Abstract
The oral and esophageal mucosa have been identified as possible sites of HIV/SIV entry following oral infection. Here, gamma/delta (γδ) T cells, a multi-functional T cell subset, were assessed at oral/esophageal mucosa and lymphoid sites at the earliest times (1–14 days) post-oral SIV inoculation utilizing quantitative RT-PCR. During these earliest times post-infection, decreased γδ TCR mRNA levels were observed at the oral gingiva and esophageal mucosa, while increased levels were observed within regional lymph nodes (cervical and retropharyngeal). Higher lymph node γδ TCR levels were associated with increased mRNA expression of the lymphoid homing chemokine/receptor (CCL21/CCR7) pair in these lymph nodes. In contrast to γδ TCR levels, CD4 mRNA expression remained relatively stable through 4 days post-infection, and depletion of CD4 T cells was only evident after 7 or 14 days post-infection. The decrease of γδ T cell mRNA from mucosal sites and the corresponding increase at lymphoid sites suggest a rapid redistribution of these immune cells at these earliest times post-SIV infection.
Keywords: gammadelta T cells, SIV, Mucosa, Acute
INTRODUCTION
Following HIV infection, disease progression is generally characterized by the depletion of peripheral CD4+ T cells. However, progression to AIDS is more complex as evidenced by reports describing the dramatic loss of CD4+ T cells at mucosal sites [1–3] along with increasing immune activation and bystander apoptosis after infection [4, 5]. In addition to CD4+ T cells, impaired functions are observed in B cells, macrophages, NK cells, and gamma/delta (γδ) T cells during chronic pathogenic HIV/SIV infection [6–14]. γδ T cells are a unique T cell subset that bridge the innate and adaptive immune response [6, 15–17]. Indeed, following oral SIV infection an increase in the levels of tonsillar γδ T cells was observed in rhesus macaques previously vaccinated with an attenuated SIV vaccine that correlated with protection [18]. In a second study, intrarectal challenge was accompanied by an increased percentage of γδ T cells expressing the β-chemokines CCL3/MIP-1α and CCL5/RANTES in those macaques previously vaccinated with purified SIVgp120 [19]. The production of β-chemokines by infiltrating γδ T cells may function to inhibit viral infection, as these chemokines are capable of blocking viral gp120-CCR5 co-receptor interactions [19]. These reports provide evidence that γδ T cells at mucosal surfaces may have important functions during the earliest times following SIV infection of macaques.
The distribution and antigenic specificity of γδ T cells are strongly dependent upon the delta variable region selected during T cell receptor (TCR) rearrangement; the Vδ1 or Vδ2 delta variable regions are the most commonly utilized [20]. T cells expressing the Vδ1 TCR are primarily located at mucosal sites and recognize non-classical MHC molecules on virally-infected or tumor cells [21, 22]. T cells expressing the Vδ2 TCR recognize non-peptide phosphoantigens independent of MHC presentation and are primarily distributed in the peripheral blood [20, 23]. Chronic HIV infections are associated with an expansion of Vδ1 γδ T cells in the peripheral blood and gut mucosa [14, 24]. Furthermore, these cells are functionally impaired as evidenced by the ability of serum from HIV+ patients to impede chemokine induced migration of CXCR3-expressing γδ T cells [25]. In situ hybridization has also revealed decreased expression of the lymph node homing chemokine and receptor pair (CCL21/6Ckine and CCR7) in the lymph nodes (LNs) of chronically SIV-infected macaques [26]. Similar to the dysfunction observed in other immune cells [6], these reports indicate that the migratory potential of γδ T cells to lymphoid and inflammatory sites may be impaired during chronic HIV/SIV infection. Here, the distribution of γδ T cells was assessed during an acute oral SIV infection of macaques (1 – 14 days). The decrease of γδ T cell mRNA from mucosal sites and the corresponding increase at lymphoid sites suggests a rapid migration of these immune cells at these earliest times post-SIV infection.
MATERIALS AND METHODS
Human and Animal subjects
Rhesus macaques (between 1–4 years of age) were cared for in accordance with NIH and local Institutional Animal Care and Use Committee guidelines (California National Primate Research Center). Prior to the initiation of the studies, all macaques were negative for SIV, simian T cell leukemia virus, and simian retrovirus. Macaques were inoculated with SIVmac251 orally as described previously [27]. Oral gingiva (mucosa adjacent to teeth) and esophageal mucosal as well as cervical, retropharyngeal, and axillary lymph node tissues were collected at necropsy (days 1, 2, 4, 7 or 14 post-oral inoculation as well as uninfected macaques) and placed into Optimal Cutting Temperature (OCT) embedding compound (Tissue-Tek/Sakura, McGaw Park, IL). Additionally tissues from uninfected macaques served as controls to identify the 95% confidence intervals depicted. Histological examination of the mucosal tissue determined that it was comprised of epithelial cells and submucosa with corresponding immune cells, and occasionally a small amount of underlying connective tissue. Peripheral blood was also assessed from macaques that had been inoculated orally with the same dose and strain of SIVmac251 but were not necropsied at the time of sampling. Human blood samples were obtained voluntarily from uninfected and chronically HIV+ donors (between 24–55 years of age) in accordance with UT Southwestern Medical Center Institutional Review Board guidelines.
RNA isolation, Complementary DNA Synthesis, and Quantitative Real-Time PCR (qRT-PCR)
RNA was isolated from 10 mg of thawed OCT-embedded tissue from both uninfected and infected macques utilizing mechanical homogenization in TRIzol (Invitrogen, Carlsbad, CA) using RNeasy Kits (Qiagen, Valencia, CA) per the manufacturer’s instructions. Contaminating DNA was removed with DNase I (Ambion, Austin, TX). Complementary DNA (cDNA) was synthesized utilizing random hexamers and the Super Script First Strand cDNA synthesis kit (Invitrogen, Carlsbad, CA). Similar qRT-PCR methods were utilized to assess gene expression as previously described [27, 28] and were always performed in duplicate. Table 2 lists the forward and reverse primer as well as probe sequences utilized for: Vδ1, Vδ2, CD4, CCR5, CCR7, CCL21/6Ckine, CCL5/RANTES, and GAPDH for normalization. The following thermocycler amplification conditions were utilized on an SDS Sequence Detector (Applied Biosystems, Foster City, CA): 1 cycle for 10 minutes at 94°C, followed by 40 cycles of 15 seconds at 94°C and 1 minute at 60°C. Gene expression from SIV+ macaques was assessed in comparison to four uninfected macaques for mucosal/lymph node tissues or seven uninfected macaques for peripheral blood. The delta Ct (ΔCt) method was performed to assess fold change in gene expression of SIV+ compared to uninfected macaques as previously described [28].
Table 2. Sequences of Primers and Probes Utilized for Quantitative Real-Time PCR.
qRT-PCR was performed utilizing primers and probes specific for rhesus macaque genes. GI# - GenBank accession number; Forward – forward primer; Reverse – reverse primer; FAM – 6’ carboxyfluorescein; TAMRA - 6-carboxytetramethylrhodamine
| Gene | GI # | Real-time PCR Sequence | |
|---|---|---|---|
| Vdelta 1 (Vδ1) TCR |
339381 | Forward | 5'-TCG CCT TAA CCA TTT TAG CC-3' |
| Reverse | 5'-ACC GGA TGG TTT GGT ATG AGG T-3' | ||
| Probe | 5'-FAM-TAC AGC TAG AAG ACT CAG CAA CAT ACT TCT GTG CTC T-TAMRA-3' |
||
| Vdelta 2 (Vδ2) TCR |
37314 | Forward | 5'-GAG AAC CAG GCT GTA CTT AAG ATC CTT-3' |
| Reverse | 5'-TGA CGA AAA CGG ATG GTT TG-3' | ||
| Probe | 5'-FAM-AGA GAG AGA TGA AGG GTC TTA CTA CTG TGC CAG TG- TAMRA-3' |
||
| CD4 | 393417 | Forward | 5'-CCA CTG GAA AAA ACT CCA ACC A-3' |
| Reverse | 5'-CGA TCG CTC AGC TTG GAT G-3' | ||
| Probe | 5'-FAM-AAA GAT TCT GGG AAT TCA GGG CTC CTT CTT AAC TAA -TAMRA-3' |
||
| CCL21 / 6CKine |
74136270 | Forward | 5'-TAC CGG AAG CAG GAA CCA AG-3' |
| Reverse | 5'-CTG CTC CAT CCC AGC TAT CCT GTT CTT G-3' | ||
| Probe | SYBR Green | ||
| CCR7 | 74136330 | Forward | 5'-GGG GAA ACC AAT GAA AAG T-3' |
| Reverse | 5-GTG ACC TCA TCT TGA CAC AGG C-3' | ||
| Probe | Probe # 77 (Roche – Universal Probe Library) | ||
| CCL5 / RANTES |
74136260 | Forward | 5'-ACC AGT GGC AAG TGC TCC A-3' |
| Reverse | 5'-GGT TGG CAC ACA CTT GGC G-3' | ||
| Probe | 5'-FAM-CAA GCA GTC GTC GTC TTT GTC ACC CGA AA-TAMRA-3' | ||
| CCR5 | 116812906 | Forward | 5'-TAC CTG CTC AAC CTG GCC AT-3' |
| Reverse | 5'-TTC CAA AGT CCC ACT GGG C-3' | ||
| Probe | 5'-FAM-CCT GCT TTT CCT TCT TAC TGT CCC CTT CTG-TAMRA-3' | ||
| GAPDH | 93004459 | Forward | 5'-GCA CCA CCA ACT GCT TAG CAC-3' |
| Reverse | 5'-TCT TCT GGG TGG CAG TGA TG-3' | ||
| Probe | 5'-FAM-TCG TGG AAG GAC TCA TGA CCA CAG TCC-TAMRA-3' | ||
Phenotypic analysis of human γδ T cells
Approximately 40 ml of whole blood was drawn from uninfected and HIV+ patients in acid-citrate-dextrose anti-coagulant tubes. Peripheral blood mononuclear cells (PBMC) were isolated utilizing a Ficoll-Hypaque gradient and stained with fluorescently-labeled antibodies. The antibodies were conjugated to fluorescein isothiocyanate [FITC; Pan-γδ TCR (clone 5A6.E9)], phycoerythrin [PE; Vδ2 γδ TCR (clone B6)], PE-Cy7 [CCR7 (clone 3D12)], allophycocyanin [APC; CD62L (clone MEL14)], and APC-Cy7 [CD3 (clone SK7)]. Following antibody staining, PBMCs were washed twice with PBS and fixed in 1% paraformaldehyde. Flow cytometric analysis was performed on a Cyan flow cytometer (Dako-Cytomation; Fort Collins, CO) and analyzed utilizing FlowJo software (Flowjo, Ashland, OR).
Statistical analysis for human samples
Statistical analyses were performed using GraphPad Prism 4.0 (GraphPad Software, San Diego, CA). A Mann-Whitney test (non-parametric, two tailed, and unpaired) was performed to determine the differences between uninfected and HIV+ patients. A p value of <0.05 (95% confidence) was considered statistically significant.
RESULTS
Decreased γδ T cell mRNA levels at mucosal sites following acute SIV infection
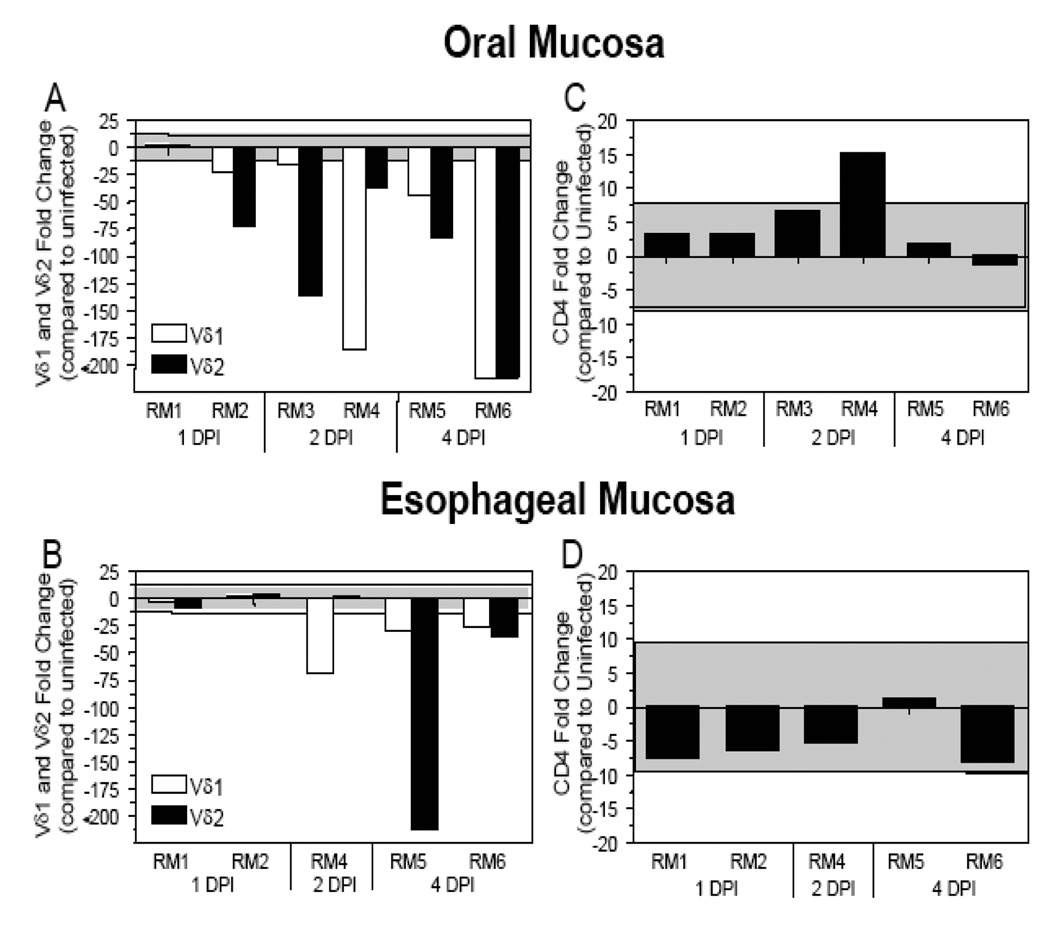
A previous study from our laboratory determined that following an oral inoculation of rhesus macaques, SIV DNA could be detected in oral gingival mucosa (adjacent to teeth) and esophageal mucosal tissues as early as 1–2 days after infection, identifying these mucosal sites as likely entry points for SIV/HIV into the host (Table 1) [27]. Here we expand upon these previous findings through an assessment of the levels of γδ T cells soon after infection (1 – 14 days) in macaques inoculated orally with SIV. The relatively small amount of frozen tissue available required that we utilize a quantitative real-time PCR based approach to detect the relatively low levels of γδ T cells present in these tissues. An assessment of γδ T cell receptor (TCR) mRNA levels at the oral gingiva and esophageal mucosa from four uninfected macaques was performed to identify a two standard deviation range away from the average gene expression. The γδ TCR levels in SIV+ macaques were evaluated to determine if there was an increase or decrease in gene expression when compared to the two standard deviation range from the uninfected macaques. At 1 day post-infection (DPI), the levels of Vδ1 and Vδ2 TCR expression in the oral gingiva mucosa were decreased in one macaque (RM2) (Fig. (1A)) while the esophageal mucosa remained within the two standard deviation range for both macaques (Fig. (1B)). In macaques assessed at 2 and 4 DPI, a decrease in both the Vδ1 and Vδ2 γδ TCR mRNA expression was observed at the oral gingiva and esophageal mucosa (Figs. (1A–1B)). Interestingly, lower levels of γδ TCR expression coincided with the detection of SIV DNA at these mucosal sites (Table 1). CD4 mRNA levels were assessed at these same mucosal sites during acute SIV infection and were generally within the normal range determined from uninfected macaques (Figs. (1C–1D)). These data indicate the decline in γδ T cell levels was relatively specific and not a reflection of changes in all T cell subsets. Although relatively few macaques were available for analysis at any one time point, the trends observed in the data obtained by macaques at consecutive time points enables a clear interpretation of these data.
Table 1. Detection of SIV DNA in mucosal and lymphoid tissues.
All tissues were assessed in carefully controlled nested PCR reactions for SIV gag DNA in 3–10 replicates. DPI – Days Post-Infection; ( − ) indicates all PCR reactions were negative; ( + ) indicates that < 50% of PCR reactions were positive; ( ++ ) indicates that >50% of PCR reactions were positive.
| RM # | Monkey ID | DPI | Mucosa | Lymph Node | |||
|---|---|---|---|---|---|---|---|
| Oral | Esophageal | Cervical | Retropharyngeal | Axillary | |||
| RM1 | 33098 | 1 | + | − | ++ | − | − |
| RM2 | 33202 | 1 | + | − | − | + | − |
| RM3 | 30379 | 2 | ++ | + | + | + | − |
| RM4 | 30381 | 2 | ++ | ++ | ++ | ++ | ++ |
| RM5 | 29976 | 4 | ++ | + | + | ++ | + |
| RM6 | 30244 | 4 | ++ | − | ++ | + | ++ |
| RM7 | 30974 | 7 | ++ | ++ | ++ | ++ | ++ |
| RM8 | 30076 | 14 | ++ | ++ | ++ | + | ++ |
Figure 1. Decreased Vδ1 and Vδ2 γδ TCR mRNA expression at the oral gingiva and esophageal mucosa during acute SIV infection.
The fold change in Vδ1, Vδ2, and CD4 gene expression was assessed in oral (A and C) and esophageal (B and D) mucosal tissues of SIV+ rhesus macaques necropsied at the designated days following oral SIV inoculation. The mRNA levels shown are reported as fold change with regard to the average of mRNA levels in matched samples of four uninfected macaques. The grey shaded area represents a two standard deviation range of the average expression in uninfected macaques for Vδ1 (white bars) and Vδ2 (black bars) γδ T cells. Bars extending beyond the grey shaded area represent samples that are increased or decreased with regard to the uninfected controls.
Increased γδ TCR mRNA expression in the cervical and retropharyngeal lymph nodes during an acute SIV infection
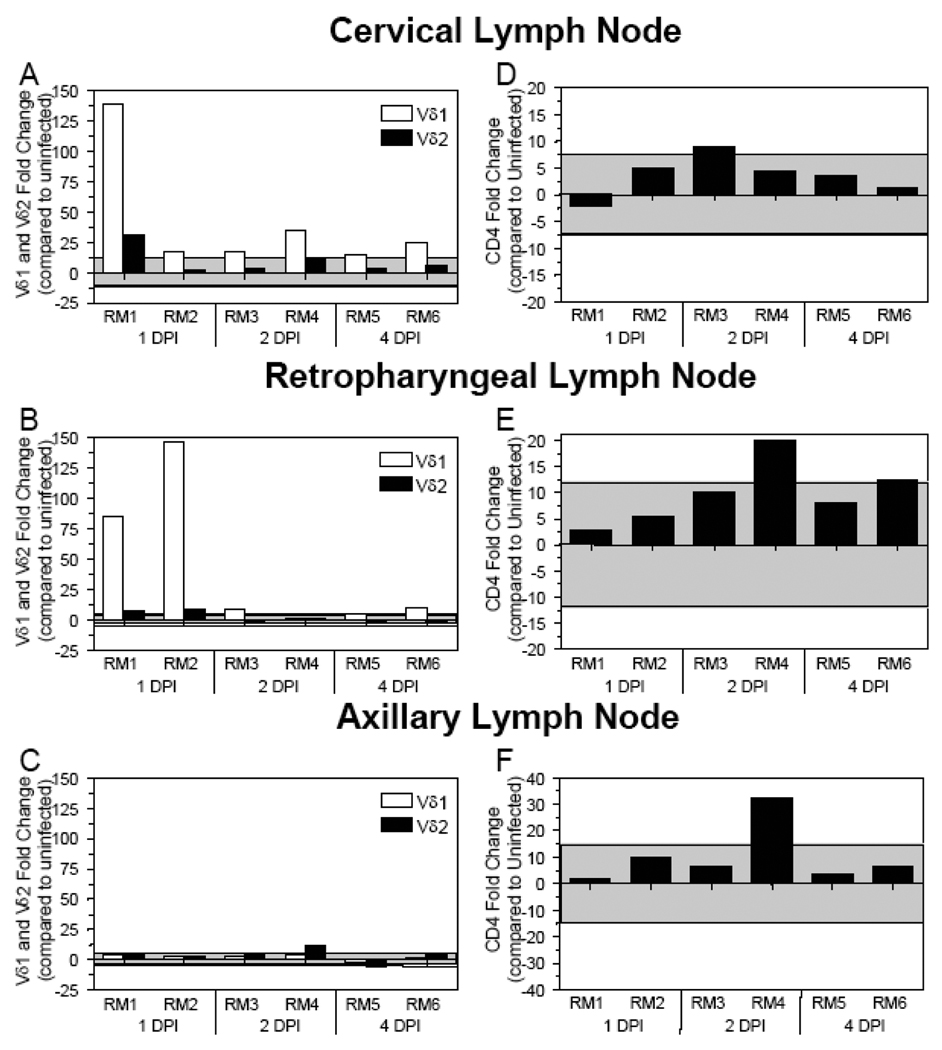
The cervical and retropharyngeal LNs drain the oral cavity where the viral inoculum was administered. SIV DNA could be detected in both of these LNs by real-time PCR at 2 DPI (Table 1) [27]. Analyses of the cervical and retropharyngeal LNs showed Vδ1 γδ TCR mRNA levels increased at 1 DPI and generally remain elevated through 2 and 4 DPI (Figs. (2A–2B)). In contrast, Vδ2 TCR and CD4 mRNA levels were generally within the two standard deviation range in both the cervical and retropharyngeal LNs (Figs. (2A–2B; 2D–2E)). To determine if lymphoid tissues distal to the inoculation site showed similar changes in γδ TCR expression, we assessed Vδ1 and Vδ2 TCR mRNA levels in the axillary LNs of the SIV+ macaques (Fig. (2C)). Within the first four DPI, neither Vδ1 nor Vδ2 TCR expression levels in the axillary LNs were altered compared to uninfected macaques (with the exception of an increase in Vδ2 in macaque RM4) (Fig. (2C)). The increased Vδ1 TCR expression (as early as 1–2 DPI) in LNs in close proximity to the oral cavity, but not in distal lymph nodes, suggest that Vδ1+ γδ T cells may be responding to the presence of SIV at the mucosa and migrating to regional LNs.
Figure 2. Increased Vδ1 and Vδ2 γδ TCR mRNA expression detected in the cervical and retropharyngeal LNs during acute SIV infection.
The fold change in Vδ1, Vδ2, and CD4 gene expression was assessed in the cervical (A and D), retropharyngeal (B and E), and axillary (C and F) lymph nodes of SIV+ rhesus macaques necropsied at the designated days following oral SIV inoculation. Changes in Vδ1 TCR expression are in white bars while changes in Vδ2 TCR expression are in black bars. The mRNA levels shown are reported as fold change with regard to the average of mRNA levels in matched samples of four uninfected macaques. The grey shaded area represents a two standard deviation range of the average expression in uninfected macaques. Bars extending beyond the grey shaded area represent samples that are increased or decreased with regard to the uninfected controls.
Assessment of γδ T cell mRNA levels at 7 and 14 days post-SIV infection
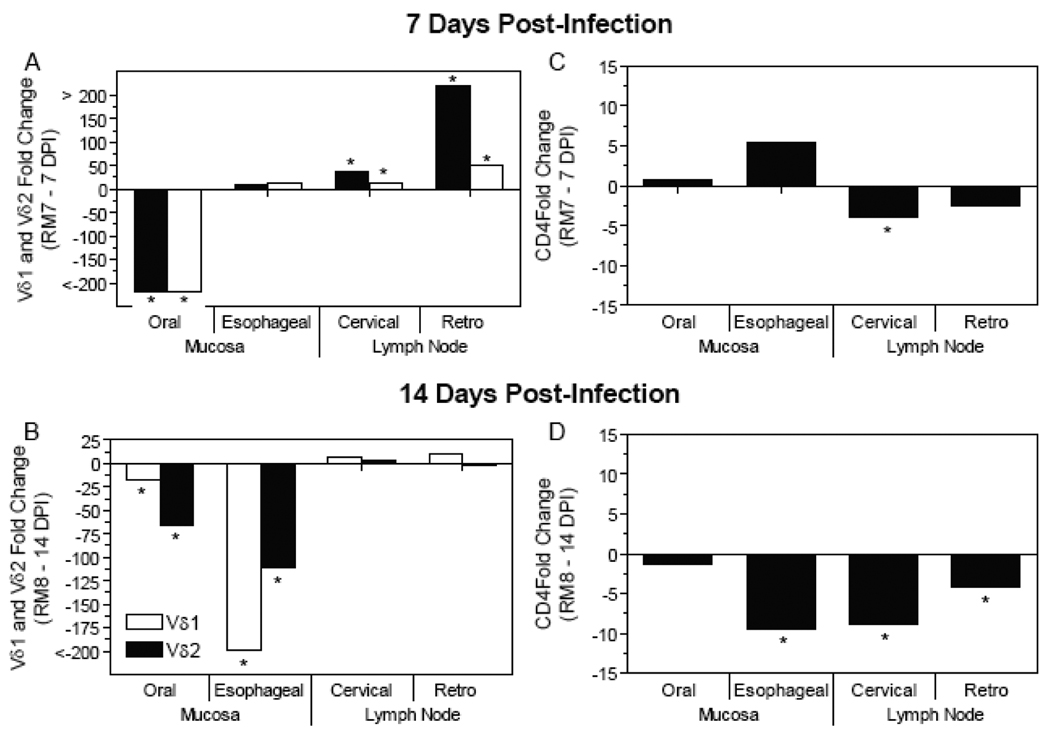
Previously, we demonstrated that SIV DNA is detectable in virtually all mucosal and lymphoid tissues assessed by 7 and 14 DPI in macaques orally inoculated with SIV (Table 1) [27]. Within the macaques necropsied at 7 DPI, Vδ1 and Vδ2 TCR mRNA expression were decreased at the oral gingival mucosa while they were increased at the regional cervical and retropharyngeal LNs (Fig. (3A)) (asterisks indicate mRNA levels that exceed the two standard deviation range of the uninfected macaques). Analysis of an orally inoculated macaque 14 DPI revealed decreased Vδ1 and Vδ2 TCR mRNA expression levels at the oral gingiva and esophageal mucosa while the levels in the cervical and retropharyngeal LNs were within the two standard deviation range (Fig. (3B)). Analysis of the CD4 expression at the mucosal and lymphoid sites identified decreased CD4 mRNA levels at the esophageal mucosa, as well as the cervical LN by 7 DPI and cervical and retropharyngeal LNs by 14 DPI (Fig. (3D)). In summary, macaques infected for 7 and 14 days revealed decreased Vδ1 and Vδ2 γδ TCR expression levels at the oral gingival mucosa indicating that the trends identified during the first four days extended through 14 days post-infection. Compared to γδ T cells, CD4 T cell depletion was generally not evident at mucosal sites until 7 to 14 DPI.
Figure 3. Decreased Vδ1 and Vδ2 γδ TCR mRNA expression at the oral gingiva and esophageal mucosa at 7 and 14 days post-oral SIV infection.
The fold change in Vδ1, Vδ, and CD4 gene expression was assessed in oral gingiva and esophageal mucosa as well as the cervical and retropharyngeal lymph nodes at 7 (A and C) and 14 (B and D) days following SIV oral inoculation from samples obtained at necropsy. Changes in Vδ1 TCR expression are in white bars while changes in Vδ2 TCR expression are in black bars (A, B), mRNA expression of CD4 is also depicted (C, D). The mRNA levels shown are reported as fold change with regard to the average of mRNA levels in matched samples of four uninfected macaques. The asterisks (*) represent gene expression that was outside of the two standard deviation range determined from uninfected macaques.
γδ T cell mRNA levels remain stable in peripheral blood
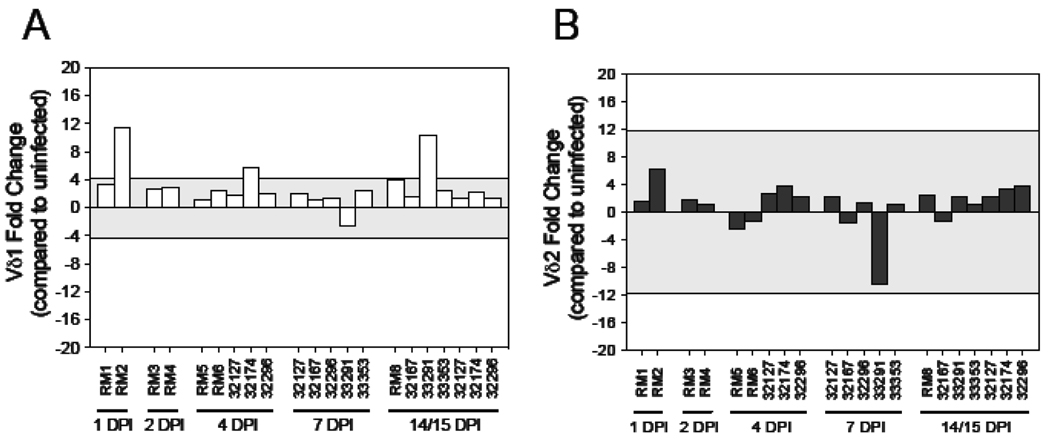
To ascertain if changes at mucosal or lymphoid sites were also manifested in the peripheral blood we assessed Vδ1 and Vδ2 expression by real-time PCR on PBMCs in SIV+ macaques at days 1 through 15 (Fig. (4)). Generally, Vδ1 and Vδ2 mRNA levels were similar at the time points assessed, and within the range of the uninfected macaques with the exception of one day 1, one day 4 and one day 14 infected macaque with higher Vδ1 mRNA levels (Fig.(4A)). These data indicate that changes at mucosal and lymphoid sites at these early times following SIV infection are generally not reflected in the peripheral blood, providing evidence that any gd T cell migration that may be occurring is via lymph and not peripheral blood.
Figure 4. Assessment of Vδ1 and Vδ2 mRNA levels within the peripheral blood during acute SIV infection.
Quantitative real-time PCR was utilized to assess the mRNA levels of (A) Vδ1 and (B) Vδ2 in PBMC obtained from macaques analyzed throughout this study (RM1 – RM6) as well as from other macaques that had been orally inoculated with the same SIV isolate from which blood samples were available. Timepoints examined include 1 day post-infection (DPI), 2 DPI, 4 DPI, 7 DPI and 14–15 DPI. The mRNA levels shown are reported as fold change with regard to the average of mRNA levels in matched samples of seven uninfected macaques. The grey shaded area represents a two standard deviation range of the average expression from uninfected macaques. Bars extending beyond the grey shaded area represent samples that are increased or decreased with regard to the uninfected controls.
Changes in lymphoid homing chemokines and chemokine receptors during SIV and HIV infection
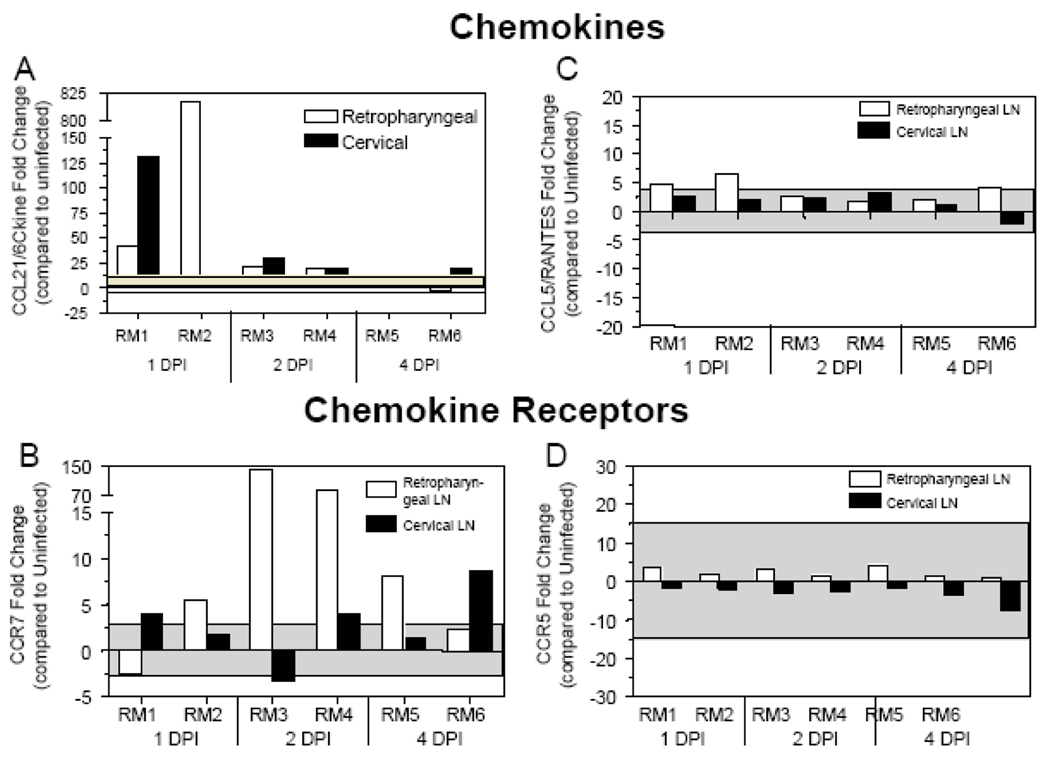
We hypothesized that the observed decrease in γδ T cell levels at mucosal sites during acute SIV infection could be due to the migration of γδ T cells away from the mucosa towards the secondary LNs in response to chemokines. To assess whether lymphoid homing chemokines could be playing a role in the redistribution of γδ T cells, we assessed the mRNA expression of the LN homing chemokine CCL21/6Ckine, and its cognate receptor CCR7, in LNs from SIV+ macaques. At 1 DPI, infected macaque RM1 exhibited increased CCL21/6Ckine mRNA expression in both the retropharyngeal and cervical LNs, while RM2 experienced increased CCL21/6Ckine in the retropharyngeal LN (Fig. (5A)). Generally, in macaques RM1 and RM2 the presence of SIV DNA in the LNs (Table 1) was associated with elevated levels of CCL21/6Ckine mRNA (Fig. (5A)). In macaques assessed at 2 DPI, CCL21/6Ckine expression remained elevated in both the cervical and retropharyngeal LNs, although reduced in comparison to 1 DPI (Fig. (5A)). However, at 4 DPI the expression of CCL21/6Ckine mRNA was generally within the levels observed in uninfected macaques (Fig. (5A)). Gene expression of the CCL21/6Ckine receptor, CCR7, was also up-regulated in at least one LN from each of the macaques at 1 DPI and a more substantial increase in expression at 2 days in the retropharyngeal LNs (Fig. (5B)), suggesting that immune cells may be migrating towards these lymphoid sites in response to the up-regulation of CCL21/6Ckine. We did not observe that these results were generally applicable to a wide range of chemokine/receptor pairs as the expression of the pro-inflammatory chemokine CCL5/RANTES and its receptor CCR5 were generally within the range observed in uninfected macaques (Figs. (5C–5D)).
Figure 5. Increased CCL21 and CCR7 mRNA expression at the cervical and retropharyngeal LNs during acute SIV infection.
Quantitative real-time PCR was utilized to assess the mRNA levels of (A) CCL21/6Ckine, (B) CCR7, (C) CCL5/RANTES and (D) CCR5 in the retropharyngeal (white bars) and cervical (black bars) LNs obtained at necropsied at the designated days following oral SIV inoculation of macaques. The mRNA levels shown are reported as fold change with regard to the average of mRNA levels in matched samples of four uninfected macaques. The grey shaded area represents a two standard deviation range of the average expression from uninfected macaques. Bars extending beyond the grey shaded area represent samples that are increased or decreased with regard to the uninfected controls.
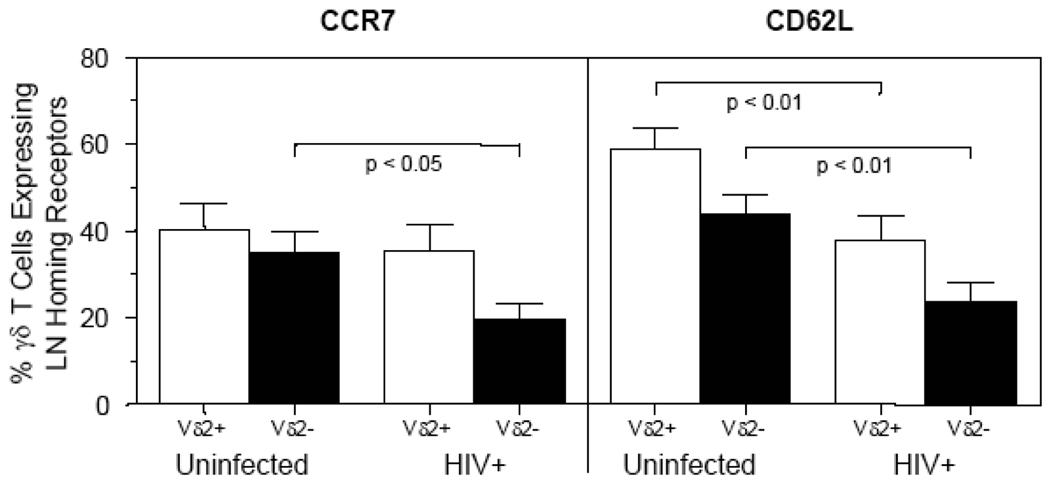
It is not possible to address these questions directly in HIV+ patients due to difficulties of identifying patients at these early time points as well as acquiring mucosal and lymph tissues. However, we can look to determine if γδ T cells have altered phenotypes in the peripheral blood of the HIV+ patients, particularly with regard to lymph node homing markers. Therefore, we undertook an assessment of CCR7 and CD62L (L-selectin) on peripheral blood γδT cells from chronically infected HIV+ patients. We observed that HIV+ patients had a significantly decreased percentage of peripheral blood Vδ2neg T cells that express CCR7 when compared to uninfected individuals (Fig. (6)). Furthermore, a decrease of expression of a second lymph node homing receptor, CD62L, was also identified in Vδ2 and Vδ2neg γδ T cells compared to uninfected individuals (Fig. (6)). These results indicate that the reduced percentages of CCR7+ and CD62L+ previously observed for alpha/beta T cells (both CD4+ or CD8+) from HIV+ patients [29–31] also occurs within the γδT cell population, indicating potential alterations in lymph node homing in the infected patients.
Figure 6. Decreased percentage of γδ T cells from HIV+ patients expressing CCR7 and CD62L.
Peripheral blood mononuclear cells from uninfected (n=13) and HIV+ (n=14) patients were assessed utilizing flow cytometry to identify the percentage of Vδ2+ and Vδ2neg γδ T cells expressing either CCR7 or CD62L. The percentage of Vδ2+ (white bars) or Vδ2neg (black bars) cells expressing either CCR7 or CD62L are shown. CCR7 expression (left panel) was significantly decreased on Vδ2neg γδ T cells in HIV+ patients compared to uninfected controls. CD62L expression (right panel) was significantly decreased on both Vδ2+ and Vδ2neg γδ T cells in HIV+ patients compared to uninfected controls. A Mann-Whitney t test at a 95% confidence interval was utilized to determine statistical significance (p < 0.05) between the uninfected and HIV+ groups.
DISCUSSION
γδ T cells have been assessed in a number of studies involving SIV+ monkeys or HIV+ humans [14, 18, 19, 32–35]. The study by Tenner-Racz et al identified increased numbers of γδ T cells at the tonsils of vaccinated macaques [18], indicating that γδ T cell levels might play an important role in mounting early immune responses at mucosal sites. Our laboratory previously identified the presence of SIV DNA in oral gingival and esophageal mucosal tissues as early as 1–2 days after oral SIV inoculation (Table 1), indicating that these mucosal sites are potential entry points for SIV/HIV [27]. Assessment of γδ T cells in this study determined that decreased Vδ1 and Vδ2 γδ T cell mRNA levels were observed at the oral gingiva and esophageal mucosa as early as one and two days after SIV infection (Fig. (1A–1B; 3A–3B)). This is in agreement with a previous report by Chen et al describing a similar loss of γδ T cells in the intestine four, five or seven days after an intrarectal SIV inoculation [32]. Our study further identified increased levels of γδ T cell mRNA at these early time points in regional cervical and retropharyngeal LNs (Fig. (2 and 3)). It is interesting that although we observed dynamic changes in γδ T cell mRNA expression, CD4 mRNA levels were generally within the range identified from uninfected macaques for both mucosal and lymphoid sites (1 to 4 DPI). A loss in CD4 mRNA was not observed until 7 to 14 DPI, in agreement with reports assessing CD4 T cell depletion at gut-associated lymphoid tissues [1–3, 36]. Therefore, these data suggest that following mucosal SIV-infection the loss of γδ T cells occurs very early from the site of inoculation (here the oral mucosal tissues), even more rapidly than depletion of CD4+ T cells.
There are a number of hypotheses that might explain the loss of mucosal γδ T cells at early times following SIV inoculation and infection. The direct infection of γδ T cells with HIV has been documented in vitro and HIV provirus has been detected in purified γδ T cells [37], therefore depletion of these cells from direct SIV infection can not be ruled out. In addition, γδ T cells may be depleted from mucosal sites as a result of activation-induced cell death (AICD), as the SIV infection can result in an increase in cytokines/chemokines at mucosal sites [28]. Also, as γδ T cells can recognize and eliminate HIV-infected CD4+ T cells in vitro [12, 34, 35], AICD might also be induced by the direct influence of virally infected cells on the γδ T cells [38, 39]. However, the rapid rate at which the γδ T cells are depleted from the mucosa most likely suggests the hypothesis that γδ T cells are induced to redistribute from the mucosa to lymphoid sites at very early times post-infection. This redistribution could be driven by the infection of CD4+ T cells and macrophages at the mucosal sites or possibly from viral products present in the inoculum.
Lymphoid homing receptors such as CCR7 and CD62L are important for proper LN migration and are up-regulated by γδ T cells upon TCR stimulation [40, 41]. In addition, in vitro γδ T cells can migrate in response to the LN homing chemokine CCL21/6Ckine [42] and have the potential to present antigens at lymphoid sites that may impact the adaptive immune response [40]. Our studies describe an up-regulation of the homeostatic lymphoid chemokine CCL21/6Ckine mRNA levels by 1 DPI in the cervical and retropharyngeal LNs of SIV-inoculated macaques (Figure 4). Though CCL21/6Ckine is constitutively expressed by stromal cells in LNs [26], higher mRNA levels detected here could potentially be induced by the presence of virus in these secondary lymphoid organs [43, 44]. These data are supported by the finding that CCR7 mRNA levels were also elevated at these same lymphoid sites 2 DPI, supporting a hypothesis in which γδ T cells and other immune cells may have migrated to these sites soon after the up-regulation of CCL21/6Ckine. Additionally, both CCL5/RANTES and CCR5 levels were generally not altered following infection, indicating that redistribution of γδ T cells was not in response to pro-inflammatory chemokines. The concept that γδ T cells home to lymph nodes within a few days following oral SIV infection is comparable to studies indicating that dendritic cells can migrate from the vaginal mucosa to secondary lymph nodes within 24-hours post-SIV infection [45, 46]. Moreover, our results provide evidence for a rapid but transient up-regulation of CCL21/6Ckine mRNA within the first 2 DPI, which is faster than previous studies describing an up-regulation of CCL21/6Ckine at 14 DPI [26]. Similarly, the increased CCR7 mRNA expression observed here at 2 and 4 days post-infection is more rapid than shown in a previous study that identified increased CCR7 mRNA levels in lymph nodes at 14 DPI [26]. However, this increase may be transient as during chronic infection CCR7 and CCL21/6Ckine mRNA expression in the LNs of SIV+ macaques was similar to or decreased compared to their uninfected counterparts [26]. The up-regulation of CCL21/6Ckine and CCR7 in the draining lymph nodes early after SIV infection provides further evidence to support the redistribution hypothesis whereby γδ T cells and probably other immune cells migrate rapidly to secondary LNs at very early time points following oral SIV infection. The increase in Vδ1 γδ T cells at lymphoid sites suggests migration from a mucosal tissue, such as the oral mucosa or intestinal mucosa, where the majority of Vδ1 gd T cells reside. Further, the generally unchanged levels of Vδ1 and Vδ2 in the peripheral blood provides evidence that any migration of γδ T cells occurs through lymphatics and not via the blood. Although further studies are needed, these data suggest that γδ T cells may be activated upon recognition of mucosal SIV, and likely HIV, infection to migrate to regional LNs and prime the adaptive immune response.
The ability of γδ T cells to home to LNs during acute SIV/HIV infection may be important for the establishment of an adaptive immune response. To ascertain if our findings in the SIV/macaque model might also be occurring in HIV infected patients we undertook an assessment of the levels of LN homing receptors on peripheral blood γδ T cells. Though our analysis was limited to peripheral blood of chronically infected HIV+ patients (not undergoing HAART) we did observe a reduced percentage of γδ T cells expressing lymph node homing receptors CCR7 and CD62L (Fig. (6)). This reduced percentage of γδ T cells expressing LN receptors in the peripheral blood of HIV+ patients could be due to increased rate of migration of these cells to LNs, or to a defect in the ability of γδ T cells from HIV+ patients to express these proteins. These results from chronically infected HIV+ patients provides evidence that the HIV infection is influencing the migratory ability of γδ T cells. While it is likely that migration of γδ T cells during the acute stage of infection might be beneficial, it is not known whether this same migration during the chronic stages of infections would be beneficial to the host or to viral replication.
The results in this study reveal a loss of γδ T cell mRNA at mucosal sites within the first four days after pathogenic SIV infection prior to the depletion of CD4+ T cells. Additionally, the migration of γδ T cells from the mucosa to LNs at these early time points may be in response to the chemokine CCL21/6Ckine. We speculate that this anti-viral response may be the result of γδ T cells processing viral antigens at the mucosal surface and then migrating towards the regional LNs to function as antigen presenting cells to CD4+ and CD8+ T cells, as observed in vitro [40]. Alternatively, the γδ T cells may home to these regional LNs in an attempt to control the rapid systemic spread of the virus, possibly through the expression of β-chemokines to interfere with viral co-receptor binding [19]. Previous studies have identified γδ T cells as playing a role during both oral [18] and rectal [19] mucosal transmission events. These studies, including ours, suggest that γδ T cells may have a protective role in preventing a pathogenic HIV/SIV infection at mucosal surfaces. Therefore, vaccine and microbicide development should consider the levels and migratory capacity of γδ T cells, and other innate immune cells, at mucosal sites to aid in the development of more effective strategies to prevent HIV infections.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to acknowledge the patients at Parkland health and Hospital Systems for their participation in this study as well as the excellent staff from the UT Southwestern HIV Research Unit, particularly Tianna Peterson and Michael Lowery. We would also like to thank Dr. Marta Marthas, Kim Schmidt and the animal care and veterinary staff at the California National Primate Research Center where the macaques were housed. We would also like to thank Elizabeth Chacko, Amanda Leone, and Kiran Mir for their contributions in the development of this manuscript.
This study was supported by the NIH Grant R01-AI035522(DLS), R21-AI060451(DLS), RR00165(Yerkes) and the James B. Pendleton Trust. DK was supported by the Integrative Immunology Training Program NIAID 5T32 AI005284 and the grant supplement NIAID AI035522-13S1.
Footnotes
These authors do not have any commercial or other considerations that might be interpreted as a conflict of interest with regard to the data presented herein.
References
- 1.Brenchley JM, Schacker TW, Ruff LE, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 3.Veazey RS, DeMaria M, Chalifoux LV, et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 4.Giorgi JV, Liu Z, Hultin LE, Cumberland WG, Hennessey K, Detels R. Elevated levels of CD38+ CD8+ T cells in HIV infection add to the prognostic value of low CD4+ T cell levels: results of 6 years of follow-up. The Los Angeles Center, Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr. 1993;6:904–912. [PubMed] [Google Scholar]
- 5.Sousa AE, Carneiro J, Meier-Schellersheim M, Grossman Z, Victorino RM. CD4 T cell depletion is linked directly to immune activation in the pathogenesis of HIV-1 and HIV-2 but only indirectly to the viral load. J Immunol. 2002;169:3400–3406. doi: 10.4049/jimmunol.169.6.3400. [DOI] [PubMed] [Google Scholar]
- 6.Alfano M, Poli G. Role of cytokines and chemokines in the regulation of innate immunity and HIV infection. Mol Immunol. 2005;42:161–182. doi: 10.1016/j.molimm.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 7.Betts MR, Gray CM, Cox JH, Ferrari G. Antigen-specific T-cell-mediated immunity after HIV-1 infection: implications for vaccine control of HIV development. Expert Rev Vaccines. 2006;5:505–516. doi: 10.1586/14760584.5.4.505. [DOI] [PubMed] [Google Scholar]
- 8.Braun DP, Kessler H, Falk L, et al. Monocyte functional studies in asymptomatic, human immunodeficiency disease virus (HIV)-infected individuals. J Clin Immunol. 1988;8:486–494. doi: 10.1007/BF00916955. [DOI] [PubMed] [Google Scholar]
- 9.Crowe SM, Vardaxis NJ, Kent SJ, et al. HIV infection of monocyte-derived macrophages in vitro reduces phagocytosis of Candida albicans. J Leukoc Biol. 1994;56:318–327. doi: 10.1002/jlb.56.3.318. [DOI] [PubMed] [Google Scholar]
- 10.Lane HC, Masur H, Edgar LC, Whalen G, Rook AH, Fauci AS. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1983;309:453–458. doi: 10.1056/NEJM198308253090803. [DOI] [PubMed] [Google Scholar]
- 11.Widney D, Gundapp G, Said JW, et al. Aberrant expression of CD27 and soluble CD27 (sCD27) in HIV infection and in AIDS-associated lymphoma. Clin Immunol. 1999;93:114–123. doi: 10.1006/clim.1999.4782. [DOI] [PubMed] [Google Scholar]
- 12.Poccia F, Boullier S, Lecoeur H, et al. Peripheral V gamma 9/V delta 2 T cell deletion and anergy to nonpeptidic mycobacterial antigens in asymptomatic HIV-1-infected persons. J Immunol. 1996;157:449–461. [PubMed] [Google Scholar]
- 13.Zhou D, Lai X, Shen Y, et al. Inhibition of adaptive Vgamma2Vdelta2+ T-cell responses during active mycobacterial coinfection of simian immunodeficiency virus SIVmac-infected monkeys. J Virol. 2003;77:2998–3006. doi: 10.1128/JVI.77.5.2998-3006.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kosub DA, Lehrman G, Milush JM, et al. Gamma/Delta T-cell functional responses differ after pathogenic human immunodeficiency virus and nonpathogenic simian immunodeficiency virus infections. J Virol. 2008;82:1155–1165. doi: 10.1128/JVI.01275-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrick DA, Schrenzel MD, Mulvania T, Hsieh B, Ferlin WG, Lepper H. Differential production of interferon-gamma and interleukin-4 in response to Th1- and Th2-stimulating pathogens by gamma delta T cells in vivo. Nature. 1995;373:255–257. doi: 10.1038/373255a0. [DOI] [PubMed] [Google Scholar]
- 16.Mak TW, Ferrick DA. The gammadelta T-cell bridge: linking innate and acquired immunity. Nat Med. 1998;4:764–765. doi: 10.1038/nm0798-764. [DOI] [PubMed] [Google Scholar]
- 17.Modlin RL, Sieling PA. Immunology. Now presenting: gammadelta T cells. Science. 2005;309:252–253. doi: 10.1126/science.1115264. [DOI] [PubMed] [Google Scholar]
- 18.Tenner-Racz K, Stahl Hennig C, Uberla K, et al. Early protection against pathogenic virus infection at a mucosal challenge site after vaccination with attenuated simian immunodeficiency virus. Proc Natl Acad Sci U S A. 2004;101:3017–3022. doi: 10.1073/pnas.0308677101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehner T, Mitchell E, Bergmeier L, et al. The role of gammadelta T cells in generating antiviral factors and beta-chemokines in protection against mucosal simian immunodeficiency virus infection. Eur J Immunol. 2000;30:2245–2256. doi: 10.1002/1521-4141(2000)30:8<2245::AID-IMMU2245>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 20.Carding SR, Egan PJ. Gammadelta T cells: functional plasticity and heterogeneity. Nat Rev Immunol. 2002;2:336–345. doi: 10.1038/nri797. [DOI] [PubMed] [Google Scholar]
- 21.Jameson J, Ugarte K, Chen N, et al. A role for skin gammadelta T cells in wound repair. Science. 2002;296:747–749. doi: 10.1126/science.1069639. [DOI] [PubMed] [Google Scholar]
- 22.Shen X, Siliciano RF. Preventing AIDS but not HIV-1 infection with a DNA vaccine. Science. 2000;290:463–465. doi: 10.1126/science.290.5491.463. [DOI] [PubMed] [Google Scholar]
- 23.Kunzmann V, Bauer E, Feurle J, Weissinger F, Tony HP, Wilhelm M. Stimulation of gammadelta T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood. 2000;96:384–392. [PubMed] [Google Scholar]
- 24.Poles MA, Barsoum S, Yu W, et al. Human immunodeficiency virus type 1 induces persistent changes in mucosal and blood gammadelta T cells despite suppressive therapy. J Virol. 2003;77:10456–10467. doi: 10.1128/JVI.77.19.10456-10467.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poggi A, Carosio R, Fenoglio D, et al. Migration of V delta 1 and V delta 2 T cells in response to CXCR3 and CXCR4 ligands in healthy donors and HIV-1-infected patients: competition by HIV-1 Tat. Blood. 2004;103:2205–2213. doi: 10.1182/blood-2003-08-2928. [DOI] [PubMed] [Google Scholar]
- 26.Choi YK, Fallert BA, Murphey-Corb MA, Reinhart TA. Simian immunodeficiency virus dramatically alters expression of homeostatic chemokines and dendritic cell markers during infection in vivo. Blood. 2003;101:1684–1691. doi: 10.1182/blood-2002-08-2653. [DOI] [PubMed] [Google Scholar]
- 27.Milush JM, Kosub D, Marthas M, et al. Rapid dissemination of SIV following oral inoculation. Aids. 2004;18:2371–2380. [PubMed] [Google Scholar]
- 28.Milush JM, Stefano-Cole K, Schmidt K, Durudas A, Pandrea I, Sodora DL. Mucosal innate immune response associated with a timely humoral immune response and slower disease progression after oral transmission of simian immunodeficiency virus to rhesus macaques. J Virol. 2007;81:6175–6186. doi: 10.1128/JVI.00042-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Douek DC, Picker LJ, Koup RA. T cell dynamics in HIV-1 infection. Annu Rev Immunol. 2003;21:265–304. doi: 10.1146/annurev.immunol.21.120601.141053. [DOI] [PubMed] [Google Scholar]
- 30.Eggena MP, Barugahare B, Jones N, et al. Depletion of regulatory T cells in HIV infection is associated with immune activation. J Immunol. 2005;174:4407–4414. doi: 10.4049/jimmunol.174.7.4407. [DOI] [PubMed] [Google Scholar]
- 31.Gray CM, Lawrence J, Ranheim EA, et al. Highly active antiretroviral therapy results in HIV type 1 suppression in lymph nodes, increased pools of naive T cells, decreased pools of activated T cells, and diminished frequencies of peripheral activated HIV type 1-specific CD8+ T cells. AIDS Res Hum Retroviruses. 2000;16:1357–1369. doi: 10.1089/08892220050140900. [DOI] [PubMed] [Google Scholar]
- 32.Chen ZW, Shen Y, Davis IC, Shen L, Letvin NL, Fultz PN. Down-regulation of macaque gammadelta + T cells in lymphoid compartments after rectal infection with SIVsmmPBj14. J Med Primatol. 2000;29:143–147. doi: 10.1034/j.1600-0684.2000.290307.x. [DOI] [PubMed] [Google Scholar]
- 33.Poccia F, Battistini L, Cipriani B, et al. Phosphoantigen-reactive Vgamma9Vdelta2 T lymphocytes suppress in vitro human immunodeficiency virus type 1 replication by cell-released antiviral factors including CC chemokines. J Infect Dis. 1999;180:858–861. doi: 10.1086/314925. [DOI] [PubMed] [Google Scholar]
- 34.Wallace M, Bartz SR, Chang WL, Mackenzie DA, Pauza CD, Malkovsky M. Gamma delta T lymphocyte responses to HIV. Clin Exp Immunol. 1996;103:177–184. doi: 10.1046/j.1365-2249.1996.d01-625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Biswas P, Ferrarini M, Mantelli B, et al. Double-edged effect of Vgamma9/Vdelta2 T lymphocytes on viral expression in an in vitro model of HIV-1/mycobacteria co-infection. Eur J Immunol. 2003;33:252–263. doi: 10.1002/immu.200390028. [DOI] [PubMed] [Google Scholar]
- 36.Li Q, Duan L, Estes JD, et al. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434:1148–1152. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- 37.Imlach S, Leen C, Bell JE, Simmonds P. Phenotypic analysis of peripheral blood gammadelta T lymphocytes and their targeting by human immunodeficiency virus type 1 in vivo. Virology. 2003;305:415–427. doi: 10.1006/viro.2002.1759. [DOI] [PubMed] [Google Scholar]
- 38.Gan YH, Lui SS, Malkovsky M. Differential susceptibility of naive and activated human gammadelta T cells to activation-induced cell death by T-cell receptor cross-linking. Mol Med. 2001;7:636–643. [PMC free article] [PubMed] [Google Scholar]
- 39.Kirkham PA, Takamatsu HH, Lam EW, Parkhouse RM. Ligation of the WC1 receptor induces gamma delta T cell growth arrest through fumonisin B1-sensitive increases in cellular ceramide. J Immunol. 2000;165:3564–3570. doi: 10.4049/jimmunol.165.7.3564. [DOI] [PubMed] [Google Scholar]
- 40.Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human gammadelta T Cells. Science. 2005;309:264–268. doi: 10.1126/science.1110267. [DOI] [PubMed] [Google Scholar]
- 41.Ebert LM, Meuter S, Moser B. Homing and function of human skin gammadelta T cells and NK cells: relevance for tumor surveillance. J Immunol. 2006;176:4331–4336. doi: 10.4049/jimmunol.176.7.4331. [DOI] [PubMed] [Google Scholar]
- 42.Wilson E, Hedges JF, Butcher EC, Briskin M, Jutila MA. Bovine gamma delta T cell subsets express distinct patterns of chemokine responsiveness and adhesion molecules: a mechanism for tissue-specific gamma delta T cell subset accumulation. J Immunol. 2002;169:4970–4975. doi: 10.4049/jimmunol.169.9.4970. [DOI] [PubMed] [Google Scholar]
- 43.Marsland BJ, Battig P, Bauer M, et al. CCL19 and CCL21 induce a potent proinflammatory differentiation program in licensed dendritic cells. Immunity. 2005;22:493–505. doi: 10.1016/j.immuni.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 44.Piqueras B, Connolly J, Freitas H, Palucka AK, Banchereau J. Upon viral exposure, myeloid and plasmacytoid dendritic cells produce 3 waves of distinct chemokines to recruit immune effectors. Blood. 2006;107:2613–2618. doi: 10.1182/blood-2005-07-2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller CJ, Shattock RJ. Target cells in vaginal HIV transmission. Microbes Infect. 2003;5:59–67. doi: 10.1016/s1286-4579(02)00056-4. [DOI] [PubMed] [Google Scholar]
- 46.Romani N, Ratzinger G, Pfaller K, et al. Migration of dendritic cells into lymphatics-the Langerhans cell example: routes, regulation, and relevance. Int Rev Cytol. 2001;207:237–270. doi: 10.1016/s0074-7696(01)07007-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.