Abstract
A major challenge associated with the development of chemopreventive polyphenols is the lack of bioavailability in vivo, which are primarily the result of coupled metabolic activities of conjugating enzymes and efflux transporters. These coupling processes are present in most of tissues and organs in mammals and are efficient for the purposes of drug metabolism, elimination and detoxification. Therefore, it was expected that these coupling processes represent a significant barrier to the oral bioavailabilities of polyphenols. In various studies of this coupling process, it was identified that various conjugating enzymes such as UGT and SULT are capable of producing very hydrophilic metabolites of polyphenols, which cannot diffuse out of the cells and needs the action of efflux transporters to pump them out of the cells. Additional studies have shown that efflux transporters such as MRP2, BCRP and OAT appear to serve as the gate keeper when there is an excess capacity to metabolize the compounds. These efflux transporters may also act as the facilitator of metabolism when there is a product/metabolite inhibition. For polyphenols, these coupled processes enable a duo recycling scheme of enteric and enterohepatic recycling, which allows the polyphenols to be reabsorbed and results in longer than expected apparent plasma half-lives for these compounds and their conjugates. Since the vast majority of polyphenols in plasma are hydrophilic conjugates, more research is needed to determine if the metabolites are active or reactive, which will help explain their mechanism of actions.
Keywords: Coupling, Efflux, Conjugating Enzymes, Polyphenols, Chemoprevention, Bioavailability, Enteric Recycling, Half-life
1. Introduction
Drug metabolizing enzymes play key roles in the metabolism, elimination and/or detoxification of xenobiotics or exogenous compounds introduced into the body [1]. Many vital organs and tissues in our body are well-equipped with diverse drug metabolizing enzymes including phase I and phase II metabolizing enzymes as well as efflux transporters, which are present in abundance either at the basal level, or at induced elevated level after xenobiotics exposure [1, 2]. Phase I enzymes consist primarily of the cytochrome P450 (CYP) superfamily of microsomal enzymes [1]. Phase II metabolizing or conjugating enzymes consist of many superfamilies of enzymes mainly including sulfotransferases (SULT) [3], UDP-glucuronosyltransferases (UGT) [4], and glutathione transferases [5]. Efflux transporters, including p-glycoprotein (p-gp) [6], multidrug resistance associated proteins (MRPs) [6], and organic anion transporter (OAT) [7] are expressed in many tissues such as the liver, intestine, kidney and brain, where they present a formidable barrier to drug penetration, and play crucial roles in drug absorption, distribution and excretion [6, 8, 9]. Drugs that undergo extensive phase I metabolism, phase II conjugation and/or efflux by various transporters have poor bioavailability and are more prone to metabolism-based drug interactions.
Polyphenolic compounds constitute a diverse group of phytochemicals that are present in the human diet such as soy, fruit, vegetables and nuts [10, 11]. Plant polyphenols are divided into several classes, i.e., hydroxybenzoic acids, hydroxycinnamic acids, anthocyanins, proanthocyanidins, flavonols, flavones, flavanols, flavanones, isoflavones, stilbenes, and lignans. The chemical structures and the dietary contents of these polyphenols have been reviewed elsewhere [12]. Natural polyphenols (Fig. 1A and 1B) such as flavonoids [11], isoflavonoids [13], resveratrol [14], curcumin [15] and tea polyphenols [16] have gathered significant interests in scientific research and public media. The aura associated with this class of compounds is that they are supposed to be non-toxic and that they have significant chemopreventive effects ranging from antiageing to cancer prevention [11, 16-21], many if not all of these problems or diseases lack conventional pharmaceutical products. For example, isoflavones such as genistein and daidzein found in soy have significant effects on bone health among postmenopausal women, together with some weak hormonal effects. Monomeric catechins found at especially high concentrations in tea have effects on plasma antioxidant biomarkers and energy metabolism [22]. The attractiveness of polyphenols as chemopreventive agents are further enhanced because humans have had long-term exposure experience with this class of compounds. Similarly, marketers have used this same scheme to promote a wide range of natural polyphenol products in pharmacies and on the internet.
Fig. 1. A. Chemical structures of polyphenols. B. Chemical structures of flavonoids.
There have been major advances in the past few years in our knowledge regarding polyphenol absorption and metabolism [23, 25, 26], and it is apparent that most classes of polyphenols are sufficiently absorbed to have the potential to exert biological effects. For instance, concentrations of quercetin after a meal containing onions, catechins after red wine consumption, or isoflavones after soy consumption can reach micromolar concentrations in the blood [25-29]. However, the circulation polyphenols are mainly in conjugated forms (i.e., glucuronides and sulfates), which may limit their target tissue exposure. Lack of bioavailability and plasma concentration also makes it difficult to correlate in vitro and in vivo mechanisms of action. Therefore, poor bioavailability, low potency that may be result of their low bioavailability and lack of exclusive patent protection are major challenges associated with developing natural polyphenols into “conventional pharmaceuticals” [22, 23]. On the other hand, even though little is known about their bioavailability, proper route and/or means of delivery, and/or pharmacological efficacy, many people are taking them daily [24]. Therefore, there are urgent needs from the public to know if and how this class of compounds is effective or toxic, which of them is better absorbed and which of them may lead to the formation of active metabolites. In the present review, we will focus on the bioavailability of natural polyphenols, the factors affecting the bioavailability of natural polyphenols in vivo. We will present the concept of enzyme-transport coupling and the barrier network responsible for the poor bioavailability of the polyphenols and then discuss what can be done to improve the bioavailability of polyphenols.
2. Bioavailability of Natural Polyphenols
It is important to realize that most common polyphenols found in our food and diet are good for our health. However, harnessing their health benefits is a big challenge because most of polyphenols have lower intrinsic activities or bioavailabilities as the result of poor absorption from the intestine, extensive metabolism following absorption, or a combination thereof. In general, the metabolites that are found in blood and target organs that result from digestive or hepatic enzymes may differ from the native substances in terms of biological activities. Therefore, extensive knowledge of the bioavailability of polyphenols is necessary if their health effects are to be clearly elucidated and pharmacologically enhanced. Many researchers have investigated the kinetics and extent of polyphenol absorption by measuring plasma concentrations and/or urinary excretion among adults after the ingestions of a single dose of polyphenol, which may be provided as a pure compound, a plant extract, or a part of whole food/beverage. Manach et al reviewed 97 studies of various classes of polyphenols, namely, anthocyanins, flavonols, flavanones, flavanol monomers, proanthocyanidins, isoflavones, hydroxycinnamic acids, and hydroxybenzoic acids (Fig. 1) [23]. In general, the aglycones of polyphenols can be absorbed from the small intestine. Whereas, most of polyphenols are present in food in the form of esters, glycosides, or polymers cannot be absorbed in their natural form. During the course of absorption, polyphenols are conjugated in the small intestine, later in the liver, and possibly in the kidneys. The conjugation of polyphenols is a metabolic detoxification process that restricts their potential toxic effects and facilitates their biliary and urinary elimination by increasing their hydrophilicity. Normally, the conjugation of polyphenols is highly efficient because their aglycones are generally either absent in blood or present in low concentrations after consumption of polyphenols. We will briefly discuss the bioavailability of polyphenols class by class as follows:
1) Anthocyanins
Anthocyanins are present in black grapes or aubergine. After single doses of 150 mg to 2 g total anthocyanins were administrated to volunteers, the concentrations of anthocyanins detected in plasma were very low, only at the range of 10-50 nmol/L [23]. Thus anthocyanins are absorbed with poor efficiency and rapidly metabolized and eliminated [23]. Glucuronides and sulfates of anthocyanins were recently identified in human urine with LC/MS/MS analyses [30, 31]. Monoglucuronides accounted for >80% of the total metabolites when analyses were performed immediately after urine collection [31]. Furthermore, all of the metabolites of the strawberry anthocyanins, except for the native glucoside, were very unstable and were extensively degraded when acidified urine samples were frozen for storage [31]. Protocatechuic acid was identified as an abundant metabolite of cysnidin-3-O-glucoside in rats, but it was also formed in vitro with simple incubation of cyanidin with rat plasma in the absence of colonic bacteria [32].
2) Flavonols
Flavonols, especially quercetin, have been extensively studied because they are widely distributed in dietary plants. Hollman et al [33, 34] showed that quercetin was indeed absorbed in humans. Moreover, they demonstrated that glucosides of quercetin were more efficiently absorbed than quercetin itself, whereas the rhamnoglucoside (rutin) was less efficiently and less rapidly absorbed [35, 36]. Quercetin is not present as an aglycone and occurs only in conjugated forms. Generally, ~20-40% of qucercetin is methylated in the 3′-position, yielding isorhamnetin [36, 37]. Quercetin-3-O-glucuronide, 3′-O-methylquercetin-3-O-glucuronide, and quercetin-3′-O-sulfate as the major conjugates were found after the ingestion of onions [37]. One characteristics of quercetin bioavailability is that the elimination of quercetin metabolites is quite slow, with reported half-lives ranging from 11 to 28 h, indicating the compound could be favorably accumulated in tissues with repeated intakes [38].
3) Flavanones
Flavanones represent a small group of compounds, including glycosides of hesperetin present in oranges and glycosides of naringenin present in grapefruit. The Cmax of flavanone metabolites measured after the ingestion of citrus fruits was shown to correlate with the time required for hydrolysis of the rhamnoglycosides of hesperidin, naringenin, and naritutin by the microflora [39]. This hydrolysis occurs before the released aglycones are absorbed in the colon since aglycone is absorbed more rapidly than their corresponding glycosides [39]. Phase I metabolites of flavanones have not yet been identified in vivo. Monoglucuronides of hesperetin were shown to be the major forms present in plasma after ingestion of orange juice [40]. The total urinary excretion of conjugated flavanones accounted for 8.6% of the intake for hesperidin and 8.8% for naringin. Plasma concentrations reach 1.3-2.2 μmol/L hesperetin metabolites with an intake of 130-220 mg given as orange juice [41] and up to 6 μmol/L naringenin metabolites with 200 mg ingested as grapefruit juice [41].
4) Isoflavones
Isoflavones are provided only by soybean-derived products. They can be present as aglycones or glycosides, depending on the soy preparation and processing. Some authors investigated the differences in bioavailability between aglycone and glycosides by using pure molecules. Izumi et al [37] found greater bioavailability of aglycone, on the basis of Cmax. Equol production was significantly higher after ingestion of daidzin than that of daidzein [42-44]. Equol is a bacterial metabolite that has been shown to be more estrogenic than its precursor daidzein in many in vitro studies and in animal models [44]. The nature of isoflavone metabolites was the same after glycoside or aglycone ingestion. Glycosides are hydrolyzed before absorption and are not recovered as such in plasma [45, 46]. Aglycone represents less than 5% of the total soy isoflavones (parent + metabolites) [47], and main metabolites are 7-O-glucuronides and 4′-O-glucuronides, with small proportions of sulfate esters [47-49]. Manach et al [22] have made a comparison on the bioavailabilities of different polyphenols, including mean values of Cmax, time to reach Cmax, area under the plasma concentration-time curve, elimination half-life, and relative urinary excretion. It was found that 1) gallic acid is far better absorbed than the other polyphenols. The Cmax values of its metabolites reached 4 μmol/L with a 50-mg intake, and the relative urinary excretion was 38%. Next are isoflavones, which are the most well-absorbed flavonoids, with Cmax values of ~2 μmol/L after a 50-mg intake and mean relative urinary excretions of 42% for daidzin and 15.6% for genistin.
5) Other polyphenols
Resveratrol, a constituent of red wine, has long been purported to have cardioprotective effects and chemopreventive effects. Regarding the pharmacokinetics of resveratol, there exist many contradictory and confusing results in the literatures. The wide range of concentrations and doses used to achieve the various effects reported for reveratrol (-32 nM-100 μM in vitro and -100 ng-1500 mg per kg (body weight) in animals) raises many questions about the concentrations that are achieved or achievable in vivo. In addition, resveratrol has a short initial half-life and is metabolized extensively in the body [50]. For other stilbenes, investigators also determined the pharmacokinetics of piceatannol, pinosylvin and rhapontigenin in male rats [51]. After single intravenous dose of 10 mg/kg of stilbene given alone, the detectable plasma half-lives of these xenobiotics appear to be relatively short. Oral bioavailability estimates suggest that these stilbenes are poorly bioavailable. Furthermore, the results showed that each stilbene was extensively glucuronidated, and predominantly eliminated via non-urinary routes. All three stilbenes were highly distributed into tissues and were highly extracted by the liver [50].
In summary, previous studies showed, although aglycone forms of natural polyphenols are rapidly absorbed since they can permeate cell membrane with ease, most of the polyphenols present in the blood are phase II conjugates [47-49]. These conjugates are formed via the action of phase II enzymes such as UDP-glucuronosyltransferases and sulfotransferases [52]. Additional metabolic pathways include methylation via the methyltransferases [53] and hydroxylation and demethylation via cytochrome P450 [54]. In any rate, it is generally believed that glucuronidation or sulfation represents the major metabolic pathway in vivo as represented by the amounts of metabolites present in the systemic circulation or blood [55]. In addition, major metabolic pathway appeared to be organ specific.
The blood concentration of intact polyphenols are usually in the 10-100 nM range following dietary exposure and can reach several hundred nM following exposure to high doses of concentrated polyphenols. For example, genistein exposure following normal soybean exposure in Asian has been reported to be 50-100 nM [56]. The AUC (0-24h) values for total genistein and conjugates were 54, 24, and 13 μM for genistein, genistin, and enriched protein soy extract, respectively. These results indicate that the bioavailability of genistein is higher for the aglycone than for its aglycoside. Genistin is partly absorbed in its glycosidic form [56]. It is concluded that bioavailability studies based on portal vein plasma levels contribute to insight into the role of the intestine and liver in deglycosylation and uptake characteristics of glycosylated flavonoids [56]. The Cmax of total genistein could reach 5.49 μmol/L when it was administrated at a single dose of 56 μmol/kg of body weight. Furthermore, when orally administrated genistein at the dose level, phase II conjugates is 20-30 times higher than that of parent compound [56]. In general, isoflavones are certainly the best absorbed flavonoids: plasma concentration of 1.4-4 μmol/L are obtained between 6 and 8h in adults who consume relatively low quantities of soy derivatives supplying ≅ 50 mg isoflavones [57-59].
Whether the major conjugates in vivo are glucuronides or sulfates are usually compound and species dependent. For example, phase II conjugates of soy isoflavones are mainly present in glucuronides in humans [60, 61], whereas phase II conjugates of resveratrol are mainly present in sulfates in humans [61, 62]. In rats, the glucuronides are the major metabolites, which is similar to humans. Unlike humans, however, sulfates are present in less relative abundance in rats. In mice, the formation of metabolites appeared to be more balanced in the intestine [63] and liver (unpublished observation).
3. Bioefficacy and Safety of Polyphenol Consumption
In the past few decades, accumulating scientific evidence has demonstrated potential beneficial effects of polyphenols such as anthocyanosides, catechins, proanthocyanidins, stilbenes and other phenolics found in fruits, vegetables or spices [22, 23]. Epidemiologic studies have also clearly shown that polyphenols rich in plant foods protect humans against degenerative diseases such as cancer and cardiovascular diseases [23]. Cooke et al have claimed that anthocyanins (glycosides) and their aglycones anthocyanidins, rich in fruits and berries, could inhibit malignant cell survival and confound many oncogenic signaling events [64]. Other epidemiological studies have shown that moderate consumption of red wine is putatively associated with lowering the risk of developing coronary heart diseases. In addition, preclinical evidence suggests that polyphenolic phytochemicals exemplified by epigallocatechin gallate from tea, curcumin from curry and isoflavones from soy possess cancer chemopreventive properties. The abundance of flavonoids and related polyphenols in plants makes it possible that several hitherto uncharacterized agents with chemopreventive efficacy are still to be identified, which may constitute attractive alternatives to currently used chemopreventive drugs [66]. However, there has been a lack of investment to fully explore the potentials of these chemopreventive agents because of weak proprietary position and definitive clinical trials are generally missing. Many large trials are run with agents that represent one type of extract from a manufacturer using one commonly used dose. Such trial, which lacks rigor, often creates more confusion than conclusion.
Despite the lack of clear clinical evidence in support of consuming concentrated polyphenols, many companies are selling various nutritional supplements enriched with polyphenols. Many recommend the daily consumption of polyphenols based on the consumption of soy products in Japan or of grapes or wine in some European countries [66, 80]. Typical doses are 50 mg per day (mg/d) for isoflavones or 100-300 mg/d grape seed extracts rich in proanthocyanidins. Others are recommending consumption doses that are without evidence, which include tablets or capsules containing 300 mg quercetin, 1 g citrus flavonoids, or 20 mg resveratrol, with suggested use of 1-6 tablets or capsules per day, are commonly found on the Internet.
Fortunately, these dietary polyphenols have been well tolerated, even when consumed at highly concentrated doses, perhaps due to their poor bioavailability. Unexpected cases of severe toxicity associated with consumption of polyphenolic phytochemicals have been rarely reported. Side-effects encountered with polyphenol consumption included nausea, abdominal pain, diarrhea, fatigue and insomnia [68]. Furthermore, consumption of polyphenols inhibits nonheme iron absorption and may lead to iron depletion in populations with marginal iron stores [69]. Another Important aspect is the fact that major sources of polyphenols, such as coffee, tea, and wine do not contain vitamin C, which is an enhancer of iron absorption [70]. Finally, polyphenols may affect drug bioavailability and pharmacokinetics. Several drugs such as benzodiazepines and terfenadine, show a up to 3-fold increases in bioavailability with grape-fruit juice, because of inhibition of CYP3A4 by polyphenol analogs [71-72]. Another recent study in animals suggest that flavonoids may inhibit the absorption of gamma-hydroxybutyrate by inhibiting the activity of monocarboxylate transporter 1 (MCT1) [73].
4. Conjugation Enzymes
Conjugating enzymes are phase II enzymes in drug metabolic pathway classification. Although drug can undergo phase I and phase II reactions simultaneously, one of the pathways typically dominates. In general, phase I metabolism acts as the first step in drug metabolic process, in that its product often but not always becomes a substrate for the phase II metabolism. For lipophilic endo- and xenobiotics that enter cells by passive diffusion or uptake transporters, phase I enzymes often represent the first enzymatic reaction that acts to clear them from body. The phase II reactions are the true ‘detoxification’ pathways and produce metabolites that are generally much more water-soluble and can be readily excreted in bile or urine, hence readily eliminated from the body.
For polyphenols, the major metabolic pathway is phase II conjugation. Once absorbed, polyphenols are subjected to 3 types of conjugation: glucuronidation, sulfation and methylation [67]. Glucuronidation and sulfation metabolize polyphenols into very hydrophilic conjugates whereas methylation produces metabolites that have essentially same if not slightly more lipophilic metabolites. Regardless if methylation occurs, the methylated products are usually conjugated subsequently into glucuronides and sulfates, as long as there is a functional group available for conjugation into more hydrophilic metabolites [74, 75]. Therefore, conjugation enzymes that catalyzed the formation of these hydrophilic metabolites significantly impact the bioavailability of polyphenols.
Two superfamilies of phase II enzymes, UDP-glucuronosyltransferases (UGTs) and sulfotransferases (SULTs), catalyze the formation of hydrophilic phase II conjugates for natural polyphenols. UGTs are membrane-bound enzymes that are located in the endoplasmic reticulum in many tissues. UGTs catalyze the transfer of a glucuronic acid from UDP-glucuronic acid to their substrates such as steroids, bile acids, polyphenols, dietary constituents and xenobiotics. Mammalian UGTs almost exclusively belong to the UGT1A and UGT2B subfamilies, although new subfamilies are being found gradually. Among the human UGT isoforms, UGT1A9, UGT1A10, UGT1A1, UGT1A6 and UGT1A8 all participate in the metabolism of natural polyphenols. UGT2Bs appear to be less active (when measured using in vitro) when comparing to UGT1As [52]. The presence of glucuronidated metabolites in the portal blood after perfusion of polyphenols in the small intestine of rats shows that glucuronidation of polyphenols first occurs in the enterocytes before further conjugation in the liver [76, 77]. This is probably the case in humans as well, because in vitro glucuronidation of quercetin and luteolin by microsomes prepared from the human intestine is markedly higher than that by microsomes prepared from the human liver [78].
SULTs are cytosolic enzymes that are soluble and highly active. They catalyze the transfer of a sulfate moiety from 3′-phosphoadenosine-5′-phosphosulfate to a hydroxyl group on various substrates. Compared to UGT isoform-catalyzed metabolism, much less is known about how SULT isoforms metabolize natural polyphenols. This is due to the fact that sulfation is often the secondary metabolic pathway (to glucuronidation) and only a handful of isoforms are commercially available [52]. Commercially available SULTs are also much more expensive than UGTs. Therefore, the isoforms that are specifically involved in the conjugation of polyphenols have yet been clearly identified. However, sulfation still can account for a large percentage of the phase II metabolism at lower concentration [75, 79], and may serve as the primary metabolic pathway for selected polyphenols such as resveratrol [80].
Unlike UGTs and SULTs, methyltransferases such as catechol-O-methyltransferase catalyze the O-methylation of several catechol-containing polyphenols, forming metabolites that are just as polar as or slightly less polar than the parent compound [81]. This O-methylation reaction is subjected to strong inhibitory regulation by S-adenosyl-L-homocyteine, which is formed in large quantities during the O-methylation of tea polyphenols. Although methylation may not be the major metabolic pathway, its importance in the mechanisms of action of tea polyphenols cannot be underestimated as methylated metabolites tend to have different activities and some may resist inactivation via UGTs and SULTs [80-84].
5. Other Metabolic Pathways
Aside from phase II enzymes, phase I enzymes, especially CYP also participate in the metabolism of natural polyphenols. For example, CYP catalyzed the formation of genistein from biochanin A [85, 86]. In animals and humans, this demethylation reaction has been detected following red clover and kaempferol administrations [87-89]. However, CYP catalyzed metabolism of polyphenols represents a minor metabolic pathway when comparing to phase II metabolism. Moreover, the products of CYP reaction are often rapidly conjugated to hydrophilic glucuronides and sulfates [90].
Another important enzyme that participates in the metabolism of natural polyphenols is lactose-phlorizin hydrolase (LPH), a glucosidase located on the brush border membrane of the small intestine. LPH catalyzes extracellular hydrolysis of polyphenol monoglucosides, which are especially important for absorption of polyphenol glucosides. This enzyme-catalyzed hydrolysis is important for bioavailability of natural occurring polyphenols (often present as glucosies and glycosides) because their absorption is severely limited by their size, polarity, large numbers of hydrogen bonds, and excretion by MRP2 [76]. Although there are literature reports of absorption of intact glucosides and glycosides [25], liberated aglycones as the result of LPH action are usually rapidly absorbed via diffusion across the brush border membrane [76, 91]. For example, quercetin 3-glucoside, which is not a substrate for cytosolic β-glucosidases, is rapidly absorbed after hydrolysis by lactase phloridzine hydrolase, whereas hydrolysis of quercetin 4′-glucoside seems to involve both hydrolases [92, 57].
Lastly, a significant number of bacteria resides in the colon appear to participate in the metabolism of polyphenols. Colonic microflora is exposed to two sources of polyphenols: dietary polyphenols (mostly in glycoside forms) that are not absorbed and hydrophilic polyphenol conjugates (sulfates and glucuronides) that are excreted by the intestine and liver. Microflora bacteria employ a large number of extracellular glycosidases (secreted by bacteria) and can break down all known glycoside bonds formed between polyphenol aglycones and sugars. These bacteria also express high concentration of glucuronidases and sulfatases, which will also release aglycones from their respective glucuronides and sulfates. The released aglycones can be taken up by the colonic cells in humans or animals, or they can be further metabolized into other products. Unlike humans and animals, bacterial metabolism can cause ring-fission and reduction reactions, producing metabolites that are drastically different from those produced by their hosts, humans or animals. These metabolites are often inactive, but occasionally, some metabolites (equol derived from daidzein metabolism by bacteria) are much more potent than their parents [93].
6. Rates of Metabolite Formation and Metabolite Excretion
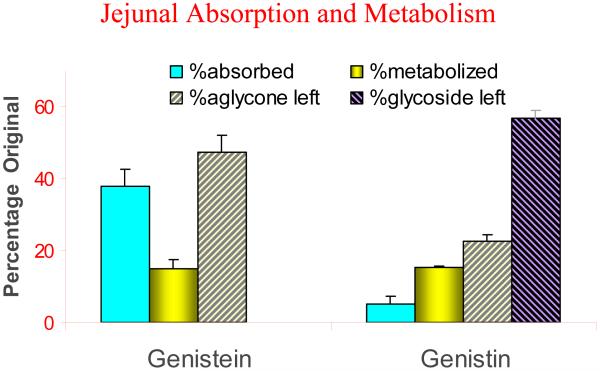
We have described in great details the importance of polyphenol metabolism and how they affect the bioavailability of natural polyphenols. In general, for naturally occurring polyphenols with glycosides, their absorption is usually poor because of their polarity and size unless glycosides are hydrolyzed via intestinal or microbial hydrolases. Hence, poor uptake contributes significantly to the poor bioavailability of intact polyphenol. In addition, poor uptake can result in more extensive metabolism as metabolic enzymes are less saturated. For example, in a rat jejunum perfusion study, we found that more metabolites were formed when we perfused genistin than when we perfused genistein, because the former has to be converted first into genistein (the aglycone) before been absorbed (Fig.2). For polyphenol aglycones, which usually are taken up rapidly, we have attributed their poor bioavailabilities to extensive metabolism after polyphenols enter the cells. In this case, solubility of polyphenols may affect their bioavailabilities. For example, higher concentration of genistein can not only cause a saturation of metabolic enzymes but also can slightly inhibit the activities of enzymes through product inhibition [94]. Taken together, these results indicate that prediction of how much metabolites will be made is highly complex and influenced by multiple factors, each of which may deserve careful examinations.
Fig.2.
Absorption and metabolism of genistein and genistin (@100 μM) in rat jejunum using single-pass perfusion (flow rate=0.191 ml/min). % Metabolized is calculated as the ratio of metabolite in outlet perfusate to parent drug concentration in inlet perfusate.
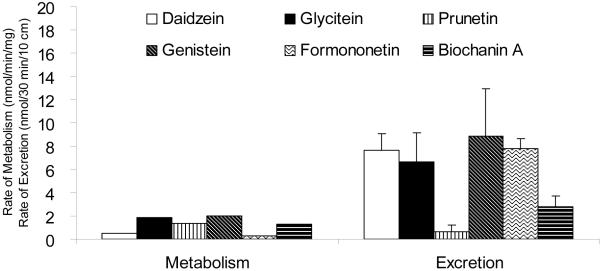
Traditionally, rates of metabolite excretion are assumed to be determined by apparent rates of metabolite formation. The formation rates are conveniently determined from studies conducted using microsomes. For polyphenols, microsomal studies have shown that they are rapidly metabolized, although the rates of metabolism varied among the natural polyphenols and between the species from which microsomes are prepared. For example, in rats the rates of metabolism varies between 0.33 nmo/min/mg protein (for prunetin) and 2.5 pmol/min/mg protein (for glycitein) in liver microsomes and 0.72 nmol/min/mg protein and 2.51 pmol/min/mg protein in jejunal microsomes among the six isoflavones (genistein, daidzein, biochanin A, daidzein, formononetin and prunetin, all @10 μM) we studied (Fig.3). We also measured the metabolism of genistein in human intestinal and liver microsomes, and found that their rates of metabolism to be significantly different. In limited comparison studies using rat and mouse S9 fractions, we found that they have similar rates of glucuronidation but significant different rates in sulfation (unpublished data). Taken together, the results of microsomal studies indicate that polyphenols can be rapidly metabolized by microsomal enzymes.
Fig.3.
Intestinal glucuronidation rates measured using rat microsomes and intestinal excretion rates measured using perfused rat intestine. Each bar is the average of four determination for perfusion experiments. The metabolism rates were calculated using kinetic parameters obtained as described in Wang et al.
The important question is then: are microsomal metabolism rates correlated with excretion rates. There is no simple answer to this question, even although we expect that rapid excretion of phase II conjugates of polyphenols will be the consequence of rapid metabolism. In rat intestinal perfusion model, the excretion rates of metabolites were not always correlated with their metabolism rates (Fig.3). However, for rapidly metabolized isoflavone such as glycitein, the percent metabolites formed may reach 10-15% following a single pass perfusion of duodenum or jejunum even though the average mean residence time is about 10 min in a 10 cm segment of the intestine (i.e., duodenum and jejunum) [95]. In mouse small intestine, the metabolism is perhaps more rapid with equal percent (10-15%) of glucuronides and sulfates. In mouse colon, only sulfates were excreted and glucuronides were not found [63, 95]. The situation in liver is similar although it is more difficult to determine the exact contribution of liver metabolism to biliary excretion of these polyphenols since studies with isoflavones given directly via portal vein have not been performed. In bile duct cannulation conducted in conjunction with rat intestinal perfusion, we found large quantities of metabolites in the biliary excretion, and rates of excretions usually matched that of the intestinal excretion in rats [95], but are usually much smaller than intestinal excretion in mice due to the much small bile flow rate in mice [63]. Even less is known about the biliary excretion of phase II conjugates in humans.
The above discussion has focused on the apical excretion from the enterocytes and hepatocytes. Metabolites of polyphenols may also be excreted via the basolateral membranes of the enterocytes or hepatocytes, which drain into blood, and are subsequent eliminated via the urinary route. Small conjugates such as monosulfates are preferentially excreted in urine [75]. Urinary excretion has often been determined in human studies. The total amount of metabolites excreted in urine is roughly correlated with maximum plasma concentrations. It is quite high for isoflavones: the percentages excreted are 16-66% for daidzein and 10-24% for genistein [57,59, 67, 96]. Interested readers to this pathway of elimination should consult recent studies [112-114].
Lastly, we would like to briefly touch the idea of recycling. Because intestinal bacteria possess β-glucuronidases and sulfatases that are able to release free aglycones from conjugated metabolites of polyphenols secreted via bile or enterocytes. Aglycone can be reabsorbed, which results in enteric and enterohepatic cycling. The recycling is sometime reflected in pharmacokinetic studies that show a second maximum plasma concentration ≅7h after genistein administration [97]. Further discussion will ensure later.
7. Lack of Correlation between Rates of Excretion versus Rates of Metabolism Derived from Microsomes
In a recent study of six isoflavones (genistein, daidzein, formononetin, glycitein, biochanin A and prunetin), we found that there were no correlations between the intestinal rates of excretion and rates of metabolism as represented by Km, Vmax, intrinsic clearance and calculated rates of metabolism at 10 μM [98]. Therefore, it is likely that excretion rates were not consistently controlled by any one of the kinetic parameters. Similarly, we did not observe correlation between amounts excreted and rate of formation in Caco-2 cells [95]. Therefore, we concluded that coupling of intestinal metabolic enzymes and efflux transporters affects the intestinal disposition of isoflavones, and structural differences of isoflavones, such as having methoxyl groups, significantly influenced their intestinal disposition.
Whereas we are quite confident about our observed lack of correlation, we do not known if these metabolism rates are correlated with efficacy of isoflavones. In a study published by Ye and co-workers, they hypothesized that high urinary isoflavone excreters would show less plasma non-HDL cholesterol than low isoflavone excreters after soy protein feeding. Their results showed that high urinary isoflavone excreters had significantly less non-HDL-C than did the low isoflavone excreters or casein-fed controls. Plasma total and non-HDL-C were negatively correlated with urinary daidzein, glycitein, and total isoflavone excretion, and urinary isoflavone excretion phenotypes predicted the cholesterol-lowering efficacy of soy protein [67]. In any rate, these data suggest higher overall exposure may be of some benefit but they do not answer the question if the exposure is to free aglycones or to conjugated polyphenols.
8. Coupling of Efflux Transporters and Conjugation Enzymes [90]
Relative concentrations of free aglycones and conjugates of polyphenols in the plasma are determined by, in addition to absorption, activities and efficacy of enzymes and efflux transporters that are of components of the various coupling processes. These processes control how much polyphenols enter into our systemic circulation, and likely how much polyphenols are eliminated into the urine.
Coupling processes are often present in the biological system to gain maximal efficiency and multi-pointed regulatory control. Coupling of enzymes and transporters are frequently observed in the biological systems. For example, digestive enzymes are often coupled to the nutrient transporters to render maximal absorption. We proposed the hypothesis that efflux transporters are coupled with conjugating enzymes to enable the best possible functions. In this coupling scheme, efflux transporters facilitate the excretion of phase II conjugates by extracting the metabolites from the intracellular domain. This is important since certain metabolites may represent the negative feedback loop which can inhibit the activities of these phase II enzymes. Phase II enzymes facilitate the excretion of phase II conjugates by providing sufficient amounts of phase II conjugates in the intracellular domain.
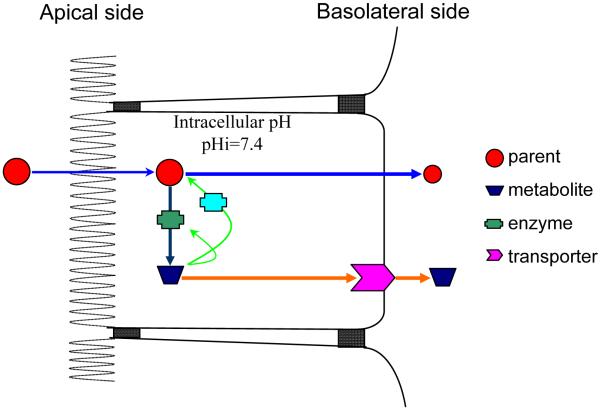
We used the scheme shown in Fig. 4 to represent cells with polarized membrane, which occur in intestine, kidney and liver, three of the most important organs for xenobiotic disposition. In the coupling process of polyphenol disposition is defined as a process where excretion or elimination process operates in concert with the metabolic process. In the process, the excretion/elimination will affect the metabolism or vice versa because these two processes interact. Hence, a change in the enzyme activities that can generate a highly hydrophilic molecule that is incapable of penetrating the cellular membrane via passive diffusion may not result in an increase in overall metabolite excretion unless efflux transporter cooperates. Furthermore, if the conjugate can be reconverted back into its corresponding aglycone or can inhibit the conjugating reaction via negative feedback, then the excretion rates of the conjugated metabolites will directly affect the amounts of metabolites excreted by the cells. This is because efflux transporters capable of extracting hydrophilic conjugated metabolites from the cytosolic domain will determine the intracellular equilibrium between aglycones and conjugated metabolites. Efficient efflux carriers that can rapidly remove the conjugated metabolites can enhance the cellular excretion of metabolites, whereas the opposite could impair it. Therefore, we believe that the coupling of conjugating enzymes and efflux transporters are not only important in the disposition of polyphenols but also could have a clear and profound impact on bioavailability, detoxification, and drug interactions.
Fig. 4.
A schematic representation of coupled efflux and metabolism.
The coupling process proposed by us [90] is different from the coupling of cytochrome P450 family of enzymes, e.g., CYP3A, and efflux transporters, e.g., p-glycoprotein, depicted by Benet and his co-workers [99, 100], in that the efflux transporter is responsible for the efflux of substrate and product of the metabolism could diffuse out the cells by passive diffusion, namely, in the coupling process, p-glycoprotein transporter and CYP3A enzyme can pair to act as a coordinated absorption barrier against drugs.
In the cellular network of absorption barriers in the intestine, there are more complex coupling processes than that depicted in Fig. 4. As has been shown by us in the previous review [90] parent compound itself may also be subjected to efflux at the apical side or basolateral side or both [101]. Moreover, some of the efflux transporters, e.g., p-glycoprotein, may be capable of sequestering metabolites in the cytosolic domain and then pump them out once the complex merged into the cell membrane [102]. Lastly, we and other investigators have found that multiple transporters, e.g., MRP2, OAT, are involved in the cellular excretion of phase II conjugates of natural polyphenols [51, 99, 103, 104]. On the other hand, knowledge of sugar absorption has been based primarily on the mechanism of Na+/glucose co-transport by SGLT1 and its long-term regulation by diet [105]. SGLT1 is also abundantly expressed in the jejunum and transports glucose into epithelial cells. It has been shown that most polyphenols had no effect on SGLT1. However, the polyphenols(+)-catechin, (-)-epicatechin gallate and (-)-epigallocatechin gallate, which are components of green tea, caused an inhibition of SGLT1, which was almost independent of glucose concentration [106]. Therefore, the actual barrier to polyphenol bioavailabilities may be more complex than those depicted in Fig. 4. Despite of these complexities, we will first examine a relatively simple coupling process called the “Revolving Door” theorem.
9. The “Revolving Door” Theorem
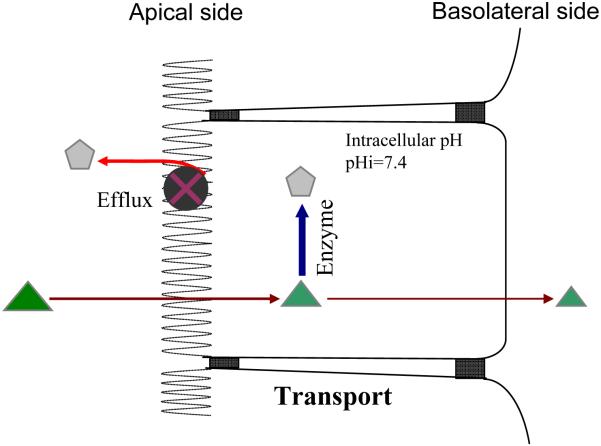
In order to account for the coupling action between conjugating enzymes and efflux transporters, we have proposed a “revolving door” theorem [67] (Fig. 5). Briefly, in the “revolving door” theorem, phase II metabolites of xenobiotics such as polyphenols depend on the efflux transporters to exit the cells because these metabolites are too hydrophilic. If the formation rate of hydrophilic metabolites exceeds that of their excretion rate, the metabolites will accumulate inside cells, which can have several consequences. If the reverse reaction occurs, e.g., glucuronidases are present in many organs including liver and intestine, the net formation rates will decrease. If the product is an inhibitor of the enzyme reaction, i.e., the classical product inhibition, the actual formation rate decreases. In any rate, an inefficient “revolving door” could result in poor excretion of the phase II metabolites, even though the potential for metabolite formation could be high. Therefore, this gating mechanism is rather unique for hydrophilic phase II conjugates. Hydrophobic phase II conjugations such as methylation do not generate hydrophilic conjugates and therefore their product outflow from the cells is not subjected to the restriction by a gating mechanism.
Fig. 5.
Schematic representation of the coupling theorem. Substrates are represented by triangles whereas products of enzymes or metabolites are represented by pentagons.
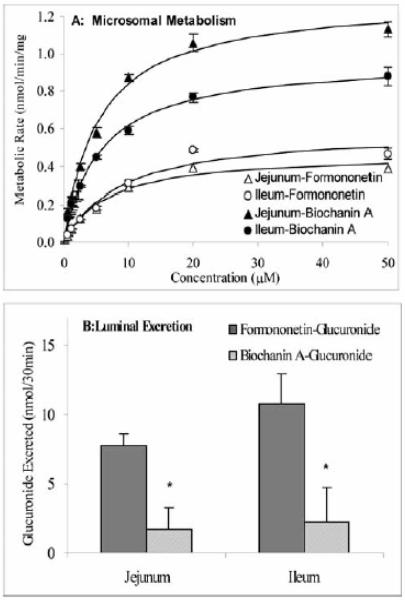
This gating mechanism represents an important concept that is based on the results of many investigations published in the literatures and those that come from our own laboratory. Traditionally, excretion of phase II metabolites is believed to be dependent only on the activities of conjugating enzymes. For instance, many studies that investigate the chemopreventive potentials of dietary chemicals routinely investigate how phase II enzymes are induced and how induction is associated with reduced carcinogen exposure [107]. However, such an approach is at best incomplete. For example, when we studied intestinal disposition of flavonoids, we found that one of isoflavones, biochanin A is metabolized much faster (more than 3 times) than formononetin in the rat intestine. When we measured the intestinal excretion of metabolites, however, much more formononetin glucuronides were excreted than biochanin A glucuronide (Fig. 6) [108]. We have also found that apigenin glucuronide formation rates were much faster than their excretion rates from the Caco-2 cells [109]. These results indicate that gating mechanisms in vivo may be quite complex since we expect differences between organs and cell types.
Fig. 6. Intestinal microsomes-catalyzed glucuronidation of formononetin and biochanin A (Panel A) glucuronides by intestine (Panel B). Concentration of isoflavone used in perfusion studies was 10 μM. Adapted from Jia et al.[86].
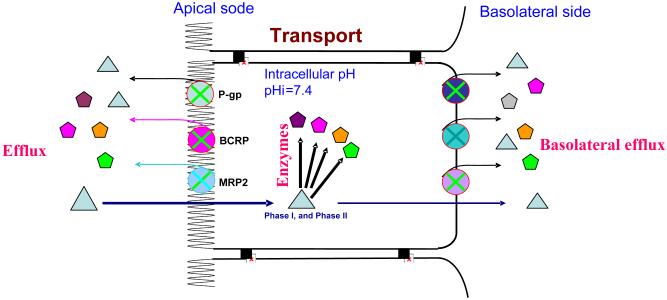
Regardless of the cell types, there may be more complex coupling processes like the one shown in Fig. 7. Based on the “revolving door” theorem, which is responsible for the transport and metabolism of polyphenols and other drugs and xenobiotics, many coupling pairs may function to elicit best outcomes for the cell type. In the depicted coupling network, the important players are at least three enzymes such as UGTs, SULTs and CYPs that metabolize the parent compound, and more than three transporters such as efflux of p-glycoprotein, MRPs (multidrug resistant proteins) and BCRP (breast cancer resistant protein), and influx of MRP3 and OATPs that excrete or pump the metabolites. In a complex network of coupled transporters and enzymes, the parent compound becomes metabolites through the action of various players. If we were to succeed in increasing bioavailability, we need to take the approach of “collapsing” the network. We should abandon the classical method of pinpointing a “player” (e.g., an enzyme) and then inhibit that player to achieve higher bioavailability. We should instead focus on the most efficient disruption of the network in order to improve bioavailability of natural polyphenols. Prompting us to be more sophisticated in designing our kinetic studies, the coupling concept may be invaluable to improve the oral bioavailability of polyphenols, desirable drugs, nutrients and phytochemicals, but to limit the oral bioavailability of dietary carcinogens.
Fig. 7.
Schematic representation of the more complex coupling theories. Substrates are represented by triangles whereas products of enzymes or metabolites are represented by other shapes. In this scheme, substances such drugs and nutrients will get in by passive diffusion or carried by transporter. The intake process at the apical side is often called uptake, whereas intake at the basolateral side is often a part of secretion. In contrast to intake, substances excreted by transporters out of the cells is called efflux. These efflux transporters can locate at either sides (apical or basolateral side). Some efflux transporters such as p-gp, MRP2, MRP4, and BCRP are generally present on the apical surface of the cells, whereas others such as MRP3, MRP5, MRP6 are localized on the basolateral of the cell. Some efflux transporters such as MRP1 and OATs are present on both apical and basolateral surfaces. Efflux transporters located at the apical side diminish absorption into the blood and facilitate excretion, whereas efflux transporters at the basolateral sides have the opposite functions.
This “Revolving Door” coupling theorem is different from the “Double Jeopardy” coupling theorem, the latter is similar to the interactive coupling theorem originally proposed by Benet et al [100]. The “Double Jeopardy” coupling theorem describes a drug efflux-metabolism alliance between an efflux transporter such as p-glycoprotein and a phase I metabolic enzyme such as CYP3A4. The process is typified by the disposition of a chemical that is the substrate of both p-glycoprotein and CYP3A4. When the chemical interacts with the enzyme when it is taken up by the enterocytes for the first time, it is subjected to metabolism (prosecution) once. If the chemical is subsequently effluxed back to where it started (e.g., intestinal lumen), then it did not escape the enzyme “prosecution”. Rather, it could be subjected to repeated exposure to the same enzyme, when it is taken up again later in lower portion of the intestine. This coupling process may result in higher extent of metabolism [99, 100]. Normally, the “Double Jeopardy” theorem involves efflux of substrate at only the apical membrane of the intestine, which is less complex than the “revolving door” theory, which could involve efflux transporter(s) at one (i.e., apical or basolateral) or both membranes. Generally, both coupling processes share the same results: higher extent of metabolism and increased potential for metabolism-based drug-drug interactions. In addition, it is difficult to sort out which player (enzyme or transporter or both) is involved clinically in the drug interactions.
10. Consequence of Enzyme-Efflux Transporter Coupling
Efficient coupling of the conjugating enzymes and efflux process in organs such as liver and intestine enables the enterohepatic and enteric recycling of polyphenols, is expected to have significant impact on the bioavailability and apparent half-life of the polyphenols. Because intestine, liver, and kidney are the major organs that play key roles in the disposition of polyphenols through phase II conjugation and subsequent elimination, we will discuss the consequence of the coupling process in intestine and liver. Interested reader should consult recent review about kidney coupling process [110].
In the intestine, after oral administration, polyphenol is absorbed into intestinal cells by passive diffusion or transporter-mediated processes. The absorbed polyphenols undergo phase II metabolism by the intestinal enzymes. In human intestine, absorbed polyphenols are subjected to intestinal conjugation to produce hydrophilic metabolites by either soluble cytosolic (e.g., SULT) or membrane bound (e.g., UGT) phase II enzymes. Once metabolized to hydrophilic metabolites, these hydrophilic conjugates have to be transported out of cells by transporter(s) that are localized either apically or basolaterally since they lose their ability to diffuse across the cells via passive diffusion. The well-known efflux transporters in the intestine mostly belong to the ABC transporter family [109]. High levels of MRP2 were expressed in the rat duodenum and jejunum and low levels in the rat ileum and colon [111]. The coupling of efflux transporters and metabolites enzyme in the intestine (e.g., coupling of CYP3A4 and p-glycoprotein and coupling of phase II enzymes and MRP and/or OAT), can affect the bioavailability of polyphenols and drugs as described by Jeong et al [99].
Polyphenols and their conjugates that are transported to liver may be subjected to additional metabolism or excretion via the bile. Liver is the main and central organ for xenobiotic disposition, especially for metabolism, and possess a variety of transporters and metabolic enzymes that are capable of rapid clearing of xenobiotics from the body [112, 113]. These transporters in the liver usually have very high capacity and can excrete flavonoids metabolites and biliary concentration can reach several mM in mice in an intestinal perfusion model [114]. The efficient biliary excretion is enabled by apical efflux transporters located at the bile cannicular membrane. These transporters in the apical side of the liver include MDR1, MDR3, MRP2, organic anion transport peptides (OATP)-A, OATP-B, OATP-C, sodium taurocholate cotransporting protein (NTCP), and human prostagrandin transporter (h-PGT). On the contrary, transporters localized to the basolateral side of the hepatocytes (MRP1 and MRP3) are involved in the efflux from hepatocytes to the blood [115]. Coupling of apical transporters and metabolic enzymes in the liver enables the enterohepatic recycling of polyphenols and drugs, because conjugated metabolites that are excreted via the bile may enter the large intestine, get hydrolyzed (by microflora), and return as absorbed aglycones from the large intestine. Our investigation has demonstrated that the disposition of flavonoids such as genistein and apigenin in the intestine may be more important than in the liver. In addition, in conjunction with enterohepatic recycling, enteric recycling may increase the apparent half-lives, which explain why flavonoids have poor systemic bioavailabilities but stay in plasma for an extended period of time (i.e., hours) [104].
Taken together, the coupling of efflux transporters and conjugating enzymes are expected to have significant impact on the bioavailability and apparent half-life of the polyphenols. This is because intestinal coupling can result in less systemic exposure to the parent compound and conjugates excreted by the intestinal cells are gradual and continuous and unlikely to produce the “double peak” phenomenon associated with the enterohepatic recycling. On the other hand, hepatic coupling has high capacity, and can render efficient enterohepatic recycling. We have proposed that repeated shuffling of flavonoids through a duo recycling scheme involving both enteric and enterohepatic recycling is the reason for extensive metabolism of flavonoids such as apigenin. They may also be the reason why polyphenols typically have decent half-lives even though they have very poor bioavailability.
11. Conclusions
Polyphenols refer to one of numerous and widely distributed group of molecules in the plant kingdom. Polyphenols have gained increasing interest in scientific research and in public media. Some polyphenols, especially flavonoids that are abundant present in vegetables, fruit, tea, flowers, wine, and soybean, have been associated with numerous beneficial biological effects including free-radical scavenging, enhancement of detoxifying enzymatic activities, and inhibition of cellular proliferation [16-21]. However, the development of polyphenols into successful chemopreventive agents is highly dependent on both their respective intakes and their bioavailability, which varied widely among polyphenols and between subjects. Mastering their disposition behavior so we can increase their bioavailability holds one of the keys to their further development.
Even though scientific researches have emphasized on studying parent polyphenols, recent studies indicate that some polyphenol metabolites may accumulate in certain target tissues rather than just equilibrate between blood and plasma [116-119]. Whether these are active metabolites, have not been carefully studies and need more of our attention since they are present at much higher concentration in vivo. In addition, very little is known about the biological properties of the conjugated derivatives present in plasma or tissues because of the lack of precise identification and commercial standards [80].
12. Expert Opinion
Over the past 10-year, researchers, and food and dietary supplement marketers have become increasingly interested in polyphenols. In particular, the flavonoids are becoming popular in our diet and food. The chief reason for this interest is the recognition of the antioxidant and chemopreventive properties of polyphenols, their probable role in the prevention of various diseases associated with oxidative stress such as cancer, cardiovascular and neurodegenerative diseases. However, a major challenge associated with the further development of chemopreventive polyphenols is the lack of bioavailability in vivo. Two factors control the production of phase II metabolites: activities of conjugating enzymes and activities of efflux transporters, which are needed to pump out hydrophilic anionic conjugates (sulfates or glucuronides) because they are too hydrophilic to penetrate the cellular membrane by passive diffusion. Recent investigations by various investigators including ourselves showed that MRP2 are important for the biliary excretion of these metabolites, whereas BCRP and MRP2 are more important in their intestinal excretion. These investigations have shown that the conjugating enzymes and efflux transporters are often coupled to achieve the optimal efficiency. In this coupling scheme, efflux transporters appear to serve one of two functions: (1) as the gate keeper when there is an excess capacity to metabolize the compounds, or (2) as the facilitator of metabolism when there is a product inhibition. Physiological functions of this coupling scheme remains to be fully elucidated, but it is known that it enables a duo recycling scheme, which are referred to as enteric recycling and enterohepatic recycling.
In enterohepatic recycling, glucuronidated and sulfated metabolites are excreted via bile, and then reach colon where they are reconverted to aglycones and reabsorbed into the blood, completing the recycling scheme. In enteric recycling, the same phase II metabolites are excreted in the intestine, including colon, with resulting conjugates undergo the same reconversion process as in the enterohepatic recycling. Because of is duo recycling scheme, polyphenols undergo repeated recycling through the digestive system, which afford them apparent plasma half-life much longer than would be predicted from their intrinsic clearance value. This long apparent half-life may facilitates the pharmacological functions of polyphenols; since conjugated polyphenols are still good antioxidants and target tissues often possess hydrolases (e.g., sulfatase) that can reconvert conjugates back to active aglycones.
Assuming aglycones are active, as shown by the vast majority of in vitro data, increasing bioavailability so that the bioavailability of polyphenols are more consistent (not necessarily higher but often time is higher) will be critical to their development into drugs. This is because large differences in bioavailability translate into large differences in drug exposure, thereby needing an even larger sample size to show statistical effects.
Lastly, our increasing understanding of polyphenol bioavailability could finally allow us to correlate total polyphenol intakes with one or several accurate measures of bioavailability (such as concentrations of key bioactive metabolites in plasma and tissues) and with their potential health-benefit effects. Knowledge of these correlations could further entice more scientists to this field, despite the difficulties with their moderate to low bioactivities, variable bioavailabilities due to high interpersonal variability in key metabolic processes, and lack of strong intellectual property protection. This is because conventional Western medicine, with its emphasis on cause and molecular mechanisms of diseases, is not very apt to prevent degenerative diseases from occurring.
In summary, coupling of efflux transporter and metabolic enzymes controls the disposition of natural polyphenols in vivo and enables duo recycling scheme that prolongs the half-life of polyphenols in vivo. In the coupling processes, the complex network of players is composed of conjugating enzymes and transporters. How a polyphenolic compound interacts with the network, not just one of its players, will determine how it is deposited in vivo. We should emphasis more on study of the metabolites of polyphenols and on studying how the coupled metabolic pathways can be manipulated to enhance their bioavailabilities.
Acknowledgements
ZQL is supported by the Faculty Research Grant of Hong Kong Baptist University, Hong Kong (FRG/0607/II-35). MH is supported by CA87779 and GM070737 from The National Institutes of Health of United States.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.MEYER UA. Overview of enzymes of drug metabolism. J. Pharmacokinet. Biopharm. 1996;24(5):449–459. doi: 10.1007/BF02353473. [DOI] [PubMed] [Google Scholar]
- 2.WANG H, LECLUYSE EL. Role of orphan nuclear receptors in the regulation of drug-metabolizing enzymes. Clin. Pharmacokinet. 2003;42(15):1331–13357. doi: 10.2165/00003088-200342150-00003. [DOI] [PubMed] [Google Scholar]
- 3.BANOGLU E. Current status of the cytosolic sulfotransferases in the metabolic activation of promutagens and procarcinogens. Curr. Drug Metab. 2000;1(1):1–30. doi: 10.2174/1389200003339234. [DOI] [PubMed] [Google Scholar]
- 4.INNOCENTI F, GRIMSLEY C, DAS S, et al. Haplotype structure of the UDP-glucuronosyltransferases 1A1 promoter in different ethnic groups. Pharmacogenetics. 2002;12:725–733. doi: 10.1097/00008571-200212000-00006. [DOI] [PubMed] [Google Scholar]
- 5.MANDLEKAR S, HONG JL, KONG AN. Modulation of metabolic enzymes by dietary phytochemicals: a review of mechanisms underlying beneficial versus unfavorable effects. Curr. Drug Metab. 2006;7(6):661–75. doi: 10.2174/138920006778017795. [DOI] [PubMed] [Google Scholar]
- 6.BRINKMANN U, EICHELBAUM M. Polymorphisms in the ABC transporter gene MDR1. Pharmacogenomics. J. 2001;1:59–64. doi: 10.1038/sj.tpj.6500001. [DOI] [PubMed] [Google Scholar]
- 7.MARZOLINI C, TIRONA RG, KIM RB. Pharmacogenomics of the OATP and OAT families. Pharmacogenomics. 2004;5(3):273–82. doi: 10.1517/phgs.5.3.273.29831. [DOI] [PubMed] [Google Scholar]
- 8.KERB R, HOFFMEYER S, BRINKMANN U. ABC drug transporters: hereditary polymorphisms and pharmacological impact in MDR1, MRP1 and MRP2. Pharmacogenomics. 2001;2(1):51–64. doi: 10.1517/14622416.2.1.51. [DOI] [PubMed] [Google Scholar]
- 9.MIZUNO N, NIWA T, YOTSUMOTO Y, et al. Impact of drug transporter studies on drug discovery and development. Pharmcol. Rev. 2003;55:425–461. doi: 10.1124/pr.55.3.1. [DOI] [PubMed] [Google Scholar]
- 10.HARNLY JM, DOHERTY RF, BEECHER GR, et al. Flavonoid content of U.S. Fruits, vegetables, and nuts. J. Agric. Food Chem. 2006;54(26):9966–9977. doi: 10.1021/jf061478a.•• This report analyzes the 20 flavonoids (as aglycones) determined for more than 60 fresh fruits, vegetables, and nut collected from four regions across the United States.
- 11.FRESCO P, BORGES F, DINIZ C, et al. New insights on the anticancer properties of dietary polyphenols. Med. Res Rev. 2006;26(6):747–766. doi: 10.1002/med.20060.• An excellent review on the anticancer mechanism of dietary polyphenols. The review summarizes the most recent advances providing new insights into the molecular mechanisms underlying the promising anticarcinogenic activity of dietary polyphenols.
- 12.RAMASSAMY C. Emerging role of polyphenolic compounds in the treatment of neurodegenerative diseases: a review of their intracellular targets. Eur. J. Pharmacol. 2006;545(1):51–64. doi: 10.1016/j.ejphar.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 13.CASSIDY A. Factors affecting the bioavailability of soy isoflavones in humans. J AOAC Int. 2006;89(4):1182–1188. [PubMed] [Google Scholar]
- 14.WHITSETT TG, JR, LAMARTINIERE CA. Genistein and resveratrol: mammary cancer chemoprevention and mechanisms of action in the rat. Expert Rev. Anticancer Ther. 2006;6(12):1699–1706. doi: 10.1586/14737140.6.12.1699. [DOI] [PubMed] [Google Scholar]
- 15.SHARMA RA, GESCHER AJ, STEWARD WP. Curcumin: the story so far. Eur. J. Cancer. 2005;41(13):1955–1968. doi: 10.1016/j.ejca.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 16.CABRERA C, ARTACHO R, GIMENEZ R. Beneficial effects of green tea- a review. J. Am. Coll. Nutr. 2006;25(2):79–99. doi: 10.1080/07315724.2006.10719518.•• This review gives a good review on beneficial effects of polyphenols in Green tea, a traditional beverage in eastern countries such China, Japan and Korea.
- 17.ADLERCREUTZ H, HONJO H, HIGASHI A, et al. Urinary excretion of lignans and isoflavonoid phytoestrogens in Japanese men and women consuming a traditional Japanese diet. Am. J. Clin. Nutr. 1991;54(6):1093–1100. doi: 10.1093/ajcn/54.6.1093. [DOI] [PubMed] [Google Scholar]
- 18.BOUKER KB, HILAKIVI-CLARKE L. Genistein: does it prevent or promote breast cancer? Environ. Health Perspect. 2000;108(8):701–708. doi: 10.1289/ehp.00108701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.SCHAFFER S, ECKERT GP, SCHMITT-SCHILLIG S, et al. Plant foods and brain aging: a critical appraisal. Forum Nutr. 2006;59:86–115. doi: 10.1159/000095209. [DOI] [PubMed] [Google Scholar]
- 20.BREINHOLT V, HOSSAINI A, SVENDSEN GW, et al. Estrogenic activity of flavonoids in mice. The importance of estrogen receptor distribution, metabolism and bioavailability. Food Chem. Toxicol. 2000;38(7):555–564. doi: 10.1016/s0278-6915(00)00046-6. [DOI] [PubMed] [Google Scholar]
- 21.BENGMARK S. Impact of nutrition on aging and disease. Curr. Opin. Clin. Nutr. Metab. Care. 2006;9(1):2–7. doi: 10.1097/01.mco.0000171129.29278.26. [DOI] [PubMed] [Google Scholar]
- 22.WILLIAMSON G, MANACH C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutri. 2005;81(1 suppl):243S–255S. doi: 10.1093/ajcn/81.1.243S.• The review discusses current understanding of bioavailabilty and bioefficacy of polyphenols in human basing on 93 intervention studies.
- 23.MANACH C, WILLIAMSON G, MORAND C, et al. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005;81(1 suppl):230S–242S. doi: 10.1093/ajcn/81.1.230S.• The review discusses current understanding of bioavailabilty and bioefficacy of polyphenols in human basing on 97 bioavailability studies.
- 24.RAPAKA RS, COATES PM. Dietary supplements and related products: a brief summary. Life Sci. 2006;78(18):2026–2032. doi: 10.1016/j.lfs.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 25.SCALBERT A, WILLIAMSON G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000;130(8S Suppl):2073S–2085S. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- 26.DAY AJ, Williamson G. Biomarkers for exposure to dietary flavonoids: a review of the current evidence for identification of quercetin glycosides in plasma. Br. J. Nutr. 2001;86(1 Suppl):S105–110. doi: 10.1079/bjn2001342. [DOI] [PubMed] [Google Scholar]
- 27.HOLLMAN PC, VAN TRIJP JM, BUYSMAN MN, et al. Relative bioavailability of the antioxidant flavonoid quercetin from various foods in man. FEBS Lett. 1997;418(12):152–156. doi: 10.1016/s0014-5793(97)01367-7. [DOI] [PubMed] [Google Scholar]
- 28.DONOVAN JL, BELL JR, KASIM-KARAKAS S, et al. Catechin is present as metabolites in human plasma after consumption of red wine. J. Nutr. 1999;129(9):1662–1668. doi: 10.1093/jn/129.9.1662. [DOI] [PubMed] [Google Scholar]
- 29.SETCHELL KD, BROWN NM, DESAI P, et al. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J. Nutr. 2001;131(4 Suppl):1362S–1375S. doi: 10.1093/jn/131.4.1362S. [DOI] [PubMed] [Google Scholar]
- 30.MANDEL LJ. Primary active sodium transport, oxygen consumption, and ATP: coupling and regulation. Kidney Int. 1986;29(1):3–9. doi: 10.1038/ki.1986.2. [DOI] [PubMed] [Google Scholar]
- 31.WU X, CAO G, PRIOR RL. Absorption and metabolism of anthocyanins in elderly women after consumption of elderberry or blueberry. J Nutr. 2002;132(7):1865–71. doi: 10.1093/jn/132.7.1865. [DOI] [PubMed] [Google Scholar]
- 32.PASSAMONTI S, VRHOVSEK U, VANZO A, et al. The stomach as a site for anthocyanins absorption from food. FEBS Lett. 2003;544:210–213. doi: 10.1016/s0014-5793(03)00504-0. [DOI] [PubMed] [Google Scholar]
- 33.GUGLER R, LESCHIK M, DENGLER HJ. Disposition of quercetin in man after single oral and intravenous doses. Eur. J. Clin. Pharmacol. 1975;9(23):229–234. doi: 10.1007/BF00614022. [DOI] [PubMed] [Google Scholar]
- 34.HOLLMAN PC, DE VRESJH VAN LEEUWEN SD, et al. Absorption of dietary quercetin glycosides and quercetin in healthy ileostomy volunteers. Am. J. Clin. Nutr. 1995;62(6):1276–1282. doi: 10.1093/ajcn/62.6.1276. [DOI] [PubMed] [Google Scholar]
- 35.ERLUND I, KOSONEN T, ALFTHAN G, et al. Pharmacokinetics of quercetin from quercetin aglycone and rutin in healthy volunteers. Eur. J. Clin. Pharmacol. 2000;56(8):545–53. doi: 10.1007/s002280000197. [DOI] [PubMed] [Google Scholar]
- 36.GRAEFE EU, QITTING J, MUELLUER S, et al. Pharmacokinetics and bioavailability of quercetin aglycone and rutin in humans. J. Clin. Pharmacol. 2001;41:492–499. doi: 10.1177/00912700122010366. [DOI] [PubMed] [Google Scholar]
- 37.DAY AJ, MELLON F, BARRON D, et al. Human metabolism of dietary flavonoids: identification of plasma metabolites of qucercetin. Free Radic Res. 2001;35(6):941–942. doi: 10.1080/10715760100301441. [DOI] [PubMed] [Google Scholar]
- 38.TSUDA T, HORIO F, OSAWA T. Absorption and metabolism of cyanidin 3-O-β-D-glucoside in rats. FEBS Lett. 1999;449:179–182. doi: 10.1016/s0014-5793(99)00407-x. [DOI] [PubMed] [Google Scholar]
- 39.BUGIANESI R, CATASTA G, SPIGNO P, et al. Naringenin from cooked tomato paste is bioavailable in men. J. Nutr. 2002;132(11):3349–3352. doi: 10.1093/jn/132.11.3349. [DOI] [PubMed] [Google Scholar]
- 40.MANACH C, MORAND C, GIL-IZQUIERDO A, et al. Bioavailability in humans of the flavanones hesperidin and narirutin after the ingestion of two doses of orange juice. Eur. J. Clin. Nutr. 2003;57(2):235–242. doi: 10.1038/sj.ejcn.1601547. [DOI] [PubMed] [Google Scholar]
- 41.ERLUND I, SILASTE ML, ALFTHAN G, et al. Plasma concentrations of the flavonoids hesperetin, naringenin and quercetin in human subjects following their habitual diets, and diets high or low in fruit and vegetables. Eur J Clin Nutr. 2002;56(9):891–8. doi: 10.1038/sj.ejcn.1601409. [DOI] [PubMed] [Google Scholar]
- 42.IZUMI T, PISKULA MK, OSAWA S, et al. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J. Nutr. 2000;130(7):1695–1699. doi: 10.1093/jn/130.7.1695. [DOI] [PubMed] [Google Scholar]
- 43.SETCHELL KD, BROWN NM, DESAI P, et al. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J. Nutr. 2001;131(4 Suppl):1362S–1375S. doi: 10.1093/jn/131.4.1362S. [DOI] [PubMed] [Google Scholar]
- 44.ZUBIK L, MEYDANI M. Bioavailability of soybean isoflavone from aglycone and glucoside forms in American women. Am. J. Clin. Nutr. 2003;77(6):1459–1465. doi: 10.1093/ajcn/77.6.1459. [DOI] [PubMed] [Google Scholar]
- 45.SETCHELL KD, BROWN NM, LYDEKING-OLSEN E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J. Nutr. 2002;132(12):3577–3584. doi: 10.1093/jn/132.12.3577. [DOI] [PubMed] [Google Scholar]
- 46.SETCHELL KD, BROWN NM, ZIMMER-NECHEMIAS L, et al. Evidence for lack of absorption of soy isoflavone glycosides in humans, supporting the crucial role of intestinal metabolism for bioavailability. Am. J. Clin. Nutr. 2002;76(2):447–453. doi: 10.1093/ajcn/76.2.447. [DOI] [PubMed] [Google Scholar]
- 47.DOERGE DR, CHANG HC, CHURCHWELL MI, et al. Analysis of soy isoflavone conjugation in vitro and in human blood using liquid chromatography-mass spectrometry. Drug. Metab. Dispos. 2000;28(3):298–307. [PubMed] [Google Scholar]
- 48.SHELNUTT SR, CIMINO CO, WIGGINS PA, et al. Pharmacokinetics of the glucuronide and sulfate conjugates of genistein and daidzein in men and women after consumption of a soy beverage. Am. J. Clin. Nutr. 2002;76(3):588–594. doi: 10.1093/ajcn/76.3.588. [DOI] [PubMed] [Google Scholar]
- 49.CLARKE DB, LLOYD AS, BOTTING NP, et al. Measurement of intact sulfate and glucuronide phytoestrogen conjugates in human urine using isotope dilution liquid chromatography-tandem mass spectrometry with [13C(3)]isoflavone internal standards. Anal. Biochem. 2002;309(1):158–172. doi: 10.1016/s0003-2697(02)00275-0. [DOI] [PubMed] [Google Scholar]
- 50.ERLUND I, MERIRINNE E, ALFTHAN G, et al. Plasma kinetics and urinary excretion of the flavanones naringenin and hesperetin in humans after ingestion of orange juice and grapefruit juice. J Nutr. 2001;131(2):235–41. doi: 10.1093/jn/131.2.235. [DOI] [PubMed] [Google Scholar]
- 51.ROUPE KA, YANEZ JA, TENG XW. Pharmacokinetics of selected stilbenes: rhapontigenin, piceatannol and pinosylvin in rats. J Pharm Pharmacol. 2006;58(11):1443–50. doi: 10.1211/jpp.58.11.0004. [DOI] [PubMed] [Google Scholar]
- 52.THOMAS HR, KONG ANT. Pharmacogenomics, regulation and signaling pathways of phase I and II drug metabolizing enzymes. Curr. Drug Metab. 2002;3(5):481–490. doi: 10.2174/1389200023337171.•• An excellent review on current knowledge on DNA microarray technology in gene expression profiling and the transduction events elicited by various xenobiotics mediated by either specific receptors or non-specific signal transduction pathways.
- 53.CAMPBELL TC. Nutrition and drug-metabolizing enzymes. Clin. Pharmacol. Ther. 1977;5(Pt 2):699–706. doi: 10.1002/cpt1977225part2699. [DOI] [PubMed] [Google Scholar]
- 54.CUPP MJ, TRACY TS. Cytochrome P-450: New nomenclature and clinical implications. Am Fam Physician. 1998;57(1):107–16. [PubMed] [Google Scholar]
- 55.WONG H, GRACE JJ, JR, WRIGHT MR, et al. Glucuronidation in the chimpanzee (Pan troglodytes): Studies with acetaminophen, oestradiol and morphine. Xenobiotica. 2006;36(12):1178–1190. doi: 10.1080/00498250600911028. [DOI] [PubMed] [Google Scholar]
- 56.STEENSMA A, FAASSEN-PETERS MA, NOTEBORN HP, et al. Bioavailability of genistein and its glycoside genistin as measured in the portal vein of freely moving unanesthetized rats. J. Agric. Food Chem. 2006;54(21):8006–8012. doi: 10.1021/jf060783t. [DOI] [PubMed] [Google Scholar]
- 57.XU X, WANG HJ, MURPHY PA, et al. Daidzein is a more bioavailable soymilk isoflavone than is genistein in adult women. J. Nutr. 1994;124(6):825–832. doi: 10.1093/jn/124.6.825. [DOI] [PubMed] [Google Scholar]
- 58.KING RA, BURSILL DB. Plasma and urinary kinetics of the isoflavones daidzein and genistein after a single soy meal in humans. Am. J. Clin. Nutr. 1998;67:867–872. doi: 10.1093/ajcn/67.5.867. [DOI] [PubMed] [Google Scholar]
- 59.WATANABE S, YAMAGUCHI M, SOBUE T, et al. Pharmacokinetics of soybean isoflavones in plasma, urine and feces of men after ingestion of 60 g baked soybean powder (kinako) J. Nutr. 1998;128(10):1710–1715. doi: 10.1093/jn/128.10.1710. [DOI] [PubMed] [Google Scholar]
- 60.RONIS MJ, LITTLE JM, BARONE GW, et al. Sulfation of the isoflavones genistein and daidzein in human and rat liver and gastrointestinal tract. J. Med. Food. 2006;9(3):348–355. doi: 10.1089/jmf.2006.9.348. [DOI] [PubMed] [Google Scholar]
- 61.GU L, HOUSE SE, PRIOR RL, et al. Metabolic phenotype of isoflavones differ among female rats, pigs, monkeys, and women. J. Nutr. 2006;136(5):1215–1221. doi: 10.1093/jn/136.5.1215. [DOI] [PubMed] [Google Scholar]
- 62.WENZEL E, SOMOZA V. Metabolism and bioavailability of trans-resveratrol. Mol. Nutr. Food Res. 2005;49(5):472–481. doi: 10.1002/mnfr.200500010. [DOI] [PubMed] [Google Scholar]
- 63.Jeong EJ, Jia X, Hu M. Disposition of formononetin via enteric recycling: metabolism and excretion in mouse intestinal perfusion and Caco-2 cell models. Mol. Pharm. 2005;2(4):319–28. doi: 10.1021/mp0498852. [DOI] [PubMed] [Google Scholar]
- 64.COOKE D, STEWARD WP, GESCHER AJ, et al. Anthocyans from fruits and vegetables--does bright colour signal cancer chemopreventive activity? Eur J Cancer. 2005;41(13):1931–40. doi: 10.1016/j.ejca.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 65.DELL’ AGLI M, BUSCIALA A, BOSISO E. Vascular effects of wine polyphenols. Cardiovasc Res. 2004;63(4):593–602. doi: 10.1016/j.cardiores.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 66.SCAlBERT A, WILLIAMSON G. Dietary intake and bioavailability of polyphenols. J Nutr. 2000;130:2073S–85S. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- 67.MANACH C, SCALBERT A, MORAND C, et al. Polyphenols: food sources and bioavailability. Am. J. Clin. Nutr. 2004;79(5):727–747. doi: 10.1093/ajcn/79.5.727.• The article gives detailed information about the sources and bioavailability of polyphenols.
- 68.THOMAS SET SC, BERRY DP, GARCEA G. Dietary polyphenolic phytochemicals--promising cancer chemopreventive agents in humans? A review of their clinical properties. Int J Cancer. 2007;120(3):451–8. doi: 10.1002/ijc.22419. [DOI] [PubMed] [Google Scholar]
- 69.MENNEN LI, WALKER R, BENNETAU-PELISSERO C, et al. Risks and safety of polyphenol consumption. Am J Clin Nutr. 2005;81(1 Suppl):326S–329S. doi: 10.1093/ajcn/81.1.326S. [DOI] [PubMed] [Google Scholar]
- 70.ZIJP IM, KORVER O, TIJBURG LBM. Effect of tea and other dietary factors on iron absorption. Crit Rev Food Sci Nutr. 2000;40:371–98. doi: 10.1080/10408690091189194. [DOI] [PubMed] [Google Scholar]
- 71.VERONESE ML, GILLEN LP, BURKE JP, et al. Exposure-dependent inhibition of intestinal and hepatic CYP3A4 in vivo by grapefruit juice. J Clin Pharmacol. 2003;43:831–9. doi: 10.1177/0091270003256059. [DOI] [PubMed] [Google Scholar]
- 72.AMEER B, WEINTRAUB RA. Drug interactions with grapefruit juice. Clin Pharmacokinet. 1997;33:103–21. doi: 10.2165/00003088-199733020-00003. [DOI] [PubMed] [Google Scholar]
- 73.WANG Q, MORRIS ME. Flavonoids modulate monocarboxylate transporter-1-mediated transport of gamma-hydroxybutyrate in vitro and in vivo. Drug Metab Dispos. 2007 Feb;35(2):201–8. doi: 10.1124/dmd.106.012369. [DOI] [PubMed] [Google Scholar]
- 74.CHEN J, LIN H, HU M. Absorption and metabolism of genistein and its isoflavone analogs in human intestinal Caco-2 cells. Cancer Chemother. Pharmacol. 2004;55(2):1591–1569. doi: 10.1007/s00280-004-0842-x. [DOI] [PubMed] [Google Scholar]
- 75.KUHNLE G, SPENCER JP, SCHROETER H, et al. Epicatechin and catechin are O-methylated and glucuronidated in the small intestine. Biochem Biophys Res Commun. 2000;277(2):507–12. doi: 10.1006/bbrc.2000.3701. [DOI] [PubMed] [Google Scholar]
- 76.LIU Y, LIU Y, DAI Y, et al. Enteric disposition and recycling of flavonoids and ginkgo flavonoids. J. Altern. Complement. Med. 2003;9:631–640. doi: 10.1089/107555303322524481. [DOI] [PubMed] [Google Scholar]
- 77.SPENCER JP, CHOWRIMOOTOO G, CHOUDHURY R, et al. The small intestine can both absorb and glucuronidate luminal flavonoids. FEBS Lett. 1999;458(2):224–230. doi: 10.1016/s0014-5793(99)01160-6. [DOI] [PubMed] [Google Scholar]
- 78.BOERSMA MG, VAN DER WOUDE H, BOGAARDS J, et al. Regioselectivity of phase II metabolism of luteolin and quercetin by UDP-glucuronosyltransferases. Chem. Res. Toxicol. 2002;15(5):662–670. doi: 10.1021/tx0101705. [DOI] [PubMed] [Google Scholar]
- 79.O’ LEARY KA, DAY AJ, NEEDS PW, et al. Metabolism of quercetin-7- and quercetin-3-glucuronides by an in vitro hepatic model: the role of human beta-glucuronidase, sulfotransferase, catechol-O-methyltransferase and multi-resistant protein 2 (MRP2) in flavonoid metabolism. Biochem Pharmacol. 2003;65(3):479–91. doi: 10.1016/s0006-2952(02)01510-1. [DOI] [PubMed] [Google Scholar]
- 80.KOSTER H, HALSEMA I, SCHOLTENS E, et al. Dose-dependent shifts in the sulfation and glucuronidation of phenolic compounds in the rat in vivo and in isolated hepatocytes. The role of saturation of phenolsulfotransferase. Biochem Pharmacol. 1981;30(18):2569–75. doi: 10.1016/0006-2952(81)90584-0. [DOI] [PubMed] [Google Scholar]
- 81.ZHU BT, PATEL UK, CAI MX, et al. O-Methylation of tea polyphenols catalyzed by human placental cytosolic catechol-O-methyltransferase. Drug Metab. Dispos. 2000;28(9):1024–1030. [PubMed] [Google Scholar]
- 82.GOLDBERG DM, YAN J, SOLEAS GJ. Absorption of three wine-related polyphenols in three different matrices by healthy subjects. Clin Biochem. 2003;36(1):79–87. doi: 10.1016/s0009-9120(02)00397-1. [DOI] [PubMed] [Google Scholar]
- 83.WU AH, TSENG CC, VAN DEN BERG D, et al. Tea intake, COMT genotype, and breast cancer in Asian-American women. Cancer Res. 2003;63(21):7526–7529. [PubMed] [Google Scholar]
- 84.LEE WJ, SHIM JY, ZHU BT. Mechanisms for the inhibition of DNA methyltransferases by tea catechins and bioflavonoids. Mol. Pharmacol. 2005;68(4):1018–1030. doi: 10.1124/mol.104.008367. [DOI] [PubMed] [Google Scholar]
- 85.NAKAJIMA M, ITOH M, YAMANAKA H, et al. Isoflavones inhibit nicotine C-oxidation catalyzed by human CYP2A6. J. Clin. Pharmacol. 2006;46(3):337–644. doi: 10.1177/0091270005285199. [DOI] [PubMed] [Google Scholar]
- 86.ROBERTS DW, DOERGE DR, CHURCHWELL MI, et al. Inhibition of extrahepatic human cytochromes P450 1A1 and 1B1 by metabolism of isoflavones found in Trifolium pratense (red clover) J. Agric. Food Chem. 2004;52(21):6623–6632. doi: 10.1021/jf049418x. [DOI] [PubMed] [Google Scholar]
- 87.TOLLESON WH, DOERGE DR, CHURCHWELL MI, et al. Metabolism of biochanin A and formononetin by human liver microsomes in vitro. J. Agric. Food Chem. 2002;50(17):4783–4790. doi: 10.1021/jf025549r. [DOI] [PubMed] [Google Scholar]
- 88.PAL D, MITRA AK. MDR- and CYP3A4-mediated drug-herbal interactions. Life Sci. 2006;78(18):2131–2145. doi: 10.1016/j.lfs.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 89.MOON YJ, WANG X, MORRIS ME. Dietary flavonoids: effects on xenobiotic and carcinogen metabolism. Toxicol. In Vitro. 2006;20(2):187–210. doi: 10.1016/j.tiv.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 90.JEONG EJ, LIU X, JIA X, et al. Coupling of conjugating enzymes and efflux transporters : impact on bioavailability and drug interactions. Curr. Drug Metab. 2005;6(5):455–468. doi: 10.2174/138920005774330657.• An excellent review on the working mechanism of coupling of conjugating enzymes and efflux transporters.
- 91.DAY AJ, CANADA FJ, DIAZ JC, et al. Dietary flavonoids and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase. FEBS Lett. 2000;468(23):166–170. doi: 10.1016/s0014-5793(00)01211-4. [DOI] [PubMed] [Google Scholar]
- 92.SESINK AL, ARTS IC, FAASSEN-PETERS M, et al. Intestinal uptake of quercetin-3-glucoside in rats involves hydrolysis by lactase phlorizin hydrolase. J. Nutr. 2003;133(3):773–776. doi: 10.1093/jn/133.3.773. [DOI] [PubMed] [Google Scholar]
- 93.MIKSITS M, MAIER-SALAMON A, AUST S, et al. Sulfation of resveratrol in human liver: evidence of a major role for the sulfotransferases SULT1A1 and SULT1E1. Xenobiotica. 2005;35(12):1101–19. doi: 10.1080/00498250500354253. [DOI] [PubMed] [Google Scholar]
- 94.ATKINSON C, FRANKENFELD CL, LAMPE JW. Gut bacterial metabolism of the soy isoflavone daidzein: exploring the relevance to human health. Exp. Biol. Med. 2005;230(3):155–70. doi: 10.1177/153537020523000302. [DOI] [PubMed] [Google Scholar]
- 95.WANG SW, CHEN J, JIA X, et al. Disposition of flavonoids via enteric recycling: structural effects and lack of correlations between in vitro and in situ metabolic properties. Drug Metab. Dispos. 2006;34(11):1837–1848. doi: 10.1124/dmd.106.009910. [DOI] [PubMed] [Google Scholar]
- 96.SETCHELL KD, FAUGHNAN MS, AVADES T, et al. Comparing the pharmacokinetics of daidzein and genistein with the use of 13C-labled tracers in premenopausal women. Am. J. Clin. Nutr. 2003;77(2):411–419. doi: 10.1093/ajcn/77.2.411. [DOI] [PubMed] [Google Scholar]
- 97.COLDHAM NG, SAUER MJ. Pharmacokinetics of [14C] genistein in the rat: gender-related differences, potential mechanisms of biological action, and implications for human health. Toxicol. Appl. Pharmacol. 2000;164(2):206–215. doi: 10.1006/taap.2000.8902. [DOI] [PubMed] [Google Scholar]
- 98.CRESPY V, MORAND C, BESSON C, et al. Comparison of the intestinal absorption of quercetin, phloretin and their glucosides in rats. J. Nutr. 2001;131(8):2109–2114. doi: 10.1093/jn/131.8.2109. [DOI] [PubMed] [Google Scholar]
- 99.CUMMINS CL, JACOBSEN W, BENET LZ. Unmasking the dynamic interplay between intestinal P-glycoprotein and CYP3A4. Pharmacol. Exp. Ther. 2002;300(3):1036–1045. doi: 10.1124/jpet.300.3.1036. [DOI] [PubMed] [Google Scholar]
- 100.BENET LZ, CUMMINS CL. The drug-metabolism alliance: biochemical aspects. Adv. Drug Deliv. Rev. 2001;50(1 Suppl):S3–S11. doi: 10.1016/s0169-409x(01)00178-8.•• The article discusses the rationale of “double jeopardy” coupling theory described a drug efflux-metabolism alliance process between p-glycoprotein and CYP3A4.
- 101.TIRONA RG, LEALE BF, WOLKOFF AW, et al. Human organic anion transporting polypeptide-C (SLC21A6) is a major determinant of rifampin-mediated pregnane X receptor activation. J. Pharmacol. Exp. Ther. 2003;304(1):223–228. doi: 10.1124/jpet.102.043026. [DOI] [PubMed] [Google Scholar]
- 102.LIU Y, HU M. P-glycoprotein and bioavailability-implication of polymorphism. Clin. Chem. Lab Med. 2000;38(9):877–881. doi: 10.1515/CCLM.2000.127. [DOI] [PubMed] [Google Scholar]
- 103.JIA X, CHEN J, LIN H, et al. Disposition of flavonoids via enteric recycling: enzyme-transporter coupling affects metabolism of biochanin A and formononetin and excretion of their phase II conjugates. J. Pharmacol. Exp. Ther. 2004;310(3):1103–1113. doi: 10.1124/jpet.104.068403.• Detailed study demonstrating the disposition of flavonoids in the polyphenolic product via recycling and enteric recycling.
- 104.CHEN J, LIN H, HU M. Metabolism of flavonoids via enteric recycling: role of intestinal disposition. J. Pharmacol. Exp. Ther. 2003;304(3):1228–1235. doi: 10.1124/jpet.102.046409. [DOI] [PubMed] [Google Scholar]
- 105.KELLETT GL, BROT-LAROCHE E. Apical GLUT2: a major pathway of intestinal sugar absorption. Diabetes. 2005;54(10):3056–62. doi: 10.2337/diabetes.54.10.3056. [DOI] [PubMed] [Google Scholar]
- 106.Hossain SJ, Kato H, Aoshima H, et al. Polyphenol-induced inhibition of the response of na(+)/glucose cotransporter expressed in Xenopus oocytes. J Agric Food Chem. 2002;50(18):5215–9. doi: 10.1021/jf020252e. [DOI] [PubMed] [Google Scholar]
- 107.ZHANG Y, HENDRICH S, MURPHY PA, et al. Glucuronides are the main isoflavone metabolites in women. J Nutr. 2003;133(2):399–404. doi: 10.1093/jn/133.2.399. [DOI] [PubMed] [Google Scholar]
- 108.JAN X, CHEN J, LIN H, et al. Disposition of flavonoids via enteric recycling: metabolism and excretion of two red clover isoflavones biochanin A and formononetin. J. Pharmacol. Exp. Ther. 2004;310(3):1103–1113. doi: 10.1124/jpet.104.068403. [DOI] [PubMed] [Google Scholar]
- 109.HU M, CHEN J, LIN H. Disposition of flavonoids via recycling: mechanistic studies of disposition of apigenin in the Caco-2 cell culture model. Pharmacol. Exp. Ther. 2003;307(1):314–321. doi: 10.1124/jpet.103.053496. [DOI] [PubMed] [Google Scholar]
- 110.YEH CT, YEN GC. Effect of vegetables on human phenolsulfotransferases in relation to their antioxidant activity and total phenolics. Free Radic. Res. 2005;39(8):893–904. doi: 10.1080/10715760500150424. [DOI] [PubMed] [Google Scholar]
- 111.GOTOH Y, SUZUKI H, KINOSHITA S, et al. Involvement of an orgainic anion transporter (canalicular multispecific organic anion transporter/multidrug resistance-associated protein 2) in gastrointestinal secretion of glutathione conjugates in rats. J. Pharmacol. Exp. Ther. 2000;292(1):433–439. [PubMed] [Google Scholar]
- 112.LIU Y, HU M. Absorption and metabolism of flavonoids in the Caco-2 cell culture model and a perfused rat intestinal model. Drug Metab. Dispos. 2002;30(4):370–377. doi: 10.1124/dmd.30.4.370. [DOI] [PubMed] [Google Scholar]
- 113.CHAN LM, LOWES S, HIRST BH. The ABCs of drug transport of drugs and nutrients: genomics of membrane transporters using expression microarrays. Eur. J. Pharm. Sci. 2004;21(1):25–51. doi: 10.1016/s0928-0987(03)00169-6. [DOI] [PubMed] [Google Scholar]
- 114.CHEN J, WANG S, JIA X, et al. Disposition of flavonoids via recycling: comparison of intestinal versus hepatic disposition. Drug Metab Dispos. 2005;33(12):1777–84. doi: 10.1124/dmd.105.003673.• Detailed study demonstrating the disposition of flavonoids via enterohepatic recycling and enteric recycling.
- 115.FABER KN, MILLER M, JANSEN PL, et al. Drug efflux transporters in the liver. Adv. Drug Deliv. Rev. 2003;55(1):107–124. doi: 10.1016/s0169-409x(02)00173-4. [DOI] [PubMed] [Google Scholar]
- 116.WALLE T, OTAKE Y, BRUBAKER JA, et al. Disposition and metabolism of the flavonoid chrysin in normal volunteers. Br. J. Clin. Pharmacol. 2001;51(2):143–146. doi: 10.1111/j.1365-2125.2001.01317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.UENO I, NAKANO N, HIRONO I. Metabolic fate of [14C] quercetin in the ACI rat. Jpn J. Exp.Med. 1983;53(1):41–50. [PubMed] [Google Scholar]
- 118.SUGANUMA M, OKABE S, ONIYAMA M, et al. Wide distribution of [3H](-)-epigallocatechin gallate, a cancer preventive tea polyphenol, in mouse tissue. Carcinogenesis. 1998;19(10):1771–1176. doi: 10.1093/carcin/19.10.1771. [DOI] [PubMed] [Google Scholar]
- 119.MULLEN W, GRAF BA, CALDWELL ST, et al. Determination of flavonol metabolites in plasma and tissue of rats by HPLC-radiocounting and tandem mass spectrometry following oral ingestion of [2-(14)C] quercetin-4′-glucoside. J. Agric. Food Chem. 2002;50(23):6902–6909. doi: 10.1021/jf020598p. [DOI] [PubMed] [Google Scholar]