Abstract
Ischaemic heart disease (IHD) is the leading cause of morbidity and mortality worldwide. While timely reperfusion of acutely ischaemic myocardium is essential for myocardial salvage, it leads to a unique type of injury known as ‘myocardial ischaemia/reperfusion (I/R) injury’. Growing evidence suggests that a defect in myocardial Ca2+ transport system with cytosolic Ca2+ overload is a major contributor to myocardial I/R injury. Progress in molecular genetics and medicine in past years has clearly demonstrated that modulation of Ca2+ handling pathways in IHD could be cardioprotective. The potential benefits of these strategies in limiting I/R injury are vast, and the time is right for challenging in vivo systemic work both at pre-clinical and clinical levels.
Keywords: Ca2+ overload, Ischaemic myocardium, Ischaemia-reperfusion, Heart failure
1. Introduction
Ischaemic heart disease (IHD) or heart attack is the single largest cause of death and disability worldwide,1,2 and acute myocardial infarction (AMI) is the fundamental clinical manifestation of heart attack following coronary thrombosis. AMI treated with early revascularization leads to myocardial reperfusion. The primary manifestations of myocardial I/R injury are myocyte death, arrhythmias, and contractile dysfunction.3–5 Patients surviving AMI are susceptible to recurrent angina, reinfarction, arrhythmias, heart failure (HF), and sudden cardiac death.6
Over the centuries, tremendous progress has been made in terms of diagnosis of MI from the post-mortem era to real-time clinical scenario of modern cardiac imaging.7,8 Management of AMI evolved in the 1960–80s from passive bed rest and symptomatic treatment to active revascularization with thrombolytic agents, angioplasty, or coronary artery bypass grafting (CABG).9–11 With these advancements and lifestyle changes, mortality from IHD has also fallen during the last three decades.12–14 While reperfusion-associated myocardial injury limits myocardial salvage, reperfusion therapies and interventions that are cardioprotective in animal models have not yet been successfully translated to achieve improved clinical outcome in patients.15 In view of this, one of the major ongoing research efforts in cardiovascular medicine has been development of approaches to prevent reperfusion injury and maximize myocardial salvage in patients with IHD.
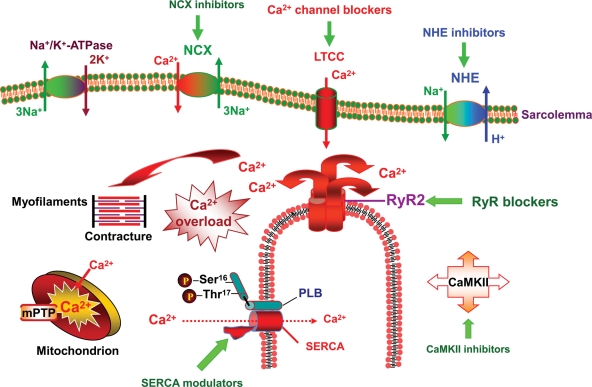
Ca2+ is a ubiquitous signal for regulating cellular function, including survival and death.16 While a small amount of Ca2+ is necessary for the optimal physiological function of the heart, growing evidence suggests that an increased cytosolic free Ca2+ overload is one of the major contributors of myocardial I/R-induced injury.17–21 Therefore, Ca2+ handling in the post-ischaemic myocardium has become a prime target to treat patients with AMI. Progress in molecular genetics has led to the cloning and characterization of cardiac Ca2+ handling proteins. Ca2+ handling in the cardiac muscle is orchestrated by a set of proteins that include: (i) L-type Ca2+ channel (LTCC), (ii) sarcoplasmic reticulum (SR) Ca2+ release channel—ryanodine receptor (RyR), (iii) sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA), (iv) Na+–Ca2+ exchanger (NCX), and (v) phospholamban (PLB) (Figure 1).
Figure 1.
Strategies to modulate Ca2+ transport in the post-ischaemic heart. The primary targets to modulate Ca2+ overload and improve post-ischaemic myocardial performance are (i) Sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA), (ii) Na+/Ca2+ exchanger (NCX), (iii) SR Ca2+ release channel RyR, (iv) L-type Ca2+ channel (LTCC), (v) Na+–H+ exchanger (NHE), and (vi) Ca2+ and calmodulin-dependent protein kinase II (CaMKII).
In this review, we will discuss the therapeutic potential and impact of targeting Ca2+ handling machinery to improve post-ischaemic myocardial function and injury.
2. Historical perspective of IHD
The history of IHD—myocardial ischaemia and angina pectoris, AMI, and sudden cardiac death belongs to atypical and interlacing presentations dated back to the 17th century.22,23 The first classic description of angina pectoris that is still valid until today was made by William Heberden in 1772 as Pectoris Dolor.24 John Hunter made the first self-diagnosis of angina in 1794 and it was confirmed in his own post-mortem by Edward Jenner.25–27 In 1910, William Osler summarized his views on angina pectoris for the Lumleian lecture based on his observation with 250 patients.28 The pathology and clinical features of MI were finally recognized in 1912 when John B. Herrick published his classic paper on the clinical features of coronary artery occlusion and MI.29 The distinction between angina and MI was clarified by Parkinson and Bedford in 1928.30 In 1933, Thomas Lewis clearly described relative ischaemia in contrast to the absolute ischaemia that follows total coronary obstruction.31 However, the worldwide morbidity and mortality with MI have led the experts to redefine MI in 2007,1 and it is now phrased that ‘the term myocardial infarction should be used when there is evidence of myocardial necrosis in a clinical setting consistent with myocardial ischemia’.
3. Ca2+ handling in cardiac muscle
The fundamental role of Ca2+ within cardiomyocyte is to enable excitation–contraction (E–C) coupling. The essential steps in this process involve membrane-potential-dependent small amount of Ca2+ entry through LTCC, subsequent large Ca2+-induced Ca2+ release (CICR) from the SR via RyR, and finally, initiation of contraction by binding of cytosolic Ca2+ to myofilaments (Figure 1). The concentration of free cytosolic Ca2+ determines the extent of muscle contraction and therefore the force development. Subsequent rapid removal of the cytosolic Ca2+ into SR by SERCA and extrusion of Ca2+ from the cell by sarcolemmal NCX and Ca2+-ATPase result in muscle relaxation. Additionally, dynamic interaction between SERCA and phospholamban plays a critical role in Ca2+ handling. Thus, E–C coupling is a finely tuned and coordinated mechanism where electrical activation is translated into cardiac contraction.32
4. Ca2+ overload hypothesis
A number of mechanisms have been proposed to mediate myocardial I/R injury. These include: intracellular Ca2+ overload; the occurrence of a no reflow phenomenon due to cell swelling, impaired vascular relaxation or the formation of white cell plugs; and the formation of oxygen radicals. The oxyradical hypothesis of myocardial I/R has been reviewed in detail.33 The Ca2+ overload hypothesis was first demonstrated by Zimmerman et al.34 in 1967 as ‘calcium paradox’ where the sudden readmission of Ca2+ to the perfusate after a brief period in which hearts are perfused with Ca2+-free media causes massive tissue disruption, enzyme release, development of contracture, and marked reductions of high-energy phosphate stores. In 1981, Nayler showed that ‘calcium paradox’ does not mimic the sequence of events that occurs when heart muscle is made ischaemic and then reperfused.35 However, it was shown that in both instances, the end result was the same: mitochondrial Ca2+ overload and depletion of tissue ATP stores. Nayler's group in 1984 showed that the gain in Ca2+ that occurs in rat hearts during Ca2+ repletion after a Ca2+-free perfusion is rapid, complex, and comprises both an early and a late phase of entry. The early phase was shown to be a Ca2+ antagonist-sensitive as well as a Ca2+ antagonist-insensitive component, and the latter was shown for NCX activity.36 Pool-Wilson highlighted the abnormalities of Ca2+ regulation and their role in possible reperfusion injury entitled ‘calcium out of control’.37 Marban's group in 1987 proposed that Ca2+ entry upon reperfusion plays a major role in the pathogenesis of myocardial stunning.38 In 1991, Opie proposed the two-stage model of Ca2+-mediated reperfusion damage, the first phase due to excess Ca2+ and the second stage the consequences of that excess Ca2+.39 The pathophysiology of myocardial stunning has been extensively reviewed where elevated Ca2+ has been shown to cause activation of protein kinase and alter Ca2+ sensitivity or maximal Ca2+-activated force through phosphorylation of contractile proteins.3,40,41 Compared with stunning, cellular mechanisms of lethal or irreversible myocardial I/R injury are complex and incremental consisting of several independent aetiologies. The pathophysiology and pharmacology of myocardial I/R injury have been elegantly reviewed by several investigators with primary emphasis on cytosolic Ca2+ control.3,4,21,42 The long-standing popular hypothesis of ‘calcium paradox’ has now been revisited with distinction that Ca2+ overload in cellular I/R is always preceded by Na+ overload, but, under the classical calcium paradox protocol Na+ overload does not occur prior to massive Ca2+ overload.43
4.1. Role of LTCC in ischaemic Ca2+ overload
Sarcolemmal LTCCs are integral component of the E–C coupling mechanism in cardiomyocytes. Membrane depolarization opens these channels, allowing rapid influx of Ca2+ into the cell. Increased Ca2+ accumulation could be augmented by ischaemia-induced depolarization of the membrane potential, which allows opening of the LTCC and further Ca2+ entry into the cell. About two decades ago, the protective effect of LTCC blockers were demonstrated in vivo canine models of I/R.44–47 and later, in a wide variety of experimental models.48–50 The expression of LTCCs was reported to be decreased51 or unchanged52 following in vitro I/R. Recently, in a rat model of chronic MI, expression of LTCC mRNA not the protein was reported to decrease 1 day after MI followed by recovery towards control values in a 4-week follow-up period.53 Although Ca2+ channel blockers initially suggested a role for LTCCs and subsequent Ca2+ overload in I/R injury, a reduction in myocardial oxygen consumption due to negative inotropic and chronotopic effects of Ca2+ channels blockers were later referred to the anti-ischaemic effects of these compounds.3 In humans, LTCC blockers have yielded conflicting results on myocardial I/R either with no beneficial effects on patient survival or with reduced cellular damage after AMI.54–56 Overall, the clinical use of LTCC blockers in MI is still in dispute because of their marked haemodynamic effects.
4.2. SERCA and Ca2+ overload
SERCA2a is primarily responsible for SR Ca2+ uptake and replenishing the SR Ca2+ load during the contraction–relaxation cycle of the heart.57–59 The SR has been shown to suffer substantial damage with impaired Ca2+ uptake function during I/R. Its nearly five decades since Stuckey and associates in 1967 demonstrated a decrease of Ca2+ uptake in isolated SR following coronary artery occlusion in a canine model of cardiopulmonary bypass.60 In 1978, a study in a canine model of MI reported that there is simultaneous decrease in the SR Ca2+ uptake rate, Ca2+–Mg+-stimulated ATPase activity and ATPase protein expression, but, all of these changes returned to normal levels by 4 weeks.61 Later, several investigators using various ischaemic durations reported a decrease in SR Ca2+ reuptake and/or SR Ca2+-ATPase activity in the ischaemic myocardium of different mammals.62–64 In humans, a significant decrease in the SR Ca2+ uptake of post-ischaemic atrial myocardium was reported after reversible I/R.65 Recently, the critical role of SR Ca2+ transport system in myocardial I/R has been consistently demonstrated both in transgenic animals and by gene therapy to improve Ca2+ handling. Hajjar and associates showed that adenoviral overexpression of SERCA2a in the heart reduces arrhythmias and improves ventricular thickening in a rat model myocardial I/R.66 Subsequently, several studies have demonstrated that increasing SR Ca2+ uptake function by SERCA overexpression or gene transfer strongly protects the heart against I/R injury and/or HF.67–69 Using both gain-of-function (SERCA overexpression) and loss-of-function (SERCA deficiency) models,70–72 we have clearly demonstrated that SERCA2a function is critical for post-ischaemic injury because sub-lethal ischaemia caused significant in vivo myocardial infarction in SERCA-deficient mice,71 whereas lethal ischaemia in SERCA overexpression mice exhibited markedly small myocardial infarction.72 Taken together, these data suggest that the reduced functional level of SERCA is one of the factors that determines intracellular Ca2+ overload and contractile dysfunction following I/R. While SERCA2a-mediated gene therapy has gained considerable attention to treat MI, studies also suggested that excessive SERCA expression might cause impaired post-ischaemic contractile function.73–75
4.3. SR Ca2+ release channel and Ca2+ overload
The cardiac SR Ca2+ release channel RyR is a key component in cardiac E–C coupling, and it is responsible for the release of Ca2+ from the SR during cardiac muscle contraction (Figure 1). The involvement of RyR in myocardial I/R injury stems from two different sets of experiments. First, several groups demonstrated that inhibition of RyR by pharmacological inhibitors, such as ryanodine, caffeine, or dantrolene, could be beneficial during I/R because it would attenuate cytosolic Ca2+ overload, improve contractile recovery, decrease myocardial infarction, and reduce the incidence of arrhythmias.76–79 Second, in contrast to the first, other investigators have reported that a decrease in the amount of RyR protein, RyR mRNA, and/or the RyR binding sites following I/R might be responsible for contractile dysfunction in the ischaemic and reperfused post-ischaemic myocardium.80,81 Studies also reported that mRNA levels of RyR were differentially expressed in the scar and viable myocardium of post-MI rat hearts.82 Recently, in a rat in vivo model of MI, the expression of RyR mRNA not the RyR proteins was found to be reduced 1 day after MI, but the expression levels recovered by 4 weeks.53 Oxidative modifications of Ca2+ handling proteins have been reported during I/R.83,84 The redox modulation of RyR activity is mediated by the redox modification of sulfhydryl groups of cysteine residues. Similarly SERCA and LTCC channel are subject to redox modulation. Interestingly, a recent in vitro report suggests that NADH oxidation and acute cytosolic redox status is involved in negative-feedback regulation of the RyR.85 Thus redox-mediated alteration of ion channels and pumps could be important in I/R injury.
4.4. NCX and Ca2+ overload
In mammalian heart, NCX plays an important role in cardiac E–C coupling by extruding Ca2+ from the cytosol during diastole. NCX is electrogenic and influenced by the membrane potential as well as by Na+ and Ca2+ gradients across cell membrane.86 The exchanger operates in the ‘forward mode’ under normal physiological conditions to extrude Ca2+ from the cell, however, it can also operate in the ‘reverse mode’ under certain conditions to transport Ca2+ into the cell. It is more than two decades when two independent groups demonstrated that Na+-dependent increase in Ca2+ during ischaemia and early reperfusion is due to reduced Ca2+ efflux by a reduced Na+ gradient or due to Ca2+ entry via reverse-mode NCX.87,88 Several investigators have consistently reported that pharmacological inhibition of reverse-mode NCX reduces cytosolic Ca2+ overload in various experimental conditions.86 This observation was subsequently verified using genetic models of mice,89,90 and it was established that inhibition or ablation of NCX function provides cardioprotection against I/R injury. Recently, administration of an NCX inhibitor at the time of reperfusion has been shown to reduce contractile dysfunction and myocardial injury in in vitro rat and in vivo swine model of I/R.91 These findings indicate that inhibition of reverse-mode NCX at the time of reperfusion could be an attractive strategy to maximize clinical benefit of reperfusion therapy in patients with AMI.
4.5. Na+/H+ exchanger activity during I/R
Myocardial NHE plays an important role in the maintenance of intracellular pH, Na+, and Ca2+ homeostasis.92 During myocardial ischaemia, intracellular acidosis develops quickly, activating the exchanger to extrude H+ into the extracellular space and bring Na+ into the cell. However, with continued ischaemia, the cell becomes unable to handle the overload of Na+, and in turn, stimulates Ca2+ influx through NCX and causes intracellular Ca2+ overload. This can lead to detrimental cardiac injury, such as contracture and necrosis. During myocardial reperfusion, these events are magnified because the blood flow lowers the extracellular Na+ concentration, stimulating NHE to extrude more intracellular H+. This leads to intracellular Na+ overload and eventually, Ca2+ overload that mediates the unfavourable sequels of myocardial I/R such as expansion of MI, myocardial stunning, and arrhythmias.92 Karmazyn93 was the first to show that the NHE inhibitor could improve the post-ischaemic contractile recovery in isolated hearts. Later, several investigators have repeatedly shown that various NHE inhibitors can prevent myocardial I/R injury in animal models.94
4.6. PLB activity during I/R
PLB is an SR membrane protein and the endogenous regulator of SERCA2a. In the dephosphorylated state, PLB inhibits the activity of SERCA2a and SR Ca2+ transport.95 Phosphorylation of PLB at Ser16 by protein kinase A (PKA) or at Thr17 by Ca2+-calmodulin protein kinase II (CaMKII) relieves its inhibitory effect on SERCA2a and SR Ca2+ transport. Thus, PLB is a critical regulator of SR function, myocardial relaxation, and contractility. A decrease in the activity of SERCA2a and/or the rate of SR Ca2+ uptake have been reported in most studies of myocardial I/R.60–65 PLB phosphorylation was found to be decreased after ≥30 min ischaemia in a porcine model of coronary ligation;96 however other reports showed increased, decreased, or unchanged phosphorylation during myocardial I/R with variable post-ischaemic recovery in different mammalian species.97,98 Interestingly, studies with PLB knockout mice demonstrated that ablation of PLB exacerbates post-ischaemic myocardial injury.99 While published data clearly indicates that phosphorylation status of PLB is altered following I/R, future long-term in vivo studies will be required to correlate the time course of phosphorylation status with post-ischaemic myocardial recovery.
4.7. CaMKII and Ca2+ overload
The multifunctional Ca2+/calmodulin-dependent protein kinase (CaMK) is an intracellular protein that is dynamically activated by intracellular Ca2+ and serves as a major downstream effector for Ca2+ signalling in the heart.100 When intracellular Ca2+ is low, CaMKII is auto-inhibited in the resting state. Activation occurs with elevation of Ca2+ and calmodulin binding to an autoinhibitory domain, and upon activation it can phosphorylate many different proteins. CaMKIIδ is the predominant isoform in the heart and it modulates an array of proteins involved in the cardiac E–C coupling and Ca2+ handling, including PLB, RyR, and L-type Ca2+ channels.101 Because of its modulatory role on different Ca2+ handling apparatus, CaMKII is postulated to play a key role in myocardial physiology and disease.102 An increased activity of myocardial CaMKII has been reported in different species following I/R.103,104 Interestingly, CaMKII activation has been shown to be beneficial on reversible (stunning) and detrimental on irreversible myocardial I/R injury.105 Importantly, whether these effects of CaMKII on myocardial I/R are present or relevant in human heart remains to be determined.
5. Therapeutic approaches to target Ca2+ overload
The clinical manifestations of IHD such as myocardial ischaemia and angina pectoris, AMI, and sudden cardiac death are the leading causes of death worldwide. During the last decades, several novel pharmacological and mechanical therapies have been developed to rapidly restore coronary artery patency.42,105–108 Currently, the single established strategy to limit infarct size in impending AMI is early reperfusion with thrombolysis or percutaneous coronary intervention. Since an acute ischaemic attack is unpredictable, majority of the patients end up with infarcts involving a large fraction of the myocardial area at risk. With this view, development of strategies aimed at limiting cell death secondary to transient coronary occlusion by directly interfering with the mechanisms of I/R injury appears to be an attractive strategy. From the preceding sections of this review, it is obvious that modulation of Ca2+ handling pathways (Figure 1) might salvage the myocardium at risk. Here, we will briefly discuss the potential therapeutic candidates for AMI.
5.1. LTCC blockers
Ca2+ channel blockers, although, proved to prevent Ca2+ overload during prolonged ischaemia and reperfusion, and limit irreversible myocardial damage in experimental models of coronary occlusions (Section 3.1), the clinical benefit of Ca2+ channel blockers in the setting of acute MI remains inconsistent because of limited clinical trials.105 One unjustifiable limitation is that, even in experimental models of I/R, Ca2+ channel blockers are cardioprotective only when administered prior to ischaemia.107 While LTCC blockers have been available for decades, there is no consistent evidence that they are protective in man and, therefore, they are not routinely recommended in the setting of AMI, unless, there is a clear indication such as hypertension or arrhythmias.108 Interestingly, two recent meta-analysis have, however, indicated that LTCC blockers may be safer with less cardiovascular complications after cardiac surgery,109 or may not increase the risk of cardiovascular death and myocardial infarction.110 Therefore, a definitive conclusion on the use of LTCC blockers awaits further evaluations.
5.2. Enhancing SERCA expression and activity
There is now convincing evidence that impaired SR calcium handling pathways are directly involved in the cardiomyocyte cell death during I/R.73,111,112 Adenoviral- and lentiviral-mediated gene transfer to increase SERCA2a expression or the use of transgenic animals with SERCA overexpression have clearly established the potent beneficial effects during I/R,67,68 and thus promoted the field of potential SERCA gene therapy. Recently, one novel drug with SERCA2a stimulating properties has emerged with great promise.113,114 Istaroxime that concomitantly inhibits Na+/K+-ATPase and stimulates SERCA2a is currently in phase IIb clinical trials.113 Istaroxime has been shown to improve cardiac performance and haemodynamics without affecting blood pressure, heart rate, and renal function, and without inducing the pro-arrhythmic consequences of intracellular Ca2+ overload in patients with chronic HF.114 The impact of myocardial SERCA upregulation requires a more highly integrated, systematic, and focused research for in vivo models.
5.3. Inhibiting SR Ca2+ release channel
Although much needs to be understood regarding the role of RyR in experimental I/R, dantrolene that selectively inhibit SR Ca2+ release channel has been shown to protect the heart from post-ischaemic injury regardless of the timing of administration.115 The advantage with dantrolene is that it is already used in humans as a therapeutic agent for malignant hyperthermia,116 and it has been used in cardiomyopathic patients without relevant side effects.117 At present, there is not enough pre-clinical and clinical data to demonstrate the potential beneficial effects of systemic inhibition of Ca2+ release channels in post-MI subjects. Therefore, future studies will justify the usefulness of dantrolene in the setting of AMI.
5.4. NCX inhibitors
Increased intracellular Ca2+ overload during myocardial I/R is due to Ca2+ influx via the reverse mode of sarcolemmal NCX and reduced SR Ca2+ uptake activity. In animal models, inhibition of the NCX or heterozygous knockout of NCX has been shown to reduce the susceptibility to I/R injury by preventing Ca2+ influx and/or diminishing the cell injury.86,89,91 NCX inhibitors are effective when they are administered at the time of reperfusion,97 and thus, it appeared to be more useful to alleviate the tissue damage in a clinical setting with AMI patients who receive reperfusion therapy. Currently, three NCX inhibitors, KB-R7943, SEA0400, and SN-6, are available with major therapeutic potential.118 However, there is no pre-clinical report or clinical trial as to whether long-term systemic inhibition is beneficial or detrimental.
5.5. NHE inhibitors
Despite highly encouraging cardioprotective effects of NHE inhibitors in pre-clinical studies,119 clinical studies in patients with evolving MI and those at risk of MI have provided mixed and largely disappointing data.120,121 In the GUARDIAN trial,120 the cardioprotective efficacy of cariporide was limited to the subset of high-risk patients who underwent CABG. In contrast, no cardioprotective benefit was observed in the ESCAMI trial in patients with acute MI.120 The failure in clinical trials could be attributed to many reasons, but the majority of pre-clinical data suggest that NHE inhibitors show most benefit when administered during ischaemia alone or during ischaemia and reperfusion.121 Interestingly, the recent EXPEDITION trial in CABG patients had revealed compelling evidence that NHE inhibitor could significantly reduce myocardial injury in patients at risk of I/R injury.122 Thus, it appears that the timing of administration plays a critical role in the positive outcome of NHE inhibitors, and it is expected that future clinical trials will extend the role of NHE inhibition in therapeutic application.
5.6. Ca2+ handling modulators
Recently, caldaret, (5-methyl-2-(1-piperazinyl) benzene sulfonic acid monohydrate; MCC-135) a novel drug having the ability to modulate intracellular Ca2+ handling has been reported. In pre-clinical studies, caldaret inhibits I/R-induced Ca2+ overload, enhances Ca2+ uptake into and inhibits Ca2+ leakage from the SR, reduces myocardial infarction, decreases cardiac markers of I/R injury, and improves LV function.123 Caldaret has been found safe and well tolerated in early clinical studies involving healthy subjects, and it has demonstrated a good safety profile as an adjunctive in patients undergoing primary percutaneous coronary intervention (PCI) for ST-elevation MI (STEMI).123 While ‘EVOLVE’ trial showed no significant benefit of caldaret on preservation of LV ejection fraction and reduction of infarct size in patients with STEMI undergoing primary PCI, ‘CASTEMI’ trial showed a significant decrease in the incidence of LV dysfunction in patients with STEMI undergoing primary PCI.124 Outcomes of current and future clinical trials will establish the therapeutic benefits of caldaret for the treatment of AMI.
6. Conclusions and perspective
In this review, we have made an attempt to identify the key players in Ca2+ homeostasis, whose function is modified during I/R. We have also attempted to highlight the ongoing efforts to develop therapeutics to treat several of these known targets to improve calcium transport. Despite significant advances in defining many of the targets that are modified during I/R injury, there are many challenges to determine which of these targets are of primary importance for developing rationale therapy in a clinical setting. In this review, we have not been able to address every aspect of calcium signalling. Apart from E–C coupling, Ca2+ plays important roles in signal transduction pathways that contribute to cardiac growth/hypertrophy, apoptosis, and HF. We should also point out that there is increasing evidence that redox modulation of ion channels, pumps, and transporters play important roles in post-ischaemic myocardial dysfunction. Therefore, the potential roles of redox modification on Ca2+ handling and mitochondrial Ca2+ overload induced apoptosis should be considered in the crucial outcome of novel therapeutic strategies. Thus, despite mounting evidence and better understanding of the Ca2+–IHD link and the benefits from potential novel therapeutic strategies (Figure 1), challenging work lies ahead both at pre-clinical and clinical levels to determine the definite importance of these interventions in limiting myocardial damage and improving patient's survival.
Conflict of interest: none declared.
Funding
This work was supported by NIH grants HL64140-08, HL080551-01 to M.P.; and HL63744, HL65608, and HL38324 to J.L.Z.
References
- 1.Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. J Am Coll Cardiol. 2007;50:2173–2195. doi: 10.1016/j.jacc.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, et al. Heart disease and stroke statistics-2008 update. A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 3.Gross GJ, Kersten JR, Warltier DC. Mechanisms of postischemic contractile dysfunction. Ann Thorac Surg. 1999;68:1898–1904. doi: 10.1016/s0003-4975(99)01035-8. [DOI] [PubMed] [Google Scholar]
- 4.Piper HM, García-Dorado D. Prime causes of rapid cardiomyocyte death during reperfusion. Ann Thorac Surg. 1999;68:1913–1919. doi: 10.1016/s0003-4975(99)01025-5. [DOI] [PubMed] [Google Scholar]
- 5.Verma S, Fedak PW, Weisel RD, Butany J, Rao V, Maitland A, et al. Fundamentals of reperfusion injury for the clinical cardiologist. Circulation. 2002;105:2332–2336. doi: 10.1161/01.cir.0000016602.96363.36. [DOI] [PubMed] [Google Scholar]
- 6.Woo KS, White HD. Factors affecting outcome after recovery from myocardial infarction. Annu Rev Med. 1994;45:325–339. doi: 10.1146/annurev.med.45.1.325. [DOI] [PubMed] [Google Scholar]
- 7.Fleming PR. A Short History of Cardiology. Amsterdam/Atlanta, GA: Rodopi Publishers; 1997. [PubMed] [Google Scholar]
- 8.Saraste A, Nekolla S, Schwaiger M. Contrast-enhanced magnetic resonance imaging in the assessment of myocardial infraction and viability. J Nucl Cardiol. 2008;15:105–117. doi: 10.1007/BF02976902. [DOI] [PubMed] [Google Scholar]
- 9.Van Der Werf JJ, Topol EJ, Sobel BE. The impact of fibrinolytic therapy for ST-segment-elevation acute myocardial infarction. J Thromb Haemost. 2009;7:14–20. doi: 10.1111/j.1538-7836.2008.03195.x. [DOI] [PubMed] [Google Scholar]
- 10.Grech ED. ABC of interventional cardiology: percutaneous coronary intervention. I: history and development. BMJ. 2003;326:1080–1082. doi: 10.1136/bmj.326.7398.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sellke F, Swanson S, del Nido PJ. Sabiston & Spencer Surgery of the Chest. Saunders, PA: Elsevier Health Sciences; 2004. [Google Scholar]
- 12.Heidenreich PA, McClellan M. Trends in treatment and outcomes for acute myocardial infarction: 1975–1995. Am J Med. 2001;110:165–174. doi: 10.1016/s0002-9343(00)00712-9. [DOI] [PubMed] [Google Scholar]
- 13.Hardoon SL, Whincup PH, Lennon LT, Wannamethee SG, Capewell S, Morris RW. How much of the recent decline in the incidence of myocardial infarction in British men can be explained by changes in cardiovascular risk factors? Evidence from a prospective population-based study. Circulation. 2008;117:598–604. doi: 10.1161/CIRCULATIONAHA.107.705947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luepker RV. Decline in incident coronary heart disease. Why are the rates falling? Circulation. 2008;117:592–593. doi: 10.1161/CIRCULATIONAHA.107.747477. [DOI] [PubMed] [Google Scholar]
- 15.Bolli R, Becker L, Gross G, Mentzer R, Jr, Balshaw D, Lathrop DA. Myocardial protection at a crossroads: the need for translation into clinical therapy. Circ Res. 2004;95:125–134. doi: 10.1161/01.RES.0000137171.97172.d7. [DOI] [PubMed] [Google Scholar]
- 16.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodeling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 17.Elz JS, Nayler WG. Contractile recovery and reperfusion-induced calcium gain after ischemia in the isolated rat heart. Lab Invest. 1988;58:653–659. [PubMed] [Google Scholar]
- 18.Steenbergen C, Fralix TA, Murphy E. Role of increased cytosolic free calcium concentration in myocardial ischemic injury. Basic Res Cardiol. 1993;88:456–470. doi: 10.1007/BF00795412. [DOI] [PubMed] [Google Scholar]
- 19.Siegmund B, Schlüter KD, Piper HM. Calcium and the oxygen paradox. Cardiovasc Res. 1993;27:1778–1783. doi: 10.1093/cvr/27.10.1778. [DOI] [PubMed] [Google Scholar]
- 20.Meissner A, Morgan JP. Contractile dysfunction and abnormal Ca2+ modulation during postischemic reperfusion in rat heart. Am J Physiol Heart Circ Physiol. 1995;268:H100–H111. doi: 10.1152/ajpheart.1995.268.1.H100. [DOI] [PubMed] [Google Scholar]
- 21.Piper HM, García-Dorado D, Ovize M. A fresh look at reperfusion injury. Cardiovasc Res. 1998;38:291–300. doi: 10.1016/s0008-6363(98)00033-9. [DOI] [PubMed] [Google Scholar]
- 22.Clarendon EH. The life of Edward, Earl of Clarendon. Oxford: Clarendon Press; 1759. [Google Scholar]
- 23.McNee JW. The clinical syndrome of thrombosis of the coronary arteries. Q J Med. 1925;19:44–52. [Google Scholar]
- 24.Heberden W. Some account of a disorder of the breast. Med Trans R Coll Physicians (Lond) 1772;2:59–67. [Google Scholar]
- 25.Parry CS. An Inquiry into the Symptoms and Causes of the Syncope Anginosa, Commonly Called Angina Pectoris. Bath: R Crutwell; 1799. [Google Scholar]
- 26.Hunter J. A Treatise in Blood, Inflammation and Gun-shot Wounds to which is Prefixed a Short Account of the Author's Life by his Brother-in-law Everard Home. London: G Nichol; 1794. [Google Scholar]
- 27.Livesley B. The spasms of John Hunder: a new interpretation. Med Hist. 1973;17:70–75. doi: 10.1017/s0025727300018226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osler W. Lumleian lecture on angina pectoris. Lancet. 1910;I:839–844. [Google Scholar]
- 29.Herrick JB. Clinical features of sudden obstruction of the coronary arteries. J Am Med Assoc. 1912;59:2015–2020. [PubMed] [Google Scholar]
- 30.Parkinson J, Bedford DE. Cardiac infarction and coronary thrombosis. Lancet. 1928;1:4–11. [Google Scholar]
- 31.Lewis T. Diseases of the Heart. London: MacMillan; 1933. [Google Scholar]
- 32.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 33.Zweier JL, Talukder MA. The role of oxidants and free radicals in reperfusion injury. Cardiovasc Res. 2006;70:181–190. doi: 10.1016/j.cardiores.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 34.Zimmerman ANE, Daems W, Hulsmann WC, Snijder J, Wisse E, Durrier D. Morphological changes of heart muscle caused by successive perfusion with calcium-free and calcium-containing solutions (calcium paradox) Cardiovasc Res. 1967;1:201–209. doi: 10.1016/s0008-6363(99)00303-x. [DOI] [PubMed] [Google Scholar]
- 35.Nayler WG. The role of calcium in the ischemic myocardium. Am J Pathol. 1981;102:262–270. [PMC free article] [PubMed] [Google Scholar]
- 36.Nayler WG, Perry SE, Elz JS, Daly MJ. Calcium, sodium and the calcium paradox. Circ Res. 1984;55:227–237. doi: 10.1161/01.res.55.2.227. [DOI] [PubMed] [Google Scholar]
- 37.Poole-Wilson PA, Harding DP, Bourdillon PD, Tones MA. Calcium out of control. J Mol Cell Cardiol. 1984;16:175–187. doi: 10.1016/s0022-2828(84)80706-3. [DOI] [PubMed] [Google Scholar]
- 38.Kusuoka H, Porterfield JK, Weisman HF, Weisfeldt ML, Marban E. Pathophysiology and pathogenesis of stunned myocardium. Depressed Ca2+ activation of contraction as a consequence of reperfusion-induced cellular calcium overload in ferret hearts. J Clin Invest. 1987;79:950–961. doi: 10.1172/JCI112906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Opie LH. Role of calcium and other ions in reperfusion injury. Cardiovasc Drugs Ther. 1991;5:237–248. doi: 10.1007/BF00054746. [DOI] [PubMed] [Google Scholar]
- 40.Bolli R, Marban E. Molecular and cellular mechanisms of myocardial stunning. Physiol Rev. 1999;79:609–634. doi: 10.1152/physrev.1999.79.2.609. [DOI] [PubMed] [Google Scholar]
- 41.Kim S-J, Depre C, Vatner SF. Novel mechanisms mediating stunned myocardium. Heart Fail Rev. 2003;8:143–153. doi: 10.1023/a:1023040718319. [DOI] [PubMed] [Google Scholar]
- 42.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008;88:581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Piper HM. The calcium paradox revisited: an artifact of great heuristic value. Cardiovasc Res. 2000;45:123–127. doi: 10.1016/s0008-6363(99)00304-1. [DOI] [PubMed] [Google Scholar]
- 44.Bush LR, Romson JL, Ash JL, Lucchesi BR. Effect of diltiazem on extent of ultimate myocardial injury resulting from temporary coronary artery occlusion in dogs. J Cardiovasc Pharmacol. 1982;4:285–296. doi: 10.1097/00005344-198203000-00018. [DOI] [PubMed] [Google Scholar]
- 45.Lamping KA, Gross GJ. Improved recovery of myocardial segment function following a short-coronary occlusion in dogs by nicorandil, a potential new antianginal agent, and nifedipine. J Cardiovasc Pharmacol. 1985;7:158–166. doi: 10.1097/00005344-198501000-00026. [DOI] [PubMed] [Google Scholar]
- 46.Przyklenk K, Ghafari GB, Eitzman DT, Kloner RA. Nifedipine administered after reperfusion ablates systolic contractile dysfunction of postischemic ‘stunned’ myocardium. J Am Coll Cardiol. 1989;13:1176–1183. doi: 10.1016/0735-1097(89)90281-7. [DOI] [PubMed] [Google Scholar]
- 47.Gross GJ, Farber NE, Pieper NE. Effects of amlodipine on myocardial ischemia-reperfusion injury in dogs. Am J Cardiol. 1989;64:94I–100I. doi: 10.1016/0002-9149(89)90966-1. [DOI] [PubMed] [Google Scholar]
- 48.duToit EF, Opie LH. Modulation of severity of reperfusion stunning in the isolated rat hearts by the agents altering calcium flux at the onset of reperfusion. Circ Res. 1992;70:960–967. doi: 10.1161/01.res.70.5.960. [DOI] [PubMed] [Google Scholar]
- 49.Hoff PT, Tamura Y, Lucchesi BR. Cardioprotective effects of amlodipine in the ischemic-reperfused heart. Am J Cardiol. 1989;64:101I–115I. doi: 10.1016/0002-9149(89)90967-3. [DOI] [PubMed] [Google Scholar]
- 50.García-Dorado D, Theroux P, Fernandez-Aviles F, Elizaga J, Solares J, Galinanes M. Diltiazem and progression of myocardial ischemic damage during coronary artery occlusion and reperfusion in porcine hearts. J Am Coll Cardiol. 1987;10:906–911. doi: 10.1016/s0735-1097(87)80287-5. [DOI] [PubMed] [Google Scholar]
- 51.Bersohn MM, Morey AK, Weiss RS. Sarcolemmal calcium transporters in myocardial ischemia. J Mol Cell Cardiol. 1997;29:2525–2532. doi: 10.1006/jmcc.1997.0487. [DOI] [PubMed] [Google Scholar]
- 52.Zucchi R, Ronca-Testoni S, Yu G, Galbani P, Ronca G, Mariani M. Are dihydropyridine receptors downregulated in the ischemic myocardium? Cardiovasc Res. 1995;30:769–774. [PubMed] [Google Scholar]
- 53.Sallinen P, Mänttäri S, Leskinen H, Ilves M, Ruskoaho H, Saarela S. Time course of changes in the expression of DHPR, RyR2, and SERCA2 after myocardial infarction in the rat left ventricle. Mol Cell Biochem. 2007;303:97–103. doi: 10.1007/s11010-007-9460-3. [DOI] [PubMed] [Google Scholar]
- 54.The HINT Research Group. Early treatment of unstable angina in the coronary care unit, a randomized, double-blind placebo controlled comparison of recurrent ischemia in patients treated with nifedipine or metoprolol or both. Br Heart J. 1986;56:400–413. doi: 10.1136/hrt.56.5.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Theroux P, Gregoire J, Chin C, Pelletier G, de Guise P, Juneau M. Intravenous diltiazem in acute myocardial infarction. DATA trial. J Am Coll Cardiol. 1998;32:620–628. doi: 10.1016/s0735-1097(98)00281-2. [DOI] [PubMed] [Google Scholar]
- 56.Sheiban I, Tonni S, Chizzoni A, Marini A, Trevi G. Recovery of left ventricular function following early reperfusion in acute myocardial infarction: a potential role for the calcium antagonist nisoldipine. Cardiovasc Drugs Ther. 1997;11:5–16. doi: 10.1023/a:1007713118941. [DOI] [PubMed] [Google Scholar]
- 57.Misquitta CM, Mack DP, Grover AK. Sarco/endoplasmic reticulum Ca2+ (SERCA)-pumps: link to heart beats and calcium waves. Cell Calcium. 1999;25:277–290. doi: 10.1054/ceca.1999.0032. [DOI] [PubMed] [Google Scholar]
- 58.Periasamy M, Huke S. SERCA pump level is a critical determinant of Ca(2+)homeostasis and cardiac contractility. J Mol Cell Cardiol. 2001;33:1053–1063. doi: 10.1006/jmcc.2001.1366. [DOI] [PubMed] [Google Scholar]
- 59.Frank KF, Bolck B, Erdmann E, Schwinger RH. Sarcoplasmic reticulum Ca2+-ATPase modulates cardiac contraction and relaxation. Cardiovasc Res. 2003;57:20–27. doi: 10.1016/s0008-6363(02)00694-6. [DOI] [PubMed] [Google Scholar]
- 60.Lee KS, Ladinsky H, Stuckey JH. Decreased Ca2+ uptake by sarcoplasmic reticulum after coronary artery occlusion for 60 and 90 min. Circ Res. 1967;21:439–444. doi: 10.1161/01.res.21.4.439. [DOI] [PubMed] [Google Scholar]
- 61.Toba K, Katagiri T, Takeyama Y. Studies of the sarcoplasmic reticulum in myocardial infarction. Jpn Circ J. 1978;42:447–453. doi: 10.1253/jcj.42.447. [DOI] [PubMed] [Google Scholar]
- 62.Krause S, Hess ML. Characterization of cardiac sarcoplasmic reticulum dysfunction during short-term, normothermic, global ischemia. Circ Res. 1984;55:176–184. doi: 10.1161/01.res.55.2.176. [DOI] [PubMed] [Google Scholar]
- 63.Kaplan P, Hendrikx M, Mattheussen M, Mubagwa K, Flameng W. Effect of ischemia and reperfusion on sarcoplasmic reticulum calcium uptake. Circ Res. 1992;71:1123–1130. doi: 10.1161/01.res.71.5.1123. [DOI] [PubMed] [Google Scholar]
- 64.Mubagwa K. Sarcoplasmic reticulum function during myocardial ischaemia and reperfusion. Cardiovasc Res. 1995;30:166–175. [PubMed] [Google Scholar]
- 65.Zucchi R, Ronca-Testoni S, Di Napoli P, Yu G, Gallina S, Bosco G, et al. Sarcoplasmic retciculum calcium uptake in human myocardium subjected to ischemia and reperfusion during cardiac surgery. J Mol Cell Cardiol. 1996;28:1693–1701. doi: 10.1006/jmcc.1996.0159. [DOI] [PubMed] [Google Scholar]
- 66.del Monte F, Lebeche D, Guerrero JL, Tsuji T, Doye AA, Gwathmey JK, et al. Abrogation of ventricular arrhythmias in a model of ischemia and reperfusion by targeting myocardial calcium cycling. Proc Natl Acad Sci USA. 2004;101:5622–5627. doi: 10.1073/pnas.0305778101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Niwano K, Arai M, Koitabashi N, Watanabe A, Ikeda Y, Miyoshi H, et al. Lentiviral vector-mediated SERCA2 gene transfer protects against heart failure and left ventricular remodeling after myocardial infarction in rats. Mol Ther. 2008;16:1026–1032. doi: 10.1038/mt.2008.61. [DOI] [PubMed] [Google Scholar]
- 68.Miyamoto MI, del Monte F, Schmidt U, DiSalvo TS, Kang ZB, Matsui T, et al. Adenoviral gene transfer of SERCA2a improves left-ventricular function in aortic-banded rats in transition to heart failure. Proc Natl Acad Sci USA. 2000;97:793–798. doi: 10.1073/pnas.97.2.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Byrne MJ, Power JM, Preovolos A, Mariani JA, Hajjar RJ, Kaye DM. Recirculating cardiac delivery of AAV2/1SERCA2a improves myocardial function in an experimental model of heart failure in large animals. Gene Ther. 2008;15:1550–1557. doi: 10.1038/gt.2008.120. [DOI] [PubMed] [Google Scholar]
- 70.Talukder MA, Kalyanasundaram A, Zuo L, Velayutham M, Nishijima Y, Periasamy M, et al. Is reduced SERCA2a expression detrimental or beneficial to postischemic cardiac function and injury? Evidence from heterozygous SERCA2a knockout mice. Am J Physiol Heart Circ Physiol. 2008;294:H1426–H1434. doi: 10.1152/ajpheart.01016.2007. [DOI] [PubMed] [Google Scholar]
- 71.Talukder MA, Yang F, Nishijima Y, Chen CA, Kalyanasundaram A, Periasamy M, et al. Reduced SERCA2a converts sub-lethal myocardial injury to infarction and affects postischemic functional recovery. J Mol Cell Cardiol. 2009;46:285–287. doi: 10.1016/j.yjmcc.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Talukder MA, Kalyanasundaram A, Xue Z, Zuo L, Bhupathy P, Babu GJ, et al. Expression of SERCA isoform with faster Ca2+ transport properties improves postischemic cardiac function, Ca2+ handling, and decreases myocardial infarction. Am J Physiol Heart Circ Physiol. 2007;293:H1418–H1428. doi: 10.1152/ajpheart.00663.2007. [DOI] [PubMed] [Google Scholar]
- 73.Piper HM, Kasseckert S, Abdallah Y. The sarcoplasmic reticulum as the primary target of reperfusion protection. Cardiovasc Res. 2006;70:170–173. doi: 10.1016/j.cardiores.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 74.Teucher N, Prestle J, Seidler T, Currie S, Elliott EB, Reynolds DF, et al. Excessive sarcoplasmic/endoplasmic reticulum Ca2+-ATPase expression causes increased sarcoplasmic reticulum Ca2+ uptake but decreases myocyte shortening. Circulation. 2004;110:3553–3559. doi: 10.1161/01.CIR.0000145161.48545.B3. [DOI] [PubMed] [Google Scholar]
- 75.Chen Y, Escoubet B, Prunier F, Amour J, Simonides WS, Vivien B, et al. Constitutive cardiac overexpression of sarcoplasmic/endoplasmic reticulum Ca2+-ATPase delays myocardial failure after myocardial infarction in rats at a cost of increased acute arrhythmias. Circulation. 2004;109:1898–1903. doi: 10.1161/01.CIR.0000124230.60028.42. [DOI] [PubMed] [Google Scholar]
- 76.Northover BJ. Effects of pretreatment with caffeine or ryanodine on the myocardial response to simulated ischaemia. Br J Pharmacol. 1991;103:1225–1229. doi: 10.1111/j.1476-5381.1991.tb12328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Du Toit EF, Opie LH. Modulation of severity of reperfusion stunning in isolated rat heart by agents altering calcium flux at onset of reperfusion. Circ Res. 1992;70:960–970. doi: 10.1161/01.res.70.5.960. [DOI] [PubMed] [Google Scholar]
- 78.Mubagwa K, Kaplan P, Flameng W. The effects of ryanodine on calcium uptake by the sarcoplasmic reticulum of ischemic and reperfused rat myocardium. Fundam Clin Pharmacol. 1997;11:315–321. doi: 10.1111/j.1472-8206.1997.tb00844.x. [DOI] [PubMed] [Google Scholar]
- 79.Zucchi R, Ronca-Testoni S. The sarcoplasmic reticulum Ca2+ channel/ryanodine receptor: modulation by endogenous effectors, drugs and disease states. Pharmacol Rev. 1997;49:1–51. [PubMed] [Google Scholar]
- 80.Zucchi R, Ronca-Testoni S, Yu G, Galbani P, Ronca G, Mariani M. Effect of ischemia and reperfusion on cardiac ryanodine receptors-sarcoplasmic reticulum Ca2+ channels. Circ Res. 1994;74:271–280. doi: 10.1161/01.res.74.2.271. [DOI] [PubMed] [Google Scholar]
- 81.Valdivia C, Hegge JO, Lasley RD, Valdivia HH, Mentzer R. Ryanodine receptor dysfunction in porcine stunned myocardium. Am J Physiol Heart Circ Physiol. 1997;273:H796–H804. doi: 10.1152/ajpheart.1997.273.2.H796. [DOI] [PubMed] [Google Scholar]
- 82.Xu Y-J, Chapman D, Dixon IMC, Sethi R, Guo X, Dhalla NS. Differential gene expression in infarct scar and viable myocardium from rat heart following coronary ligation. J Cell Mol Med. 2004;8:85–92. doi: 10.1111/j.1582-4934.2004.tb00262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dixon IM, Kaneko M, Hata T, Panagia V, Dhalla NS. Alterations in cardiac membrane Ca2+ transport during oxidative stress. Mol Cell Biochem. 1990;99:125–133. doi: 10.1007/BF00230342. [DOI] [PubMed] [Google Scholar]
- 84.Zima AV, Blatter LA. Redox regulation of cardiac calcium channels and transporters. Cardiovasc Res. 2006;71:310–321. doi: 10.1016/j.cardiores.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 85.Cherednichenko G, Zima AV, Feng W, Schaefer S, Blatter LA, Pessah IN. NADH oxidase activity of rat cardiac sarcoplasmic reticulum regulates calcium-induced calcium release. Circ Res. 2004;94:478–486. doi: 10.1161/01.RES.0000115554.65513.7C. [DOI] [PubMed] [Google Scholar]
- 86.Blaustein M, Lederer J. Sodium/calcium exchange: its physiological implications. Physiol Rev. 1999;79:763–854. doi: 10.1152/physrev.1999.79.3.763. [DOI] [PubMed] [Google Scholar]
- 87.Tani M, Neely JR. Role of intracellular Na+ in Ca2+ overload and depressed recovery in ventricular function of reperfused ischemic rat hearts. Possible involvement of H+-Na+ and Na+-Ca2+ exchange. Circ Res. 1989;65:1045–1056. doi: 10.1161/01.res.65.4.1045. [DOI] [PubMed] [Google Scholar]
- 88.Cross HR, Lu L, Steenbergen C, Philipson KD, Murphy E. Overexpression of the cardiac Na+/Ca2+ exchanger increases susceptibility to ischemia/reperfusion in male, but not female, transgenic mice. Circ Res. 1998;83:1215–1223. doi: 10.1161/01.res.83.12.1215. [DOI] [PubMed] [Google Scholar]
- 89.Ohtsuka M, Takano H, Suzuki M, Zou Y, Akazawa H, Tamagawa M, et al. Role of Na+-Ca2+ exchanger in myocardial ischemia/reperfusion injury: evaluation using a heterozygous Na+-Ca2+ exchanger knockout mouse model. Biochem Biophys Res Commun. 2004;314:849–853. doi: 10.1016/j.bbrc.2003.12.165. [DOI] [PubMed] [Google Scholar]
- 90.Imahashi K, Christian P, Goldhaber JI, Steenberger C, Philipson KD, Murphy E. Cardiac-specific ablation of the Na+-Ca2+ exchanger confers protection against ischemia/reperfusion injury. Circ Res. 2005;97:916–921. doi: 10.1161/01.RES.0000187456.06162.cb. [DOI] [PubMed] [Google Scholar]
- 91.Inserte J, Garcia-Dorado D, Ruiz-Meana M, Padilla F, Barrabés JA, Pina P, et al. Effect of inhibition of the Na+/Ca2+ exchanger at the time of myocardial reperfusion on hypercontracture and cell death. Cardiovasc Res. 2002;55:739–748. doi: 10.1016/s0008-6363(02)00461-3. [DOI] [PubMed] [Google Scholar]
- 92.Karmazyn M, Gan XT, Humphrevs RA, Yoshida H, Kusumoto K. The myocardial Na(+)-H(+) exchange: structure, regulation, and its role in heart disease. Circ Res. 1999;85:777–786. doi: 10.1161/01.res.85.9.777. [DOI] [PubMed] [Google Scholar]
- 93.Karmazyn M. Amiloride enhances postischemic ventricular recovery: possible role of Na+-H+ exchange. Am J Physiol Heart Circ Physiol. 1988;255:H608–H615. doi: 10.1152/ajpheart.1988.255.3.H608. [DOI] [PubMed] [Google Scholar]
- 94.Levitsky J, Gurell D, Frishman WH. Sodium ion/hydrogen ion inhibition: a new pharmacologic approach to myocardial ischemia and reperfusion injury. J Clin Pharmacol. 1998;38:887–897. doi: 10.1002/j.1552-4604.1998.tb04383.x. [DOI] [PubMed] [Google Scholar]
- 95.MacLennan DH, Kranias EG. Phospholamban: a crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol. 2003;4:566–577. doi: 10.1038/nrm1151. [DOI] [PubMed] [Google Scholar]
- 96.Schoutsen B, Blom JJ, Verdouw PD, Lamers JM. Calcium transport and phospholamban in sarcoplasmic reticulum of ischemic myocardium. J Mol Cell Cardiol. 1989;21:719–727. doi: 10.1016/0022-2828(89)90613-5. [DOI] [PubMed] [Google Scholar]
- 97.Vittone L, Mundina-Weilenmann C, Mattiazzi A. Phospholamban phosphorylation by CaMKII under pathophysiological conditions. Front Biosci. 2008;13:5988–6005. doi: 10.2741/3131. [DOI] [PubMed] [Google Scholar]
- 98.Weber T, Neumann J, Meißner A, Hartlage MG, Aken HV, Hanske G, et al. Reduced serine-16 and threonine-17 phospholamban phosphorylation in stunning of conscious dogs. No evidence for any involvement of protein kinase A or protein phosphatases. Basic Res Cardiol. 2006;101:253–260. doi: 10.1007/s00395-005-0577-9. [DOI] [PubMed] [Google Scholar]
- 99.Cross HR, Kranias EG, Murphy E, Steenbergen C. Ablation of PLB exacerbates ischemic injury to a lesser extent in female than male mice: protective role of NO. Am J Physiol Heart Circ Physiol. 2003;284:H683–H690. doi: 10.1152/ajpheart.00567.2002. [DOI] [PubMed] [Google Scholar]
- 100.Maier LS, Bers DM. Role of Ca2+/calmodulin-dependent protein kinase (CaMK) in excitation-contraction coupling in the heart. Cardiovasc Res. 2007;73:631–640. doi: 10.1016/j.cardiores.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 101.Anderson ME. Calmodulin kinase signaling in heart: an intriguing candidate target for therapy of myocardial dysfunction and arrhythmias. Pharmacol Ther. 2005;106:39–55. doi: 10.1016/j.pharmthera.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 102.Couchonnal LF, Anderson ME. The role of calmodulin kinase II in myocardial physiology and disease. Physiol. 2008;23:151–159. doi: 10.1152/physiol.00043.2007. [DOI] [PubMed] [Google Scholar]
- 103.Vila-Petroff M, Salas MA, Said M, Valverde CA, Sapia L, Portiansky E, et al. CaMKII inhibition protects against necrosis and apoptosis in irreversible ischemia-reperfusion. Cardiovasc Res. 2007;73:689–698. doi: 10.1016/j.cardiores.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 104.Currie S, Smith GL. Calcium/calmodulin-dependent protein kinase II activity is increased in sarcoplasmic reticulum from coronary artery ligated rabbit hearts. FEBS Lett. 1999;459:244–248. doi: 10.1016/s0014-5793(99)01254-5. [DOI] [PubMed] [Google Scholar]
- 105.Dirksen MT, Laarman GJ, Simoons ML, Duncker DJGM. Reperfusion injury in humans: a review of clinical trials on reperfusion injury inhibitory strategies. Cardiovasc Res. 2007;74:343–355. doi: 10.1016/j.cardiores.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 106.Moens AL, Claeys MJ, Timmermans JP, Vrints CJ. Myocardial ischemia/reperfusion-injury, a clinical view on a complex pathophysiological process. Intl J Cardiol. 2005;100:179–190. doi: 10.1016/j.ijcard.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 107.Wang Q-D, Pernow J, Sjöquist P-O, Rydén L. Pharmacological possibilities for protection against myocardial reperfusion injury. Cardiovasc Res. 2002;55:25–37. doi: 10.1016/s0008-6363(02)00261-4. [DOI] [PubMed] [Google Scholar]
- 108.Kloner RA, Rezkalla SH. Cardiac protection during acute myocardial infarction: where do we stand in 2004? J Am Coll Cardiol. 2004;44:276–286. doi: 10.1016/j.jacc.2004.03.068. [DOI] [PubMed] [Google Scholar]
- 109.Wijeysundera DN, Beattie WS, Rao V, Karski J. Calcium antagonist reduce cardiovascular complications after cardiac surgery: a meta-analysis. J Am Coll Cardiol. 2003;41:1496–1505. doi: 10.1016/s0735-1097(03)00191-8. [DOI] [PubMed] [Google Scholar]
- 110.Costanzo P, Perrone-Filardi P, Petretta M, Marciano C, Vassallo E, Gargiulo P, et al. Calcium channel blockers and cardiovascular outcomes: a meta-analysis of 175634 patients. J Hypertens. 2009;27:1136–1151. doi: 10.1097/HJH.0b013e3283281254. [DOI] [PubMed] [Google Scholar]
- 111.Zucchi R, Ronca F, Ronca-Testoni S. Modulation of sarcoplasmic reticulum function: a new strategy in cardioprotection? Pharmacol Ther. 2001;89:47–65. doi: 10.1016/s0163-7258(00)00103-0. [DOI] [PubMed] [Google Scholar]
- 112.Vangheluwe P, Sipido KR, Raeymaekers L, Wuytack F. New perspectives on the role of SERCA2's Ca2+ affinity in cardiac function. Biochim Biophys Acta. 2006;1763:1216–1228. doi: 10.1016/j.bbamcr.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 113.Ghali JK, Smith WB, Torre–Amione G, Haynos W, Rayburn BK, Amato A, et al. A phase 1-2 dose-escalating study evaluating the safety and tolerability of Istaroxime and specific effects on electrocardiographic and hemodynamic parameters in patients with chronic heart failure with reduced systolic function. Am J Cardiol. 2007;99(suppl.):47A–56A. doi: 10.1016/j.amjcard.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 114.Micheletti R, Palazzo F, Barassi P, Giacalone G, Ferrandi M, Schiavone A, et al. Istaroxime, a stimulator of sarcoplasmic reticulum calcium adenosine triphosphatase isoform 2a activity, as a novel therapeutic approach to heart failure. Am J Cardiol. 2007;99(suppl.):24A–32A. doi: 10.1016/j.amjcard.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 115.Yu G, Zucchi R, Ronca-Testoni S, Ronca G. Protection of ischemic rat heart by dantrolene, an antagonist of the sarcoplasmic reticulum calcium release channel. Basic Res Cardiol. 2000;95:137–143. doi: 10.1007/s003950050175. [DOI] [PubMed] [Google Scholar]
- 116.Ward A, Chaffman MO, Sorkin EM. Dantrolene. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic use in malignant hyperthermia, the neuroleptic malignant syndrome and an update of its use in muscle spasticity. Drugs. 1986;32:130–168. doi: 10.2165/00003495-198632020-00003. [DOI] [PubMed] [Google Scholar]
- 117.Koehntop DE, Beebe DS, Belani KG. The safety of dantrolene in a patient with severe cardiomyopathy requiring a heart transplant. Anesth Analg. 1997;85:229–230. doi: 10.1097/00000539-199707000-00049. [DOI] [PubMed] [Google Scholar]
- 118.Lee C, Dhalla NS, Hryshko LV. Therapeutic potential of novel Na+-Ca2+ exchange inhibitors in attenuating ischemia-reperfusion injury. Can J Cardiol. 2005;21:509–516. [PubMed] [Google Scholar]
- 119.Karmazyn M. Pharmacology and clinical assessment of cariporide for the treatment coronary artery disease. Exp Opin Invest Drugs. 2000;9:1099–1108. doi: 10.1517/13543784.9.5.1099. [DOI] [PubMed] [Google Scholar]
- 120.Avkiran M, Marber MS. Na+/H+ exchange inhibitors for cardioprotective therapy: progress, problems and prospects. J Am Coll Cardiol. 2002;39:747–753. doi: 10.1016/s0735-1097(02)01693-5. [DOI] [PubMed] [Google Scholar]
- 121.Murphy E, Allen DG. Why did the NHE inhibitor clinical trial fail? J Mol Cell Cardiol. 2009;46:137–141. doi: 10.1016/j.yjmcc.2008.09.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mentzer RM, Bartels C, Bolli R, Boyce S, Buckberg GD, Chaitman B, et al. Sodium-hydrogen exchange inhibition by cariporide to reduce the risk of ischemic cardiac events in patients undergoing coronary artery bypass grafting: results of the EXPEDITION study. Ann Thorac Surg. 2008;85:1261–1270. doi: 10.1016/j.athoracsur.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 123.Jang I-K, Pettigrew V, Picard MH, Kowey PR, Demmel V, Zile MR, et al. A randomized, double-blind, placebo-controlled study of the safety and efficacy of intravenous MCC-135 as an adjunct to primary percutaneous coronary intervention in patients with acute myocardial infarction: rationale and design of the evaluation of MCC-135 for left ventricular salvage in acute MI (EVOLVE) study. J Thromb Thrombolysis. 2005;20:147–153. doi: 10.1007/s11239-005-3267-4. [DOI] [PubMed] [Google Scholar]
- 124.Tzivoni D, Balkin J, Bär FW, Hibberd M, Reiber JHC, Cowing G. Effect of caldaret on the incidence of severe left ventricular dysfunction in patients with ST-elevation myocardial infarction undergoing primary coronary intervention. Am J Cardiol. 2009;103:1–4. doi: 10.1016/j.amjcard.2008.08.047. [DOI] [PubMed] [Google Scholar]