Abstract
Oxidative stress and inflammation are implicated in the pathogenesis of many age-related diseases. We have demonstrated previously that oxidative inactivation of the proteasome is a molecular link between oxidative stress and overexpression of interleukin (IL)-8. Here, we elucidated a novel signaling cascade that leads to up-regulation of IL-8 in response to proteasome inactivation. The sequence of events in this cascade includes proteasome inactivation, activation of mitogen-activated protein kinase kinase (MKK)3/MKK6, activation of p38 mitogen-activated protein kinase (MAPK), epidermal growth factor receptor phosphorylation, phosphatidylinositol 3-kinase (PI3K) activation and increased IL-8 expression. Blocking any of these signaling pathways abolished the up-regulation of IL-8 induced by proteasome inhibition. Although Akt is also activated in response to proteasome inactivation, we found that the PI3K-dependent up-regulation of IL-8 is independent of 3-phosphoinositide-dependent protein kinase (PDK)1 and Akt. Inhibition of PDK1 and Akt with chemical inhibitors or expression of constitutive active Akt had little effects on IL-8 expression in response to proteasome inactivation. In contrast, inhibition of interleukin 2-inducible T cell kinase, a kinase downstream of PI3K, significantly reduced the expression and secretion of IL-8 in response to proteasome inactivation. Together, these data elucidate a novel signaling network that leads to increased IL-8 production in response to proteasome inactivation.
INTRODUCTION
Oxidative stress, which refers to cellular damage caused by reactive oxygen species, has been implicated in the onset and progression of many age-related diseases, including age-related macular degeneration (AMD), arthritis, atherosclerosis, and certain types of cancer (Beatty et al., 2000; Lavrovsky et al., 2000; Chung et al., 2006). Inflammatory events are also known to participate in the pathogenesis of these age-related diseases. However, the molecular links between oxidative stress and inflammation are not fully understood.
Retina has the highest metabolic rate and oxygen consumption in the body. The high metabolic rate and oxygen consumption is usually accompanied by generation of reactive oxygen species. Chronic exposure to light may further increase the production of reactive oxygen species (Beatty et al., 2000; Liang and Godley, 2003). Therefore, the retinal pigment epithelium (RPE) is a primary target of oxidative stress.
An increasing body of literature indicates that oxidative stress and dysfunction of RPE are associated with the pathogenesis of AMD (Boulton et al., 2004; Zarbin, 2004; Zhou et al., 2005), the leading cause of blindness in industrialized countries. Recent studies indicate that inflammation is an important component of AMD (Zarbin, 2004; McGeer et al., 2005; Donoso et al., 2006) and that oxidative stress in RPE can trigger the activation of the complement system (Zhou et al., 2006). Moreover, complement activation is associated with enhanced expression of interleukin (IL)-8, an important inflammatory cytokine (Fukuoka and Medof, 2001; Fukuoka et al., 2003). Increased expression of IL-8 was also reported when RPE were fed with oxidized photoreceptor outer segments (Higgins et al., 2003). The increased expression of IL-8 may account, at least in part, for the inflammatory reactions during the development of AMD (Higgins et al., 2003; Kalayoglu et al., 2005). However, the molecular mechanisms that regulate IL-8 expression under these conditions remain to be elucidated.
The ubiquitin–proteasome pathway (UPP) is the major nonlysosomal proteolytic pathway within cells (Glickman and Ciechanover, 2002; Ciechanover, 2003; Shang and Taylor, 2004). Proteins destined for degradation are first conjugated with a polyubiquitin chain by the sequential action of three classes of enzymes: E1, E2, and E3. The ubiquitin-protein conjugates are then recognized and degraded by a large protease complex called the proteasome (Pickart, 2001; Glickman and Ciechanover, 2002). The UPP is involved in a myriad of cellular processes (Shang and Taylor, 2004; Welchman et al., 2005), including regulation of immune response and inflammation (Kloetzel, 2004; Qureshi et al., 2005). Dysfunction of the UPP has been implicated in the pathogenesis of many age-related degenerative diseases, such as Alzheimer's disease (Hope et al., 2003), Parkinson's disease (Dawson and Dawson, 2003), diabetic retinopathy (Fernandes et al., 2006b), and cataracts (Jahngen-Hodge et al., 1992; Shang et al., 1997; Shang et al., 2001; Dudek et al., 2005).
Consistent with an age-related decline in proteasome activity in many other tissues, a decline in proteasome activity upon ageing also was reported in the neural retina (Louie et al., 2002; Kapphahn et al., 2007). Our preliminary data also indicate that there is an age-dependent decline in proteasome activity in both RPE and neuronal retina of rats (Shang, Zhang, and Taylor, unpublished data). However, it remains to be determined whether the age-related decline in proteasome activity in the retina plays a role in the pathogenesis of AMD. Furthermore, a recent study showed that advanced AMD is associated with transformation of the proteasome into immunoproteasome in the retina (Ethen et al., 2007). The transformation into immunoproteasome may reflect retinal response to local inflammation, a causal factor for AMD.
We have demonstrated previously that the proteasome is a target of oxidative damage in cultured RPE (Zhang et al., 2008) and that oxidative inactivation of the proteasome is a mechanistic link between oxidative stress and up-regulation of IL-8 production in RPE. We demonstrated that inactivation of the proteasome for 8 h or longer up-regulates IL-8 production through the activation of the p38 mitogen-activated protein kinase (MAPK) pathway (Fernandes et al., 2008). However, the signaling events downstream of p38 MAPK activation that lead to the increased IL-8 production upon prolonged (≥8 h) proteasome inactivation remain elusive. The data presented in this work suggest that the increased IL-8 production upon prolonged inactivation of the proteasome is controlled by putative sequential events of activation of p38 MAPK, phosphorylation of epidermal growth factor receptor (EGFR), and activation of the phosphatidylinositol 3-kinase (PI3K) pathway. Furthermore, we identify interleukin 2-inducible T cell kinase (Itk) as a novel regulator of IL-8 expression. Together, this study revealed a novel signaling network that links proteasome inactivation and overproduction of IL-8 in RPE.
MATERIALS AND METHODS
Materials
DMEM was obtained from Invitrogen (Carlsbad, CA). Fetal bovine serum (FBS) was purchased from HyClone Laboratories (Logan, UT). Epoxomicin was obtained from Boston Biochem (Cambridge, MA). MG132, SB203580, LY294002, Akt inhibitor IV, Akt inhibitor X, and AG1478 were purchased from Calbiochem (San Diego, CA). Epidermal growth factor (EGF) and platelet-derived growth factor (PDGF) were obtained from PeproTech (Rocky Hill, NJ). BX912 was purchased from Axon Medchem BV (Groningen, The Netherlands). The synthesis of BMS509744 was performed as described previously (Readinger et al., 2008). The SuperFect Transfection Reagent was obtained from QIAGEN (Valencia, CA). Insulin, sodium orthovanadate, and the monoclonal antibody (mAb) to β-actin were purchased from Sigma-Aldrich (St. Louis, MO). The nitrocellulose membrane for Western blot was obtained from Bio-Rad Laboratories (Hercules, CA). Rabbit polyclonal antibodies to phospho-p38 MAPK, phospho-Akt, phospho-EGF receptor (Tyr1045), phospho-EGF receptor (Tyr1068), total p38 MAPK, total Akt, and total EGF receptor were purchased from Cell Signaling Technology (Danvers, MA). Goat anti-rabbit immunoglobulin G (IgG) and sheep anti-mouse IgG were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA). The SuperSignal chemiluminescent detection kit was purchased from Pierce Chemical (Rockford, IL). The DuoSet enzyme-linked immunosorbent assay (ELISA) kit for IL-8 was obtained from R&D Systems (Minneapolis, MN).
Plasmids
The plasmids expressing a constitutively active (pCMV6.Myr.Akt.HA) and a mutant (pCMV6.HA.Akt.K/M) form of Akt were kindly provided by Dr. Alex Toker (Harvard Medical School, Boston, MA). The plasmids expressing constitutively active forms of mitogen-activated protein kinase kinase (MKK)3 [MKK3b (E)-pcDNA3] and MKK6 [MKK6b (E)-pcDNA3] and the mutant forms of MKK3 [MKK3b (A)-pcDNA3] and MKK6 [MKK6b (A)-pcDNA3] were generous gifts from Dr. Jiahuai Han (The Scripps Research Institute, La Jolla, CA).
Cell Culture and Treatments
The retinal pigment epithelial cell line ARPE-19 (Dunn et al., 1996) was obtained from American Type Culture Collection (Manassas, VA). The cells were routinely maintained at 37°C under 5% CO2 and cultured in DMEM supplemented with 10% FBS and containing 100 U/ml penicillin G and 100 μg/ml streptomycin. Before treatments, confluent cell monolayers were rinsed once with phosphate-buffered saline (PBS), and fresh medium was added. For proteasome inhibition studies, MG132 and epoxomicin were prepared in dimethyl sulfoxide (DMSO) at 10 mM (MG132) or 5 mM (epoxomicin) and diluted to 10 μM (MG132) and 5 μM (epoxomicin) in the cell medium immediately before use. For Akt phosphorylation studies (see Figure 2A), cells were incubated with proteasome inhibitors for different times as indicated in the figure legend. In the other experiments, ARPE-19 cells were incubated with epoxomicin or MG132 for 8 h. The p38 MAPK inhibitor SB203580 was prepared in DMSO at 10 mM and then diluted to 10 μM in the cell medium immediately before use. The Akt inhibitors Akt IV and Akt X were prepared in DMSO at 20 mM and then diluted to 10 μM (Akt IV) and 5 μM (Akt X) in the cell medium just before use. The PI3K inhibitor LY294002 was prepared in DMSO at 20 mM and then diluted to 10 μM in the cell medium immediately before use. The EGFR inhibitor AG1478 was prepared in DMSO at 16 mM and then diluted to 1 μM in the cell medium just before use. The 3-phosphoinositide-dependent protein kinase (PDK)1 inhibitor BX912 was prepared in DMSO at 10 mM and then diluted to 10 μM in the cell medium immediately before use. The Itk inhibitor BMS509744 was prepared in DMSO at 10 mM and then diluted to 20 μM in the cell medium just before use. EGF was prepared in water, and PDGF was prepared in 10 mM acetic acid at a concentration of 100 μg/ml and then diluted to 1 ng/ml (EGF) and 10 ng/ml (PDGF) in the cell medium immediately before use. Insulin was prepared in PBS at 4 μg/μl and then diluted to 1 μg/ml in the medium just before use.
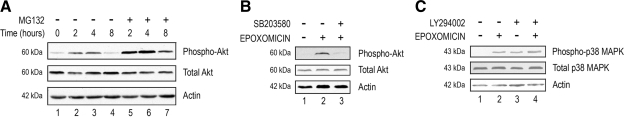
Figure 2.
p38 MAPK activation precedes Akt activation after proteasome inactivation in RPE. (A) ARPE-19 cells were cultured in the presence or absence of MG132, a proteasome inhibitor, for 0, 2, 4, and 8 h. (B) ARPE-19 cells were cultured in the presence or absence of epoxomicin and epoxomicin plus SB203580 for 8 h. (C) ARPE-19 cells were cultured in the presence or absence of epoxomicin, LY294002, and epoxomicin plus LY294002 for 8 h. Levels of endogenous phospho-p38 MAPK, total p38 MAPK, phospho-Akt, total Akt, and actin were detected by Western blot by using polyclonal (to phosphorylated and total p38 MAPK and phosphorylated and total Akt) and monoclonal antibodies (to actin). The figures are representative of three independent experiments with similar results.
Cell Transfection
Subconfluent (70% confluence) ARPE-19 cells were used to perform transfection experiments. Cells were grown on 60-mm dishes. On the day of transfection, 6 μg of the DNA of interest was mixed with 30 μl of SuperFect transfection reagent and DMEM without serum or antibiotics to a total volume of 150 μl and incubated at room temperature for 20 min to allow transfection-complex formation. While complex formation took place, the cell medium was aspirated from the dishes, and cells were washed once with PBS. One milliliter of DMEM containing 10% FBS and antibiotics was then added to each dish before addition of 150 μl of the transfection complexes. The cells were incubated at 37°C for 4 h, and the medium was replaced with fresh DMEM containing 10% FBS. The cells were then incubated at 37°C for 40 h before they were used for treatments.
Western Blot Analysis
Whole cell lysates were prepared for Western Blot analysis. After treatment, cells were rinsed once with ice-cold PBS supplemented with 2 mM sodium orthovanadate, a phosphatase inhibitor, and immediately collected in SDS loading buffer. Cell lysates were then denatured at 100°C for 5 min. Equal amounts of protein (50 or 100 μg/lane) were resolved on 7.5–12% SDS-polyacrylamide gels and transferred to nitrocellulose membranes. Membranes were then probed with rabbit polyclonal antibodies against phospho-p38 MAPK, phospho-Akt, phospho-EGF receptor (Tyr1045), phospho-EGF receptor (Tyr1068), total p38 MAPK, total Akt, and total EGF receptor, or mouse mAb against β-actin. After incubation with the corresponding horse radish peroxidase-conjugated secondary antibodies, the specific bound antibody was visualized using SuperSignal chemiluminescent detection kit.
ELISA
Levels of IL-8 secreted into the medium by RPE were determined by ELISA. All ELISAs were performed according to the manufacturer's instructions. IL-8 levels in untreated cells were defined as 100%, and the IL-8 levels measured in the different experimental conditions are expressed as relative levels compared with the untreated cells.
Real-Time Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Total RNA extraction from ARPE-19 cells and real time RT-PCR analysis were performed as described previously (Fernandes et al., 2006a). For IL-8, the forward primer was 5′-AAACCACCGGAAGGAACCAT-3′ and the reverse primer was 5′-CCTTCACACAGAGCTGCAGAAA-3′. Levels of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used for normalization of the total mRNA amount. For quantification of GAPDH mRNA, the forward primer was 5′-ATCACCATCTTCCAGGAGCGA-3′ and the reverse primer was 5′-CCTTCTCCATGGTGGTGAAGAC-3′.
Statistical Analyses
Statistical analysis was performed using Student's t test assuming equal variances for all data points.
RESULTS
Proteasome Inhibition Up-Regulates IL-8 Production in a PI3K-dependent Manner
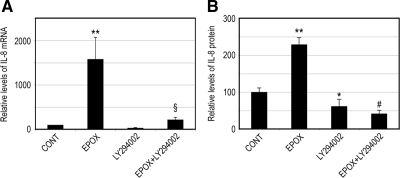
We have shown previously that oxidative stress can up-regulate IL-8 production in RPE by inactivating the proteasome and activating the p38 MAPK pathway (Fernandes et al., 2008). However, the downstream events underlying the enhanced IL-8 production in response to proteasome inhibition are still unclear. It has been shown that the PI3K/Akt pathway is also involved in the regulation of IL-8 (Newcomb et al., 2005; Kim et al., 2006). To assess the involvement of this signaling pathway in the regulation of IL-8 expression in response to proteasome inhibition, we tested the effect of a PI3K inhibitor on IL-8 production after 8 h of proteasome inhibition. As shown in Figure 1A, proteasome inhibition up-regulated IL-8 mRNA by 15-fold. LY294002, a PI3K inhibitor, was able to prevent the IL-8 mRNA up-regulation induced by proteasome inhibitors. Even in control cells, LY294002 led to a decrease in IL-8 gene expression. Consistent with the effects on mRNA levels, proteasome inhibitors also dramatically increased secretion of IL-8 by RPE cells, and this effect was abolished in the presence of PI3K inhibitor (Figure 1B).
Figure 1.
Proteasome inhibition up-regulates IL-8 production in a PI3K-dependent manner. ARPE-19 cells were cultured in the presence or absence of epoxomicin, LY294002, and epoxomicin plus LY294002 for 8 h. (A) Levels of mRNA for IL-8 were assessed by real-time RT-PCR analysis. GAPDH mRNA was used as a control to normalize the total mRNA levels. (B) IL-8 protein levels in the medium were determined by ELISA. The results are the mean ± SD of three independent experiments. p < 0.05 and **p < 0.01 compared with the control; #p < 0.001 and §p = 0.05 compared with epoxomicin alone.
p38 MAPK Activation Precedes Akt Activation after Proteasome Inactivation
The above-mentioned data suggest that the PI3K pathway is involved in the up-regulation of IL-8 upon prolonged (8 h or longer) proteasome inhibition. To further prove this hypothesis, we evaluated the effect of proteasome inhibition on PI3K activation using phosphorylated Akt as a marker of PI3K activity (Hawkins et al., 2006). Figure 2A shows that Akt was phosphorylated by replacing the medium and that its phosphorylation levels peaked between 2 and 4 h after addition of fresh medium. Proteasome inhibition resulted in the accumulation of phosphorylated Akt compared with the respective controls (Figure 2A, compare lanes 5 and 6 with lanes 2 and 3, respectively). This accumulation was observed as early as 2 h. By 8 h, phosphorylated Akt was barely detectable in control cells, whereas it could readily be detected in cells treated with proteasome inhibitors (Figure 2A, compare lane 7 with lane 4). Densitometry scanning of the Western blots showed that the levels of phosphorylated Akt in cells treated with proteasome inhibitors were two- to fourfold higher than those observed in the control cells at the corresponding time points.
Together with our previous observations (Fernandes et al., 2008), these results suggest that proteasome impairment in RPE activates p38 MAPK and PI3K and up-regulates IL-8 production. However, how proteasome inhibition affects these two pathways is not clear. For example, it is not clear whether activation of the p38 MAPK and PI3K pathways are two separated events or two related sequential events. To assess a possible cross-talk between these two pathways in response to proteasome inactivation, we tested the effect of p38 MAPK and PI3K inhibitors on the phosphorylation of Akt and p38 MAPK, respectively. As shown in Figure 2B, proteasome inhibition resulted in PI3K activation, as indicated by the increased levels of phosphorylated Akt (compare lane 2 with lane 1). This activation seems to be p38 MAPK dependent, because the presence of a p38 MAPK inhibitor almost prevented (>90%) the phosphorylation of Akt induced by proteasome inhibition (Figure 2B, compare lane 3 with lane 2). To further determine the putative cross-talk between the p38 MAPK and PI3K pathways in response to proteasome inhibition, we assessed the effect of a PI3K inhibitor on the activation of p38 MAPK. As shown previously (Fernandes et al., 2008), prolonged proteasome inhibition resulted in the activation of p38 MAPK as indicated by the increased levels of phosphorylated p38 MAPK (Figure 2C, compare lane 2 with lane 1). However, inhibition of PI3K by LY294002 did not prevent the activation of p38 MAPK induced by proteasome inhibition, as indicated by the levels of phosphorylated p38 MAPK (Figure 2C, compare lane 4 with lane 2). In fact, LY294002, by itself, was able to activate p38 MAPK (Figure 2C, compare lane 3 with lane 1). Together, these data suggest that proteasome inhibition in RPE results in p38 MAPK activation, which, in turn, promotes the activation of the PI3K pathway.
EGFR Is Involved in the IL-8 Production in Response to Proteasome Inhibition
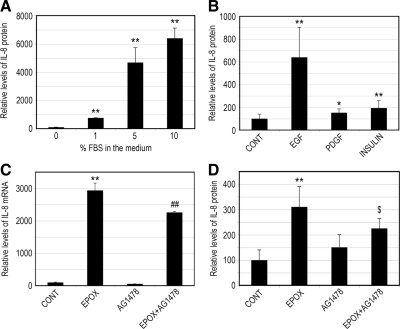
The above-mentioned data indicate that PI3K is activated after proteasome inactivation and that this activation is required for IL-8 production in RPE. To further verify the involvement of PI3K in the production of IL-8 in RPE, we incubated ARPE-19 cells with culture medium containing different amounts of FBS for 8 h and assessed the effect of serum concentrations on IL-8 secretion. As shown in Figure 3A, FBS increased secretion of IL-8 in a dose-dependent manner, with the culture medium containing 10% FBS inducing a dramatic increase (>60-fold) in the secretion of IL-8 compared with serum-free medium.
Figure 3.
The EGFR is involved in the IL-8 production in response to proteasome inhibition. (A) ARPE-19 cells were cultured in the presence of 0, 1, 5, and 10% FBS for 8 h. (B) ARPE-19 cells were cultured in the presence of different growth factors (EGF, PDGF, and insulin) in serum-free medium for 8 h. (C and D) ARPE-19 cells were cultured in the presence or absence of epoxomicin, AG1478, and epoxomicin plus AG1478 in serum-free medium for 8 h. Levels of mRNA for IL-8 were assessed by real-time RT-PCR analysis (C). GAPDH mRNA was used as a control to normalize the total mRNA levels. IL-8 protein levels in the medium were determined by ELISA (A, B, and D). The results are the mean ± SD of three independent experiments. *p < 0.05 and **p < 0.01 compared with the control; $p < 0.05 and ##p < 0.09 compared with epoxomicin alone.
PI3K is activated by a large number of receptor tyrosine kinases (Schlessinger, 2000), which are triggered by many growth factors present in the serum. To evaluate the contribution of different growth factors to IL-8 production, we incubated ARPE-19 cells for 8 h with various growth factors known to activate the PI3K, and then we assessed their effects on IL-8 secretion. Figure 3B shows that the EGF greatly enhanced IL-8 production in RPE cells. Incubation of ARPE-19 cells with this growth factor resulted in a sixfold increase in the secretion of IL-8 (Figure 3B). Insulin and PDGF, two growth factors also known to activate PI3K (Gschwind et al., 2004), led to modest increases in IL-8 secretion (Figure 3B). These data suggest that EGF plays a major role in IL-8 production, presumably by activation of the PI3K pathway.
Given that the EGFR is known to activate PI3K (Gschwind et al., 2004) and is regulated by the UPP (Ettenberg et al., 1999; Levkowitz et al., 1999), we hypothesized that the increase in IL-8 production after long-term proteasome inhibition could be mediated, at least in part, by the activation of EGFR. To test this hypothesis, we assessed the effect of AG1478, a specific EGFR inhibitor, on IL-8 production induced by proteasome inhibition. As shown previously, proteasome inhibition induced a dramatic increase in IL-8 mRNA levels (Figure 3C). Treatment of cells with AG1478 partially prevented this effect. Consistently, treatment of cells with AG1478 also partially prevented the increase in IL-8 secretion induced by proteasome inhibition (Figure 3D). Collectively, these data imply that activation of EGFR is involved in the increased IL-8 production after long-term proteasome inactivation in RPE.
EGFR Activation Induced by Proteasome Inhibition Is p38 MAPK Dependent
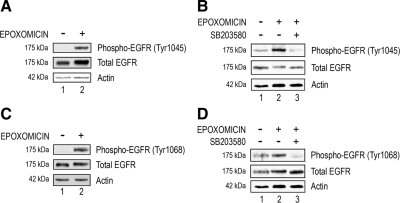
The data obtained with AG1478 suggest that EGFR is involved in the up-regulation of IL-8 induced by proteasome inhibition. To further support this hypothesis, we determined the effect of proteasome inhibition on EGFR phosphorylation. EGFR can be phosphorylated at several residues, and these different phosphorylations result in different outcomes (Bishayee, 2000). Because p38 MAPK was shown to phosphorylate EGFR at tyrosine 1045 (Frey et al., 2006) and our data show that proteasome inhibition activates p38 MAPK, we determined the effect of prolonged proteasome inhibition on the phosphorylation of EGFR at tyrosine 1045. To preclude the possibility that the EGFR is being activated in response to the growth factors present in the medium, we used serum-free and growth factor-free medium during the incubation with proteasome inhibitors. As shown in Figure 4A, prolonged proteasome inhibition resulted in increased phosphorylation of tyrosine 1045 of EGFR (Figure 4, A and B, compare lane 2 with lane 1). The extent of this increase varied from experiment to experiment, ranging from two- to fivefold, but the increase was consistently observed in every experiment. Moreover, this phosphorylation seems to be p38 MAPK dependent, because inhibition of p38 MAPK by SB203580 abolished the phosphorylation of EGFR induced by proteasome inhibition (Figure 4B, compare lane 3 with lane 2). Phosphorylation of tyrosine 1068 in EGFR works as a binding site for Grb2-associated protein 1 and this docking protein links EGFR signaling to the PI3K pathway (Rodrigues et al., 2000). Therefore, phosphorylation of tyrosine 1068 may contribute to PI3K activation. We found that proteasome inhibitors also increased phosphorylation of EGFR at tyrosine 1068 by two- to fivefold (Figure 4, C and D, compare lane 2 with lane 1). Just like the phosphorylation at tyrosine 1045, the phosphorylation at tyrosine 1068 in the EGFR in response to prolonged proteasome inhibition is also p38 MAPK dependent, because it was abrogated in the presence of SB203580 (Figure 4D, compare lane 3 with lane 2). We also observed a slight shift in the molecular weight of total EGFR when the proteasome was inhibited compared with the controls (Figure 4, compare lane 2 with lane 1 in all panels). This shift may be due to phosphorylation by p38 MAPK. Consistently, this shift was abolished when SB203580 was used (Figure 4, B and D, compare lane 3 with lane 2). Together, these data indicate that prolonged proteasome inhibition activates EGFR via activation of p38 MAPK.
Figure 4.
EGFR phosphorylation upon proteasome inhibition is p38 MAPK dependent. ARPE-19 cells were cultured in the presence or absence of epoxomicin (A and C) and epoxomicin plus SB203580 (B and D) in serum-free medium for 8 h. Levels of endogenous phospho-EGFR (Tyr1045 and Tyr1068), total EGFR, and actin were detected by Western blot using polyclonal (to phosphorylated and total EGFR) and monoclonal antibodies (to actin). (A and B) EGFR phosphorylation at Tyr1045 position is shown. (C and D) EGFR phosphorylation at Tyr1068 position is shown. The figures are representative of three independent experiments with similar results.
MKK3 and MKK6 Increase IL-8 Production in an EGFR- and PI3K-dependent Manner
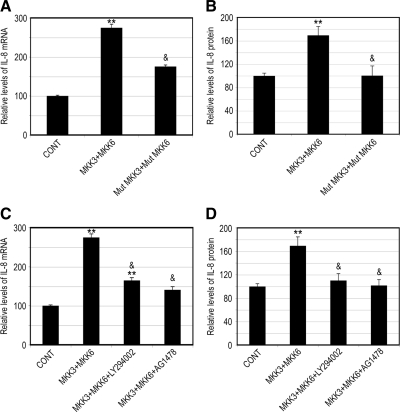
We have shown previously that proteasome inhibition activates MKK3 and MKK6, the upstream kinases involved in the activation of p38 MAPK (Fernandes et al., 2008). To test whether activation of these kinases is sufficient for induction of IL-8 production, we assessed the effects of overexpression of MKK3 and MKK6 on IL-8 production. To rule out the participation of other signaling pathways activated by growth factors present in the serum, we used serum-free and growth factor-free medium in these experiments. Cells transfected with vectors encoding a constitutively active form of MKK3 and MKK6 (MKK3 + MKK6) expressed higher levels of IL-8 (∼2.75-fold), compared with RPE cells transfected with empty vectors (Figure 5A). Expression of a mutant form of MKK3 and MKK6 (Mut MKK3 + Mut MKK6) had a subtle effect on IL-8 gene expression. Consistent with the effect on mRNA levels, overexpression of MKK3 and MKK6 also increased IL-8 secretion (Figure 5B), and only a slight effect was observed when mutant forms of MKK3 and MKK6 were expressed (Figure 5B). These data indicate that activation of MKK3 and MKK6 is sufficient to trigger the production of IL-8. To further determine whether EGFR and PI3K are involved in the signaling cascade downstream of MKK3 and MKK6 responsible for the enhanced IL-8 expression, we determined the effect of EGFR and PI3K inhibitors on the increased IL-8 production induced by MKK3 and MKK6. The data show that the enhanced levels of mRNA for IL-8 in the presence of a constitutively active form of MKK3 and MKK6 were diminished when LY294002 or AG1478 (inhibitors of the PI3K and EGFR, respectively) were added (Figure 5C). Consistently, the increase in IL-8 protein levels after MKK3 and MKK6 overexpression was also blocked by EGFR or PI3K inhibitors (Figure 5D).
Figure 5.
MKK3 and MKK6 activation increases IL-8 production in an EGFR- and PI3K-dependent manner. ARPE-19 cells were transfected with an empty vector (CONT) or cotransfected with vectors encoding constitutively active (MKK3 + MKK6) and mutant (Mut MKK3+Mut MKK6) forms of MKK3 and MKK6. After 40 h of transfection, ARPE-19 cells were incubated in fresh serum-free medium for 8 h (A and B). Alternatively, ARPE-19 cells were incubated in the presence of LY294002 or AG1478 in serum-free medium for 8 h (C and D). Levels of mRNA for IL-8 were assessed by real-time RT-PCR analysis (A and C). GAPDH mRNA was used as a control to normalize the total mRNA levels. IL-8 protein levels in the medium were determined by ELISA (B and D). The results are the mean ± SD of three independent experiments. **p < 0.01 compared with the control; &p < 0.01 compared with MKK3 + MKK6 alone.
Up-Regulation of IL-8 in Response to Proteasome Inhibition Is Independent of Akt Activity
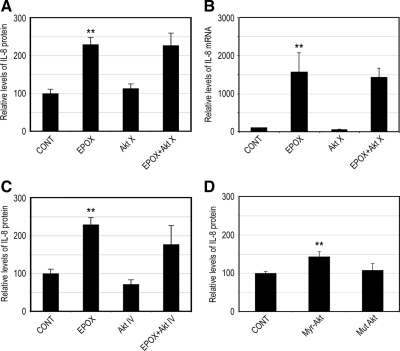
The above-mentioned data presented along with our previous data (Fernandes et al., 2008) suggest that proteasome inhibition activates p38 MAPK, the EGFR, and PI3K and that activation of these pathways is required for the enhanced IL-8 production under these conditions. Akt is a classic downstream effector of PI3K (Hawkins et al., 2006), and it was shown to be involved in the regulation of IL-8 in other cell types (Newcomb et al., 2005; Wen et al., 2006). To test whether activation of Akt is responsible for the increased IL-8 production in response to prolonged proteasome inhibition, we evaluated the effect of a specific Akt inhibitor on the increased IL-8 production induced by proteasome inhibition. Figure 6A shows that proteasome inhibition dramatically increased IL-8 secretion in RPE. Treatment with Akt X, a specific inhibitor of Akt, was not able to prevent the effect of proteasome inhibition on IL-8 production. Consistent with the protein levels, treatment with Akt X did not prevent the up-regulation of IL-8 mRNA induced by proteasome inhibitors (Figure 6B). Treatment of ARPE-19 cells with Akt IV, another specific inhibitor of Akt, also failed to prevent the effect of proteasome inhibition on the secretion of IL-8 (Figure 6C). These data suggest that, although Akt is activated upon proteasome inhibition, Akt activity is not required for the up-regulation of IL-8 induced by proteasome inhibition. To further corroborate this hypothesis, we manipulated the levels of activated Akt by overexpressing a constitutively active or a mutant Akt and tested their effects on IL-8 production. Cells transfected with a vector encoding a constitutively active form of Akt (Myr-Akt) produced slightly higher levels of IL-8 (Figure 6D), compared with the empty vector. Overexpression of a mutant form of Akt (Mut Akt) had no detectable effect on IL-8 expression (Figure 6D). Together, these data indicate that the increased IL-8 production in response to prolonged proteasome inhibition is independent of Akt activity.
Figure 6.
Up-regulation of IL-8 in response to proteasome inhibitors is independent of activation of Akt. (A–C) ARPE-19 cells were cultured in the presence or absence of epoxomicin, Akt X, and epoxomicin plus Akt X (A and B) or Akt IV and epoxomicin plus Akt IV (C) for 8 h. (D) ARPE-19 cells were transfected with an empty vector (CONT) or vectors encoding a constitutively active (Myr-Akt) and a mutant (Mut Akt) form of Akt. After 40 h of transfection, ARPE-19 cells were incubated in fresh serum-free medium for 8 h. Levels of mRNA for IL-8 were assessed by real-time RT-PCR analysis (B). GAPDH mRNA was used as a control to normalize the total mRNA levels. IL-8 protein levels in the medium were determined by ELISA (A, C, and D). The results are the mean ± SD of three independent experiments. **p < 0.01 compared with the control.
Itk Regulates IL-8 Production upon Proteasome Inhibition
Our data demonstrate that proteasome inactivation increases IL-8 production in a PI3K-dependent, but Akt-independent manner. Although surprising, these data are consistent with the existence of PI3K-dependent, Akt-independent pathways (Vivanco and Sawyers, 2002). To identify the pathways downstream PI3K responsible for the enhanced IL-8 production in response to proteasome inhibition, we first evaluated the effect of a specific PDK1 inhibitor on the increased IL-8 production induced by proteasome inhibition. PDK1 is a PI3K-dependent serine/threonine kinase and is responsible for phosphorylation and activation of PI3K effectors, such as Akt (Vivanco and Sawyers, 2002).
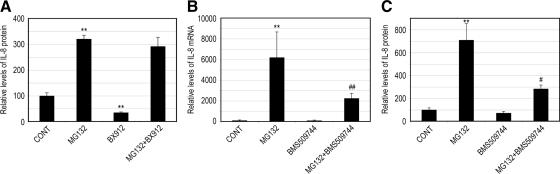
As shown in Figure 7A, although the presence of a specific PDK1 inhibitor (BX912) reduced IL-8 production in control cells, it did not prevent the IL-8 production induced by proteasome inhibition (Figure 7A), suggesting that the increased IL-8 production under these conditions is independent of PDK1 activity.
Figure 7.
Itk regulates IL-8 production upon proteasome inhibition. ARPE-19 cells were cultured in the presence or absence of MG132, BX912, MG132 plus BX912 (A), or BMS509744 and MG132 plus BMS509744 (B and C) for 8 h. Levels of mRNA for IL-8 were assessed by real-time RT-PCR analysis (B). GAPDH mRNA was used as a control to normalize the total mRNA levels. IL-8 protein levels in the medium were determined by ELISA (A and C). The results are the mean ± SD of three independent experiments. **p < 0.01 compared with the control; #p < 0.001 and ##p = 0.08 compared with MG132 alone.
To search for the effectors downstream of PI3K that are responsible for the up-regulation of IL-8 upon proteasome inhibition, we tested the effect of Itk inhibition on IL-8 production in response to proteasome inactivation. Itk is a nonreceptor tyrosine kinase belonging to the tyrosine kinase expressed in hepatocellular carcinoma (Tec) family (Readinger et al., 2009). This kinase is highly expressed in T cells and its activation is PI3K dependent. Data in Figure 7B show that treatment of the cells with BMS509744 (a specific and potent inhibitor of Itk) had little effects on IL-8 expression. However, the presence of BMS509744 significantly diminished the up-regulation of IL-8 induced by proteasome inhibition. Consistent with the effects on mRNA levels for IL-8, the presence of an Itk inhibitor did not alter IL-8 secretion in control cells but suppressed the enhancement of IL-8 secretion by RPE cells after proteasome inhibition (Figure 7C). These data indicate that Itk is one of the regulators downstream of PI3K that are responsible for the IL-8 overproduction upon proteasome inactivation in RPE cells.
DISCUSSION
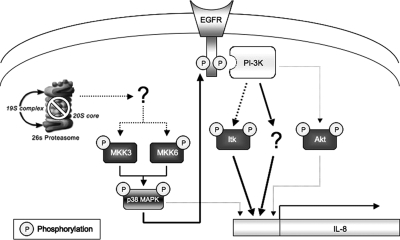
We have demonstrated previously that oxidative inactivation of the proteasome is a molecular link between oxidative stress and overproduction of IL-8 (Fernandes et al., 2008). In the present work, we elucidate a novel signaling pathway through which proteasome inhibition triggers the overproduction of IL-8. This work shows that exposing ARPE-19 cells to specific proteasome inhibitors resulted in a dramatic increase in the expression and secretion of IL-8 (Figures 1, 3, 6, and 7). Proteasome inhibition in RPE also activated p38 MAPK, leading to EGFR phosphorylation (Figure 4) and PI3K activation (Figure 2). Consistently, inhibition of any of these signaling pathways abolished the enhanced IL-8 production in response to proteasome inhibition (Figures 1 and 3). These data indicate that the increase in IL-8 production in response to proteasome inhibition is regulated by a sequence of signaling events, as illustrated in Figure 8. In this model, proteasome impairment results in the activation of MKK3 and MKK6, the upstream kinases involved in the activation of p38 MAPK. Activation of p38 MAPK results in EGFR phosphorylation, which in turn leads to the activation of downstream signaling pathways, such as PI3K. The PI3K then activates Itk, as well as other unidentified effectors, leading to up-regulation of IL-8.
Figure 8.
Schematic model of a possible molecular mechanism regulating IL-8 production after prolonged proteasome inhibition. In this model, the impairment of proteasome activity will result in the activation of MKK3 and MKK6, the upstream kinases involved in the activation of p38 MAPK. Activation of p38 MAPK will result in EGFR phosphorylation. This phosphorylation allows docking proteins to bind the receptor, leading to the activation of PI3K. The PI3K then activates Itk, as well as other unidentified effectors, which in turn will lead to an increase in IL-8 gene expression. Akt can also be activated by PI3K, but it may only play a minor role on IL-8 production. Alternatively, p38 MAPK may regulate IL-8 production through other mechanisms, such as stabilization of its mRNA.
IL-8 expression is regulated by many signaling pathways and transcription factors, such as p38 MAPK (Hoffmann et al., 2002; Kumar et al., 2003), PI3K (Newcomb et al., 2005; Kim et al., 2006), nuclear factor-κB, activator protein (AP)-1 (Roebuck, 1999; Hoffmann et al., 2002), extracellular signal-related kinase, c-Jun NH2-terminal kinase (Wu et al., 2002; Joshi-Barve et al., 2003), and hypoxia-inducible factor (HIF)-1 (Kim et al., 2006; Natarajan et al., 2007). This work indicates that there is a cross-talk between some of these signaling pathways and that they can be activated in a sequential manner. For example, proteasome inhibition activates p38 MAPK, which in turn phosphorylates the EGFR and leads to PI3K activation. A similar interrelationship between different signaling pathways was also reported previously. For example, activation of the PI3K pathway triggers the activation of different transcription factors, including AP-1 and HIF-1 (Bachelor et al., 2005; Belaiba et al., 2007).
It seems that the activation of p38 MAPK is a key event for the enhanced IL-8 production in response to prolonged proteasome inhibition. The data presented here indicate that MKK3 and MKK6 can increase IL-8 production in RPE cells. Given that these kinases are the upstream kinases for p38 MAPK and that they can be activated upon proteasome inhibition in RPE cells (Fernandes et al., 2008), it is likely that activation of MKK3 and MKK6 is an important signaling event upstream of p38 MAPK in response to prolonged proteasome inhibition. However, proteasome inhibition may also activate p38 MAPK by a different mechanism. Transforming growth factor-β–activated protein kinase 1 (TAK1)-binding protein 1 has also been reported to activate p38 MAPK (Ge et al., 2002), and TAK1 is regulated by the UPP (Adhikari et al., 2007).
Our data indicate that proteasome inhibition promotes the p38 MAPK-dependent activation of the PI3K pathway. This observation is consistent with a previous study that reported the p38 MAPK-dependent activation of Akt (Rane et al., 2001). In addition, there is a report of reciprocal cross-talk between p38 MAPK and PI3K pathways (Gonzalez et al., 2004). However, we found that the cross-talk between p38 MAPK and PI3K/Akt pathways in RPE is not reciprocal, because inhibition of the PI3K did not abolish the p38 MAPK activation (Figure 2C). The p38 MAPK-dependent activation of EGFR and PI3K seems to be the main signaling event leading to increased IL-8 production in response to prolonged proteasome inhibition. However, p38 MAPK may also regulate IL-8 production by other mechanisms, such as stabilization of IL-8 mRNA (Winzen et al., 1999; Sparkman and Boggaram, 2004). This may explain why the enhanced levels of mRNA and protein for IL-8 in the presence of a constitutively active form of MKK3 and MKK6 were only partially blocked by EGFR and PI3K inhibitors (Figure 5, B and D).
Activation of PI3K is required for IL-8 production in response to proteasome inhibition (Figure 1, A and B). This signaling pathway can be activated by the EGFR, as well as by other growth factor receptors, such as the PDGF receptor and the insulin receptor (Schlessinger, 2000; Gschwind et al., 2004). Although activation of these receptors by the respective ligands also increased IL-8 production, stimulation of cells with EGF resulted in the greatest increase on IL-8 production (Figure 3B), indicating that activation of the EGFR plays a major role in regulating IL-8 production. Consistently, inhibition of EGFR significantly reduced IL-8 production induced by proteasome inhibitors (Figure 3, C and D).
The best studied downstream target of PI3K is Akt (Hawkins et al., 2006). Although inhibition of the proteasome activated Akt (Figure 2, A and B), blocking this kinase with two different inhibitors did not prevent IL-8 up-regulation induced by proteasome inactivation (Figure 6). Overexpression of a constitutively active Akt only marginally increased IL-8 production (Figure 6D), indicating that Akt plays a minor role in regulating IL-8 production in RPE. This suggests that PI3K is signaling through an alternative effector to up-regulate IL-8 levels in response to proteasome inhibition. This hypothesis is consistent with the existence of PI3K-dependent, but Akt-independent, pathways (Vivanco and Sawyers, 2002). We also demonstrate that the increased IL-8 production induced by proteasome inhibition in RPE cells is independent of PDK1 (Figure 7A). This is in accordance with the minor role of Akt in IL-8 production under these conditions, because PDK1 is the kinase responsible for the phosphorylation of Akt on threonine 308 (Vanhaesebroeck and Alessi, 2000). Thr308 phosphorylation is necessary and sufficient for Akt activation (Stokoe et al., 1997).
Furthermore, we identify Itk as a novel regulator of IL-8 expression in response to proteasome inhibition. Inhibition of Itk activity significantly blocked the IL-8 expression and secretion stimulated by proteasome inhibition (Figure 7, B and C). Itk is a nonreceptor tyrosine kinase belonging to the Tec family (Readinger et al., 2009) and has been shown to regulate the expression of cytokines and inflammatory mediators (Wong, 2005; Felices and Berg, 2008). Moreover, Itk activation is PI3K dependent (Lu et al., 1998; Shan et al., 2000). Therefore, we propose that Itk plays an important role in the PI3K-dependent up-regulation of IL-8 in response to proteasome inhibition. To our knowledge, this is the first report describing a role for Itk in the regulation of IL-8 gene expression.
Together, the results presented in this work elucidate a novel signaling network that leads to overproduction of IL-8 in response to proteasome inactivation. Although some individual events of the cross-talk between signaling pathways have been reported previously in isolation, this is the first comprehensive demonstration of a cross-talk between signaling pathways that leads to a functional consequence. This information not only sheds light onto understanding how proteasome impairment triggers IL-8 production but also provides clues about how to control IL-8 production to reduce inflammation and inflammation-related diseases.
ACKNOWLEDGMENTS
We thank Drs. Alex Toker and Jiahuai Han for providing the plasmids. This work was supported by National Institutes of Health grants EY11717 (to F. S.) and EY13250 (to A. T.); a grant from the Portuguese Foundation for Science and Technology, PTDC/SAU-OSM/67498/2006 (to P. P.); and USDA/Current Research Information System 1950-51000-060-02S (to A. T.) and by the Molecular Libraries Initiative of the National Institutes of Health Roadmap for Medical Research (to J.-K.J. and C.J.T.). A.F.F. is a recipient of a Fellowship from the Portuguese Foundation for Science and Technology (SFRH/BD/19039/2004).
Abbreviations used:
- AP
activator protein
- AMD
age-related macular degeneration
- AP-1
activator protein-1
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- HIF
hypoxia-inducible factor
- IL-8
interleukin-8
- Itk
interleukin-2–inducible T cell kinase
- MAPK
mitogen-activated protein kinase
- MKK
mitogen-activated protein kinase kinase
- PDGF
platelet-derived growth factor
- PDK1
3-phosphoinositide-dependent protein kinase-1
- PI3K
phosphatidylinositol 3-kinase
- RPE
retinal pigment epithelial cells
- Tec
tyrosine kinase expressed in hepatocellular carcinoma
- UPP
ubiquitin–proteasome pathway.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-10-1068) on July 1, 2009.
REFERENCES
- Adhikari A., Xu M., Chen Z. J. Ubiquitin-mediated activation of TAK1 and IKK. Oncogene. 2007;26:3214–3226. doi: 10.1038/sj.onc.1210413. [DOI] [PubMed] [Google Scholar]
- Bachelor M. A., Cooper S. J., Sikorski E. T., Bowden G. T. Inhibition of p38 mitogen-activated protein kinase and phosphatidylinositol 3-kinase decreases UVB-induced activator protein-1 and cyclooxygenase-2 in a SKH-1 hairless mouse model. Mol. Cancer Res. 2005;3:90–99. doi: 10.1158/1541-7786.MCR-04-0065. [DOI] [PubMed] [Google Scholar]
- Beatty S., Koh H., Phil M., Henson D., Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv. Ophthalmol. 2000;45:115–134. doi: 10.1016/s0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- Belaiba R. S., Bonello S., Zahringer C., Schmidt S., Hess J., Kietzmann T., Gorlach A. Hypoxia up-regulates hypoxia-inducible factor-1alpha transcription by involving phosphatidylinositol 3-kinase and nuclear factor κB in pulmonary artery smooth muscle cells. Mol. Biol. Cell. 2007;18:4691–4697. doi: 10.1091/mbc.E07-04-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishayee S. Role of conformational alteration in the epidermal growth factor receptor (EGFR) function. Biochem. Pharmacol. 2000;60:1217–1223. doi: 10.1016/s0006-2952(00)00425-1. [DOI] [PubMed] [Google Scholar]
- Boulton M., Roanowska M., Wess T. Ageing of the retinal pigment epithelium: implications for transplantation. Graefes Arch. Clin. Exp. Ophthalmol. 2004;242:76–84. doi: 10.1007/s00417-003-0812-8. [DOI] [PubMed] [Google Scholar]
- Chung H. Y., Sung B., Jung K. J., Zou Y., Yu B. P. The molecular inflammatory process in aging. Antioxid. Redox. Signal. 2006;8:572–581. doi: 10.1089/ars.2006.8.572. [DOI] [PubMed] [Google Scholar]
- Ciechanover A. The ubiquitin proteolytic system and pathogenesis of human diseases: a novel platform for mechanism-based drug targeting. Biochem. Soc. Trans. 2003;31:474–481. doi: 10.1042/bst0310474. [DOI] [PubMed] [Google Scholar]
- Dawson T. M., Dawson V. L. Molecular pathways of neurodegeneration in Parkinson's disease. Science. 2003;302:819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- Donoso L. A., Kim D., Frost A., Callahan A., Hageman G. The role of inflammation in the pathogenesis of age-related macular degeneration. Surv. Ophthalmol. 2006;51:137–152. doi: 10.1016/j.survophthal.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek E. J., Shang F., Valverde P., Liu Q., Hobbs M., Taylor A. Selectivity of the ubiquitin pathway for oxidatively modified proteins: relevance to protein precipitation diseases. FASEB J. 2005;19:1707–1709. doi: 10.1096/fj.05-4049fje. [DOI] [PubMed] [Google Scholar]
- Dunn K. C., Aotaki-Keen A. E., Putkey F. R., Hjelmeland L. M. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp. Eye Res. 1996;62:155–169. doi: 10.1006/exer.1996.0020. [DOI] [PubMed] [Google Scholar]
- Ethen C. M., Hussong S. A., Reilly C., Feng X., Olsen T. W., Ferrington D. A. Transformation of the proteasome with age-related macular degeneration. FEBS Lett. 2007;581:885–890. doi: 10.1016/j.febslet.2007.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg S. A., Keane M. M., Nau M. M., Frankel M., Wang L. M., Pierce J. H., Lipkowitz S. cbl-b inhibits epidermal growth factor receptor signaling. Oncogene. 1999;18:1855–1866. doi: 10.1038/sj.onc.1202499. [DOI] [PubMed] [Google Scholar]
- Felices M., Berg L. J. The Tec kinases Itk and Rlk regulate NKT cell maturation, cytokine production, and survival. J. Immunol. 2008;180:3007–3018. doi: 10.4049/jimmunol.180.5.3007. [DOI] [PubMed] [Google Scholar]
- Fernandes A. F., Guo W., Zhang X., Gallagher M., Ivan M., Taylor A., Pereira P., Shang F. Proteasome-dependent regulation of signal transduction in retinal pigment epithelial cells. Exp. Eye Res. 2006a;83:1472–1481. doi: 10.1016/j.exer.2006.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes A. F., Zhou J., Zhang X., Bian Q., Sparrow J., Taylor A., Pereira P., Shang F. Oxidative inactivation of the proteasome in retinal pigment epithelial cells. A potential link between oxidative stress and up-regulation of interleukin-8. J. Biol. Chem. 2008;283:20745–20753. doi: 10.1074/jbc.M800268200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes R., Ramalho J., Pereira P. Oxidative stress upregulates ubiquitin proteasome pathway in retinal endothelial cells. Mol. Vis. 2006b;12:1526–1535. [PubMed] [Google Scholar]
- Frey M. R., Dise R. S., Edelblum K. L., Polk D. B. p38 kinase regulates epidermal growth factor receptor downregulation and cellular migration. EMBO J. 2006;25:5683–5692. doi: 10.1038/sj.emboj.7601457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuoka Y., Medof E. M. C5a receptor-mediated production of IL-8 by the human retinal pigment epithelial cell line, ARPE-19. Curr. Eye Res. 2001;23:320–325. doi: 10.1076/ceyr.23.5.320.5437. [DOI] [PubMed] [Google Scholar]
- Fukuoka Y., Strainic M., Medof M. E. Differential cytokine expression of human retinal pigment epithelial cells in response to stimulation by C5a. Clin. Exp. Immunol. 2003;131:248–253. doi: 10.1046/j.1365-2249.2003.02087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge B., Gram H., Di Padova F., Huang B., New L., Ulevitch R. J., Luo Y., Han J. MAPKK-independent activation of p38alpha mediated by TAB1-dependent autophosphorylation of p38alpha. Science. 2002;295:1291–1294. doi: 10.1126/science.1067289. [DOI] [PubMed] [Google Scholar]
- Glickman M. H., Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol. Rev. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- Gonzalez I., Tripathi G., Carter E. J., Cobb L. J., Salih D. A., Lovett F. A., Holding C., Pell J. M. Akt2, a novel functional link between p38 mitogen-activated protein kinase and phosphatidylinositol 3-kinase pathways in myogenesis. Mol. Cell Biol. 2004;24:3607–3622. doi: 10.1128/MCB.24.9.3607-3622.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gschwind A., Fischer O. M., Ullrich A. The discovery of receptor tyrosine kinases: targets for cancer therapy. Nat. Rev. Cancer. 2004;4:361–370. doi: 10.1038/nrc1360. [DOI] [PubMed] [Google Scholar]
- Hawkins P. T., Anderson K. E., Davidson K., Stephens L. R. Signalling through Class I PI3Ks in mammalian cells. Biochem. Soc. Trans. 2006;34:647–662. doi: 10.1042/BST0340647. [DOI] [PubMed] [Google Scholar]
- Higgins G. T., Wang J. H., Dockery P., Cleary P. E., Redmond H. P. Induction of angiogenic cytokine expression in cultured RPE by ingestion of oxidized photoreceptor outer segments. Invest. Ophthalmol. Vis. Sci. 2003;44:1775–1782. doi: 10.1167/iovs.02-0742. [DOI] [PubMed] [Google Scholar]
- Hoffmann E., Dittrich-Breiholz O., Holtmann H., Kracht M. Multiple control of interleukin-8 gene expression. J. Leukoc. Biol. 2002;72:847–855. [PubMed] [Google Scholar]
- Hope A. D., de Silva R., Fischer D. F., Hol E. M., van Leeuwen F. W., Lees A. J. Alzheimer's associated variant ubiquitin causes inhibition of the 26S proteasome and chaperone expression. J. Neurochem. 2003;86:394–404. doi: 10.1046/j.1471-4159.2003.01844.x. [DOI] [PubMed] [Google Scholar]
- Jahngen-Hodge J., Cyr D., Laxman E., Taylor A. Ubiquitin and ubiquitin conjugates in human lens. Exp. Eye Res. 1992;55:897–902. doi: 10.1016/0014-4835(92)90016-l. [DOI] [PubMed] [Google Scholar]
- Joshi-Barve S., Barve S. S., Butt W., Klein J., McClain C. J. Inhibition of proteasome function leads to NF-kappaB-independent IL-8 expression in human hepatocytes. Hepatology. 2003;38:1178–1187. doi: 10.1053/jhep.2003.50470. [DOI] [PubMed] [Google Scholar]
- Kalayoglu M. V., Bula D., Arroyo J., Gragoudas E. S., D'Amico D., Miller J. W. Identification of Chlamydia pneumoniae within human choroidal neovascular membranes secondary to age-related macular degeneration. Graefes Arch. Clin. Exp. Ophthalmol. 2005;243:1080–1090. doi: 10.1007/s00417-005-1169-y. [DOI] [PubMed] [Google Scholar]
- Kapphahn R. J., Bigelow E. J., Ferrington D. A. Age-dependent inhibition of proteasome chymotrypsin-like activity in the retina. Exp. Eye Res. 2007;84:646–654. doi: 10.1016/j.exer.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. S., Rajagopal V., Gonsalves C., Johnson C., Kalra V. K. A novel role of hypoxia-inducible factor in cobalt chloride- and hypoxia-mediated expression of IL-8 chemokine in human endothelial cells. J. Immunol. 2006;177:7211–7224. doi: 10.4049/jimmunol.177.10.7211. [DOI] [PubMed] [Google Scholar]
- Kloetzel P. M. Generation of major histocompatibility complex class I antigens: functional interplay between proteasomes and TPPII. Nat. Immunol. 2004;5:661–669. doi: 10.1038/ni1090. [DOI] [PubMed] [Google Scholar]
- Kumar S., Boehm J., Lee J. C. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nat. Rev. Drug Discov. 2003;2:717–726. doi: 10.1038/nrd1177. [DOI] [PubMed] [Google Scholar]
- Lavrovsky Y., Chatterjee B., Clark R. A., Roy A. K. Role of redox-regulated transcription factors in inflammation, aging and age-related diseases. Exp. Gerontol. 2000;35:521–532. doi: 10.1016/s0531-5565(00)00118-2. [DOI] [PubMed] [Google Scholar]
- Levkowitz G., Waterman H., Ettenberg S. A., Katz M., Tsygankov A. Y., Alroy I., Lavi S., Iwai K., Reiss Y., Ciechanover A., Lipkowitz S., Yarden Y. Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Mol. Cell. 1999;4:1029–1040. doi: 10.1016/s1097-2765(00)80231-2. [DOI] [PubMed] [Google Scholar]
- Liang F. Q., Godley B. F. Oxidative stress-induced mitochondrial DNA damage in human retinal pigment epithelial cells: a possible mechanism for RPE aging and age-related macular degeneration. Exp. Eye Res. 2003;76:397–403. doi: 10.1016/s0014-4835(03)00023-x. [DOI] [PubMed] [Google Scholar]
- Louie J. L., Kapphahn R. J., Ferrington D. A. Proteasome function and protein oxidation in the aged retina. Exp. Eye Res. 2002;75:271–284. [PubMed] [Google Scholar]
- Lu Y., Cuevas B., Gibson S., Khan H., LaPushin R., Imboden J., Mills G. B. Phosphatidylinositol 3-kinase is required for CD28 but not CD3 regulation of the TEC family tyrosine kinase EMT/ITK/TSK: functional and physical interaction of EMT with phosphatidylinositol 3-kinase. J. Immunol. 1998;161:5404–5412. [PubMed] [Google Scholar]
- McGeer E. G., Klegeris A., McGeer P. L. Inflammation, the complement system and the diseases of aging. Neurobiol. Aging. 2005;26(suppl 1):94–97. doi: 10.1016/j.neurobiolaging.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Natarajan R., Fisher B. J., Fowler A. A., 3rd. Hypoxia inducible factor-1 modulates hemin-induced IL-8 secretion in microvascular endothelium. Microvasc. Res. 2007;73:163–172. doi: 10.1016/j.mvr.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Newcomb D. C., Sajjan U., Nanua S., Jia Y., Goldsmith A. M., Bentley J. K., Hershenson M. B. Phosphatidylinositol 3-kinase is required for rhinovirus-induced airway epithelial cell interleukin-8 expression. J. Biol. Chem. 2005;280:36952–36961. doi: 10.1074/jbc.M502449200. [DOI] [PubMed] [Google Scholar]
- Pickart C. M. Mechanisms underlying ubiquitination. Annu. Rev. Biochem. 2001;70:503–533. doi: 10.1146/annurev.biochem.70.1.503. [DOI] [PubMed] [Google Scholar]
- Qureshi N., Vogel S. N., Van Way C., 3rd, Papasian C. J., Qureshi A. A., Morrison D. C. The proteasome: a central regulator of inflammation and macrophage function. Immunol. Res. 2005;31:243–260. doi: 10.1385/IR:31:3:243. [DOI] [PubMed] [Google Scholar]
- Rane M. J., Coxon P. Y., Powell D. W., Webster R., Klein J. B., Pierce W., Ping P., McLeish K. R. p38 Kinase-dependent MAPKAPK-2 activation functions as 3-phosphoinositide-dependent kinase-2 for Akt in human neutrophils. J. Biol. Chem. 2001;276:3517–3523. doi: 10.1074/jbc.M005953200. [DOI] [PubMed] [Google Scholar]
- Readinger J. A., Mueller K. L., Venegas A. M., Horai R., Schwartzberg P. L. Tec kinases regulate T-lymphocyte development and function: new insights into the roles of Itk and Rlk/Txk. Immunol. Rev. 2009;228:93–114. doi: 10.1111/j.1600-065X.2008.00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Readinger J. A., Schiralli G. M., Jiang J. K., Thomas C. J., August A., Henderson A. J., Schwartzberg P. L. Selective targeting of ITK blocks multiple steps of HIV replication. Proc. Natl. Acad. Sci. USA. 2008;105:6684–6689. doi: 10.1073/pnas.0709659105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues G. A., Falasca M., Zhang Z., Ong S. H., Schlessinger J. A novel positive feedback loop mediated by the docking protein Gab1 and phosphatidylinositol 3-kinase in epidermal growth factor receptor signaling. Mol. Cell Biol. 2000;20:1448–1459. doi: 10.1128/mcb.20.4.1448-1459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roebuck K. A. Regulation of interleukin-8 gene expression. J. Interferon Cytokine Res. 1999;19:429–438. doi: 10.1089/107999099313866. [DOI] [PubMed] [Google Scholar]
- Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- Shan X., Czar M. J., Bunnell S. C., Liu P., Liu Y., Schwartzberg P. L., Wange R. L. Deficiency of PTEN in Jurkat T cells causes constitutive localization of Itk to the plasma membrane and hyperresponsiveness to CD3 stimulation. Mol. Cell Biol. 2000;20:6945–6957. doi: 10.1128/mcb.20.18.6945-6957.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang F., Gong X., Palmer H. J., Nowell T. R., Jr., Taylor A. Age-related decline in ubiquitin conjugation in response to oxidative stress in the lens. Exp. Eye Res. 1997;64:21–30. doi: 10.1006/exer.1996.0176. [DOI] [PubMed] [Google Scholar]
- Shang F., Nowell T. R., Jr., Taylor A. Removal of oxidatively damaged proteins from lens cells by the ubiquitin-proteasome pathway. Exp. Eye Res. 2001;73:229–238. doi: 10.1006/exer.2001.1029. [DOI] [PubMed] [Google Scholar]
- Shang F., Taylor A. Function of the ubiquitin proteolytic pathway in the eye. Exp. Eye Res. 2004;78:1–14. doi: 10.1016/j.exer.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Sparkman L., Boggaram V. Nitric oxide increases IL-8 gene transcription and mRNA stability to enhance IL-8 gene expression in lung epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2004;287:L764–L773. doi: 10.1152/ajplung.00165.2004. [DOI] [PubMed] [Google Scholar]
- Stokoe D., Stephens L. R., Copeland T., Gaffney P. R., Reese C. B., Painter G. F., Holmes A. B., McCormick F., Hawkins P. T. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science. 1997;277:567–570. doi: 10.1126/science.277.5325.567. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B., Alessi D. R. The PI3K-PDK1 connection: more than just a road to PKB. Biochem. J. 2000;346:561–576. [PMC free article] [PubMed] [Google Scholar]
- Vivanco I., Sawyers C. L. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat. Rev. Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- Welchman R. L., Gordon C., Mayer R. J. Ubiquitin and ubiquitin-like proteins as multifunctional signals. Nat. Rev. Mol. Cell Biol. 2005;6:599–609. doi: 10.1038/nrm1700. [DOI] [PubMed] [Google Scholar]
- Wen X. F., Yang G., Mao W., Thornton A., Liu J., Bast R. C., Jr., Le X. F. HER2 signaling modulates the equilibrium between pro- and antiangiogenic factors via distinct pathways: implications for HER2-targeted antibody therapy. Oncogene. 2006;25:6986–6996. doi: 10.1038/sj.onc.1209685. [DOI] [PubMed] [Google Scholar]
- Winzen R., Kracht M., Ritter B., Wilhelm A., Chen C. Y., Shyu A. B., Muller M., Gaestel M., Resch K., Holtmann H. The p38 MAP kinase pathway signals for cytokine-induced mRNA stabilization via MAP kinase-activated protein kinase 2 and an AU-rich region-targeted mechanism. EMBO J. 1999;18:4969–4980. doi: 10.1093/emboj/18.18.4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong W. S. Inhibitors of the tyrosine kinase signaling cascade for asthma. Curr. Opin. Pharmacol. 2005;5:264–271. doi: 10.1016/j.coph.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Wu H. M., Wen H. C., Lin W. W. Proteasome inhibitors stimulate interleukin-8 expression via Ras and apoptosis signal-regulating kinase-dependent extracellular signal-related kinase and c-Jun N-terminal kinase activation. Am. J. Respir. Cell Mol. Biol. 2002;27:234–243. doi: 10.1165/ajrcmb.27.2.4792. [DOI] [PubMed] [Google Scholar]
- Zarbin M. A. Current concepts in the pathogenesis of age-related macular degeneration. Arch. Ophthalmol. 2004;122:598–614. doi: 10.1001/archopht.122.4.598. [DOI] [PubMed] [Google Scholar]
- Zhang X., Zhou J., Fernandes A. F., Sparrow J. R., Pereira P., Taylor A., Shang F. The proteasome: a target of oxidative damage in cultured human retina pigment epithelial cells. Invest. Ophthalmol. Vis. Sci. 2008;49:3622–3630. doi: 10.1167/iovs.07-1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Cai B., Jang Y. P., Pachydaki S., Schmidt A. M., Sparrow J. R. Mechanisms for the induction of HNE- MDA- and AGE-adducts, RAGE and VEGF in retinal pigment epithelial cells. Exp. Eye Res. 2005;80:567–580. doi: 10.1016/j.exer.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Zhou J., Jang Y. P., Kim S. R., Sparrow J. R. Complement activation by photooxidation products of A2E, a lipofuscin constituent of the retinal pigment epithelium. Proc. Natl. Acad. Sci. USA. 2006;103:16182–16187. doi: 10.1073/pnas.0604255103. [DOI] [PMC free article] [PubMed] [Google Scholar]