compound from a metabolic waste product to an important intracellular signaling molecule (Stone and Yang, 2006; D'Autreaux and Toledano, 2007).
The mitogen-activated protein kinase kinase kinase 5 (MAPKKK5), better known as apoptosis signal-regulated kinase 1 (ASK1), is a major convergence point of signal transduction induced by H2O2. ASK1 lies upstream of the stress-activated kinase, p38 and the Jun NH2-terminal kinase, JNK and is essential for their H2O2-dependent activation (Ichijo et al., 1997; Tobiume et al., 2001). Glucose deprivation, extracellular ATP, accumulation of amyloid β peptides in Alzheimer disease, and TNF all stimulate the production of reactive oxygen species (ROS), such as H2O2, and lead to apoptosis by an ASK1-dependent process (Gotoh and Cooper, 1998; Liu et al., 2000; Song et al., 2002; Song and Lee, 2003b; Kadowaki et al., 2005; Noguchi et al., 2008). Many mechanisms have been proposed to regulate the H2O2-dependent activation of ASK1 such as the binding of cofactors TRAF2 or PKD and the dissociation of the inhibitors 14-3-3, Hsp70, glutaredoxin-1 and thioredoxin-1 (Trx1) (Nishitoh et al., 1998; Saitoh et al., 1998; Liu et al., 2000; Park et al., 2002; Song et al., 2002; Song and Lee, 2003b; Goldman et al., 2004; Zhang et al., 2004a; Noguchi et al., 2005; Zhang et al., 2005). Among these negative regulators, Trx1 is undoubtedly the most studied.
The canonical function of Trx1 is to reduce intra- or intermolecular disulfide bonds within or between proteins (Powis and Montfort, 2001). Trx1-mediated disulfide reduction in ribonucleotide reductase, TNFRSF8, NF-κB, and PTEN has demonstrated a role for Trx1 in DNA synthesis, inflammation, transcription, and signal transduction regulation, respectively (Laurent et al., 1964; Matthews et al., 1992; Lee et al., 2002; Meuillet et al., 2004; Schwertassek et al., 2007).
The thiol reductase activity of Trx1 is provided by cysteines 32 (C32) and 35 (C35) which form a redox catalytic CXPC motif. First, C32 forms an intermediate disulfide bond with one of the two cysteines of the disulfide bond found on the substrate. Then, C35 reduces the intermediate disulfide bond resulting in the formation of an intramolecular disulfide bond between C32 and C35 and the release of the reduced substrate (Holmgren, 1995; Powis and Montfort, 2001).
Many studies have shown that Trx1 maintains ASK1 inactive by direct association and that the dissociation of Trx1 from ASK1 corresponds with the activation of the kinase function of ASK1 (Saitoh et al., 1998; Liu et al., 2000). This interaction involves the catalytic cysteines C32 or C35 of Trx1 because mutation of either is sufficient to inhibit the dissociation of Trx1 from ASK1 upon H2O2 treatment and subsequent activation of downstream ASK1-dependent pathways (Liu and Min, 2002). Moreover, TNF stimulation and glucose deprivation lead to increased cellular H2O2 levels, and antioxidants such as catalase or N-acetylcysteine (NAC) inhibit the release of Trx1 from ASK1 (Liu and Min, 2002; Song and Lee, 2003a, b). These results led to a model in which H2O2 triggers Trx1 oxidation on C32 and C35 and the formation of an intramolecular disulfide bond between these two cysteines. This would allow the dissociation of Trx1 from ASK1 and its subsequent activation. Intriguingly, this Trx1-ASK1 regulation model does not involve the classical thiol-reductase function of Trx1 (Liu and Min, 2002).
We recently showed an alternative mechanism for the function of Trx1 in the regulation of ASK1 disulfide-bonded multimers (DBM) which was more in line with its canonical thiol reductase function. More precisely, we found that cell exposure to H2O2 induced the oxidation of specific ASK1 cysteine residues and the consequent formation of ASK1 DBM (Nadeau et al., 2007). We proposed a functional role for ASK1 DBM by showing that changing all oxidation-sensitive cysteines responsible for DBM formation prevented the activation of JNK and the induction of apoptosis. Furthermore, ASKl DBM were reduced by Trx1 in the recovery period. This result explained the known function of Trx1 as a negative regulator of ASK1 activity and was, at the same time, more consistent with the classical thiol reductase function of Trx1. However, the precise molecular mechanism by which Trx1 regulated each oxidation-sensitive cysteine of ASK1 remained unknown.
In the present study, we clarify the regulation of ASK1 by oxidoreduction. First, we identifiy the cysteines of ASK1 associated with Trx1 and characterize the function of Trx1 on these cysteines. Second, we analyze the role of these cysteines in H2O2-dependent JNK activation and apoptosis. Thus, we report two types of thiol reductase activity between Trx1 and the oxidation-sensitive cysteines of ASK1. Moreover, we show the critical involvement of Cys250 of ASK1 in H2O2-dependent signal transduction.
MATERIALS AND METHODS
Plasmids and Reagents
pcDNA3-ASK1-HA encodes the full-length sequence of human ASK1 and an hemagglutinin (HA) epitope (Ichijo et al., 1997). The various cysteine to alanine ASK1 mutants were constructed by PCR (PCR) after site-directed mutagenesis (QuickChange mutagenesis kit, Stratagene, La Jolla, CA). All newly developed constructs were validated by automated DNA sequencing (Plateforme de séquençage et de génotypage du genome, CRCHUQ, Québec, Canada). pCMV5-Myc-Trx1 expressed a Myc-tagged human Trx1 (Liu et al., 2000), whereas pRK-Flag-Trx1, pRK-Flag-Trx1C35S, and pRK-Flag-Trx1C32S encode Flag-tagged human Trx1 and cysteine 35 or 32 to serine substitutions Trx1 mutants, respectively (Liu and Min, 2002). Hydrogen peroxide (H2O2), thapsigargin, Iodoacetamide, 2-mercaptoethanol, and poly-L-lysine were purchased from Sigma Diagnostics Canada (Mississauga, ON, Canada).
Cell Culture and Transfection
HeLa, Human embryonic kidney (HEK) 293 and 293T cells (variant of HEK293 transformed with the SV40 large T antigen) were cultivated in DMEM (catalogue no. 12100-046; Invitrogen Canada, Burlington, ON, Canada) containing 2.2 g/l NaHCO3 and 0.4 g/l NaCl and supplemented with 10% fetal bovine serum (Sigma Diagnostics Canada). The cultures were maintained at 37°C in a 5% CO2 humidified atmosphere. Before transfection, the cells were seeded onto six-well plates or 35-mm Petri dishes (for immunofluorescence) coated (293T) or not coated (HEK293, HeLa) with 1 mg/ml poly-L-lysine. Twenty-four hours after plating, cells were transfected with 0.01–10 μg of DNA plasmid by calcium phosphate precipitation or with Lipofectamine 2000 (Invitrogen). Calcium phosphate precipitation was performed as previously described (Landry et al., 1989) with the exception that 25 μM chloroquine was added for the first 4 h of transfection. The Lipofectamine 2000 transfection was performed according to the manufacturer's protocol with the exception that the DNA(μg):Lipofectamine(μl) ratio was 1.5:1 with a maximum of 1.5 μl Lipofectamine 2000 per condition.
Antibodies
Mouse monoclonal antibodies detecting the HA (HA.11) and the Flag (Flag M2) epitopes were purchased from Sigma Diagnostics Canada. Anti-MYC (9E10) was from American Type Culture Collection. Rabbit polyclonal antibody against Trx1 and JNK were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-phospho JNK (P-JNK) was obtained from Cell Signaling Technology (Danvers, MA). Anti-phospho ASK1 was described previously (Nadeau et al., 2007).
Cells Extracts, Coimmunoprecipitation, and Western Blot Analysis
For experiments in Figures 2B, 3B, 4B, and S1, cells were washed once with ice-cold PBS and lyzed in SDS-sample buffer lacking 2-mercaptoethanol and supplemented with 100 mM iodoacetamide. For experiments in Figures 2D and 4D and for coimmunoprecipitations, cells were washed once with ice-cold PBS, incubated on ice with lysis buffer (20 mM Tris pH 7.4, 100 mM KCl, 1.25 mM MgCl2, 0.5% Igepal, 3.3% glycerol, Complete protease inhibitors [Roche Diagnostics, Indianapolis, IN] and 100 mM iodoacetamide) for 5 min with agitation and centrifuged at 15,000g for 10 min at 4°C. Supernatants were incubated with anti-HA affinity matrix (Roche Diagnostics) for 5h at 4°C, washed three times with 20 mM Tris-HCl pH 7.4, 150 mM NaCl, 0.5% Igepal, and 3.3% glycerol, resuspended in SDS-sample buffer with or without 5% 2-mercaptoethanol. Western blot analyses were essentially done as previously described (Nadeau et al., 2007).
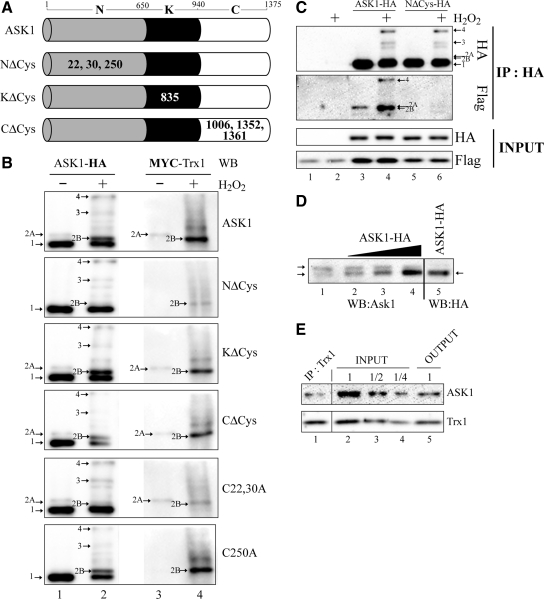
Figure 2.
Identification of the cysteines in ASK1 responsible for the covalent association with Trx1. (A) Schematic representation of the different cysteine substitution mutants used. Represented are the N-terminal (N), kinase (K), and C-terminal (C) domain of ASK1. Residue numbers at domain boundaries are indicated. Numbers in bold or in white within each domain indicate which cysteine residue(s) have been replaced by alanine residue(s). (B and C) 293T cells were transfected with pRK-Flag-Trx1 alone (C), or pRK-Flag-Trx1 or pCMV5-Myc-Trx1 together with the various ASK1 constructs defined in A or other labeled “Cx,yA” (the x and y indicates the positions of ASK1 cysteines residues which have been replaced by alanine residues) as indicated at the right (B) or the top (C) of panels. Twenty-four hours after transfection, the cells were left untreated (−) or exposed (+) to 1 mM H2O2 for 3 min. Total cell extracts were migrated on nonreducing (B) or reducing (INPUT in C) SDS gels, and anti-HA immunoprecipitated extracts were migrated under nonreducing conditions (IP:HA in C). Western blot (WB) analysis were performed either with anti-HA (ASK1-HA in B and HA in C), anti-MYC (MYC-Trx1 in B), or anti-Flag (Flag in C). Band 1 indicates the expected positions of monomeric ASK1 (150 kDa), bands 3 and 4 point the position of ASK1 disulfide-bonded complexes, and bands 2A and 2B indicate the positions of Trx1-ASK1 covalent complexes. (D) HEK293 cell extract (− was combined with increasing amounts of extracts from HEK293 cells transfected with pcDNA3-ASK1-HA. Total μg of proteins was equivalent between each lane. Extracts were migrated on SDS gels under nonreducing conditions and analyzed by Western blot with either anti-ASK1 (WB:ASK1) or anti-HA (WB:HA). The pcDNA3-ASK1-HA extracts in lanes 4 and 5 are the same. Arrows indicate the expected positions of monomeric endogenous ASK1. (E) 293T cells extracts were immunoprecipitated with anti-Trx1. Total cell (INPUT), anti-Trx1 immunoprecipitated (IP:Trx1), and anti-Trx1 depleted (OUTPUT) extracts were migrated on reducing SDS gels and analyzed with either anti-ASK1 (ASK1) or anti-Trx1 (Trx1). The third (labeled “1/2”) and fourth (labeled “1/4”) lanes were loaded with two and four times less protein than the second lane. They serve as a scale to determine the quantity of Trx1 and ASK1 depleted from the INPUT when compared with the OUTPUT. Lane numbers are referred to in the text.
Figure 3.
Function of Trx1 on Cys22,30 and Cys250 of ASK1. (A) Schematic representation of the different cysteine substitution mutants used. Represented are the N-terminal (N), kinase (K), and C-terminal (C) domain of ASK1. Residue numbers at domain boundaries are indicated. Numbers in bold or in white within each domain indicate which cysteine residue(s) have been replaced by alanine residue(s). (B through D) 293T cells were transfected with pRK-Flag-Trx1, pRK-Flag-Trx1C35S, pRK-Flag-Trx1C32S, pcDNA3-ASK1-HA, or pcDNA3-NΔCys-HA alone, or pRK-Flag-Trx1, pRK-Flag-Trx1C35S, or pRK-Flag-Trx1C32S together with either pcDNA3-ASK1-HA, pcDNA3-NΔCys-HA, pcDNA3-C250CΔCys-HA, or pcDNA3-C22,30CΔCys-HA. Twenty-four hours after transfection, the cells were left untreated (−) or exposed (+) to 100 μM (B), 1 mM (C), or 1,5 mM (D) H2O2 for 1 min (D) or the time indicated (B and C). Total cell (B and INPUT in C and D) or anti-HA immunoprecipitated (IP:HA in C and D) extracts were analyzed by Western blot with either anti-HA (HA) or anti-Flag (Flag). All samples were migrated on nonreducing SDS gel except for the INPUT samples in C and D and the anti-HA immunoprecipitated extracts analyzed with anti-HA labeled “reducing” in D. Band 1 indicates the expected positions of monomeric ASK1 (150 kDa). In B, bands 3 and 4 point the positions of ASK1 DBM as in C and D, together with bands 2A and 2B, they indicate the positions of Trx1 or Trx1C35S covalent associations with either or NΔCys, C250CΔCys or C22,30CΔCys.
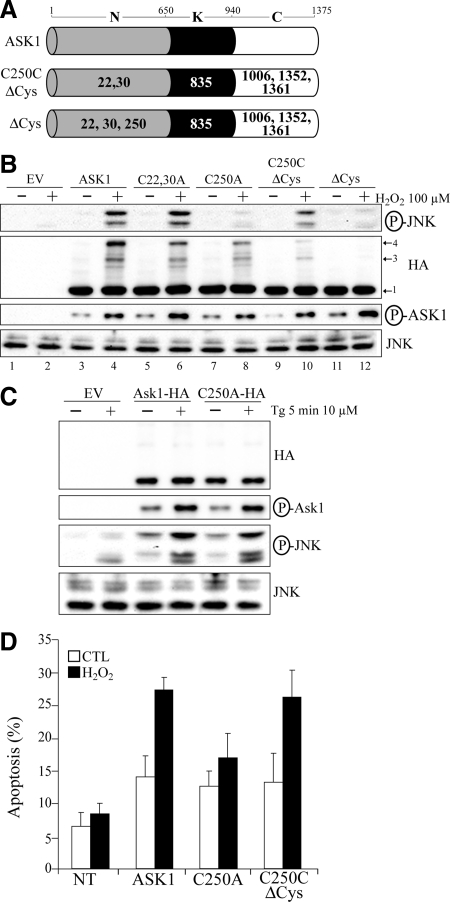
Figure 4.
ASK1 Cys250 is essential for ASK1-dependent activation of JNK and induction of apoptosis by H2O2 in vivo. (A) Schematic representation of the different cysteine substitution mutants used. Represented are the N-terminal (N), kinase (K), and C-terminal (C) domain of ASK1. Residue numbers at domain boundaries are indicated. Numbers in bold or in white within each domain indicate which cysteine residue(s) have been replaced by alanine residue(s). (B through D) 293T (B and C) or HeLa (D) were transfected with the empty vector pcDNA3 (EV in C), various ASK1 constructs defined in A or with other ASK1 mutants labeled “Cx,yA” where the x and y indicates the positions of cysteines residues replaced by alanine residues. Twenty-four hours after transfection, cells were left untreated ([−] in B and C or CTL in D) or exposed ([+] in B and C) to 100 μM H2O2 for 3 min in (B), 500 μM H2O2 for 3 h in (D), or 10 μM thapsigargin (Tg) for 5 min in (C). In B and C, total cell extracts were migrated on nonreducing gels and immnunoblotted with anti-HA or migrated on reducing gel and probed with anti-JNK or either anti-phospho specific ASK1 (P-ASK1) or JNK (P-JNK). Band 1 indicates the expected positions of monomeric ASK1 (150 kDa) and bands 3 and 4 point the position of ASK1 DBM. In D, the percentage of HA-positive cells showing condensed or fragmented nuclei was determined by immunofluorescent microscopy. The nontransfected condition (NT) consist of cells showing no signal for anti-HA by immunofluorescent microscopy in conditions where ASK1-HA was transfected. The data are means±SEM from three distinct experiments. P values of <0.05 were obtained (two-tailed paired t test) when percentages of apoptosis from cells transfected with C250A were compared with those of cells transfected with ASK1 or C250CΔCys after H2O2 treatment.
Immunofluorescence Microscopy
HeLa cells were fixed in 3.7% formaldehyde and permeabilized for 15 min in 0.1% saponin. After blocking for 30 min with 3% bovine serum albumin, samples were incubated for 1 h with anti-HA.11 at 37°C. Antigen-antibody complexes were revealed by the addition of Alexa Fluor 488-labeled anti-mouse IgG for 1 h at RT. Cell nuclei were stained with DAPI (1.6 μg/ml). All solutions were made in PBS. Observations were made with a Nikon Eclipse E600 upright microscope equipped with a 40 × 0.75 NA objective lens.
RESULTS
Trx1 Covalently Associates with Different ASK1 Complexes
In resting cells, ASK1 is found in a high-molecular-weight complex of 1500 to 3000 kDa termed the “ASK1 signalosome” (Noguchi et al., 2005). In this complex, the proteins are not covalently associated. Indeed, when separated under nonreducing conditions, an anti-HA Western blot performed on anti-HA immunoprecipitates from ASK1-HA and Flag-Trx1–expressing cells revealed ASK1-HA migrating as a unique species at a predicted molecular weight of 150 kDa (Figure 1A, left panel, band 1). In H2O2-treated cells, two other bands (band 3 and 4) migrating above the 200-kDa marker were also detected. As reported before, these bands correspond to the DBM of ASK1-HA induced during oxidative stress (Nadeau et al., 2007). The same Western blot revealed with anti-Flag showed the presence of Flag-Trx1 in bands 3 and 4 (Figure 1A and B, lane 8). This confirmed a previously described covalent association between ASK1 DBM and Trx1 which was suggested to result from the temporary interaction of Trx1 with ASK1 during Trx1-mediated disulfide reduction (Nadeau et al., 2007). Interestingly, two other bands were also detected with anti-Flag: bands 2A and 2B in nontreated and H2O2-treated cells, respectively. Band 2A migrated just above band 1 and below the 200-kDa molecular weight marker, suggesting an association between Flag-Trx1 and monomeric ASK1 (lane 7). Band 2B was more intense and migrated slightly faster than band 2A (lane 8 and also Figures 2 and 3). Bands 2A and 2B were also faintly visible in the anti-HA Western blot (Figure 1, A and B, lanes 3 and 4). However, they were much more apparent when anti-HA Western blots were performed on total cell extracts rather than HA immunoprecipitates (Figure 2B, top panel). Actually, in extracts of untreated cells coexpressing ASK1-HA and MYC-Trx1, ASK1 distributed in two bands, ≈87.5% being in band 1 and 12.5% in band 2A (see Figure S1 in supplementary data section for explanation of the quantification procedure). In H2O2-treated cells, the ASK1 distribution pattern was similar between bands 1 and 2B. Both bands 2A and 2B also labeled with anti-MYC demonstrating again that MYC-Trx1 and ASK1 were covalently associated. Notably, the difference in mobility between band 1 and bands 2A and 2B is consistent with a 14-kDa increase in molecular weight predicted for the covalent association of Trx1 to ASK1. The faster migration of band 2B as compared with band 2A may be attributable to further intramolecular disulfide bonds induced by H2O2 in ASK1 or Trx1.
Figure 1.
Trx1 covalently associates with different ASK1 complexes. (A) 293T cells were transfected with pRK-Flag-Trx1 with or without pcDNA3-ASK1-HA for 24 h and left untreated (−) or exposed (+) to 1 mM H2O2 for 1 min. Anti-HA immunoprecipitates (IP:HA) and total cell extracts (Input) were migrated on nonreducing and reducing SDS gels, respectively, and analyzed by Western blot with anti-HA (WB:HA) or anti-Flag (WB:Flag). Band 1 indicates the expected positions of monomeric ASK1 (150 kDa), and bands 2A, 2B, 3, and 4 point the positions of covalent Trx1-ASK1 complexes, which are described. The dot indicates a nonspecific band recognized by the anti-HA. Numbers on the left indicate the positions of molecular weight standards migrated under nonreducing conditions. Lane numbers are referred to in the text. (B) Areas in A (indicated by the dotted lines) of lanes 3 and 7 (upper panel) or 4 and 8 (lower panel) are shown magnified to help with the visualization of bands 1, 2A, 2B, 3, and 4.
The functional relevance of these findings was evaluated by studying the distribution of endogenous ASK1 extracted from untransfected cells in nonreducing gels (Figure 2D, lane 1). Endogenous ASK1 was also found as a doublet (arrows). However, in contrast to transfected cells, endogenous ASK1 was mostly found in the slowest migrating band. To determine the similarity between the migration of endogenous ASK1 and the above described band 2A and band 1, increased concentrations of extract from cells transfected with ASK1-HA were added to the untransfected cell extract (lanes 2 to 4, note that the HA epitope had no detectable effect on the migration of ASK1). In these conditions, only the faster migrating band was increased. This suggests that endogenous ASK1 migrates in a greater proportion at a distance equivalent of band 2A for exogenous ASK1, and that the concentration of endogenous Trx1 might be a limiting factor in the formation of the ASK1-Trx1 complex. To confirm that the majority of endogenous ASK1 was associated with Trx1, the latter was immunoprecipitated and the amount of coimmunoprecipitated ASK1 was determined (Figure 2E). In these conditions, more than 50% of ASK1 was depleted in the extract. This result was consistent with the hypothesis that a large proportion of endogenous ASK1 is constitutively associated with Trx1 in control cells. Moreover, based on results in Figures 1 and 2, this association between the two endogenous proteins is most likely covalent.
ASK1 N-Terminal Cysteines 22, 30, and 250 Covalently Bind Trx1 in Different Conditions
As previously mentioned, the covalent association observed between Trx1 and ASK1 in band 3 and 4 could be the results of transient interactions of Trx1 when it reduces ASK1 DBM. However, the other associations seen between Trx1 and monomeric ASK1 in bands 2 (2A and 2B), both in control and H2O2-treated cells, suggested another type of functional interaction between the two proteins.
Seven potential oxidation-sensitive cysteines in ASK1 were previously identified (Nadeau et al., 2007). In this study, we sought to identify which of these cysteines were involved in the covalent association of ASK1 with Trx1. To achieve this goal, wild-type ASK1-HA and various ASK1-HA cysteine to alanine mutants were cooverexpressed with MYC-Trx1 in 293T cells treated or not with H2O2. Total cell extracts were migrated on nonreducing SDS-PAGE gels, and Western blot analyses were performed with either anti-HA or anti-MYC. The detection of bands migrating at the same relative molecular weight with the two antibodies would indicate a possible direct covalent association of Trx1 with the indicated ASK1 construct. First, NΔCys, KΔCys, and CΔCys mutants in which all oxidation-sensitive cysteines were mutated within a specific domain of ASK1 were analyzed (Figure 2A). Interestingly, only NΔCys, in which the N-terminal domain cysteines 22, 30, and 250 were replaced by alanines, showed differences in anti-HA and anti-MYC signals when compared with wild-type ASK1 (Figure 2B). Indeed, for both antibodies, no signal for band 2A was detected in untreated cells and only a very faint signal for band 2B in H2O2-treated cells was observed. These results demonstrate that oxidation-sensitive cysteines found in the N-terminal domain of ASK1 are involved in its association with Trx1.
The role of ASK1 N-terminal Cys22, Cys30, and Cys250 in the covalent association of ASK1 with Trx1 was investigated further. C22,30A-HA (C22,30A), a ASK1 mutant in which both Cys22 and Cys30 were replaced by Ala or C250A-HA (C250A), a Cys to Ala mutant of Cys250, were cooverexpressed with MYC-Trx1 and their interaction analyzed as described above (Figure 2B). In nontreated cells, C250A showed no band 2A with either anti-MYC or anti-HA but still accumulated band 2B in H2O2-treated cells. On the other hand, the C22,30A distributed normally in band 2A as revealed with anti-HA and anti-MYC, but the induction of band 2B after H2O2-treatment was highly reduced. This residual signal of anti-HA and anti-MYC for band 2B in H2O2-treated cells with C22,30A, and previously with NΔCys, could be explained for anti-HA by the association of C22,30A or NΔCys-HA with endogenous Trx1 or for anti-MYC by the association of MYC-Trx1 with endogenous ASK1. Nevertheless, these results clearly show that the detected covalent associations of Trx1 in resting and H2O2-treated cells are on Cys250 and Cys22,30 of ASK1, respectively.
In Figure 2B, although covalent associations of Trx1 were observed with monomeric ASK1 (bands 2), none were detected with ASK1 DBM (bands 3 and 4) when whole cell extracts were analyzed. As shown in Figure 1, enrichment of ASK1 by immunoprecipitation was necessary to detect Trx1 within ASK1 DBM. Thus, based on the result of Figure 2B, immunoprecipitation was used to examine whether Cys22, Cys30, and Cys250 of ASK1 were implicated in the detection of Trx1 with the ASK1 DBM. NΔCys-HA was cotransfected with Flag-Trx1 and the cell lysates were immunoprecipitated with anti-HA, and Western blot analyses were performed with either anti-HA or anti-MYC (Figure 2C). As expected, the results with anti-HA confirm previous results that bands 3 and 4 were produced normally with this mutant, because the N-terminal cysteines contribute little to the formation of the DBM (Nadeau et al., 2007). Results with anti-Flag confirmed the data of Figure 2B that the bands 2A and 2B are not produced by this mutant and furthermore indicated no association between Trx1 and NΔCys in the DBM (bands 3 and 4). This finding shows that the detection of Trx1 with ASK1 DBM is also dependent on the N-terminal domain cysteines of ASK1, most likely cysteines 22 or 30; Figure 2B demonstrates that they are essential for the detection of Trx1 with monomeric ASK1 after cell exposure to H2O2.
Taken together, these results suggest that in resting cells, Trx1 is covalently bound to ASK1 through Cys250. On treatment with H2O2, increased covalent interaction between Trx1 and ASK1 on Cys22 or 30 occurs. The binding to one site is independent of the other, since the mutation at one site did not affect the binding at the other site.
Function of Trx1 on Cys22, 30, and Cys250 of ASK1
We showed previously that Trx1 was involved in the reduction of the DBM species (Nadeau et al., 2007). Because the NΔCys was still oxidized to DBM after H2O2 treatment (Figure 2C) but did not show interaction with Trx1, we asked whether reduction of the DBM by Trx1 proceeded normally. ASK1-HA or NΔCys-HA were overexpressed in 293T cells treated with or without H2O2. Extracts were analyzed with anti-HA to detect the induction pattern and the reduction of DBM (Figure 3B). No difference was observed between the wild type and the mutant. In both cases, induction of DBM (band 3 and 4) was maximal at 3 min and disappeared at 30 min, being completely reduced to the monomeric 150 kDa ASK1 or NΔCys forms (band 1). These results indicated that ASK1 cysteines 22, 30, or 250 were not the target of Trx1 during the reduction of ASK1 DBM.
Although no interaction was detected between Trx1 and NΔCys DBM, we investigated whether NΔCys DBM were still reduced by Trx1. The approach used here was based on the Trx1 reduction mechanism. During this reduction process, the disulfide bond in the Trx1 substrate is first attacked by Cys32 of Trx1 and then resolved by the action of Cys35. Thus, a mutant of Trx1 in which Cys35 is mutated would lack thiol reductase activity and would not dissociate from its substrates (Figure 3C). We tested the effect of the Flag-Trx1C35S (Trx1C35S) mutant, in which Cys35 of Trx1 was replaced by a serine, and wild-type Flag-Trx1 (Trx1WT) on the formation and the reduction of NΔCys DBM. As expected, the formation of NΔCys DBM (bands 3 and 4) upon H2O2 treatment was stronger and lasted longer in cells cotransfected with Trx1C35S as compared with cells cotransfected with Trx1WT. Furthermore, immunoprecipitation of total cell extracts with anti-HA followed by an anti-Flag Western blot detected a covalent association of Trx1C35S with NΔCys DBM. No signal was detected for Trx1WT with NΔCys, supporting the results shown in Figure 2C. The interaction of NΔCys DBM with Trx1C35S but not with Trx1WT indicated that a Trx1 disulfide reductase-mediated interaction truly occurred with ASK1 DBM but is likely highly transient and therefore difficult to detect using a functional Trx1. Based on this interpretation, the detection of Trx1WT with cysteines 22, 30, and 250 in Figure 2 suggests that the interaction with the Cysteines 22, 30, and 250 is much more long lasting.
To determine the type of interaction between Trx1 and ASK1 on Cysteines 22,30 and 250, we studied the interaction of ASK1 mutants C22,30CΔCys-HA or C250CΔCys-HA, in which all oxidation-sensitive cysteines were substituted to alanine except for Cys22,30 or Cys250, respectively, with Flag-Trx1C32S (Trx1C32S) and Flag-Trx1C35S (Figure 3D). The interactions of the Trx1 mutants with ASK1 cysteine 22,30 (Flag, lower panels) after treatment with H2O2 and with Cys250 (Flag, upper panels) in untreated cells were consistent with a thiol reductase interaction of Trx1. Indeed, the Cys32 to Ser mutation in Trx1 completely abolished its association with C250CΔCys and highly diminished its association with C22,30CΔCys. The remaining interaction of Trx1C32S mutant with C22,30CΔCys could result from the association of other Trx1 cysteines (Cys62, 69 or 73), which are also known to regulate Trx1 activity and to be sensitive to oxidation (Haendeler, 2006). Less obvious but still strongly suggesting a thiol reductase interaction of Trx1 with Cys22,30 or Cys250 of ASK1, the Cys35 to Ser mutation in Trx1 likely enhanced its interaction with both ASK1 mutants. The higher expression (INPUT) of Trx1C35S as compared with Trx1 does not allow us to firmly state on their degree of association with ASK1 mutants. Interestingly, in cells overexpressing C22,30CΔCys and Trx1C35S, the anti-Flag Western blot performed on the anti-HA immunoprecipitate revealed a doublet above band 2B. The shift of molecular weight suggests this could correspond to a covalently bound dimer of Trx1 or to two monomers of Trx1 covalently bound to ASK1.
Taken together, these results show that Trx1 has two different thiol reductase activities on ASK1. One is transient and involved in the reduction of ASK1 DBM. The other, on Cys 22,30 and Cys250 of ASK1 in control and H2O2-treated cells, respectively, are much more stable or long lasting.
ASK1 Cys250 Is Essential for ASK1-Dependent Activation of JNK and Induction of Apoptosis by H2O2 In Vivo
Because we previously demonstrated that the replacement of all oxidation-sensitive cysteines of ASK1 to alanine (including 22,30 and 250) inhibited the capacity of ASK1 to activate JNK and to induce apoptosis in response to H2O2 treatment (Nadeau et al., 2007), we decided here to evaluate whether cysteines 22,30 and 250 have a specific role in the activation of JNK and in apoptosis induction.
First, various cysteine to alanine mutants of ASK1 (Figure 4A) were tested for their capacity to phosphorylate JNK after treatment with H2O2. It is known that H2O2 induces a strong activation of JNK for which the presence of ASK1 is essential and rate-limiting (Tobiume et al., 2001; Nadeau et al., 2007). Hence, transfection of wild-type ASK1 caused a marked increased in the phosphorylation of JNK in response to H2O2 (Figure 4B, lane 2). As shown previously, transfection of the ΔCys mutant, in which all oxidation-sensitive cysteine of ASK1 were replaced by alanine, did not increase JNK phosphorylation (lane 10; Nadeau et al., 2007). Similarly, transfection of the C250A mutant severely reduced the phosphorylation of JNK but had only a minimal effect on the induction of DBM (lane 6). The C22, 30A mutant did not effect either JNK activation or induction of DBM (lane 4). These results suggest that Cys250 is essential for JNK activation. Moreover, Cys250 was sufficient among the other oxidation-sensitive cysteines because the transfection of C250CΔCys was almost as efficient as ASK1WT to activate JNK (lane 8). Because Cys250 is the only oxidation-sensitive cysteine remaining in C250CΔCys, it was not surprising to observe diminished DBM formation. All tested mutants, including C250A, were normally phosphorylated on T838, a marker of ASK1 activation (Figure 4B, P-ASK1). This suggests that Cys250 is not required for upstream ASK1 activation signals but is necessary for activation of its downstream substrates. The incapacity of the ASK1 C250A mutant to induce phosphorylation of JNK was not attributable to a structural defect of the mutant. Indeed, JNK activation was equivalent with ASK1WT or C250A in cells treated with thapsigargin (Tg), another potent activator ASK1 (Figure 4C; Nishitoh et al., 2002). These results clearly reveal that Cys250 of ASK1 plays a critical and specific role in ASK1-dependent signal transduction upon H2O2 treatment.
Finally, the biological function of Cys250 was investigated by studying its role in ASK1-dependent apoptosis after H2O2 treatment. Transfection of ASK1WT markedly increased apoptosis in cells treated with H2O2 (Figure 4D). However expression of C250A mutant showed decreased potential to activate apoptosis, which was totally restored by the C250CΔCys mutant. These results indicate that Cys250 is essential for ASK1 to induce apoptosis in response to H2O2.
In conclusion, these findings show that Cys250 alone is required for ASK1 to properly regulate the JNK signaling pathway and to induce apoptosis in response to oxidative stress.
DISCUSSION
In this work, we demonstrate that Cys250 of ASK1 is essential for the H2O2-dependent activation of JNK and induction of apoptosis. Moreover, our results suggest that Trx1 prevents inappropriate activation of ASK1 via its thiol reductase activity on Cys250 of ASK1 in resting cells. Our results give new insights on the regulation of ASK1 upon oxidative stress and highlight the importance of thiol-mediated H2O2 signaling in mammalian cell responses.
Cys250 of ASK1 Is Essential for the H2O2-Dependent Activation of JNK
Oxidative, endoplasmic reticulum (ER), and genotoxic stress as well as heat shock, UV radiation, and ligand-receptor binding (e.g., TNF/TNFR, FasL/FasR) are clear inducers of ASK1 activity (Ichijo et al., 1997; Chang et al., 1998; Chen et al., 1999; Noguchi et al., 2001; Dorion et al., 2002; Nishitoh et al., 2002). Posttranslational modifications of specific ASK1 residues or the association/dissociation of activators/inhibitors from specific ASK1 domains can be promoted by specific stimuli to selectively activate ASK1. For example, heat shock treatment induces the dissociation of GSTM1-1 from the N-terminal domain of ASK1 (Dorion et al., 2002). The TNF/TNFR or FasL/FasR ligand-receptor associations promote the binding of TRAF2 and Daxx, respectively, to the N-terminal domain of ASK1 (Chang et al., 1998; Nishitoh et al., 1998; Liu et al., 2000; Fujino et al., 2007). The phosphorylation of Ser83, Ser967, and Ser1034 inhibits the stress-induced proapoptotic activity of ASK1 (Zhang et al., 1999; Kim et al., 2001; Fujii et al., 2004; Goldman et al., 2004). Commonly to all stimuli, the phosphorylation of ASK1 at Thr838 is essential for its kinase activity (Tobiume et al., 2002). We show here that another residue, specifically the Cys250 of ASK1, is essential for downstream JNK activation and induction of apoptosis specifically by H2O2. This mechanism is specific to H2O2 stimulation because thapsigargin, an ER stress inducing agent, induced ASK1-dependent JNK phosphorylation independently of Cys250 of ASK1.
The event occurring on Cys250 of ASK1 which leads to JNK activation and induction of apoptosis was unclear. We showed that Trx1 dissociates from Cys250 upon H2O2 treatment, as further described below. In the previous model of ASK1 regulation by Trx1, the latter negatively regulates ASK1 by direct association and its dissociation from ASK1 induces the kinase activity (Saitoh et al., 1998). According to this model, inhibiting Trx1 association with ASK1 in unstimulated cells would result in a constitutively activated ASK1. Our results demonstrate that the C250A mutant, which cannot bind Trx1, is not constitutively phosphorylated on Thr838 and does not constitutively activate JNK. These results suggest that simple dissociation of Trx1 from Cys250 is not sufficient to activate the ASK1/JNK pathway. However, we show that Cys250 of ASK1 is essential for JNK activation upon H2O2 treatment. The precise role of Cys250 in this context remains unclear. However, the dissociation of Trx1 from Cys250 could induce a structural change in ASK1 ternary or quaternary structure which depends on Cys250 (e.g., disulfide bond) and is necessary for ASK1-dependent downstream signaling. Additionally, the binding of an unidentified cofactor (Factor X, see Figure 5) for downstream signaling, could be essential for ASK1 to activate JNK. Possible candidates for Factor X include H2O2-dependent activators of ASK1, such as PKD and TRAF2 (Gotoh and Cooper, 1998; Liu et al., 2000; Zhang et al., 2005). Finally, studies suggest that Daxx could possibly activate ASK1 by a glucose deprivation–induced upregulation of H2O2 (Song and Lee, 2003a). Daxx and TRAF2 bind to the N-terminal domain of ASK1 and evidence suggests that Daxx and TRAF2 bind ASK1 near or possibly directly on Cys250 (Chang et al., 1998; Fujino et al., 2007). Whether Trx1 dissociation allows these interactors to bind Cys250 or whether factor X association induces the dissociation of Trx1 from Cys250 remains unknown.
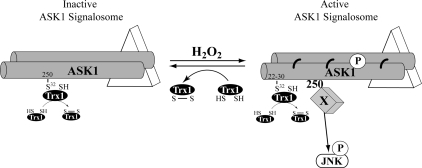
Figure 5.
A new model for the regulation of the ASK1-dependent signaling pathway by H2O2. In untreated cells, ASK1 oligomers are associated with other interactors (triangle) in a complex of 1500 to 3000 kDa called the ASK1 signalosome. In this complex, Cys32 of Trx1 binds to and masks Cys250 of ASK1 likely via a slower thiol reductase activity (see text for details). Stimulation of cells with H2O2 induces the formation of the active ASK1 signalosme (<3000 kDa) in which ASK1 is phosphorylated (P). This occurs simultaneously with the lost of thiol reductase activity of Trx1 and its dissociation from Cys250. This cysteine is essential for JNK activation after H2O2 treatment. The exact role of Cys250 in this mechanism is unknown. One possible scenario, illustrated in this figure, shows the Cys250-dependent association of a H2O2-specific interactor (Factor X) with ASK1. In addition, H2O2 induces an association between Cys32 of Trx1 on Cys22,30 of ASK1, also via a slower thiol reductase activity, like on Cys250 of ASK1. Moreover, disulfide-bonded (thick curved line) multimers of ASK1 are formed and are reduced by Trx1.
Our conclusions are in contrast with results previously published by Zhang and colleagues. They showed that transfection of an ASK1 mutant in which cysteine 250 was replaced by serine (C250S) increased JNK-dependent transcriptional activity and apoptosis in unstimulated endothelial cells (Zhang et al., 2004b). In our work, neither JNK phosphorylation nor induction of apoptosis was different between ASK1WT and the C250A mutant in untreated epithelial cells. Differences between the two studies could be explained because different cell lines and times of transfection were tested as well as different types of mutation (replacement of cysteine by alanine or serine). Nevertheless, all these results clearly demonstrate the involvement of cysteine 250 in the function of Ask1.
Our recent results raise further questions on the functional role of ASK1 DBM because only Cys250 is required for JNK activation, and mutation of Cys250 does not affect the induction of ASK1 DBM. On the other hand, because endogenous ASK1 can form DBM and Trx1 clearly reduces these forms of ASK1 (see below), DBM likely have a functional role. One can imagine that DBM are also required to sustain the activation of ASK1 by structural modification or facilitate the cellular relocation of ASK1. Further studies will be needed to get a clearer image of the role of ASK1 DBM in H2O2 signaling.
Different Thiol Reductase Activities of Trx1 on ASK1
Trx1 is a thiol reductase, meaning it reduces intra- or intermolecular disulifide bonds within or between other proteins (Powis and Montfort, 2001). In 1998, Saitoh et al. observed, in two-hybrid screenings, that Trx1 strongly interacted with ASK1. They proposed that Trx1 binds to ASK1 in unstressed cells and that treating cells with H2O2 induces the formation of an intramolecular disulfide bond between Cys32 and Cys35 of Trx1, allowing its dissociation from and activation of ASK1 (Saitoh et al., 1998). Indirectly, this mechanism implied that Trx1 was a target of H2O2 and identified it as a peroxidase instead of a thiol reductase. Since then, little progress has been made to understand the precise mechanism involved in the regulation of ASK1 by Trx1. Recently, we reported that a thiol reductase activity of Trx1 negatively regulated the activity of ASK1 by reducing ASK1 DBM (Nadeau et al., 2007). Here, we enrich the model by proposing that this thiol reductase interaction with ASK1 DBM is very rapid and transient because it can only be detected with a thiol reductase-deficient mutant of Trx1 that remains trapped to its substrate (Figure 5).
In addition, our results show that Trx1 also has thiol reductase activity on cysteine 22,30 and 250 of ASK1 (Figure 5). For cysteines 22 and 30, Trx1 could reduce an intra- or intermolecular disulfide bond, which is formed upon cell exposure to H2O2. We show that cysteines 22, 30 have no function on ASK1 activity. Thus, the role of Trx1 on these residues needs further investigation.
In unstressed cells, more than 50% of Trx1 is bound to ASK1 and this major interaction occurs between Cys250 of ASK1 and the nucleophilic Cys32 of Trx1. This result suggests that Cys250 is highly sensitive to oxidation. The accumulation of an inactive thiol reductase Trx1 mutant (C35S) on Cys250 of ASK1 suggests that Trx1 has a thiol reductase activity on Cys250, possibly on an intra- or intermolecular disulfide bond being continuously formed in basal conditions.
The thiol reductase activity of Trx1 on its substrate is normally very rapid and transient (Holmgren, 1995). This was illustrated with the DBM of the NΔCys mutant. Only the inactive thiol reductase Trx1 mutant (C35S), which is trapped and accumulates on its substrate, but not wild-type Trx1 was detected with DBM of the NΔCys mutant. As we mentioned earlier, Trx1 (C35S) accumulates on Cys250 and Cys22,30 of ASK1, which also suggests a thiol reductase activity of Trx1 on these cysteines. However, wild-type Trx1 was also detected (at a lower level than C35S) bound to Cys250 or Cys22,30 of ASK1. This could suggest (1) a lower turnover rate of Trx1 thiol reductase activity on Cys250 and Cys22,30 than on ASK1 DBM, thus a more stable interaction or (2) that Cys250 and Cys22,30 are highly sensitive to oxidation in specific conditions, in resting cells and after H2O2 treatment, respectively, and thus continuously reduced by Trx1.
ASK1 Thiols: Potential Targets for New Drug Development
Our findings show that a specific thiol-containing residue of ASK1, Cys250, is essential for its activation and proapoptotic function in cells exposed to oxidative stress. Previous studies have also proposed this kind of cysteine-dependent regulation of ASK1. Treatment of cells with N-ethylmaleimide (NEM), a carboxylating agent of cysteine residues, induces ASK1 activation in vitro (Cross and Templeton, 2004). Glutaredoxin, a thiol reductase which has a main function of reducing S-glutathionylated proteins (protein-SS-G), negatively regulates ASK1 by direct binding (Song et al., 2002). Furthermore, nitric oxide (NO) production in response to interferon-gamma stimulation induces nitrosylation of cysteine 869 of ASK1 and its inhibition (Park et al., 2004). From a therapeutic point a view, thiols are already the target for anti-cancer drugs. For example, PMX464, a benzothiazole substituted quinol compound, inhibits the proproliferative and antiapototic functions of Trx1, which is upregulated in many malignant cell types, by targeting the catalytically active cysteines (Cys32 and Cys35; Mukherjee and Martin, 2008). In the case of ASK1, its oxidative stress–induced proapoptotic and proinflammatory functions play important roles in the initiation or the development of several diseases such as cardiopathy, neuropathy, genetic disorders, and cancer (Saadatzadeh et al., 2004; Kadowaki et al., 2005; Watanabe et al., 2005; Iriyama et al., 2009). Thus, targeting specific cysteine residues important in the regulation of ASK1, such as Cys250, looks promising for the development of new drugs or tools against these pathologies. Ultimately, because ASK1 is a converging point of signaling induced by oxidative stress in mammalian cells, efforts must be made to improve the knowledge of its thiol-dependent regulation.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Hidenori Ichijo (University of Tokyo), John Kyriakis (Tufts University School of Medicine), and Wang Min (Yale University) for providing plasmids, Dr Michel Toledano for his critical advice and comments, and Dr. Darren E. Richard for carefully reading the manuscript. This work was supported by the Canadian Institutes of Health Research and the Canada Research Chair in Stress Signal Transduction (to J.L.).
Abbreviations used:
- ASK1
apoptosis signal-regulated kinase 1
- Trx1
thioredoxin-1
- DBM
disulfide-bonded multimer
- JNK
c-Jun NH2-terminal kinase
- TNF
tumor necrosis factor
- TRAF
tumor receptor-associated factor.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-03-0211) on July 1, 2009.
REFERENCES
- Chang H. Y., Nishitoh H., Yang X., Ichijo H., Baltimore D. Activation of apoptosis signal-regulating kinase 1 (ASK1) by the adapter protein Daxx. Science. 1998;281:1860–1863. doi: 10.1126/science.281.5384.1860. [DOI] [PubMed] [Google Scholar]
- Chen Z., Seimiya H., Naito M., Mashima T., Kizaki A., Dan S., Imaizumi M., Ichijo H., Miyazono K., Tsuruo T. ASK1 mediates apoptotic cell death induced by genotoxic stress. Oncogene. 1999;18:173–180. doi: 10.1038/sj.onc.1202276. [DOI] [PubMed] [Google Scholar]
- Cross J. V., Templeton D. J. Oxidative stress inhibits MEKK1 by site-specific glutathionylation in the ATP-binding domain. Biochem. J. 2004;381:675–683. doi: 10.1042/BJ20040591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Autreaux B., Toledano M. B. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- Dorion S., Lambert H., Landry J. Activation of the p38 signaling pathway by heat shock involves the dissociation of glutathione S-transferase Mu from Ask1. J. Biol. Chem. 2002;277:30792–30797. doi: 10.1074/jbc.M203642200. [DOI] [PubMed] [Google Scholar]
- Fujii K., Goldman E. H., Park H. R., Zhang L., Chen J., Fu H. Negative control of apoptosis signal-regulating kinase 1 through phosphorylation of Ser-1034. Oncogene. 2004;23:5099–5104. doi: 10.1038/sj.onc.1207668. [DOI] [PubMed] [Google Scholar]
- Fujino G., Noguchi T., Matsuzawa A., Yamauchi S., Saitoh M., Takeda K., Ichijo H. Thioredoxin and TRAF family proteins regulate reactive oxygen species-dependent activation of ASK1 through reciprocal modulation of the N-terminal homophilic interaction of ASK1. Mol. Cell. Biol. 2007;27:8152–8163. doi: 10.1128/MCB.00227-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman E. H., Chen L., Fu H. Activation of apoptosis signal-regulating kinase 1 by reactive oxygen species through dephosphorylation at serine 967 and 14-3-3 dissociation. J. Biol. Chem. 2004;279:10442–10449. doi: 10.1074/jbc.M311129200. [DOI] [PubMed] [Google Scholar]
- Gotoh Y., Cooper J. A. Reactive oxygen species- and dimerization-induced activation of apoptosis signal-regulating kinase 1 in tumor necrosis factor-alpha signal transduction. J. Biol. Chem. 1998;273:17477–17482. doi: 10.1074/jbc.273.28.17477. [DOI] [PubMed] [Google Scholar]
- Haendeler J. Thioredoxin-1 and posttranslational modifications. Antioxid Redox Signal. 2006;8:1723–1728. doi: 10.1089/ars.2006.8.1723. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin structure and mechanism: conformational changes on oxidation of the active-site sulfhydryls to a disulfide. Structure. 1995;3:239–243. doi: 10.1016/s0969-2126(01)00153-8. [DOI] [PubMed] [Google Scholar]
- Ichijo H., Nishida E., Irie K., ten Dijke P., Saitoh M., Moriguchi T., Takagi M., Matsumoto K., Miyazono K., Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- Iriyama T., et al. ASK1 and ASK2 differentially regulate the counteracting roles of apoptosis and inflammation in tumorigenesis. EMBO J. 2009;28:843–853. doi: 10.1038/emboj.2009.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki H., et al. Amyloid beta induces neuronal cell death through ROS-mediated ASK1 activation. Cell Death Differ. 2005;12:19–24. doi: 10.1038/sj.cdd.4401528. [DOI] [PubMed] [Google Scholar]
- Kim A. H., Khursigara G., Sun X., Franke T. F., Chao M. V. Akt phosphorylates and negatively regulates apoptosis signal-regulating kinase 1. Mol. Cell. Biol. 2001;21:893–901. doi: 10.1128/MCB.21.3.893-901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry J., Chretien P., Lambert H., Hickey E., Weber L. A. Heat shock resistance conferred by expression of the human HSP27 gene in rodent cells. J. Cell Biol. 1989;109:7–15. doi: 10.1083/jcb.109.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent T. C., Moore E. C., Reichard P. Enzymatic Synthesis of Deoxyribonucleotides. Iv. Isolation and Characterization of Thioredoxin, the Hydrogen Donor from Escherichia Coli B. J. Biol. Chem. 1964;239:3436–3444. [PubMed] [Google Scholar]
- Lee S. R., Yang K. S., Kwon J., Lee C., Jeong W., Rhee S. G. Reversible inactivation of the tumor suppressor PTEN by H2O2. J. Biol. Chem. 2002;277:20336–20342. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- Liu H., Nishitoh H., Ichijo H., Kyriakis J. M. Activation of apoptosis signal-regulating kinase 1 (ASK1) by tumor necrosis factor receptor-associated factor 2 requires prior dissociation of the ASK1 inhibitor thioredoxin. Mol. Cell. Biol. 2000;20:2198–2208. doi: 10.1128/mcb.20.6.2198-2208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Min W. Thioredoxin promotes ASK1 ubiquitination and degradation to inhibit ASK1-mediated apoptosis in a redox activity-independent manner. Circ. Res. 2002;90:1259–1266. doi: 10.1161/01.res.0000022160.64355.62. [DOI] [PubMed] [Google Scholar]
- Matthews J. R., Wakasugi N., Virelizier J. L., Yodoi J., Hay R. T. Thioredoxin regulates the DNA binding activity of NF-kappa B by reduction of a disulphide bond involving cysteine 62. Nucleic Acids Res. 1992;20:3821–3830. doi: 10.1093/nar/20.15.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuillet E. J., Mahadevan D., Berggren M., Coon A., Powis G. Thioredoxin-1 binds to the C2 domain of PTEN inhibiting PTEN′s lipid phosphatase activity and membrane binding: a mechanism for the functional loss of PTEN′s tumor suppressor activity. Arch Biochem. Biophys. 2004;429:123–133. doi: 10.1016/j.abb.2004.04.020. [DOI] [PubMed] [Google Scholar]
- Mukherjee A., Martin S. G. The thioredoxin system: a key target in tumour and endothelial cells. Br. J. Radiol. 2008;81:S57–S68. doi: 10.1259/bjr/34180435. Spec No 1. [DOI] [PubMed] [Google Scholar]
- Nadeau P. J., Charette S. J., Toledano M. B., Landry J. Disulfide bond-mediated multimerization of Ask1 and its reduction by thioredoxin-1 regulate H2O2-induced c-Jun NH2-terminal kinase activation and apoptosis. Mol. Biol. Cell. 2007;18:3903–3913. doi: 10.1091/mbc.E07-05-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitoh H., Matsuzawa A., Tobiume K., Saegusa K., Takeda K., Inoue K., Hori S., Kakizuka A., Ichijo H. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 2002;16:1345–1355. doi: 10.1101/gad.992302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitoh H., Saitoh M., Mochida Y., Takeda K., Nakano H., Rothe M., Miyazono K., Ichijo H. ASK1 is essential for JNK/SAPK activation by TRAF2. Mol. Cell. 1998;2:389–395. doi: 10.1016/s1097-2765(00)80283-x. [DOI] [PubMed] [Google Scholar]
- Noguchi K., Kokubu A., Kitanaka C., Ichijo H., Kuchino Y. ASK1-signaling promotes c-Myc protein stability during apoptosis. Biochem. Biophys. Res. Commun. 2001;281:1313–1320. doi: 10.1006/bbrc.2001.4498. [DOI] [PubMed] [Google Scholar]
- Noguchi T., Ishii K., Fukutomi H., Naguro I., Matsuzawa A., Takeda K., Ichijo H. Requirement of reactive oxygen species-dependent activation of ASK1–p38 MAPK pathway for extracellular ATP-induced apoptosis in macrophage. J. Biol. Chem. 2008;283:7657–7665. doi: 10.1074/jbc.M708402200. [DOI] [PubMed] [Google Scholar]
- Noguchi T., Takeda K., Matsuzawa A., Saegusa K., Nakano H., Gohda J., Inoue J., Ichijo H. Recruitment of tumor necrosis factor receptor-associated factor family proteins to apoptosis signal-regulating kinase 1 signalosome is essential for oxidative stress-induced cell death. J. Biol. Chem. 2005;280:37033–37040. doi: 10.1074/jbc.M506771200. [DOI] [PubMed] [Google Scholar]
- Park H. S., Cho S. G., Kim C. K., Hwang H. S., Noh K. T., Kim M. S., Huh S. H., Kim M. J., Ryoo K., Kim E. K. Heat shock protein hsp72 is a negative regulator of apoptosis signal-regulating kinase 1. Mol. Cell. Biol. 2002;22:7721–7730. doi: 10.1128/MCB.22.22.7721-7730.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H. S., Yu J. W., Cho J. H., Kim M. S., Huh S. H., Ryoo K., Choi E. J. Inhibition of apoptosis signal-regulating kinase 1 by nitric oxide through a thiol redox mechanism. J. Biol. Chem. 2004;279:7584–7590. doi: 10.1074/jbc.M304183200. [DOI] [PubMed] [Google Scholar]
- Powis G., Montfort W. R. Properties and biological activities of thioredoxins. Annu Rev. Biophys. Biomol. Struct. 2001;30:421–455. doi: 10.1146/annurev.biophys.30.1.421. [DOI] [PubMed] [Google Scholar]
- Saadatzadeh M. R., Bijangi-Vishehsaraei K., Hong P., Bergmann H., Haneline L. S. Oxidant hypersensitivity of Fanconi anemia type C-deficient cells is dependent on a redox-regulated apoptotic pathway. J. Biol. Chem. 2004;279:16805–16812. doi: 10.1074/jbc.M313721200. [DOI] [PubMed] [Google Scholar]
- Saitoh M., Nishitoh H., Fujii M., Takeda K., Tobiume K., Sawada Y., Kawabata M., Miyazono K., Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwertassek U., Balmer Y., Gutscher M., Weingarten L., Preuss M., Engelhard J., Winkler M., Dick T. P. Selective redox regulation of cytokine receptor signaling by extracellular thioredoxin-1. EMBO J. 2007;26:3086–3097. doi: 10.1038/sj.emboj.7601746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J. J., Lee Y. J. Catalase, but not MnSOD, inhibits glucose deprivation-activated ASK1-MEK-MAPK signal transduction pathway and prevents relocalization of Daxx: hydrogen peroxide as a major second messenger of metabolic oxidative stress. J. Cell. Biochem. 2003a;90:304–314. doi: 10.1002/jcb.10619. [DOI] [PubMed] [Google Scholar]
- Song J. J., Lee Y. J. Differential role of glutaredoxin and thioredoxin in metabolic oxidative stress-induced activation of apoptosis signal-regulating kinase 1. Biochem. J. 2003b;373:845–853. doi: 10.1042/BJ20030275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J. J., Rhee J. G., Suntharalingam M., Walsh S. A., Spitz D. R., Lee Y. J. Role of glutaredoxin in metabolic oxidative stress. Glutaredoxin as a sensor of oxidative stress mediated by H2O2. J. Biol. Chem. 2002;277:46566–46575. doi: 10.1074/jbc.M206826200. [DOI] [PubMed] [Google Scholar]
- Stone J. R., Yang S. Hydrogen peroxide: a signaling messenger. Antioxid. Redox. Signal. 2006;8:243–270. doi: 10.1089/ars.2006.8.243. [DOI] [PubMed] [Google Scholar]
- Tobiume K., Matsuzawa A., Takahashi T., Nishitoh H., Morita K., Takeda K., Minowa O., Miyazono K., Noda T., Ichijo H. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001;2:222–228. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobiume K., Saitoh M., Ichijo H. Activation of apoptosis signal-regulating kinase 1 by the stress-induced activating phosphorylation of pre-formed oligomer. J. Cell. Physiol. 2002;191:95–104. doi: 10.1002/jcp.10080. [DOI] [PubMed] [Google Scholar]
- Watanabe T., et al. Apoptosis signal-regulating kinase 1 is involved not only in apoptosis but also in non-apoptotic cardiomyocyte death. Biochem. Biophys. Res. Commun. 2005;333:562–567. doi: 10.1016/j.bbrc.2005.05.151. [DOI] [PubMed] [Google Scholar]
- Zhang L., Chen J., Fu H. Suppression of apoptosis signal-regulating kinase 1-induced cell death by 14–3-3 proteins. Proc. Natl. Acad Sci. USA. 1999;96:8511–8515. doi: 10.1073/pnas.96.15.8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Zhang R., Luo Y., D'Alessio A., Pober J. S., Min W. AIP1/DAB2IP, a novel member of the Ras-GAP family, transduces TRAF2-induced ASK1-JNK activation. J. Biol. Chem. 2004a;279:44955–44965. doi: 10.1074/jbc.M407617200. [DOI] [PubMed] [Google Scholar]
- Zhang R., Al-Lamki R., Bai L., Streb J. W., Miano J. M., Bradley J., Min W. Thioredoxin-2 inhibits mitochondria-located ASK1-mediated apoptosis in a JNK-independent manner. Circ Res. 2004b;94:1483–1491. doi: 10.1161/01.RES.0000130525.37646.a7. [DOI] [PubMed] [Google Scholar]
- Zhang W., Zheng S., Storz P., Min W. Protein kinase D specifically mediates apoptosis signal-regulating kinase 1-JNK signaling induced by H2O2 but not tumor necrosis factor. J. Biol. Chem. 2005;280:19036–19044. doi: 10.1074/jbc.M414674200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.