Abstract
Background
Increased systemic cytokine levels, modulators of the immune system, have been repeatedly documented in adult and adolescent major depressive disorder (MDD). This preliminary study extends this work to test the role of cytokines in suicidal symptomatology in adolescent MDD. Hypotheses were that acutely suicidal depressed adolescents would have: (1) increased plasma levels of interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and IL-1β, and (2) a proinflammatory/antiinflammatory cytokine imbalance (indexed by plasma IFN-γ/IL-4), compared to nonsuicidal depressed adolescents and healthy controls.
Methods
Twelve suicidal adolescents with MDD (7 females [58%]; 5 medication-free/naïve), 18 nonsuicidal adolescents with MDD (12 females [67%]; 8 medication-free/naïve), and 15 controls (8 females [53%]) were enrolled. MDD had to be of at least 6 weeks duration, with a minimum severity score of 40 on the Children's Depression Rating Scale–Revised. Plasma cytokines were examined using enzyme-linked immunosorbent assays. Nonparametric tests were used to compare subject groups.
Results
Unexpectedly, suicidal adolescents with MDD had significantly decreased plasma TNF-α concentrations compared to nonsuicidal adolescents with MDD (1.33 ± 2.95 pg/mL versus 30.9 ± 110.9 pg/mL; p = 0.03). IFN-γ was increased in both suicidal and nonsuicidal adolescents with MDD compared to controls (2.14 ± 6.22 and 4.20 ± 14.48 versus 0.37 ± 0.64; p < 0.02, p = 0.005). Findings remained evident when controlled for age and gender.
Conclusions
Our preliminary findings suggest that immune system dysregulation may be associated with suicidal symptomatology in adolescent MDD. These findings should be replicated in larger samples with medication-free adolescents.
Introduction
Suicide is a leading cause of death among teenagers. Although major depressive disorder (MDD) is a major risk factor for suicide in adolescence (Shaffer et al. 1996), most depressed adolescents do not attempt or commit suicide. The recent Food and Drug Administration (FDA) warnings linking antidepressant treatment to suicide, specifically in children and young adults, further highlight the need for specific biological research in adolescent suicide to illuminate patient vulnerability and improve risk assessment. However, to date, little research has examined the biological correlates of suicidal behavior in adolescents with MDD.
Mounting evidence, derived from epidemiological, clinical, and basic science research, has linked immune system activation to a wide variety of neuropsychiatric illnesses, including MDD (Cohen et al. 1998; Peterson et al. 2000; Dantzer et al. 2008; Leslie et al. 2008). One possible pathway implicating the immune system is through the action of cytokines, signaling molecules that mediate key steps in cellular and humoral immunity and that can cross the blood–brain barrier and influence complex brain functions (Dantzer et al. 2008).
Cytokines are divided into two broad subsets: (1) T-helper 1 (Th1) cytokines induce cellular-mediated immunity and include interleukin-12 (IL-12) and interferon-γ (IFN-γ); (2) Th2 cytokines promote humoral immunity and include IL-4 and IL-10. Cytokines are also characterized as proinflammatory (e.g., IFN γ) or antiinflammatory (e.g., IL-4). Clinical case reports worldwide have noted the emergence of depressive symptoms and death by suicide in patients treated with cytokines (Denicoff et al. 1987; Meyers 1999; Capuron et al. 2000; Capuron et al. 2001; Maes et al. 2001; Bonaccorso et al. 2002; Van Gool et al. 2003). In addition, studies in adult MDD have reported increased plasma levels of cytokines, including IFN-γ, tumor necrosis factor-α (TNF-α), IL-6, IL-1β, and IL-12 (Maes et al. 1997; Song et al. 1998; Myint et al. 2005; Irwin and Miller 2007; Kim et al. 2007; Simon et al. 2008).
The few studies that have examined systemic cytokines in suicidal MDD have been conducted in adult patients. Mendlovic et al. (1999) found increased IFN-γ plasma levels in outpatient suicidal adults with MDD compared to controls, and decreased IL-4 and IL-5 plasma levels compared to outpatient nonsuicidal adults with MDD. Kim et al. (2008) found decreased plasma IL-6 levels in suicidal patients with MDD compared to nonsuicidal patients with MDD. Additionally, the Th1/Th2 ratio, as indexed by IL-2/IL-4, was significantly lower in the suicidal group compared to the nonsuicidal MDD group and the control group (Kim et al. 2008). A parallel line of research involving postmortem studies found increased microglial density in specific brain regions in suicide victims (Steiner et al. 2008) and increased mRNA expression of IL-4 and IL-13 in the orbitofrontal cortex of women and men, respectively, who committed suicide (Tonelli et al. 2008). These findings support the notion that immunological regulation may differ between suicidal and nonsuicidal patients with MDD.
Cytokine research in adolescent MDD has been sparse. Recently, we reported increased IFN-γ and IFN-γ/IL-4 in adolescents with MDD relative to controls, suggesting the role of immune system dysregulation with a proinflammatory/antiinflammatory imbalance in this age group as well (Gabbay et al. 2009). The aim of this study was to extend analyses in the previously reported sample of adolescents with MDD and controls (Gabbay et al. 2009) to test the hypothesis that suicidal adolescents with MDD would have elevated IFN-γ, TNF-α, IL-6, and IL-1β plasma levels and a proinflammatory/antiinflammatory cytokine imbalance shifted toward the proinflammatory subset (indexed by plasma IFN-γ/IL-4) compared to nonsuicidal MDD adolescents and healthy controls.
Materials and Methods
Subjects
Subjects are described in a previous report by our laboratory that focuses on immunological indicators of dysregulation in adolescents with MDD (Gabbay et al. 2009). This study extends those findings by examining specific cytokine differences between acutely suicidal adolescents with MDD, nonsuicidal adolescents with MDD, and healthy controls. Briefly, three groups were compared: 12 adolescents with MDD (7 female, 58%), ages 14–19 (mean = 16.6 ± 2.0) with active suicidality, defined as expressing a highly lethal plan for committing suicide that was perceived by the clinician as presenting an imminent risk to self. The second group consisted of 18 nonsuicidal adolescents with MDD (12 female, 67%), ages 12–19 (mean = 16.0 ± 2.0). MDD had to have a minimum duration of 6 weeks and a minimum severity score of 40 on the Children's Depression Rating Scale–Revised (CDRS-R). Adolescents with MDD (suicidal and nonsuicidal) were enrolled from the New York University (NYU) Child Study Center, the NYU Tisch inpatient psychiatric unit, and the Bellevue Department of Psychiatry. The third group consisted of 15 healthy comparison subjects (8 females, 53%), ages 12–19 (mean = 15.5 ± 3.0), recruited from families of NYU staff. Participants ages 18 and 19 (n = 11) provided signed informed consent; those under age 18 provided assent, and a parent or guardian provided signed consent. Consent included authorization for the results of the research to be published. This study was approved by the NYU School of Medicine Institutional Review Board.
A child psychiatrist interviewed all adolescents (patients and controls) and a parent using the Schedule for Affective Disorders and Schizophrenia–Present and Lifetime Version for Children (K-SADS-PL) (Kaufman et al. 1997), a semistructured psychiatric interview. On the basis of interview, the psychiatrist rated severity of depression on the CDRS-R, and overall adjustment on the Children's Global Assessment Scale (CGAS). Participants completed the Beck Depression Inventory, 2nd edition (BDI-II), and the Beck Scale for Suicidal Ideation (BSS). Baseline medical assessments included medical history and laboratory tests, including complete blood count, metabolic panel, liver, and thyroid function tests, urine toxicology test, and a pregnancy test for females. For the medical history assessment, both the parent and the adolescent were interviewed by the clinician to assess for positive indication of an infectious illness in the month prior to enrollment, including the common cold.
Exclusion criteria for all subjects included: immune-affecting medications taken in the past 6 months, any immunological or hematological disorder, chronic fatigue syndrome, any infection during the month prior to the blood draw (including the common cold), significant medical or neurological disorders, and, in females, a positive urine pregnancy test.
The following psychiatric disorders were exclusionary for subjects with MDD: (1) bipolar disorder, (2) schizophrenia, (3) pervasive developmental disorder, (4) posttraumatic stress disorder, (5) obsessive-compulsive disorder, (6) Tourette's disorder, (7) eating disorder, and (8) a substance-related disorder in the past 12 months (based on history and urine toxicology test). Control subjects could not meet criteria for any major current or past Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) (American Psychiatric Association 1994) diagnosis, and could not be taking psychotropic medications.
Cytokine measurements
To address diurnal variability of cytokine production, all blood samples (10 mL) were drawn between 08:00 and 09:00 a.m. after an overnight fast; the EDTA blood was processed within 20 minutes of collection. Aliquots of the plasma samples were stored at −80°C for appropriate serial immunoassay analysis.
The quantitative determination of the plasma levels of IFN-γ, TNF-α, IL-6, IL-1β, and IL-4 was performed in duplicate for each of the serial aliquots by commercial enzyme-linked immunosorbent assays (ELISA) in accordance with the manufacturer's instructions. All thawed samples for the respective cytokines were run simultaneously. The IFN-γ assay utilized was the IFN-γ, HumanHigh-Sensitivity Biotrak Assay (GE-Biosciences, Piscataway, NJ). For TNF-α, also known as cachectin and TNFSF1A, the Quantikine Human TNF-α/TNFSF1A assay (R&D Systems, Minneapolis, MN) was used; IL-6 was assayed with High Sensitivity Human Quantikine IL-6 (R&D Systems, Minneapolis, MN); for IL-1β, also known as IL-1F2, the Quantikine HS Human IL-1β/ILF2 immunoassay (R&D Systems, Minneapolis, MN) was used; and for IL-4, the Quantikine High Sensitivity Human IL-4 assay (R&D Systems, Minneapolis, MN) was used. According to the manufacturers, the lower limits of detection of the assays are: 0.1 pg/mL, 0.038 pg/mL, 0.039 pg/mL, 0.057 pg/mL, and 0.11 pg/mL, respectively. The intraassay variability was less than 10%. The mean of the duplicate sample values was used. All assays were performed by CG, who was blind to the subjects' clinical status.
Statistical analysis
Because data were not normally distributed, the nonparametric Mann–Whitney test was used to compare subject groups. Additionally, to control for possible covariates, analysis of covariance based on ranks was used to compare the groups while adjusting for age and gender. For each outcome measure, the observed levels were converted to ranks, which were then used as the dependent variable. Spearman rank correlation coefficients were used to characterize the association of cytokine levels with the number of past suicide attempts, CDRS-R, BDI-II, and BSS scores, as well as duration of depressive illness.
All reported p values are exact two-sided significance levels. Statistical significance was defined as p ≤ 0.05. SAS version 9.0 (SAS Institute, Cary, NC) was used for all statistical computations.
Results
Participants
Demographics, diagnoses, and treatment profiles are compiled in Table 1.
Table 1.
Demographic and Clinical Characteristics of Suicidal Adolescents with MDD, Nonsuicidal Adolescents with MDD, and Healthy Controls
| Characteristic | Suicidal adolescents with MDD n = 12 | Nonsuicidal adolescents with MDD n = 18 | Healthy controls n = 15 |
|---|---|---|---|
| Age (years) | 16.6 ± 2.0 | 16.0 ± 2.0 | 15.5 ± 3.0 |
| Gender (male/female) | 5/7 (42/58%)a | 6/12 (33/67%)a | 7/8 (47/53%)a |
| Illness history | |||
| Duration of Illness (months) | 23.3 ± 22.3 (3–84)b | 15.5 ± 10.5 (1.5–36)b | 0 |
| History of suicide attempts | 1.6 ± 1.1 (0–3)b | 0.7 ± 0.8 (0–3)b | 0 |
| Medication-naïve/Medication-free/Medicated | 3/2/7 (25/17/58%)a | 7/1/10 (39/6/56%)a | 15/0/0 (100/0/0%)a |
| CDRS-R | 74.2 ± 13.0 (51–97)b | 53.6 ± 12.0 (40–81)b | 17.9 ± 1.7 (17–23)b |
| BDI-II | 32.3 ± 9.7 (19–53)b | 20.2 ± 12.1 (4–50)b | 2.5 ± 3.2 (0–11)b |
| BSS | 21.0 ± 6.6 (6–33)b | 4.0 ± 4.2 (0–13)b | 0.1 ± 0.4 (0–1)b |
| Current co-morbidity | |||
| ADHD | 0 | 4 (22.0%)a | 0 |
| ODD | 1 (8.0%)a | 1 (6.0%)a | 0 |
| Any anxiety disorder | 1 (8.0%)a | 10 (56.0%)a | 0 |
| Any co-morbidity | 2 (17.0%)a | 14 (78.0%)a | 0 |
Respective percentages (may not add up to 100% due to rounding).
Range.
Abbreviations: MDD = major depressive disorder; CDRS-R = children's depression rating scale–revised; BDI-II = Beck Depression Inventory-II; BSS = Beck Scale for Suicidal Ideation; ADHD = attention-deficit/hyperactivity disorder; ODD = oppositional defiant disorder.
Suicidal MDD group
All 12 patients, including 6 patients who had attempted suicide in the previous week, were actively suicidal and were perceived by the clinician as presenting an imminent risk to self at the time of the blood draw. Suicidal plan/methods included medication overdose (prescription and over-the-counter), jumping from the roof, running into a train, hanging, cutting the carotid artery, cutting wrists, and drinking cleaning agents.
Five patients in the suicidal MDD group (42%) were medication free for at least 1 year, 3 of whom were medication naïve. Two patients (17%) had recently initiated psychotropic medications (4 and 7 days prior to the study), and 5 patients (42%) had been treated with medications for periods ranging from 1 month to 2 years. Medications included fluoxetine, sertraline, citalopram, mirtazapine, lithium, lamotrigine, and quetiapine.
Nonsuicidal MDD group
Eight of the 18 patients (44%) were medication-free for at least 1 year, 7 of whom were medication naive, and 10 patients (56%) had been treated with medications for periods ranging from 1 month to 2.5 years. All patients on medication had failed to respond to treatment. Medications included fluoxetine, sertraline, bupropion, lamotrigine, lithium, risperidone, methylphenidate, and mixed amphetamine salt.
Cytokine findings
Means and standard deviations of plasma cytokine levels are summarized in Table 2.
Table 2.
Mean (SD) Levels of Plasma Cytokines in Suicidal Adolescents with MDD, Nonsuicidal Adolescents with MDD, and Controls
| |
|
Adolescents with MDD |
|
|---|---|---|---|
| Cytokine measure | Healthy controls (n = 15) | Suicidal (n = 12) | Nonsuicidal (n = 18) |
| Interferon-γ (IFN-γ) | 0.37 (0.64) | 2.14 (6.22)a | 4.20 (14.48)b |
| Tumor necrosis factor-α (TNF-α) | 4.13 (13.44) | 1.33 (2.95)c | 30.90 (110.9) |
| Interleukin-6 (IL-6) | 0.49 (0.90) | 0.61 (0.72) | 2.12 (3.58) |
| IL-1β | 0.16 (0.05) | 0.18 (0.10) | 0.15 (0.06) |
| IL-4 | 0.28 (0.35) | 0.23 (0.09) | 0.19 (0.08) |
| IFN-γ/interleukin-4 | 1.76 (2.28) | 13.34 (39.98) | 18.82 (66.33)d |
Analysis of covariance is based on ranks adjusting for age and gender.
IFN-γ: suicidal versus controls (Mann–Whitney, p < 0.02; ANCOVA, p < 0.03).
IFN-γ: nonsuicidal versus controls (Mann–Whitney, p = 0.005; ANCOVA, p = 0.005).
TNF-α: suicidal versus nonsuicidal (Mann–Whitney, p = 0.03; ANCOVA, p < 0.03).
IFN-γ/IL-4: nonsuicidal versus controls (Mann–Whitney, p = 0.005; ANCOVA, p = 0.007).
Abbreviations: SD = standard deviation; MDD = major depressive disorder; ANCOVA = analysis of covariance.
Suicidal adolescents with MDD versus nonsuicidal adolescents with MDD
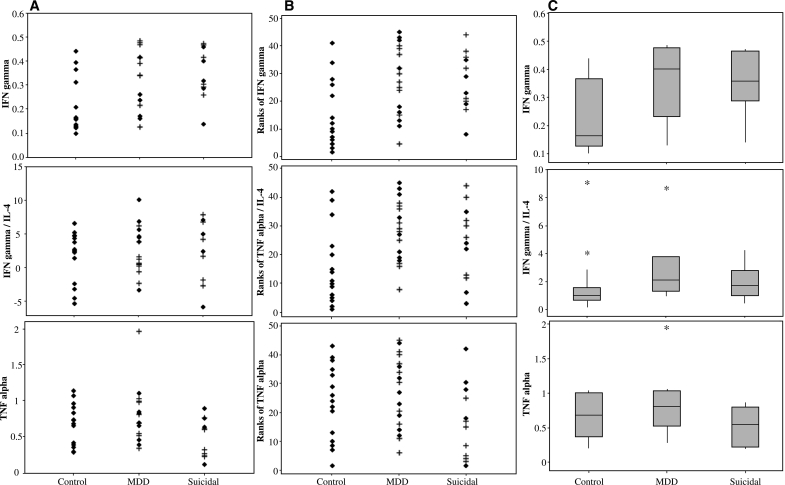
Suicidal adolescents with MDD had significantly decreased plasma levels of TNF-α compared to nonsuicidal adolescents with MDD (1.33 ± 2.95 pg/mL versus 30.90 ± 110.9 pg/mL, rank statistic = 136; p = 0.03). Findings remained evident when analysis was adjusted for age and gender (t = 2.29, degrees of freedom [df ] = 40; p < 0.03). The groups did not differ significantly on any other cytokine measure (Fig. 1 and Table 2).
FIG. 1.
(A) Actual plasma cytokine levels (in pg/mL) for control, nonsuicidal major depressive disorder (MDD), and suicidal adolescents (+, medicated; ♦, medication free/naïve); outliers were deleted to enhance resolution, which did not affect results because a nonparametric test was used for analysis. (B) Ranks of plasma cytokine levels for control, nonsuicidal MDD, and suicidal adolescents (+, medicated; ♦, medication free/naïve) including all data points, with no outliers deleted. (C) Box plots displaying the 25th and 75th percentiles (box), median (line within box), 95% range (whiskers), and moderate outliers (asterisks) of the cytokine data in control, nonsuicidal MDD, and suicidal adolescents; Interferon-γ (IFN-γ) (top), IFN-γ/interleukin-4 (IL-4) ratio (middle), and tumor necrosis factor-α (TNF-α (bottom).
Suicidal adolescents with MDD versus controls
IFN-γ was significantly increased in the suicidal MDD group compared to the control group (2.14 ± 6.22 pg/mL versus 0.37 ± 0.64 pg/mL, rank statistic = 218; p < 0.02). Findings remained evident when analysis was adjusted for age and gender (t = −2.31, df = 40; p < 0.03). No other significant group differences were found.
Nonsuicidal adolescents with MDD versus controls
IFN-γ was significantly increased in the nonsuicidal MDD group compared to the control group (4.20 ± 14.48 pg/mL versus 0.37 ± 0.64 pg/mL, rank statistic = 180; p = 0.005), even when controlled for age and gender (t = −2.94, df = 40; p = 0.005). The IFN-γ/IL-4 ratio was also significantly elevated in the nonsuicidal MDD group compared to the control group (18.82 ± 66.33 versus 1.76 ± 2.28, rank statistic = 179; p = 0.005), even when controlled for age and gender (t = −2.83, df = 40; p = 0.007).
Medicated versus nonmedicated adolescents with MDD
IFN-γ, TNF-α, and IFN-γ/IL-4 levels did not differ significantly between unmedicated and medicated adolescents with MDD (p = 0.5, 0.4, 0.9, respectively).
Correlations of MDD features and cytokine measures
No significant correlations were found between MDD clinical features and cytokine plasma levels.
Discussion
Plasma levels of TNF-α were significantly decreased in suicidal adolescents with MDD compared to the nonsuicidal MDD group. IFN-γ was significantly elevated in the suicidal and nonsuicidal groups compared to controls. Findings remained evident when controlled for age and gender.
Our finding of decreased TNF-α in suicidal adolescents compared to controls was unexpected. TNF-α is produced by macrophages and circulating monocytes and plays an important role in a wide range of immune reactions, including autoimmune conditions (Vassalli 1992). TNF-α has been shown to affect central processes through stimulation of vagal afferents and acts as a central nervous system modulator (Rothwell and Hopkins 1995). Most studies in adult MDD have reported increased TNF-α compared to controls (Tuglu et al. 2003; Leo et al. 2006; Pavon et al. 2006; Kim et al. 2007). By contrast, many studies have reported decreased TNF-α in adults with obsessive compulsive disorder (OCD) (Brambilla et al. 1997; Monteleone et al. 1998; Denys et al. 2004). The interpretation of the similarity between our finding of decreased TNF-α in suicidal adolescents with MDD and parallel findings in adult OCD will require further study.
With respect to the effect on monoamines, systemic administration of TNF-α was shown to result in increased serotonin (5-HT) levels in the prefrontal cortex (PFC) and decreased 5-HT levels in the paraventricular nucleus (Brebner et al. 2000). Relatedly, multiple postmortem studies have implicated the PFC serotonergic system in suicide in adolescents and adults (Mann et al. 1989; Pandey et al. 2002), providing a possible pathway linking decreased TNF-α to suicidality. It is important to note that, to date, cytokine studies have not included assessment of TNF-α, excluding examination of its influence on brain function in suicidal adults (Mendlovic et al. 1999; Kim et al. 2008). Also contributing to the interpretation of the TNF-α finding is the observation that TNF-α knockout mice exhibit increased emotional responses when exposed to stressful conditions (which respond to benzodiazepine treatment), and increased grooming activity compared to wild-type mice (Yamada et al. 2000).
Our second finding of increased plasma IFN-γ in suicidal adolescents with MDD compared to controls is consistent with one prior study of cytokines in suicidal adult patients compared to controls (Mendlovic et al. 1999). However, because we found IFN-γ to be elevated in nonsuicidal MDD patients as well, consistent with many adult MDD studies (Maes et al. 1994; Seidel et al. 1995; Myint et al. 2005; Tsao et al. 2006; Simon et al. 2008), it seems that elevated plasma IFN-γ may be related to major depression rather than to active suicidality. One possible link between IFN-γ, MDD, and suicide is the enzyme indoleamine 2,3-dioxygenase (IDO), the rate-limiting enzyme in the tryptophan-kynurenine pathway that converts tryptophan (TRP) to kynurenine (KYN), and is mainly induced by IFN-γ (Carlin et al. 1989; Takikawa et al. 1991; Taylor and Feng 1991; Daubener and MacKenzie 1999; Currier et al. 2000; Fujigaki et al. 2001). In cases of overstimulation, induction of this pathway may lead to lower TRP concentrations (serotonin substrate), which have been implicated in MDD (Maes et al. 1993; Wirleitner et al. 2003). Of relevance to suicide, low peripheral levels of tryptophan were found in children who had made a recent suicide attempt (Pfeffer et al. 1998). KYN itself can be further metabolized to N-methyl-D-aspartate (NMDA) receptor agonist and antagonist (Stone and Darlington 2002), and alterations in NMDA receptors have also been noted in the PFC of suicide victims (Nowak et al. 1995). However, while IDO is mainly activated by IFN-γ, TNF-α can also synergistically increase IDO transcriptional activation in response to IFN-γ (Robinson et al. 2005), suggesting that IDO activation may be linked to the depressive state rather than to the suicidal state of the patient sample.
Our findings of immune system dysregulation in suicidal adolescents with MDD should be considered preliminary in light of several limiting factors: (1) the cohort size was relatively modest; (2) a substantial proportion of patients (57%) were receiving psychotropic medications, which have been reported to induce negative immunoregulatory effects in adults with MDD. This possible confound may have limited our ability to discern other group differences between cytokines and contributed to a Type II error (false negative). Importantly, there were no statistical differences between medicated and nonmedicated patients with MDD. Furthermore, due to the small sample, we did not apply a multiple comparison correction in order to preserve statistical power. As such, our significant findings may include Type I errors (false positive). Additionally, it would be important to collect data on a larger number of cytokines and to measure cytokine activity across multiple time points.
In summary, our results support the notion that suicidal behavior in the context of adolescent MDD entails immune system dysregulation. Specifically, the role of decreased TNF-α is suggested. These findings require replication in a larger sample of medication-free adolescents.
Footnotes
James S. Babb, Ph.D., New York University School of Medicine, Department of Radiology, Research served as statistical consultant.
This study was supported by grants from the National Institutes of Health (NIH) (AT002395, MH077072), the American Foundation for Suicide Prevention, the NYU School of Medicine General Clinical Research Center grant (M01-RR00096), and generous gifts from the Leon Levy Foundation, the Anita Saltz Foundation, and from Bruce and Claude Wasserstein.
Disclosures
Drs. Vilma Gabbay, Rachel G. Klein, Carmen M. Alonso, Melissa Nishawala, Marta R. Gaite, and Charles J. Gonzalez have no conflicts of interest or financial ties to report. Leah E. Guttman and Yisrael Katz have no conflicts of interest or financial ties to report. At the time of the data collection, Dr. James S. Babb had consulting contracts with E-Z-EM, Inc. and Applied NeuroSolutions, Inc.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington (DC): American Psychiatric Association; 1994. [Google Scholar]
- Bonaccorso S. Marino V. Biondi M. Grimaldi F. Ippoliti F. Maes M. Depression induced by treatment with interferon-alpha in patients affected by hepatitis C virus. J Affect Disord. 2002;72:237–241. doi: 10.1016/s0165-0327(02)00264-1. [DOI] [PubMed] [Google Scholar]
- Brambilla F. Perna G. Bellodi L. Arancio C. Bertani A. Perini G. Carraro C. Gava F. Plasma interleukin-1 beta and tumor necrosis factor concentrations in obsessive-compulsive disorders. Biol Psychiatry. 1997;42:976–981. doi: 10.1016/s0006-3223(96)00495-7. [DOI] [PubMed] [Google Scholar]
- Brebner K. Hayley S. Zacharko R. Merali Z. Anisman H. Synergistic effects of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha: Central monoamine, corticosterone, and behavioral variations. Neuropsychopharmacology. 2000;22:566–580. doi: 10.1016/S0893-133X(99)00166-9. [DOI] [PubMed] [Google Scholar]
- Capuron L. Ravaud A. Dantzer R. Early depressive symptoms in cancer patients receiving interleukin 2 and/or interferon alfa-2b therapy. J Clin Oncol. 2000;18:2143–2151. doi: 10.1200/JCO.2000.18.10.2143. [DOI] [PubMed] [Google Scholar]
- Capuron L. Ravaud A. Gualde N. Bosmans E. Dantzer R. Maes M. Neveu PJ. Association between immune activation and early depressive symptoms in cancer patients treated with interleukin-2-based therapy. Psychoneuroendocrinology. 2001;26:797–808. doi: 10.1016/s0306-4530(01)00030-0. [DOI] [PubMed] [Google Scholar]
- Carlin JM. Borden EC. Sondel PM. Byrne GI. Interferon-induced indoleamine 2,3-dioxygenase activity in human mononuclear phagocytes. J Leukoc Biol. 1989;45:29–34. doi: 10.1002/jlb.45.1.29. [DOI] [PubMed] [Google Scholar]
- Cohen P. Pine DS. Must A. Kasen S. Brook J. Prospective associations between somatic illness and mental illness from childhood to adulthood. Am J Epidemiol. 1998;147:232–239. doi: 10.1093/oxfordjournals.aje.a009442. [DOI] [PubMed] [Google Scholar]
- Currier AR. Ziegler MH. Riley MM. Babcock TA. Telbis VP. Carlin JM. Tumor necrosis factor-alpha and lipopolysaccharide enhance interferon-induced antichlamydial indoleamine dioxygenase activity independently. J Interferon Cytokine Res. 2000;20:369–376. doi: 10.1089/107999000312306. [DOI] [PubMed] [Google Scholar]
- Dantzer R. O'Connor JC. Freund GG. Johnson RW. Kelley KW. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubener W. MacKenzie CR. IFN-gamma activated indoleamine 2,3-dioxygenase activity in human cells is an antiparasitic and an antibacterial effector mechanism. Adv Exp Med Biol. 1999;467:517–524. doi: 10.1007/978-1-4615-4709-9_64. [DOI] [PubMed] [Google Scholar]
- Denicoff KD. Rubinow DR. Papa MZ. Simpson C. Seipp CA. Lotze MT. Chang AE. Rosenstein D. Rosenberg SA. The neuropsychiatric effects of treatment with interleukin-2 and lymphokine-activated killer cells. Ann Intern Med. 1987;107:293–300. doi: 10.7326/0003-4819-107-2-293. [DOI] [PubMed] [Google Scholar]
- Denys D. Fluitman S. Kavelaars A. Heijnen C. Westenberg H. Decreased TNF-alpha and NK activity in obsessive-compulsive disorder. Psychoneuroendocrinology. 2004;29:945–952. doi: 10.1016/j.psyneuen.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Fujigaki S. Saito K. Sekikawa K. Tone S. Takikawa O. Fujii H. Wada H. Noma A. Seishima M. Lipopolysaccharide induction of indoleamine 2,3-dioxygenase is mediated dominantly by an IFN-gamma-independent mechanism. Eur J Immunol. 2001;31:2313–2318. doi: 10.1002/1521-4141(200108)31:8<2313::aid-immu2313>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Gabbay V. Klein RG. Alonso CM. Babb JS. Nishawala M. De Jesus G. Hirsch GS. Hottinger-Blanc PM. Gonzalez CJ. Immune system dysregulation in adolescent major depressive disorder. J Affect Disord. 2009;115:177–182. doi: 10.1016/j.jad.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR. Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav Immun. 2007;21:374–383. doi: 10.1016/j.bbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Kaufman J. Birmaher B. Brent D. Rao U. Flynn C. Moreci P. Williamson D. Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kim YK. Na KS. Shin KH. Jung HY. Choi SH. Kim JB. Cytokine imbalance in the pathophysiology of major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1044–1053. doi: 10.1016/j.pnpbp.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Kim YK. Lee SW. Kim SH. Shim SH. Han SW. Choi SH. Lee BH. Differences in cytokines between non-suicidal patients and suicidal patients in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:356–361. doi: 10.1016/j.pnpbp.2007.08.041. [DOI] [PubMed] [Google Scholar]
- Leo R. Di Lorenzo G. Tesauro M. Razzini C. Forleo GB. Chiricolo G. Cola C. Zanasi M. Troisi A. Siracusano A. Lauro R. Romeo F. Association between enhanced soluble CD40 ligand and proinflammatory and prothrombotic states in major depressive disorder: Pilot observations on the effects of selective serotonin reuptake inhibitor therapy. J Clin Psychiatry. 2006;67:1760–1766. doi: 10.4088/jcp.v67n1114. [DOI] [PubMed] [Google Scholar]
- Leslie DL. Kozma L. Martin A. Landeros A. Katsovich L. King RA. Leckman JF. Neuropsychiatric disorders associated with streptococcal infection: A case-control study among privately insured children. J Am Acad Child Adolesc Psychiatry. 2008;47:1166–1172. doi: 10.1097/CHI.0b013e3181825a3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M. Bonaccorso S. Marino V. Puzella A. Pasquini M. Biondi M. Artini M. Almerighi C. Meltzer H. Treatment with interferon-alpha (IFN alpha) of hepatitis C patients induces lower serum dipeptidyl peptidase IV activity, which is related to IFN alpha-induced depressive and anxiety symptoms and immune activation. Mol.Psychiatry. 2001;6:475–480. doi: 10.1038/sj.mp.4000872. [DOI] [PubMed] [Google Scholar]
- Maes M. Meltzer HY. Scharpe S. Bosmans E. Suy E. De MI. Calabrese J. Cosyns P. Relationships between lower plasma L-tryptophan levels and immune-inflammatory variables in depression. Psychiatry Res. 1993;49:151–165. doi: 10.1016/0165-1781(93)90102-m. [DOI] [PubMed] [Google Scholar]
- Maes M. Scharpe S. Meltzer HY. Okayli G. Bosmans E. D'Hondt P. Vanden Bossche BV. Cosyns P. Increased neopterin and interferon-gamma secretion and lower availability of L-tryptophan in major depression: Further evidence for an immune response. Psychiatry Res. 1994;54:143–160. doi: 10.1016/0165-1781(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Maes M. Bosmans E. De Jongh R. Kenis G. Vandoolaeghe E. Neels H. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine. 1997;9:853–858. doi: 10.1006/cyto.1997.0238. [DOI] [PubMed] [Google Scholar]
- Mann JJ. Arango V. Marzuk PM. Theccanat S. Reis DJ. Evidence for the 5-HT hypothesis of suicide. A review of post-mortem studies. Br J Psychiatry. 1989;155:7–14. [PubMed] [Google Scholar]
- Mendlovic S. Mozes E. Eilat E. Doron A. Lereya J. Zakuth V. Spirer Z. Immune activation in non-treated suicidal major depression. Immunol Lett. 1999;67:105–108. doi: 10.1016/s0165-2478(98)00145-x. [DOI] [PubMed] [Google Scholar]
- Meyers CA. Mood and cognitive disorders in cancer patients receiving cytokine therapy. Adv Exp Med Biol. 1999;461:75–81. doi: 10.1007/978-0-585-37970-8_5. [DOI] [PubMed] [Google Scholar]
- Monteleone P. Catapano F. Fabrazzo M. Tortorella A. Maj M. Decreased blood levels of tumor necrosis factor-alpha in patients with obsessive-compulsive disorder. Neuropsychobiology. 1998;37:182–185. doi: 10.1159/000026500. [DOI] [PubMed] [Google Scholar]
- Myint AM. Leonard BE. Steinbusch HW. Kim YK. Th1, Th2, and Th3 cytokine alterations in major depression. J Affect Disord. 2005;88:167–173. doi: 10.1016/j.jad.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Nowak G. Ordway GA. Paul IA. Alterations in the N-methyl-D-aspartate (NMDA) receptor complex in the frontal cortex of suicide victims. Brain Res. 1995;675:157–164. doi: 10.1016/0006-8993(95)00057-w. [DOI] [PubMed] [Google Scholar]
- Pandey GN. Dwivedi Y. Rizavi HS. Ren X. Pandey SC. Pesold C. Roberts RC. Conley RR. Tamminga CA. Higher expression of serotonin 5-HT(2A) receptors in the postmortem brains of teenage suicide victims. Am J Psychiatry. 2002;159:419–429. doi: 10.1176/appi.ajp.159.3.419. [DOI] [PubMed] [Google Scholar]
- Pavon L. Sandoval-Lopez G. Eugenia Hernandez M. Loria F. Estrada I. Perez M. Moreno J. Avila U. Leff P. Anton B. Heinze G. Th2 cytokine response in Major Depressive Disorder patients before treatment. J Neuroimmunol. 2006;172:156–165. doi: 10.1016/j.jneuroim.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Peterson BS. Leckman JF. Tucker D. Scahill L. Staib L. Zhang H. King R. Cohen DJ. Gore JC. Lombroso P. Preliminary findings of antistreptococcal antibody titers and basal ganglia volumes in tic, obsessive-compulsive, and attention deficit/hyperactivity disorders. Arch Gen Psychiatry. 2000;57:364–372. doi: 10.1001/archpsyc.57.4.364. [DOI] [PubMed] [Google Scholar]
- Pfeffer CR. McBride PA. Anderson GM. Kakuma T. Fensterheim L. Khait V. Peripheral serotonin measures in prepubertal psychiatric inpatients and normal children: Associations with suicidal behavior and its risk factors. Biol Psychiatry. 1998;44:568–577. doi: 10.1016/s0006-3223(98)00020-1. [DOI] [PubMed] [Google Scholar]
- Robinson CM. Hale PT. Carlin JM. The role of IFN-gamma and TNF-alpha-responsive regulatory elements in the synergistic induction of indoleamine dioxygenase. J Interferon Cytokine Res. 2005;25:20–30. doi: 10.1089/jir.2005.25.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell NJ. Hopkins SJ. Cytokines and the nervous system II: Actions and mechanisms of action. Trends Neurosci. 1995;18:130–136. doi: 10.1016/0166-2236(95)93890-a. [DOI] [PubMed] [Google Scholar]
- Seidel A. Arolt V. Hunstiger M. Rink L. Behnisch A. Kirchner H. Cytokine production and serum proteins in depression. Scand J Immunol. 1995;41:534–538. doi: 10.1111/j.1365-3083.1995.tb03604.x. [DOI] [PubMed] [Google Scholar]
- Shaffer D. Gould MS. Fisher P. Trautman P. Moreau D. Kleinman M. Flory M. Psychiatric diagnosis in child and adolescent suicide. Arch Gen Psychiatry. 1996;53:339–348. doi: 10.1001/archpsyc.1996.01830040075012. [DOI] [PubMed] [Google Scholar]
- Simon NM. McNamara K. Chow CW. Maser RS. Papakostas GI. Pollack MH. Nierenberg AA. Fava M. Wong KK. A detailed examination of cytokine abnormalities in Major Depressive Disorder. Eur Neuropsychopharmacol. 2008;18:230–233. doi: 10.1016/j.euroneuro.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C. Lin A. Bonaccorso S. Heide C. Verkerk R. Kenis G. Bosmans E. Scharpe S. Whelan A. Cosyns P. De Jongh R. Maes M. The inflammatory response system and the availability of plasma tryptophan in patients with primary sleep disorders and major depression. J Affect Disord. 1998;49:211–219. doi: 10.1016/s0165-0327(98)00025-1. [DOI] [PubMed] [Google Scholar]
- Steiner J. Bielau H. Brisch R. Danos P. Ullrich O. Mawrin C. Bernstein HG. Bogerts B. Immunological aspects in the neurobiology of suicide: Elevated microglial density in schizophrenia and depression is associated with suicide. J Psychiatr Res. 2008;42:151–157. doi: 10.1016/j.jpsychires.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Stone TW. Darlington LG. Endogenous kynurenines as targets for drug discovery and development. Nat Rev Drug Discov. 2002;1:609–620. doi: 10.1038/nrd870. [DOI] [PubMed] [Google Scholar]
- Takikawa O. Habara-Ohkubo A. Yoshida R. Induction of indoleamine 2,3-dioxygenase in tumor cells transplanted into allogeneic mouse: Interferon-gamma is the inducer. Adv Exp Med Biol. 1991;294:437–444. doi: 10.1007/978-1-4684-5952-4_40. [DOI] [PubMed] [Google Scholar]
- Taylor MW. Feng GS. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991;5:2516–2522. [PubMed] [Google Scholar]
- Tonelli LH. Stiller J. Rujescu D. Giegling I. Schneider B. Maurer K. Schnabel A. Moller HJ. Chen HH. Postolache TT. Elevated cytokine expression in the orbitofrontal cortex of victims of suicide. Acta Psychiatr Scand. 2008;117:198–206. doi: 10.1111/j.1600-0447.2007.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao CW. Lin YS. Chen CC. Bai CH. Wu SR. Cytokines and serotonin transporter in patients with major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:899–905. doi: 10.1016/j.pnpbp.2006.01.029. [DOI] [PubMed] [Google Scholar]
- Tuglu C. Kara SH. Caliyurt O. Vardar E. Abay E. Increased serum tumor necrosis factor-alpha levels and treatment response in major depressive disorder. Psychopharmacology (Berl) 2003;170:429–433. doi: 10.1007/s00213-003-1566-z. [DOI] [PubMed] [Google Scholar]
- Van Gool AR. Fekkes D. Kruit WH. Mulder PG. Ten Hagen TL. Bannink M. Maes M. Eggermont AM. Serum amino acids, biopterin and neopterin during long-term immunotherapy with interferon-alpha in high-risk melanoma patients. Psychiatry Res. 2003;119:125–132. doi: 10.1016/s0165-1781(03)00113-6. [DOI] [PubMed] [Google Scholar]
- Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10:411–452. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- Wirleitner B. Neurauter G. Schrocksnadel K. Frick B. Fuchs D. Interferon-gamma-induced conversion of tryptophan: Immunologic and neuropsychiatric aspects. Curr Med Chem. 2003;10:1581–1591. doi: 10.2174/0929867033457179. [DOI] [PubMed] [Google Scholar]
- Yamada K. Iida R. Miyamoto Y. Saito K. Sekikawa K. Seishima M. Nabeshima T. Neurobehavioral alterations in mice with a targeted deletion of the tumor necrosis factor-alpha gene: Implications for emotional behavior. J Neuroimmunol. 2000;111:131–138. doi: 10.1016/s0165-5728(00)00375-1. [DOI] [PubMed] [Google Scholar]