Abstract
Aims
Although cardiac alternans is a known predictor of lethal arrhythmias, its underlying causes remain largely undefined in disease settings. The potential role of, and mechanisms responsible for, beat-to-beat alternations in the amplitude of systolic Ca2+ transients (Ca2+ alternans) was investigated in a canine post-myocardial infarction (MI) model of sudden cardiac death (SCD).
Methods and results
Post-MI dogs had preserved left ventricular (LV) function and susceptibility to ventricular fibrillation (VF) during exercise. LV wedge preparations from VF dogs were more susceptible to action potential (AP) alternans and the frequency-dependence of Ca2+ alternans was shifted towards slower rates in myocytes isolated from VF dogs relative to controls. In both groups of cells, cytosolic Ca2+ transients ([Ca2+]c) alternated in phase with changes in diastolic Ca2+ in sarcoplasmic reticulum ([Ca2+]SR), but the dependence of [Ca2+]c amplitude on [Ca2+]SR was steeper in VF cells. Abnormal ryanodine receptor (RyR) function in VF cells was indicated by increased fractional Ca2+ release for a given amplitude of Ca2+ current and elevated diastolic RyR-mediated SR Ca2+ leak. SR Ca2+ uptake activity did not differ between VF and control cells. VF myocytes had an increased rate of reactive oxygen species production and increased RyR oxidation. Treatment of VF myocytes with reducing agents normalized parameters of Ca2+ handling and shifted the threshold of Ca2+ alternans to higher frequencies.
Conclusion
Redox modulation of RyRs promotes generation of Ca2+ alternans by enhancing the steepness of the Ca2+ release–load relationship and thereby providing a substrate for post-MI arrhythmias.
Keywords: Cardiac alternans, Ryanodine receptor, Redox modification, Ca2+ release, Myocardial infarction
1. Introduction
Sudden cardiac death (SCD) due to sustained ventricular arrhythmias [ventricular tachycardia or ventricular fibrillation (VF)] is a major cause of mortality in patients following myocardial infarction (MI).1–3 In these patients, ventricular ectopy appears to be part of the pathogenic mechanism of arrhythmias, whereas electrical heterogeneity and dispersion of repolarization are thought to be essential for the arrhythmogenic substrate.4 One important source of repolarization dispersion is cardiac alternans, a beat-to-beat alternation in action potential duration (APD) and contractile force or intracellular Ca2+ transient.5,6 Ca2+ dynamics, and in particular regulation of Ca2+ release from the sarcoplasmic reticulum (SR), has been recognized as a key factor in the genesis of electromechanical alternans.6–9
Several factors have been implicated in the disturbances of Ca2+ signalling that result in alternans. These include slowed SR Ca2+ uptake,10,11 incomplete recovery of ryanodine receptor (RyR) from inactivation,12,13 and increased steepness of the Ca2+ release–SR Ca2+ content relationship.11,14 However, the relative importance of these factors in the genesis of alternans remains a subject of debate. Moreover, most studies of alternans have been performed in myocytes from healthy hearts using various experimental interventions or computer simulations using mathematical models of Ca2+ cycling. Thus, the mechanisms of alternans in clinically relevant disease models remain largely unexplored.
Altered redox balance and redox-mediated changes in Ca2+ handling are increasingly recognized as important pathogenic factors in different cardiac diseases, including both ischaemic and non-ischaemic disease processes.15 Reactive oxygen species (ROS) have the capability to affect Ca2+ handling via redox modification of components of the excitation–contraction (EC) coupling machinery, including RyRs.16 However, the potential role of redox-mediated alterations of Ca2+ signalling in the genesis of alternans and arrhythmias in general remains to be investigated.
In the present study, the potential role of, and mechanisms responsible for, Ca2+ alternans was investigated using a canine post-MI model of SCD. This well-characterized model is known to recapitulate many aspects of humans with healed MIs and a residual risk of SCD (recently reviewed in Billman17) including altered repolarization, altered autonomic balance, and responses to pharmacological interventions. The present study reports susceptibility to Ca2+ alternans at the tissue and myocyte levels in post-MI animals with a demonstrated vulnerability to sustained ventricular tachycardia and VF. Ca2+ alternans resulted from the increased steepness of the SR Ca2+ release–content relationship and is attributable to enhanced RyR activity secondary to redox modification of the channel protein.
2. Methods
Expanded Methods section is given in Supplementary material online.
2.1. Canine model of sustained ventricular tachyarrhythmia
All in vivo procedures18,19 were approved by the Ohio State University Institutional Animal Care and Use Committee and conformed with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). An anterior wall MI was induced by ligation of the left anterior descending coronary artery, and a vascular occluder was placed on the left circumflex coronary artery at the time of surgery. A minimum of 4 weeks after surgery and the MI, the dogs were risk-stratified for arrhythmia susceptibility by an exercise plus ischaemia test as described previously.17 This test reliably induced ventricular flutter that deteriorated into VF. A minimum of 7 days elapsed between the exercise plus ischaemia test and tissue or myocyte isolation to avoid any acute effects of recent ischaemia. Only dogs with VF in response to the exercise plus ischaemia test were included in the present study (VF animals, n = 13 female, age 2–3 years, weight 18–26 kg); a group of 20 normal dogs (male/female, age 1–4 years, weight 15–25 kg) served as controls. Left ventricle (LV) function was assessed in a subset of control and VF animals using echocardiography. For the dogs used in the present study, LV fractional shortening was 41 ± 2 and 41 ± 1% in control (n = 10) and VF (n = 5) animals, respectively.20
2.2. Optical AP mapping in the canine wedge preparation
Canine wedge preparations were isolated from normal (n = 4) and VF (excluding both infracted and border zone tissue, n = 4) dogs and placed in an imaging chamber for optical mapping while being perfused with Tyrode solution. Wedges were stained with the voltage-sensitive dye, di-4-ANEPPS (15 µM, Molecular Probes, OR, USA) and then administered cytochalasin D (6 µM) to ensure that motion artefact was prevented. Optical APs were recorded with high spatial (0.9 mm), temporal (1.0 ms), and voltage (0.5 mV) resolutions from cells spanning the entire LV wall. AP alternans was induced by decreasing pacing cycle lengths until 1-to-1 capture was lost.
2.3. Ca2+ imaging in myocytes
Myocytes were isolated from LV mid-myocardial wall by using standard techniques as described previously.20 In the VF group, cells were isolated from region located at least 6–8 cm from the centre of the infarct to exclude necrotic and border zone area. Whole-cell patch-clamp recordings of AP and Ca2+ currents (ICa) were performed with an Axopatch 200B amplifier (Axon Instruments, CA, USA). Intracellular Ca2+ imaging was performed using an Olympus Fluoview 1000 confocal microscope in line-scan or XY mode. Intra-SR Ca2+ levels were studied by loading myocytes with 10 µM Fluo-5N AM (Molecular Probes) for 3–4 h at 37°C. Rhod-2 or Fluo-3 Ca2+ indicators were used to monitor cytosolic Ca2+. To facilitate [Ca2+]SR measurements, voltage-clamp experiments involving [Ca2+]SR recordings were performed in the presence of 100 nM isoproterenol (Iso, Calbiochem, CA, USA), a β-adrenergic agonist, which enhances SR Ca2+ release by increasing ICa and stimulating SERCA activity in both control and VF myocytes (see Supplementary material online, Figure S1).
2.4. Measurements of ROS production and RyR free thiol content
Changes in ROS production were measured with the fluorescent indicator 5-(and-6) chloromethyl-2′,7′-dichlorodihydrofluoroscein diacetate (DCFDA, 10 µM, Molecular Probes).21 After subtraction of background fluorescence, the signal (ΔF) was normalized to maximum fluorescence attained by application of 10 mM H2O2 (FMAX).
The content of free thiols in RyRs was determined with monobromobimane (mBB, Calbiochem) fluorescence method.22 Heavy SR vesicles were prepared from fresh tissue samples from control and VF hearts under non-reducing conditions.23 Samples were incubated with 400 µM mBB for 1 h in the dark at room temperature; subsequently, proteins were acetone precipitated and subjected to SDS–PAGE (4–20% gradient gel, Bio-Rad, CA, USA). mBB fluorescence was normalized to RyR amount determined using Coomassie Blue staining of the gels run in parallel.
2.5. Data analysis
Results are presented as mean ± SE. Statistical significance was evaluated either by the appropriate Student's t-test or by one-way ANOVA where appropriate. A P-value of <0.05 was considered significant.
3. Results
3.1. AP alternans in wedge preparations
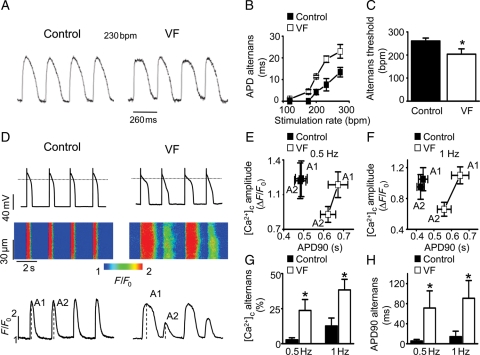
In order to examine potential arrhythmogenic mechanisms in the post-infarction hearts of dogs susceptible to VF, optical APs were recorded from the transmural wall of LV wedge preparations while alternans was induced. The rate-dependence of APD alternans was significantly shifted towards slower heart rates, and the alternans magnitude was increased in wedges from VF animals compared with controls, indicating greater susceptibility to APD alternans in these preparations (Figure 1A–C). Furthermore, spatially discordant alternans occurred in all VF wedge preparations but in only 50% of the control. The presence of spatially discordant alternans significantly increased maximum repolarization gradients in VF wedge preparations from 4.3 ± 2.0 to 13.5 ± 3.3 mm/ms (P < 0.03). In addition, rapid pacing-induced VF was only observed in the VF preparations, never in control (see Supplementary material online, Figure S2). Baseline APD was similar in wedges from hearts of both control (254 ± 15 ms) and VF (262 ± 14 ms, P= NS) animals. Conduction velocity in control wedges (0.43 ± 0.06 m/s) was also similar to that in VF wedges (0.41 ± 0.06 m/s, P= NS). These results suggest that cardiac alternans is a potential mechanism for arrhythmogenesis in the myocardium of VF animals.
Figure 1.
Increased susceptibility to AP and Ca2+ alternans in VF hearts. (A) Representative mid-myocardial APs recorded at a stimulation rate of 230 bpm from a control and VF heart. (B) Dependence of the amplitude of APD alternans on stimulation rate recorded in VF and control wedge preparations. (C) APD alternans threshold in VF hearts (203 ± 23 bpm) is significantly lower than the threshold recorded in control (261 ± 12 bpm; *P < 0.05 vs. control). (D) Representative recordings of membrane potential with corresponding line-scan images and temporal profiles of Fluo-3 fluorescence in control and VF myocytes. (E and F) Average amplitudes of [Ca2+]c and duration of corresponding APs were measured with consecutive stimuli in control and VF cells paced with 0.5 and 1 Hz, respectively. A1 and A2 are [Ca2+]c amplitudes of two consecutive stimuli, as shown in (D). Average amplitudes of [Ca2+]c (G) and APD90 (H) alternans in control and VF myocytes were measured at indicated stimulation frequencies. *P < 0.05, vs. control.
3.2. AP and Ca2+ alternans in isolated cardiomyocytes myocytes
Experiments performed on isolated myocytes demonstrated that APD alternans was paralleled by, and occurred in phase with, alternations in the amplitude of Ca2+ transients, i.e. Ca2+ alternans (Figure 1D–H). Consistent with the results in wedge preparations, myocytes from VF hearts showed AP alternans at slower stimulation frequencies than controls.
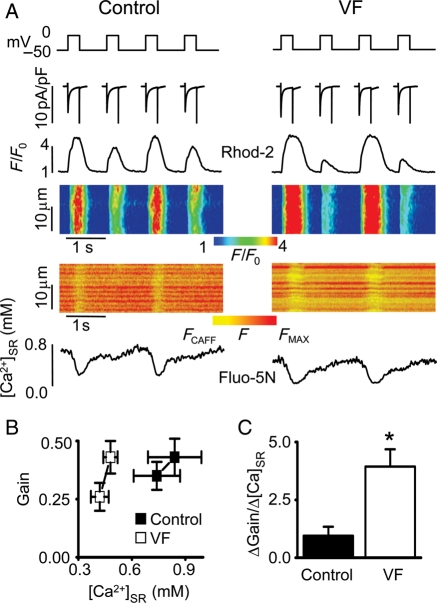
To explore the mechanisms of Ca2+ alternans, the next series of experiments were performed under voltage-clamp conditions (Figure 2). Additionally, in these experiments, the role of changes in intra-SR [Ca2+] in the generation of alternans was examined by measuring [Ca2+]SR simultaneously with cytosolic [Ca2+] using SR-entrapped Fluo-5N and cytosolic Rhod-2. Representative cytosolic Ca2+ and [Ca2+]SR measurements in a control and VF myocyte paced at 1 Hz are shown in Figure 2A. Cytosolic Ca2+ alternans in both control and VF cells occurred in phase with changes in diastolic [Ca2+]SR. Alternans was more pronounced and occurred at substantially lower [Ca2+]SR in VF cells than in controls. These results obtained at a fixed membrane potential showed that changes in Ca2+ handling alone could produce Ca2+ and, therefore, APD alternans. These data also point to a role for [Ca2+]SR fluctuations in the genesis of Ca2+ alternans.
Figure 2.
Properties of SR Ca2+ release in control and VF myocytes displaying Ca2+ alternans during 1 Hz stimulation. (A) Representative ICa traces and corresponding line-scan images and temporal profiles of Rhod-2 and Fluo-5N fluorescence recorded in voltage-clamped control and VF cells. Upper traces show voltage protocol used. (B) Dependence of the SR Ca2+ release gain function ([Ca2+]c amplitude/density of peak ICa) on [Ca2+]SR measured with consecutive stimuli in control and VF cells paced at 1 Hz. (C) The slope of gain–[Ca2+]SR function, calculated from the data presented in (B), was 1.0 ± 0.4 (n = 9) in control and 3.9 ± 0.8 (n = 10) in VF myocytes, respectively. *P < 0.05 vs. control.
Increased steepness of the Ca2+ release–load relationship is thought to be a key factor contributing to the development of Ca2+ alternans,11,14 although the contribution of this mechanism has been recently challenged.12 We measured directly the steepness of release–load relationships in control vs. VF myocytes exhibiting Ca2+ alternans. EC coupling gain, defined as the Ca2+ transient amplitude normalized to the peak of corresponding ICa density, was plotted as a function of the end-diastolic [Ca2+]SR of the preceding Ca2+ release cycle for the large and small releases in control and VF myocytes (Figure 2). As shown in Figure 2B and C, the steepness of Ca2+ release–load relationship was markedly increased in VF cells compared with control cells.
3.3. Ca2+ release during EC coupling
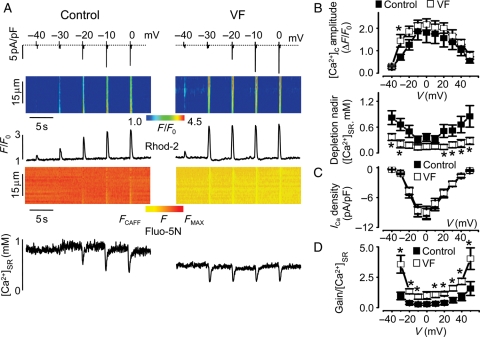
To better understand the mechanism(s) responsible for altered cellular Ca2+ dynamics in myocytes from the hearts of animals susceptible to VF, SR Ca2+ release in control vs. VF myocytes was characterized in more detail. Figure 3A shows representative recordings of changes in cytosolic and SR Ca2+ concentrations along with ICa during steps to varying membrane potentials in a control and a VF myocyte. The results are summarized in Figure 3B and C that plot the voltage dependencies of the peak amplitude of cytosolic Ca2+ transients, the nadir of the luminal Ca2+ depletion signals, and ICa amplitude for the control and VF groups. Peak and decay of ICa measured at 0 mV were similar between the two groups (see Supplementary material online, Table S1). The amplitude of maximum cytosolic Ca2+ transients (at 0 mV) was also preserved in VF myocytes, although diastolic [Ca2+]SR was markedly reduced from 1.1 ± 0.3 (n = 7) observed in control to 0.4 ± 0.1 mM (n = 8, P < 0.05). Notably, the magnitudes of both the cytosolic and luminal Ca2+ signals increased with small depolarizations, resulting in flattened and broadened voltage-dependence curves. Figure 3D shows that the ratio of gain of Ca2+-induced Ca2+ release to [Ca2+]SR is significantly increased in VF myocytes, suggesting that RyR functional activity was increased in VF myocytes during EC coupling.
Figure 3.
Voltage-dependent characteristics of Ca2+-induced Ca2+ release in control and VF myocytes. (A) Representative traces of ICa, line-scan images and temporal profiles of Rhod-2 and Fluo-5N fluorescence recorded in control and VF cells. ICa and corresponding [Ca2+]c were evoked by depolarizing steps from a holding potential of −50 mV to the indicated potentials. (B) Voltage-dependence of the amplitude of [Ca2+]c and nadir of SR Ca2+ depletion in control and VF cells.*P < 0.05 vs. control. Voltage-dependence of the peak ICa (C) and of the gain/diastolic [Ca2+]SR ratio (D). *P < 0.05 vs. control.
3.4. Ca2+ sparks and SR Ca2+ leak
In order to examine further the relationship between susceptibility to VF and SR Ca2+ release, Ca2+ sparks were measured in permeabilized myocytes at a constant baseline cytosolic [Ca2+] (see Supplementary material online, Figure S3). Ca2+ spark frequency was significantly increased, whereas the amplitude of the Ca2+ sparks was significantly decreased in VF myocytes compared with controls. These effects were associated with decreased SR Ca2+ content, as determined by applications of caffeine (see Supplementary material online, Figure S3D and E). Increased Ca2+ spark frequency observed at reduced SR Ca2+ content suggested that RyR functional activity was enhanced, resulting in leaky SR Ca2+ stores in VF myocytes.
3.5. SERCA-mediated SR Ca2+ uptake and Na+/Ca2+ exchanger activity
In addition to elevated SR Ca2+ leak, the reduced SR Ca2+ content in VF myocytes could result from the inhibition of SR Ca2+ uptake. SERCA-mediated SR Ca2+ uptake was measured in permeabilized control and VF myocytes using SR-entrapped Fluo-5N in the presence of the RyR-antagonist ruthenium red (RR). In these experiments, the SR was first depleted in a Ca2+-free solution using caffeine (10 mM) and then SR Ca2+ uptake was initiated by addition of 500 nM Ca2+ to the bath solution. As shown in Supplementary material online, Figure S3F and G, the rate of SR Ca2+ uptake measured as an increase in Fluo-5N fluorescence did not significantly differ between control and VF myocytes. Thus, the intrinsic Ca2+ transport activity of SERCA was not altered in VF myocytes.
In large animals, Na+/Ca2+ exchanger (NCX) contributes significantly to intracellular Ca2+ dynamic and hence it could contribute to the generation of Ca2+ alternans. NCX activity was assessed by analysing decay kinetics of caffeine-induced Ca2+ transients in control and VF myocytes. As shown in Supplementary material online, Figure S4, the rate of decay of caffeine-induced Ca2+ transients was not different between control and VF myocytes.
3.6. Role of redox modification of RyR
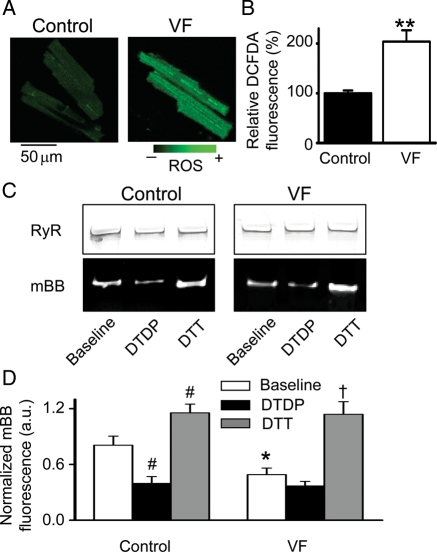
Redox modification of Ca2+ regulatory proteins, including RyRs, is increasingly recognized as a potential factor in the pathophysiology of cardiac diseases, including arrhythmias.16,24 The levels of ROS in VF and control myocytes were assessed using the ROS-sensitive indicator DCFDA. As shown in Figure 4A and B, ROS production was increased approximately two-fold in VF myocytes compared with controls. Furthermore, measurements with mBB revealed decreased levels of free thiols in RyRs isolated from VF hearts, providing direct evidence of enhanced oxidation of RyRs in VF hearts. RyRs from each group were treated with a specific oxidizer of sulfhydryl groups, 2,2′-dithiodipyridine (DTDP, 200 µM), to assess susceptibility to oxidation or the reducing agent dithiothreitol (DTT, 5 mM) to assess reversibility of oxidation (Figure 4C and D).
Figure 4.
Increased production of ROS and RyR oxidation in VF hearts. (A) Representative images of ROS-sensitive indicator DCFDA loaded into myocytes from normal and VF hearts. (B) Relative normalized DCFDA fluorescence in myocytes from control (n = 25) and VF hearts (n = 31). **P < 0.01 vs. control. Fluorescence values were normalized to the signal measured in the presence of 10 mM of H2O2 and presented relative to control (100%). (C) Representative Coomassie Blue-stained gels (upper panels) and corresponding mBB fluorescence intensity (lower panels) of RyR from normal and VF hearts measured under baseline conditions and after 30 min incubation with of 0.2 mM DTDP or 5 mM DTT. (D) Relative free thiol content of RyRs from control vs. VF samples obtained by normalizing mBB fluorescence to RyR amount determined using Coomassie Blue staining of the gels run in parallel. *P < 0.05 baseline VF (n = 7) vs. baseline control (n = 9); #P < 0.05 vs. baseline control; †P < 0.05 vs. baseline VF.
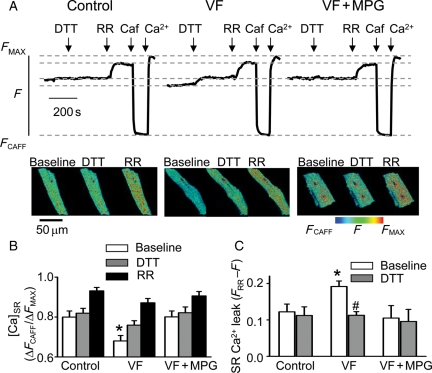
To determine the functional impact of redox modifications on altered SR Ca2+ regulation in VF myocytes, the effects of the reducing agent DTT on SR Ca2+ leak were examined in permeabilized VF vs. control myocytes (Figure 5). In these experiments, SR Ca2+ leak was estimated as the difference in intra-SR [Ca2+] measured with SR-entrapped Fluo-5N in the presence and absence of RR. Since at steady state, [Ca2+]SR is determined by the balance between SR Ca2+ leak via RyRs and Ca2+ uptake by SERCA, inhibition of RyRs by RR must raise [Ca2+]SR to a degree proportional to the level of Ca2+ leak via these channels.25 Consistent with the results of the Ca2+ spark measurements, SR Ca2+ leak was significantly higher in VF myocytes than in control (Figure 5C). However, treatment of VF cells with the reducing agents DTT or mercaptopropionylglycine (MPG) produced a substantial decrease in leak towards normal levels (Figure 5A–C). These results suggest that RyR-mediated SR Ca2+ leak was in part attributable to oxidation of RyRs. Moreover, incubation of intact VF myocytes with the antioxidant MPG led to a marked reduction in the amplitude of Ca2+ alternans, restoration of diastolic [Ca2+]SR (Figure 6A–C), and normalization of the steepness of Ca2+ release–[Ca2+]SR relationship (Figure 6D and E).
Figure 5.
Reducing agents normalize [Ca2+]SR in permeabilized VF myocytes by inhibiting RR-sensitive SR Ca2+ leak. (A) Time-dependent profiles of intra-SR Fluo-5N signals recorded before and after the application of 1 mM DTT and 30 µmol/L RR in control, VF cells, and in VF cells pre-treated with 1 mM N-(2-mercapto-propionyl)glycine (MPG). Representative XY-images of Fluo-5N signal of a control, a VF cell, and a VF cell pre-treated with MPG recorded under specified conditions. (B) Average data of normalized Fluo-5N signal in control (n = 11), VF cells (n = 12), and VF cells pre-treated with MPG (n = 9) recorded in the absence and presence of DTT and RR. *P < 0.05 baseline VF vs. baseline control and VF with MPG. (C) SR Ca2+ leak, defined as the difference in Fluo-5N fluorescence recorded with (FRR) and without (F) RR, under baseline conditions and in the presence of DTT in control, VF, and MPG-treated VF myocytes. *P < 0.05 vs. baseline control; #P < 0.05 vs. baseline VF.
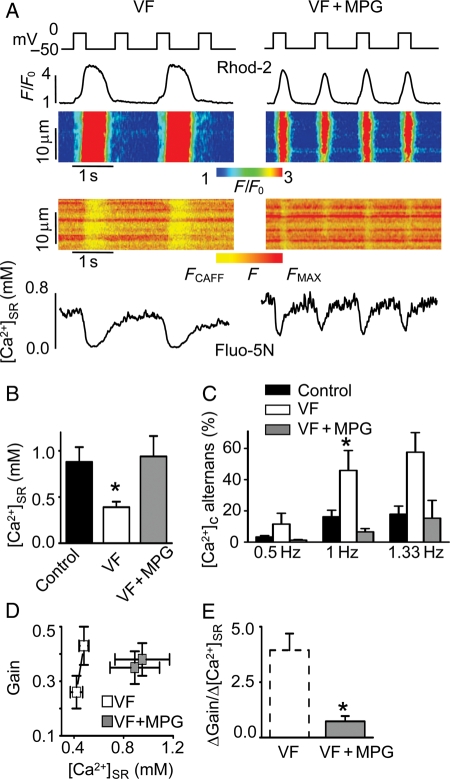
Figure 6.
A reducing agent normalizes [Ca2+]SR and lessens the amplitude of Ca2+ alternans in patch-clamped VF myocytes. (A) Representative line-scan images and temporal profiles of Rhod-2 and Fluo-5N fluorescence recorded in voltage-clamped VF cells recorded in the absence and presence of MPG, a reducing agent. Cells were stimulated at 1 Hz frequency. Upper traces show voltage protocol used. (B) Average values of end-diastolic [Ca2+]SR were 0.88 ± 0.16, 0.41 ± 0.06, and 0.94 ± 0.22 mM in control (n = 8), in VF myocytes (n = 10), and in VF myocytes treated with 750 µM MPG (n = 9), respectively. *P < 0.05 vs. control. (C) Average amplitude of [Ca2+]c alternans recorded at the indicated frequency of stimulation in voltage-clamped control cells (n = 4–13), VF cells (n = 8–11), and VF cells treated with MPG (n = 6–9). *P < 0.05 vs. 1 Hz control and 1 Hz VF cells treated with MPG. (D) Dependence of the SR Ca2+ release gain function ([Ca2+]c amplitude/density of peak ICa) on [Ca2+]SR measured for consecutive stimuli in VF cells in the absence and presence of MPG. Cells were paced at 1 Hz. (E) The slope of gain–[Ca2+]SR function, calculated from the data presented (D), was 0.7 ± 0.2 (n = 9) in VF myocytes treated with MPG. *P < 0.05 vs. untreated VF.
4. Discussion
In the present study, the role of, and mechanisms responsible for, abnormal intracellular Ca2+ handling in arrhythmogenesis was investigated using a canine model of post-MI SCD with a combination of methodological approaches, including AP mapping in cardiac tissue and monitoring Ca2+ levels in the cytosolic and SR compartments of isolated patch-clamped myocytes. The major findings of the present study are as follows: (i) myocytes and tissue obtained from the hearts of animals susceptible to VF exhibited an increased predisposition to arrhythmogenic AP and Ca2+ alternans; (ii) these alternans were associated with increased steepness of the Ca2+ release–[Ca2+]SR load relationship and hyperactive RyRs; and (iii) the pathological changes in RyRs and in alternans were at least partly due to redox modification(s) of the RyR channel.
4.1. The model
A well-characterized canine model of SCD was used in the present study.17 This model confers significant advantages over other models, in that it is a large animal model of chronic duration. The model mimics many of the pathological conditions that have been associated with a high risk of SCD including pre-existing ischaemic myocardial injury (i.e. anterior wall MI), acute MI at a site distant from previous ischaemic injury, and altered cardiac autonomic balance (decreased parasympathetic regulation coupled with enhanced sympathetic activation).17 In addition, it is essential that the lethal arrhythmias must be reliably and reproducibly induced before an accurate assessment of the mechanism responsible for these malignant rhythm disorders can be determined. In this model, VF is reproducibly induced in 95% of animals.17 Thus, the model used in this study provides the opportunity to investigate the relationship between pathological alterations in Ca2+ regulation and the propensity for lethal ventricular arrhythmias following MI in the absence of heart failure.
4.2. Role and mechanisms of Ca2+ alternans
Ca2+ alternans is increasingly recognized as an important factor in the development of cardiac arrhythmias by generating a substrate for re-entrant excitation.6,26,27 In the present study, myocytes and tissue obtained from the hearts of animals susceptible to VF exhibited an increased propensity for APD and Ca2+ alternans. The underlying factors responsible for Ca2+ alternans have not been fully determined, especially in the setting of cardiac disease. Several mechanisms have been suggested, including slowed SERCA-mediated SR Ca2+ uptake,10,11 incomplete recovery of RyR from inactivation,12,13 and increased steepness of the Ca2+ release–SR Ca2+ load relationship.11,14 The relative importance of these mechanisms is currently subject to debate. The effect of slowed Ca2+ uptake and prolonged refractoriness would be to decrease Ca2+ cycling such that normal Ca2+ release from the SR could be obtained only on alternating beats. With a steep Ca2+ release–load relationship, which, in general, reflects the stimulatory effects of luminal Ca2+ on RyR open probability,28 small differences in [Ca2+]SR would be expected to lead to substantial differences in the amplitude of Ca2+ transients, i.e. alternans. In our study, VF-related Ca2+ alternans was not attributable to slowed SR Ca2+ uptake. SR Ca2+ uptake rates were similar in control and VF myocytes (see Supplementary material online, Figure S3F and G). Moreover, Ca2+ alternans persisted in VF cells even after exposure to isoproterenol, which induced a significant acceleration of Ca2+ transient decay indicative of accelerated SR Ca2+ uptake (Figures 2 and 6). Similarly, a slowed recovery of RyR from inactivation did not contribute to alternans. Indeed, the present study demonstrated that both diastolic SR Ca2+ release and RyR functional activity were enhanced rather than decreased, consistent with reduced rather than increased inactivation of RyRs. At the same time, supporting a central role for [Ca2+]SR-dependent mechanisms in the genesis of alternans, cytosolic Ca2+ transients always alternated in phase with changes in end-diastolic [Ca2+]SR. Moreover, during Ca2+ alternans at a given pacing rate, smaller alterations in end-diastolic [Ca2+]SR resulted in larger deviations in cytosolic Ca2+ transients (i.e. steeper Ca2+ release–[Ca2+]SR relationship) in VF myocytes than in control myocytes. Thus, altered [Ca2+]SR-dependence of release contributed to the increased predisposition to alternans in VF myocytes. Although these studies demonstrate the role of abnormal Ca2+ release in the genesis of alternans in our particular arrhythmia model, they do not rule out the possibility that other mechanisms, including defective SR Ca2+-reuptake, contribute to alternans in other disease settings. It is also important to note that even though we show significant abnormalities of Ca2+ regulation that can create a substrate for arrhythmogenesis, this does not exclude the possibility that repolarization abnormalities (independent of Ca2+) also contribute to arrhythmogenic events.20,29
4.3. Alterations in Ca2+ release properties
The present investigation of EC coupling and SR Ca2+ release properties provides further insights as to the abnormalities of SR Ca2+ signalling in VF myocytes. Our experiments showed that while maximum Ca2+ transient amplitude (at 0 mV) was retained, the amplitude of Ca2+ transients at low membrane depolarizations was significantly increased, resulting in distinct broadening and flattening of the voltage-dependence of SR Ca2+ release in VF myocytes (Figure 3A and B). Furthermore, diastolic [Ca2+]SR was significantly reduced in VF cells resulting in a significant increase in EC gain at a given intra-SR [Ca2+] (Figure 3D). Additionally, VF myocytes exhibited a significantly higher RyR-mediated diastolic SR Ca2+ leak measured both as Ca2+ sparks (see Supplementary material online, Figure S3) and as the ruthenium red-sensitive component of baseline [Ca2+]SR (Figure 5). These results indicate that SR Ca2+ release via RyRs was potentiated in VF myocytes during both systole and diastole. RyRs are known to respond positively to elevated luminal Ca2+.28 Our finding that the increase in RyR functional activity occurred against the backdrop of reduced [Ca2+]SR is consistent with the hypothesis that RyR responsiveness to luminal Ca2+ was increased in VF myocytes. These changes in RyRs could account for, or contribute to, the increased steepness of release–[Ca2+]SR relationship in VF myocytes during Ca2+ alternans.
In previous studies, severe and/or large infarctions resulted in diminished rather than enhanced SR Ca2+ release. For example, myocytes from the border zone of infarcted hearts and myocytes from hearts with ischaemic cardiomyopathy30–33 have been reported to have reduced and slowed Ca2+ transients, lowered EC coupling gain, and decreased contractility at all membrane potentials in stark contrast to our present findings. These different results suggest that responses to infarction are more heterogeneous and diverse than previously thought and may reflect different stages of cardiac remodelling following infarction and/or qualitatively different types of responses to infarctions of different severities. It is also possible that changes in RyR in the previous studies were masked by other changes such as disruption of the membrane system in models with more severe/larger infarction that induced heart failure. Notably, our model does not exhibit impaired LV systolic function and is a model of arrhythmogenesis without concomitant heart failure.17,20
4.4. Role of RyR redox modification in altered SR Ca2+ release
In the present study, we investigated the hypothesis that abnormal RyR function in VF hearts results from redox modification of the channel protein. This hypothesis was based on the following notions. Sulfhydryl oxidation of reactive cysteine molecules by various oxidizing agents is known to increase RyR open time probability, thus producing ‘leaky’ RyR channels.16 Previous studies demonstrated increased ROS production and oxidative stress in various ischaemic and non-ischemic cardiac diseases states15 including models of healed infarction (up to 16 weeks post-MI).34 In the present study, the following findings are consistent with redox-mediated alterations of Ca2+ signalling in VF hearts: (i) ROS generation was enhanced in VF myocytes (Figure 4A and B); (ii) the number of free thiols on RyRs decreased in VF hearts compared with control (Figure 4C and D); and (iii) reducing agents normalized Ca2+ leak and Ca2+ cycling in VF myocytes (Figures 5 and 6).
Even though treatment with antioxidants resulted in significant improvements in intracellular Ca2+ handling, the results of the present study do not exclude the possibility that other types of post-translational modification(s), such as phosphorylation by cAMP-dependent protein kinase35,36 and/or Ca2+/calmodulin kinase II,37 also contribute to abnormal Ca2+ handling in VF cells. Moreover, these pathways may be linked at the level of CAMKII, which is reportedly activated by increased oxidative stress and contributes to ischaemic myocardial apoptosis38 and arrhythmogenesis.39
4.5. Arrhythmia mechanisms
Previous work illustrated a role for re-entry in the pathogenesis of ventricular tachyarrhythmias in this model.40 In addition, Ca2+ chelators (BAPTA-AM) and modulators of Ca2+ entry (e.g. L-type Ca2+ channel blockers) have demonstrated in vivo efficacy in preventing tachyarrhythmias in this model.17,40,41 Importantly, both have also been shown to inhibit Ca2+ alternans.5 The results from the present study are consistent with these previous in vivo observations and provide further insights into arrhythmogenic mechanisms in the post-MI heart. Our tissue and myocyte studies demonstrated an increased predisposition to APD and Ca2+ alternans in arrhythmic hearts following MI. APD alternans is arrhythmogenic because it can amplify spatial heterogeneities of repolarization, creating a substrate for re-entrant excitation.42 Of note, the VF wedge preparations in our study showed increased predisposition to the development of spatially discordant alternans that significantly increased spatial gradients of repolarization and is a known precursor to VF.6,9
4.6. Limitations
Caution should be used when extrapolating our findings to in vivo settings. Our experiments on isolated cardiac myocytes were performed at the room temperature and ambient O2 tension (20%) as opposed to physiological temperature (37°C) and O2 (5%) levels which are known to effect both myocyte Ca2+ handling and redox reactions. Although our data suggest that alternans is a potential mechanism of arrhythmogenesis in these VF hearts, our study does not preclude the possibility that other mechanisms such as ectopic activity or conduction block due to preserved structural damage (scar) contribute to arrhythmogenesis.
4.7. Conclusions
Collectively, our data suggest that redox-dependent changes in RyRs contribute to arrhythmogenesis by promoting Ca2+ cycling-induced repolarization alternans. Therefore, in the post-infarction heart, normalizing Ca2+ release, potentially via targeted antioxidant therapies, may be beneficial in patients at risk for SCD.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This work was supported by the National Institutes of Health Grants [HL074045 and HL063043 to S.G., HL086700 and HL68609 to G.E.B., and HL84142 to K.R.L.].
Supplementary Material
References
- 1.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 2.Bunch TJ, Hohnloser SH, Gersh BJ. Mechanisms of sudden cardiac death in myocardial infarction survivors: insights from the randomized trials of implantable cardioverter-defibrillators. Circulation. 2007;115:2451–2457. doi: 10.1161/CIRCULATIONAHA.106.683235. [DOI] [PubMed] [Google Scholar]
- 3.Clements-Jewery H, Andrag E, Curtis MJ. Druggable targets for sudden cardiac death prevention: lessons from the past and strategies for the future. Curr Opin Pharmacol. 2009;9:146–153. doi: 10.1016/j.coph.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Janse MJ, Wit AL. Electrophysiological mechanisms of ventricular arrhythmias resulting from myocardial ischemia and infarction. Physiol Rev. 1989;69:1049–1169. doi: 10.1152/physrev.1989.69.4.1049. [DOI] [PubMed] [Google Scholar]
- 5.Walker ML, Rosenbaum DS. Repolarization alternans: implications for the mechanism and prevention of sudden cardiac death. Cardiovasc Res. 2003;57:599–614. doi: 10.1016/s0008-6363(02)00737-x. [DOI] [PubMed] [Google Scholar]
- 6.Weiss JN, Karma A, Shiferaw Y, Chen PS, Garfinkel A, Qu Z. From pulsus to pulseless: the saga of cardiac alternans. Circ Res. 2006;98:1244–1253. doi: 10.1161/01.RES.0000224540.97431.f0. [DOI] [PubMed] [Google Scholar]
- 7.Huser J, Wang YG, Sheehan KA, Cifuentes F, Lipsius SL, Blatter LA. Functional coupling between glycolysis and excitation-contraction coupling underlies alternans in cat heart cells. J Physiol. 2000;524(Pt. 3):795–806. doi: 10.1111/j.1469-7793.2000.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eisner DA, Li Y, O'Neill SC. Alternans of intracellular calcium: mechanism and significance. Heart Rhythm. 2006;3:743–745. doi: 10.1016/j.hrthm.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 9.Laurita KR, Rosenbaum DS. Mechanisms and potential therapeutic targets for ventricular arrhythmias associated with impaired cardiac calcium cycling. J Mol Cell Cardiol. 2008;44:31–43. doi: 10.1016/j.yjmcc.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kameyama M, Hirayama Y, Saitoh H, Maruyama M, Atarashi H, Takano T. Possible contribution of the sarcoplasmic reticulum Ca2+ pump function to electrical and mechanical alternans. J Electrocardiol. 2003;36:125–135. doi: 10.1054/jelc.2003.50021. [DOI] [PubMed] [Google Scholar]
- 11.Shiferaw Y, Watanabe MA, Garfinkel A, Weiss JN, Karma A. Model of intracellular calcium cycling in ventricular myocytes. Biophys J. 2003;85:3666–3686. doi: 10.1016/S0006-3495(03)74784-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Picht E, DeSantiago J, Blatter LA, Bers DM. Cardiac alternans do not rely on diastolic sarcoplasmic reticulum calcium content fluctuations. Circ Res. 2006;99:740–748. doi: 10.1161/01.RES.0000244002.88813.91. [DOI] [PubMed] [Google Scholar]
- 13.Restrepo JG, Weiss JN, Karma A. Calsequestrin-mediated mechanism for cellular calcium transient alternans. Biophys J. 2008;95:3767–3789. doi: 10.1529/biophysj.108.130419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diaz ME, O'Neill SC, Eisner DA. Sarcoplasmic reticulum calcium content fluctuation is the key to cardiac alternans. Circ Res. 2004;94:650–656. doi: 10.1161/01.RES.0000119923.64774.72. [DOI] [PubMed] [Google Scholar]
- 15.Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005;115:500–508. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zima AV, Blatter LA. Redox regulation of cardiac calcium channels and transporters. Cardiovasc Res. 2006;71:310–321. doi: 10.1016/j.cardiores.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 17.Billman GE. A comprehensive review and analysis of 25 years of data from an in vivo canine model of sudden cardiac death: implications for future anti-arrhythmic drug development. Pharmacol Ther. 2006;111:808–835. doi: 10.1016/j.pharmthera.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Billman GE, Schwartz PJ, Stone HL. Baroreceptor reflex control of heart rate: a predictor of sudden cardiac death. Circulation. 1982;66:874–880. doi: 10.1161/01.cir.66.4.874. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz PJ, Billman GE, Stone HL. Autonomic mechanisms in ventricular fibrillation induced by myocardial ischemia during exercise in dogs with healed myocardial infarction. An experimental preparation for sudden cardiac death. Circulation. 1984;69:790–800. doi: 10.1161/01.cir.69.4.790. [DOI] [PubMed] [Google Scholar]
- 20.Sridhar A, Nishijima Y, Terentyev D, Terentyeva R, Uelmen R, Kukielka M, et al. Repolarization abnormalities and afterdepolarizations in a canine model of sudden cardiac death. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1463–R1472. doi: 10.1152/ajpregu.90583.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terentyev D, Gyorke I, Belevych AE, Terentyeva R, Sridhar A, Nishijima Y, et al. Redox modification of ryanodine receptors contributes to sarcoplasmic reticulum Ca2+ leak in chronic heart failure. Circ Res. 2008;103:1466–1472. doi: 10.1161/CIRCRESAHA.108.184457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu L, Eu JP, Meissner G, Stamler JS. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science. 1998;279:234–237. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- 23.Hidalgo C, Aracena P, Sanchez G, Donoso P. Redox regulation of calcium release in skeletal and cardiac muscle. Biol Res. 2002;35:183–193. doi: 10.4067/s0716-97602002000200009. [DOI] [PubMed] [Google Scholar]
- 24.Hidalgo C, Donoso P. Crosstalk between calcium and redox signaling: from molecular mechanisms to health implications. Antioxid Redox Signal. 2008;10:1275–1312. doi: 10.1089/ars.2007.1886. [DOI] [PubMed] [Google Scholar]
- 25.Belevych A, Kubalova Z, Terentyev D, Hamlin RL, Carnes CA, Gyorke S. Enhanced ryanodine receptor-mediated calcium leak determines reduced sarcoplasmic reticulum calcium content in chronic canine heart failure. Biophys J. 2007;93:4083–4092. doi: 10.1529/biophysj.107.114546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiss JN, Chen PS, Wu TJ, Siegerman C, Garfinkel A. Ventricular fibrillation: new insights into mechanisms. Ann N Y Acad Sci. 2004;1015:122–132. doi: 10.1196/annals.1302.010. [DOI] [PubMed] [Google Scholar]
- 27.Laurita KR, Rosenbaum DS. Cellular mechanisms of arrhythmogenic cardiac alternans. Prog Biophys Mol Biol. 2008;97:332–347. doi: 10.1016/j.pbiomolbio.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gyorke S, Terentyev D. Modulation of ryanodine receptor by luminal calcium and accessory proteins in health and cardiac disease. Cardiovasc Res. 2008;77:245–255. doi: 10.1093/cvr/cvm038. [DOI] [PubMed] [Google Scholar]
- 29.Biliczki P, Virag L, Iost N, Papp JG, Varro A. Interaction of different potassium channels in cardiac repolarization in dog ventricular preparations: role of repolarization reserve. Br J Pharmacol. 2002;137:361–368. doi: 10.1038/sj.bjp.0704881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Litwin SE, Bridge JH. Enhanced Na+-Ca2+ exchange in the infarcted heart. Implications for excitation-contraction coupling. Circ Res. 1997;81:1083–1093. doi: 10.1161/01.res.81.6.1083. [DOI] [PubMed] [Google Scholar]
- 31.Kim YK, Kim SJ, Kramer CM, Yatani A, Takagi G, Mankad S, et al. Altered excitation-contraction coupling in myocytes from remodeled myocardium after chronic myocardial infarction. J Mol Cell Cardiol. 2002;34:63–73. doi: 10.1006/jmcc.2001.1490. [DOI] [PubMed] [Google Scholar]
- 32.Gomez AM, Guatimosim S, Dilly KW, Vassort G, Lederer WJ. Heart failure after myocardial infarction: altered excitation-contraction coupling. Circulation. 2001;104:688–693. doi: 10.1161/hc3201.092285. [DOI] [PubMed] [Google Scholar]
- 33.Heinzel FR, Bito V, Biesmans L, Wu M, Detre E, von Wegner F, et al. Remodeling of T-tubules and reduced synchrony of Ca2+ release in myocytes from chronically ischemic myocardium. Circ Res. 2008;102:338–346. doi: 10.1161/CIRCRESAHA.107.160085. [DOI] [PubMed] [Google Scholar]
- 34.Hill MF, Singal PK. Right and left myocardial antioxidant responses during heart failure subsequent to myocardial infarction. Circulation. 1997;96:2414–2420. doi: 10.1161/01.cir.96.7.2414. [DOI] [PubMed] [Google Scholar]
- 35.Lindegger N, Niggli E. Paradoxical SR Ca2+ release in guinea-pig cardiac myocytes after beta-adrenergic stimulation revealed by two-photon photolysis of caged Ca2+ J Physiol. 2005;565:801–813. doi: 10.1113/jphysiol.2005.084376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 37.Livshitz LM, Rudy Y. Regulation of Ca2+ and electrical alternans in cardiac myocytes: role of CAMKII and repolarizing currents. Am J Physiol Heart Circ Physiol. 2007;292:H2854–H2866. doi: 10.1152/ajpheart.01347.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, et al. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xie LH, Chen F, Karagueuzian HS, Weiss JN. Oxidative stress-induced afterdepolarizations and calmodulin kinase II signaling. Circ Res. 2009;104:79–86. doi: 10.1161/CIRCRESAHA.108.183475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Billman GE, Hamlin RL. The effects of mibefradil, a novel calcium channel antagonist on ventricular arrhythmias induced by myocardial ischemia and programmed electrical stimulation. J Pharmacol Exp Ther. 1996;277:1517–1526. [PubMed] [Google Scholar]
- 41.Billman GE, McIlroy B, Johnson JD. Elevated myocardial calcium and its role in sudden cardiac death. FASEB J. 1991;5:2586–2592. doi: 10.1096/fasebj.5.11.1714409. [DOI] [PubMed] [Google Scholar]
- 42.Pastore JM, Girouard SD, Laurita KR, Akar FG, Rosenbaum DS. Mechanism linking T-wave alternans to the genesis of cardiac fibrillation. Circulation. 1999;99:1385–1394. doi: 10.1161/01.cir.99.10.1385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.