Abstract
Background
Pentraxin-3 (PTX3) may be a useful biomarker in sepsis, but its regulatory mechanisms are still unclear. Oxidative stress is well defined in patients with sepsis and has a role in regulation of inflammatory pathways which may include PTX3. We undertook an in vitro study of the effect of antioxidants on regulation of PTX3 in endothelial cells combined with a prospective observational pilot study of PTX3 in relation to markers of antioxidant capacity and oxidative stress in patients with sepsis.
Methods
Human endothelial cells were cultured with lipopolysaccharide 2 µg ml−1, peptidoglycan G 20 µg ml−1, tumour necrosis factor (TNF) α 10 ng ml−1, interleukin-1 (IL-1) β 20 ng ml−1, or killed Candida albicans yeast cells plus either N-acetylcysteine (NAC) 25 mM, trolox 100 mM, or idebenone 1 µM. Plasma samples were obtained from 15 patients with sepsis and 11 healthy volunteers.
Results
PTX3 levels in plasma were higher in patients with sepsis than in healthy people [26 (1–202) ng ml−1 compared with 6 (1–12) ng ml−1, P=0.01]. Antioxidant capacity was lower in patients with sepsis than healthy controls [0.99 (0.1–1.7) mM compared with 2.2 (1.3–3.3) mM, P=0.01]. In patients with sepsis, lipid hydroperoxide levels were 3.32 (0.3–10.6) nM and undetectable in controls. We found no relationship between PTX3 and antioxidant capacity or lipid hydroperoxides. Cell expression of PTX3 increased with all inflammatory stimulants but was highest in cells treated with TNFα plus IL-1β. PTX3 concentrations were lower in cells co-treated with antioxidants (all P<0.05), associated with lower nuclear factor κB expression for NAC and trolox (P<0.05).
Conclusions
PTX3 expression is down-regulated in vitro by antioxidants. Plasma levels of PTX3 are elevated in sepsis but seem to be unrelated to markers of oxidant stress or antioxidant capacity.
Keywords: immune response; infection, bacterial; infection, fungal; metabolism, protein, acute phase
Pentraxins are a group of acute phase proteins which are produced in response to inflammatory conditions in vivo and which play a key role in the innate immune system.1,2 C-reactive protein (CRP) is a classical short pentraxin routinely used in the diagnosis and monitoring of inflammation and infection. Pentraxin-3 (PTX3) is the first member of the long pentraxin family group and is expressed in several cell types after exposure to pro-inflammatory stimuli, including specific microbial constituents and cytokines.3–5
Sepsis is the systemic generalized activation of the innate immune response due to infection, involving cellular inflammatory responses triggered by intracellular oxidative stress. Clinically, causative organisms are predominantly bacterial, but the use of indwelling central venous catheters and treatment with broad-spectrum antibiotics are additional risk factors for fungal infection and Candida spp. is now the fourth-most-common organism to be isolated from the bloodstream of hospitalized patients6 with attributable mortality of candidiasis of around 38%.7
The promoter region of PTX3 contains binding sites for the redox-sensitive transcription factor nuclear factor κB (NFκB),8,9 which is involved in the regulation pathway of many inflammatory mediators important in innate immunity. Oxidative stress has consistently been demonstrated in patients with sepsis10–14 and acts as a trigger for inflammation. We hypothesized that antioxidants may affect expression of PTX3. Using bacterial cell components, cytokines, or killed Candida albicans cells to mimic different conditions of sepsis, we determined the effect of antioxidant treatment on PTX3 expression in human endothelial cells. In addition, we measured PTX3 in relation to total antioxidant capacity and lipid hydroperoxides in plasma from patients with severe sepsis.
Methods
Patient study
In this pilot study, 20 consecutive patients were recruited from the intensive care unit (ICU) within 24 h of fulfilling the criteria for sepsis, after local ethical approval and obtaining written informed consent from the patient or assent from a close relative. The criteria used were those recommended by the Consensus Meeting of the American Thoracic Society and the American Society of Critical Care Medicine,15 namely clinical suspicion of infection plus two of the following: tachycardia (>100 beats min−1), tachypnoea (>20 bpm or ventilated), or leucocyte count <4 or >12×109 litre−1. Patients <16 yr old, who were pregnant or lactating, HIV positive, on steroid or immunosuppression therapy, who had any form of cancer or autoimmune disease or who were taking statins, were excluded. Five patients were subsequently excluded; two were found subsequently not to have sepsis and another three were taking statins. Heparinized blood was obtained from an indwelling cannula and immediately centrifuged and the plasma stored at −80°C for PTX3 analysis. Acute physiological and chronic health evaluation (APACHE) II score was also recorded. Blood samples were also obtained with consent from 11 healthy laboratory and research staff (age range 25–50 yr) using heparinized vacutainers and samples were treated as for patients.
In vitro study
All reagents were obtained from Sigma Aldrich Ltd, Poole, Dorset, UK, unless stated otherwise. The human umbilical vein endothelial cell line HUVEC-C was used (American Type Culture Collection, Manassas, VA, USA). Cells were cultured in 6-well plates as we have previously described15 in Dulbecco's Modified Eagle's Medium (Lonza Wokingham Ltd, Berkshire, UK) containing heat-inactivated fetal calf serum 10%, gentamicin 50 µg ml−1, and amphotericin B 250 µg ml−1.
Yeast cells of the human pathogenic fungus C. albicans wild-type derivative strain NGY152 were used.16 Candida albicans was grown in Sabouraud broth overnight at 30°C with shaking. The overnight culture was diluted 1:100 in fresh broth, grown until spectrophotometry showed an absorbance of 0.5 at 600 nm, then harvested. The pellet was washed three times in phosphate-buffered saline (PBS) and resuspended to a final concentration of 1×108 yeast cells ml−1 in PBS. The cells were heat-killed by incubation at 56°C for 2 h.
Endothelial cells were cultured for 24 h at 37°C in the presence of lipopolysaccharide (LPS) 2 µg ml−1 plus peptidoglycan G (PepG) 20 µg ml−1, tumour necrosis factor α (TNFα, PeproTech EC Ltd, London, UK) 10 ng ml−1, interleukin-1β (IL-1β, PeproTech) 20 ng ml−1, TNFα and IL-1β combined, or killed C. albicans cells at a multiplicity of infection (MOI) of 1–10, along with the antioxidants 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (trolox, a water-soluble vitamin E analogue, 100 mM), N-acetylcysteine (NAC, a glutathione precursor, 25 mM) or 2,3-dimethoxy-5-methyl-6-(10-hydroxydecyl)-1,4-benzoquinone (idebenone, a water-soluble co-enzyme Q10 analogue, 1 µM, a kind gift from Dr M.P. Murphy, MRC-Dunn Nutrition Unit, Cambridge, UK), or appropriate solvent controls. Culture supernatants were stored at –80°C for subsequent PTX3 measurement. In separate experiments, endothelial cells were treated with IL-1β and TNFα plus antioxidants as above and nuclear extracts were prepared for NFκB assay.
All measurements on cells were corrected for viable cell number which was determined using acid phosphatase activity.17 The precision of this assay (% coefficient of variation) was 1.06%.
Pentraxin-3 and CRP measurement
PTX3 expression from plasma or culture supernatants was measured by enzyme immunoassay (R&D Systems Europe Ltd, Abingdon, Oxon, UK). Briefly, 96-well plates were coated with anti-PTX3 monoclonal antibody and incubated overnight at 4°C. After incubation, plates were washed with PBS containing Tween 20 0.05% and blocked with skim milk powder 10% for 1 h at 37°C. Plates were washed again and recombinant human PTX3 as a calibration standard, or plasma or culture supernatant was added to each well. After 2 h incubation at 37°C, plates were washed and biotinylated anti-PTX3 polyclonal antibody was added for a further hour at 37°C followed by 40 min incubation with streptavidin–horseradish peroxidase (HRP) then a chromogen substrate. The reaction was stopped using sulphuric acid and quantified spectrophotometrically. The precision of this assay (% coefficient of variation) was 2.9%.
CRP was measured colorimetrically using a Siemens ADVIA 2400 autoanalyzer (Siemens Diagnostics, Tarrytown, NY, USA).
Nuclear factor κB
Nuclear extraction was performed using the NucBuster kit (Novagen, Merk Chemicals, Nottingham, UK) according to the manufacturer's instructions. Briefly, cells were suspended in NucBuster extraction reagent 1 and vortexed for 15 s, incubated on ice for 5 min and vortexed again. Nuclei were sedimented by centrifugation at 13 000g at 4°C, for 5 min, the supernatant was removed and the nuclei pellet was resuspended in Nucbuster extraction reagent 2 containing protease inhibitor cocktail and dithiothreitol 1.28 mM. The samples were then vortexed, incubated on ice, and centrifuged as before. The supernatant (nuclear extract) was then used in the NFκB assay.
NFκB was measured using the NoShift transcription factor immunoassay kit (Novagen, Merk Chemicals). First, nuclear extract was incubated on ice for 30 min with binding buffer containing poly(dI-dC), salmon sperm DNA, and wild-type DNA (biotinylated, 10 pmol ml−1). After incubation, the reaction mixture was made up to 100 µl with binding buffer before being transferred to a streptavidin-coated 96-well plate and incubated at 37°C for 1 h. The plate was then washed, detection antibody added, and incubated again at 37°C for 1 h. The plate was again washed before the addition of goat-anti-mouse IgG HRP conjugate for 30 min before being washed five times. Chromogen substrate was then added to the wells and incubated at room temperature until colour developed. This reaction was stopped with the addition of hydrochloric acid, quantified spectrophotometrically, and expressed in terms of nuclear protein content, measured using the Bradford reagent. The precision of this assay (% coefficient of variation) was 1.4%.
Total antioxidant capacity and lipid peroxides
Total antioxidant capacity was measured using a commercially available kit (Cayman Chemical, Ann Arbor, MI, USA) based on the ability of plasma antioxidants to inhibit oxidation of 2,2′-azino-di-[3-ethylbenzothiaolinesulphonate] (ABTS) to the ABTS radical by metmyoglobin.18 The capacity of plasma to prevent oxidation of ABTS is compared with that of trolox and is quantified as molar trolox equivalents. The precision of this assay (% coefficient of variation) was 3.4%.
Total lipid hydroperoxides were measured using a spectrophotometric technique.12 Lipid hydroperoxides are extracted into chloroform, which eliminates interference by hydrogen peroxide or endogenous ferric ions in the sample. The precision of this assay (% coefficient of variation) was 0.63%.
Data analysis
For the in vitro study, four replicate independent experiments in triplicate were performed. No assumptions were made about the distribution of data which were analysed using the Kruskal–Wallis with Mann–Whitney post hoc testing and Bonferroni's correction as appropriate. Patients' plasma PTX3 and total antioxidant capacity were compared with those of healthy subjects using the Mann–Whitney U-test. All data are presented as median (range). A P-value of <0.05 was considered to be significant.
Results
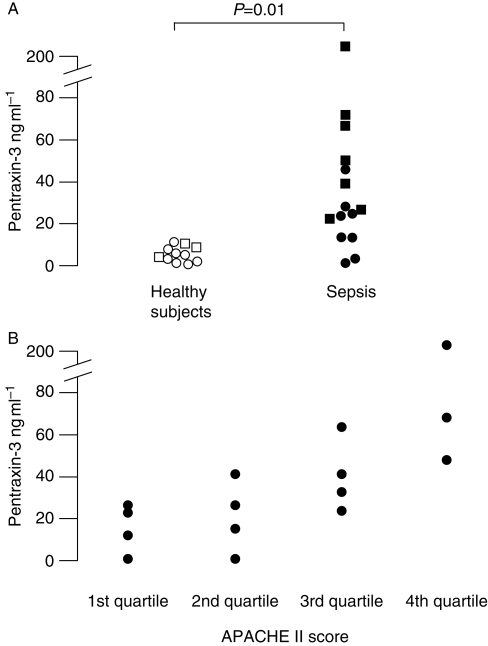
Fifteen patients were included (six females and nine males, aged 22–85 yr) (Table 1). The median (range) APACHE II score was 21 (9–32). PTX3 levels in plasma were significantly higher in patients with sepsis than in healthy people [26 (1–202) ng ml−1 compared with 6 (1–12) ng ml−1, P=0.01, Fig. 1a]. PTX3 levels were also significantly lower in men than in women [16.7 (1–42) ng ml−1 compared with 55 (24–202) ng ml−1, P<0.003, Fig. 1a]. When PTX3 levels in patients were plotted according to APACHE II score quartile, PTX3 levels were related to APACHE score quartile (Fig. 1b). Median (range) CRP was 164 (20–370) mg ml−1 and was unrelated to APACHE II score quartile.
Table 1.
Characteristics of patients with sepsis. UTI, urinary tract infection; CBD, common bile duct
| Sex M/F | Age (yr) | APACHE II score | ICU survival | Hospital survival | Infection site |
|---|---|---|---|---|---|
| F | 75 | 15 | N | N | Pancreatic abscess |
| F | 56 | 24 | Y | Y | UTI |
| F | 70 | 32 | Y | N | Pneumonia |
| M | 49 | 17 | Y | Y | Pneumonia |
| M | 45 | 31 | Y | Y | Pneumonia |
| M | 73 | 19 | N | N | Pneumonia |
| F | 54 | 30 | Y | Y | Pneumonia |
| F | 81 | 32 | Y | N | UTI |
| M | 74 | 18 | Y | Y | Sepsis CBD stent |
| M | 85 | 27 | N | N | Peritonitis |
| M | 32 | 25 | Y | Y | Pneumonia |
| M | 83 | 21 | Y | Y | Pneumonia |
| M | 39 | 12 | Y | Y | Pneumonia |
| M | 22 | 9 | Y | Y | Pneumonia |
| F | 55 | 24 | Y | Y | Pneumonia |
Fig 1.
(a) Plasma PTX3 concentrations in 15 patients with sepsis and 11 healthy subjects. Squares are females, circles are males. (b) Data sets compared using Mann–Whitney U-test. Plasma PTX3 concentrations according to APACHE II score quartile in 15 patients with sepsis.
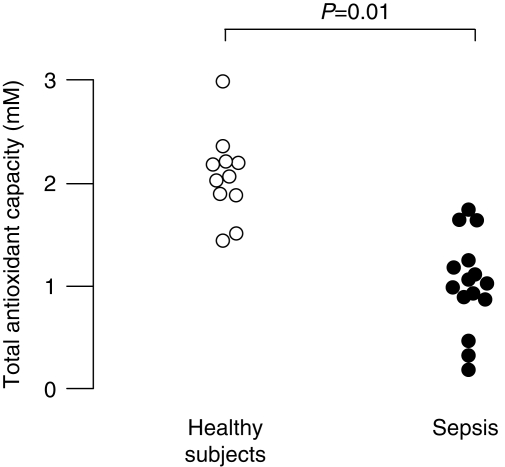
To assess oxidative stress, we measured total plasma antioxidant capacity and plasma lipid hydroperoxides. Antioxidant capacity was significantly lower in patients with sepsis than healthy controls [0.99 (0.1–1.7) mM compared with 2.2 (1.3–3.3) mM, P=0.01, Fig. 2]. Lipid hydroperoxides were below the limit of detection in plasma from all healthy subjects. In patients with sepsis, lipid hydroperoxide levels were 3.32 (0.3–10.6) nM. There was no relationship between PTX3 and either antioxidant capacity or lipid hydroperoxides. Total antioxidant capacity and lipid hydroperoxide were not different between males and females.
Fig 2.
Plasma total antioxidant capacity in 15 patients with sepsis and 11 healthy subjects. Data sets compared using Mann–Whitney U-test.
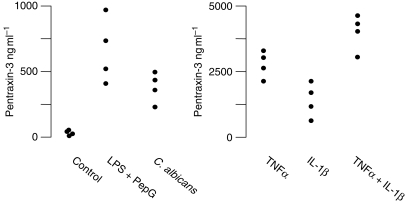
We also measured PTX3 secretion from human endothelial cells in vitro. In the absence of an inflammatory stimulus, endothelial cells produced detectable basal levels of PTX3 (Fig. 3). When we exposed cells to either LPS plus PepG, TNFα, IL-1β, TNFα plus IL-1β, or C. albicans, PTX3 levels in culture medium were significantly higher than in untreated cells (Fig. 3). There was no difference in PTX3 expression in endothelial cells exposed to C. albicans between 1 and 10 MOI (data not shown). PTX3 expression was greatest in cells exposed to both TNFα and IL-1α and was ∼10-fold higher than in control cells (P<0.001, Fig. 3). When we treated cells concurrently with antioxidants, PTX3 levels were lower, independent of the cell stimulus, than without antioxidants.
Fig 3.
The effect of different inflammatory stimuli on PTX3 expression by human endothelial cells in vitro. Note the different scales on the two graphs. Data are from four replicate experiments conducted in triplicate. LPS, lipopolysaccharide (2 µg ml−1); PepG, peptidoglycan G (20 µg ml−1); TNFα, tumour necrosis factor α (10 ng ml−1); IL-1β, interleukin-1β (20 ng ml−1); C. albicans, heat-killed Candida albicans cells (MOI=3).
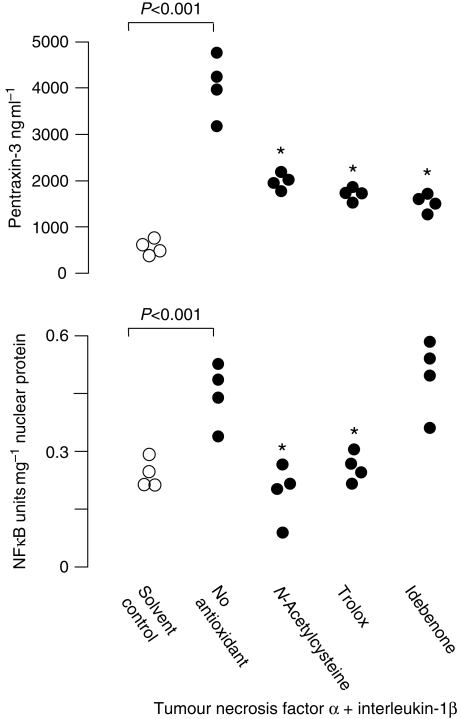
Nuclear expression of NFκB was higher in cells treated with TNFα and IL-1β than controls (P<0.001, Fig. 4), and was lower in cells also treated with trolox or NAC, but not idebenone, compared with those treated with TNFα plus IL-1β alone (Fig. 4).
Fig 4.
The effect of antioxidants on PTX3 and NFκB expression in human endothelial cells treated with TNFα and IL-1β. Data are from four replicate experiments conducted in triplicate and were analysed by the Kruskal–Wallis with Mann–Whitney U post hoc testing. *Significantly lower than cells without antioxidant (P<0.001).
Discussion
We showed that PTX3 was up-regulated in human endothelial cells in response to bacterial cell proteins, heat-killed Candida cells, or cytokines, with the greatest response occurring with a combination of TNFα plus IL-1β. Endothelial PTX3 response to an inflammatory stimulus was significantly reduced by treatment with NAC, trolox, or idebenone, and was associated with lower nuclear NFκB expression in NAC and trolox-treated cells. Idebenone had no effect on NFκB expression. We also showed that circulating PTX3 concentrations are higher in patients with sepsis compared with healthy subjects and are higher in female patients than males. PTX3 was also related to APACHE II score quartile. In addition, total antioxidant capacity was low and lipid hydroperoxide levels were elevated in the patients with sepsis, indicating oxidative stress. However, PTX3 concentrations in patients with sepsis were not related to antioxidant capacity, despite the regulation of PTX3 expression by antioxidants in vitro.
PTX3 is a member of the pentraxin superfamily, a family of proteins characterized by a multimeric, usually pentameric structure.1,2 CRP, the prototype of the pentraxin family, is an acute-phase protein produced in the liver in response to inflammatory signals such as IL-6. PTX3 is the prototype of the long pentraxin family and has some similarities with the short pentraxins but differs with respect to cellular source, and ligand recognition. PTX3 is produced in response by a variety of cell types, including macrophages and monocytes, dendritic cells, fibroblasts, epithelial, and endothelial cells, in response to inflammatory signals such as TNFα, IL-1β, microbial proteins including LPS, lipoarabinomannans, and PepG, but not IL-6.1–5
We found that exposure of endothelial cells to LPS, PepG, TNFα, or IL-1β resulted in PTX3 up-regulation, with an additive response when cells were treated with both TNFα and IL-1β, as previously reported.3–5,7 We also found that a ratio of between 1 and 10 heat-killed Candida cells to one endothelial cell resulted in an up-regulation of PTX3 expression by endothelial cells. Infection of mice with intra-cerebroventricular injection of live C. albicans was previously shown to result in up-regulation of PTX3 in the brain,19 but PTX3 expression by endothelial cells in response to C. albicans has not been described. Endothelial cells phagocytose live Candida cells, leading to secretion of cytokines including TNFα and IL-1β.20–23 The up-regulation of PTX3 expression found in our study may be related to TNFα and IL-1α produced as a result of phagocytosis of heat-killed Candida cells by the endothelial cells, although it has been reported that Candida cells killed with sodium periodate, although phagocytosed, are unable to elicit this cytokine response.20,21 However, in another study, heat-killed Candida cells were able to stimulate monocytes to release pro-inflammatory cytokines,24 suggesting that the means of killing the cells may be relevant.
PTX3 blood levels are usually <10 ng ml−1 in healthy subjects and increase rapidly during inflammation and infection, leading to investigation of the potential of PTX3 as an early biomarker for sepsis. In a heterogeneous group of critically ill patients ranging from those with no infection to those with septic shock, it was reported that PTX3 levels were higher in the more severely ill patients.25 We also found that PTX3 levels were related to severity of illness as assessed by APACHE II score. The APACHE scores were not presented in that report,25 making further comparisons difficult. PTX3 has also been found to be increased in patients with acute respiratory distress syndrome.26 In a recent study in children with meningococcal disease, high PTX3 and low CRP concentrations at admission discriminated between the presence and the absence of shock.27 PTX3 did not correlate with paediatric risk of mortality, whereas CRP correlated negatively. PTX3 blood levels increase more rapidly than CRP and only loose25,26 or no27 correlations are found between circulating levels of PTX3 and CRP. PTX3 has also been shown to be up-regulated in patients with dengue virus infection.28
Marked oxidative stress has been consistently reported in patients with sepsis8,20,29,30 and acts as a trigger for up-regulation of many inflammatory responses. The human PTX3 proximal promoter contains binding sites for several transcription factors, including activator protein-1 (AP-1), NFκB, and nuclear factor-IL-6. The NFκB proximal site is essential for induction by IL-1β and TNFα,6,7 and PTX3 was shown to be regulated by NFκB.31 NFκB is redox-sensitive32 and has been shown to be involved in regulation of inflammatory responses in patients with sepsis: we and others have found elevated activation of NFκB linked to increased mortality33,34 and inhibitable by NAC treatment, resulting in down-regulation of inflammatory mediators.35 We therefore investigated whether antioxidants were able to down-regulate PTX3 expression by endothelial cells in vitro. We found that NAC, trolox, and idebenone treatment all resulted in lower PTX3 levels on exposure to an inflammatory stimulus. In the case of NAC and trolox, this was accompanied by lower nuclear expression of NFκB. In a study using macrophages, the antioxidant pyrrolidine dithiocarbamate inhibited LPS-induced PTX3 expression in murine macrophages via an NFκB-dependent mechanism.31 However, there are other redox-sensitive transcription factors besides NFκB, such as AP-1,36 which may also be important for regulation of PTX3. Exogenous and endogenous antioxidants have been shown to be effective in blocking activation of NFκB either directly or indirectly, including NAC and trolox.35,36 Although co-enzyme Q10 can inhibit NFκB,37 we can find no reports of the effect of idebenone on transcription, and so the mechanism of the down-regulation of PTX3 expression by idebenone remains unknown.
Oxidative stress can be defined as an imbalance between the production of reactive oxygen species and their removal by protective antioxidants. We measured lipid hydroperoxide levels and total antioxidant capacity in our patients with sepsis. Lipid peroxidation is the oxidative degradation of lipids and has been reported previously to be elevated in critically ill patients.8,10,12,30 Total antioxidant capacity reflects the ability of plasma to resist oxidative damage in vitro and has been widely used to assess the antioxidant status of patients with sepsis.8,9,29 We found that lipid hydroperoxides were increased and total antioxidant capacity was decreased in our patients. The classical view of lipid peroxidation products is that they are essentially harmful, inducing and propagating chronic inflammatory reactions. However, it has been suggested that lipid peroxide breakdown products may promote cellular defence mechanisms,38,39 such as induction of signalling pathways, resulting in up-regulation of anti-inflammatory mediators, and inhibition of signalling pathways which control pro-inflammatory mediator expression. Thus, the role of lipid hydroperoxides in inflammatory responses in sepsis is unclear. We found that neither lipid hydroperoxides nor antioxidant capacity was related to PTX3 levels, despite the effect of antioxidants on PTX3 expression in vitro. Although this was a small pilot study, there was a range of PTX values and oxidative stress was apparent, and so we would have expected some evidence of a relationship. These results may reflect the complex nature of the role of redox state involved in regulation of inflammatory pathways in vivo.
Other factors also influence PTX3 levels. In a study of more than 2000 healthy Japanese subjects, plasma PTX3 levels were reported to be lower in men than in women, higher in older age groups, and inversely correlated with triglycerides.40 We also found that PTX3 levels in patients with sepsis were highest in the female patients, but the skewed age range of patients with sepsis did not allow assessment of any age. The previous study of critically ill patients25 did not report sex distribution. It remains unclear as to the importance of the contribution of these confounding factors.
In conclusion, we report that PTX3 expression in endothelial cells can be up-regulated by a variety of inflammatory mediators, including bacterial cell proteins, cytokines, and C. albicans and is down-regulated by NAC and trolox through an NFκB-mediated process. PTX3 levels are increased in patients with sepsis, and related to APACHE II score. Although we found evidence of oxidative stress in our patients, the regulation of PTX3 by antioxidants in vitro was not reflected clinically in this pilot study. Further work is needed to clarify regulatory mechanisms for PTX3 and its use as a biomarker in sepsis.
Funding
A.L.H. was an Anaesthetic Research Society vacation scholar. N.R.W. and H.F.G. are funded by the Medical Research Council, the British Journal of Anaesthesia, and the Intensive Care Society. N.A.R.G. acknowledges funding from the Wellcome Trust.
References
- 1.Mantovani A, Garlanda C, Doni A, Bottazzi B. Pentraxins in innate immunity: from C-reactive protein to the long pentraxin PTX3. J Clin Immunol. 2008;28:1–13. doi: 10.1007/s10875-007-9126-7. [DOI] [PubMed] [Google Scholar]
- 2.Bottazzi B, Bastone A, Doni A, et al. The long pentraxin PTX3 as a link among innate immunity, inflammation, and female fertility. J Leukoc Biol. 2006;79:909–12. doi: 10.1189/jlb.1005557. [DOI] [PubMed] [Google Scholar]
- 3.Breviario F, d'Aniello EM, Golay J, et al. Interleukin-1-inducible genes in endothelial cells. Cloning of a new gene related to C-reactive protein and serum amyloid P component. J Biol Chem. 1992;267:22190–7. [PubMed] [Google Scholar]
- 4.Lee GW, Lee TH, Vilcek J. TSG-14, a tumor necrosis factor- and IL-1-inducible protein, is a novel member of the pentraxin family of acute phase proteins. J Immunol. 1993;150:1804–12. [PubMed] [Google Scholar]
- 5.Vidal Alles V, Bottazzi B, Peri G, Golay J, Introna M, Mantovani A. Inducible expression of PTX3, a new member of the pentraxin family, in human mononuclear phagocytes. Blood. 1994;84:3483–93. [PubMed] [Google Scholar]
- 6.Pfaller M, Jones RN, Messer SA, Edmond MB, Wenzel RP. National surveillance of nosocomial blood stream infection due to Candida albicans: frequency of occurrence and antifungal susceptibility in the SCOPE Program. Diagn Microbiol Infect Dis. 1998;31:327–32. doi: 10.1016/s0732-8893(97)00240-x. [DOI] [PubMed] [Google Scholar]
- 7.Wey SB, Mori M, Pfaller MA, Woolson RF, Wenzel RP. Hospital-acquired candidemia. The attributable mortality and excess length of stay. Arch Intern Med. 1988;148:2642–5. doi: 10.1001/archinte.148.12.2642. [DOI] [PubMed] [Google Scholar]
- 8.Basile A, Sica A, D'Aniello E, et al. Characterization of the promoter for the human long pentraxin PTX3. J Biol Chem. 1997;272:8172–8. doi: 10.1074/jbc.272.13.8172. [DOI] [PubMed] [Google Scholar]
- 9.Altmeyer A, Klampfer L, Goodman AR, Vilcek J. Promoter structure and transcriptional activation of the murine TSG-14 gene encoding a tumor necrosis factor interleukin-1-inducible pentraxin protein. J Biol Chem. 1995;270:25584–90. doi: 10.1074/jbc.270.43.25584. [DOI] [PubMed] [Google Scholar]
- 10.Goode HF, Cowley HC, Walker BE, Webster NR. Decreased antioxidant status and increased lipid peroxidation in patients with sepsis and secondary organ dysfunction. Crit Care Med. 1995;23:646–51. doi: 10.1097/00003246-199504000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Cowley HC, Bacon PJ, Goode HF, Webster NR, Jones JG, Menon DK. Plasma antioxidant potential in severe sepsis: a comparison of survivors and non-survivors. Crit Care Med. 1996;24:1179–83. doi: 10.1097/00003246-199607000-00019. [DOI] [PubMed] [Google Scholar]
- 12.Galley HF, Howdle PD, Walker BE, Webster NR. The effects of intravenous antioxidants in patients with septic shock. Free Rad Biol Med. 1997;23:768–74. doi: 10.1016/s0891-5849(97)00059-2. [DOI] [PubMed] [Google Scholar]
- 13.Berger MM, Chioléro RL. Antioxidant supplementation in sepsis and systemic inflammatory response syndrome. Crit Care Med. 2007;35:S584–90. doi: 10.1097/01.CCM.0000279189.81529.C4. [DOI] [PubMed] [Google Scholar]
- 14.Andresen M, Regueira T, Bruhn A, et al. Lipoperoxidation and protein oxidative damage exhibit different kinetics during septic shock. Mediators Inflamm. 2008 doi: 10.1155/2008/168652. doi:10.1155/2008/168652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–74. [PubMed] [Google Scholar]
- 16.Lowes DA, Thottakam BMVJ, Webster NR, Murphy MP, Galley HF. The mitochondria-targeted antioxidant MitoQ protects against organ damage in a lipopolysaccharide–peptidoglycan model of sepsis. Free Rad Biol Med. 2008;45:1559–65. doi: 10.1016/j.freeradbiomed.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Walker LA, MacCallum DM, Bertram G, Gow NA, Odds FC, Brown AJ. Genome-wide analysis of Candida albicans gene expression patterns during infection of the mammalian kidney. Fungal Gen Biol. 2009;46:210–9. doi: 10.1016/j.fgb.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goode HF, Richardson N, Myers DS, Walker BE, Howdle PD, Webster NR. The effect of anticoagulant choice on apparent total antioxidant capacity using three different methods. Ann Clin Biochem. 1995;32:413–6. doi: 10.1177/000456329503200410. [DOI] [PubMed] [Google Scholar]
- 19.Polentarutti N, Bottazzi B, Di Santo E, et al. Inducible expression of the long pentraxin PTX3 in the central nervous system. J Neuroimmunol. 2000;106:87–94. doi: 10.1016/s0165-5728(00)00214-9. [DOI] [PubMed] [Google Scholar]
- 20.Filler SG, Pfunder AS, Spellberg BJ, Spellberg JP, Edwards JE., Jr Candida albicans stimulates cytokine production and leukocyte adhesion molecule expression by endothelial cells. Infect Immun. 1996;64:2609–17. doi: 10.1128/iai.64.7.2609-2617.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Filler SG, Swerdloff JN, Hobbs C, Luckett PM. Penetration and damage of endothelial cells by Candida albicans. Infect Immun. 1995;63:976–83. doi: 10.1128/iai.63.3.976-983.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phan QT, Filler SG. Endothelial cell stimulation by Candida albicans. Methods Mol Biol. 2009;470:313–6. doi: 10.1007/978-1-59745-204-5_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orozco AS, Zhou X, Filler SG. Mechanisms of the proinflammatory response of endothelial cells to Candida albicans infection. Infect Immun. 2000;68:1134–41. doi: 10.1128/iai.68.3.1134-1141.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rapponi G, Ghezzi MC, Mancini C, Filadoro F. Culture filtrates and whole heat-killed Candida albicans stimulate human monocytes to release interleukin-6. Microbiologica. 1993;16:267–74. [PubMed] [Google Scholar]
- 25.Muller B, Peri G, Doni A, et al. Circulating levels of the long pentraxin PTX3 correlate with severity of infection in critically ill patients. Crit Care Med. 2001;29:1404–7. doi: 10.1097/00003246-200107000-00017. [DOI] [PubMed] [Google Scholar]
- 26.Mauri T, Coppadoro A, Bellani G, et al. Pentraxin 3 in acute respiratory distress syndrome: an early marker of severity. Crit Care Med. 2008;36:2302–8. doi: 10.1097/CCM.0b013e3181809aaf. [DOI] [PubMed] [Google Scholar]
- 27.Sprong T, Peri G, Neeleman C, et al. Pentraxin 3 and C-reactive protein in severe meningococcal disease. Shock. 2009;31:28–32. doi: 10.1097/SHK.0b013e31817fd543. [DOI] [PubMed] [Google Scholar]
- 28.Mairuhu AT, Peri G, Setiati TE, et al. Elevated plasma levels of the long pentraxin, pentraxin 3, in severe dengue virus infections. J Med Virol. 2005;76:5. doi: 10.1002/jmv.20397. [DOI] [PubMed] [Google Scholar]
- 29.Roth E, Manhart N, Wessner B. Assessing antioxidative status in critically ill patients. Curr Opin Clin Nutr Metab Care. 2004;7:161–8. doi: 10.1097/00075197-200403000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Ogilvie AC, Groeneveld AB, Straub JP, Thijs LG. Plasma lipid peroxides and antioxidants in human septic shock. Intensive Care Med. 1991;17:40–4. doi: 10.1007/BF01708408. [DOI] [PubMed] [Google Scholar]
- 31.Goodman AR, Levy DE, Reis LF, Vilcek J. Differential regulation of TSG-14 expression in murine fibroblasts and peritoneal macrophages. J Leukoc Biol. 2000;67:387–95. doi: 10.1002/jlb.67.3.387. [DOI] [PubMed] [Google Scholar]
- 32.Sun Y, Oberley LW. Redox regulation of transcriptional activators. Free Rad Biol Med. 1996;21:335–48. doi: 10.1016/0891-5849(96)00109-8. [DOI] [PubMed] [Google Scholar]
- 33.Paterson RL, Galley HF, Dhillon JK, Webster NR. Increased nuclear factor kappa B activation in critically ill patients who die. Crit Care Med. 2000;28:1047–51. doi: 10.1097/00003246-200004000-00022. [DOI] [PubMed] [Google Scholar]
- 34.Böhrer H, Qiu F, Zimmermann T, et al. Role of NFkappaB in the mortality of sepsis. J Clin Invest. 1997;100:972–85. doi: 10.1172/JCI119648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paterson RL, Galley HF, Webster NR. The effect of N-acetylcysteine on nuclear factor κB activation, interleukin-6, interleukin-8 and intercellular adhesion molecule-1 expression in patients with sepsis. Crit Care Med. 2003;31:2574–8. doi: 10.1097/01.CCM.0000089945.69588.18. [DOI] [PubMed] [Google Scholar]
- 36.MacDonald J, Galley HF, Webster NR. Oxidative stress and gene expression in sepsis. Br J Anaesth. 2003;90:221–32. doi: 10.1093/bja/aeg034. [DOI] [PubMed] [Google Scholar]
- 37.Ebadi M, Sharma SK, Wanpen S, Amornpan A. Coenzyme Q10 inhibits mitochondrial complex-1 down-regulation and nuclear factor-kappa B activation. J Cell Mol Med. 2004;8:213–22. doi: 10.1111/j.1582-4934.2004.tb00276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bochkov VN, Leitinger N. Anti-inflammatory properties of lipid oxidation products. J Mol Med. 2003;81:613–26. doi: 10.1007/s00109-003-0467-2. [DOI] [PubMed] [Google Scholar]
- 39.Uchida K. Lipid peroxidation and redox-sensitive signaling pathways. Curr Atheroscler Rep. 2007;9:216–21. doi: 10.1007/s11883-007-0022-7. [DOI] [PubMed] [Google Scholar]
- 40.Yamasaki K, Kurimura M, Kasai T, Sagara M, Kodama T, Inoue K. Determination of physiological plasma pentraxin 3 (PTX3) levels in healthy populations. Clin Chem Lab Med. 2009;47:471–7. doi: 10.1515/CCLM.2009.110. [DOI] [PubMed] [Google Scholar]