Abstract
The selective delivery of small interfering RNA (siRNA) to metastatic tumors remains a challenging task. We have developed a nanoparticle (NP) formulation composed of siRNA, a carrier DNA, a polycationic peptide, and cationic liposomes. The NP was obtained by a self-assembling process, followed by surface modification with a polyethylene glycol (PEG)-conjugated ligand, anisamide. The NP was PEGylated and a ligand was presented to target sigma receptor–expressing murine melanoma cells, B16F10. The lung metastasis model was established by intravenous (IV) injection of the B16F10 cells into C57BL/6 mice. A mixture of siRNA against MDM2, c-myc, and vascular endothelial growth factor (VEGF) co-formulated in the targeted NP caused simultaneous silencing of each of the oncogenes in the metastatic nodules. Two consecutive IV injections of siRNA in the targeted NP significantly reduced the lung metastasis (~70–80%) at a relatively low dose (0.45 mg/kg), whereas free siRNA and the nontargeted NP showed little effect. This targeted NP formulation significantly prolonged the mean survival time of the animals by 30% as compared to the untreated controls. At the therapeutic dose, the targeted NP showed little local and systemic immunotoxicity and did not decrease the body weight or damage the major organs.
INTRODUCTION
The lung is a common location of a secondary tumor that has metastasized from the primary source tumor.1–4 To date, when pulmonary metastases are diagnosed, aggressive surgical resection is usually performed in order to provide the best chance of a long-term cure.2 Unfortunately, a high relapse rate (~70%) results in a low survival rate (30%) even after complete resection.2 The relapse of lung metastases usually occurs during chemotherapy,2 thereby indicating that conventional anticancer drugs are no longer effective against the metastasis. Such a life-threatening condition urgently needs a new systemic treatment.
Among anticancer agents, small interfering RNA (siRNA) has drawn much attention because of its high specificity, high efficiency, and low toxicity. By targeting the oncogene, siRNA can be applied as a therapeutic agent in cancer therapy5; however, the effective delivery of siRNA remains a challenging task. Despite the development of various delivery carriers for siRNA, few successful cases have been reported of treating metastatic tumor with systemically delivered siRNA.6–9
We have earlier shown that our targeted nanoparticle (NP) could deliver significant amounts of siRNA into the cytoplasm of a xenograft tumor in a nude mouse model, leading to the silencing of epidermal growth factor receptor, tumor growth inhibition, and chemosensitization.10 In order to determine whether the targeted NP can also deliver siRNA to a metastatic tumor, we used an established experimental lung metastasis model by intravenous (IV) injecting murine melanoma cells (B16F10 cells stably transduced with the luciferase gene) into C57BL/6 mice. We have performed several feasibility studies which showed that the targeted NP improved the delivery and efficacy of the siRNA.11 First, the targeted NP showed an enhanced delivery of cy3-siRNA into the metastatic nodules. The luciferase gene in the metastatic nodules could be effectively silenced by anti-luciferase siRNA formulated in the targeted NP; ~70–80% of the luciferase activity was silenced by a single IV injection (0.15 mg/kg).11 ED50 was only 0.075 mg/kg. The preliminary data showed the potential of achieving siRNA-based tumor therapy using this targeted NP formulation.
A combination treatment with three different siRNA sequences [MDM2, c-myc, and vascular endothelial growth factor (VEGF)] has been shown to have a synergistic antiproliferation effect on the B16 cells.12 We therefore used a combination of these sequences (MDM2/c-myc/VEGF = 1:1:1, weight ratio) in our therapeutic studies.
RESULTS
In vivo gene silencing study
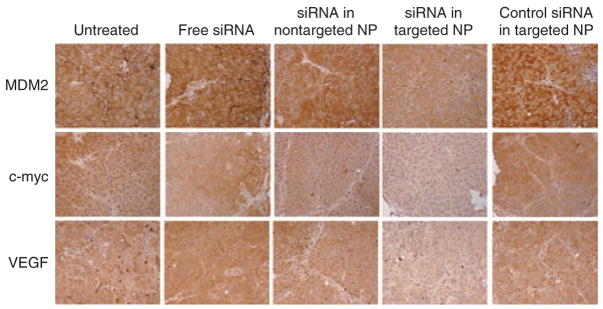
We encapsulated the combined siRNA sequences into different formulations, treated the lung metastases-bearing mice on days 10 and 11 with two consecutive IV injections (dose = 0.45 mg/kg), and examined the gene silencing activity using immunohistochemistry (Figure 1) and western blotting (Figure 2). As shown in Figure 1, only siRNA formulated in the targeted NP was able to effect simultaneously silencing of MDM2, c-myc, and VEGF in the B16F10 lung metastases (Figure 1). The data from the western blotting analysis (Figure 2) confirmed the data obtained from the immunohistochemical analysis.
Figure 1. Immunohistochemical analysis of the lung metastasis.
Immunohistochemical staining of MDM2, c-myc, and vascular endothelial growth factor (VEGF) in the B16F10 lung metastatic nodules after treatment with small interfering RNA (siRNA) in different formulations. Original magnification = ×100. NP, nanoparticle.
Figure 2. Western blot analysis of the tumor-loaded lung.
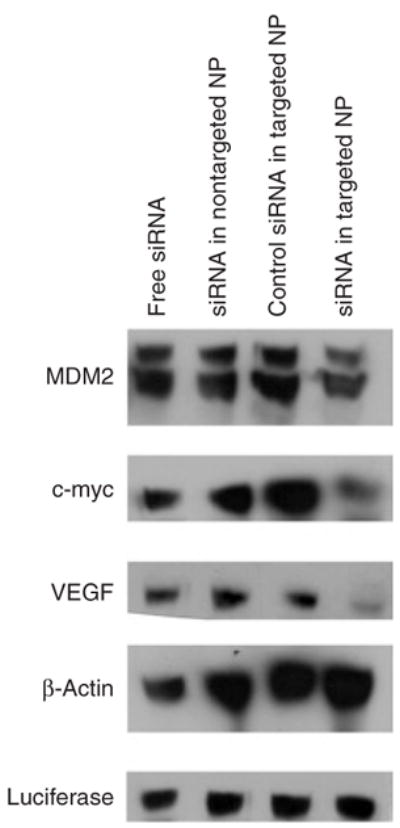
Western blot analysis of MDM2, c-myc, and vascular endothelial growth factor (VEGF) in the B16F10 tumor-loaded lung after treatment with small interfering RNA (siRNA) in different formulations. NP, nanoparticle.
In vivo antitumor/antimetastasis study
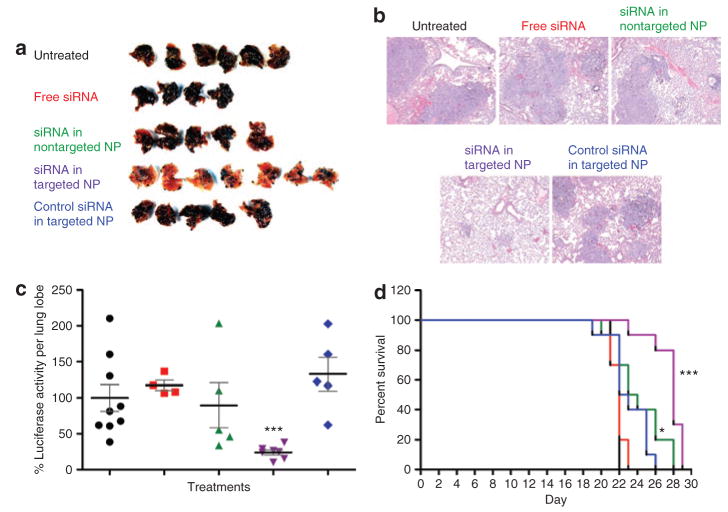
In order to evaluate the therapeutic outcomes, we administered two consecutive injections of siRNA (0.45 mg/kg, MDM2/c-myc/VEGF = 1:1:1, weight ratio) in different formulations 10 days after IV injection of B16F10 cells, which were stably transduced with a luciferase gene. As shown in Figure 3a, metastasis nodules were significantly reduced in lungs removed from the mice treated with siRNA formulated in the targeted NP. Other control treatments, including free siRNA, siRNA in the nontargeted NP, and control siRNA in the targeted NP, had very little therapeutic effect (Figure 3a). The hematoxylin and eosin–stained tissue sections also showed that the lung treated with siRNA in the targeted NP had only a few small nodules, and most of the lung was free of tumor (Figure 3b). In contrast, metastasis nodules occupied most of the lung when mice were given other control treatments (Figure 3b). In order to quantify the B16F10 nodules in the lung, we took one lobe of the excised lung and assayed for the luciferase activity. As indicated in Figure 3c, siRNA in the targeted NP significantly reduced the tumor load to 20–30% as compared to the untreated control (P < 0.01), whereas other control treatments showed no significant effect. The therapeutic outcome was also analyzed in terms of animal survival (Figure 3d). On day 23, the survival rates for untreated controls, for mice treated with free siRNA, siRNA in the nontargeted NP, siRNA in the targeted NP, and control siRNA in the targeted NP were 0, 0, 50, 90, and 40%, respectively (Figure 3d), and the mean survival times were 22, 22, 23.5, 28, and 22.5 days, respectively. Only siRNA in nontargeted or targeted NP significantly improved survival of the animal compared to the free siRNA formulation (P < 0.05). Control siRNA in the targeted NP did not improve animal survival compared to the free siRNA (P > 0.05). Although two injections of siRNA in the nontargeted NP did not reduce the tumor metastasis (Figure 3a–c), the administration of four such injections resulted in significant improvement in animal survival (Figure 3d).
Figure 3. Antimetastasis efficacy of different small interfering RNA (siRNA) formulations.
(a) Images of lungs excised from the tumor-bearing mice on day 17 after two consecutive treatments. (b) Photographs of the hematoxylin and eosin–stained tissue sections processed from the excised lungs. Original magnification = ×40. (c) Luciferase activity in the tumor-loaded lungs on day 17 after two consecutive intravenous (IV) injections of siRNA in different formulations on days 10 and 11. n = 4–9. ***P < 0.0001 as compared to the siRNA in phosphate-buffered saline (PBS) formulation. Formulations from left to right: untreated control (black), siRNA in PBS (red), siRNA in nontargeted nanoparticle (NP) (green), siRNA in targeted NP (purple), and control siRNA in targeted NP (blue). (d) Survival analysis of B16F10 lung metastases–bearing mice. Tumor-bearing mice were IV injected with different siRNA formulations on days 10, 11, 17, and 18. n = 10. *P < 0.05, ***P < 0.0001 as compared to the siRNA in PBS formulation. Formulations: untreated control (black), siRNA in PBS (red), siRNA in nontargeted NP (green), siRNA in targeted NP (purple), and control siRNA in targeted NP (blue).
Toxicity study
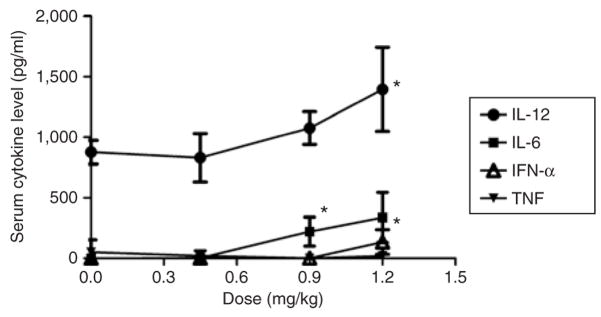
At the therapeutic dose (0.45 mg/kg), the targeted NP did not induce significant production of all analyzed cytokines, including interleukin-6 (IL-6), IL-12, tumor necrosis factor, and interferon-α (Figure 4). Even after two consecutive injections, the IL-6 and IL-12 levels were not significantly elevated (data not shown). However, when the dose was increased, the inflammatory toxicity was significantly enhanced (Figure 4), although still moderate. At the therapeutic dose, none of the formulations caused elevation in cytokine production in the lung (Supplementary Figure S1a) (method described in Supplementary Materials and Methods). Additionally, the body weight did not significantly decrease during treatment at the therapeutic dose (Supplementary Figure S1b) (method described in Supplementary Materials and Methods). The targeted NP formulation did not damage the major organs (heart, liver, spleen, lung, and kidney) that were examined using hematoxylin and eosin staining of the respective tissue sections (Supplementary Figure S2) (method described in Supplementary Materials and Methods).
Figure 4. Serum cytokine analysis.
Serum cytokine levels of C57BL/6 mice 2 hours after receiving intravenous injections of small interfering RNA in targeted nanoparticle at different doses. The data are given as mean values ± SD, n = 4. *P < 0.05; **P < 0.01 as compared to the untreated control. IFN-α, interferon-α; IL, interleukin; TNF, tumor necrosis factor.
DISCUSSION
siRNA and the carrier DNA (calf thymus DNA) were complexed by protamine into a compact core, which was further coated with the cationic lipids to form the NP. The surface of the NP was then modified with a ligand-conjugated polyethylene glycol (PEG)-lipid for targeting the sigma receptor–expressing B16F10 cells. The preliminary data showed that the siRNA delivery efficiency into the B16F10 cells was enhanced fourfold by the targeted NP as compared to the nontargeted NP (NP modified with PEG but without ligand) and could be partially inhibited by the presence of free ligand.11 In addition, the NP was prepared with a self-assembling method in a mild condition. Calf thymus DNA was used for improving the particle complexation, given its high molecular weight. Its limited CpG motif content provides low immunotoxicity, representing a significant advantage over the plasmid DNA.10,11,13
In order to improve the antitumor/antimetastasis effect, we used combined siRNA sequences (MDM2/c-myc/VEGF = 1:1:1, weight ratio) so as to attack multiple oncogene pathways. MDM2 is an inactivator for p53,14 and c-myc serves as an activated transcription factor that promotes cell proliferation.15 VEGF is a growth factor secreted by the tumor and mediates angiogenesis and metastasis.16 According to the literature, simultaneous silencing of these oncogenes by siRNA results in enhanced antiproliferation on the B16 cells.12 Recently, it was demonstrated that combined delivery of different siRNA sequences resulted in siRNA competition for incorporation into RNA-induced silencing complex and reduced the efficacy of RNA interference.17,18 In order to determine whether a combination of the three siRNA sequences interferes with the gene silencing activity of each sequence, we employed a quantitative reverse transcription–PCR method to quantify the RNA interference activity of a single sequence and pooled sequences delivered by the targeted NP (method described in Supplementary Materials and Methods and Table S1). We did find a sequence competition effect, with the pooled siRNA formulation showing significantly reduced gene silencing activity on the c-myc and MDM2 genes but not on the VEGF gene, as compared to the single siRNA formulations (Supplementary Figure S3) (method described in Supplementary Materials and Methods). However, the competition effect was only moderate, and significant gene knockdown (45–85%) was observed in all the genes when 100 nmol/l pooled siRNA was used. The data suggest that combined delivery of these three sequences may be acceptable. We have also checked the RNA interference activity in protein levels by immunocytochemistry. As shown in Supplementary Figure S4 (method described in Supplementary Materials and Methods), pooled siRNA in the targeted NP was able to silence MDM2, c-myc, and VEGF in the B16F10 cells when delivered at a concentration of 100 nmol/l, whereas other control treatments, including free siRNA, siRNA in nontargeted NP, and control siRNA in targeted NP showed little effect. There was an enhanced antiproliferation effect on the B16F10 cells by the targeted NP (data not shown). Both the in vitro and in vivo gene silencing results (Supplementary Figure S4 and Figures 1 and 2) showed that the gene silencing activity was highly formulation- and siRNA-sequence-dependent, thereby suggesting that only the delivery of a sufficient dose of the correct sequences of siRNA results in significant activity. The antitumor activities of the different formulations were highly correlated to their RNA interference activities, and only the targeted NP significantly reduced the tumor load (Figure 3a–c).
Although two injections of siRNA in the nontargeted NP did not reduce the tumor load in the lung (Figure 3a–c), four such injections significantly improved survival of the animal (Figure 3d, P < 0.05). The results suggest that multiple dosing may compensate for the poor delivery of the nontargeted formulation. However, the prolongation of survival on account of the nontargeted NP was moderate, although statistically significant. Targeted NP, on the other hand, with superior delivery efficiency for metastasis,11 not only significantly reduced the tumor load in the lung (Figure 3a–c), but also prolonged survival by ~30% (Figure 3d). Targeted NP showed significantly improved therapeutic effects as compared to any of the other siRNA formulations (Figure 3). Although it is unlikely, it is possible that the off-target effect of the combined siRNA contributed to the in vivo reduction of tumor load. The off-target effect of siRNA causing nonspecific gene downregulation was first reported by Scacheri et al.19 It is speculated that a partial complementary sequence of the siRNA matches with the off-target genes, and the genes are silenced through the micro-RNA mechanism. This is of particular concern when multiple siRNA sequences are used. Whether the off-target effect of the siRNA contributed to the antitumor effect of our formulation is not clear at the present time. However, the primary goal of this research is to develop a delivery system. The extent to which the off-target effect contributes to the antitumor activity requires future study. Additionally, the toxicity study showed that the targeted NP was safe at the therapeutic dose.
In summary, with the improved delivery of siRNA by the targeted NP,11 the corresponding oncogenes were simultaneously silenced, the mass of lung metastasis was greatly reduced, and animal survival was significantly prolonged with a relatively low and nontoxic dose (0.45 mg/kg). We therefore conclude that siRNA formulated in the targeted NP has the potential to become a useful tool in cancer therapy.
MATERIALS AND METHODS
Materials
DOTAP, cholesterol, and DSPE-PEG2000 were obtained from Avanti Polar Lipids (Alabaster, AL). Protamine sulfate (fraction X from salmon) and calf thymus DNA (for hybridization, phenol–chloroform extracted and ethanol precipitated) were purchased from Sigma-Aldrich (St. Louis, MO). DSPE-PEG2000-anisamide was synthesized in our laboratory using the methods described earlier.20
B16F10 cells, sigma receptor–positive21 and commonly used for establishing an experimental lung metastasis model,22 were used in this study. The cells were obtained from American Type Culture Collection and stably transduced with GL3 firefly luciferase gene using a retroviral vector, in Dr. Pilar Blancafort’s laboratory at the University of North Carolina at Chapel Hill. The cells were maintained in Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA).
Primary antibodies (mouse monoclonal antibodies against mouse MDM2, c-myc, luciferase, β-actin, and rabbit polyclonal antibodies against VEGF) and secondary antibodies conjugated with horseradish peroxidase (HRP) (goat anti-mouse IgG-HRP and goat anti-rabbit IgG-HRP) were purchased from Santa Cruz Biotechnologies (Santa Cruz, CA).
siRNA
MDM2 siRNA (target sequence: 5′-GCUUCGGAACAAGAG ACUC-3′), c-myc siRNA (target sequence: 5′-GAACAUCAUCAUC CAGGAC-3′), VEGF siRNA (target sequence: 5′-CGAUGAAGCCCUGG AGUGC-3′), and control siRNA (target sequence: 5′-AATTCTCCGAA CGTGTCACGT-3′) were purchased from Dharmacon (Lafayette, CO) in deprotected, desalted, and annealed form. The sequences were adopted from the literature.12,23
Experimental animals
Female C57BL/6 mice of ages 6–7 weeks (weights 16–18 g) were purchased from Charles River Laboratories (Wilmington, MA). All experiments performed on the animals were in accordance with and approved by the Institutional Animal Care and Use Committee at University of North Carolina. Murine model of lung metastasis was established by IV injection of 2 × 105 B16F10 cells into the C57BL/6 mice.
Preparation of siRNA containing NP
NP were prepared as described earlier.11 Each component (siRNA, calf thymus DNA, protamine, and cationic liposome) of the formulation was dissolved or prepared in cloning-grade water with nondetectable endotoxin (Sigma-Aldrich, St. Louis, MO). The solution or suspension was then sterile filtered (0.22 μm), and the formulations were prepared in a laminar flow hood using autoclaved tubes and pipette tips. The characteristics of the formulations were similar to those of formulations reported earlier,11 with a mean diameter of ~110 nm and a zeta potential of 20–25 mV. The siRNA encapsulation efficiency was >95% as determined using size exclusion chromatography described earlier.24 The endotoxin level in the formulation was 0.46 endotoxin units (EU)/ml [determined using an endotoxin detection kit (Cambrex Bio Science, Walkersville, MD)], and ~2.8 EU/kg was injected into each mouse at the dose of 0.45 mg siRNA/kg, which is lower than the upper limit (5 EU/kg) suggested by the US Food and Drug Administration. The source of the endotoxin was likely from the calf thymus DNA.
In vivo gene silencing study
Tumor-bearing mice were given IV injections of total siRNA (MDM2/c-myc/VEFG = 1:1:1, weight ratio) at the dose of 0.45 mg/kg formulated in phosphate-buffered saline, the nontargeted NP, or the targeted NP, on days 10 and 11. One day after the second injection, the mice were killed and the tumor-loaded lungs were collected for the preparation of paraffin embedded sections (5-μm thick). Expressions of MDM2, c-myc, and VEGF in the sections were examined immunohistochemically using the antibodies from a kit [DakoCytomation Envision + Dual Link System-HRP (DAB+); DakoCytomation, Carpinteria, CA] in accordance with the product protocol. The nuclei were counterstained with hematoxylin, and the samples were imaged using a Nikon phase contrast light microscope. Total protein (10 μg) isolated from the tumor-loaded lung was resolved on a polyacrylamide/sodium dodecyl sulfate gel and then transferred on to a polyvinylidene difluoride membrane. The membranes were blocked with 5% nonfat milk in phosphate-buffered saline for 1 hour and then incubated overnight with primary antibody at 4 °C. After the membrane had been washed with Tris-buffered saline (1% Tween 20 in phosphate-buffered saline) five times, it was incubated with the HRP-conjugated secondary antibody for 1 hour. The peroxidase activity associated with the protein bands was detected with enhanced chemiluminescence using ECL plus (GE Health Care, Buckinghamshire, UK) followed by autoradiography.
In vivo metastasis inhibition study and survival analysis
Tumor-bearing mice were given IV injections of total siRNA (MDM2/c-myc/VEFG = 1:1:1, weight ratio) at the dose of 0.45 mg/kg formulated in phosphate-buffered saline, the nontargeted NP, or the targeted NP, on days 10 and 11. Control siRNA formulated in the targeted NP was also prepared as a negative control. On day 17, the mice were killed and the tumor-loaded lungs were removed. One lobe of each lung was analyzed for luciferase activity in order to quantify the lung metastasis nodules. The lobe was homogenized in 1 ml of lysis buffer (0.05% Triton X-100 and 2 mmol/l EDTA in 0.1 mol/l Tris–HCl) followed by centrifugation at 5,000 rpm for 5 minutes. Ten microliter of the supernatant was mixed with one hundred microliter of luciferase substrate (Luciferase Assay System; Promega, Madison, WI), and the luciferase activity was measured using a plate reader (PLATE CHAMELEON Multilabel Detection Platform; Bioscan, Washington, DC). The rest of the lung was fixed in 3.6% formalin solution overnight and imaged. The fixed lung was processed for paraffin sections followed by hematoxylin and eosin staining. For the survival analysis study, tumor-bearing mice were treated on days 10, 11, 17, and 18. The mice were killed if they exhibited a weight loss >10% or showed ruffled fur.
Cytokine induction assay
The C57BL/6 mice were IV injected with siRNA (MDM2/c-myc/VEGF = 1:1:1) formulated in the targeted NP at various doses, and the serum cytokine level was determined using the method described earlier.10,11
Statistical analysis
The data are presented as mean values ± SD. The statistical significance was determined using one-way analysis of variance. The animal survival data were analyzed using Kaplan–Meier survival analysis. P values <0.05 were considered significant.
Supplementary Material
Acknowledgments
We thank Joyeeta Sen [University of North Carolina (UNC)] for synthesizing the DSPE-PEG2000-anisamide, and the Histopathology Department as well as Michael Hooker Microscopy Facility at UNC for their assistance in tissue section preparation and microscopy imaging. We also thank Elizabeth Vasievich (UNC) for her help in preparing the article. The B16F10 cells were transduced with luciferase gene in Pilar Blancafort’s laboratory at UNC.
Footnotes
Figure S1. Toxicity assay.
Materials and Methods.
Figure S2. Tissue section examination.
Table S1. Primer sequences for qRT-PCR.
Figure S3. Relative mRNA level in the B16F10 cells 12 hours after treatment with single or combined sequences formulated in the targeted NP.
Figure S4. Immunocytochemical analysis.
References
- 1.Geschwind JF, Dagli MS, Vogel-Claussen J, Seifter E, Huncharek MS. Metastatic breast carcinoma presenting as a large pulmonary embolus: case report and review of the literature. Am J Clin Oncol. 2003;26:89–91. doi: 10.1097/00000421-200302000-00017. [DOI] [PubMed] [Google Scholar]
- 2.Ashley AC, Deschamps C, Alberts SR. Impact of prognostic factors on clinical outcome after resection of colorectal pulmonary metastases. Clin Colorectal Cancer. 2006;6:32–37. doi: 10.3816/CCC.2006.n.018. [DOI] [PubMed] [Google Scholar]
- 3.Bacci G, Avella M, Picci P, Briccoli A, Dallari D, Campanacci M. Metastatic patterns in osteosarcoma. Tumori. 1988;74:421–427. doi: 10.1177/030089168807400408. [DOI] [PubMed] [Google Scholar]
- 4.Murakami T, Cardones AR, Hwang ST. Chemokine receptors and melanoma metastasis. J Dermatol Sci. 2004;36:71–78. doi: 10.1016/j.jdermsci.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Jana S, Chakraborty C, Nandi S, Deb JK. RNA interference: potential therapeutic targets. Appl Microbiol Biotechnol. 2004;65:649–657. doi: 10.1007/s00253-004-1732-1. [DOI] [PubMed] [Google Scholar]
- 6.Hu-Lieskovan S, Heidel JD, Bartlett DW, Davis ME, Triche TJ. Sequence-specific knockdown of EWS-FLI1 by targeted, nonviral delivery of small interfering RNA inhibits tumor growth in a murine model of metastatic Ewing’s sarcoma. Cancer Res. 2005;65:8984–8992. doi: 10.1158/0008-5472.CAN-05-0565. [DOI] [PubMed] [Google Scholar]
- 7.Pirollo KF, Rait A, Zhou Q, Hwang SH, Dagata JA, Zon G, et al. Materializing the potential of small interfering RNA via a tumor-targeting nanodelivery system. Cancer Res. 2007;67:2938–2943. doi: 10.1158/0008-5472.CAN-06-4535. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi Y, Nishikawa M, Kobayashi N, Takakura Y. Gene silencing in primary and metastatic tumors by small interfering RNA delivery in mice: quantitative analysis using melanoma cells expressing firefly and sea pansy luciferases. J Control Release. 2005;105:332–343. doi: 10.1016/j.jconrel.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Takeshita F, Minakuchi Y, Nagahara S, Honma K, Sasaki H, Hirai K, et al. Efficient delivery of small interfering RNA to bone-metastatic tumors by using atelocollagen in vivo. Proc Natl Acad Sci USA. 2005;102:12177–12182. doi: 10.1073/pnas.0501753102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li SD, Chen YC, Hackett MJ, Huang L. Tumor-targeted delivery of siRNA by self-assembled nanoparticles. Mol Ther. 2008;16:163–169. doi: 10.1038/sj.mt.6300323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li SD, Chono S, Huang L. Efficient gene silencing in metastatic tumor by siRNA formulated in surface-modified nanoparticles. J Control Release. 2008;126:77–84. doi: 10.1016/j.jconrel.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song E, Zhu P, Lee SK, Chowdhury D, Kussman S, Dykxhoorn DM, et al. Antibody mediated in vivo delivery of small interfering RNAs via cell-surface receptors. Nat Biotechnol. 2005;23:709–717. doi: 10.1038/nbt1101. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz DA, Quinn TJ, Thorne PS, Sayeed S, Yi AK, Krieg AM. CpG motifs in bacterial DNA cause inflammation in the lower respiratory tract. J Clin Invest. 1997;100:68–73. doi: 10.1172/JCI119523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halaby MJ, Yang DQ. p53 translational control: a new facet of p53 regulation and its implication for tumorigenesis and cancer therapeutics. Gene. 2007;395:1–7. doi: 10.1016/j.gene.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 15.de Nigris F, Balestrieri ML, Napoli C. Targeting c-Myc, Ras and IGF cascade to treat cancer and vascular disorders. Cell Cycle. 2006;5:1621–1628. doi: 10.4161/cc.5.15.3138. [DOI] [PubMed] [Google Scholar]
- 16.Grothey A. Future directions in vascular endothelial growth factor-targeted therapy for metastatic colorectal cancer. Semin Oncol. 2006;33:S41–S49. doi: 10.1053/j.seminoncol.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Castanotto D, Sakurai K, Lingeman R, Li H, Shively L, Aagaard L, et al. Combinatorial delivery of small interfering RNAs reduces RNAi efficacy by selective incorporation into RISC. Nucleic Acids Res. 2007;35:5154–5164. doi: 10.1093/nar/gkm543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vickers TA, Lima WF, Nichols JG, Crooke ST. Reduced levels of Ago2 expression result in increased siRNA competition in mammalian cells. Nucleic Acids Res. 2007;35:6598–6610. doi: 10.1093/nar/gkm663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scacheri PC, Rozenblatt-Rosen O, Caplen NJ, Wolfsberg TG, Umayam L, Lee JC, et al. Short interfering RNAs can induce unexpected and divergent changes in the levels of untargeted proteins in mammalian cells. Proc Natl Acad Sci USA. 2004;101:1892–1897. doi: 10.1073/pnas.0308698100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banerjee R, Tyagi P, Li S, Huang L. Anisamide-targeted stealth liposomes: a potent carrier for targeting doxorubicin to human prostate cancer cells. Int J Cancer. 2004;112:693–700. doi: 10.1002/ijc.20452. [DOI] [PubMed] [Google Scholar]
- 21.Pham TQ, Berghofer P, Liu X, Greguric I, Dikic B, Ballantyne P, et al. Preparation and biologic evaluation of a novel radioiodinated benzylpiperazine, 123I-MEL037, for malignant melanoma. J Nucl Med. 2007;48:1348–1356. doi: 10.2967/jnumed.107.041673. [DOI] [PubMed] [Google Scholar]
- 22.Li S, Dong W, Zong Y, Yin W, Jin G, Hu Q, et al. Polyethylenimine-complexed plasmid particles targeting focal adhesion kinase function as melanoma tumor therapeutics. Mol Ther. 2007;15:515–523. doi: 10.1038/sj.mt.6300072. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Chen ZG, Choe MS, Lin Y, Sun SY, Wieand HS, et al. Tumor growth inhibition by simultaneously blocking epidermal growth factor receptor and cyclooxygenase-2 in a xenograft model. Clin Cancer Res. 2005;11:6261–6269. doi: 10.1158/1078-0432.CCR-04-2102. [DOI] [PubMed] [Google Scholar]
- 24.Li SD, Huang L. Surface-modified LPD nanoparticles for tumor targeting. Ann NY Acad Sci. 2006;1082:1–8. doi: 10.1196/annals.1348.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.