Abstract
The cholinergic system is important for learning, memory and responses to novel stimuli. Exposure to novel, but not familiar, tastes increases extracellular acetylcholine (ACh) levels in insular cortex (IC). To further examine whether cholinergic activation is a critical signal of taste novelty the present studies infused carbachol, a direct cholinergic agonist, into IC prior to conditioned taste aversion (CTA) training with a familiar taste. By mimicking the cholinergic activation generated by novel taste exposure, it was hypothesized that a familiar taste would be treated as “novel”, and therefore a salient target for aversion learning. As predicted, rats infused with the agonist were able to acquire CTAs to familiar saccharin. Effects of carbachol infusion on patterns of neuronal activation during CS-US pairing were assessed using Fos-like immunoreactivity (FLI). Familiar taste-illness pairing following carbachol, but not vehicle, induced significant elevations of FLI in amygdala, a region with reciprocal connections to IC that is also important for CTA learning. These results support the view that IC ACh activity provides a critical signal of taste novelty which facilitates CTA acquisition.
Keywords: acetylcholine, immunoreactivity, amygdala, insular cortex, learning and memory, Pavlovian conditioning, novelty
The cholinergic system has long been implicated in arousal and attentional processes (Sarter & Bruno, 1997), response to novelty (Giovannini, Rakovska, Benton, Pazzagli, Bianchi & Pepeu, 2001), and learning and memory (Decker & McGaugh, 1991; Dunnett, Everitt & Robbins, 1991; Everitt & Robbins, 1997), among other functions. In humans, memory deficits associated with normal aging and Alzheimer’s disease are related to degeneration of neurons in the cholinergic basal forebrain (Muir, 1997). Behavioral studies in animals have shown that destruction of the nucleus basalis magnocellularis (NBM) or its cholinergic projections to neocortex results in dramatic impairments of learning and memory for a range of tasks including delayed matching-to-position (Dunnett, 1993), passive avoidance retention (Leanza, Nilsson, Wiley & Bjorklund, 1995) and the Morris water maze (Berger-Sweeney et al., 1994). More recently, the cholinergic system has been investigated for its role in another learning paradigm, conditioned taste aversion (CTA).
Taste aversions are formed by pairing a novel taste (conditioned stimulus, CS) with administration of an illness-inducing agent (unconditioned stimulus, US) such as lithium chloride (LiCl). After this CS-US association is made, subjects will avoid consuming the CS during subsequent presentations (Bernstein, 1991; Chambers & Bernstein, 1995; Garcia, Hankins & Rusiniak, 1974). An important characteristic of CTA is that novel tastes are extremely effective CSs, while familiar tastes show considerable resistance to the development of aversions (Kalat & Rozin, 1973; Revusky & Bedarf, 1967). In their investigation of the role of the cholinergic system in taste processing, Miranda, Ramirez-Lugo and Bermúdez-Rattoni (2000) used in vivo microdialysis to measure extracellular levels of acetylcholine (ACh) in the insular cortex (IC), an area known to be involved in CTA learning, following exposure to taste stimuli. They found that novel tastes significantly elevated ACh levels, while familiar tastes did not. The more familiar a taste was (i.e., the more exposures an animal had received), the less ACh release was observed (Miranda et al., 2000). Furthermore, inactivation of the NBM (which sends strong cholinergic projections to IC) prior to presentation of a novel taste blocked the normal increase in cortical ACh release and, more importantly, impaired CTA acquisition (Miranda & Bermúdez-Rattoni, 1999).
Further evidence for the involvement of cortical ACh in CTA learning comes from pharmacological studies. Normally, exposure of rats to a novel taste is characterized by cautious consumption (taste neophobia), since the post-ingestive consequences of the taste are not yet known (Bures, 1998). If no ill effects occur, subsequent presentations of the taste will lead to increased consumption, also termed attenuation of neophobia (Domjan, 1977), as the novel taste becomes a familiar, safe taste (Rozin, 1977). However, infusion of scopolamine, a direct cholinergic antagonist, into IC prior to novel taste presentation disrupts attenuation of neophobia (i.e., prevents the novel taste from becoming familiar) and impairs CTA acquisition (Gutiérrez, Rodriguez-Ortiz, De La Cruz, Núñez-Jaramillo, & Bermúdez-Rattoni, 2003). This suggests that ACh activity in IC is critical for taste processing and for CTA acquisition. Such activity may serve as a cortical taste novelty signal, which can be blocked through pharmacological antagonism or through inactivation of major cholinergic inputs to cortex.
If ACh in IC is a critical neurochemical signal of taste novelty, mimicking this signal by infusing a cholinergic agonist should render a familiar taste “novel,” hence a salient target for aversion learning. The present studies investigated this possibility by infusing carbachol, a direct cholinergic agonist, into IC prior to CTA training with a familiar taste. As predicted, rats infused with the agonist were able to acquire CTAs to a familiar saccharin solution. Normally, CTA acquisition to a novel, but not a familiar taste is associated with increased expression of Fos-like immunoreactivity (FLI), a marker of neuronal activation, in several brain regions (Koh & Bernstein, 2005). One such region, the amygdala, is known to be involved in CTA learning (Schafe & Bernstein, 1996) and is reciprocally connected to IC (Shi & Cassell, 1998). Since increased FLI expression is seen here after novel, but not familiar, CS-US pairing (Koh & Bernstein, 2005) we investigated whether carbachol infusion into IC would also alter patterns of neuronal activation in amygdala triggered by familiar CS-US pairing. In the present study, infusions of carbachol prior to familiar CS-US pairing produced significant FLI elevations throughout amygdala, similar to patterns shown in other studies after novel CS-US pairing.
General Methods
Subjects
All procedures were approved by the University of Washington Institutional Animal Care and Use Committee. Adult male Long-Evans rats (n=22 and n=24 for Experiments 1 and 2, respectively; Charles River, Raleigh, NC) were housed individually and maintained on a 12:12 h light/dark cycle with free access to Teklad rodent chow and tap water unless otherwise specified.
Intracranial cannula implantation
One week before behavioral training, rats were anesthetized and implanted with bilateral guide cannulas (26 gauge stainless steel and plastic, Plastics One, Roanoke, VA) for delivery of drug to the IC. Coordinates for bilateral IC implantations were 1.2 mm anterior to bregma, 5.2 mm lateral to midline, and 5.6 mm ventral to the skull surface (Paxinos & Watson, 1997). The cannulas were anchored to the skull with stainless steel screws and dental cement. A 33-gauge dummy cannula was inserted into each guide cannula to prevent clogging.
Safe taste training
After recovery from surgery, rats were habituated for 2 d to a fluid-restriction schedule consisting of 30-min water access each morning and afternoon. On the third and fourth days, a 0.5% saccharin solution was substituted for water during the morning session in order to familiarize rats to the tastant. Intake of the solution was recorded on both days.
Experiment 1: Effects of Carbachol Infusion into IC on CTA Acquisition with a Familiar Taste
Ingestion of a novel taste is associated with elevated levels of ACh in IC (Miranda et al., 2000), which are blocked by TTX inactivation of the NBM (Miranda & Bermúdez-Rattoni, 1999), suggesting that ACh activity may signal taste novelty during cortical taste processing. The main purpose of this experiment was to determine if infusion of a cholinergic agonist into IC would mimic this novelty signal and enable rats to acquire a CTA to a familiar taste, effectively treating the taste as though it were novel.
Methods
CTA training and testing with familiar saccharin
Prior to conditioning, rats were administered microinfusions of either carbachol (carbamylcholine, a direct cholinergic agonist) or physiological saline (vehicle control) through a 33-gauge injector cannula connected to a Hamilton microsyringe. The injector cannula extended 1.0 mm beyond the tip of the guide cannula. During infusion, rats were freely moving in a small, plastic tub while the drug was administered over the course of one minute via an infusion pump. The injector cannula was left in place for an additional minute. Carbachol was dissolved in physiological saline at a concentration of 50 μM in 0.5 μl per side. Approximately 20 min after infusion, rats were given 30 min to consume up to 5 ml of the 0.5% saccharin solution in the home cage. Duration of time to consume 5 ml was recorded as an index of taste recognition, since rapid consumption indicates recognition of a familiar, safe taste, while slower consumption is a mark of neophobia or caution when confronted with an unfamiliar taste. Consumption durations were determined visually by an experimenter blind to the animal’s experimental condition. After this 30-min period, rats were injected with either 0.15 M LiCl (0.5% bodyweight, ip; Carbachol-LiCl, n=6; Vehicle-LiCl, n=5) or a comparable volume of saline (Carbachol-saline, n=6; Vehicle-saline, n=5). Single-bottle CTA testing in the home cage occurred the next day, with saccharin intake measured over the course of 30 min. Food was freely available throughout training and testing.
CTA training and testing with novel NaCl
The observation of striking effects of carbachol on CTAs to a familiar taste led us to ask whether carbachol would strengthen all CTAs or only those to a familiar taste. To test carbachol’s effect on CTAs to a novel taste, a subset of animals that received CTA training with the familiar saccharin solution were subsequently given CTA training with a novel 0.9% NaCl solution. Subjects who had previously received the CS paired with LiCl were placed into one of the saline control groups (Carbachol-saline, n=4; Vehicle-saline, n=4), while those who had received saline were placed into one of the LiCl paired groups (Carbachol-LiCl, n=4; Vehicle-LiCl, n=4). Infusion group (carbachol vs. vehicle) was randomly assigned. CTA training and testing followed the same procedure described in the previous section, with the only difference being the absence of pre-exposure to the NaCl solution prior to conditioning.
Results
Carbachol infusion into IC enables CTA acquisition to a familiar CS
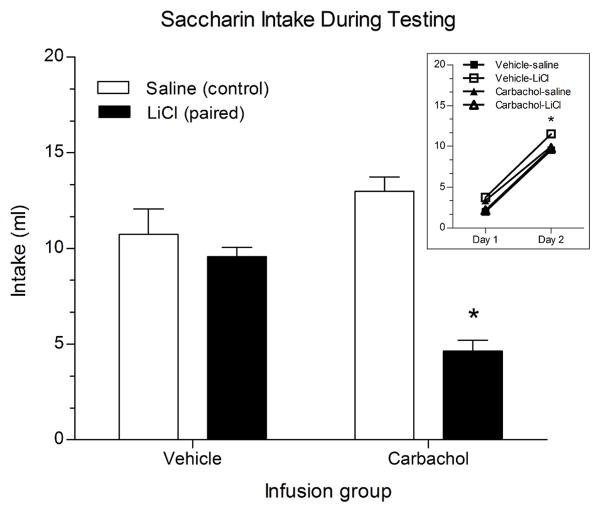
Animals were given two days of safe taste training to familiarize them to the saccharin solution prior to conditioning. Daily intakes are shown in Figure 1 (inset). Paired t-tests showed that all groups significantly increased their intake of the solution from Day 1 to Day 2, indicating normal attenuation of neophobia (all ps < .001). When tested 24 hr after CS-US pairing, rats that had received an infusion of carbachol prior to conditioning showed strong aversions to saccharin despite two days of safe taste training, which had essentially blocked any evidence of conditioning in vehicle-infused controls. A two-way ANOVA revealed a main effect of infusion group, F(1,18) = 26.50, p < .05; a main effect of pairing, F(1,18) = 33.08, p < .001; and an interaction of infusion group x pairing, F(1,18) = 18.89, p < .001 (Figure 1). The Carbachol-LiCl group drank significantly less saccharin during the 30-min test than the Carbachol-saline group (p < .01). The vehicle-infused groups did not differ significantly in their intake of saccharin (p = .778) indicating that two exposures to the saccharin solution prior to conditioning were sufficient for the development of latent inhibition in the Vehicle-LiCl group.
Figure 1.
Mean (± SEM) intake of familiar 0.5% saccharin during two days of safe taste training (inset) and during a 30-min, one-bottle test (main graph). Testing occurred 24 hr after CTA training with the familiar CS in rats receiving infusion of either vehicle or carbachol into IC. Main graph: *p<.001 between carbachol-infused groups; inset: *p<.001 between Day 1 and Day 2 for all groups.
Carbachol infusion into IC does not influence initial taste recognition processing
The finding that rats receiving carbachol infusion into IC learned aversions to a familiar taste could indicate that the cholinergic signal mimicked the signal generated by a novel taste. Alternatively, the infusion could have blocked retrieval of the taste memory. We have the data to evaluate this issue because on conditioning day rats drank the saccharin solution after infusions, and the duration of time it took for them to consume the 5 ml is an index of their neophobia. Familiar, palatable tastes are readily consumed by thirsty rats while rats show hesitation in consuming novel tastes (Clark & Bernstein, 2009).
Consumption duration during conditioning with a familiar saccharin CS is shown in Figure 2. All groups drank the solution in less than 4 min (range of means = 2.46–3.06 min) and there were no significant differences between groups (Mann-Whitney U-test; p > .05 for all comparisons) indicating no effect of carbachol on retrieval of the memory of prior experience with saccharin. Despite the fact that the Carbachol-LiCl group would later acquire a strong aversion to the saccharin after its pairing with LiCl, initial rapid consumption of the solution indicates that carbachol-infused rats recognized the saccharin as a familiar taste. This is an important observation and one that suggests that cholinergic activity does not play a role in the initial stages of taste recognition that influence gustatory neophobia.
Figure 2.
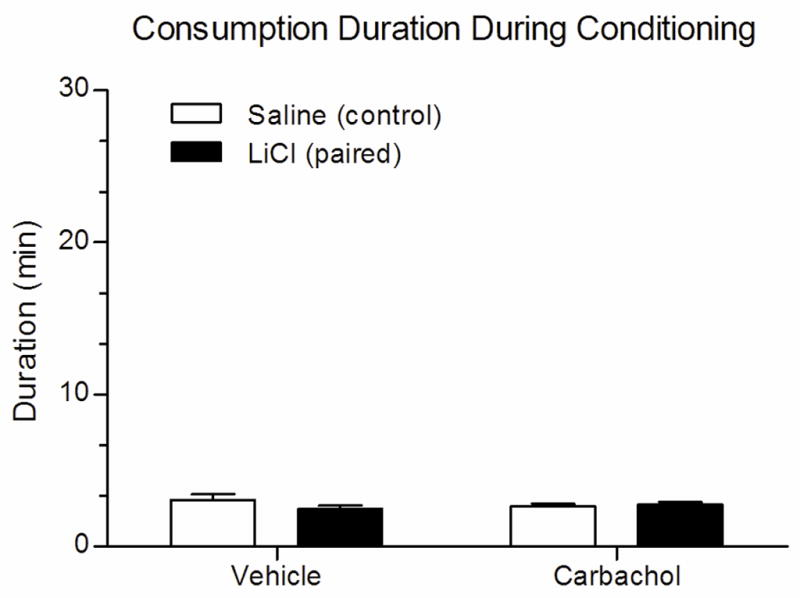
Mean (± SEM) duration of time to consume 5 ml of familiar 0.5% saccharin during CTA training after infusion of either vehicle or carbachol into IC. All groups took less than 5 min, and no differences in consumption duration were found between the groups during CS presentation.
Carbachol infusion into IC does not affect CTA acquisition to a novel CS
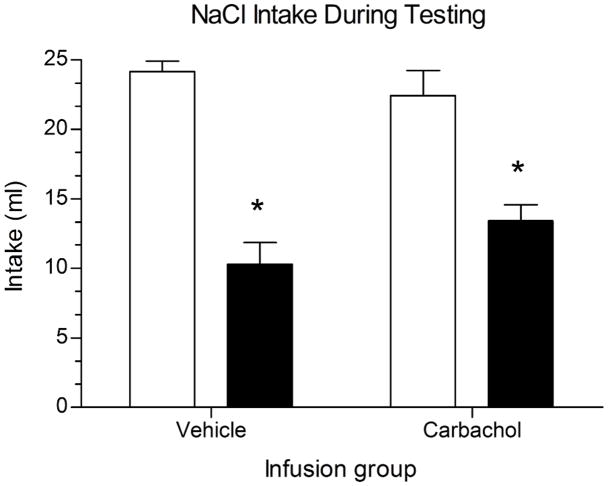
These results support the hypothesis that enhancing cholinergic activity can render a familiar taste an effective target for conditioning. However, it was possible that carbachol enhanced the potency of the LiCl, or strengthened learning in a general way. To assess this issue the effects of the same carbachol infusions on aversions to a novel taste CS were examined. A subset of the same rats were infused with either carbachol or vehicle prior to CTA training with a novel CS, 0.9% NaCl. In contrast with the findings using a familiar taste, no effects of carbachol on CTA severity were observed when the CS was novel. Both groups receiving LiCl injections developed aversions of similar magnitude regardless of whether they were infused with carbachol or vehicle. A two-way ANOVA revealed a main effect of pairing, F(1,12) = 47.60, p < .001; but no main effect of infusion drug and no infusion drug x pairing interaction. Post hoc comparisons showed the Carbachol-LiCl and Vehicle-LiCl groups drank significantly less NaCl during a 30-min test than their saline-injected control groups (p < .01 for both comparisons). Intakes did not differ between the two paired groups (p = .401), which indicates that carbachol did not enhance the effectiveness of LiCl or act as a US on its own. (Figure 3).
Figure 3.
Mean (± SEM) intake of 0.9% NaCl during a 30-min, one-bottle test. Testing occurred 24 hr after CTA training with the novel CS in rats receiving infusion of either vehicle or carbachol into IC. *p<.01 between paired and control groups in both infusion conditions.
Experiment 2: Effect of Carbachol Infusion into IC on Amygdala FLI Expression after Familiar CS-US Pairing
The next experiment examined the patterns of neuronal activation in amygdala associated with the learning of CTA to a familiar taste facilitated by carbachol. FLI expression in the basolateral (including lateral; BLA) and central (CNA) subnuclei of the amygdala are elevated after novel but not familiar CS-US pairing (Koh and Bernstein, 2005). We determined whether the carbachol infusions which rendered a familiar CS taste effective in one-trial CTA learning also affected patterns of neuronal activation in amygdala.
Methods
FLI patterns in amygdala following familiar CS-US pairing were assessed in rats receiving either carbachol (Carbachol-LiCl, n=6; Carbachol-saline, n=6) or vehicle infusion into IC (Vehicle-LiCl, n=6; Vehicle-saline, n=6) prior to conditioning. IC cannula surgery, saccharin pre-exposure, drug infusion, and conditioning methods were identical to Experiment 1, except that rats were sacrificed 90 min after receiving the familiar saccharin paired with either LiCl or saline.
Immunohistochemistry and FLI analysis
Rats were deeply anesthetized with sodium pentobarbital and transcardially perfused with isotonic phosphate-buffered saline (PBS) followed by 4% paraformaldehyde. Brain regions of interest were sectioned into 50-μm slices in the transverse plane. Slices were rinsed in PBS, incubated for 20 min in 0.3% hydrogen peroxide in absolute methanol to quench endogenous peroxidase, rinsed in PBS, and incubated for 1 hr in 1% gelatin-3 % normal goat serum in PBS. Slices were then transferred directly to the primary antibody solution, containing 1:20k c-fos polyclonal rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA). After incubating for 48 hr at approximately 4° C, slices were rinsed in PBS and processed using the standard ABC method (Vector Laboratories, Burlingame, CA) and 3,3′-diaminobenzidine (DAB) with nickel chloride enhancement techniques to visualize the presence of FLI. Sections were then mounted onto slides and counterstained with neutral red. Regions examined were basolateral (including the lateral amygdala) and central subnuclei of the amygdala (BLA and CNA). The hippocampus, which is thought to be minimally involved in CTA (Welzl, D’Adamo, & Lipp, 2001), was included as a control region. Atlas coordinates (relative to bregma) were, approximately, −2.56 mm for amygdala and hippocampus (Paxinos & Watson, 1997). FLI counts were based on an average of four sections per region for each rat. Sections were chosen on the basis of anatomical landmarks and were plotted by an observer blind to experimental groups. FLI-positive neurons were defined as cells with nuclei containing solid black reaction product that covered at least half of the nucleus.
Histology
For both experiments, IC cannula placement was verified by examining brain sections containing the cannula tracks, which were sectioned in 50-μm slices, stained with cresyl violet, and examined under a microscope.
Results
Carbachol infusion into IC increases FLI expression in amygdala following familiar CS-US pairing
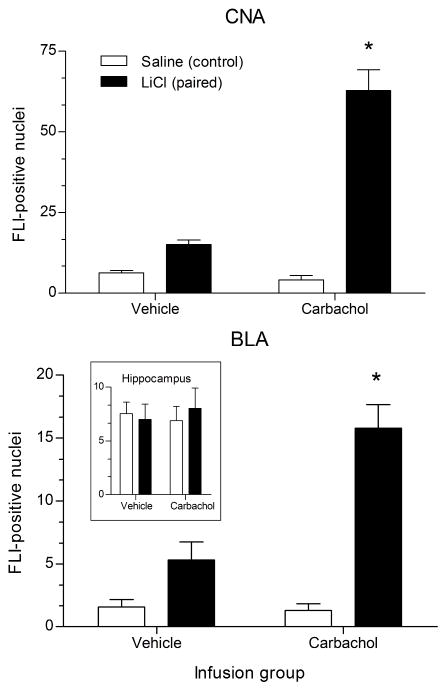
As shown in Figures 4 and 5, carbachol infusion into IC prior to conditioning with a familiar saccharin CS was associated with a dramatic elevation of FLI expression in amygdala in the Carbachol-LiCl group. Two-way ANOVAs with infusion group (carbachol or vehicle) and pairing (LiCl or saline control) as between-subjects factors were conducted for each subregion of amygdala, as well as hippocampus, followed (when appropriate) by post hoc analysis using Tukey’s honestly significant difference (HSD) with the Bonferroni correction for multiple comparisons. In CNA there was a main effect of infusion group, F(1,20) = 44.89, p < .001; a main effect of pairing, F(1,20) = 97.90, p < .001; and an interaction of infusion group × pairing, F(1,20) = 53.83, p < .001 (Figure 4, top panel). BLA showed a similar pattern of results, with a main effect of infusion group, F(1,20) = 16.71, p < .01; a main effect of pairing, F(1,20) = 53.86, p < .001; and an infusion group x pairing interaction, F(1,20) = 18.69, p < .001 (Figure 4, bottom panel). Post hoc comparisons showed the Carbachol-LiCl group expressed significantly more FLI-positive nuclei than their saline controls in both CNA and BLA (p < .001 for both subregions). In contrast, FLI expression in the Vehicle-LiCl group, though slightly elevated above the Vehicle-saline group, did not reach significance (p = .299 for CNA; p = .177 for BLA). This slight elevation was most likely due to the LiCl injection, since LiCl alone has been shown to increase FLI expression in amygdala (Koh & Bernstein, 2005; Lamprecht & Dudai, 1995). In contrast to the amygdala results, FLI expression in the hippocampus did not differ between the four groups (F < 1; Figure 4, bottom panel inset).
Figure 4.
Mean (± SEM) number of FLI-positive nuclei in CNA (top panel), BLA (bottom panel), and hippocampus (bottom panel inset) after CTA training with familiar 0.5% saccharin in rats receiving infusion of either vehicle or carbachol into IC prior to conditioning. *p<.001 between carbachol-infused groups in both amygdala subregions. CNA = central nucleus of amygdala; BLA = basolateral nucleus of amygdala.
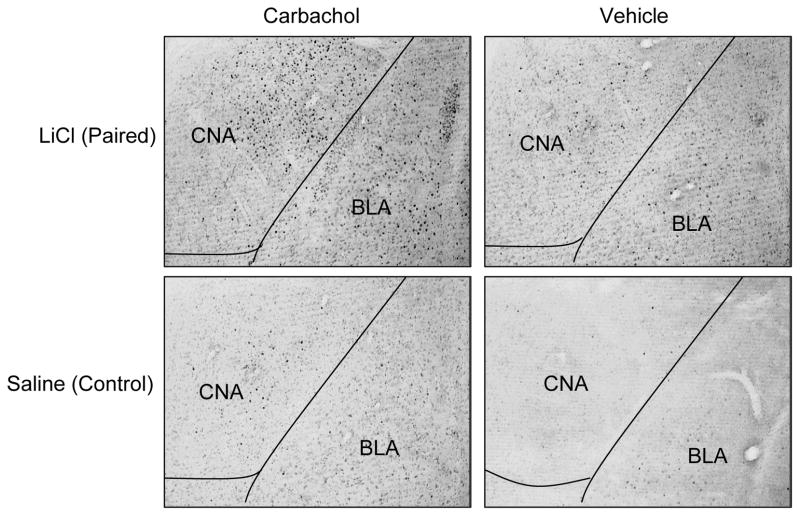
Figure 5.
Photomicrographs of FLI expression in amygdala after pairing of familiar saccharin with either LiCl (paired condition) or saline (control) following carbachol or vehicle infusions into IC. Carbachol-LiCl rats showed significant FLI elevation after taste-illness pairing (top left panel) relative to Carbachol-saline controls (top right panel). Vehicle-infused rats did not show differential FLI expression, regardless of pairing condition (bottom panels). CNA= central nucleus of amygdala; BLA= basolateral nucleus of amygdala.
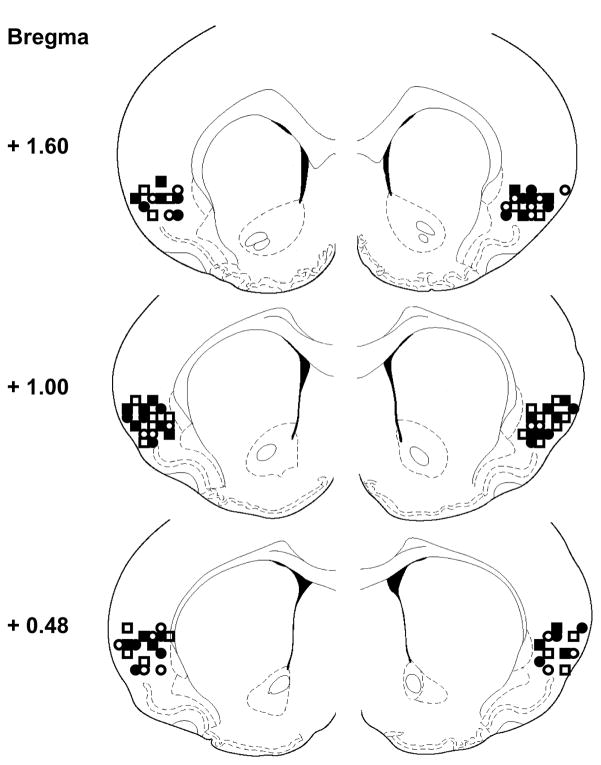
Cannula placements for Experiments 1 and 2 are shown in Figure 6. Only rats with cannula tips within the boundaries of IC were included in the data analysis.
Figure 6.
Cannula tip placements in insular cortex on coronal brain sections adapted from Paxinos & Watson (1997). Rats were infused with 50 μM carbachol or vehicle 20 min prior to consumption of a familiar saccharin solution paired with injection of either LiCl (Carbachol-LiCl, filled squares; Vehicle-LiCl, filled circles) or saline (Carbachol-saline, open squares; Vehicle-saline, open circles).
Discussion
Infusions of carbachol, a direct cholinergic agonist, into IC prior to familiar CS-US pairing enabled rats to acquire a CTA despite two sessions of safe taste training with the saccharin solution. Unlike vehicle-infused rats, which did not learn the CS-US association due to latent inhibition, the Carbachol-LiCl group acquired a strong aversion in a single trial, suggesting that they treated the saccharin like a novel CS. In addition to these behavioral results, FLI analysis in amygdala showed that carbachol infusions prior to familiar CS-US pairing dramatically elevated FLI levels in both CNA and BLA, relative to saline-paired controls. In contrast, familiar CS-US pairing following vehicle infusions failed to elevate FLI above the low level seen in controls. This differential FLI expression parallels the behavioral effects, since the Carbachol-LiCl group developed strong aversions and also showed greater amygdala FLI expression, while the Vehicle-LiCl group neither acquired aversions nor showed any FLI increase. Differential FLI expression has been shown in a number of studies where training conditions normally yield strong CTA learning. For example, Koh and Bernstein (2005) found significant FLI elevations in BLA and CNA after CTA training with novel, but not familiar saccharin. When viewed in this context, the behavioral and FLI results of the current study suggest that intra-IC carbachol infusion changes a normally weak learning situation (pairing of LiCl-induced illness with a familiar saccharin CS) into one in which the CS-US association forms rapidly (akin to novel saccharin paired with illness), and that such a change is reflected as increased FLI expression in amygdala.
The behavioral results are not likely to be due to carbachol acting to intensify effects of the US or to a general strengthening of learned associations. Infusing carbachol prior to conditioning with a novel NaCl solution did not yield stronger aversion learning. The lack of effect on aversions to novel NaCl would not appear to be due to a floor effect since intakes in the vehicle group were well above what is often seen with higher LiCl doses, and the minor difference between vehicle and carbachol conditioned groups was in the opposite direction from what would be predicted if carbachol intensified the learning. It should be noted that the present studies were not aimed at a systematic examination of the effects of carbachol on novel taste learning. The same animals were used in both studies, which was an effective way to control for cannula position but necessitated the use of a different tastant, NaCl, as a novel CS. Despite these caveats, no evidence was found for either an enhancement or a disruption by carbachol of CTA learning with novel NaCl.
Another possible explanation for the behavioral results of Experiment 1 was that carbachol blocked retrieval of the saccharin taste memory, instead of mimicking the cortical cholinergic signal associated with novel taste processing. However, analysis of consumption durations during conditioning showed that all groups drank the required 5 ml of familiar saccharin in under 4 min, regardless of whether they had received an infusion of carbachol or vehicle prior to saccharin presentation. Such short durations indicate recognition of a familiar, safe taste. If carbachol had blocked retrieval of the saccharin memory, those groups would have taken much closer to the 30-min time limit to consume the 5 ml (Clark & Bernstein, 2009). The fact that carbachol infusion does not seem to affect immediate recognition of a familiar taste, yet enables animals to acquire a CTA to the taste as though it were novel, poses an interesting paradox. We interpret these findings as indicating that the cortical cholinergic system is not involved in the earliest stages of taste recognition which influence gustatory neophobia, but does exert a potent effect on the formation of CS-US associations. In addition to our own findings, this view is supported by studies involving the cholinergic antagonist, scopolamine. Infusions of the drug into IC prior to taste presentation do not disrupt the normal neophobic response to a novel taste, yet do prevent attenuation of this neophobic response over days and do impair CTA acquisition when the taste is later paired with illness (Gutiérrez et al., 2003). Further evidence that neophobia and CTA learning involve separate, but related processes comes from a recent study in which crossed-disconnection lesions of IC and parabrachial nucleus (PBN) were shown to impair CTA learning with a novel CS, while leaving the neophobic response intact (Clark & Bernstein, 2009).
Taken together these results suggest that initial taste recognition can occur without cholinergic activity but that cholinergic activation is needed to generate a novelty signal which permits rapid CTA associations, presumably involving transmission to and from regions such as amygdala and PBN. Blockade of cholinergic receptors decreases the novelty of the taste signal, while activation of these receptors enhances novelty. The absence of an enhancement effect of carbachol on CTAs to a novel taste indicates that the carbachol signal is permissive but that increases in its strength do not necessarily lead to increases in the strength of the learning. In fact, high concentrations of carbachol have been shown to impair CTA acquisition to novel tastes (Naor & Dudai, 1996), and extremely high concentrations may cause motor seizures (Stivers, Skirboll, Long & Crawley, 1988) or lead to fluid imbalance or dysregulation (Fitts & Simpson, 1986; Fitts, Thunhorst, & Simpson, 1985), which would obviously interfere with learning. Our findings support a role for cholinergic activity within some optimal range.
Given that the current study did not explicitly compare novel and familiar saccharin, it is not possible to conclude definitively that our carbachol treatment actually duplicated the cortical signal generated by a novel taste. However, these studies support and extend the perspective that the cortical cholinergic system is critically involved in taste processing and CTA acquisition. Activity in the NBM leads to greater ACh release in IC following novel taste consumption, and the novelty of a given taste may be modulated through manipulation of cholinergic receptors. Artificial enhancement of ACh levels in IC enables learning when a familiar taste would normally be resistant to rapid CTA acquisition and alters related gene expression in regions known to be involved in CTA acquisition.
Acknowledgments
This research was supported by National Institutes of Health Grant NS37040 to Ilene L. Bernstein.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/journals/bne.
References
- Berger-Sweeney J, Heckers S, Mesulam MM, Wiley RG, Lappi DA, Sharma M. Differential effects on spatial navigation of immunotoxin-induced cholinergic lesions on the medial septal area and nucleus basalis magnocellularis. Journal of Neuroscience. 1994;14:4507–4519. doi: 10.1523/JNEUROSCI.14-07-04507.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein IL. Flavor aversion. In: Getchell TV, Doty RL, Bartoshuk LM, Snow JB, editors. Smell and taste in health and disease. New York: Raven Press; 1991. pp. 417–428. [Google Scholar]
- Braun JJ, Rosenthal B. Relative salience of saccharin and quinine in long-delay taste aversion learning. Behavioral Biology. 1976;16:341–352. doi: 10.1016/s0091-6773(76)91473-5. [DOI] [PubMed] [Google Scholar]
- Bures J. Ethology, physiological psychology, and neurobiology of CTA. In: Bures J, Bermúdez-Rattoni F, Yamamoto T, editors. Conditioned taste aversion: Memory of a special kind. New York: Oxford University Press; 1998. pp. 1–13. [Google Scholar]
- Chambers KC, Bernstein IL. Conditioned flavor aversions. In: Doty RL, editor. Handbook of medical olfaction and taste. New York: Marcel Dekker; 1995. pp. 745–773. [Google Scholar]
- Clark EW, Bernstein IL. Establishing aversive, but not safe, taste memories requires lateralized pontine-cortical connections. Behavioural Brain Research. 2009;197:356–363. doi: 10.1016/j.bbr.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker MW, McGaugh JL. The role of interactions between the cholinergic system and other neuromodulatory systems in learning and memory. Synapse. 1991;7:151–168. doi: 10.1002/syn.890070209. [DOI] [PubMed] [Google Scholar]
- Domjan M. Attenuation and enhancement of neophobia for edible substances. In: Barker LM, Best MA, Domjan M, editors. Learning mechanisms in food selection. TX: Baylor University Press; 1977. pp. 151–179. [Google Scholar]
- Dunnett SB. The role and repair of forebrain cholinergic systems in short-term memory. Studies using the delayed matching-to-position task in rats. Advances in Neurology. 1993;59:53–65. [PubMed] [Google Scholar]
- Dunnett SB, Everitt BJ, Robbins TW. The basal forebrain-cortical cholinergic system: Interpreting the functional consequences of excitotoxic lesions. Trends in Neuroscience. 1991;14:494–501. doi: 10.1016/0166-2236(91)90061-x. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annual Review of Psychology. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- Fitts DA, Simpson JB. Drinking and natriuresis during volume expansion and intracranial angiotensin or carbachol. American Journal of Physiology. 1986;251:R381–R387. doi: 10.1152/ajpregu.1986.251.2.R381. [DOI] [PubMed] [Google Scholar]
- Fitts DA, Thunhorst RL, Simpson JB. Fluid intake, distribution, and excretion during lateral ventricular infusions of carbachol in rats. Brain Research. 1985;332:237–245. doi: 10.1016/0006-8993(85)90593-1. [DOI] [PubMed] [Google Scholar]
- Garcia J, Hankins WG, Rusiniak KW. Behavioral regulation of the milieu interne in man and rat. Science. 1974;185:824–831. doi: 10.1126/science.185.4154.824. [DOI] [PubMed] [Google Scholar]
- Giovannini MG, Rakovska A, Benton RS, Pazzagli M, Bianchi L, Pepeu G. Effects of novelty and habituation on acetylcholine, GABA, and glutamate release from the frontal cortex and hippocampus of freely moving rats. Neuroscience. 2001;106:43–53. doi: 10.1016/s0306-4522(01)00266-4. [DOI] [PubMed] [Google Scholar]
- Gutiérrez R, Rodriguez-Ortiz CJ, De La Cruz V, Núñez-Jaramillo L, Bermúdez-Rattoni F. Cholinergic dependence of taste memory formation: Evidence of two distinct processes. Neurobiology of Learning and Memory. 2003;80:323–331. doi: 10.1016/s1074-7427(03)00066-2. [DOI] [PubMed] [Google Scholar]
- Kalat JW, Rozin P. “Learned safety” as a mechanism in long-delay taste-aversion learning in rats. Journal of Comparative and Physiological Psychology. 1973;83:198–207. doi: 10.1037/h0034424. [DOI] [PubMed] [Google Scholar]
- Koh MT, Bernstein IL. Mapping conditioned taste aversion associations using c-Fos reveals a dynamic role for insular cortex. Behavioral Neuroscience. 2005;119:388–398. doi: 10.1037/0735-7044.119.2.388. [DOI] [PubMed] [Google Scholar]
- Lamprecht R, Dudai Y. Differential modulation of brain immediate early genes by intraperitoneal LiCl. Neuroreport. 1995;7:289–293. [PubMed] [Google Scholar]
- Leanza G, Nilsson OG, Wiley RG, Bjorklund A. Selective lesioning of the basal forebrain cholinergic system by intraventricular 192 IgG-saporin: Behavioural, biochemical and stereological studies in the rat. European Journal of Neuroscience. 1995;7:329–343. doi: 10.1111/j.1460-9568.1995.tb01068.x. [DOI] [PubMed] [Google Scholar]
- Miranda MI, Bermúdez-Rattoni F. Reversible inactivation of the nucleus basalis magnocellularis induces disruption of cortical acetylcholine release and acquisition, but not retrieval, of aversive memories. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:6478–6482. doi: 10.1073/pnas.96.11.6478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda MI, Ramirez-Lugo L, Bermúdez-Rattoni F. Cortical cholinergic activity is related to the novelty of the stimulus. Brain Research. 2000;882:230–235. doi: 10.1016/s0926-6410(00)00050-1. [DOI] [PubMed] [Google Scholar]
- Muir JL. Acetylcholine, aging, and Alzheimer’s disease. Pharmacology, Biochemistry, and Behavior. 1997;56:687–696. doi: 10.1016/s0091-3057(96)00431-5. [DOI] [PubMed] [Google Scholar]
- Nachman M, Ashe JH. Learned taste aversions over long delays in rats: The role of learned safety. Journal of Comparative and Physiological Psychology. 1973;86:949–956. [Google Scholar]
- Naor C, Dudai Y. Transient impairment of cholinergic function in the rat insular cortex disrupts the encoding of taste in conditioned taste aversion. Behavioral Brain Research. 1996;79:61–67. doi: 10.1016/0166-4328(95)00262-6. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates, compact. 3. San Diego, CA: Academic Press; 1997. [Google Scholar]
- Revusky SH, Bedarf EW. Association of illness with prior ingestion of novel foods. Science. 1967;155:219–210. doi: 10.1126/science.155.3759.219. [DOI] [PubMed] [Google Scholar]
- Rozin P. The significance of learning mechanisms in food selection: Some biology, psychology and sociology of science. In: Barker LM, Best MA, Domjan M, editors. Learning mechanisms in food selection. TX: Baylor University Press; 1977. pp. 557–589. [Google Scholar]
- Sarter M, Bruno JP. Cognitive functions of cortical acetylcholine: Toward a unifying hypothesis. Brain Research. Brain Research Reviews. 1997;23:28–46. doi: 10.1016/s0165-0173(96)00009-4. [DOI] [PubMed] [Google Scholar]
- Schafe GE, Bernstein IL. Forebrain contribution to the induction of a brainstem correlate of conditioned taste aversion: I. The amygdala. Brain Research. 1996;741:109–116. doi: 10.1016/s0006-8993(96)00906-7. [DOI] [PubMed] [Google Scholar]
- Shi CJ, Cassell MD. Cortical, thalamic, and amygdaloid connections of the anterior and posterior insular cortices. Journal of Comparative Neurology. 1998;399:440–468. doi: 10.1002/(sici)1096-9861(19981005)399:4<440::aid-cne2>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Stivers JA, Skirboll LR, Long R, Crawley JN. Anatomical analysis of frontal cortex sites at which carbachol induces motor seizures in the rat. Pharmacology, Biochemistry and Behavior. 1988;30:129–136. doi: 10.1016/0091-3057(88)90435-2. [DOI] [PubMed] [Google Scholar]
- Welzl H, D’Adamo P, Lipp HP. Conditioned taste aversion as a learning and memory paradigm. Behavioural Brain Research. 2001;125:205–213. doi: 10.1016/s0166-4328(01)00302-3. [DOI] [PubMed] [Google Scholar]