Abstract
Cerebral lateralization is a fundamental property of the human brain and a marker of successful development. Here we provide evidence that multiple mechanisms control asymmetry for distinct brain systems. Using intrinsic activity to measure asymmetry in 300 adults, we mapped the most strongly lateralized brain regions. Both men and women showed strong asymmetries with a significant, but small, group difference. Factor analysis on the asymmetric regions revealed 4 separate factors that each accounted for significant variation across subjects. The factors were associated with brain systems involved in vision, internal thought (the default network), attention, and language. An independent sample of right- and left-handed individuals showed that hand dominance affects brain asymmetry but differentially across the 4 factors supporting their independence. These findings show the feasibility of measuring brain asymmetry using intrinsic activity fluctuations and suggest that multiple genetic or environmental mechanisms control cerebral lateralization.
Keywords: fMRI, functional connectivity, laterality
A fundamental property of brain organization is the presence of structural and functional asymmetries between the hemispheres (1–4). Lateralization of function is thought to contribute to the evolution of human language and reasoning by providing an axis for specialization of cortical systems. Estimates based on sodium amobarbital injection (5), task-based functional MRI (fMRI) (6), and perfusion (7) suggest considerable variability among people in the degree of brain asymmetry with language predominantly left-lateralized in most healthy right-handed adults; 2%–8% show reversed right-lateralized dominance (7). Left-handed individuals show a shift with a greater percentage demonstrating a dominance reversal (5). At the population level, atypical lateralization is present in neuropsychiatric disorders, including autism (8, 9) and schizophrenia (10, 11), presumably as a reflection of aberrant development (12).
Suggesting that lateralization is partly controlled by genetic factors, prominent structural asymmetries are present at birth (13, 14) and show high heritability estimates in twin studies (15). Most leading theories of lateralization emphasize a single factor that controls brain lateralization, often with the additional assumption that the factor also gives rise to hand dominance. For example, the prominent “right-shift theory” of cerebral dominance by Annett (16) suggests the existence of a single gene with 2 alleles, one of which influences the distribution of asymmetries. Though recognizing that multiple factors contribute to the development of lateralization, the influential Geschwind-Galaburda hypothesis proposed that brain asymmetries are dependent upon circulating testosterone levels in the intrauterine environment (3, 17). Debate persists as to whether sex is a factor contributing to cerebral lateralization (18). Men with lateralized brain lesions are more prone to language impairment than women (19), but later studies have qualified this observation (20). Similarly, imaging studies have observed sex differences in language lateralization, but these effects have not been uniformly observed (21, 22).
Here we show strong evidence that multiple factors associate with asymmetry of distinct brain systems and provide a method to measure the degree of lateralization of each of these factors in individual subjects. We first developed an approach to quantify functional laterality based on intrinsic activity fluctuations using fMRI (23, 24). Factor analysis was then performed to explore whether all lateralized brain systems arise from a common factor or through multiple, distinct factors. Clear evidence was obtained that showed separate factors influence the lateralization of distinct brain systems.
Results
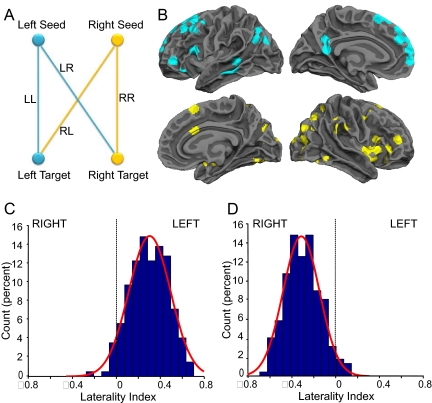
We found that the brain's intrinsic activity at rest is sufficient to measure functional asymmetry. Our analyses began by selecting 400 equally spaced spherical seed regions (7 mm in radius, 200 in each hemisphere) covering the entire cerebral cortex but excluding the white matter and cerebellum (Fig. S1). For each pair of seed regions within one hemisphere, the homologous regions in the opposite hemisphere were identified and used to derive a laterality index based on the relative functional correlation strengths among the 4 regions (see Fig. 1A, Fig. S2, and Materials and Methods). We refer to this measure as the intrinsic laterality index (iLI). iLI estimates for all 200 × 199 possible seed pairs were computed in an exploratory sample of 100 subjects (50 men, 50 women). Those regions revealing the highest level of asymmetry (iLI > 0.3 or iLI < −0.3) were combined into one metric for the most left-lateralized regions (37 regions) and another for the most right-lateralized regions (47 regions; see Fig. 1B and Materials and Methods). The most left-lateralized regions included certain traditional language regions as well as distributed regions along the cortical midline. The most strongly right-lateralized regions were localized in the visual cortex, the occipital-parietal junction, the angular gyrus, and the insula (Fig. 1B).
Fig. 1.
Intrinsic activity identifies lateralized brain regions. (A) Functional laterality was computed using intrinsic (spontaneous) activity by examining relative correlation strengths between seed and target regions in the 2 hemispheres. LL is the strength of correlation between the left hemisphere target region and the left hemisphere seed; LR represents the strength of correlation between the left seed and the right target; and RR and RL represent the contralateral homologues. The intrinsic laterality index (iLI), defined in Eq. 1 (see Materials and Methods), represents the relative correlation strength difference between the left and right hemispheres. (B) Using resting-state fMRI data from 100 right-handed subjects, the iLI of 39,800 pairwise correlations were computed and ranked. The 37 most left-lateralized regions (iLI > 0.3, top row) and 47 most right-lateralized regions (iLI < −0.3, bottom row) are projected onto a surface representation of the brain. (C) The laterality distribution for left-lateralized regions is displayed for an independent sample of 200 right-handed subjects and fit by a Gaussian. The iLI was defined as the mean laterality index of the 37 left-lateralized regions shown in (B). Positive iLI reflects left dominance. (D) The laterality distribution based on the 47 right-lateralized regions is displayed.
To explore the distribution of laterality across people in an unbiased manner, the regions identified in the initial sample of 100 subjects were examined in an independent sample of 200 subjects (Fig. 1 C and D). Dominance for both left- and right-lateralized regions was found to be on a continuum rather than a dichotomy (7). A significant correlation was also found between the iLI and a language laterality index determined using language task performance (see SI Text, Fig. S3, and Fig. S4).
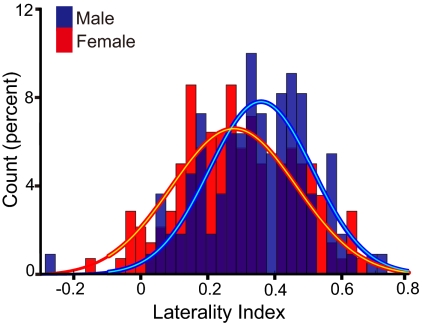
Sex was found to be associated with functional asymmetry, though the effect was small. Previous imaging studies have reported that language is more strongly lateralized in men than women, though some other studies have not detected sex differences (21, 22). Here we asked if cerebral lateralization differs between right-handed men and women using intrinsic laterality. We calculated iLI for all 300 subjects as described above and compared the distributions for the men (n = 131) and women (n = 169) (Fig. 2). Significant sex differences were found for both left-lateralized and right-lateralized systems, with stronger laterality in men than women [left, P < 0.01 (Fig. 2); right, P < 0.05]. Though statistically reliable, the effects were small, with both sexes showing strong functional asymmetry.
Fig. 2.
Sex differences are present but small. Sex differences of the laterality index distribution for left-lateralized regions (blue regions in Fig. 1) are shown. The distribution for left-lateralized regions is displayed split by men (blue bars) and women (red bars). Overlap is shown in dark blue. The distributions are fit by Gaussian curves revealing that women show more symmetric functional organization than men (Kolmogorov-Smirnov test, P < 0.01). A similar effect is present for the right-lateralized regions.
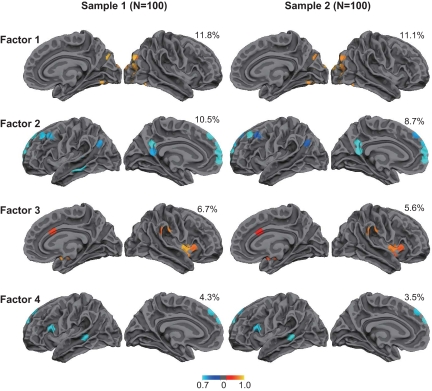
Intrinsic laterality provides a means to ask directly how cerebral lateralization is organized by examining variance across subjects and asking whether the laterality of all systems track together as a single factor or whether multiple factors emerge (3). We found evidence that multiple factors control functional lateralization. To perform this analysis, the 84 regions from Fig. 1 were subjected to a factor analysis in the initial sample of 100 subjects (see Materials and Methods). The number of extracted factors was determined by principal component with the criterion that eigenvalues equal or exceed 1. The cortical topographies of the 3 major factors that each explained >5% of the variance and a 4th factor linked to frontal and temporal regions associated with language (which explained 4.3% of the variance) are illustrated in Fig. 3.
Fig. 3.
Cerebral lateralization is controlled by multiple, distinct mechanisms. Factor analysis derived from the lateralized regions of Fig. 1 reveals 4 factors that are replicable across independent data samples (color intensity represents loading value of the factor, blue and yellow color schemes reflect the different hemispheres). Factors tended to center around individual regions and involve their correlated partner regions. Each sample consists of 50 men and 50 women. The top 3 factors each account for >5% of the between-subject variance (explained variance is shown next to each plot). A factor that included putative language regions (ranked 5th) is also shown. Results were replicated in terms of the cortical topography, ranking, and explained variance associated with each factor. The presence of distinct factors suggests that cerebral lateralization in humans arises from multiple genetic or environmental mechanisms.
To determine whether these 4 factors were reliable, we replicated the data-driven analysis in an independent sample of 100 subjects consisting of 50 men and 50 women. The factors that emerged contained almost identical cortical topographies to the first sample. The factors were also found to yield stable within-subject estimates over multiple scanning sessions. A control group (n = 22) was imaged on a second occasion within 3 months of the initial session. Between-session correlations (Pearson's r) for the 4 factors were 0.79, 0.55, 0.58, and 0.59.
The distributed anatomy of each factor roughly corresponded to a well-studied brain system, although more detailed analysis of the factors in relation to task-based studies will be required to verify correspondence. The first factor included regions within the visual system. The second largest factor was associated with a network linked to internal thought often referred to as the “default network” (25, 26). The third factor was a right-lateralized network including the angular gyrus and the insula that has previously been associated with an attentional system important for detecting unattended events (27). The last left-lateralized factor included frontal and temporal regions associated with language—in particular, controlled semantic processing.
Expanding the analysis to include factors that capture smaller proportions of the variance showed that 69% and 71% of the variance can be explained by 20 factors in the first and second datasets, respectively. The 4 factors illustrated in Fig. 3 capture significant, reproducible contributions to functional asymmetry, so we conservatively conclude that there are at least 4 major factors that determine cerebral lateralization in the human brain.
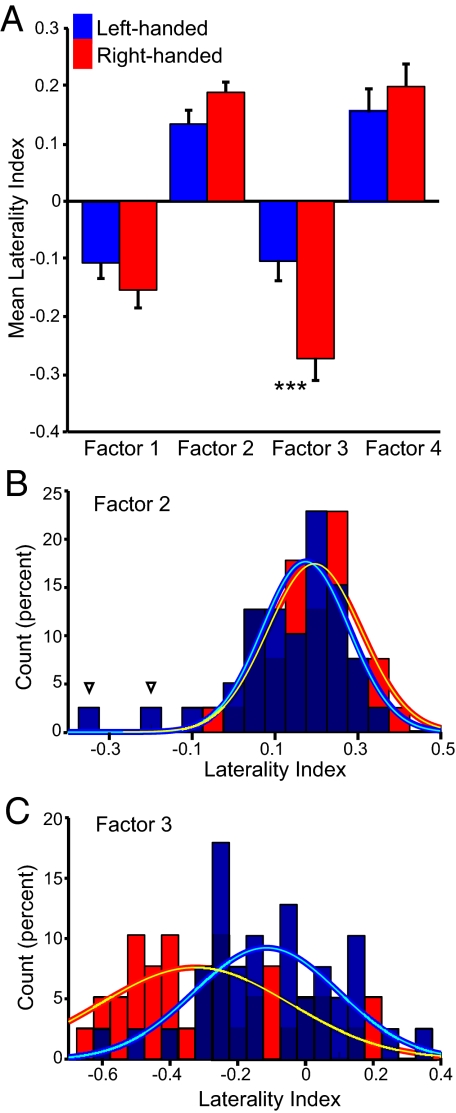
As a final analysis, we explored whether the 4 factors could be dissociated. For this analysis, the factors were measured in an independent sample that included handedness as a factor (38 left-handed and 38 right-handed age- and sex-match individuals). A significant interaction of hand dominance and the lateralization factors was observed (P < 0.005; Fig. 4A). Factor 3, linked to attention, showed markedly stronger asymmetry in the right-handed individuals (P < 0.001). Factor 2 also showed a trend for an effect of handedness (P = 0.07). However, the factor 2 effect was carried by 2 left-handed individuals with anomalous dominance (Fig. 4B). These 2 left-handed individuals may reflect examples of “cerebral situs invertus” (3) as they had right-dominant factor 2 estimates greater than any right-handed subject in the initial 300-person sample. The factor 3 effect was not carried by a few individuals but rather reflected a shift in the distribution of laterality scores (Fig. 4C). Fig. S5 displays the laterality distributions for all 4 factors.
Fig. 4.
Handedness differentially affects asymmetry across distinct brain systems. Mean laterality estimates for each of the 4 factors in Fig. 3 are plotted for independent data samples of right-handed (n = 38) and left-handed (n = 38) individuals. (A) A significant interaction between hand dominance and factor is observed (P < 0.005) with an effect of handedness observed for factor 3 (***, P < 0.001). Bars represent standard error of the mean. (B) The laterality index distribution for factor 2 is plotted split by handedness. Though the distributions overlap, there are 2 left-handed individuals with complete reversal of asymmetry. (C) The distributions for factor 3 show a marked effect of handedness with right-handed individuals demonstrating greater asymmetry.
Discussion
Understanding the genetic and developmental basis of brain asymmetry will illuminate cerebral specialization and shed light on neuropsychiatric, neurologic, and other developmental disorders that alter brain laterality (8–12, 28, 29). Genetic models have been proposed to account for cerebral dominance (15, 16), and anatomical asymmetries are likely influenced by genetic factors (30). However, so far no gene or pathway has been identified as a determinant of lateralization, although there are a number of candidates (12, 28, 31).
The present findings indicate that brain asymmetries unlikely arise from a single mechanism, but rather that multiple separate factors contribute to the development of cerebral lateralization. A single gene, as proposed by Annett (16), cannot explain the multiple effects observed but might explain a subset of brain asymmetries such as that observed for the factor linked to handedness (factor 3). Similarly, a single environmental factor associated with hormonal levels (3, 17) cannot account for the effects, especially considering the modest contribution of sex, although intrauterine testosterone levels could still play an important role. It is presently unclear whether, or to what degree, local anatomical asymmetries contribute to the present findings.
Our findings suggest that models of brain asymmetry should seek to identify multiple, distinct factors that lead to individual differences in cortical lateralization perhaps through their influence on early developmental events that promote cortical specialization. Providing insight into mechanisms that may give rise to functional lateralization, molecular studies of transcription have revealed an array of asymmetric gene expression patterns in multiple forebrain structures during early human fetal development (12, 28).
The present observations also show the feasibility and efficiency of estimating functional brain asymmetries using intrinsic activity fluctuations. Rest-state laterality indices were computed within individual subjects from rapidly acquired MRI data (≈10 min). Within-subject estimates showed moderate test-retest reliability. Thus, the present methods provide an approach to explore determinants of lateralization in large human samples, such as required for genetic explorations, as well as in patients where determination of functional dominance is clinically relevant (e.g., neurosurgical planning).
Materials and Methods
Participants.
Three hundred healthy right-handed adults participated for payment in the main MRI study (131 men, age 22.3 ± 3.2) and 38 right- and 38 left-handed adults participated in the second study that examined effects of handedness (17 men in each group, age 20.8 ± 1.7). Handedness was assessed by the Edinburgh handedness inventory (32). All participants performed between 1 and 4 rest runs to estimate intrinsic functional lateralization. Thirty-five participants additionally performed 3 runs of a language task. All participants were native English speakers and had normal or corrected-to-normal vision. Participants were screened to exclude individuals with a history of neurologic or psychiatric conditions as well as those using psychoactive medications.
MRI Acquisition Procedures.
Scanning was performed on 3 Tesla TimTrio systems (Siemens) using the 12-channel phased-array head coil supplied by the vendor. Structural images were acquired using a sagittal MP-RAGE three-dimensional T1-weighted sequence (TR = 2,530 ms, TE = 3.44 ms, FA = 7°, 1.0 mm isotropic voxels; FOV 256 × 256).
For the main group of 300 subjects, the fixation (rest) runs were 390 s (156 time points) or 370 s (148 time points) in duration, and a black visual crosshair (plus sign) was centered on a white screen during the entire run. Subjects were instructed to stay awake and look at the crosshair; no other task instruction was provided. Subjects were also instructed to minimize head movement. Images were acquired using the gradient-echo echo-planar pulse sequence (TR = 2,500 ms, TE = 30 ms, flip angle = 90°, 3-mm isotropic voxels). For the 76 subjects that contributed to the analysis of the effect of handedness, 2 rest runs were acquired (each 372 s, 120 TP, TR = 3,000 ms). For these runs, subjects rested with their eyes open.
Thirty-five subjects within the main subject cohort also performed 3 runs of a language task involving semantic classification of words previously used to determine language dominance (6, 33). Images were acquired using an echo planar imaging (EPI) gradient-echo sequence sensitive to blood oxygenation level-dependent (BOLD) contrast (TR = 2,000 ms, TE = 30 ms, FA = 90°, slice number = 33, 3-mm isotropic voxels). Each run consisted of three 36-s block of the task, four 28-s blocks of fixation. During the task blocks, 12 words (6 concrete and 6 abstract words in random order) were presented for 2 s each with a 1-s interstimulus interval. In total, 108 stimuli were presented. The visual stimuli were generated on an Apple PowerBook G4 computer (Apple, Inc.) using Matlab (Mathworks, Inc.) and the Psychophysics Toolbox extensions (34). Stimuli were projected onto a screen positioned at the head of the magnet bore. Participants were asked to indicate if the word was concrete or abstract. They were instructed to respond quickly and accurately, and indicate their response by key press (left-hand key press for abstract words, right-hand key press for concrete words).
MRI Preprocessing.
Both the resting-state data and the task data were preprocessed using the following steps: (1) slice timing correction (SPM2, Wellcome Department of Cognitive Neurology, London), (2) rigid body correction for head motion, (3) normalization for global mean signal intensity across runs, and (4) transformation of the data into a standard atlas space. The second step provided a record of head position that was later used as a nuisance regressor for correlation analysis. Atlas registration was achieved by computing affine transforms connecting the first image volume of the first functional run with the T2*-weighted functional image target (RIB, Univ of Oxford, Oxford, U.K.). The atlas representative template conformed to the Montreal Neurological Institute (MNI) atlas (MNI152). Motion correction and atlas transformation were combined into a single step to yield a motion-corrected time series resampled to 2 mm isotropic voxels.
The resting-state data were analyzed using region-based correlation analysis, often referred to as functional connectivity MRI (fcMRI) analysis. The present methods extend from Biswal et al. (23) and are described in detail in Vincent et al. (35) and Buckner et al. (36). Task data were analyzed using the general linear model as implemented in SPM2 (Wellcome Department of Cognitive Neurology, London). Regressors of no interest included motion correction parameters and low-frequency drift. The task blocks used a gamma function convolved with a boxcar function to model the hemodynamic response function.
The Quantitative Intrinsic Laterality Index (iLI).
The present exploration required the development of a quantitative measure of a region's intrinsic laterality. Functional mapping studies based on intrinsic activity have observed lateralization within the attention system in normal subjects (37) and the memory system in patients (38). Building from these earlier observations, we developed a generic approach to explore lateralization across all regions of cortex simultaneously. To compute an intrinsic laterality index (iLI), the relative strengths of correlations between seed and target region pairs were measured between the hemispheres (see Fig. 1). Two hundred spherical regions were defined in each hemisphere, covering the gray matter of the cerebral cortex (Fig. S1). All possible estimates were computed (e.g., 200 × 199 pairwise combinations). From these, the regions with the strongest lateralization were identified (iLI > 0.3 or < −0.3).
For illustration purposes, an example of asymmetric correlations is shown in Fig. S2. Functional correlation maps are displayed for a seed region in the left hemisphere and its homologous region in the right hemisphere (see top 2 rows in Fig. S2; circles in the frontal region indicate the seed regions). The map associated with the right hemisphere seed is subtracted from the map associated with the left hemisphere seed to derive a difference map (third row in Fig. S2). In this example, the left temporal region (marked by the circles in the third row), among other regions, is more strongly correlated with the left frontal seed region than the mirroring correlations in the right hemisphere. This asymmetry is easily appreciated in the difference map; the laterality index in Eq. 1 below quantifies this asymmetry.
Specifically, for each seed region, the iLI was defined based on the relative correlation differences between the seed and target regions across the 2 hemispheres. Fig. 1A illustrates the correlation values used in this calculation, where LL is the strength of the correlation between the left hemisphere seed and the left hemisphere target regions; LR represents the strength of the correlation between the left hemisphere seed and right hemisphere target regions; and RR and RL represent the contralateral homologues. From these 4 seed-target correlations, iLI is then calculated according to the following equation:
Note that LL–RL corresponds to the left target region on the difference map (see the temporal region in Fig. S2 as an example), and RR–LR corresponds to the right target region on the difference map. When the denominator fell below 0.2, iLI was set to zero. iLI was computed for all 200 seed regions in each hemisphere against the 199 possible target regions, yielding 39,800 pairwise correlations for each subject.
For each one of these pairwise correlations, we averaged the corresponding iLI values across the exploratory dataset of 100 individuals. The resulting 39,800 mean iLIs were then sorted to determine those regions showing the strongest levels of lateralization. The most left-lateralized correlations (iLI > 0.3, 37 regions) and most right-lateralized correlations (iLI < −0.3, 47 regions) were combined together into a single iLI metric (Fig. 1B). The threshold is somewhat arbitrary but was selected to reduce the number of regions to a number appropriate to factor analysis. A level of 0.3 ensured no >100 regions would be selected for further factor analysis. Alternative threshold values do not change the results for these strongly lateralized regions but may lead to differences for weakly lateralized regions. The iLIs values for the left- and right-lateralized regions were then calculated on an independent sample of 200 subjects to derive an unbiased estimate of the distribution of lateralization (Fig. 2). Note also that the data sample used to derive regions for analysis included an equal number of men and women, allowing unbiased analysis of sex differences.
Factor Analysis.
Principal axis factoring was used for the factor analysis (39). The iLIs of the 84 regions were used as the observed variables. The number of extracted factors was determined by principal component with the criterion that eigenvalues equal or exceed 1. The resulting factor loading values were rotated using normalized varimax rotation. Factor analysis was performed on the first sample of 100 subjects and repeated on an independent sample of 100 subjects. The factor loading values for the top 3 factors are shown in Fig. S6. The results on 2 independent data samples show similar clusters of regions corresponding to each factor, indicating that these factors are highly reproducible across data samples. Local anatomic asymmetries may contribute to the factors.
Supplementary Material
Acknowledgments.
We thank Abraham Snyder, Jessica Andrews-Hanna, Itamar Kahn, Ting Ren, and Tanveer Talukdar for assistance with functional connectivity; Marisa Hollinshead and Betsy Hemphill for data collection; Timothy O'Keefe and Gabriele Fariello for neuroinformatics; and the Harvard Center for Brain Science and the Athinoula A. Martinos Center for Biomedical Imaging for imaging support. This work was supported by National Institutes of Health Grants R01AG021910, R01AG034556, P41RR14074, and K08MH067966; the Simons Foundation; and the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908073106/DCSupplemental.
References
- 1.Geschwind N, Levitsky W. Human brain: Left-right asymmetries in temporal speech region. Science. 1968;161(3837):186–187. doi: 10.1126/science.161.3837.186. [DOI] [PubMed] [Google Scholar]
- 2.Milner B. Interhemispheric differences in the localization of psychological processes in man. Br Med Bull. 1971;27(3):272–277. doi: 10.1093/oxfordjournals.bmb.a070866. [DOI] [PubMed] [Google Scholar]
- 3.Geschwind N, Galaburda AM. Cerebral lateralization. Biological mechanisms, associations, and pathology: I. A hypothesis and a program for research. Arch Neurol. 1985;42(7):428–459. doi: 10.1001/archneur.1985.04060050026008. [DOI] [PubMed] [Google Scholar]
- 4.Toga AW, Thompson PM. Mapping brain asymmetry. Nat Rev Neurosci. 2003;4(1):37–48. doi: 10.1038/nrn1009. [DOI] [PubMed] [Google Scholar]
- 5.Rasmussen T, Milner B. The role of early left-brain injury in determining lateralization of cerebral speech functions. Ann N Y Acad Sci. 1977;299:355–369. doi: 10.1111/j.1749-6632.1977.tb41921.x. [DOI] [PubMed] [Google Scholar]
- 6.Desmond JE, et al. Functional MRI measurement of language lateralization in Wada-tested patients. Brain. 1995;118:1411–1419. doi: 10.1093/brain/118.6.1411. [DOI] [PubMed] [Google Scholar]
- 7.Knecht S, et al. Language lateralization in healthy right-handers. Brain. 2000;123:74–81. doi: 10.1093/brain/123.1.74. [DOI] [PubMed] [Google Scholar]
- 8.Herbert MR, et al. Abnormal asymmetry in language association cortex in autism. Ann Neurol. 2002;52(5):588–596. doi: 10.1002/ana.10349. [DOI] [PubMed] [Google Scholar]
- 9.Kleinhans NM, Müller RA, Cohen DN, Courchesne E. Atypical functional lateralization of language in autism spectrum disorders. Brain Res. 2008;1221:115–125. doi: 10.1016/j.brainres.2008.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crow TJ, et al. Schizophrenia as an anomaly of development of cerebral asymmetry: A postmortem study and a proposal concerning the genetic basis of the disease. Arch Gen Psychiatry. 1989;46(12):1145–1150. doi: 10.1001/archpsyc.1989.01810120087013. [DOI] [PubMed] [Google Scholar]
- 11.Sommer IEC, Ramsey NF, Kahn RS. Language lateralization in schizophrenia, an fMRI study. Schizophr Res. 2001;52:57–67. doi: 10.1016/s0920-9964(00)00180-8. [DOI] [PubMed] [Google Scholar]
- 12.Sun T, et al. Early asymmetry of gene transcription in embryonic human left and right cerebral cortex. Science. 2005;308(5729):1794–1798. doi: 10.1126/science.1110324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Witelson SF, Pallie W. Left hemisphere specialization for language in the newborn: Neuroanatomical evidence of asymmetry. Brain. 1973;96(3):641–646. doi: 10.1093/brain/96.3.641. [DOI] [PubMed] [Google Scholar]
- 14.Gilmore JH, et al. 3 Tesla magnetic resonance imaging of the brain in newborns. Psychiatry Res. 2004;132(1):81–85. doi: 10.1016/j.pscychresns.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Geschwind DH, Miller BL, DeCarli C, Carmelli D. Heritability of lobar brain volumes in twins supports genetic models of cerebral laterality and handedness. Proc Natl Acad Sci USA. 2002;99(5):3176–3181. doi: 10.1073/pnas.052494999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Annett M. A model of the inheritance of handedness and cerebral dominance. Nature. 1964;204:59–60. doi: 10.1038/204059a0. [DOI] [PubMed] [Google Scholar]
- 17.Geschwind N, Galaburda AM. Cerebral lateralization. Biological mechanisms, associations, and pathology: III. A hypothesis and a program for research. Arch Neurol. 1985;42(7):634–654. doi: 10.1001/archneur.1985.04060070024012. [DOI] [PubMed] [Google Scholar]
- 18.Kimura D. Sex and Cognition. Cambridge, MA: MIT Press; 2000. [Google Scholar]
- 19.McGlone J. Sex differences in human brain asymmetry: A critical survey. Behav Brain Sci. 1980;3:215–227. [Google Scholar]
- 20.Damasio H, Tranel D, Spradling J, Alliger R. Aphasia in men and women. In: Galaburda AM, editor. From Reading to Neurons. Cambridge, MA: MIT Press; 1989. pp. 307–325. [Google Scholar]
- 21.Shaywitz BA, et al. Sex differences in the functional organization of the brain for language. Nature. 1995;373(6515):607–609. doi: 10.1038/373607a0. [DOI] [PubMed] [Google Scholar]
- 22.Sommer IEC, Aleman A, Bouma A, Kahn RS. Do women really have more bilateral language representation than men? A meta-analysis of functional imaging studies. Brain. 2004;127(8):1845–1852. doi: 10.1093/brain/awh207. [DOI] [PubMed] [Google Scholar]
- 23.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 24.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 25.Gusnard DA, Raichle ME. Searching for a baseline: Functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2(10):685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- 26.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 27.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 28.Sun T, Walsh CA. Molecular approaches to brain asymmetry and handedness. Nat Rev Neurosci. 2006;7(8):655–662. doi: 10.1038/nrn1930. [DOI] [PubMed] [Google Scholar]
- 29.Escalante-Mead PR, Minshew NJ, Sweeney JA. Abnormal brain lateralization in high-functioning autism. J Autism Dev Disord. 2003;33(5):539–543. doi: 10.1023/a:1025887713788. [DOI] [PubMed] [Google Scholar]
- 30.Thompson PM, et al. Genetic influences on brain structure. Nat Neurosci. 2001;4(12):1253–1258. doi: 10.1038/nn758. [DOI] [PubMed] [Google Scholar]
- 31.Lai CSL, Fisher SE, Hurst JA, Vargha-Khadem F, Monaco AP. A forkhead-domain gene is mutated in a severe speech and language disorder. Nature. 2001;413(6855):519–523. doi: 10.1038/35097076. [DOI] [PubMed] [Google Scholar]
- 32.Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 33.Demb JB, et al. Semantic encoding and retrieval in the left inferior prefrontal cortex: A functional MRI study of task difficulty and process specificity. J Neurosci. 1995;15(9):5870–5878. doi: 10.1523/JNEUROSCI.15-09-05870.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10(4):433–436. [PubMed] [Google Scholar]
- 35.Vincent JL, et al. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J Neurophysiol. 2006;96(6):3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- 36.Buckner RL, et al. Cortical hubs revealed by intrinsic functional connectivity: Mapping, assessment of stability, and relation to Alzheimer's disease. J Neurosci. 2009;29(6):1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci USA. 2006;103(26):10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Z, Mechanic-Hamilton D, Pluta J, Glynn S, Detre JA. Function lateralization via measuring coherence laterality. Neuroimage. 2009;47(1):281–288. doi: 10.1016/j.neuroimage.2009.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harman HH. Modern Factor Analysis. Chicago: Univ of Chicago Press; 1976. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.