Abstract
The C3H/HeJ inbred mouse strain and the Dundee Experimental Bald Rat (DEBR) strain spontaneously develop adult onset alopecia areata (AA), a cell mediated disease directed against actively growing hair follicles. The low frequency of AA and the inability to predict the stage of AA as it evolves in the naturally occuring C3H/HeJ model of AA can be converted into a highly predictable system by grafting full thickness skin from AA affected mice to normal haired mice of the same strain. The rat DEBR model develops spontaneous AA at a higher frequency than in the mouse model but they are more expensive to use in drug studies due to their larger size. Regardless of the shortcomings of either model, these rodent models can be used succesfully to screen novel or approved drugs for efficacy to treat human AA. Since the pathogenesis of AA follows the canonical lymphocytic co-stimulatory cascade in the mouse AA model, it can be used to screen compounds potentially useful to treat a variety of cell mediated diseases. Efficacy of various agents can easily be screened by simply observing the presence, rate, and cosmetic acceptability of hair regrowth. More sophisticated assays can refine how the drugs induce hair regrowth and evaluate the underlying pathogenesis of AA. Some drugs commonly used to treat human AA patients work equally as well in both rodent models validating their usefulness as models for drug efficacy and safety for human AA.
Keywords: alopecia areata, treatment, animal model, review, diphenylcyclopropenone, squaric acid dibutyl esterase, dinotrochlorobenzene
Introduction
Alopecia areata (AA) is a relatively common disfiguring human cell mediated disease that targets hair follicles in the actively growing (anagen) phase of the hair cycle. It has a complex genetic basis (1) and is often associated with other systemic cell mediated diseases (2). AA is relatively common in the general population, with a lifetime risk of 1.7% (3). Human AA involves patchy hair loss from any hair-bearing region of the body that may progress to total body hair loss. AA may wax or wane on different sites, or the same site, or frequently it will spontaneously resolve with no treatment. AA most commonly affects the scalp but other body regions may also be affected. Extensive or total hair loss involving the scalp is termed alopecia totalis (AT). AA affecting sites in addition to the bald scalp is termed AT/AU or alopecia universalis (AU) if the entire body is affected. This spontaneous, patchy, potentially reversible, non-scarring, hair loss has been the focus of medical research for over 100 years (4). Only recently however, with access to many new biomedical and molecular tools, have concerted attempts been made to understand the susceptibility, activation, pathogenesis, and treatment of AA (5). Progress in understanding the pathogenesis and genetics of AA and developing new therapies was severely hampered until relatively recently due to the lack of appropriate animal models.
In the last decade, AA-like diseases have been recognized in a variety of mammals, in addition to humans, including laboratory rodents, dogs, cats, horses, cattle, and non-human primates (2, 6, 7). A feather loss syndrome in chickens also has many characteristics of AA (8). The most extensively studied mammalian models are spontaneous AA-like diseases in the C3H/HeJ inbred mouse strain (Fig. 1-3), the Dundee Experimental Bald Rat (DEBR; Fig. 4) and, to a lesser extent, several inbred and congenic mouse strains (6, 7, 9-16). These models have been used effectively to work out the immunologic mechanisms and genetic basis of AA, as well as to test the efficacy and safety of drugs known to work to varying degrees on humans with AA. These studies showed that AA is a cell mediated disease targeting anagen stage hair follicles. AA progresses through the canonical lymphocyte costimulatory cascade (17), but many other aspects of its pathogenesis have yet to be elucidated. In particular, the critical first step, identifying the disease initiating antigen(s), has not yet been determined. Regardless, having 2 homologous rodent model systems makes it possible to systematically test compounds for therapeutic efficacy and safety. Recently completed longitudinal gene expression profiles in the mouse model identified many gene pathways and potential drug targets making future efficacy screening studies analytical rather than empirical (Sundberg et al., unpublished data).
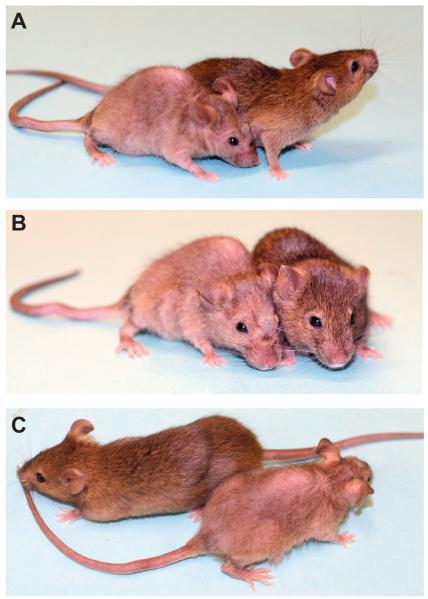
Figure 1.
C3H/HeJ mice with full thickness skin graft-induced alopecia areata compared to the haired control that received normal skin from a normal C3H/HeJ mouse 20 weeks after skin grafts. Here are 3 views of the same mice (A-C) to illustrate the extent of the hair loss. The pale patch on the dorsal thorax is the healed full thickness skin graft from an affected C3H/HeJ mouse.
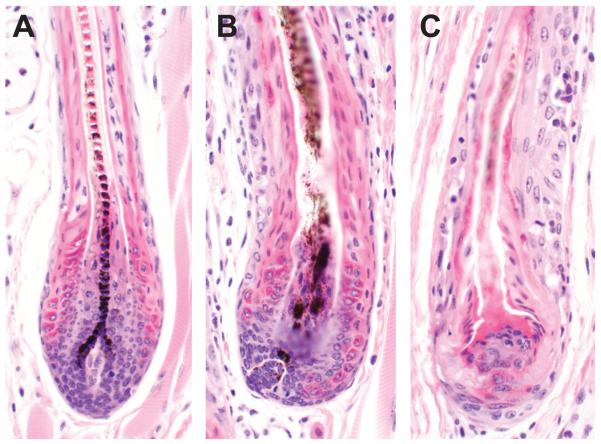
Figure 3.
Bulbs of late anagen hair follicles in normal C3H/HeJ mice form a well organized, pigmented hair fiber (A). By contrast, there are various degrees of follicular dystrophy in the bulb of mice with AA associated with lymphocyte infiltration (B, C).
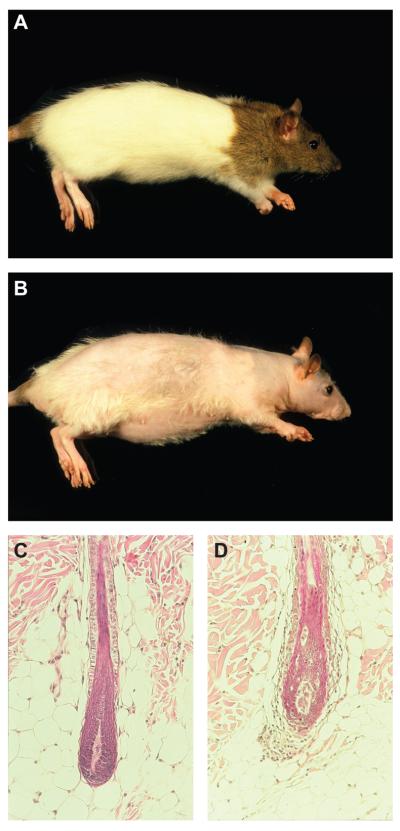
Figure 4.
Normal DEBR rats have a pigmented “hood” around their head and neck (A). By contrast, rats with alopecia areata have a scant hair coat. The pigmented “hood” is still visible as a consequence of melanin incontinence in the dystrophic hair follicles (B). Histologically, late anagen hair follicles in non-AA affected rats are similar to those in mice (C) while those affected with AA have lymphocytes in and around the follicles with various degrees of follicular dystrophy (D).
Concerns remain that the C3H/HeJ and DEBR rat AA models are not the best for human AA (Table 1), although most researchers in the field do consider them to be the best tools currently availabe, especially for novel drug efficacy trials (18). It is always difficult to compare monomorphic rodent models maintained on inbred backgrounds (genetically identical) in highly controlled, pathogen free environments with human patients who have NONE of these advantages. Human AA, like many other diseases that affect this species, consists of numerous very similar diseases lumped together clinically, which makes comparisons to the rodent models controversial at best for those who do not recognize these fundamental differences. The rodent models provide a distinct advantage over human patients as they are highly controlled models and can be reproduced to generate adequate numbers to generate reproducible results.
Table 1.
Comparison of human alopecia areata with similar diseases in the mouse and rat (alopecia areata, AA; alopecia totalis, AT; alopecia universalis, AU).
| Features | Human AA | C3H/HeJ Mouse AA | DEBR Rat AA |
|---|---|---|---|
| Clinical forms | AA, AT, AU | AA, AT, AU | AA, AT, AU |
| Wax and wane | Yes | Yes | Yes |
| Spontaneous hair regrowth | Yes | Yes | Yes |
| Hair fiber defects | Yes | Yes | Yes |
| Nail defects | ≤ 10% of patients | Not C3H but 1/10 strains w/AA | Under investigation |
| Sequella: white hair | Yes, variable | Yes, variable | Yes, variable |
| Hair follicle autoantibodies | Yes, many types | Yes, many types | Yes, many types |
| Dermal/hair follicle lymphocytes | CD4+>CD8+ | CD4+<CD8+ | CD4+>CD8+ |
| Location of lymphocytes | Bulb to sebaceous duct | Suprabulbar to sebaceous duct | Bulb to sebaceous duct |
| Sexual dichotomy | Yes, age dependent | Yes | Yes |
| Response to treatment | Variable | See Table 2 | See Table 2 |
| Research cost | Very expensive | Relatively cheap | More expensive than mice |
| Number of types of AA | 4+ | 1 (slightly different forms in other inbred strains and congenics) |
1 |
| Inheritance | Yes - Unknown | Yes – Polygenic dominant with incomplete penetrance |
Yes – Polygenic dominant with incomplete penetrance |
A number of drugs used to treat human and rodent AA with various degrees of efficacy were tested in several different laboratories. How the efficacy and safety of these drugs were assessed as well as the methodology and formulation of these agents have not been systematically investigated. In order to demonstrate that these rodent AA models AA can be effectively used to screen therapeutic agents for AA, we have summarized the methodology, routes of administration, and results of these trials. This summary is to encourage the use of these rodent AA models to test drugs that are approved by the United States Food and Drug Administration (FDA) for other indications (off-label use) and agents found by analysis of gene array (transcript) data to be potential new therapies for human AA. The ultimate goal of using these rodent models of AA is to devise effective and safe new treatments for human AA patients.
Therapeutic approaches to alopecia areata
Many drugs have been tried in small to large clinical trials on human AA patients (19, 20). The most effective agents are classified as: contact sensitizers, immunosuppressive agents, monoclonal antibodies to block specific molecular pathways, alkylating agents, and hair growth promoters. We summarize the results of these agents used in the rodent AA models below (Table 2).
Table 2.
Drugs tested in alopecia areata rodent models.
| Agent/ Therapy | Route/dose/ Time | Response – Gross Analysis (immunophenotypic analysis) |
Ref. | |
|---|---|---|---|---|
| DPCP | Mouse | Topical unilaterally 16 wks | 9/14 mice regrew hair | (37) |
| Rat | Topical unilaterally 16 wks | 10/10 rats regrew hair, no shifts of lymphocytes |
(37) | |
| SADBE Mouse | 1. Topical 0.1, 0.5, 1% SADBE for 7~ 21wks 2. Topical 0.1-1.0% SADBE weekly for 22 wks |
1. 9/12 (75%) mice successfully treated 2. 9/11 (81.8%) mice regrew hair |
(31) (32) |
|
| FK506 | Mouse | Topical 0.1% FK506 (Tacrolimus) | 4/6 (66.67%) of mice had complete hair regrowth |
(56) |
| Rat | Topical 0.25, 0.1% FK506 for 8 wks | All treated rats regrew hair within 14-21 days | (11) | |
| CsA (Cyclosporin A) Rat |
1. Oral 10mg/kg days/week for 7 wks 2. Topical 0.5% CsA twice a day for 6 wks |
1. Regrowth began after 10 days and whole body and full pelt by 5 wks 2. Regrowth is visible in 2 wks with maximum density at 6 wks |
(55) (48) |
|
| Corticosteroids Mouse | Intralesional 0.5 mg of triamcinolone acetonide |
11 experimental mice developed a short, fine hair coat |
(6) | |
| Anti-B7.1/ B7.2 (CD80/CD86) Mouse |
IP Injection anti-B7.1/ B 7.2, 100μg/ mouse/time/ week/4 wks |
AA onset inhibited in skin-grafted mice by anti-B7.1- and B7.2- specific monoclonal antibodies |
(17) | |
| Anti-CD44v10 Mouse |
IP injection, 150μg -200μg CD44v10 in PBS-buffered saline, 2Xweek for 11 wks |
Anti-CD44v10 inhibited AA onset | (74) | |
| Mechlorethamine Mouse |
Topical 0.02% mechloretha-mine 5 consecutive days/week for 10wks. |
All 24 experimental mice regrew a full pelage | (80) | |
| Anthralin | Mouse | Topical 0.2% anthralin daily for 5 days/ week for 10 wks |
On treated side hair regrew in 9/14 (64.3%) mice with 4 mice had near normal hair density and length |
(111) |
| Rat | Topical 0.1% anthralin for 10 wks | Nearly all rats regrew hair | (58) | |
| Dietary soy oil/ genistein Mouse |
1/3 diets containing 1%, 5% or 20% soy oil 1mg IP genistein 3x week/10 wks |
Of mice on 1%, 5%, and 20% soy oil diets, 43/50 (86%), 11/28 (39%), and 2/11 (18%) developed AA respectively 4/10 (40%) mice injected with genistein and 9/10 (90%) controls developed AA |
(90) | |
| T-box21 (Tbx21) Mouse |
1. Antisense Tbx21, 0.5 mg/kg/day, 0.2 ml subcutaneously every 3 days into 6 alopecic regions 2. Non-sense oligonucleotides (same as above) 3. Tbx21 siRNA, 10 ug subcutaneously every 7 days for 3 times into 8 alopecic lesions 4. Non-sense siRNA 5. Cationized gelatine conjugated Tbx21 siRNA |
1/2. Significantly more effective than non- sense oligonucleotide 3-5. Cationized gelatine-conjugated Tbx21 siRNA injections more effective than naked Tbx21 siRNA or non-sense siRNA conjugated with cationized gelatin |
(18) | |
| Rat anti-mouse IFNG | 0.4 mg/kg in 0.1 ml PBS subcutaneously into 10 alopecic lesions daily for one week |
Improved the hair growth index more efficiently than the negative control |
(18) | |
| Recombinant mouse IL4 | 0.1 ug/0.2 ml subcutaneously daily for 21 days into 10 alopecic lesions intralesional daily, 3 weeks |
Significant and prolonged response compared to control |
(18) | |
Contact Sensitizers used as immunotherapy agents
Topical immunotherapy with contact sensitizers is considered the most effective therapeutic modality (21, 22). The contact sensitizers used are all effective to treat human AA patients with various rates of success (23-26). Contact sensitizers that have been used include diphencyprone (DPCP), squaric acid dibutylester (SADBE), and dinitrochlorobenzene (DNCB) that is no longer used due to its potential mutagenic properties. Topical sensitization to treat AA relies on four characteristics of the contact sensitizer for efficacy and safety: predictable immunomodulation (sensitization of the immune system), absence from the natural environment, lack of cross-reactivity with other substances, and safety (27).
The mechanism by which contact sensitizing agents act to induce hair re-growth is unknown but the hypothetical phenomenon of “antigenic competition” has been proposed. An immune reaction to a given antigen may inhibit the development of the immune response to another unrelated antigen (28). One of the more popular theories is that they redirect the inflammatory response in AA away from the hair follicle and direct it towards the exogenous chemical sensitizer (28). Other hypotheses include a tolerization of AA-specific T cells and the induction of a non-permissive milieu for these cells, or induction by contact sensitizing agent treatment alters trafficking/homing of leukocytes in the affected skin (29, 30). Other mechanisms suggest contact sensitizers alter the AA-associated cytokine profile and that this non-specifically mediates beneficial effects on AA (29, 30).
Squaric acid dibutylester (SADBE)
Freyschmidt-Paul et al. (31) reported that AA-like hair loss in C3H/HeJ mice responded to treatment with SADBE analogous to human AA. Hair regrowth was observed only on the treated side of the dorsal skin in 9 of 12 mice. Histopathologic examination revealed a change in the distribution of the inflammatory infiltrate around the mid and lower regions of hair follicles in untreated skin to a uniform presence in the upper dermis in treated skin. Immunohistomorphometric studies revealed that treatment with SADBE altered the CD4+/CD8+ ratio from approximately 1:2 in untreated AA to 1:1 in treated AA. SADBE treatment also changed the aberrant expression of major histocompatibility complex class I and II in a way similar to that observed in human AA. A similar report by Gardner (32) showed 9 of 11 experimental mice with AA regrew hair on the treated side only and that this was associated with a reduction in peri/intrafollicular inflammatory cell infiltrates, hair follicle dystrophy, melanin incontinence/clumping, and an increase in the numbers of hair follicles in full anagen.
Diphencyprone (DPCP)
Diphencyprone (DPCP) was reported to be a highly variable, but effective, topical immunotherapy for extensive human AA (25, 26, 33-35). However, the mechanisms of DPCP action to induce hair growth in AA patients are still unknown (23). Topical DPCP treatment for AA is effective, well-tolerated, and provides prolonged therapeutic benefits in treatment responsive patients. The two rodent AA models were used to determine if topical DPCP was efficacious (36, 37). Similar to human AA, DPCP induced hair regrowth on the treated skin regions in the majority of the animals. Mice with AA that regrew hair exhibited intrafollicular infiltration of CD4+and CD8+ T lymphocytes prior to hair regrowth. Successful treatment with DPCP was associated with a reduction of the peribulbar lymphocytic infiltrate and development of inflammation in the upper dermis. In rats, there was a paucity of lymphocytic infiltrates in both the untreated and the treated skin and thus no significant shift of lymphocyte localization was apparent. These two models of AA can be used as a tool to further our understanding of human AA and the therapeutic actions of DPCP (36).
Dinitrochlorobenzene (DNCB)
Dinitrochlorobenzene exerts a significant immunomodulatory effect on antigen presenting cells in vitro and in vivo (38, 39). The effect of topically applied DNCB appears to be systemic in nature, with cellular immune responses appearing at distant sites following DNCB application (40, 41). Dendritic cells exposed to DNCB in vivo and in vitro stimulate CD8+ T-cells and natural killer cells (42, 43). A significant finding was that topical DNCB application did not result in change of CD4+ T-cells counts, on the contrary, CD8+ T- cells and natural killer cells counts increased significantly which was presumed via dendritic cell modulation (44). Dearman and Kimber (45) proposed that topical DNCB stimulates Th1 responses in mice. One study obtained more than 75% hair regrowth at the end of 20 weeks of DNCB therapy in 65% of treated human AA patients. However, Summer et al. (46) reported that DNCB was mutagenic against Salmonella typhimurium in the Ames test and should no longer be used. DNCB was tested at one point in DEBR rats with a hair re-growth response, however, the inflammatory response was so severe that the investigators abandoned the study after a few applications. The work was never published but results were listed in a review (15).
Recombinant mouse interleukin 4 (IL4)
Recent studies confirmed that in human AA Th1 reactions are dominant (18). Intraperitoneal injections of recombinant IL4 were used successfully to suppress delayed type hypersensitivity reactions (47). To determine if this approach would have an effect on spontaneous C3H/HeJ mouse AA, intralesional injects of mouse recombinant IL4 were given daily for 3 weeks which significantly restored hair growth which persisted during the 2 month observation period (18).
Gene therapy with T-box 21 (Tbx21)
T-box 21 is a transcription factor involved in Th1 differentiation, the dominant immunological process in AA as mentioned above. TBX21 binds to and augments the expression of interferon gamma (IFNG), which is significantly upregulated in AA skin. Using the sponteneous C3H/HeJ mouse model, antisense Tbx21 oligonucleotide was significantly more effective for resolving AA that the non-sense oligonucleotide. Furthermore, the cationized gelatin-conjugated Tbx21 siRNA intralesional injections were more effective than naked siRNA. Resolution of AA without recurrence persisted during the 2 month observation period. These results suggest that molecular downregulation of the Th1 response can be effectively accomplished, at least in the mouse model, and that modifications in formulation and delivery can improve efficacy (18).
Immunosuppressive Treatment
Cyclosporin A (CsA)
Cyclosporin A (CsA), a cyclic endecapeptide, is a T cell-specific immunosuppressant and, due to its lack of bone marrow toxicity, has assumed a leading role in the therapy of organ transplant rejection (48). CsA is used to treat severe psoriasis and cell mediated diseases such as systemic lupus erythematosus. More recently, it has been used experimentally to treat AA (49, 50). A common side effect of oral CsA is induction of hypertrichosis suggesting that CsA may be an effective treatment for different forms of hair loss, not just for the treatment of AA, but also for androgenetic alopecia (AGA). It also stimulates mouse telogen follicles to enter anagen (51, 52). CsA inhibits T cell activation by inhibition of phosphatase 3, catalytic subunit, alpha isoform (PPP3CA, obsolete name: calcineurin) (53). Because CsA is a specific inhibitor of lymphocyte activation, it may be useful in treating patients with AA (50). However, whether CsA is an effective treatment for AA is controversial (49). Recent studies in mice suggest that CsA may induce hair growth by inhibiting PPP3CA which is needed for nuclear localization of the protein nuclear factor of activated T-cells, cytoplasmic, calcineurin-dependent 1 (NFATC1) in the bulge region. NFATC1 is activated by bone morphogenic protein (BMP) signaling which results in transcriptional repression of cyclin-dependent kinase 4 (Cdk4) and stem cell quiescence (54).
Oliver (55) studied the effect of orally administered CsA (10 mg/kg; 5 days/week for 7 weeks) on established and extensive areas of AA lesions on DEBR rats. New hairs appeared after 10 days of treatment and there was simultaneous regrowth of hair over the whole body with restoration of a full pelt by 5 weeks. Histological examination of CsA treated DEBR rats confirmed the transition from short, dystrophic anagen follicles to long, hair-producing anagen follicles. Concomittantly, renewed hair growth following systemic CsA treatment coincided with depletion of the mononuclear cell infiltrate around lesional follicles as seen in human AA patients (50). Because oral CsA has serious side effects, such as nephrotoxicity, topical CsA potentially could be an effective way to reduce side effects if skin barrier penetration problems are overcome. Verma et al. (48) evaluated the efficacy of topical 0.5% CsA in a liposomal formulation to treat AA in the DEBR model. 15 Rats in all the groups exhibited visible hair regrowth on the CsA treated site after drug application. Histological examination revealed a reduced inflammatory infiltrate and improved hair follicle morphology within the topically treated CsA skin area as compared to the contralateral vehicle treated skin. The results of this proof of concept preliminary study suggested that CsA in lipid vesicle formulations, with and without penetration enhancers, showed some promise as a topical treatment for human AA.
Tacrolimus (FK506)
Recently, a number of publications reported topical Tacrolimus was very efficacious in treating experimental bald animal models, such as mice, rats, and hamsters. Freyschmidt-Paul, et al. (56) reported treating C3H/HeJ mice with AA using topical 0.1% Tacrolimus ointment. Four of six of these Tacrolimus-treated mice showed complete hair regrowth, whereas 1/4 vehicle-treated mice regrew hair. The mice with AA who responded to Tacrolimus had reduced perifollicular infiltrates of CD4+ and CD8+ cells and a decreased expression of MHC class I and II and ICAM1 on hair follicle epithelium as compared to control mice (56). These results suggested that topical Tacrolimus was able to induce hair regrowth in the C3H/HeJ AA model, most likely by suppressing the T cell mediated immune responses. Another study (11) examined the efficacy of the topically applied Tacrolimus in the DEBR rat model. All Tacrolimus-treated DEBR rats regrew hair at the site of drug application within 14-21 days. Growth continued for 3 weeks beyond termination of treatment after which gradual hair loss was observed. No hair growth was seen as a result of vehicle application and hair loss continued on untreated areas and in the untreated control group. Even though Tacrolimus proved effective in inducing hair regrowth in rodent AA models, it has not yet been shown to induce cosmetically acceptable hair regrowth in human AA (57). It is assumed that there is insufficient penetration into human skin due to thickness and barrier differences between human skin and the rodent models' skin.
Anthralin
Anthralin is a widely used topical anti-psoriatic drug that may have an immunomodulating effect on human AA as it does in psoriasis (58). Anthralin remains one of the most effective and most widely used therapeutic agents for psoriasis (59). At the molecular level, anthralin inhibits pro-inflammatory cytokines such as granulocyte macrophage colony stimulating factor (GMCSF), interferon-g (IFNG), interleukin-6 (IL6), interleukin-8 (IL8), and tumor necrosis factor-a (TNFA) produced by activated monocytes and epidermal keratinocytes (59, 60). The mechanism of anthralin's potential beneficial effect in AA is speculative at this time. AA is believed to be an cell mediated disease and a number of pro-inflammatory cytokines have been indicated in the pathogenesis of AA including IL1B, TNFA, and IFNG (22, 61, 62). The inhibitory effects of anthralin on the production of the proinflammatory cytokines might be the mechanism responsible for hair regrowth in AA which was identified by using the DEBR rats (58). When AA affected C3H/HeJ mice were treated daily for 10 weeks on half of the dorsal skin with 0.2% anthralin hair regrowth was observed in 9/14 mice on the treated sides only. Similarly, AA-affected DEBR rats were treated on the dorsal surface using 0.1% anthralin ointment. After six to eight weeks of treatment, follicular activity was reversed with clear regrowth over nearly all of the treated side in all rats whereas the control side remained bald (63). The results indicated that anthralin may be an effective therapy for AA-affected C3H/HeJ mice or DEBR rats promoting a hair growth response similar to that seen with anthralin treatment of human AA. The results also imply that certain cytokines may be involved in the therapeutic effects of anthralin on restoring hair regrowth in these two animal models.
Corticosteroids
Corticosteroids are most often used to treat human AA and intralesional glucocorticosteroids are consistently effective in many studies (64-67). Using the mouse model, 11 AA mice received 0.5 mg of triamcinolone acetonide intralesionally. These mice developed a short, fine hair coat. Microscopically, mice showed either normal, uninvolved telogen follicles or biopsies contained anagen follicles with few inflammatory cell infiltrates (6).
Interferons
It was postulated that upregulation of MHC I in hair follicles was one of the initiating factors in the pathogenesis of AA (68). IFNG can cause increased expression of Major Histocompatibility Complex I (MHC I) and it has been proposed that this could induce AA. To test this hypothesis, two independent labs used recombinant IFNG in unmanipulated mice. One group, using a variety of strains, found alopecia developed in C3H/HeJ but not C3H/HeN mice or other strains evaluated (69). These results were surprising since several C3H substrains, including both C3H/HeJ and C3H/HeN, develop AA with similar frequency (7). This study was repeated independently using much larger study groups of C3H/HeJ (AA susceptible) and C57BL/6J (AA resistant) mice. The second study found that AA occurred with a frequency well within what was considered to be normal background levels for the C3H/HeJ mouse strain and concluded that injection of exogenous IFNG into normal haired mice had no significant AA promoting effect (70). Although induction of AA in mice using various forms of recombinant IFNG yield inconsistent results (69, 71), this remains a reasonable target. Injections of rat anti-mouse IFNG improved the hair growth index more efficiently than control rat IgG in C3H/HeJ mice (18) suggesting this may be a useful approach but more importantly supports IFNG as a target for future drug therapy.
Monoclonal antibodies to block lymphocyte co-stimulatory pathways
Monoclonal antibodies (MoAbs) were used in rodents to test hypotheses not specifically as therapeutic approaches. While they offer interesting new options, MoAb therapy can have complications. These studies summarized below define potential drug targets for future therapeutic developments.
Anti-CD44v 10
The splice variant v10 (CD44v10) is expressd on leukocytes in several cell mediated skin diseases. It is thought to be involved in leukocyte homing into the skin. CD44v10 is also expressed on T cells in AA lesional skin (72). A mouse CD44v10-neutralizing antibody (anti-CD44v10) was reported to impair the delayed-type hypersensitivity (DTH) reaction (73). Using this antibody, Freyschmidt-Paul et al. (74) demonstrated that anti-CD44v10 monoclonal antibodiy (MoAb) was able to inhibit the onset of AA in the C3H/HeJ AA model. Mice were injected intraperitoneally twice a week for 11 weeks with anti-CD44v10. These data showed that anti-CD44v10 inhibited the onset of AA in AA graft induced C3H/HeJ mice. Also the AA graft induced mice treated with anti-CD44v10 had a marked reduction of perifollicular CD8+ lymphocytes and, to a lesser degree, CD4+ cells, as well as a decreased expression of MHC I on hair follicle epithelium as compared to untreated control mice. It is presumed that the anti-CD44v10 antibody impaired immune cell homing (e.g., CD8+ T cells), thereby decreasing the immunocytes in the target tissues.
Anti-B7.1/B7.2 (CD80/CD86); mCTLA-4-mIgG2am; 53±6.72 MoAb/ GK1.5 MoAb
To examine the kinetics of cellular and molecular events leading to hair loss in rodent models of AA, the spontaneous and skin graft induced AA mouse models were used (12). Agents specific for antagonizing lymphocyte co-stimulation pathways were employed in the mouse skin graft model to determine whether interfering with these pathways was sufficient to prevent disease onset. Carroll et al. (17) reported three types of monoclonal antibodies blocked T cell activation through co-stimulatory pathways which prevented onset of AA in mice.
Anti-B7.1/B7.2 (CD80/CD86)
One pathway of T cell activation requires a costimulatory signal via CD28 on the T cell surface interacting with B7 family members on the antigen presenting cell (APC) surface (17). Monoclonal antibodies directed against the antigen-presenting cell (APC) surface markers B7.1 (CD80) and B7.2 (CD86) were predicted to prevent onset of AA in mice. In this study, C3H/HeJ mice that received skin grafts from AA affected mice of the same strain were injected with B7.1-/B7.2-specific monoclonal antibodies intraperitoneally (100 mg per mouse). These antibodies effectively inhibited AA onset in all five skin-grafted mice (17).
mCTLA-4-mIgG2am
Similarly, this study confirmed that mCTLA-4-mIgG2am delayed AA onset. 15 mice that received AA skin grafts and 15 that received normal skin grafts were all injected intraperitoneally with 100 mg of mCTLA-4-mIgG2am per mouse. All mice were injected 1 day prior to receiving the full thickness graft and then three times a week for 1 week after surgery. Because mCTLA-4-mIgG2am effectively competed with CD28 to interact with B7.2, the onset of AA was prevented in 13 of 15 and 14 of 15 mice receiving AA grafts when administered for 1 or 4 weeks, respectively (17).
53±6.72 MoAb/ GK1.5 MoAb
Rat IgG2b isotype MoAb from clones 53±6.72 and GK1.5 target and deplete mouse CD8+ or CD4+ cells, respectively (17). Of 21 spontaneous AA-affected mice, seven received 53±6.72 MoAb, seven received GK1.5 MoAb, and seven received rat IgG. Each mouse received 100 mg of antibody per injection on days 0, 3, 7, and 10 injected intraperitoneally. Mice were permitted to regenerate their CD4+ and CD8+ cell numbers. Of seven AA affected mice depleted of CD4+ cells, three showed significant hair regrowth with the first observation for one mouse on day 27. All AA affected mice responding to CD4+ cell depletion showed hair re-growth by day 34 after initiation of the study. Pelage regeneration reached a maximum by day 41. For seven AA affected mice depleted of CD8+ cells, significant hair regeneration was noted in one and sparse hair growth in three. Three mice showed no re-growth. First hair regrowth was noted by day 31 and all responding mice showed re-growth by day 41. These results suggest that both CD4+ and CD8+ cells are involved in AA.
All the inhibition or modification of AA onset studies described above confirmed the crucial role of T cell activation via APC co-stimulation, blockade of CD28±B7 interactions and CTLA-4-mIgG2am competition with CD28 delayed AA onset. These data are consistent with earlier studies where blockade of T cell migration using an anti-CD44v10 MoAb prevented the onset of AA in the mouse graft model (74). Blockade of parts of the lymphocyte co-stimulatory cascade prevents onset of AA in the mouse AA graft-induced model suggesting that similar approaches in human patients may prevent the development of new AA lesions (17).
Alkylating agents
Mechlorethamine
Mechlorethamine was the first alkylating agent to be tested to treat human malignancies and is still being used to treat a variety of human cancers including leukemia, lymphoma, and other solid tumors (75, 76). The topical form is widely used in the treatment of cutaneous T cell lymphoma (77) and Langerhans cell histiocytosis (78). The biochemical basis of a differential response to mechlorethamine in different cell types is currently unknown. The therapeutic effects of mechlorethamine is primarily due to apoptotic cell death caused by DNA damage through production of free oxygen radicals (79). Tang (80) used 0.02% mechlorethamine to fully restore regrowth of cell mediated-arrested follicles in the C3H/HeJ AA model. Mechlorethamine appears to specifically target activated T lymphocytes in AA affected skin and may down-regulate interleukin-12 (IL12), TNFA, TNFB, and IFNG. It was proposed that these effects may be responsible for mechlorethamine's therapeutic effects in C3H/HeJ mice with AA-like disease. Mechlorethamine may be a promising therapeutic reagent for human AA except for concerns over the long-term effects of DNA damage potentially inducing neoplasia.
Hair growth promotion drugs
Minoxidil
Minoxidil was introduced in the early 1970s as a treatment for hypertension. Hypertrichosis was a common side-effect in those taking minoxidil tablets (81, 82) and included the re-growth of hair in male pattern baldness (83). How minoxidil, a pyrimidine derivative (2, 4-diamino-6-piperidinopyrimidine-3-oxide), promotes hair growth in AA and protects against radiation induced alopecia is currently unknown, but may be due to stimulation of prostaglandin PGE2 or an indirect effect as proposed for AGA (84, 85) In one study, topical minoxidil solutions supplemented with tocopheryl polyethylene glycol succinate (TPGS) in cosolvent systems of various compositions, were designed. These compounds were used to evaluate the efficacy of promoting hair growth after topical application, and the safety in terms of the amount of minoxidil absorbed through the skin into the circulation, using C57BL/6J mice as a model. The results revealed that the addition of 0.5% TPGS was able to enhance hair growth, but an increase in the amount of TPGS to 2% led to deterioration of this effect (86). Although small pilot studies using minoxidil to treat human AA showed possible efficacy, no definitive trials of minoxidil in the rodent models of AA have been reported and should be tested as minoxidil is widely used off-label to treat human AA.
Gonadal steroid hormone regulator agents
Circumstantial observations suggest changes in sex hormone levels may be a modifying factor for susceptibility to mouse AA (6). Studies show that gonadal steroid hormones may have a profound impact on autoimmune disease. Estrogens can either increase or decrease immune system sensitivity depending on hormone levels and the observed immune mechanism (87, 88). McElwee et al. reported that gonadectomy conferred complete resistance to AA for a subset of both females and males and delayed the onset of induced AA by up to 4 weeks in 70% of females and 55% of males (89). The experiment was repeated with gonadectomized female and male mice plus non-gonadectomized mice subcutaneously implanted with capsules containing estradiol or dihydrotestosterone. Their study suggested that gonadal steroid hormones could modulate C3H/HeJ mouse AA with estradiol promoting rapid progression of AA while dihydrotestosterone increased resistance to AA onset. The accelerated AA development in this study may reflect an overwhelming promotion of immune system activation by estradiol (E2). It may also be possible that hair follicles in estrogen supplemented rodents are more likely to enter a prolonged telogen state under adverse conditions such as when challenged by an inflammatory infiltrate. These studies suggest the estrogen receptor pathway regulates both transition of hair follicles from anagen to telogen and modifies immune system activity. Both of these mechanisms may be significant in rodent susceptibility to AA. McElwee et al. also reported that adding phytoestrogens (soy oil) in the diet reduced the numbers of C3H/HeJ mice developing AA after they received AA skin grafts (90).
Chanda et al. (91) found that 17b-Estradiol and ICI-182780 regulated the hair follicle cycle in mice through an estrogen receptor pathway. Estradiol (E2) applied topically twice weekly to mouse skin at doses as low as 1 nmol inhibited hair growth by blocking the transition of the hair follicle from the resting phase (telogen) to the growth phase (anagen). By contrast, topical treatment with the estrogen receptor (ER) antagonist ICI-182780 reversed the effects of E2, and caused a telogen-to-anagen transition and initiate hair growth (91). Furthermore, two other similar studies reported an estrogen receptor anatagonist induced onset of anagen in a dose dependent manner (92, 93).
Alternatively, Kim et al. (94) suggested that the central hypothalamic-pituitary-adrenal axis is involved in alopecia areata. Psychological stress may play a role in AA (95, 96). Kim et al. (94) found AA human patients had higher corticotropin releasing hormone and alpha melanocyte stimulating hormone expression in their epidermis and pilosebaceous units and adrenocorticotropic hormone strongly expressed in the upper epidermis compared to controls. They hypothesized that 1) defects of corticosterone production in AA patients may lead to compensatory increases in corticotrophin releasing hormone, adrenocorticotropic hormone, and alpha melanocyte stimulating hormone, or 2) AA patients may over-react psychological stress that controls. These studies suggest that regulation of the hair follicle cycle in both mice and humans involves an complicated hormonal pathway but also suggests new approaches or drug targets to treat human AA in the future.
Approaches to using rodent models for testing new treatments for alopecia areata
C3H/HeJ Mouse model of AA
Sources
Alopecia areata occurs naturally in up to 20% of C3H/HeJ females by 18 months of age and to a lesser extent in males (6). Inbred C3H/HeJ mice and other C3H substrains can be obtained from many of the commercial vendors. While it has been confirmed as a low penetrance, strain-specific disease in the C3H/HeJ strain (The Jackson Laboratory, Bar Harbor, ME) (6), AA appears to occur spontaneously in several other C3H substrains including the relatively common C3H/HeN strain (7).
Skin graft
Alopecia areata can be reproducibly induced in C3H/HeJ mice by transferring full thickness skin grafts from affected C3H/HeJ mice to 10 week old female recipients (12, 90) These mice develop patchy alopecia consistently within 10 weeks of engraftment with large areas of AA by 20 weeks after surgery. C3H/HeJ mice with AA induced by the skin graft method are available from The Jackson Laboratory (West Sacramento, CA; http://jaxmice.jax.org/services/alopecia_areata.html, http://jaxmice.jax.org/library/notes/504/504b.html).
DEBR rat model
Sources
Approximately 70% of female DEBR develop spontaneous AA lesions covering 50% or more of the skin surface by 18 months of age. The frequency of AA in males is much less, though the hair loss pattern and extent of AA in both male and female rats with AA is similar (97). The unusually high disease phenotype presence in female DEBR makes the spontaneous model a viable option for pharmacologic research and consequently an induced AA rat model has not been developed from this strain.
Attempts have been made to find a commercial vendor or public repository willing to accept and breed the DEBR, but so far without success. Breeding pairs of DEBR rats are available from surviving research colonies (contact: KJ. McElwee for current colony locations).
Routes of Administration
There are various ways to administer drugs to rodents to test for efficacy. These are summarized below with references to provide more specific details on how to conduct these types of studies.
Oral Routes
Mice can be dosed using a gavage tube (98). It is also possible to mix the drug with jelly and let the rodents eat measured portions, a method that was used to treat DEBR rats orally with CsA.
Injectable Routes
Several routes of administration are available. Frequency will depend on route, volume, and tolerance of the compound all depending upon Institutional Animal Care and Use Committee (IACUC) approval. Compounds can be administered intravenously (via the tail vein), intramuscularly, subcutaneously, intradermally, or intraperitoneally. However, due to the extremely thin skin in mice, intradermal injections are difficult to conduct successfully and the result is often subcutaneous injection. This latter method can be done if the mouse is anesthetized and it is done into anagen stage skin where there is just enough thickness to do it. Success can be determined by the degree of resistance to injection and the nature of the epithelial “blebbing” that results. These approaches require relatively minimal restraint and are not necessarily labor intensive. Details on how to perform these methods are provided elsewhere (98).
Topical Routes
Although topical applications are the most practical for human patients, it can be problematic for mice depending upon the vehicle. As such, a variety of approaches may be needed. Compounds in volatile vehicles (such as acetone), represent minor issues. The compound can be applied with a micropipettor, allowed to spread over a marked area (the site can be tattooed to ensure the compound is repeatedly applied to the same site and spread over the same unit area of skin) and allowed to dry. Small amounts can be applied repeatedly allowing for evaporation between applications to maintain volume in a defined area. Aqueous or ointment vehicles are more problematic. We have tried a variety of stick on bubble chambers, compression bandages, and “Elizabethan collars” for mice that are available from various vendors with disasterous results. These should be avoided. Bandaging the graft surgical site has proven to be very effective. A nonstick (Telfa) type pad is placed over the site and a small custom cut vest-shaped elastic bandage is used to hold this in place. The compound is applied under the bandage daily and the bandages are changed weekly or if untoward effects are noted (ulcers, swelling, etc.) (90).
Discussion
Two rodent AA models, the C3H/HeJ mouse (6) and DEBR rat (99), are useful for testing drug efficacy and safety screening. A variety of drugs have been tested to date in both the mouse and rat models, most of which cause some degree of hair growth response in human AA patients. The positive results, although sometimes limited, correlate closely with human trials and serve as a proof of concept that these rodent strains can serve as useful models in drug efficacy trials. Since both mice and rats are used extensively in toxicology studies, they will also be useful for preclinical safety evaluation when using totally novel compounds in the future. Furthermore, the availability and small size of these two rodent models makes them practical tools for screening drugs for efficacy. Lastly, since AA is a T cell mediated cell disease, these models are potentially very useful to screen for effective agents used to treat other T cell mediated diseases. It is very easy to screen a therapeutic response to a specific agent by observing regrowth of hair clinically. Histopathology, immunocytochemistry, and gene expression analysis are but a few of the many additional assays available to further validate results.
Compounds based upon promotion of immunoregulatory mechanisms used to treat human AA are also effective in the rodent AA models as described above. Many prior studies indicate that AA is a polygenic disease with certain genes correlated to AA susceptibility while other genes modify disease severity (1, 16). Loss of hair during active disease is associated with an infiltrate of activated CD4+ cells around the hair follicles, along with a CD8+ peri- and intra-follicular infiltrate (100). The interaction of host antigen presenting cells with injected, AA antigen-specific CD4+ T cells may stimulate naive, endogenous CD8+ cytotoxic T cells, which may then function as specific effector cells and target hair follicles (101, 102). Transfer of CD4+/CD25+ cells has been shown to prevent autoimmune disease in several models (103, 104). The depressed frequency of CD4+/CD25+ cells in AA-affected mice significantly limited the availability of cells for use in AA transfer studies (105). However, McElwee et al. (106) reported an increased resistance to AA development in mice injected with CD4+/CD25+ cells while mice injected with CD4+/CD25− cells developed AA. This study strongly suggested that the prevention of AA development after the transfer/co-transfer of CD4+/CD25+ cells at least partly relied on the high-level of regulatory IL10 cytokine expression.
Two other studies provided evidence that follicular melanocytes may be the targets for activated T cells in AA (107, 108). Control of certain melanocytic peptides is linked to immune response MHC loci, providing another possible point of control of AA by the MHC (109). However, since AA can develop in other substrains of mice with no obvious melanization of hair (white hair) (2), and in regions of white hair induced by dry ice branding in the AA graft model (89), it is unclear if targeting melanin related antigens is critical to the pathogenesis of all types of AA.
There are several potential targets within the mechanism of an autoimmune disease pathogenesis at which new treatment development could be directed. New and improved AA therapies might be based upon down-regulation of autoreactive cells in the lymphocytic infiltrate using an immunosuppressive or immunomodulatory agent. Another approach would be to promote anergy or depletion of autoreactive T cell clones. Protecting hair follicles from injurious effects due to inflammation or assisting hair follicles to block or attenuate the adverse inflammatory activity could be used. Blocking antigen recognition by masking target antigens, specific autoreactive T cell receptors, down- regulating co-stimulatory signalling pathways associated with antigen presentation, or modifying target antigen(s) or their expression to become non-stimulatory to autoreactive T cells are also approaches (110). Finding new drug targets for AA and other human cell mediated diseases by using rodent and other animal models has recently become more feasible with the rapid progress in developing gene array technology to define the genetic basis of signalling networks. This summary of rodent models of AA provides baseline data and approaches to speed up the search for new drugs for human AA.
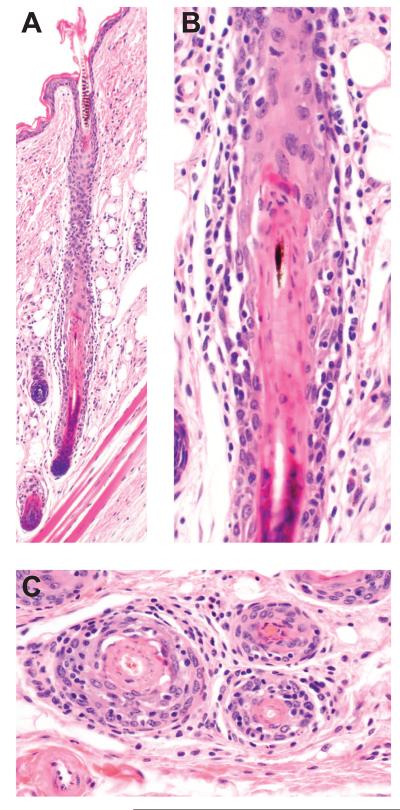
Figure 2.
Histologic features of graft induced alopecia areata in C3H/HeJ mice. A late anagen hair follicle is surrounded and invaded by lymphocytes (A). Disruption of the root sheaths is seen in the higher magnification of A (B) and in cross sections of other affected follicles (C). Earlier stages of anagen and catagen can be affected (not shown).
Acknowledgements
This work was supported by grants from the National Alopecia Areata Foundation (JPS, LEK, KJM), the National Institutes of Health (AR43801, RR173, CA34196 to JPS and 5P30AR041943-13 to LEK), Council for Nail Disorders (JPS), and the Dermatology Foundation/Glaxo Dermatology Fellowship (KJM).
Abbreviations
- AA
alopecia areata
- AT
alopecia totalis
- AU
alopecia universalis
- B7.1/B7.2
currently CD80/CD86
- C3H/HeJ, C3H/HeN, and C57BL/6J
3 inbred mouse strains
- CD28
CD28 antigen
- CD80
CD80 antigen formerly B7-1, B7.1, CD28l, or Ly53
- CD86
CD86 antigen formerly B7-2, B7.2, B70, CD28l2, LY58, or MB7-2
- CsA
cyclosporin A
- CTLA4
cytotoxic T-lymphocyte-associated protein 4
- DEBR
Dundee experimental bald rat
- DPCP
diphenylcyclopropenone
- E2
estradiol
- ER
estrogen receptor
- FK506
Tacrolimus
- GMCSF
granulocyte macrophage colony stimulating factor
- IC-182780
estrogen receptor antagonist
- IL1, 6, 8, 10, and 12
interleukin-1, 6, 8, 10, and 12
- IFNG
interferon gamma
- MHC I
major histocompatibility complex I
- SADBE
squaric acid dibutyl ester
- DNCB
dinitrochlorobenzene
- Tbx21
T-box 21
- TNFA
tumor necrosis factor alpha
- TPGS
tocopheryl polyethylene glycol succinate
Footnotes
Conflict of Interest
This review was written in part to set up guidelines for a National Alopecia Areata Foundation funded preclinical drug program that involves all the authors.
References
- 1.Martinez-Mir A, Zlotogorski A, Gordon D, et al. Genomewide scan for linkage reveals evidence of several susceptibility loci for alopecia areata. Am J Human Genetics. 2007;80:316–328. doi: 10.1086/511442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McElwee KJ, Boggess D, Miller J, King LE, Jr., Sundberg JP. Spontaneous alopecia areata-like hair loss in one congenic and seven inbred laboratory mouse strains. J Investig Dermatol Symp Proc. 1999;4:202–206. doi: 10.1038/sj.jidsp.5640211. [DOI] [PubMed] [Google Scholar]
- 3.Safavi KH, Muller SA, Suman VJ, Moshell AN, Melton LJ., 3rd. Incidence of alopecia areata in Olmsted County, Minnesota, 1975 through 1989. Mayo Clin Proc. 1995;70:628–633. doi: 10.4065/70.7.628. [DOI] [PubMed] [Google Scholar]
- 4.Duckworth D, H VD. Case of area. Celsi (porrigo. decalvans) in which the parts were examined after death. Trans Pathol Soc. 1882:6. [Google Scholar]
- 5.Lu W, Shapiro J, Yu M, et al. Alopecia areata: pathogenesis and potential for therapy. Expert Rev Mol Med. 2006;8:1–19. doi: 10.1017/S146239940601101X. [DOI] [PubMed] [Google Scholar]
- 6.Sundberg JP, Cordy WR, King LE., Jr. Alopecia areata in aging C3H/HeJ mice. J Invest Dermatol. 1994;102:847–856. doi: 10.1111/1523-1747.ep12382416. [DOI] [PubMed] [Google Scholar]
- 7.McElwee KJ, Boggess D, Olivry T, et al. Comparison of alopecia areata in human and nonhuman mammalian species. Pathobiology. 1998;66:90–107. doi: 10.1159/000028002. [DOI] [PubMed] [Google Scholar]
- 8.Smyth JR, Jr., McNeil M. Alopecia areata and universalis in the Smyth chicken model for spontaneous autoimmune vitiligo. J Investig Dermatol Symp Proc. 1999;4:211–215. doi: 10.1038/sj.jidsp.5640213. [DOI] [PubMed] [Google Scholar]
- 9.McElwee KJ, Hoffmann R. Alopecia areata - animal models. Clin Exp Dermatol. 2002;27:410–417. doi: 10.1046/j.1365-2230.2002.01075.x. [DOI] [PubMed] [Google Scholar]
- 10.McElwee KJ, Pickett P, Oliver RF. The DEBR rat, alopecia areata and autoantibodies to the hair follicle. Br J Dermatol. 1996;134:55–63. [PubMed] [Google Scholar]
- 11.McElwee KJ, Rushton DH, Trachy R, Oliver RF. Topical FK506: a potent immunotherapy for alopecia areata? Studies using the Dundee experimental bald rat model. Br J Dermatol. 1997;137:491–497. doi: 10.1111/j.1365-2133.1997.tb03777.x. [DOI] [PubMed] [Google Scholar]
- 12.McElwee KJ, Boggess D, King LE, Jr., Sundberg JP. Experimental induction of alopecia areata-like hair loss in C3H/HeJ mice using full-thickness skin grafts. J Invest Dermatol. 1998;111:797–803. doi: 10.1046/j.1523-1747.1998.00380.x. [DOI] [PubMed] [Google Scholar]
- 13.McElwee KJ, Freyschmidt-Paul P, Sundberg JP, Hoffmann R. The pathogenesis of alopecia areata in rodent models. J Investig Dermatol Symp Proc. 2003;8:6–11. doi: 10.1046/j.1523-1747.2003.12164.x. [DOI] [PubMed] [Google Scholar]
- 14.McElwee KJ, Freyschmidt-Paul P, Zoller M, Hoffmann R. Alopecia areata susceptibility in rodent models. J Investig Dermatol Symp Proc. 2003;8:182–187. doi: 10.1046/j.1087-0024.2003.00806.x. [DOI] [PubMed] [Google Scholar]
- 15.Sundberg JP, Oliver RF, McElwee KJ, King LE., Jr. Alopecia areata in humans and other mammalian species. J Invest Dermatol. 1995;104:32S–33S. doi: 10.1038/jid.1995.51. [DOI] [PubMed] [Google Scholar]
- 16.Sundberg JP, Silva KA, Li R, Cox GA, King LE. Adult-onset Alopecia areata is a complex polygenic trait in the C3H/HeJ mouse model. J Invest Dermatol. 2004;123:294–297. doi: 10.1111/j.0022-202X.2004.23222.x. [DOI] [PubMed] [Google Scholar]
- 17.Carroll JM, McElwee KJ, L E K, Byrne MC, Sundberg JP. Gene array profiling and immunomodulation studies define a cell-mediated immune response underlying the pathogenesis of alopecia areata in a mouse model and humans. J Invest Dermatol. 2002;119:392–402. doi: 10.1046/j.1523-1747.2002.01811.x. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura M, Jo J, Tabata Y, Ishikawa O. Controlled delivery of T-box21 small interfering RNA ameliorates autoimmune alopecia (alopecia areata) in a C3H/HeJ mouse model. Am J Pathol. 2008;172:650–658. doi: 10.2353/ajpath.2008.061249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shapiro J, Lui H, Tron V, Ho V. Systemic cyclosporine and low-dose prednisone in the treatment of chronic severe alopecia areata: a clinical and immunopathologic evaluation. J Am Acad Dermatol. 1997;36:114–117. doi: 10.1016/s0190-9622(97)70342-6. [DOI] [PubMed] [Google Scholar]
- 20.Shapiro J, Tan J, Ho V, Abbott F, Tron V. Treatment of chronic severe alopecia areata with topical diphenylcyclopropenone and 5% minoxidil: a clinical and immunopathologic evaluation. J Am Acad Dermatol. 1993;29:729–735. doi: 10.1016/0190-9622(93)70238-o. [DOI] [PubMed] [Google Scholar]
- 21.Shapiro J, Price VH. Hair regrowth. Therapeutic agents. Dermatol Clin. 1998;16:341–356. doi: 10.1016/s0733-8635(05)70017-6. [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann R, Eicheler W, Huth A, Wenzel E, Happle R. Cytokines and growth factors influence hair growth in vitro. Possible implications for the pathogenesis and treatment of alopecia areata. Arch Dermatol Res. 1996;288:153–156. doi: 10.1007/BF02505825. [DOI] [PubMed] [Google Scholar]
- 23.Aghaei S. Topical immunotherapy of severe alopecia areata with diphenylcyclopropenone (DPCP): experience in an Iranian population. BMC Dermatol. 2005;5:6. doi: 10.1186/1471-5945-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schuttelaar ML, Hamstra JJ, Plinck EP, et al. Alopecia areata in children: treatment with diphencyprone. Br J Dermatol. 1996;135:581–585. [PubMed] [Google Scholar]
- 25.Wiseman MC, Shapiro J, MacDonald N, Lui H. Predictive model for immunotherapy of alopecia areata with diphencyprone. Arch Dermatol. 2001;137:1063–1068. [PubMed] [Google Scholar]
- 26.Galadari I, Rubaie S, Alkaabi J, Galadari H. Diphenylcyclopropenone (diphencyprone, DPCP) in the treatment of chronic severe alopecia areata (AA) Allerg Immunol (Paris) 2003;35:397–401. [PubMed] [Google Scholar]
- 27.McMichael AJ, Henderson RL., Jr. Topical sensitizers in alopecia areata. Dermatol Nurs. 2004;16:333–336. [PubMed] [Google Scholar]
- 28.Happle R. Antigenic competition as a therapeutic concept for alopecia areata. Arch Dermatol Res. 1980;267:109–114. doi: 10.1007/BF00416931. [DOI] [PubMed] [Google Scholar]
- 29.Hoffmann R, Wenzel E, Huth A, et al. Growth factor mRNA levels in alopecia areata before and after treatment with the contact allergen diphenylcyclopropenone. Acta Derm Venereol. 1996;76:17–20. doi: 10.2340/00015555761720. [DOI] [PubMed] [Google Scholar]
- 30.Hoffmann R, Happle R. Topical immunotherapy in alopecia areata. What, how, and why? Dermatol Clin. 1996;14:739–744. doi: 10.1016/s0733-8635(05)70400-9. [DOI] [PubMed] [Google Scholar]
- 31.Freyschmidt-Paul P, Sundberg JP, Happle R, et al. Successful treatment of alopecia areata-like hair loss with the contact sensitizer squaric acid dibutylester (SADBE) in C3H/HeJ mice. J Invest Dermatol. 1999;113:61–68. doi: 10.1046/j.1523-1747.1999.00640.x. [DOI] [PubMed] [Google Scholar]
- 32.Gardner S, Freyschmidt-Paul P, Hoffmann R, et al. Normalisation of hair follicle morphology in C3H/HeJ alopecia areata mice after treatment with squaric acid dibutylester. Eur J Dermatol. 2000;10:443–450. [PubMed] [Google Scholar]
- 33.Bolduc C, Shapiro J. DPCP for the treatment of alopecia areata. Skin Therapy Lett. 2000;5:3–4. [PubMed] [Google Scholar]
- 34.Pericin M, Trueb RM. Topical immunotherapy of severe alopecia areata with diphenylcyclopropenone: evaluation of 68 cases. Dermatology. 1998;196:418–421. doi: 10.1159/000017935. [DOI] [PubMed] [Google Scholar]
- 35.van der Steen PH, Boezeman JB, Happle R. Topical immunotherapy for alopecia areata: re-evaluation of 139 cases after an additional follow-up period of 19 months. Dermatology. 1992;184:198–201. doi: 10.1159/000247540. [DOI] [PubMed] [Google Scholar]
- 36.Tang L, Lui H, Sundberg JP, Bissonnette R, McLean DI, Shapiro J. Restoration of hair growth with topical diphencyprone in mouse and rat models of alopecia areata. J Am Acad Dermatol. 2003;49:1013–1019. doi: 10.1016/s0190-9622(03)02141-8. [DOI] [PubMed] [Google Scholar]
- 37.Shapiro J, Sundberg JP, Bissonnette R, et al. Alopecia areata-like hair loss in C3H/HeJ mice and DEBR rats can be reversed using topical diphencyprone. J Investig Dermatol Symp Proc. 1999;4:239. doi: 10.1038/sj.jidsp.5640219. [DOI] [PubMed] [Google Scholar]
- 38.Stricker RB, Elswood BF. Dendritic cells and dinitrochlorobenzene (DNCB): a new treatment approach to AIDS. Immunol Lett. 1991;29:191–196. doi: 10.1016/0165-2478(91)90169-b. [DOI] [PubMed] [Google Scholar]
- 39.Hanau D, Fabre M, Schmitt DA, et al. ATPase and morphologic changes in Langerhans cells induced by epicutaneous application of a sensitizing dose of DNFB. J Invest Dermatol. 1989;92:689–694. doi: 10.1111/1523-1747.ep12696879. [DOI] [PubMed] [Google Scholar]
- 40.Lee S, Cho CK, Kim JG, Chun SI. Therapeutic effect of dinitrochlorobenzene (DNCB) on verruca plana and verruca vulgaris. Int J Dermatol. 1984;23:624–626. doi: 10.1111/j.1365-4362.1984.tb05705.x. [DOI] [PubMed] [Google Scholar]
- 41.Mills LB. Stimulation of T-cellular immunity by cutaneous application of dinitrochlorobenzene. J Am Acad Dermatol. 1986;14:1089–1090. doi: 10.1016/s0190-9622(86)80189-x. [DOI] [PubMed] [Google Scholar]
- 42.McKinney EC, Streilein JW. On the extraordinary capacity of allogeneic epidermal Langerhans cells to prime cytotoxic T cells in vivo. J Immunol. 1989;143:1560–1564. [PubMed] [Google Scholar]
- 43.Young JW, Steinman RM. Dendritic cells stimulate primary human cytolytic lymphocyte responses in the absence of CD4+ helper T cells. J Exp Med. 1990;171:1315–1332. doi: 10.1084/jem.171.4.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stricker RB, Zhu YS, Elswood BF, et al. Pilot study of topical dinitrochlorobenzene (DNCB) in human immunodeficiency virus infection. Immunol Lett. 1993;36:1–6. doi: 10.1016/0165-2478(93)90060-f. [DOI] [PubMed] [Google Scholar]
- 45.Dearman RJ, Kimber I. Differential stimulation of immune function by respiratory and contact chemical allergens. Immunology. 1991;72:563–570. [PMC free article] [PubMed] [Google Scholar]
- 46.Summer KH, Goggelmann W, Greim H. Glutathione and glutathione S-transferases in the Salmonella mammalian-microsome mutagenicity test. Mutat Res. 1980;70:269–278. doi: 10.1016/0027-5107(80)90018-4. [DOI] [PubMed] [Google Scholar]
- 47.Biedermann T, Mailhammer R, Mai A, et al. Reversal of established delayed type hypersensitivity reactions following therapy with IL-4 or antigen-specific Th2 cells. Eur J Immunol. 2001;31:1582–1591. doi: 10.1002/1521-4141(200105)31:5<1582::AID-IMMU1582>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 48.Verma DD, Verma S, McElwee KJ, Freyschmidt-Paul P, Hoffman R, Fahr A. Treatment of alopecia areata in the DEBR model using Cyclosporin A lipid vesicles. Eur J Dermatol. 2004;14:332–338. [PubMed] [Google Scholar]
- 49.Rallis E, Nasiopoulou A, Kouskoukis C, et al. Oral administration of cyclosporin A in patients with severe alopecia areata. Int J Tissue React. 2005;27:107–110. [PubMed] [Google Scholar]
- 50.Gupta AK, Ellis CN, Cooper KD, et al. Oral cyclosporine for the treatment of alopecia areata. A clinical and immunohistochemical analysis. J Am Acad Dermatol. 1990;22:242–250. doi: 10.1016/0190-9622(90)70032-d. [DOI] [PubMed] [Google Scholar]
- 51.Paus R, Handjiski B, Czarnetzki BM, Eichmuller S. A murine model for inducing and manipulating hair follicle regression (catagen): effects of dexamethasone and cyclosporin A. J Invest Dermatol. 1994;103:143–147. doi: 10.1111/1523-1747.ep12392542. [DOI] [PubMed] [Google Scholar]
- 52.Sawada M, Terada N, Taniguchi H, Tateishi R, Mori Y. Cyclosporin A stimulates hair growth in nude mice. Lab Invest. 1987;56:684–686. [PubMed] [Google Scholar]
- 53.Yamamoto S, Kato R. Hair growth-stimulating effects of cyclosporin A and FK506, potent immunosuppressants. J Dermatol Sci. 1994;7(Suppl):S47–54. doi: 10.1016/0923-1811(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 54.Horsley V, Aliprantis AO, Polak L, Glimcher LH, Fuchs E. NFATc1 balances quiescence and proliferation of skin stem cells. Cell. 2008;132:299–310. doi: 10.1016/j.cell.2007.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oliver RF, Lowe JG. Oral cyclosporin A restores hair growth in the DEBR rat model for alopecia areata. Clin Exp Dermatol. 1995;20:127–131. doi: 10.1111/j.1365-2230.1995.tb02669.x. [DOI] [PubMed] [Google Scholar]
- 56.Freyschmidt-Paul P, Ziegler A, McElwee KJ, et al. Treatment of alopecia areata in C3H/HeJ mice with the topical immunosuppressant FK506 (Tacrolimus) Eur J Dermatol. 2001;11:405–409. [PubMed] [Google Scholar]
- 57.Price VH, Willey A, Chen BK. Topical tacrolimus in alopecia areata. J Am Acad Dermatol. 2005;52:138–139. doi: 10.1016/j.jaad.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 58.Tang L, Cao L, Pelech S, Lui H, Shapiro J. Cytokines and signal transduction pathways mediated by anthralin in alopecia areata-affected Dundee experimental balding rats. J Investig Dermatol Symp Proc. 2003;8:87–90. doi: 10.1046/j.1523-1747.2003.12178.x. [DOI] [PubMed] [Google Scholar]
- 59.Muller K. Antipsoriatic and proinflammatory action of anthralin. Implications for the role of oxygen radicals. Biochem Pharmacol. 1997;53:1215–1221. doi: 10.1016/s0006-2952(96)00732-0. [DOI] [PubMed] [Google Scholar]
- 60.Mrowietz U, Jessat H, Schwarz A, Schwarz T. Anthralin (dithranol) in vitro inhibits human monocytes to secrete IL-6, IL-8 and TNF-alpha, but not IL-1. Br J Dermatol. 1997;136:542–547. [PubMed] [Google Scholar]
- 61.Carroll JM, Crompton T, Seery JP, Watt FM. Transgenic mice expressing IFN-gamma in the epidermis have eczema, hair hypopigmentation, and hair loss. J Invest Dermatol. 1997;108:412–422. doi: 10.1111/1523-1747.ep12289702. [DOI] [PubMed] [Google Scholar]
- 62.Philpott MP, Sanders DA, Bowen J, Kealey T. Effects of interleukins, colony-stimulating factor and tumour necrosis factor on human hair follicle growth in vitro: a possible role for interleukin-1 and tumour necrosis factor-alpha in alopecia areata. Br J Dermatol. 1996;135:942–948. doi: 10.1046/j.1365-2133.1996.d01-1099.x. [DOI] [PubMed] [Google Scholar]
- 63.Tang L, Sundberg JP, Lui H, Shapiro J. Old wine in new bottles: reviving old therapies for alopecia areata using rodent models. J Investig Dermatol Symp Proc. 2003;8:212–216. doi: 10.1046/j.1087-0024.2003.00812.x. [DOI] [PubMed] [Google Scholar]
- 64.Kubeyinje EP, C'Mathur M. Topical tretinoin as an adjunctive therapy with intralesional triamcinolone acetonide for alopecia areata. Clinical experience in northern Saudi Arabia. Int J Dermatol. 1997;36:320. doi: 10.1111/j.1365-4362.1997.tb03060.x. [DOI] [PubMed] [Google Scholar]
- 65.Kubeyinje EP. Intralesional triamcinolone acetonide in alopecia areata amongst 62 Saudi Arabs. East Afr Med J. 1994;71:674–675. [PubMed] [Google Scholar]
- 66.Porter D, Burton JL. A comparison of intra-lesional triamcinolone hexacetonide and triamcinolone acetonide in alopecia areata. Br J Dermatol. 1971;85:272–273. doi: 10.1111/j.1365-2133.1971.tb07230.x. [DOI] [PubMed] [Google Scholar]
- 67.Abell E, Munro DD. Intralesional treatment of alopecia areata with triamcinolone acetonide by jet injector. Br J Dermatol. 1973;88:55–59. doi: 10.1111/j.1365-2133.1973.tb06672.x. [DOI] [PubMed] [Google Scholar]
- 68.Paus R, Slominski A, Czarnetzki BM. Is alopecia areata an autoimmune-response against melanogenesis-related proteins, exposed by abnormal MHC class I expression in the anagen hair bulb? Yale J Biol Med. 1993;66:541–554. [PMC free article] [PubMed] [Google Scholar]
- 69.Gilhar A, Kam Y, Assy B, Kalish RS. Alopecia areata induced in C3H/HeJ mice by interferon-gamma: evidence for loss of immune privilege. J Invest Dermatol. 2005;124:288–289. doi: 10.1111/j.0022-202X.2004.23580.x. [DOI] [PubMed] [Google Scholar]
- 70.Sundberg JP, Silva KA, Edwards K, Black S, Jenson AB, King LE. Failure to induce alopecia areata in C3H/HeJ mice with exogenous interferon gamma. J Exp Anim Sci. 2007;43:265–270. [Google Scholar]
- 71.Siebenhaar F, Sharov AA, Peters EM, et al. Substance P as an immunomodulatory neuropeptide in a mouse model for autoimmune hair loss (alopecia areata) J Invest Dermatol. 2007;127:1489–1497. doi: 10.1038/sj.jid.5700704. [DOI] [PubMed] [Google Scholar]
- 72.Wagner SN, Wagner C, Reinhold U, Funk R, Zoller M, Goos M. Predominant expression of CD44 splice variant v10 in malignant and reactive human skin lymphocytes. J Invest Dermatol. 1998;111:464–471. doi: 10.1046/j.1523-1747.1998.00302.x. [DOI] [PubMed] [Google Scholar]
- 73.Rosel M, Seiter S, Zoller M. CD44v10 expression in the mouse and functional activity in delayed type hypersensitivity. J Cell Physiol. 1997;171:305–317. doi: 10.1002/(SICI)1097-4652(199706)171:3<305::AID-JCP9>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 74.Freyschmidt-Paul P, Seiter S, Zoller M, et al. Treatment with an anti-CD44v10-specific antibody inhibits the onset of alopecia areata in C3H/HeJ mice. J Invest Dermatol. 2000;115:653–657. doi: 10.1046/j.1523-1747.2000.00113.x. [DOI] [PubMed] [Google Scholar]
- 75.Engert A, Wolf J, Diehl V. Treatment of advanced Hodgkin's lymphoma: standard and experimental approaches. Semin Hematol. 1999;36:282–289. [PubMed] [Google Scholar]
- 76.Tesch H, Sieber M, Diehl V. Treatment of advanced stage Hodgkin's disease. Oncology. 2001;60:101–109. doi: 10.1159/000055305. [DOI] [PubMed] [Google Scholar]
- 77.Esteve E, Bagot M, Joly P, et al. A prospective study of cutaneous intolerance to topical mechlorethamine therapy in patients with cutaneous T-cell lymphomas. French Study Group of Cutaneous Lymphomas. Arch Dermatol. 1999;135:1349–1353. doi: 10.1001/archderm.135.11.1349. [DOI] [PubMed] [Google Scholar]
- 78.Hoeger PH, Nanduri VR, Harper JI, Atherton DA, Pritchard J. Long term follow up of topical mustine treatment for cutaneous langerhans cell histiocytosis. Arch Dis Child. 2000;82:483–487. doi: 10.1136/adc.82.6.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moller P, Wassermann K, Damgaard J, Nexo BA, Wallin H. Sensitivity to nitrogen mustard relates to the ability of processing DNA damage in Chinese hamster ovary cells. Pharmacol Toxicol. 2000;86:169–177. doi: 10.1034/j.1600-0773.2000.d01-31.x. [DOI] [PubMed] [Google Scholar]
- 80.Tang L, Cao L, Bernardo O, et al. Topical mechlorethamine restores autoimmune-arrested follicular activity in mice with an alopecia areata-like disease by targeting infiltrated lymphocytes. J Invest Dermatol. 2003;120:400–406. doi: 10.1046/j.1523-1747.2003.12059.x. [DOI] [PubMed] [Google Scholar]
- 81.Limas CJ, Freis ED. Minoxidil in severe hypertension with renal failure. Effect of its addition to conventional antihypertensive drugs. Am J Cardiol. 1973;31:355–361. doi: 10.1016/0002-9149(73)90268-3. [DOI] [PubMed] [Google Scholar]
- 82.Mehta PK, Mamdani B, Shansky RM, Mahurkar SD, Dunea G. Severe hypertension. Treatment with minoxidil. Jama. 1975;233:249–252. [PubMed] [Google Scholar]
- 83.Zappacosta AR. Reversal of baldness in patient receiving minoxidil for hypertension. N Engl J Med. 1980;303:1480–1481. doi: 10.1056/nejm198012183032516. [DOI] [PubMed] [Google Scholar]
- 84.Shapiro J. Hair loss: principles of diagnosis and management of alopecia. Martin Dunitz Ltd; London: 2002. [Google Scholar]
- 85.Uno H, Cappas A, Brigham P. Action of topical minoxidil in the bald stump-tailed macaque. J Am Acad Dermatol. 1987;16:657–668. doi: 10.1016/s0190-9622(87)70084-x. [DOI] [PubMed] [Google Scholar]
- 86.Chen CH, Sheu MT, Wu AB, Lin KP, Ho HO. Simultaneous effects of tocopheryl polyethylene glycol succinate (TPGS) on local hair growth promotion and systemic absorption of topically applied minoxidil in a mouse model. Int J Pharm. 2005;306:91–98. doi: 10.1016/j.ijpharm.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 87.Wilder RL. Hormones, pregnancy, and autoimmune diseases. Ann N Y Acad Sci. 1998;840:45–50. doi: 10.1111/j.1749-6632.1998.tb09547.x. [DOI] [PubMed] [Google Scholar]
- 88.Gilmore W, Weiner LP, Correale J. Effect of estradiol on cytokine secretion by proteolipid protein-specific T cell clones isolated from multiple sclerosis patients and normal control subjects. J Immunol. 1997;158:446–451. [PubMed] [Google Scholar]
- 89.McElwee KJ, Silva K, Beamer WG, King LE, Jr., Sundberg JP. Melanocyte and gonad activity as potential severity modifying factors in C3H/HeJ mouse alopecia areata. Exp Dermatol. 2001;10:420–429. doi: 10.1034/j.1600-0625.2001.100605.x. [DOI] [PubMed] [Google Scholar]
- 90.McElwee KJ, Niiyama S, Freyschmidt-Paul P, et al. Dietary soy oil content and soy-derived phytoestrogen genistein increase resistance to alopecia areata onset in C3H/HeJ mice. Exp Dermatol. 2003;12:30–36. doi: 10.1034/j.1600-0625.2003.120104.x. [DOI] [PubMed] [Google Scholar]
- 91.Chanda S, Robinette CL, Couse JF, Smart RC. 17beta-estradiol and ICI-182780 regulate the hair follicle cycle in mice through an estrogen receptor-alpha pathway. Am J Physiol Endocrinol Metab. 2000;278:E202–210. doi: 10.1152/ajpendo.2000.278.2.E202. [DOI] [PubMed] [Google Scholar]
- 92.Oh HS, Smart RC. An estrogen receptor pathway regulates the telogen-anagen hair follicle transition and influences epidermal cell proliferation. Proc Natl Acad Sci U S A. 1996;93:12525–12530. doi: 10.1073/pnas.93.22.12525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Smart RC, Oh HS. On the effect of estrogen receptor agonists and antagonists on the mouse hair follicle cycle. J Invest Dermatol. 1998;111:175. doi: 10.1046/j.1523-1747.1998.00257.x. [DOI] [PubMed] [Google Scholar]
- 94.Kim HS, Cho DH, Kim HJ, Lee JY, Cho BK, Park HJ. Immunoreactivity of corticotropin-releasing hormone, adrenocorticotropic hormone and alpha-melanocyte-stimulating hormone in alopecia areata. Exp Dermatol. 2006;15:515–522. doi: 10.1111/j.1600-0625.2006.00443.x. [DOI] [PubMed] [Google Scholar]
- 95.De Weert J, Temmerman L, Kint A. Alopecia areata: a clinical study. Dermatologica. 1984;168:224–229. doi: 10.1159/000249708. [DOI] [PubMed] [Google Scholar]
- 96.Muller SA, Winkelmann RK. Alopecia Areata. an Evaluation of 736 Patients. Arch Dermatol. 1963;88:290–297. doi: 10.1001/archderm.1963.01590210048007. [DOI] [PubMed] [Google Scholar]
- 97.McElwee K. Alopecia areata: Spontaneous DEBR rat model. In: Chan LS, editor. Animal models of human inflammatory skin diseases. Boca Ranton; USA: 2003. pp. 451–468. [Google Scholar]
- 98.Boggess D, Silva, KA, Landel, CP, Mobraaten L, Sundberg JP. Approaches to handling, breeding, strain preservation, genotyping and using drug administration for mouse models of cancer. In: Holland EC, editor. Mouse models of human cancer. John Wiley & Sons, Inc.; N.J.: Hoboken: 2004. pp. 3–14. [Google Scholar]
- 99.Michie HJ, Jahoda CA, Oliver RF, Johnson BE. The DEBR rat: an animal model of human alopecia areata. Br J Dermatol. 1991;125:94–100. doi: 10.1111/j.1365-2133.1991.tb06054.x. [DOI] [PubMed] [Google Scholar]
- 100.Todes-Taylor N, Turner R, Wood GS, Stratte PT, Morhenn VB. T cell subpopulations in alopecia areata. J Am Acad Dermatol. 1984;11:216–223. doi: 10.1016/s0190-9622(84)70152-6. [DOI] [PubMed] [Google Scholar]
- 101.Gilhar A, Ullmann Y, Berkutzki T, Assy B, Kalish RS. Autoimmune hair loss (alopecia areata) transferred by T lymphocytes to human scalp explants on SCID mice. J Clin Invest. 1998;101:62–67. doi: 10.1172/JCI551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gilhar A, Shalaginov R, Assy B, Serafimovich S, Kalish RS. Alopecia areata is a T-lymphocyte mediated autoimmune disease: lesional human T-lymphocytes transfer alopecia areata to human skin grafts on SCID mice. J Investig Dermatol Symp Proc. 1999;4:207–210. doi: 10.1038/sj.jidsp.5640212. [DOI] [PubMed] [Google Scholar]
- 103.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 104.Suri-Payer E, Amar AZ, Thornton AM, Shevach EM. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J Immunol. 1998;160:1212–1218. [PubMed] [Google Scholar]
- 105.Zoller M, McElwee KJ, Engel P, Hoffmann R. Transient CD44 variant isoform expression and reduction in CD4(+)/CD25(+) regulatory T cells in C3H/HeJ mice with alopecia areata. J Invest Dermatol. 2002;118:983–992. doi: 10.1046/j.1523-1747.2002.01745.x. [DOI] [PubMed] [Google Scholar]
- 106.McElwee KJ, Freyschmidt-Paul P, Hoffmann R, et al. Transfer of CD8(+) cells induces localized hair loss whereas CD4(+)/CD25(–) cells promote systemic alopecia areata and CD4(+)/CD25(+) cells blockade disease onset in the C3H/HeJ mouse model. J Invest Dermatol. 2005;124:947–957. doi: 10.1111/j.0022-202X.2005.23692.x. [DOI] [PubMed] [Google Scholar]
- 107.Tobin DJ, Sundberg JP, King LE, Jr., Boggess D, Bystryn JC. Autoantibodies to hair follicles in C3H/HeJ mice with alopecia areata-like hair loss. J Invest Dermatol. 1997;109:329–333. doi: 10.1111/1523-1747.ep12335848. [DOI] [PubMed] [Google Scholar]
- 108.Bystryn JC, Tamesis J. Immunologic aspects of hair loss. J Invest Dermatol. 1991;96:88S–89S. doi: 10.1111/1523-1747.ep12858178. [DOI] [PubMed] [Google Scholar]
- 109.Norris DA. How close are we to solving the puzzle? Review of the Alopecia Areata Research Workshop David Norris. J Investig Dermatol Symp Proc. 2003;8:222–225. doi: 10.1046/j.1087-0024.2003.00815.x. [DOI] [PubMed] [Google Scholar]
- 110.Freyschmidt-Paul P, Hoffmann R, Levine E, Sundberg JP, Happle R, McElwee KJ. Current and potential agents for the treatment of alopecia areata. Curr Pharm Des. 2001;7:213–230. doi: 10.2174/1381612013398266. [DOI] [PubMed] [Google Scholar]
- 111.Tang L, Cao L, Sundberg JP, Lui H, Shapiro J. Restoration of hair growth in mice with an alopecia areata-like disease using topical anthralin. Exp Dermatol. 2004;13:5–10. doi: 10.1111/j.0906-6705.2004.00098.x. [DOI] [PubMed] [Google Scholar]