Abstract
Aims
Hearts of mice expressing K258stop in place of connexin43 (Cx43) protein were subjected to acute myocardial infarction in order to assess the importance of Cx43 regulation on infarct size and arrhythmia susceptibility. This mutation K258stop prevents chemical regulation of Cx43 channels, including by low intracellular pH.
Methods and results
Langendorff-perfused hearts of mice harbouring one Cx43 knockout (KO) allele and one K258stop or Cx43 allele (K258stop/KO; Cx43/KO as control) were subjected to 1 h of ischaemia and 4 h of reperfusion by reversibly occluding the left anterior descending (LAD) coronary artery. Inducibility of ventricular tachyarrhythmias (VTs) was tested by applying an endocardial burst-pacing protocol during LAD occlusion. Separately, time course and the extent of acidification-induced closure of gap junction channels were tested by dual-voltage clamp. Infarct volume (as per cent of area at risk) was significantly larger in K258stop/KO hearts compared with Cx43/KO controls (42.2 ± 3 vs. 30.4 ± 1.7%, P = 0.004, n = 8 each). During LAD occlusion, K258stop/KO hearts had a higher incidence of pacing-induced VT and a higher frequency of occurrence of spontaneous premature ventricular beats. The occurrence of ventricular arrhythmias was also significantly larger in the K258stop/KO hearts during reperfusion. In separate experiments, we demonstrated reduced sensitivity to acidification-induced uncoupling in cell pairs obtained from K258stop/KO hearts.
Conclusion
Loss of the regulatory domain of Cx43 leads to an increase in infarct size and increased susceptibility to arrhythmias following acute coronary occlusion.
Keywords: Gap junctions, Cx43, Genetic models of arrhythmia, Transgenic mouse models, Ventricular arrhythmia
1. Introduction
Gap junctions allow for passage of ions and small molecules between cells; in the heart, these structures are essential for electrical synchronization of the heartbeat. Previous studies suggest that myocardial infarction (MI) leads to gap junction channel closure,1 and that the consequent loss of intercellular communication may affect both infarct size and arrhythmogenesis.2,3 Indeed, Kanno et al.2 demonstrated that left anterior descending (LAD) coronary artery occlusion in mice having reduced expression of connexin43 (Cx43) resulted in significantly smaller infarcts than in hearts from wild-type (WT) controls, likely because of a reduction in gap junction-mediated spread of toxic ischaemic metabolites.4,5 Yet, although reducing intercellular communication may protect the integrity of cardiac tissue, it may also disrupt electrical propagation and set the stage for life-threatening arrhythmias. Murine models have shown that loss of Cx43 expression greatly increases the risk of ventricular tachycardia and sudden death.6,7 Acute MI in heterozygous Cx43 knockout (KO) hearts, expressing only 50% of WT Cx43 protein, resulted in increased incidence of ventricular arrhythmias.8 Yet, the alternative hypothesis, that preventing the regulation of gap junctions in the acute stages of MI may decrease arrhythmia susceptibility, remains to be assessed.
Cx43 channels can be regulated by a variety of factors, many of which are present in the setting of acute MI (e.g. low intracellular pH,9 or kinase activation10,11). The ability of Cx43 to be regulated by the intracellular environment is thought to be essential for the gap junction-related changes that occur during an acute MI. Studies from this and other laboratories have shown that Cx43 regulation requires the integrity of the carboxyl terminal (CT) domain of Cx4312,13. Thus, we have utilized a genetically modified murine model to directly assess whether preventing Cx43 chemical gating (by deleting the Cx43CT domain) impacts infarct size and arrhythmogenesis in the setting of acute MI.
Characterization of the murine model under study has been published.14,15 In these animals, the coding region of the Cx43 gene was replaced with a mutation lacking most of the CT domain (K258stop). While homozygous K258stop animals died shortly after birth owing to a disruption in epidermal differentiation, decreasing the K258stop gene dosage by cross-breeding with Cx43-deficient animals (connexin43 del allele, genotype referred to as K258stop/KO) rescued the lethal epidermal phenotype.15,16 Further studies showed that hearts of K258stop/KO adult animals were morphologically normal, and the cardiomyocytes were functionally coupled, though differences were noted in the total amount of ventricular gap junction protein and on the number, size, and localization of ventricular gap junctions, when compared with hearts of Cx43/KO littermates.14 Here, we show that loss of the Cx43CT domain leads to an increase in infarct size and an increase in the susceptibility to ventricular arrhythmias in these animals. This study has important implications to the overall understanding of the role of cardiac gap junctions in ischaemia-related disturbances of the cardiac rhythm.
2. Methods
All experiments were conducted with the approval from the Committee for the Humane Use of Animals of SUNY Upstate Medical University (protocol 936) and conformed with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication 58-23, revised 1996).
Additional details on methods can be found in Supplementary material online.
2.1. Animal resources
Cx43/KO and K258stop/KO littermates were obtained as described.15 Mice were kept under standard housing conditions with a fixed 12/12 h light/dark cycle. Hearts of 12 Cx43/KO and K258stop/KO adult animals (3–6 months of age) were subjected to the study.
2.2. Acute MI and reperfusion of isolated, perfused mouse hearts
Experimental protocol was adapted from Lerner et al.8 Hearts were perfused retrogradely with oxygenated modified Krebs–Henseleit buffer at 37°C. After 30 min stabilization, the LAD was occluded for 1 h, and reperfusion allowed for 4 h. Hearts were stained with 1% triphenyltetrazolium chloride (vital stain) and 1% Evans Blue (perfused area) to distinguish between areas perfused and not perfused during LAD occlusion (area at risk, AAR). Coronal sections of left ventricle were weighed and photographed. Left ventricular mass, mass of the AAR, and mass of the infarct were quantified using Image J software. Infarct size was expressed as per cent of the mass of the AAR.
2.3. Electrophysiology of isolated perfused mouse hearts
An eight-polar electrophysiology catheter connected to a Pulsar 6i stimulator was used for pacing and recording of intracavitary electrograms. Signals were digitized, monitored, and recorded as waveforms with a Sinus Rhythm Analyzer™ 400 unit (Micro-Med, Inc.). Burst-pacing series consisted of 18 S1 stimuli (interpulse interval: 80, 60, 40, and 20 ms; 2.5× threshold). Each train of S1 stimuli was carried out three consecutive times for each cycle length (inter-train interval, 5 s). This protocol was repeated at 15 (n = 6 hearts), 30, 45, and 60 min after LAD occlusion and during equilibration (n = 12 hearts). Ventricular tachyarrhythmia (VT) was defined as a minimum of three consecutive ectopic beats. Spontaneous and burst-pacing-induced VTs were analysed for morphology (mono- or polymorphic), number of beats, and duration of episodes. Electrophysiological data were analysed offline (DMSI Version 1.12 software).
2.4. Cell isolation and dual-patch electrophysiology
Adult mouse ventricular myocytes were obtained by enzymatic dissociation following standard procedures.17 Cells were used for recording within 8 h after isolation. Details on recording of gap junctional currents can be found in Shibayama et al.18 and Lewandowski et al.19 and in Supplementary material online. Estimation of macroscopic junctional conductance (Gj) and series resistance correction followed methods previously described.20
2.5. Statistical analysis
Statistical significance of incidences was assessed by Fisher's exact test. Statistical analysis of sample populations was performed using ANOVA with subsequent post hoc Tukey test (Origin Version 7.0; Origin Lab Corporation, Northampton, MA, USA) and, if necessary, corrected for multiple measurements Values are shown as mean ± SEM. Statistical significance was set at P < 0.05. Significant differences (P < 0.05) within the same genotype group are indicated by the symbol #; significance between experimental groups is indicated by *.
3. Results
3.1. Analysis of infarct size
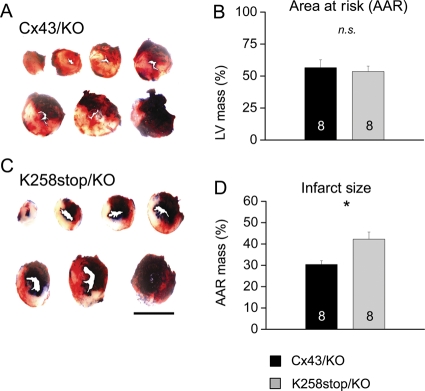
Closure of gap junctions in response to intracellular acidification depends on the presence of the Cx43CT domain.12 Here, we tested whether loss of the Cx43CT domain influences cardiac infarct size consequent to LAD occlusion. Infarct and AAR volumes after 1 h LAD occlusion and 4 h reperfusion were measured for K258stop/KO hearts; Cx43/KO hearts were used as control (Figure 1). Quantification of AAR as a fraction of left ventricular volume revealed no significant differences between genotypes (Figure 1B). AAR was 53.5 ± 6.4% of left ventricular mass for K258stop/KO hearts and 56.5 ± 5.8% for Cx43/KO hearts (n = 8 in both groups; P = 0.73). However, there was a significant difference between genotypes regarding infarct size (Figure 2B). In K258stop/KO hearts, infarct mass represented 42.2 ± 3% of the hypo-perfused mass of the left ventricle, whereas in Cx43/KO hearts, the infarct comprised only 30.4 ± 1.7% of the AAR (P = 0.004).
Figure 1.
Quantification of infarct size. (A) Representative sections of Cx43/KO (top) and K258stop/KO (bottom) left ventricles after 1 h of LAD occlusion and 4 h of reperfusion. Perfused area: blue; area at risk (AAR): red and white. Bar: 5 mm. AAR volume expressed as percentage of total left ventricular mass (B); infarct volume as percentage of AAR (C). No difference in the AAR, but infarct volume was significantly increased in K258stop/KO hearts (grey) compared with WT/KO (black).
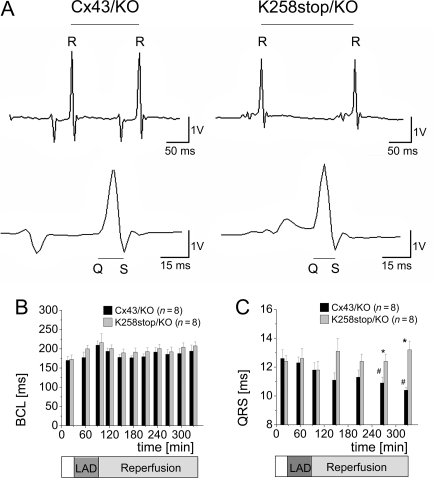
Figure 2.
Cycle length and QRS duration. (A) Cycle length (RR intervals; top) and QRS duration (bottom). (B) No significant differences in basic cycle length (BCL) between genotypes. (C) QRS interval steadily decreased in Cx43/KO hearts during reperfusion, but not in K258stop/KO hearts.
3.2. Electrophysiological stability: cycle length and QRS duration
Although burst-pacing-induced and spontaneous VTs were observed (see what follows), none of these arrhythmias degenerated into ventricular fibrillation or asystole. RR and QRS intervals (Figure 2A, n = 8 each) were collected throughout the experiment. In both groups, there was a tendency towards higher cycle lengths during LAD occlusion, which recovered during reperfusion (see Figure 2B). Although cycle lengths of K258stop/KO hearts were slightly longer throughout, there were no significant differences between both genotypes (P = 0.15).
QRS duration was measured after 20 min of equilibration and before the initial burst pacing, 20 min after the onset of LAD occlusion, and 10 min, 1, 2, 3, and 4 h after the onset of reperfusion (data in Figure 2C). Two-way ANOVA detected a difference between both genotypes (P < 0.001) due to the significant shortening (P < 0.05) of QRS duration in control hearts as the experiment progressed in time (in Cx43/KO: 12.6 ± 0.6 ms at start; 10.9 ± 0.4 ms at 3.5 h; and 10.4 ± 0.2 ms at 4 h of reperfusion; in K258stop/KO hearts: 12.4 ± 04 ms at start; 12.4 ± 0.5 ms at 3.5 h; and 13.2 ± 0.6 at 4 h of reperfusion).
3.3. Susceptibility to burst-pacing-induced VT during ischaemia
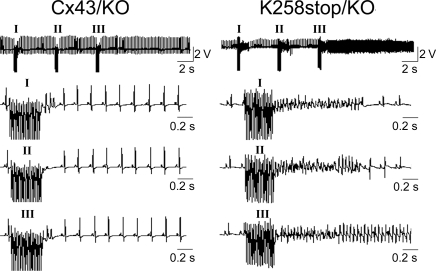
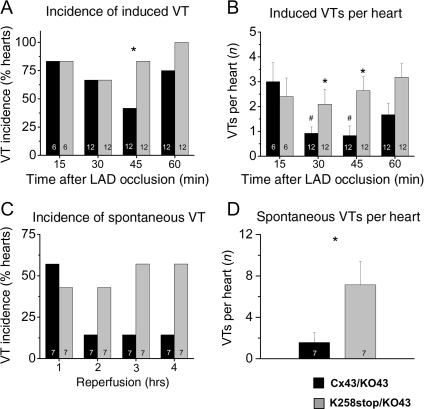
To compare susceptibility to ischaemia-induced arrhythmogenesis, Langendorff-perfused hearts were subjected to burst pacing after 15, 30, 45, and 60 min of LAD occlusion (Figure 3). As a control, hearts were also initially tested at the end of the 30 min equilibration phase. One K258stop/KO heart displayed two short burst-pacing-induced VT events; all other hearts were free of spontaneous or pacing-induced arrhythmias during equilibration. Figure 4A shows the fraction of all tested hearts (per genotype) in which VT was induced by burst pacing, at each point in time. There was no difference in inducibility of VTs between hearts of both groups at 15 min (83.3%; n = 6 in both groups) and 30 min of LAD occlusion (66.7%; n = 12 in both groups). In the second half-hour of the ischaemic event, however, K258stop/KO hearts were more prone to develop VTs after burst pacing (83.8 vs. 41.7% for Cx43/KO hearts at 45 min, P = 0.049; 100% compared with 75% of Cx43/KO hearts at 60 min, P = NS; n = 12 each). Increased susceptibility to pacing-induced ventricular arrhythmias in the K258stop/KO hearts was further quantified by comparing the average number of VT events at each time point, regardless of burst-pacing frequency (Figure 4B). The average number of pacing-induced VTs was similar between groups (3.0 ± 0.8 Cx43/KO vs. 2.4 ± 0.7 K268stop/KO) 15 min after LAD occlusion. For the Cx43/KO group, this number significantly decreased as time progressed (0.9 ± 0.3 at 30 min; 0.8 ± 0.4 at 45 min; P < 0.05 each). Yet, a time-dependent decrease in the number of pacing-induced VT events was not observed in the K258stop/KO group (2.3 ± 0.6 at 30 min and 2.8 ± 0.6 at 45 min; Figure 4B). Consequently, there was a statistically significant difference in the number of pacing-induced VT events when data obtained from K258stop/KO hearts were compared with those obtained from control (P = 0.007). Moreover, although pacing-induced VT episodes in Cx43/KO hearts were mostly of short duration (3–20 ectopic beats), 20 or more ectopic beats were often observed in the K258stop/KO group. Table 1 segregates data according to the duration of the VT episode. Only 8.8% of events observed in Cx43/KO hearts lasted >1 s (3.5% of total events lasted >4 s). In contrast, 35.6% of all events in the K258stop/KO hearts lasted more than 1 s (P < 0.001), with 22.6% lasting >4 s (P = 0.006). Finally, to assess whether the occurrence of the pacing-induced arrhythmias in K258stop/KO hearts was due to the ischaemic event, sham-operated hearts were also tested. As opposed to the results obtained from the group in which the LAD was occluded, no pacing-induced ventricular arrhythmias were observed in sham-operated animals (n = 3). Overall, truncation of the Cx43CT domain led to an increase in the incidence and duration of VT episodes occurring as a result of fast pacing in the setting of LAD occlusion.
Figure 3.
Ventricular tachycardia during LAD occlusion and reperfusion. Burst pacing at 20 ms cycle length 1 h after the onset of LAD occlusion. Top: examples showing three burst-pacing events (I, II, III) for representative K258stop/KO (right) and Cx43/KO control hearts (left). Horizontal bars: 5 s; vertical bars: 2.5 V. Bottom: burst-pacing events at expanded time scale. VTs were induced by three sets of burst stimuli in K258stop/KO hearts, and duration of VT episode was longer than that in Cx43/KO hearts.
Figure 4.
Ventricular tachycardia susceptibility. (A) Percentage of hearts susceptible to burst-pacing-induced tachyarrhythmias at each time point for Cx43/KO (black columns) and K258stop/KO (grey columns). K258stop-expressing hearts displayed higher inducibility of pacing-induced tachyarrhythmias at 45 min (83.3 vs. 41.6% in WT) and 1 h of LAD occlusion (100 vs. 77.6%). (B) K258stop/KO hearts (grey columns) showed higher number of VT episodes compared with Cx43/KO controls (black columns) at 30 and 45 min of ischaemia. (C) Percentage of hearts displaying spontaneous VTs during each hour of reperfusion. Cx43/KO, black bars; K258stop/KO, grey bars. K258stop-expressing hearts displayed trend towards higher incidence of tachyarrhythmias during second (42.9 vs. 14.3% in control), third (57.1 vs. 14.3%), and fourth hours of reperfusion (57.1 vs. 14.3%). Because of small sample size, difference was not statistically significant (Fisher's exact test). (D) K258stop/KO hearts (grey columns) presented with significantly more episodes of tachyarrhythmias than controls (black columns). To assess this variable, spontaneous VT episodes during the entire reperfusion were counted for each animal and averaged per genotype (1.57 ± 0.95 in Cx43/KO hearts vs. 7.14 ± 2.27 in K258stop/KO hearts; P = 0.043).
Table 1.
Duration of burst-pacing-induced VTs
| Cx43/KO (n = 57) | K258stop/KO (n = 115) | P-value | |
|---|---|---|---|
| VT duration >1 s | 8.8% | 35.6% | <0.001 |
| VT duration >4 s | 3.5% | 22.6% | 0.006 |
VTs, ventricular tachyarrhythmias; n, total number of VT events (12 hearts and 576 burst-pacing events per group).
3.4. Incidence of spontaneous episodes of VT and premature ventricular complexes
Electrical traces with clear atrial deflections throughout recording were analysed off-line for the occurrence of spontaneous VT (Supplementary material online, Figure S1) and isolated premature ventricular complexes (PVCs) (idioventricular events occurring between two sinus beats; Supplementary material online, Figure S2; n = 7 for both genotypes). No episodes of spontaneous VT, on the hearts of either genotype, were observed during the period of LAD occlusion. Yet, spontaneous VT was frequently observed during reperfusion. The fraction of hearts displaying spontaneous VT during the first hour of reperfusion was slightly smaller for the K258stop/KO group (42.9 vs. 57.1% in the Cx43/KO group). However, this trend reversed during the second hour and persisted for the duration of the recording (Figure 4C). Additionally, individual K258stop/KO hearts developed more episodes of spontaneous VT. Spontaneous VT episodes during reperfusion were counted for each animal, and then averaged for total number of animals with a genotype. Whereas Cx43/KO hearts developed 1.57 ± 0.95 episodes of VT during reperfusion, K258stop/KO hearts showed an average of 7.14 ± 2.27 episodes per heart (P = 0.043; Figure 4D).
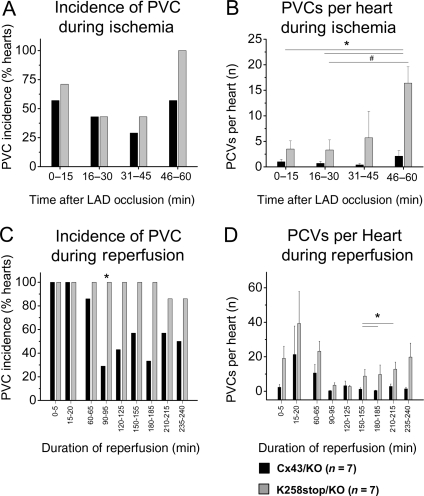
PVCs of individual animals were counted throughout the ischaemic period, as well as for the first 20 min of reperfusion and subsequently for 5 min every 30 min. Whereas the percentage of hearts with PVCs was comparable and the average number of PVCs per individual heart was not significantly different during the first 45 min of LAD occlusion, K258stop/KO hearts displayed increased propensity for PVCs in the last 15 min of LAD occlusion (Figure 5A). Only 57% of the Cx43/KO hearts developed PVCs during this stage, in contrast to 100% of the K258stop/KO hearts. Comparison of average number of PVCs for 15 min intervals during ischaemia showed significant difference between genotypes (Figure 5B; P = 0.0006). Moreover, the average total number of PVCs was nine times larger in those hearts expressing the truncated Cx43 protein (4.3 ± 0.9 in Cx43/KO vs. 40.7 ± 15.4 in K258stop/KO hearts; P = 0.035). As also seen for spontaneous VTs, the incidence of PVCs stayed high in the K258stop/KO group throughout reperfusion (between 80 and 100% of sampled hearts), whereas it dropped from 100% in the first hour of reperfusion to <60% in the second, third, and fourth hours of reperfusion in the Cx43/KO group (Figure 5C). The number of PVC events per heart was also higher for the K258stop/KO group, particularly in late stages of reperfusion (Figure 5D). Overall, Cx43CT truncation led to an increase in the incidence of PVCs starting in the second hour of reperfusion and remaining for the duration of recording (4 h in total).
Figure 5.
PVC during ischaemia and reperfusion. Seven Langendorff-perfused hearts per genotype were monitored for PVCs during LAD occlusion. (A) Percentage of all hearts displaying spontaneous PCVs during each 15 min interval. Cx43/KO (black columns) and K258stop/KO (grey columns). K258stop-expressing hearts displayed a trend towards higher incidence of tachyarrhythmias during the fourth 15 min interval (100 vs. 50%). Because of small sample size, the difference was not statistically significant (Fisher's exact test). (B) K258stop/KO hearts displayed increasingly more episodes of PVC than the hearts of Cx43/KO animals. This trend was significant in the last 15 min of LAD occlusion (Cx43/KO: 2 ± 1.3 vs. K258stop/KO: 16.8 ± 3.8). The same seven hearts per genotype were also monitored for PVCs during the 4 h of reperfusion. (C) Percentage of all hearts displaying PVCs during each hour of reperfusion Cx43/KO (black columns) and K258stop/KO. K258stop-expressing hearts displayed a higher incidence of PVCs during the second, third, and fourth hours of reperfusion. (D) K258stop/KO hearts tended to display more PVCs than the hearts of Cx43/KO animals during reperfusion. This trend was significant during the third and fourth hours of reperfusion.
3.5. Effects of intracellular acidification on adult cardiomyocyte K258stop gap junctions
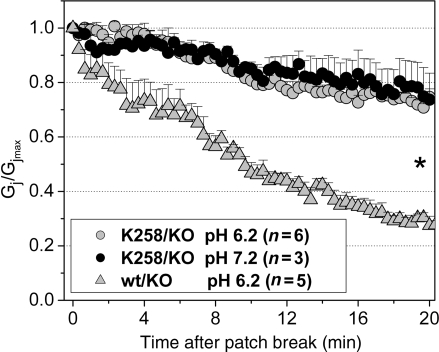
To demonstrate that Cx43CT truncation interferes with chemical gating of cardiac gap junctions, we carried out dual whole-cell voltage clamp analysis in pairs of adult cardiac myocytes internally dialysed with a pipette solution buffered at pH 6.2.19 Initial whole-cell conductances were not significantly different between cardiomyocyte pairs of either genotype (Cx43/KO: 27.2 ± 5.2, NS, n = 5; K258stop/KO: 25.3 ± 3.6, NS, n = 6; P > 0.05). Cardiomyocyte pairs expressing Cx43 displayed the previously described reduction in junctional conductance (Gj) upon intracellular acidification. Within 20 min after patch break, Gj dropped to 20% of the initial value (Figure 6, grey triangles). In accordance with the impairment of chemical gating observed in exogenously expressed, CT-truncated Cx43 channels,12 cell pairs from hearts harbouring the K258stop/KO genotype remained coupled throughout the period of intracellular acidification, with junctional conductance decreasing to an average 80% from control at the end of recording (Figure 6; grey circles). The latter was attributable to channel rundown, since a similar decrease was observed in cell pairs with the same genotype and recorded with an internal pipette solution buffered at pH 7.2 (black circles). Overall, these data support the notion that K258stop gap junction channels may remain open during the ischaemic event.
Figure 6.
Acidification-induced uncoupling in pairs of cardiomyocytes. Changes in junctional conductance (Gj) as a function of time after patch break. Cells recorded in dual-patch clamp configuration. Pipettes were filled with an internal pipette solution buffered to pH 7.2 (closed circles) or 6.2 (grey symbols). Notice the progressive reduction in Gj in cell pairs obtained from Cx43/KO hearts (grey triangles), not apparent in cells with the K258stop/KO genotype (grey circles and closed circles; P << 0.001; Student's t-test).
4. Discussion
Cx43-mediated intercellular communication is highly susceptible to changes in the intracellular environment. This ‘chemical gating’ of Cx43 requires the integrity of the CT domain.13 As such, K258stop mice offer, for the first time, an experimental system to analyse the role of Cx43 regulation on infarct size and arrhythmogenesis in the setting of LAD occlusion. Because of high postnatal lethality of homozygous K258stop mice, our study was carried out in the hearts of adult K258stop/KO mice, using Cx43/KO hearts as controls.
4.1. Increase in infarct size after acute MI in K258stop/KO hearts
An important goal was to define whether the truncation of the CT domain of Cx43 associated with changes in infarct size. To minimize a possible role of the inflammatory response on affecting infarct size in vivo, 21 we focused on the acute ischaemia in a Langendorff-perfused preparation. As a first step, we determined ‘area at risk’, that is, the area of the ventricles that was deprived of coronary circulation following LAD occlusion (see ‘Methods’; see also Michael et al.22). Our analysis indicated that AAR in K258stop/KO mice was not different from previously reported for either WT22 or mice deficient in Cx43.8 This was consistent with gross anatomical observations indicating no apparent change in the morphology of the coronary circulation and provided us with a parameter for the normalization of infarct size for each individual case studied.
Our data show that Cx43CT truncation led to a significant increase in infarct size (Figure 2B). The latter occurred despite an almost 90% reduction in total Cx43 content14 and suggests that loss of the CT domain prevented ischaemia-induced closure of cardiac gap junctions. This is consistent with previous observations in exogenous expression systems, and in cell culture preparations, indicating that the Cx43CT domain is involved in intra-12,23 and intermolecular interactions necessary for chemical gating of Cx43.13,24 The result is also consistent with patch clamp experiments demonstrating that intracellular acidification (a component of myocardial ischaemia9) did not lead to uncoupling between cell pairs from hearts with the K258stop/KO genotype. Overall, our data support the hypothesis that Cx43 chemical gating plays a fundamental role in the containment of necrosis consequent to the acute ischaemic insult, likely by preventing the passage of toxic metabolites and apoptotic signals between cells.24,25
4.2. Increased arrhythmogenesis in ischaemic K258stop/KO hearts
The studies of Lerner et al.8 showed that the occurrence of VT in Langendorff-perfused heterozygous Cx43 knockout (Cx43/KO) hearts subjected to 1 h of acute MI was significantly higher than in the WT animals. Here, we used a similar experimental protocol to demonstrate that the incidence of ventricular arrhythmias increases when the CT domain of Cx43 is truncated (Figures 3 and 4). The mechanism for this increase in arrhythmia susceptibility is unclear. It is worth noting that the amount of K258stop protein in the hearts of these animals is only 22% of the amount of Cx43 found in Cx43/KO controls, and gap junction plaques are less abundant.14 Previous studies have shown that a reduction of >50% of total cardiac Cx43 protein in mice leads to severe ventricular arrhythmias and sudden death.6,7 As such, it could be argued that the increased arrhythmogenesis is consistent with reduced levels of connexin expression, as seen in conditional Cx43KO mice. Yet, previous studies have shown that macroscopic Gj between cardiac myocyte pairs obtained from K258stop/KO hearts is not significantly reduced.14 Data in this paper are consistent with this observation (our measurements of Gj did not reveal differences between groups). Cx43-truncated unitary conductance values are similar to those of the WT channel.14 Our previous studies have shown a loss of the residual state in the truncated form, though this modification is likely to not reflect on the extent of cell–cell coupling in the functioning heart, given the very limited extent of transjunctional voltage difference between cardiac cells in the syncitium. Overall, our data show that Gj in K258stop/KO hearts subjected to ischaemia is larger than in the control group, given the loss of chemical gating. Finally, if the reduced amount of gap junction protein was fundamental to the increased incidence of arrhythmias that we observed, this difference would have been noticeable without ischaemia, or in the first few minutes of the ischaemic event (Figure 4B). Overall, decreased electrical coupling seems a highly unlikely explanation for the higher incidence of ischaemia-induced arrhythmias observed in the K258stop/KO animals.
An alternative explanation might be found in the paradoxical improvement of impulse propagation observed in cardiac tissue following partial uncoupling.26 Reduction of cell coupling has been shown to suppress unidirectional conduction block27,28 and improve propagation.26,27 Chemical Cx43 gating in the normal heart may therefore provide, even if temporarily, an improvement of the sink-to-source match between the normal and damaged myocardia and in doing so, prevent formation of areas of block. The large sink-to-source mismatch between unaffected myocardium and a well-coupled area of injury would, on the other hand, act as a substrate for ventricular tachycardia in the setting of MI.
Increased infarct size may in itself act as an arrhythmogenic substrate. In that regard, it is important to emphasize the heightened susceptibility to spontaneous ventricular arrhythmias observed after the first hour of reperfusion in the K258stop/KO group. Previous studies have shown that gap junction-mediated intercellular communication is a key determinant of infarct size2 and that re-establishment of coupling during reperfusion can be arrhythmogenic.29 We speculate that the larger infarct size in K258stop hearts would provide for larger anatomical obstacles, as well as a larger source of toxic metabolites associated with cell injury. Both factors would have a pro-arrhythmic effect.
Finally, although we speculate that the observed incidence of arrhythmias is a direct result of changes in Cx43 function, an indirect involvement of other molecules, which may be affected secondary to genetic manipulation, cannot be excluded. From that perspective, extrapolation of our results to the setting of gap junction pharmacology should be done with caution. Previous studies indicate that chemical agents that improve electrical coupling may have an anti-arrhythmic effect.30 In apparent contrast, we show that a manipulation likely to cause loss of Cx43 chemical gating leads to increased incidence of arrhythmias. Yet, loss of the CT domain affects a variety of functions including protein trafficking,31 gap junction plaque size and number,14,32 intermolecular interactions with kinases,33,34 scaffolding proteins35 and other molecules of the intercalated disc,14,36 and intramolecular interactions within the Cx43 molecule.23,37 As such, disruption of these interactions may not have the same impact as the use of a chemical agent that preserves or improves coupling. It should be noted, however, that holding gap junctions open in the setting of intracellular acidification supported slow conduction,19 which can facilitate re-entrant arrhythmias.38,39
In summary, we have used a genetically modified murine model to directly characterize, for the first time, the role of the CT domain of Cx43 on cardiac infarct size and arrhythmia susceptibility. Our results strongly support the notion that Cx43 regulation plays an important role in the containment of myocardial damage during the ischaemic event. Our data further suggest that Cx43 chemical gating is involved in preserving electrical stability in the infarcted heart. Our results suggest that a complete isolation of Cx43 from its molecular partners (by deletion of its regulatory domain) may have a negative impact on heart function in the setting of MI.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by the National Institutes of Health (HL39707, GM GM057691 to M.D.). K.M. was recipient of the Kenneth Rosen Fellowship awarded by the Heart Rhythm Society.
Supplementary Material
Acknowledgements
We thank Deborah L. Lerner and Richard B. Schuessler for their initial help in the technique of LAD occlusion in Langendorff-perfused mouse hearts.
Conflict of interest: none declared.
References
- 1.Beardslee MA, Lerner DL, Tadros PN, Laing JG, Beyer EC, Yamada KA, et al. Dephosphorylation and intracellular redistribution of ventricular connexin43 during electrical uncoupling induced by ischemia. Circ Res. 2000;87:656–662. doi: 10.1161/01.res.87.8.656. [DOI] [PubMed] [Google Scholar]
- 2.Kanno S, Kovacs A, Yamada KA, Saffitz JE. Connexin43 as a determinant of myocardial infarct size following coronary occlusion in mice. J Am Coll Cardiol. 2003;41:681–686. doi: 10.1016/s0735-1097(02)02893-0. [DOI] [PubMed] [Google Scholar]
- 3.Cascio WE, Yang H, Muller-Borer BJ, Johnson TA. Ischemia-induced arrhythmia: the role of connexins, gap junctions, and attendant changes in impulse propagation. J Electrocardiol. 2005;38:55–59. doi: 10.1016/j.jelectrocard.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 4.Ruiz-Meana M, Garcia-Dorado D, Lane S, Pina P, Inserte J, Mirabet M, et al. Persistence of gap junction communication during myocardial ischemia. Am J Physiol Heart Circ Physiol. 2001;280:H2563–H2571. doi: 10.1152/ajpheart.2001.280.6.H2563. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Sinovas A, Garcia-Dorado D, Ruiz-Meana M, Soler-Soler J. Protective effect of gap junction uncouplers given during hypoxia against reoxygenation injury in isolated rat hearts. Am J Physiol Heart Circ Physiol. 2006;290:H648–H656. doi: 10.1152/ajpheart.00439.2005. [DOI] [PubMed] [Google Scholar]
- 6.van Rijen HV, Eckardt D, Degen J, Theis M, Ott T, Willecke K, et al. Slow conduction and enhanced anisotropy increase the propensity for ventricular tachyarrhythmias in adult mice with induced deletion of connexin43. Circulation. 2004;109:1048–1055. doi: 10.1161/01.CIR.0000117402.70689.75. [DOI] [PubMed] [Google Scholar]
- 7.Gutstein DE, Morley GE, Tamaddon H, Vaidya D, Schneider MD, Chen J, et al. Conduction slowing and sudden arrhythmic death in mice with cardiac-restricted inactivation of connexin43. Circ Res. 2001;88:333–339. doi: 10.1161/01.res.88.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lerner DL, Yamada KA, Schuessler RB, Saffitz JE. Accelerated onset and increased incidence of ventricular arrhythmias induced by ischemia in Cx43-deficient mice. Circulation. 2000;101:547–552. doi: 10.1161/01.cir.101.5.547. [DOI] [PubMed] [Google Scholar]
- 9.Noma A, Tsuboi N. Dependence of junctional conductance on proton, calcium and magnesium ions in cardiac paired cells of guinea-pig. J Physiol. 1987;382:193–211. doi: 10.1113/jphysiol.1987.sp016363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lau AF. c-Src: bridging the gap between phosphorylation- and acidification-induced gap junction channel closure. Sci STKE. 2005;2005:e33. doi: 10.1126/stke.2912005pe33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Axelsen LN, Stahlhut M, Mohammed S, Larsen BD, Nielsen MS, Holstein-Rathlou NH, et al. Identification of ischemia-regulated phosphorylation sites in connexin43: a possible target for the antiarrhythmic peptide analogue rotigaptide (ZP123) J Mol Cell Cardiol. 2006;40:790–798. doi: 10.1016/j.yjmcc.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Morley GE, Taffet SM, Delmar M. Intramolecular interactions mediate pH regulation of connexin43 channels. Biophys J. 1996;70:1294–1302. doi: 10.1016/S0006-3495(96)79686-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delmar M, Coombs W, Sorgen P, Duffy HS, Taffet SM. Structural bases for the chemical regulation of connexin43 channels. Cardiovasc Res. 2004;62:268–275. doi: 10.1016/j.cardiores.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 14.Maass K, Shibayama J, Chase SE, Willecke K, Delmar M. C-terminal truncation of connexin43 changes number, size, and localization of cardiac gap junction plaques. Circ Res. 2007;101:1283–1291. doi: 10.1161/CIRCRESAHA.107.162818. [DOI] [PubMed] [Google Scholar]
- 15.Maass K, Ghanem A, Kim JS, Saathoff M, Urschel S, Kirfel G, et al. Defective epidermal barrier in neonatal mice lacking the C-terminal region of connexin43. Mol Biol Cell. 2004;15:4597–4608. doi: 10.1091/mbc.E04-04-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Theis M, Mas C, Doring B, Kruger O, Herrera P, Meda P, et al. General and conditional replacement of connexin43-coding DNA by a lacZ reporter gene for cell-autonomous analysis of expression. Cell Commun Adhes. 2001;8:383–386. doi: 10.3109/15419060109080758. [DOI] [PubMed] [Google Scholar]
- 17.Cerrone M, Noujaim SF, Tolkacheva EG, Talkachou A, O'Connell R, Berenfeld O, et al. Arrhythmogenic mechanisms in a mouse model of catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2007;101:1039–1048. doi: 10.1161/CIRCRESAHA.107.148064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shibayama J, Lewandowski R, Kieken F, Coombs W, Shah S, Sorgen PL, et al. Identification of a novel peptide that interferes with the chemical regulation of connexin43. Circ Res. 2006;98:1365–1372. doi: 10.1161/01.RES.0000225911.24228.9c. [DOI] [PubMed] [Google Scholar]
- 19.Lewandowski R, Procida K, Vaidyanathan R, Coombs W, Jalife J, Nielsen MS, et al. RXP-E: a connexin43-binding peptide that prevents action potential propagation block. Circ Res. 2008;103:519–526. doi: 10.1161/CIRCRESAHA.108.179069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin X, Zemlin C, Hennan JK, Petersen JS, Veenstra RD. Enhancement of ventricular gap-junction coupling by rotigaptide. Cardiovasc Res. 2008;79:416–426. doi: 10.1093/cvr/cvn100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarieddine MZ, Scheckenbach KL, Foglia B, Maass K, Garcia I, Kwak BR, et al. Connexin43 modulates neutrophil recruitment to the lung. J Cell Mol Med. 2009 doi: 10.1111/j.1582-4934.2008.00654.x. epub ahead of print 23 January 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michael LH, Entman ML, Hartley CJ, Youker KA, Zhu J, Hall SR, et al. Myocardial ischemia and reperfusion: a murine model. Am J Physiol. 1995;269:H2147–H2154. doi: 10.1152/ajpheart.1995.269.6.H2147. [DOI] [PubMed] [Google Scholar]
- 23.Duffy HS, Sorgen PL, Girvin ME, O'Donnell P, Coombs W, Taffet SM, et al. pH-dependent intramolecular binding and structure involving Cx43 cytoplasmic domains. J Biol Chem. 2002;277:36706–36714. doi: 10.1074/jbc.M207016200. [DOI] [PubMed] [Google Scholar]
- 24.Duffy HS, Ashton AW, O'Donnell P, Coombs W, Taffet SM, Delmar M, et al. Regulation of connexin43 protein complexes by intracellular acidification. Circ Res. 2004;94:215–222. doi: 10.1161/01.RES.0000113924.06926.11. [DOI] [PubMed] [Google Scholar]
- 25.Zucchi R, Ghelardoni S, Evangelista S. Biochemical basis of ischemic heart injury and of cardioprotective interventions. Curr Med Chem. 2007;14:1619–1637. doi: 10.2174/092986707780831014. [DOI] [PubMed] [Google Scholar]
- 26.Rohr S, Kucera JP, Fast VG, Kleber AG. Paradoxical improvement of impulse conduction in cardiac tissue by partial cellular uncoupling. Science. 1997;275:841–844. doi: 10.1126/science.275.5301.841. [DOI] [PubMed] [Google Scholar]
- 27.Rohr S. Role of gap junctions in the propagation of the cardiac action potential. Cardiovasc Res. 2004;62:309–322. doi: 10.1016/j.cardiores.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 28.Fast VG, Kleber AG. Block of impulse propagation at an abrupt tissue expansion: evaluation of the critical strand diameter in 2- and 3-dimensional computer models. Cardiovasc Res. 1995;30:449–459. [PubMed] [Google Scholar]
- 29.Cascio WE, Yang H, Johnson TA, Muller-Borer BJ, Lemasters JJ. Electrical properties and conduction in reperfused papillary muscle. Circ Res. 2001;89:807–814. doi: 10.1161/hh2101.098612. [DOI] [PubMed] [Google Scholar]
- 30.Kjolbye AL, Haugan K, Hennan JK, Petersen JS. Pharmacological modulation of gap junction function with the novel compound rotigaptide: a promising new principle for prevention of arrhythmias. Basic Clin Pharmacol Toxicol. 2007;101:215–230. doi: 10.1111/j.1742-7843.2007.00123.x. [DOI] [PubMed] [Google Scholar]
- 31.TenBroek EM, Lampe PD, Solan JL, Reynhout JK, Johnson RG. Ser364 of connexin43 and the upregulation of gap junction assembly by cAMP. J Cell Biol. 2001;155:1307–1318. doi: 10.1083/jcb.200102017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hunter AW, Barker RJ, Zhu C, Gourdie RG. Zonula occludens-1 alters connexin43 gap junction size and organization by influencing channel accretion. Mol Biol Cell. 2005;16:5686–5698. doi: 10.1091/mbc.E05-08-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toyofuku T, Akamatsu Y, Zhang H, Kuzuya T, Tada M, Hori M. c-Src regulates the interaction between connexin-43 and ZO-1 in cardiac myocytes. J Biol Chem. 2001;276:1780–1788. doi: 10.1074/jbc.M005826200. [DOI] [PubMed] [Google Scholar]
- 34.Solan JL, Marquez-Rosado L, Sorgen PL, Thornton PJ, Gafken PR, Lampe PD. Phosphorylation at S365 is a gatekeeper event that changes the structure of Cx43 and prevents down-regulation by PKC. J Cell Biol. 2007;179:1301–1309. doi: 10.1083/jcb.200707060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giepmans BN, Moolenaar WH. The gap junction protein connexin43 interacts with the second PDZ domain of the zona occludens-1 protein. Curr Biol. 1998;8:931–934. doi: 10.1016/s0960-9822(07)00375-2. [DOI] [PubMed] [Google Scholar]
- 36.Wei CJ, Francis R, Xu X, Lo CW. Connexin43 associated with an N-cadherin-containing multiprotein complex is required for gap junction formation in NIH3T3 cells. J Biol Chem. 2005;280:19925–19936. doi: 10.1074/jbc.M412921200. [DOI] [PubMed] [Google Scholar]
- 37.Hirst-Jensen BJ, Sahoo P, Kieken F, Delmar M, Sorgen PL. Characterization of the pH-dependent interaction between the gap junction protein connexin43 carboxyl terminus and cytoplasmic loop domains. J Biol Chem. 2007;282:5801–5813. doi: 10.1074/jbc.M605233200. [DOI] [PubMed] [Google Scholar]
- 38.Peters NS, Coromilas J, Severs NJ, Wit AL. Disturbed connexin43 gap junction distribution correlates with the location of reentrant circuits in the epicardial border zone of healing canine infarcts that cause ventricular tachycardia. Circulation. 1997;95:988–996. doi: 10.1161/01.cir.95.4.988. [DOI] [PubMed] [Google Scholar]
- 39.de Bakker JM, van Capelle FJ, Janse MJ, Tasseron S, Vermeulen JT, de Jonge N, et al. Slow conduction in the infarcted human heart. ‘Zigzag’ course of activation. Circulation. 1993;88:915–926. doi: 10.1161/01.cir.88.3.915. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.