Abstract
Aims
Current efforts to treat myocardial infarction include the delivery of cells and matrix scaffolds. Bone marrow-derived mesenchymal stem cells (BM-MSCs) are multipotent stem cells that secrete angiogenic growth factors, and fibrin has been shown to be a biomaterial that provides structural support to cells and tissues. The objective of this study was to characterize the attachment and viability of BM-MSCs in fibrin in vitro, and then to assess the efficacy of treatment with BM-MSCs in fibrin for promoting neovascularization in the chronically infarcted myocardium.
Materials & methods
BM-MSCs were cultured in fibrin and assessed for cell attachment and viability by using immunofluorescence staining for actin filaments and Live/Dead® viability assays, respectively. To determine the efficacy of BM-MSCs in fibrin in vivo, chronically infarcted rat hearts were treated with either cells, cells in fibrin, fibrin or saline (n = 9). After 5 weeks, the infarct scar tissues were assessed for neovascularization.
Results
BM-MSCs exhibited robust cell attachment and viability when cultured in fibrin in vitro. Furthermore, when injected together into the infarcted tissue, BM-MSCs in fibrin could enhance neovasculature formation by increasing capillary density, in comparison to treatment by cells or fibrin separately. Concomitant to significant improvement in capillary density was an increase in the levels of VEGF in the infarct scar.
Conclusion
This study demonstrates the angiogenic potential of the combined delivery of BM-MSCs and fibrin, and highlights the advantage of stem cell-matrix approaches for myocardial repair.
Keywords: angiogenesis, cardiac tissue engineering, extracellular matrix, fibrin, mesenchymal stem cell, myocardial infarction
Despite advancements in treating myocardial infarction (MI), therapies that improve the structural and functional capacities of the myocardium remain lacking. To replace cells in damaged myocardium, numerous cell types have been studied for therapeutic transplantation, including fetal cardiomyocytes [1], skeletal myoblasts [2], embryonic stem cells [3] and bone marrow-derived mesenchymal stem cells (BM-MSCs) [4]. In particular, BM-MSCs are multipotent stem cells that have shown tremendous therapeutic potential. It has been demonstrated that BM-MSC transplantation after MI can improve cardiac performance, regenerate the infarcted myocardium and promote angiogenesis in the ischemic infarct area [4-6]. When applied to asynchronously beating cardiomyocytes, BM-MSCs could restore synchronization, most likely by connexin-43 coupling [7]. Recent data show that BM-MSCs secrete numerous angiogenic growth factors that implicate a paracrine mechanism of involvement in therapeutic repair in vivo [8]. Among the growth factors expressed by BM-MSCs, VEGF is known to promote endothelial cell proliferation and migration and can enhance postischemic neovascularization [9,10].
Concomitant with cell delivery, biomaterials are another emerging area of research in cardiac repair. When used alone or in combination with cells, biomaterials have been shown to prevent the negative remodeling associated with the MI [3,11-13]. For example, Zimmermann et al. demonstrated that the grafting of tissue constructs containing neonatal heart cells, collagen I and Matrigel™ to the infarcted heart could improve cardiac function and wall thickening [13]. Biomaterials can serve as delivery vehicles that mediate cell alignment, cell migration and growth factor release [14-17]. One potential biomaterial for myocardial repair is fibrin, a US FDA-approved biomaterial that is commercially available as a sealant and adhesive. Fibrin is bioresorbable and serves as a provisional matrix for cellular ingrowth during wound repair.
Previous studies have characterized the in vitro effect of fibrin on BM-MSC survival [18] and differentiation towards vascular or osteogenic lineages [18,19]. In addition, their therapeutic effect has been assessed for repair in the setting of acute MI [20], which is shortly after the induction of MI, during which the infarct zone actively undergoes a remodeling process characterized by the infiltration of inflammatory cells and elaboration of cytokines [21]. Compared with acute MI, a more clinically relevant setting is the model of chronic MI, which occurs several weeks following the induction of MI when the pathological remodeling process is complete and myocardial pumping capacity is compromised, predisposing the heart to congestive heart failure [22,23]. However, the angiogenic effect of BM-MSCs in fibrin for treatment of chronic MI remains to be elucidated.
In this study, we characterized the attachment and viability of human BM-MSCs in fibrin in vitro, and then assessed the efficacy of treatment with BM-MSCs in fibrin for promoting neovascularization in the chronically infarcted myocardium.
Materials & methods
Fibrin characterization
To characterize the porous structure of fibrin (Tisseel, Baxter Healthcare Corp., CA, USA), the fibrinogen and thrombin components were prepared according to our previous studies [12,14]. In brief, fibrinogen and thrombin were briefly mixed at a 1:1 ratio to form 100 μl fibrin matrices. For scanning electron microscopy (SEM), the fibrin samples were sputter-coated with gold:palladium (40:60) particles to a thickness of 10-15 nm. Fibrin pore sizes were visualized using a Hitachi S-5000 cold field emission scanning electron microscope [24]. For histological analysis of pore size, fibrin was embedded in OCT compound (Sakura Finetek, CA, USA) and cryosectioned for routine hematoxylin and eosin (H&E) staining [25]. Pore sizes were quantified by measurement of the pore diameter of H&E-stained fibrin cross-sections using Image J software (NIH, MD, USA) (n = 3). At least 40 pores were assessed in each sample.
Cell morphology & survival in fibrin in vitro
Human BM-MSCs (Lonza, MD, USA) were cultured in MSC growth medium (MSCGM, Lonza). These cells were tested by the manufacturer for positive expression of BM-MSC phenotypic markers including CD105, CD166 and CD44. To characterize cell morphology and survival in 3D fibrin gels in vitro, the BM-MSCs were resuspended in 100 μl thrombin, transferred to tissue-culture dishes and mixed with 100 μl fibrinogen. After solidification of fibrin, the gels were incubated with MSCGM. As a control, BM-MSCs were cultured on tissue-culture dishes. After 2 days, cell viability in fibrin was assessed by incubation with 2 μM calcein-AM and 4 μM ethidium homodimer (Live/Dead® viability assay; Molecular Probes, OR, USA) for 1 h before visualization. To track cell migration in 3D fibrin, a sprouting assay was carried out in which BM-MSC aggregates were formed by hanging drops for 24 h, and then embedded within 200 μl fibrin. After 1 h, the gels were immersed in MSCGM. As a control, cell aggregates was cultured on tissue-culture dishes in MSCGM. After 2 days, the aggregates were evaluated for cell migration by bright field microscopy and then fluorescently stained with Alexafluor-488-conjugated phalloidin (Molecular Probes). The samples were visualized with a Zeiss Axioskop 2 fluorescent microscope.
MI model & injections
This study was performed in accordance with the Committee for Animal Research of the University of California, San Francisco. Female nude (rnu homozygous) rats aged 8-12 weeks underwent occlusion of the left anterior descending (LAD) coronary artery for 17 min, followed by reperfusion, according to our previous studies [26]. A midline thoracotomy was performed 5 weeks post-MI [14,25] and the infarct scar was injected intramuscularly with 50 μl of one of four treatments, namely 0.5% bovine serum albumin (BSA) in phosphate-buffered saline, 2 × 106 cells in 0.5% BSA, fibrin or 2 × 106 cells in fibrin (n = 9 per group).
For treatment of fibrin, the fibrinogen and thrombin components were maintained in separate syringes in liquid form until injecting simultaneously using the supplied Duploject applicator into the infarct area, where the two components solidified into a 3D fibrin matrix. For treatment of BM-MSCs in fibrin, the cells were briefly suspended in thrombin before joining with fibrinogen using the Duploject applicator and then solidifying within the infarct. The BM-MSCs used in this study were cultured in MSCGM prior to in vivo studies and used within eight passages. At 2 days or 5 weeks after treatment, the animals were euthanized by pentobarbital overdose (200 mg/kg) and the hearts were freshly frozen in OCT compound for histology and immunohistochemical analysis (n = 9 per group at each time point).
Histology & immunohistochemistry
The hearts were cryosectioned into 10-μm transverse slices. Representative cryosections spanning the infarct were processed for routine H&E staining [26] for quantification of infarct size. The infarct size was measured as the (infarct scar area)/(total left ventricular area), according to our previous studies [26]. Neovascularization was evaluated by immunohistochemical staining of anti-CD31 (BD Biosciences, CA, USA) for the identification of endothelial cells and anti-smooth muscle α-actin (SMA, Sigma-Aldrich, MO, USA) for smooth muscle cells, as previously described [14]. Capillary and arteriole densities were analyzed according to our previous studies [14]. Briefly, for measurement of capillary density, five fields within the infarct of each tissue section were imaged under 40× objectives, and capillaries with less than 10 μm diameters were counted. The capillary density was quantified as the (total CD31-positive microvessels)/mm2. Arteriole density was quantified as the (total SMA-positive microvessels)/mm2 within the infarct area. Vascular densities were averaged out of at least four tissue sections spanning the infarct region for each animal.
In addition, the level of infarct scar VEGF expression was semiquantified by immunohistochemical staining of VEGF using a human- and rat-reactive polyclonal antibody (Santa Cruz Biotechnology, CA, USA) and the Vectastain ABC kit (Vector Labs, CA, USA). For semiquantification of VEGF expression, five fields within the infarct area of each tissue section were imaged under 40× objectives, and the area of positive VEGF expression was measured by Image J software and expressed as a percentage of the total infarct area [27]. The expression for each animal was averaged out of at least four tissue sections spanning the infarct region.
BM-MSC retention & differentiation in infarct scar
Bone marrow-derived mesenchymal stem cells were identified in cryosections of the infarcted myocardium by immunofluorescence staining of nuclear matrix antigen (NuMA, Calbiochem, CA, USA) and vimentin (Dako, CA, USA) using human-specific antibodies that did not cross-react with rat tissue. Gap junctional proteins were assessed by anti-connexin-43 (Sigma) antibody. Differentiation towards cardiovascular lineages was assessed by the expression of SMA for SMC phenotype and Nkx2.5 (Santa Cruz Biotechnology) for cardiomyocyte lineage. Fluorescein or tetramethyl rhodamine isothiocyanate-conjugated secondary antibodies (Jackson ImmunoResearch, PA, USA) were then applied. Cell nuclei were dyed with Hoechst 33342 (Molecular Probes) before visualizing with a Zeiss Axioskop 2 microscope or Leica TCS SL confocal microscope. Human and rat samples were used as positive and negative control tissues, respectively, for the human-specific antibodies. For quantification of BM-MSC survival in the infarct scar at 2 days or 5 weeks after cell delivery using human-specific NuMA antibody, at least four fields within the infarct area of each tissue section were imaged using 40× objectives. The number of NuMA-expressing nuclei in each section was counted and averaged out of at least four tissue sections for each animal.
Statistical analysis
Results are shown as mean ± standard error of the mean. Vascular densities among multiple groups followed normal distributions and were compared by analysis of variance (ANOVA) with Holm’s adjustment. Statistical analysis of cell retention between delivery in saline or fibrin was carried out by an unpaired t-test. Significance was accepted at p < 0.05.
Results
In vitro characterization of cell viability on fibrin
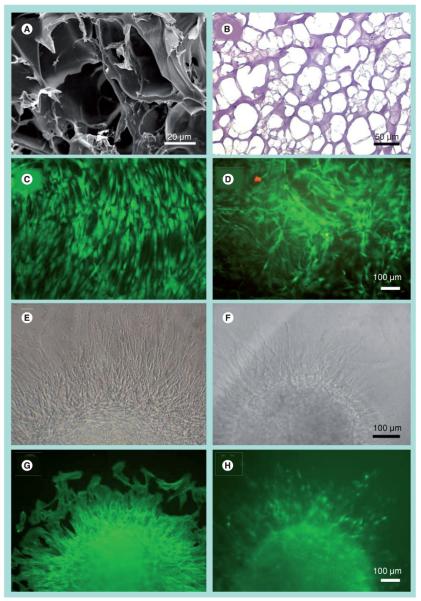
Scanning electron microscopy images showed that fibrin was a porous 3D matrix (Figure 1A). From H&E-stained cross-sections, the average pore size was 84 ± 4 μm in diameter (Figure 1B). After 2 days in culture, Live/Dead viability assay demonstrated that the BM-MSCs in fibrin gels were comparable to those on the control substrate (Figures 1C & 1D), based on the abundance of viable cells (green) and dead cells (red). In fibrin gels, the BM-MSCs retained an elongated morphology. To assay their ability to migrate within fibrin, we performed a sprouting assay in which aggregates of BM-MSCs were embedded in fibrin or cultured on control tissue-culture substrates. After 2 days, sprouting BM-MSCs could be observed migrating in the radial direction from the aggregates on the control substrate (Figure 1E) as well as within fibrin (Figure 1F). The outgrowths from both substrates had organized F-actin assembly in the radial direction (Figures 1G & 1H). Taken together, these data demonstrated that the fibrin formulation was porous and did not interfere with cell attachment, migration and viability in vitro.
Figure 1. In vitro characterization of bone marrow-derived mesenchymal stem cell morphology and survival in 3D fibrin after 2 days.
Visualization of porous fibrin structure by (A) scanning electron microscopy and (B) hematoxylin and eosin of a cross-sectional slice. Live/Dead® viability assay depicts viable (green) and dead (red) cells on (C) control tissue culture dishes or in (D) fibrin gels. Migration of bone marrow-derived mesenchymal stem cells from aggregates on (E & G) control or (F & H) fibrin gels as shown by bright field (E & F) or by immunofluorescence staining of F-actin (G & H).
Cell engraftment in vivo
The therapeutic effects of delivering BM-MSCs in fibrin were then tested in vivo for repair of chronic MI. To assess the retention of BM-MSCs in the infarct area, we utilized human-specific antibodies against NuMA and vimentin to detect the cells. Figures 2A & 2B show the nuclear localization of NuMA and cytoskeletal assembly of vimentin in vitro as positive sample controls for these antibodies. The BM-MSCs were also shown to express connexin-43 gap junctions robustly. To verify successful intramuscular injections of fibrin and cells 2 days after injection, H&E-stained cross-sections showed the presence of fibrin in ischemic tissue (Figure 2C), and BM-MSCs could be visualized in an adjacent tissue section by immunofluorescence staining for NuMA (Figure 2D). Quantification of NuMA-expressing cells in the infarct after 2 days revealed an average of 46 ± 8 BM-MSCs per high power field when delivered in BSA, which was not significantly different from 47 ± 6 cells when delivered in fibrin. The BM-MSC survival was estimated to be less than 1% 5 weeks after injection, based on the average of 33 ± 23 NuMA-expressing cells found in each 10 μm thick section within the infarct, regardless of delivery in BSA or fibrin. The surviving BM-MSCs in the infarct showed elongated NuMA-positive nuclei and vimentin-positive filaments (Figures 2E & 2F). In addition, they expressed connexin-43 gap junctions that were often within the vicinity of the gap junctions of the host myocardium, as demonstrated by double staining of connexin-43 with NuMA (Figure 2E) or with vimentin (Figure 2F). However, double immunofluorescence staining for NuMA with cardiovascular differentiation markers Nkx2.5 and SMA demonstrated no co-localization of staining among the cells assessed, suggesting that the BM-MSCs did not differentiate into cardiovascular cell types after 5 weeks of any treatment. In addition, the BM-MSCs did not appear to differentiate into osteogenic, adipogenic or chondrogenic lineages based on histological analysis of H&E-stained transverse sections of the infarcted tissue (data not shown).
Figure 2. In vivo delivery of bone marrow-derived mesenchymal stem cells in fibrin into the infarcted myocardium.
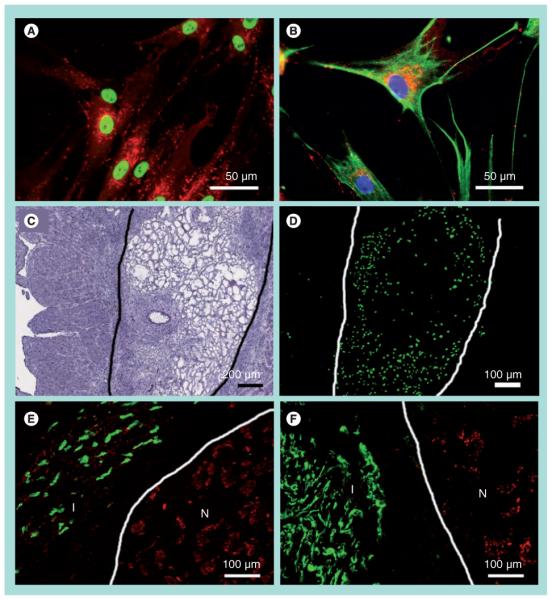
Immunofluorescence staining of (A) human-specific nuclear matrix antigen (NuMA, green) and connexin-43 (red) and (B) human-specific vimentin (green), connexin-43 (red) and Hoechst nuclear dye (blue). At 2 days postinjection, fibrin was visible by (C) hematoxylin and eosin staining, and bone marrow-derived mesenchymal stem cells (BM-MSCs) were visualized by (D) NuMA-positive BM-MSCs in an adjacent tissue section. The boundary of the fibrin matrix is marked for clarity. BM-MSCs persisted in the infarct after 5 weeks of injection, as shown by immunofluorescence staining of (E) NuMA (green) and connexin-43 (red), and (F) vimentin (green) and connexin-43 (red). The boundaries between infarct and normal myocardium are marked for clarity.
I: Infarct; N: Normal.
Immunohistochemical analysis of neovascularization
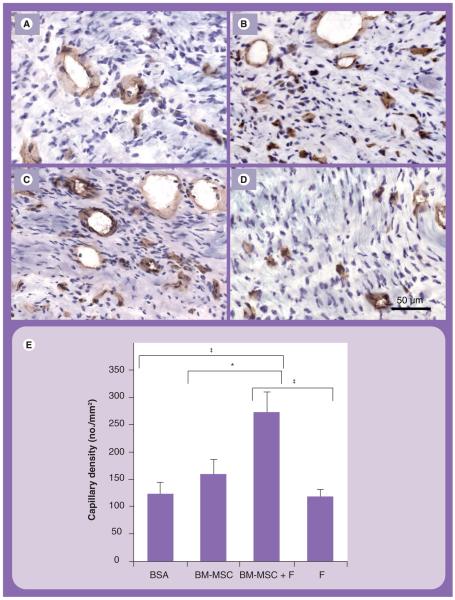
After demonstrating BM-MSC survival in the infarct in the absence of differentiation at 5 weeks after delivery, we assessed whether BM-MSCs had a paracrine effect by enhancing neovasculature formation, and whether the effect could be augmented by fibrin. Indeed, quantification of capillaries revealed that the BM-MSCs in fibrin group had significantly higher capillary density (273 ± 38 microvessels/mm2), in comparison to the BM-MSC (159 ± 28 microvessels/mm2) (p = 0.015), fibrin (118 ± 14 microvessels/mm2) (p < 0.0001) and BSA (123 ± 22 microvessels/mm2) groups (p < 0.0001) (Figures 3A-3E). This finding indicated that the combined delivery of BM-MSCs in fibrin could augment capillary density, when compared with delivery of each component separately.
Figure 3. Capillary density assessment in the infarct area.
Immunohistochemical staining of CD31 for endothelial cells in (A) BSA, (B) BM-MSC, (C) BM-MSC in fibrin and (D) fibrin groups. Samples were counterstained with hematoxylin. (E) Quantification of capillary density.
*p = 0.015; ‡p < 0.0001.
BM-MSC: Bone marrow-derived mesenchymal stem cell; BSA: Bovine serum albumin; F: Fibrin.
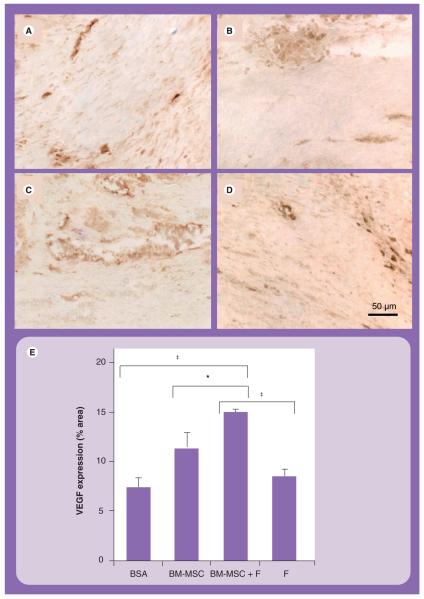
To understand the mechanism governing the enhanced capillary formation due to the combined delivery of BM-MSCs in fibrin, we examined whether VEGF, an angiogenic cytokine known to be secreted by BM-MSCs, was differentially expressed by BM-MSCs between treatment groups [8]. Indeed, semiquantification of the infarct scar VEGF expression (Figures 4A-4E), as assessed by a human-reactive VEGF antibody, demonstrated that the VEGF staining occupied 15 ± 1% of the infarct area in the BM-MSCs in fibrin group, which was significantly higher than that of the BSA group (7 ± 1%; p < 0.0001), BM-MSCs group (11 ± 2%; p = 0.029) and fibrin group (8 ± 1%; p < 0.0001). VEGF staining was more pronounced in regions near vasculature. These data suggested that the augmented VEGF expression level may be involved in the enhanced capillary formation in the BM-MSCs in fibrin group.
Figure 4. VEGF expression in the infarct area.
Immunohistochemical staining of VEGF in (A) BSA, (B) BM-MSC, (C) BM-MSC in fibrin and (D) fibrin groups. (E) Semiquantification of VEGF expression area.
*p = 0.029; ‡p < 0.0001.
BM-MSC: Bone marrow-derived mesenchymal stem cell; BSA: Bovine serum albumin; F: Fibrin.
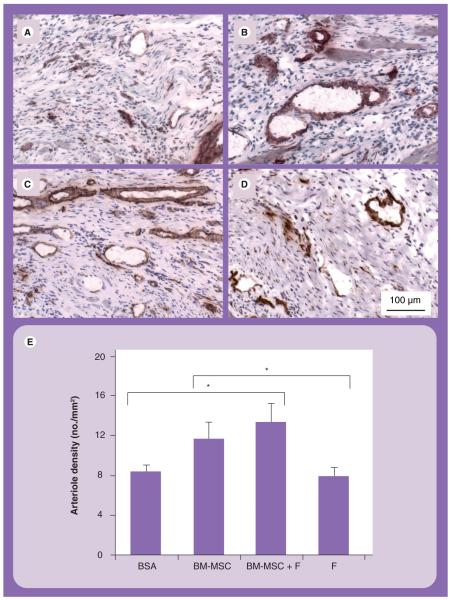
Since arterioles are mature vessels that can contribute to blood perfusion, SMA staining for arterioles was carried out and quantified. Arteriole density analysis revealed that the BM-MSCs in fibrin group had significantly higher arteriole density (13 ± 2 arterioles/mm2), when compared with both the BSA (8 ± 1 arterioles/mm2; p = 0.025) and fibrin (8 ± 1 arterioles/mm2; p = 0.016) groups, but not when compared with the BM-MSCs group (12 ± 2 arterioles/mm2) (Figures 5A-5E). Based on these data, the combined treatment of BM-MSCs and fibrin could enhance capillary formation and VEGF levels, when compared with all other treatment groups, as well as improve arteriole formation when compared with the fibrin or BSA groups.
Figure 5. Arteriogenesis in the infarct area.
Immunohistochemical staining of smooth muscle α-actin for smooth muscle cells in (A) BSA, (B) BM-MSC, (C) BM-MSC in fibrin and (D) fibrin groups. Samples were counterstained with hematoxylin. (E) Quantification of arteriole density.
*p = 0.025, BSA versus BM-MSCs in fibrin; p = 0.016, fibrin versus BM-MSCs in fibrin.
BM-MSC: Bone marrow-derived mesenchymal stem cell; BSA: Bovine serum albumin; F: Fibrin.
Morphometric analysis of infarct size at 5 weeks after therapeutic injections revealed nonsignificant comparisons between treatment groups. The infarct size of the BSA group was 26 ± 9%, which was not significantly different from other treatment groups. These data suggested that treatment of BM-MSCs in fibrin could enhance angiogenesis but could not reduce the infarct size.
Discussion
In the present study, the feasibility of augmenting the angiogenic effect of BM-MSCs using fibrin was demonstrated. We showed that BM-MSCs had robust cell attachment, migration and viability when cultured in fibrin in vitro, a finding in concurrence with published literature [18,19,28]. When delivered together as an injectable therapy to the infarct scar, the BM-MSCs in fibrin treatment could enhance capillary formation. A significant improvement in capillary density was associated with increased levels of VEGF expressed in the infarct scar. Although the present method used for VEGF assessment can only be semiquantified, this technique provided spatial information regarding VEGF localization within the infarct tissue.
The enhancement of VEGF expression after treatment of BM-MSCs in fibrin can provide cardioprotective effects to cardiomyocytes. This finding is supported by in vivo studies in which treatment of BM-MSCs overexpressing VEGF improved cardiac function [29]. In related studies, Markel et al. demonstrated that knockdown of VEGF in BM-MSCs by siRNA impaired VEGF production and diminished stem cell-mediated postischemic myocardial recovery [30].
Although the BM-MSCs demonstrated low cell survival and no evidence of cardiovascular differentiation at 5 weeks after delivery - a finding supported by other reports [31,32] - the transient retention of BM-MSCs may be sufficient to promote the paracrine release of angiogenic cytokines such as VEGF. Moreover, our findings suggest that fibrin could modulate the angiogenic effect of BM-MSCs by promoting significantly increased neovascularization, when compared with the treatment by each individual component. However, the inability of BM-MSCs and fibrin to reduce the infarct size in the present study suggests that other additional treatment factors may be required, such as prosurvival cues or cell-cell interaction [33,34].
The ability of fibrin to regulate BM-MSC behavior has been demonstrated in previous studies [18,28], in which fibrin stimulated BM-MSC proliferation and migration and could modulate BM-MSC phenotype towards cardiovascular lineages. The effect of fibrin on BM-MSCs could be due to factors such as matrix elasticity [35] or specific integrin binding [36]. These potential factors are interesting and warrant further investigation.
An advantage of this approach of delivering BM-MSCs in fibrin is the ability to enhance VEGF levels in the infarct without relying on viral approaches to induce VEGF overexpression [30,37]. Furthermore, the treatment of BM-MSCs in fibrin can potentially become an off-the-shelf therapy. BM-MSCs can be easily isolated from numerous sources and expanded in vitro one million-fold while sustaining multilineage potential [38-40]. Also, BM-MSCs lack MHC II antigens that are responsible for immune rejection, making them a favorable candidate for allogeneic cell transplantation [41]. In addition, fibrin is already an FDA-approved material. Therefore, the delivery of BM-MSCs and fibrin could provide a treatment strategy for patients nearing congestive heart failure who require immediate therapeutic intervention.
In contrast to previous studies that demonstrated angiogenic effects of fibrin treatment alone [14,26], the current study showed no enhancement in neovascularization in the fibrin group. This finding is supported by others and by our previous work showing that fibrin alone does not affect capillary formation or microvascular density [26,42]. The difference in results may also be related to the type of animal model used. Earlier reports suggest that fibrin’s ability to modulate angiogenesis may, in part, be related to its ability to recruit inflammatory cells that secrete angiogenic factors [43]. However, since the animals used in this study were immunocompromised, they may have had a limited ability to respond to the treatment of fibrin. Instead, our results suggest that it may be necessary to deliver both BM-MSCs and fibrin in order to augment capillary formation and VEGF expression for repair of chronic MI in the immunocompromised setting.
Earlier reports on the combined delivery of cells with fibrin for myocardial repair remain limited. Previous studies of delivering myoblasts in fibrin in an acute MI model also demonstrated significant increases in neovascularization and cardiac functional improvement [12,26], but myoblasts lack the ability to form electrical connections with cardiomyocytes and therefore pose the risk of arrhythmia [44-46]. Transplantation of endothelial cells with fibrin into the ischemic myocardium has been shown to improve ejection fraction and neovascularization [47], but endothelial cells also have limited ability to transdifferentiate into cardiomyocytes. The therapeutic effect of delivering bone marrow mononuclear cells alone or in conjunction with fibrin resulted in significantly higher microvascular density and improvement in cardiac function [42,48,49]. In addition, Zhang et al. demonstrated that controlled release of stromal cell-derived factor-1α conjugated to a poly(ethylene glycol) fibrin patch could improve recruitment of c-kit-expressing cells and improve cardiac function [50]. With respect to BM-MSCs and fibrin delivery for myocardial repair, Liu et al. transplanted patches containing BM-MSCs in fibrin onto acutely infarcted porcine hearts and reported enhanced angiogenesis when compared with acellular fibrin constructs [20]. However, the patch approach relied on migration of the BM-MSCs from the patch into the myocardium, a process reported by the authors as only 10% efficient. By contrast, the current study utilizes an injectable delivery system that efficiently delivers the cells locally into the myocardium. To date, this is the only study that has investigated the injectable delivery of BM-MSCs in fibrin using a chronic model of MI.
Conclusion
In summary, we have demonstrated that BM-MSCs have robust cell attachment and viability when cultured in fibrin in vitro. When injected into the chronically infarcted myocardium, the BM-MSCs and fibrin treatment could enhance neovasculature formation by the formation of a greater number of capillaries when compared with delivery of cells or fibrin alone. Concomitant to significant improvement in neovascularization was an increase in VEGF expression in the infarct scar, in comparison to delivery by cells or fibrin alone. This study highlights the role of stem cell-matrix approaches for myocardial repair.
Executive summary.
Introduction
-
□
Bone marrow-derived mesenchymal stem cells (BM-MSCs) are multipotent stem cells that secrete angiogenic factors.
-
□
Fibrin is a biomaterial that provides structural support to cells and tissues.
-
□
The objective of this study was to characterize the attachment and viability of BM-MSCs in fibrin in vitro, and then to assess the efficacy of treatment with BM-MSCs in fibrin for promoting neovascularization in the chronically infarcted myocardium.
Materials & methods
-
□
BM-MSCs were cultured in fibrin and assessed for cell attachment and viability by immunofluorescence staining for F-actin and Live/Dead® viability assays, respectively.
-
□
Chronically infarcted rat hearts were treated with either cells, cells in fibrin, fibrin or saline. After 5 weeks, the infarct scar tissues were assessed for neovascularization.
Results
-
□
BM-MSCs exhibited robust cell attachment and viability when cultured in fibrin in vitro.
-
□
BM-MSCs in fibrin could enhance neovasculature formation by increasing capillary density, in comparison to treatments by cells or fibrin separately.
-
□
BM-MSCs in fibrin could enhance infarct VEGF expression, in comparison to treatments by cells or fibrin separately.
Conclusion
-
□
This study demonstrates the angiogenic potential of combined delivery of BM-MSCs and fibrin, and highlights the advantage of stem cell-matrix approaches for myocardial repair.
Acknowledgments
The authors thank A Wong, P Leng Leong, D Kim and J Chu for technical assistance.
Financial & competing interests disclosure
This work was supported in part by research grants from the NIH (HL078534 and HL083900) to S Li, and research grants from the California Institute of Regenerative Medicine and Nora Eccles Treadwell Foundation to R Lee. N Huang was supported by a graduate fellowship from the National Science Foundation. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Footnotes
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investi gations involving human subjects, informed consent has been obtained from the participants involved.
Bibliography
- 1.Soonpaa MH, Koh GY, Klug MG, Field LJ. Formation of nascent intercalated disks between grafted fetal cardiomyocytes and host myocardium. Science. 1994;264:98–101. doi: 10.1126/science.8140423. [DOI] [PubMed] [Google Scholar]
- 2.Murry CE, Wiseman RW, Schwartz SM, Hauschka SD. Skeletal myoblast transplantation for repair of myocardial necrosis. J. Clin. Invest. 1996;98:2512–2523. doi: 10.1172/JCI119070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kofidis T, Lebl DR, Martinez EC, Hoyt G, Tanaka M, Robbins RC. Novel injectable bioartificial tissue facilitates targeted, less invasive, large-scale tissue restoration on the beating heart after myocardial injury. Circulation. 2005;112:I173–I177. doi: 10.1161/CIRCULATIONAHA.104.526178. [DOI] [PubMed] [Google Scholar]
- 4.Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 5.Shake JG, Gruber PJ, Baumgartner WA, et al. Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. Ann. Thorac. Surg. 2002;73:1919–1925. doi: 10.1016/s0003-4975(02)03517-8. discussion 1926. [DOI] [PubMed] [Google Scholar]
- 6.Nagaya N, Fujii T, Iwase T, et al. Intravenous administration of mesenchymal stem cells improves cardiac function in rats with acute myocardial infarction through angiogenesis and myogenesis. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H2670–H2676. doi: 10.1152/ajpheart.01071.2003. [DOI] [PubMed] [Google Scholar]
- 7.Beeres SL, Atsma DE, van der Laarse A, et al. Human adult bone marrow mesenchymal stem cells repair experimental conduction block in rat cardiomyocyte cultures. J. Am. Coll. Cardiol. 2005;46:1943–1952. doi: 10.1016/j.jacc.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 8.Kinnaird T, Stabile E, Burnett MS, et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ. Res. 2004;94:678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 9.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 10.Keck PJ, Hauser SD, Krivi G, et al. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989;246:1309–1312. doi: 10.1126/science.2479987. [DOI] [PubMed] [Google Scholar]
- 11.Kutschka I, Chen IY, Kofidis T, et al. Collagen matrices enhance survival of transplanted cardiomyoblasts and contribute to functional improvement of ischemic rat hearts. Circulation. 2006;114:I167–I173. doi: 10.1161/CIRCULATIONAHA.105.001297. [DOI] [PubMed] [Google Scholar]
- 12.Christman KL, Fok HH, Sievers RE, Fang Q, Lee RJ. Fibrin glue alone and skeletal myoblasts in a fibrin scaffold preserve cardiac function after myocardial infarction. Tissue Eng. 2004;10:403–409. doi: 10.1089/107632704323061762. [DOI] [PubMed] [Google Scholar]
- 13.Zimmermann WH, Melnychenko I, Wasmeier G, et al. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat. Med. 2006;12:452–458. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]
- 14.Huang NF, Yu J, Sievers R, Li S, Lee RJ. Injectable biopolymers enhance angiogenesis after myocardial infarction. Tissue Eng. 2005;11:1860–1866. doi: 10.1089/ten.2005.11.1860. [DOI] [PubMed] [Google Scholar]
- 15.Davis ME, Hsieh PC, Grodzinsky AJ, Lee RT. Custom design of the cardiac microenvironment with biomaterials. Circ. Res. 2005;97:8–15. doi: 10.1161/01.RES.0000173376.39447.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat. Biotechnol. 2001;19:1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 17.Engelmayr GC, Jr, Cheng M, Bettinger CJ, Borenstein JT, Langer R, Freed LE. Accordion-like honeycombs for tissue engineering of cardiac anisotropy. Nat. Mater. 2008;7:1003–1010. doi: 10.1038/nmat2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang G, Wang X, Wang Z, Zhang J, Suggs L. A PEGylated fibrin patch for mesenchymal stem cell delivery. Tissue Eng. 2006;12:9–19. doi: 10.1089/ten.2006.12.9. [DOI] [PubMed] [Google Scholar]
- 19.Catelas I, Sese N, Wu BM, Dunn JC, Helgerson S, Tawil B. Human mesenchymal stem cell proliferation and osteogenic differentiation in fibrin gels in vitro. Tissue Eng. 2006;12:2385–2396. doi: 10.1089/ten.2006.12.2385. [DOI] [PubMed] [Google Scholar]
- 20.Liu J, Hu Q, Wang Z, et al. Autologous stem cell transplantation for myocardial repair. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H501–H511. doi: 10.1152/ajpheart.00019.2004. [DOI] [PubMed] [Google Scholar]
- 21.Jugdutt BI. Ventricular remodeling after infarction and the extracellular collagen matrix: when is enough enough? Circulation. 2003;108:1395–1403. doi: 10.1161/01.CIR.0000085658.98621.49. [DOI] [PubMed] [Google Scholar]
- 22.Anversa P, Nadal-Ginard B. Myocyte renewal and ventricular remodelling. Nature. 2002;415:240–243. doi: 10.1038/415240a. [DOI] [PubMed] [Google Scholar]
- 23.Strauer BE, Brehm M, Zeus T, et al. Regeneration of human infarcted heart muscle by intracoronary autologous bone marrow cell transplantation in chronic coronary artery disease: the IACT Study. J. Am. Coll. Cardiol. 2005;46:1651–1658. doi: 10.1016/j.jacc.2005.01.069. [DOI] [PubMed] [Google Scholar]
- 24.Huang NF, Patel S, Thakar RG, et al. Myotube assembly on nanofibrous and micropatterned polymers. Nano Lett. 2006;6:537–542. doi: 10.1021/nl060060o. [DOI] [PubMed] [Google Scholar]
- 25.Huang NF, Sievers RE, Park JS, Fang Q, Li S, Lee RJ. A rodent model of myocardial infarction for testing the efficacy of cells and polymers for myocardial reconstruction. Nat. Protocols. 2006;1:1598–1609. doi: 10.1038/nprot.2006.188. [DOI] [PubMed] [Google Scholar]
- 26.Christman KL, Vardanian AJ, Fang Q, Sievers RE, Fok HH, Lee RJ. Injectable fibrin scaffold improves cell transplant survival, reduces infarct expansion, and induces neovasculature formation in ischemic myocardium. J. Am. Coll. Cardiol. 2004;44:654–660. doi: 10.1016/j.jacc.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 27.Tang YL, Zhao Q, Zhang YC, et al. Autologous mesenchymal stem cell transplantation induce VEGF and neovascularization in ischemic myocardium. Regul. Pept. 2004;117:3–10. doi: 10.1016/j.regpep.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Ho W, Tawil B, Dunn JC, Wu BM. The behavior of human mesenchymal stem cells in 3D fibrin clots: dependence on fibrinogen concentration and clot structure. Tissue Eng. 2006;12:1587–1595. doi: 10.1089/ten.2006.12.1587. [DOI] [PubMed] [Google Scholar]
- 29.Matsumoto R, Omura T, Yoshiyama M, et al. Vascular endothelial growth factor-expressing mesenchymal stem cell transplantation for the treatment of acute myocardial infarction. Arterioscler. Thromb. Vasc. Biol. 2005;25:1168–1173. doi: 10.1161/01.ATV.0000165696.25680.ce. [DOI] [PubMed] [Google Scholar]
- 30.Markel TA, Wang Y, Herrmann JL, et al. VEGF is critical for stem cell-mediated cardioprotection and a crucial paracrine factor for defining the age threshold in adult and neonatal stem cell function. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H2308–H2314. doi: 10.1152/ajpheart.00565.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grinnemo KH, Mansson-Broberg A, Leblanc K, et al. Human mesenchymal stem cells do not differentiate into cardiomyocytes in a cardiac ischemic xenomodel. Ann. Med. 2006;38:144–153. doi: 10.1080/07853890500422982. [DOI] [PubMed] [Google Scholar]
- 32.Noiseux N, Gnecchi M, Lopez-Ilasaca M, et al. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol. Ther. 2006;14:840–850. doi: 10.1016/j.ymthe.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 33.Mangi AA, Noiseux N, Kong D, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat. Med. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 34.Wang CC, Chen CH, Lin WW, et al. Direct intramyocardial injection of mesenchymal stem cell sheet fragments improves cardiac functions after infarction. Cardiovasc. Res. 2008;77:515–524. doi: 10.1093/cvr/cvm046. [DOI] [PubMed] [Google Scholar]
- 35.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 36.Schneller M, Vuori K, Ruoslahti E. αvβ3 integrin associates with activated insulin and PDGFβ receptors and potentiates the biological activity of PDGF. EMBO J. 1997;16:5600–5607. doi: 10.1093/emboj/16.18.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X, Hu Q, Mansoor A, et al. Bioenergetic and functional consequences of stem cell-based VEGF delivery in pressure-overloaded swine hearts. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H1393–H1405. doi: 10.1152/ajpheart.00871.2005. [DOI] [PubMed] [Google Scholar]
- 38.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 39.Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ. Res. 2004;95:9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- 40.Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 41.Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringden O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp. Hematol. 2003;31:890–896. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 42.Zhang G, Hu Q, Braunlin EA, Suggs LJ, Zhang J. Enhancing efficacy of stem cell transplantation to the heart with a PEGylated fibrin biomatrix. Tissue Eng. Part A. 2008;14:1025–1036. doi: 10.1089/ten.tea.2007.0289. [DOI] [PubMed] [Google Scholar]
- 43.Zacharowski K, Zacharowski P, Reingruber Petzelbauer P. Fibrin(ogen) and its fragments in the pathophysiology and treatment of myocardial infarction. J. Mol. Med. 2006;84:469–477. doi: 10.1007/s00109-006-0051-7. [DOI] [PubMed] [Google Scholar]
- 44.Menasche P, Hagege AA, Vilquin JT, et al. Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J. Am. Coll. Cardiol. 2003;41:1078–1083. doi: 10.1016/s0735-1097(03)00092-5. [DOI] [PubMed] [Google Scholar]
- 45.Leobon B, Garcin I, Menasche P, Vilquin JT, Audinat E, Charpak S. Myoblasts transplanted into rat infarcted myocardium are functionally isolated from their host. Proc. Natl Acad. Sci. USA. 2003;100:7808–7811. doi: 10.1073/pnas.1232447100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reinecke H, Minami E, Poppa V, Murry CE. Evidence for fusion between cardiac and skeletal muscle cells. Circ. Res. 2004;94:e56–e60. doi: 10.1161/01.RES.0000125294.04612.81. [DOI] [PubMed] [Google Scholar]
- 47.Chekanov V, Akhtar M, Tchekanov G, et al. Transplantation of autologous endothelial cells induces angiogenesis. Pacing Clin. Electrophysiol. 2003;26:496–499. doi: 10.1046/j.1460-9592.2003.00080.x. [DOI] [PubMed] [Google Scholar]
- 48.Ruger BM, Breuss J, Hollemann D, et al. Vascular morphogenesis by adult bone marrow progenitor cells in three-dimensional fibrin matrices. Differentiation. 2008;76:772–783. doi: 10.1111/j.1432-0436.2007.00259.x. [DOI] [PubMed] [Google Scholar]
- 49.Ryu JH, Kim IK, Cho SW, et al. Implantation of bone marrow mononuclear cells using injectable fibrin matrix enhances neovascularization in infarcted myocardium. Biomaterials. 2005;26:319–326. doi: 10.1016/j.biomaterials.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 50.Zhang G, Nakamura Y, Wang X, Hu Q, Suggs LJ, Zhang J. Controlled release of stromal cell-derived factor-1 α in situ increases c-kit+ cell homing to the infarcted heart. Tissue Eng. 2007;13:2063–2071. doi: 10.1089/ten.2006.0013. [DOI] [PubMed] [Google Scholar]