Abstract
The bisanthraquinone antibiotic BE-43472B [(+)-1] was isolated by Rowley and coworkers from a streptomycete strain found in a green algae associated with the ascidian Ecteinascidia turbinata and has shown promising antibacterial activity against clinically derived isolates of methicillin-susceptible, methicillin-resistant, and tetracyclin-resistant Staphylococcus aureus (MSSA, MRSA, and TRSA, respectively), and vancomycin-resistant Enterococcus faecalis (VRE). Described herein is the first total synthesis of both enantiomers of this bisanthraquinone antibiotic, the determination of its absolute configuration, as well as the biological evaluation of these and related compounds. The developed synthesis relies on a highly efficient cascade sequence involving an intermolecular Diels–Alder reaction between diene (R)-61 and dienophile 55 followed by an intramolecular nucleophilic aromatic ipso substitution. Late stage transformations included a remarkable photochemical α,β-epoxyketone rearrangement [80 → (+)-1]. Interestingly, the unnatural enantiomer [(–)-1] of antibiotic BE-43472B exhibited comparable antibacterial properties to those of the natural enantiomer [(+)-1].
Introduction
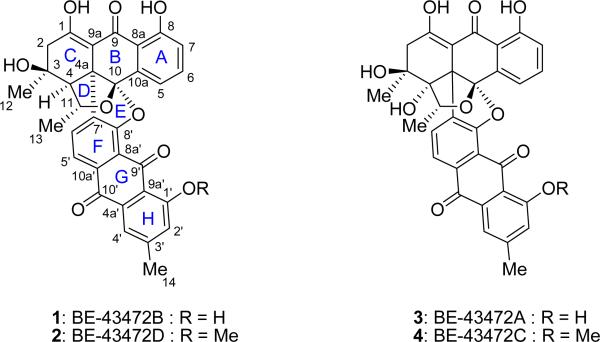
Bisanthraquinones 1 and 3 (Figure 1) were reported by Rowley and co-workers as two naturally occurring antibiotics with biological activities against drug resistant bacteria.1,2 Possessing striking molecular architectures, these compounds were isolated by these researchers from Streptomyces strain #N1-78-1 which was isolated from cultured cells of an unidentified unicellular green algae (URI strain #N36-11-10) extracted from the ascidian Ecteinascidia turbinata, collected from La Parguera, Puerto Rico. Interestingly, the gross structures of these compounds, and their mono-methylated siblings 2 and 4 (Figure 1), were previously reported in a Japanese patent as anti-tumor agents.3 Isolated from Streptomyces sp. A43472, these compounds were designated in this patent as BE-43472A (3), BE-43472B (1), BE-43472C (4) and BE-43472D (2). Although the Rowley group was able to assign the complete relative stereochemistry of the bisanthraquinone antibiotics BE-43472B (1) and BE-43472A (3) on the basis of NMR spectroscopic analysis, and to point out their likely identities to those isolated by the Japanese workers, they left their absolute configuration unassigned.1,2
Figure 1.
Bisanthraquinone antibiotics BE-43472A–D (1–4).
These bisanthraquinone antibiotics exhibited, in addition to anti-tumor properties, impressive inhibitory activities against a variety of bacteria.1,2 Antibiotic BE-43472B (1) demonstrated potent inhibitory activities against an expanded panel of clinical bacterial isolates of Gram-positive pathogens, including methicillin-susceptible Staphylococcus aureus [MSSA, MIC50 = 0.11 μM (range 0.054–0.22, 25 isolates], methicillin-resistant Staphylococcus aureus [MRSA, MIC50 = 0.23 μM (range 0.11–0.90, 25 isolates)], vancomycin-resistant Enterococcus faecalis [VRE, MIC50 = 0.90 μM (range 0.22–3.6), 25 isolates] and tetracycline-resistant Staphylococcus aureus [TRSA, MIC50 = 0.11 μM (range 0.11–0.23, 11 isolates)]. Most significantly, in a time-kill study, BE-43472B (1) exhibited strong bactericidal activity (>99.9% kill) against MSSA, MRSA and VRE.2 This compound was found to possess no activity against the Gram-negative pathogens Klebsiella pneumoniae (ATCC 700603) and Escherichia coli (ATCC 35218) while it demonstrated significant potency (IC50 = 2.0 μM) against the human colon cancer cell line HCT-116.
In view of the continuing search for new antibacterial agents to combat infections due to drug-resistant bacteria,4 and because of the unprecedented structures of these new antibiotics, their chemical synthesis was deemed important.5 In a recent communication, we reported the first total synthesis and absolute configuration of bisanthraquinone antibiotic BE-43472B (1) and its enantiomer (Figure 2).6,7 In this article, we provide the full account of our work in this area that includes the evolution of the synthetic strategy toward both enantiomers of 1 and the construction of a number of analogues of this unusual antibiotic.
Figure 2.
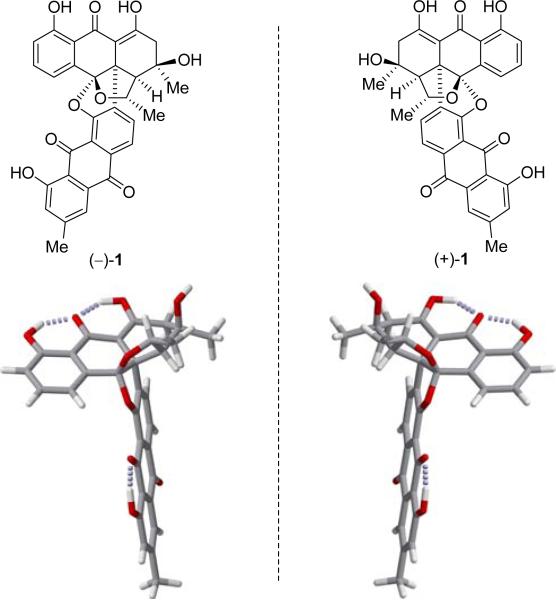
Enantiomers of bisanthraquinone antibiotic BE-43472B [(–)-1 and (+)-1] (top) and their computer-generated molecular models (bottom, fully optimized at the B3LYB/6-31G* level, Spartan ’06 suite of programs, carbon: gray, hydrogen: white, oxygen: red, H-bonds: dotted gray).
Results and Discussion
General Retrosynthetic Analysis. The Michael Reaction Approach
The T-shaped molecular architecture of the bisanthraquinone antibiotics as exemplified by BE-43472B (1) is both unprecedented and challenging from the synthetic point of view. Its C32H24O9 formula reveals its highly conjugated/unsaturated nature with two non-identical anthraquinone moieties serving as its two dominant regions. These domains are fused together through a bicyclic system comprised of two 5-membered rings, each containing an oxygen atom and featuring a ketal functionality. The two anthraquinone structural motifs are held together by a crowded carbon–carbon bond and a carbon-oxygen-carbon bridge. Its five stereogenic centers reside in a cluster resulting in considerable distortion of the molecule's otherwise flat regions. Figure 2 presents the two enantiomeric forms of BE-43472B (1) and their computer energy-minimized frame models. Since the absolute stereochemistry of the target molecule was unknown at the outset of our work, our synthetic plan had to accommodate both enantiomers, one at a time, from readily available starting materials and with subsequent stereocontrol.
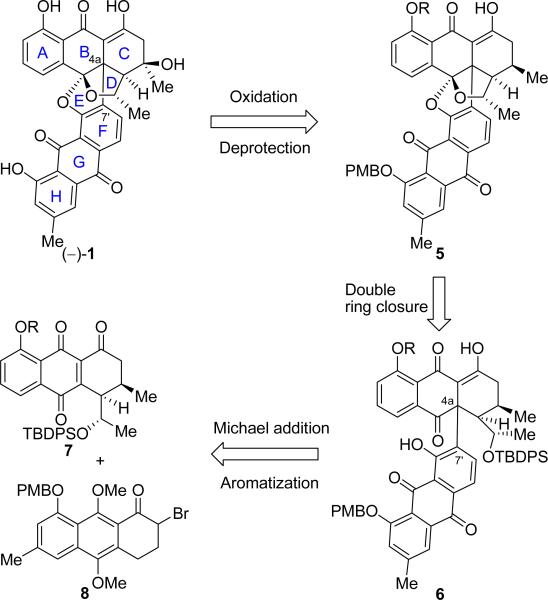
In our initial retrosynthetic analysis, shown in Scheme 1 (with the originally depicted1,2 absolute configuration, which eventually turned out to be the antipodal to that of the natural product), we first removed the rather labile C-3 hydroxyl group1 and protected the two phenolic groups of the BE-43472B (1) molecule to reveal structure 5 as a potential precursor to our target. By removing the C-3 hydroxyl group, we ensured stability for the intermediates on the way toward the final stages where we would seek an opportunity to install it. The ketal functionality within the advanced precursor 5 was then dismantled, unraveling intermediate 6, protected at the secondary alcohol site. Holding the two, now conspicuous anthraquinone moieties together in 6 was only a single bond (C-4a–C-7′). This key carbon–carbon bond was envisioned to be formed through a Michael-type addition of the enolate derived from bromoketone 8 (or its debromo analogue) to enetrione 7, followed by HBr elimination and aromatization (or oxidation/aromatization) to generate the requisite phenolic structural motif of 6 (R = H or protecting group in 5–7).
Scheme 1.
Initial Retrosynthetic Analysis of Bisanthraquinone (–)-1
Initial Model Studies. Formation of Key Carbon–Carbon Bond and Aromatization
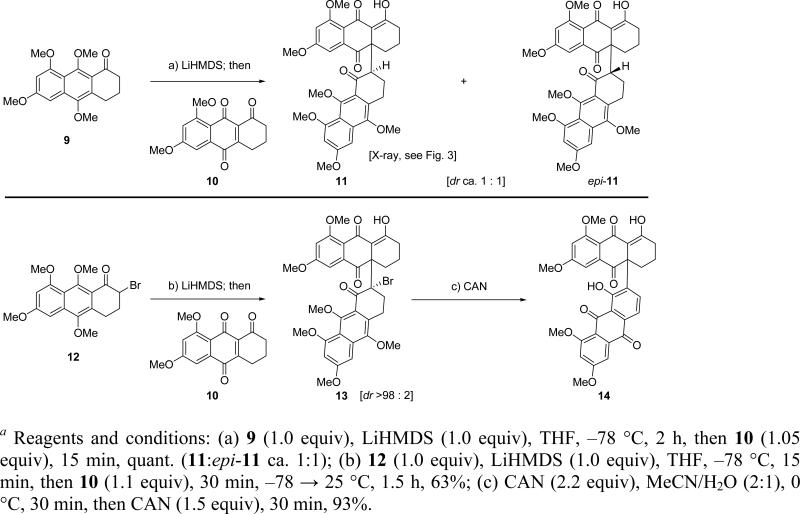
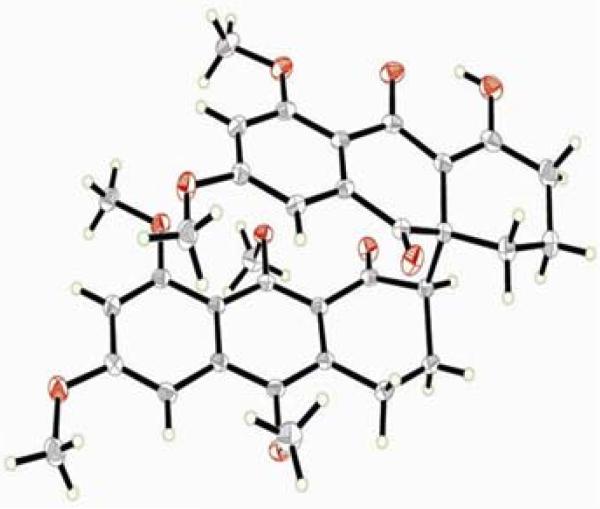
In order to test the feasibility of the key carbon–carbon bond (C-4a–C-7′) forming step of our designed strategy toward antibiotic BE-43472B (1), a model study was undertaken using simplified aryl ketone 9 as the donor and quinone 10 as the acceptor in the projected Michael reaction (Scheme 2). Thus, exposure of the known aryl ketone 98 to LiHMDS (1.0 equiv, THF, –78 °C) followed by quenching of the resulting enolate with enetrione 108,9 furnished, in quantitative yield, the desired racemic Michael adducts 11 and epi-11, which upon flash column chromatography (silica) equilibrated to ca. 1:1 mixture of inseparable epimers. Fortunately, crystallization from acetone led to preferential formation of crystals of 11 (m.p. = 188–190 °C, acetone), whose structure was proven by X-ray crystallographic analysis (see ORTEP drawing, Figure 3). This crystallographic analysis not only proved the identity of the two epimers, but also confirmed the regiochemical outcome of the addition of the enolate of 9 to the enetrione system (10).
Scheme 2.
Initial Model Studies. Investigation of the Key Michael Reaction Stepa
Figure 3.
X-Ray derived ORTEP drawing of compound 11.
Having achieved the requisite carbon–carbon bond formation, we then turned our attention to the oxidation/aromatization of the so-obtained product (11). However, the seemingly simple task of aromatizing ring F of this product proved much more challenging than expected. Thus, and much to our dismay, all attempts (e.g. exposure to acids, bases, heat, microwave conditions) at aromatizing and/or oxidizing ring F of adduct 11 proved in vain, leading only to decomposition (mainly retro-Michael products) or non-selective oxidations to an array of unidentified products. In the end, a way forward was found by abandoning ketone 9 and resorting to bromoketone 12 as a “pre-oxidized” substrate for the Michael reaction with enetrione 10 (Scheme 2). Thus, treatment of bromoketone 12 (prepared from 9 by exposure to NBS) with LiHMDS (1.0 equiv, THF, –78 °C), followed by reaction of the generated enolate with enetrione 10 (1.1 equiv), resulted in the formation of Michael adduct 13, albeit in lower yield than before (63%). It was interesting, however, to observe in this case that only a single diastereomer (stereochemistry unassigned) of 13 was observed (by 1H NMR spectroscopy). Exposure of this adduct to CAN in MeCN:H2O (2:1) at 0 °C led to concurrent oxidation of the protected hydroquinone ring G, HBr elimination, and aromatization of ring F to furnish the desired phenolic quinone 14 in a pleasing 93% yield. It was with these encouraging results that we embarked on our first attempt to synthesize BE-43472B (1) using the Michael reaction as the key step to unite the two bisanthraquinone fragments of the molecule.
Initial Approach Toward BE-43472B. Synthesis of Building Blocks
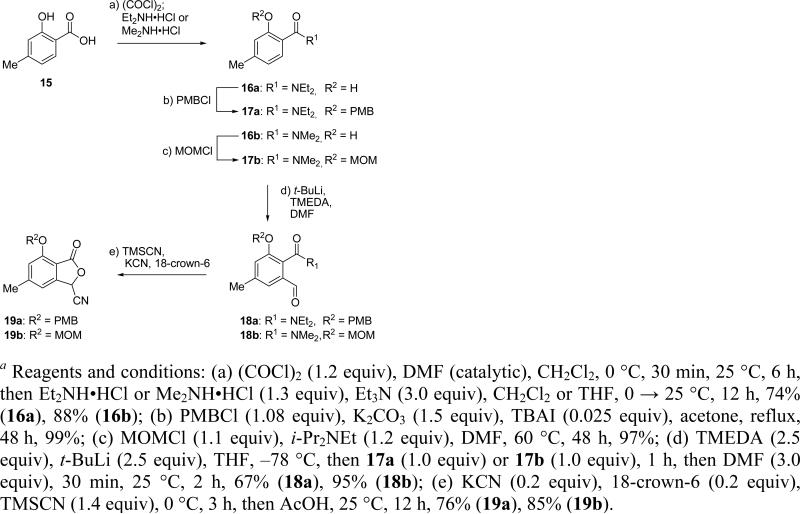
Having completed successfully the model study for the projected Michael addition/aromatization approach to antibiotic BE-43472B (1), we then proceeded to construct the required building blocks for the natural product, quinone 7 and bromoketone 8 (Scheme 1). For building block 8 and related fragments, we developed an improved synthesis through modification of our previous route8 based on the Hauser annulation reaction. The required nitrile components 19a and 19b were prepared as shown in Scheme 3. Thus, exposure of 4-methylsalicylic acid (15) to oxalyl chloride (1.2 equiv) in the presence of catalytic amounts of DMF in CH2Cl2 followed by reaction of the resulting acid chloride with either Et2NH•HCl (1.3 equiv), or Me2NH•HCl (1.3 equiv) afforded phenolic amides 16a (74% yield) or 16b (88% yield), respectively. These intermediates (16a or 16b) served as precursors to the corresponding protected derivatives (17a or 17b) which were prepared by protection of the free phenolic hydroxy group either with a PMB (PMBCl, K2CO3, n-Bu4NI cat., 99% yield of 17a), or a MOM (MOMCl, i-Pr2NEt, 97% yield of 17b) group. o-Lithiation10 of aryl amides 17a or 17b (t-BuLi, THF, –78 °C) followed by quenching with freshly distilled DMF furnished aldehydes 18a or 18b in 67 and 95% yield, respectively. Treatment of aldehydes 18a or 18b with TMSCN in the presence of catalytic amounts of KCN and 18-crown-6, followed by quenching with AcOH, then led to nitriles 19a or 19b in 76 and 85% yield, respectively, as shown in Scheme 3.
Scheme 3.
Synthesis of Nitriles 19a and 19ba
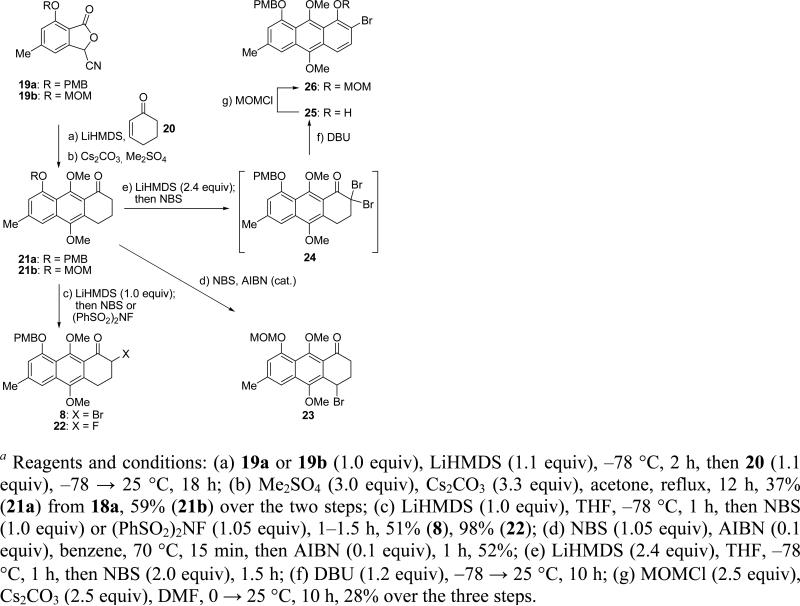
The conversions of nitriles 19a and 19b to the desired bromoketone 8, hydroquinone derivatives 21–23 and bromoanthracene 26 (which were also called upon to act as Michael-donors later on in the campaign, vide infra) through a Hauser annulation11 are shown in Scheme 4. Thus, treatment of 19a or 19b with LiHMDS in THF at –78 °C followed by addition of cyclohex-2-enone (20) gave the corresponding dihydroquinones, which upon methylation (Cs2CO3, Me2SO4) led to methylated ketones 21a (37% yield from aldehyde 18a over the three steps) or 21b (59% yield from nitrile 19a over the two steps), respectively. Treatment of ketone 21a with 1.0 equiv of LiHMDS in THF at –78 °C, followed by quenching with 1.0 equiv of NBS, furnished the desired bromoketone 8 in 51% yield. On the other hand, the use of 2.4 equiv of LiHMDS and 2.0 equiv of NBS in the above reaction led to dibromoketone 24, which upon exposure to DBU furnished bromoanthracene 25, demonstrating that the aromatization of such systems is possible, and providing access to aryl bromide precursors for coupling reactions with suitable Michael acceptors. Finally, protection of the phenolic group within 25 with MOMCl (Cs2CO3) led to MOM derivative 26 in 28% overall yield for the three steps.
Scheme 4.
Synthesis of Aryl Fragments 8, 21, 22, 23, and 26a
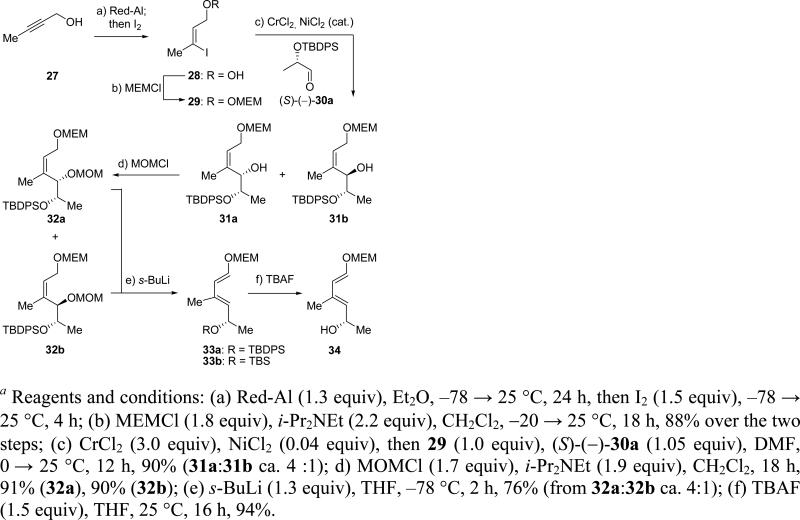
The required quinone fragment 7a and related compounds 7b and 7c were envisaged to be constructed through a route featuring a diastereoselective Diels–Alder reaction between chiral diene 33a and juglone (35) as the dienophile.12 The requisite diene 33a was synthesized starting from (S)-ethyl lactate and 2-butyn-1-ol (27) as outlined in Scheme 5.13 Thus, addition of Red-Al to alkynol 27 in ether at –78 → 25 °C,14 followed by quenching the resulting aluminate with iodine furnished, upon protection with MEMCl, vinyl iodide MEM ether 29 in 88% overall yield. A Nozaki–Hiyama–Kishi coupling15 of 29 with known lactaldehyde (S)-(–)-30a16 mediated by CrCl2-NiCl2 afforded a mixture of syn and anti alcohols 31a and 31b (ca. 4:1 ratio) in 90% yield. Reaction of this mixture (or each of the chromatographically separated diastereomers) with MOMCl and i-Pr2NEt led to the corresponding MOM derivatives (32a and 32b, 91 and 90% yield, respectively), which underwent 1,4-elimination on exposure to s-BuLi in THF at –78 °C to afford diene 33a, in 76% yield.17 Finally, removal of the TBDPS group from the latter compound with TBAF gave hydroxy diene 34 in 94% yield, as shown in Scheme 5.
Scheme 5.
Synthesis of Dienes 33 and 34a
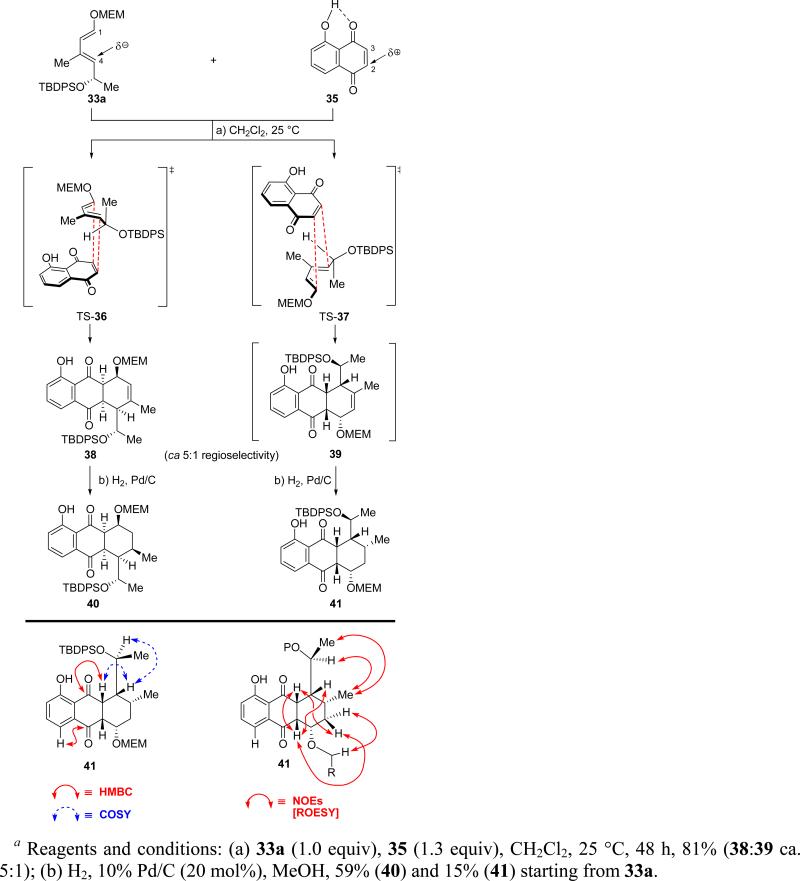
As depicted in Scheme 6, reaction of diene 33a with juglone (35, 1.3 equiv) proceeded smoothly in CH2Cl2 (ambient temperature, 48 h) to afford Diels–Alder adduct 38 as the major product, together with its regioisomer 39 (81% combined yield, ca. 5:1 ratio). These compounds proved to be rather labile due to their susceptibility toward oxidation by atmospheric oxygen and partial decomposition on silica gel through elimination of the OMEM group. Stereoselective hydrogenation (10% Pd/C, MeOH) of the crude Diels–Alder reaction mixture gave the more stable adducts 40 and 41 in 59 and 15% yield starting from 33a, respectively. While the regioselectivity of this Diels–Alder reaction was expected on the basis of electronic complementarity [dominating FMO interactions between the dienophile (C-2, δ+) and the diene (C-4, δ−), see structures 35 and 33a, Scheme 6], its facial selectivity required consideration of steric factors in order to trace its origins. Thus, minimization of 1,3-allylic strain within the conformation of diene 33a and minimization of steric repulsion in the transition states TS-36 and TS-37 may explain the exquisite diastereoselectivity in the formation of both regioisomers 38 and 39.
Scheme 6.
Diels–Alder Reaction of Diene 33a with Juglone (35) and Subsequent Hydrogenationa
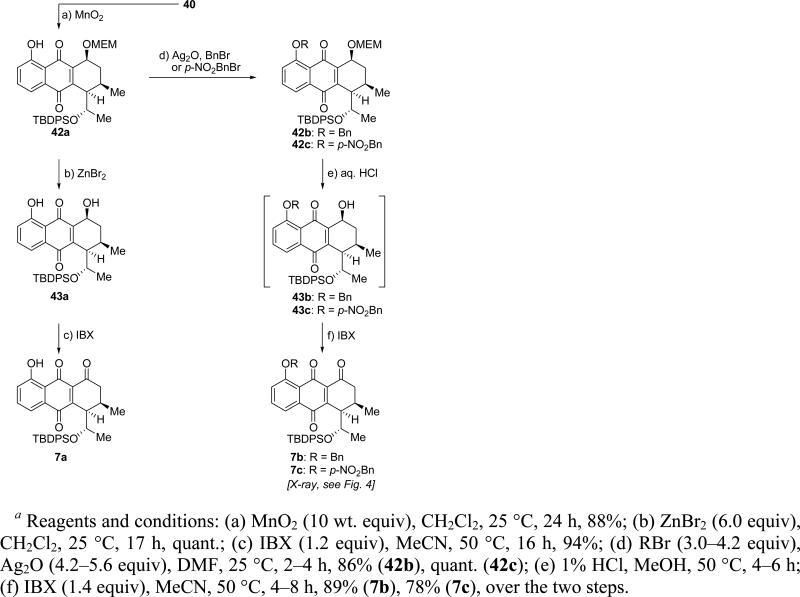
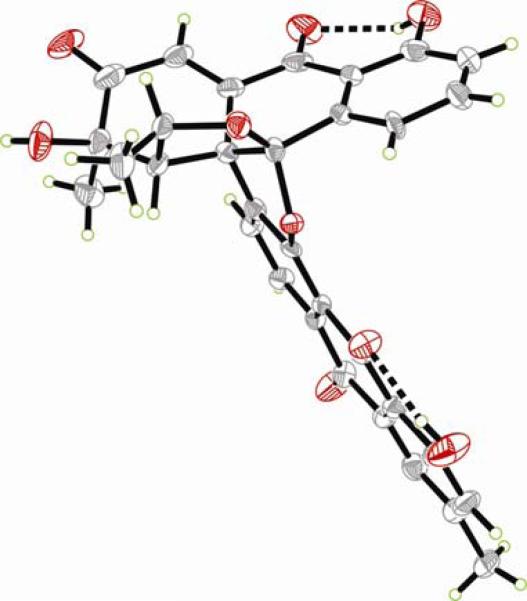
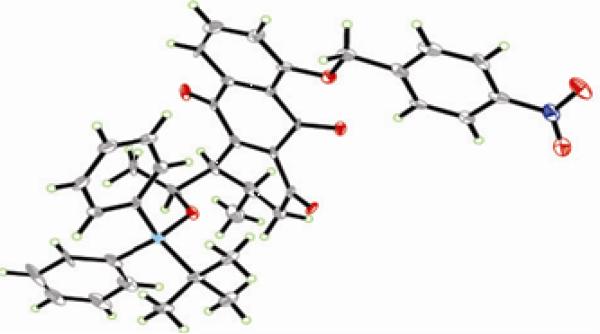
Proceeding with the synthesis, exposure of the major regioisomer 40 to MnO2 in CH2Cl2 at ambient temperature furnished the desired quinone 42a in 88% yield (Scheme 7). Treatment of the latter compound with ZnBr2 (CH2Cl2, 25 °C) furnished, in quantitative yield, hydroxy compound 43a, whose oxidation to the targeted quinone 7a was accomplished with IBX (MeCN, 50 °C, 94% yield). Alternatively, phenolic quinone 42a was first protected, either as benzyl ether (BnBr, Ag2O, DMF, in the dark) to afford 42b (86% yield), or p-nitrobenzyl ether (p-NO2BnBr, Ag2O, DMF, in the dark), to furnish 42c (quantitative yield). Exposure of the latter compounds to aqueous HCl in MeOH led to the corresponding alcohols (43b or 43c), which were subsequently oxidized with IBX (MeCN, 50 °C) to afford quinone 7b (89% yield) or 7c (78% yield), respectively. p-Nitrobenzyl quinone derivative 7c crystallized in beautiful yellow-orange crystals (m.p. = 159–161 °C, H2O/MeCN) whose X-ray crystallographic analysis (see ORTEP drawing, Figure 5) proved its absolute configuration and confirmed the initial structural assignment of the Diels–Alder adduct 38 (Scheme 6).
Scheme 7.
Synthesis of Quinones 7a–ca
Figure 5.
ORTEP drawing of enone 75 derived from X-ray crystallographic analysis.
Coupling of the Two Fragments Through Michael-Type Reaction and Further Exploration Toward the Target Molecule
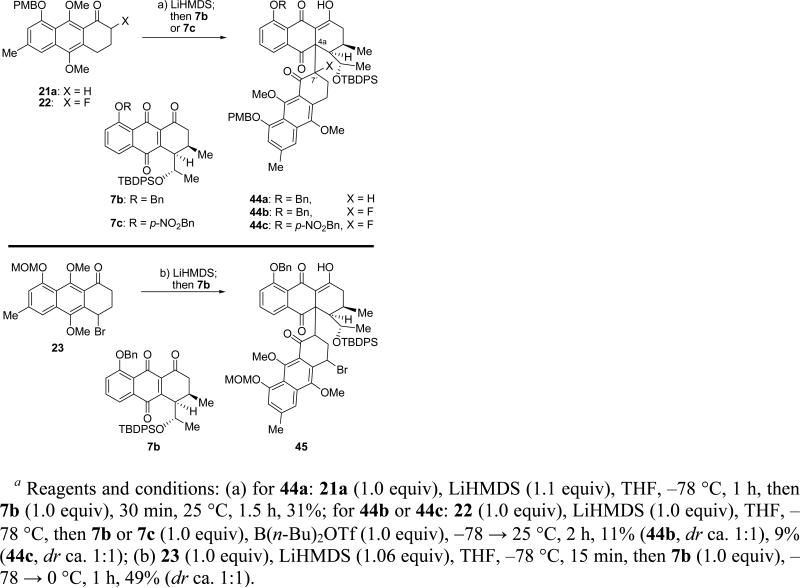
With the two key requisite building blocks for the construction of the target molecule now available, we set out to explore their coupling through the intended Michael addition/aromatization sequence along the pathway to antibiotic BE-43472B (1). Scheme 8 summarizes our advances and setbacks as we attempted to break through certain barriers toward the target molecule. Initial reaction of the enolate of 21a with Michael acceptor anthraquinone 7a (R =H, see Scheme 7) had shown to result in low conversion, presumably due to protonation of the enolate by the free phenol moiety of 7a. Thus, treatment of aryl ketone 21a with LiHMDS (1.0 equiv) in THF at –78 °C, followed by addition of benzyl-protected quinone 7b (–78 → 25 °C), furnished adduct 44a in 31% yield as a mixture of diastereomers (ca. 1:1), isomerizing over time to a single diastereomer (stereochemistry unassigned).
Scheme 8.
Michael Reaction/Aromatization Attempts To Construct the Crowded Quaternary C–C Bonda
Unfortunately, exposure of bromoketone 8 (see Scheme 4) to the same conditions as those shown in Scheme 8 failed to produce any coupling product, presumably due to the steric bulk of the bromine residue. This hypothesis prompted us to synthesize the corresponding fluoroketone 22 (X = F, Scheme 8) by quenching the enolate of ketone 21a (LiHMDS) with (PhSO2)NF (98% yield, see Scheme 4) in order to explore its coupling with Michael acceptor 7b. In the event, the enolate of 22 reacted with enetrione 7b to afford the desired product (44b), albeit in only 11% yield, as a mixture of diastereomers (ca. 1:1 ratio) as shown in Scheme 8. A number of other partners, including p-nitrobenzyl quinone 7c and regioisomeric bromoketone 23 (prepared from ketone 21b by heating at 70 °C with NBS in the presence of AIBN in benzene, 52% yield, see Scheme 4), were also explored for their reactivity toward the desired goal. Thus, partnering fluoroketone 22 with quinone 7c under the developed coupling conditions led to a very low yield (9%) of adduct 44c (ca. 1:1 mixture of diastereomers), while the union of the regioisomeric bromoketone 23 with quinone 7b under the standard conditions led to 45 in 49% yield (ca. 1:1 mixture of diastereoisomers), as shown in Scheme 8. However, aromatization attempts to convert these adducts to the desired anthraquinone systems 6 (Scheme 1) proved in vain, as only decomposition and undesired products, primarily due to retro-Michael reactions, were observed. In order to avoid the problem of the competing retro-Michael reactions, the coupling of the aromatized bromoanthracene 26 (see Scheme 4) and enetrione 7b via metalation and various cross-coupling type reactions was investigated. Disappointingly, all attempts to form the challenging C-7′–C-4a bond failed. Faced with low yields in the Michael coupling reactions and the inability to form the desired anthraquinone 6 from the resulting products, we decided to abort this approach and seek, instead, an entirely new strategy for the set goal of synthesizing BE-43472B (1).
Second Generation Diels–Alder Approach. The All-Carbon Skeleton Encompassing Strategy
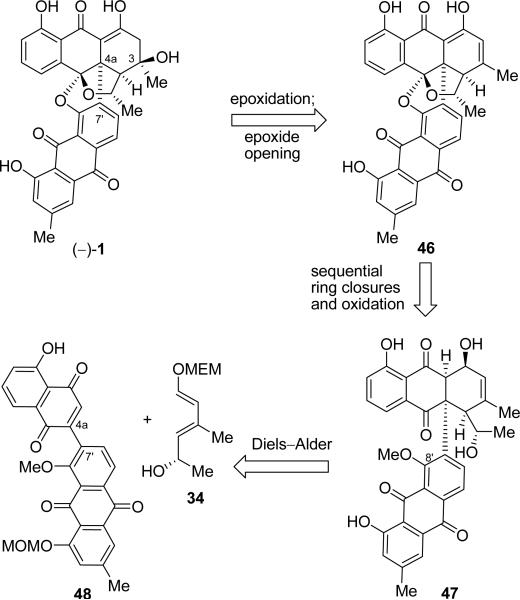
Despite our failure to reach the desired framework of the BE-43472B molecule (1) through the Michael reaction/aromatization approach, during these studies we came to recognize the usefulness of the Diels–Alder reaction in forming the C-ring of our target. In a speculative, but daring move we considered generating the entire carbon skeleton of the antibiotic 1 through a Diels–Alder reaction-based strategy between the appropriate components. This second generation Diels–Alder approach is shown retrosynthetically in Scheme 9 using the structure depicted by (–)-1 (although at this stage we did not know the absolute stereochemistry of the natural product as mentioned above). Thus, dehydrating (–)-1 retrosynthetically toward the enol moiety led to conjugated system 46, whose forward manipulation would require regio- and stereoselective epoxidation, followed by regioselective opening of the resulting oxirane, to afford the natural product. Dismantling of the two 5-membered ether rings within the latter intermediate through sequential C–O bond disconnections, insertion of a MeO group at C-8′, and reduction of the enol moiety revealed structure 47 as a possible precursor to 46. Applying a retro Diels–Alder reaction on 47 unraveled diene 34 and dienophile 48 as the required building blocks for this strategy. The adoption of diene 34 was based on our findings regarding its regiochemical reactivity toward juglone (35) as discussed above (Scheme 6), which was expected to favor the desired regioisomeric Diels–Alder product. This expectation was in part based on wishful thinking, since at this stage we were cognizant of the uncertainty surrounding the regio- and stereochemical outcome of the designed Diels–Alder reaction because we were unsure about the overall electronic effect of the anthraquinone moiety attached onto the juglone structural motif within dienophile 48. The answer would come through experimentation as we shall describe below.
Scheme 9.
Second Generation Retrosynthetic Analysis of (–)-BE-43472B [(–)-1]. The Diels–Alder Approach
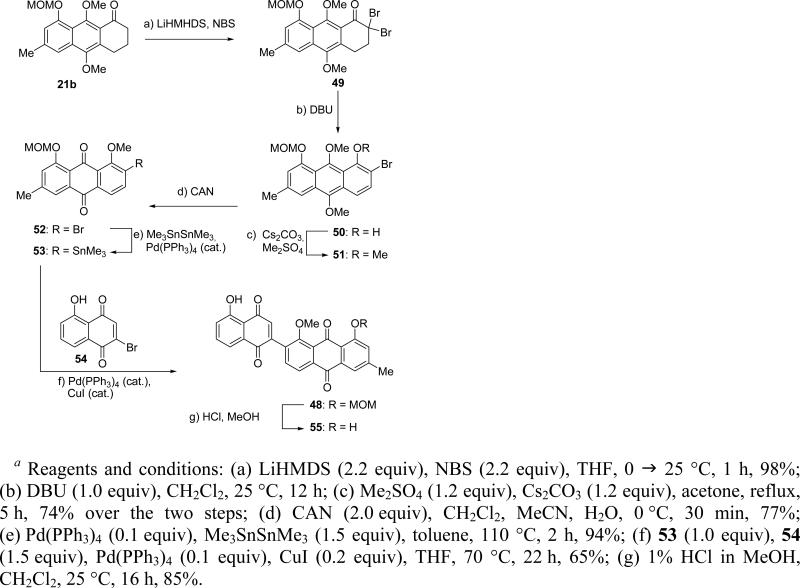
The construction of dienophile 48 commenced from hydroanthracene 21b (see Scheme 4 for preparation) and proceeded as summarized in Scheme 10. Thus, treatment of 21b with LiHMDS in the presence of NBS produced dibromoketone 49 in 98% yield, whose aromatization (DBU) and methylation (Cs2CO3, Me2SO4) furnished aryl bromide 51 (74% overall yield over two steps) through intermediate phenol 50. Oxidation of 51 with CAN afforded anthraquinone 52 (77% yield), which reacted with Me3SnSnMe3 in the presence of Pd(PPh3)4 catalyst to afford trimethyl stannane 53 in 94% yield. Stille coupling18,19 of 53 with bromoquinone 5420 proceeded smoothly in the presence of catalytic amounts of Pd(PPh3)4 and CuI, furnishing the desired dienophile 48 in 65% yield. Later on, the MOM group was removed from the latter compound (1% HCl in MeOH, 85% yield) to generate the diphenolic compound 55, which was subsequently also called upon to act as a dienophile in these studies.
Scheme 10.
Construction of Quinone Dienophiles 48 and 55a
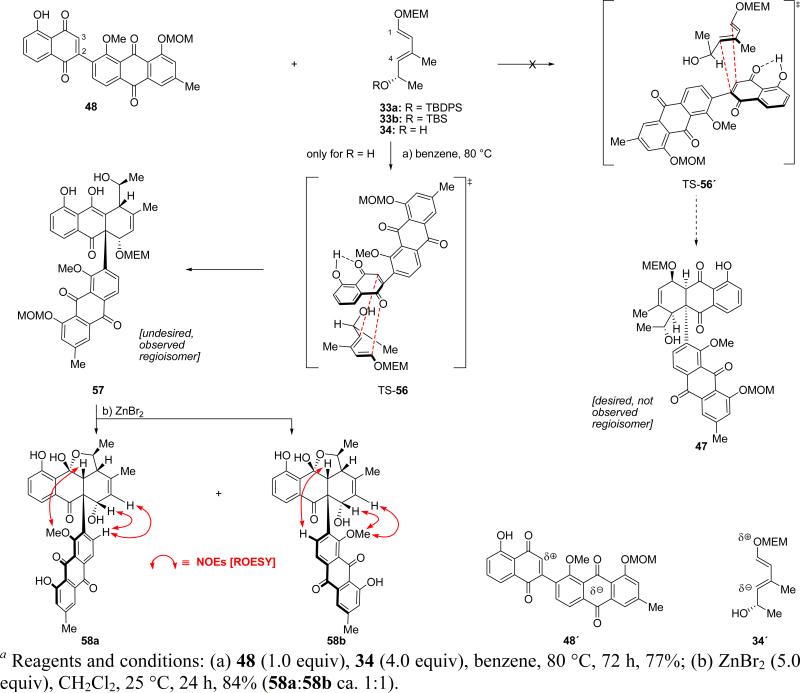
Scheme 11 presents the results of the Diels–Alder reaction between diene 34 and dienophile 48. Thus, heating a mixture of 34 (4.0 equiv) with 48 (1.0 equiv) in benzene at 80 °C for 72 h furnished a single Diels–Alder adduct whose structure was determined, through subsequent chemistry and NMR spectroscopy, to be that of the undesired regioisomer 57.21,22 Interestingly, the silylated dienes 33a and 33b (prepared through a similar route as that shown in Scheme 5 for 33a) failed to react with dienophile quinone 48 under various conditions, presumably due to steric hindrance, underscoring the relevance of the free secondary alcohol in the diene system to the success of the Diels–Alder reaction. Exposure of adduct 57 to the action of ZnBr2 resulted in the simultaneous removal of the MOM and MEM protecting groups and the formation of the isomeric heptacyclic lactols 58a and 58b (84% yield, ca. 1:1 ratio, not separable by chromatography). Their structures were determined through 1H NMR ROESY studies that revealed the NOEs indicated on their structures in Scheme 11. Manual molecular models are supportive of the rotational barrier between these two atropisomeric structures (58a and 58b). These studies also provided the crucial evidence for the regioisomeric nature of the Diels–Alder adduct 57, apparently formed through endo transition state TS-56 which is favored over its regioisomeric transition state TS-56′ that was expected to form the desired adduct 47 as shown in Scheme 11. The stereochemistry of compounds 57 and 58a/58b could not be completely discerned through NMR spectroscopy. It was presumed to be as shown on the basis of the most favorable facial orientation of diene 34 and dienophile 48 as they merge through transition state TS-56 (Scheme 11) to form the Diels–Alder product 57. However, from these failed experiments we draw the conclusion that the electronic (–I) effect of the electron withdrawing anthraquinone substituent attached onto the juglone dienophile overrides the polarizing effect of the intramolecular H-bond within this system,23 enlarging the C-3 orbital coefficient of the LUMO of the juglone system. The overall polarized characters of diene 34 and dienophile 48 (as shown in 34′ and 48′, Scheme 11) dictate the regiochemical outcome of their union to afford cycloadduct 57. This path-pointing study led us to the next phase of our campaign toward the total synthesis of antibiotic BE-43472B (1).
Scheme 11.
Diels–Alder Reaction of Diene 34 with Dienophile 44 Leading to Undesired Regioisomeric Adduct 57 and Atropisomers 58a and 58ba
Final Diels–Alder Approach. Total Synthesis of Antibiotic BE-43472B
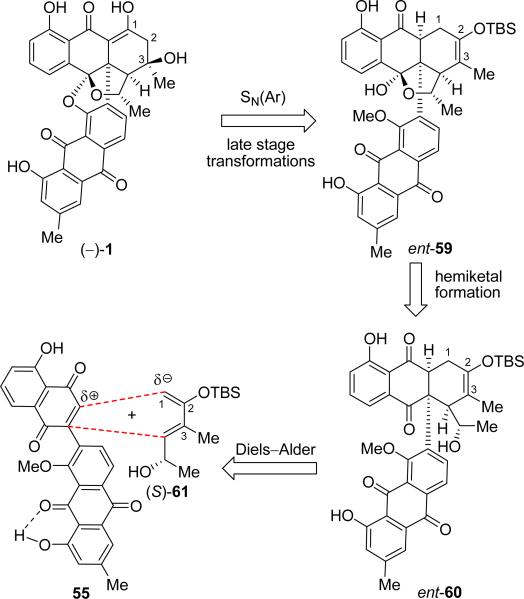
Based on the latest intelligence gathering, we redesigned our second generation Diels–Alder strategy toward the target molecule in order to accommodate the realities of the electronics within the dienophile component of the cycloaddition reaction. Scheme 12 outlines, in retrosynthetic format, the newly devised synthetic strategy. Note that in this Scheme we again use the antipodal structure [(–)-1] of the natural product (we will switch to the structure of the enantiomer (+)-1 in our final drive toward BE-43472B). The main provision in this plan was the adoption of diene (S)-61 (with a 2-oxa as opposed to a 1-oxa substituent), which was expected to possess the reverse polarity (enlargement of the C-1 orbital coefficient of the HOMO) from that of the originally used diene (34), and therefore match the demonstrated polarity of dienophile 55 (see Scheme 12). The adoption of (S)-61, in turn, required placement of an oxygen atom at C-2 within the Diels–Alder product (ent-60) that would have to be subsequently removed. Additionally, two new oxygen atoms will have to be introduced into the emerging structures (ent-60 and ent-59) at C-3 and C-1 before reaching (–)-1. Another interesting feature of the new synthetic design was the choice of the naked dienophile 55, whose anthraquinone intramolecular H-bonding was expected to facilitate the intended intramolecular ipso substitution in order to cast the last bond of the ring framework of the target molecule. These new design modifications highlighted our expectations for success with the possibility of a cascade sequence, beginning with the Diels–Alder reaction and ending with an octacyclic structure, whose molecular complexity would be impressively close to that of the targeted natural product.
Scheme 12.
Final Retrosynthetic Analysis of (–)-BE-43472B [(–)-1]
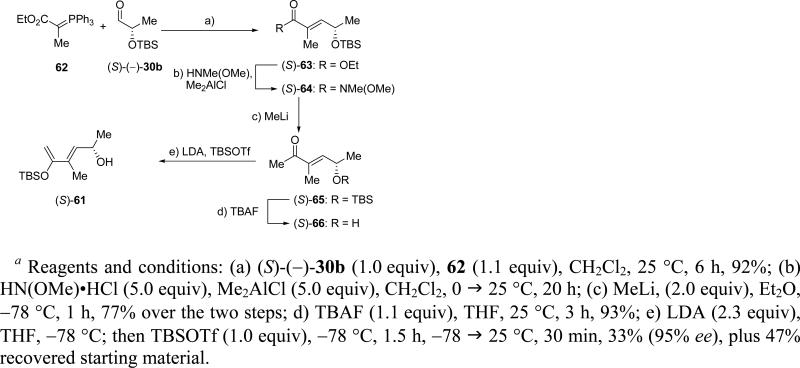
The required diene (S)-61 became readily available through the concise synthetic route summarized in Scheme 13. Reaction of commercially available phosphorane 62 with known aldehyde (S)-(–)-30b24 gave α,β-unsaturated ester (S)-63 in 92% yield (E:Z >98:2), which was first converted to Weinreb amide (S)-64 through the action of HNMe(OMe) and Me2AlCl and then to methyl ketone (S)-65 by reaction with MeLi (77% yield for the two steps).25 Desilylation of the latter compound with TBAF led to hydroxy ketone (S)-66 (93% yield), which was treated with 2.3 equiv of LDA, to afford, after quenching the resulting dianion with 1.0 equiv TBSOTf, diene (S)-61 in 33% yield and 95% ee, as determined by HPLC analysis using a chiral column (plus 47% recovered starting material).
Scheme 13.
Synthesis of Diene (S)-61a
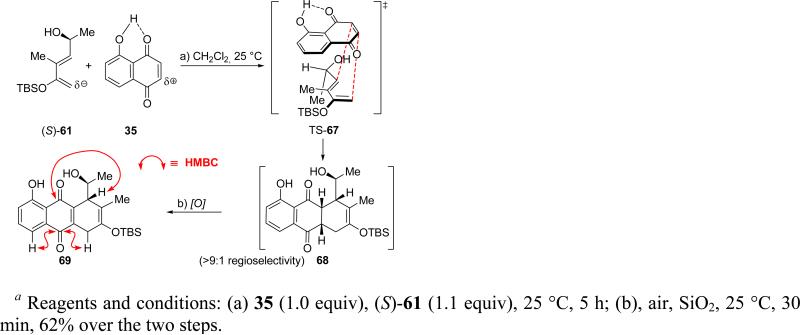
At this stage, and in order to obtain insight into the behavior of (S)-61 toward relevant quinone-type dienophiles, this diene was allowed to react with juglone (35). As shown in Scheme 14, the reaction proceeded in CH2Cl2 at room temperature, leading, as expected, to the opposite regioisomer (i.e. 68, >9:1 regioselectivity) to that obtained previously with the terminally (1-oxa) substituted diene (33a, see Scheme 6). However, in this case the resulting Diels–Alder product (68) was found to be rather labile, undergoing facile oxidation by air in the presence of silica gel, forming quinone 69 (62% yield overall from 35). The regiochemical identity of 69 was based on HMBC NMR correlations as designated in Scheme 14, while the stereochemistry of its precursor (68) was based on transition state TS-67 as the sterically most favorable (1,3-allylic strain). This regio- and stereochemical outcome pointed to the high likelihood that diene (S)-61 will react with dienophile 55 with the desired regio- and diastereoselectivity.
Scheme 14.
Diels–Alder Model Study with Diene (S)-61 and Juglone (35)a
Indeed, the Diels–Alder reaction between diene (S)-61 and dienophile 55 this time proceeded as planned, and was followed by a pleasing sequence of transformations that eventually led to a successful total synthesis (vide infra). However, the obtained synthetic BE-43472B exhibited the opposite optical rotation to that exhibited by the natural BE-43472B, an observation that led to the true configurational identity of the natural product. Thus, by utilizing diene (S)-61 we reached the enantiomer of BE-43472B [(–)-1, Figure 2]. We then synthesized diene (R)-61 starting with aldehyde (R)-(+)-30b and entered it into the developed synthetic sequence, arriving at BE-43472B in its naturally occurring enantiomeric form [(+)-1, Figure 2]. It is this sequence that we now describe below.
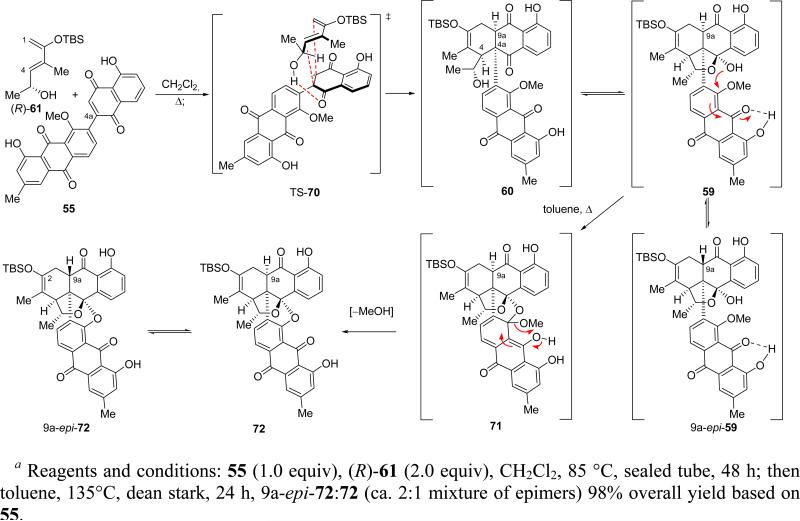
Scheme 15 depicts the first phase of the reaction sequence that was initiated upon mixing (R)-61 and dienophile 55. Thus, heating (R)-61 (2.0 equiv) with 55 (1.0 equiv) in CH2Cl2 solution in a sealed tube (85 °C external temperature) for 48 h, followed by addition of toluene and refluxing with a Dean–Stark apparatus for another 24 h, led, through a remarkable cascade sequence,26 to an inconsequential mixture of the two C-9a epimers 72 and 9a-epi-72 (9a-epi-72:72 ca. 2:1) in a 98% combined yield. This aesthetically pleasing cascade became possible only because the initial Diels–Alder cycloaddition reaction proceeded, through transition state TS-70, regio- and stereoselectively, as desired. The resulting cycloadduct 60 was observed by NMR spectroscopic analysis to exist in equilibrium with its hemiketal form 59 (59:60 ca. 4:1, benzene-d6). Inspection of manual molecular models indicates that hemiketal 59 finds itself poised and in a favorable conformation to undergo an intramolecular aromatic ipso substitution [SN(Ar)-type intramolecular transesterification], expelling a molecule of MeOH to form octacycle 72 through tetrahedral intermediate 71 as shown in Scheme 15. The presence of the adjacent β-phenolic quinone moiety apparently facilitates this novel cyclization through internal H-bonding, which activates the vinylogous-type ester for the initiating attack by the hemiketal hydroxyl group as shown in structures 59 and 71. Under the toluene azeotropic refluxing conditions that removed the released MeOH from the reaction mixture, product 72 suffers epimerization at C-9a to afford 9a-epi-72, a phenomenon also observed on silica gel. In refluxing benzene, we also observed (by 1H NMR spectroscopy) epimerization of 59 to 9a-epi-59 as shown in Scheme 15. With ample amounts of 72 and 9a-epi-72 in hand, the stage was now set for an attempt to reach the final target, antibiotic BE-43472B [(+)-1].
Scheme 15.
Diels–Alder Reaction/Double THF Cyclization Cascadea
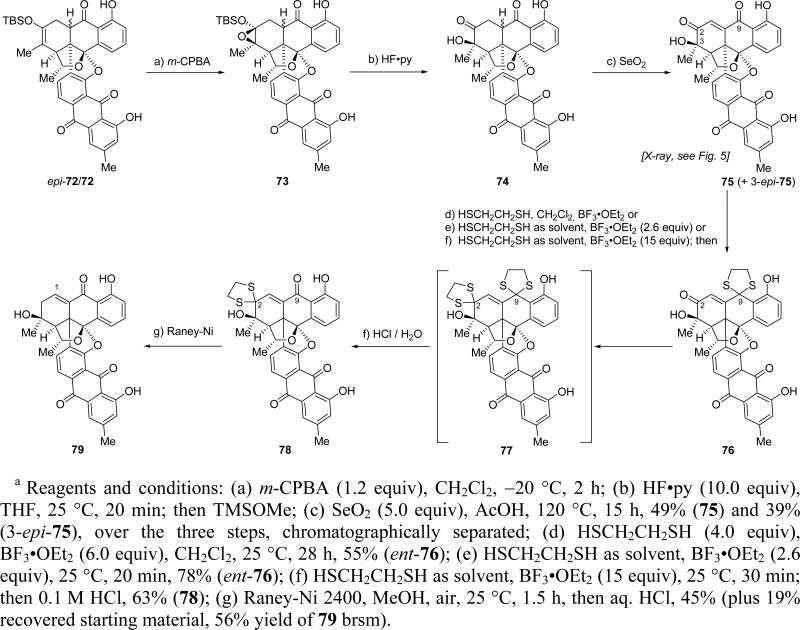
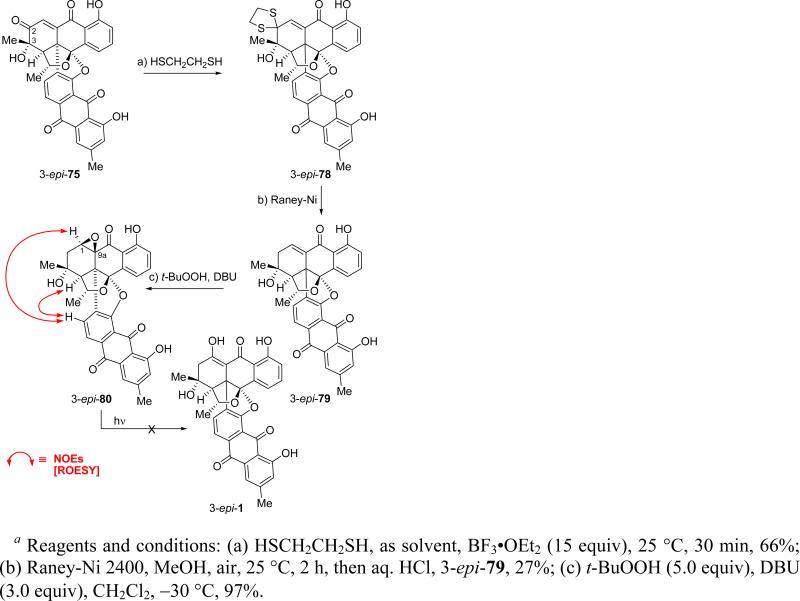
The obligatory employment of diene (R)-61 in our synthesis necessitated excision of the superfluous oxygen atom from the C-2 position of the growing molecule; furthermore, two new oxygen atoms, one at C-1 and one at C-3, had to be introduced in their proper positions before the final target could be reached. These objectives were achieved as shown in Scheme 16. Thus, treatment of the mixture of 9a-epi-72 and 72 (ca. 2:1 ratio)27 with m-CPBA at –20 °C led to a mixture of four oxirane epimers (ca. 4:1 ratio of β:α oxirane pairs, α-epimers not shown). Desilylation of these oxiranes as a mixture (HF•py, TMSOMe) was accompanied by ring opening to afford a mixture of four hydroxy ketones (74, ca. 4:1 β:α ratio, α-epimers not shown), which were subsequently, and without isolation, subjected to SeO2 oxidation in AcOH (120 °C) to afford enone 75 (49% overall for the three steps) together with its C-3 epimer (3-epi-75, 39%, chromatographically separated), indicating that epimerization of the C-3 stereogenic center took place under the oxidation conditions. Crystallization of 75 from CH2Cl2 led to single crystals (m.p. >250 °C, CH2Cl2/hexanes), whose X-ray crystallographic analysis (see ORTEP drawing, Figure 5) revealed its relative configuration and confirmed the stereochemical outcomes of both the Diels–Alder and the epoxidation reactions. Having accomplished the installation of the C-3 hydroxyl group in its desired configuration, we then proceeded to address the challenging task of selectively removing the superfluous oxygen atom from C-2. To this end, and as shown in Scheme 16, we subjected enone 75 to the action of ethane-1,2-dithiol under various conditions. After considerable experimentation we found that reaction of the enantiomeric ketone ent-75 with 4.0 equiv ethane-1,2-dithiol and 6.0 equiv BF3•OEt2 in CH2Cl2 led to dithioketal ent-76 (55% yield), in which the C-9 carbonyl group (activated through intramolecular H-bonding) was preferentially protected out of the four carbonyl moieties present in the substrate (i.e. ent-75). Using ethane-1,2-dithiol as solvent and 2.6 equiv of BF3•OEt2 improved the efficiency of this reaction, furnishing ent-76 in 78% yield. However, when the reaction was carried out with enone 75 under more forcing conditions (ethane-1,2-dithiol as solvent, 15 equiv BF3•OEt2), followed by quenching with 0.1 M HCl, the desired C-2 dithioketal 78 was isolated in 63% yield, presuambly through intermediate bis-dithioketal 77, which apparently collapses upon exposure to H2O by hydrolysis of the C-9 dithiolane. The latter process is rendered facile and selective, presumably through intramolecular H-bonding of the phenolic OH with the neighboring sulfur atom, now made possible by the increased electron density on the latter as a result of the suppression of the electron withdrawing effect of the C-2 carbonyl moiety (upon its engagement as a dithiolane system). This remarkably selective reaction offered the sought-after window of opportunity for the desired C-2 deoxygenation, which was accomplished through reductive desulfurization (Raney-Ni 2400) to afford compound 79 in 45% yield (plus 19% recovered starting material; 56% yield brsm). With this operation behind us, all that remained to reach the target molecule was the insertion of an oxygen atom in the C–H bond of the enone moiety within 79.
Scheme 16.
Late Stage Functional Group Transformations and Synthesis of Enone 79a
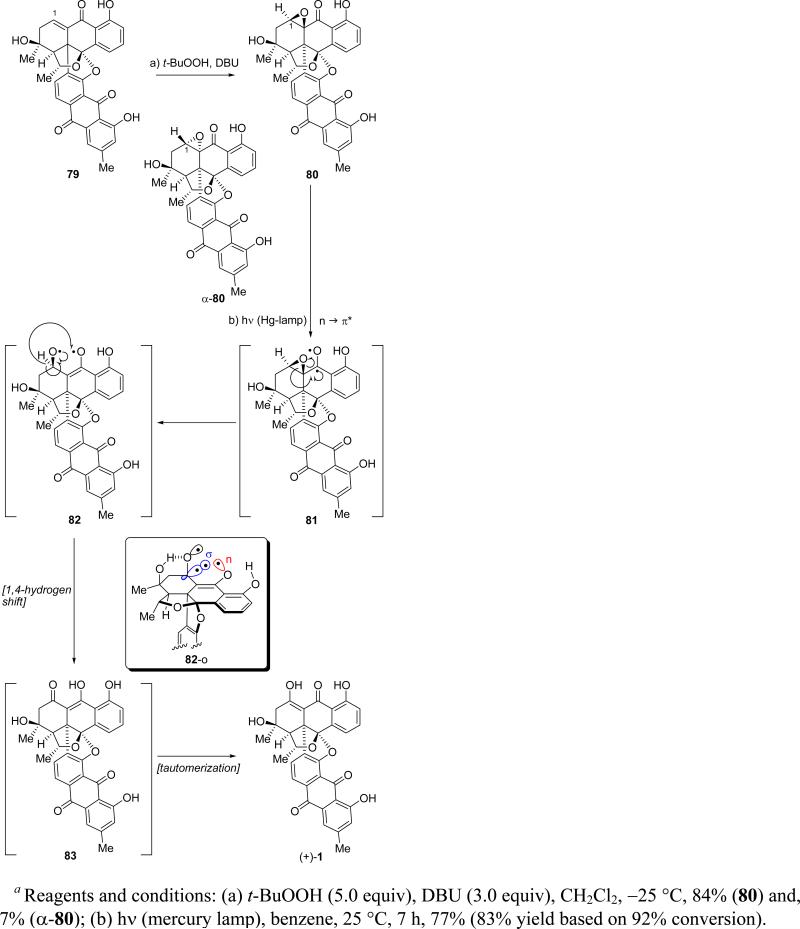
Having failed to achieve a direct Wacker-type oxidation28 of enone 79, and due to the ease of elimination of the axial C-3 hydroxyl group of the natural product [(+)-1],1 we resorted to the mild two-step procedure involving epoxidation/oxirane rearrangement29 as shown in Scheme 17. Thus, epoxidation of 79 with t-BuOOH and DBU furnished the β-oxirane 80 as the major product (84% yield), together with small amounts of the epimeric α-oxirane (α-80, 7% yield, chromatographically separated). While a number of metal- and Lewis acid-catalyzed protocols failed to induce the desired transformation of epoxy ketone 80 to the natural product, simple irradiation of this precursor in benzene with UV light at ambient temperature (and in the absence of any sensitizer) smoothly converted it to bisanthraquinone antibiotic BE-43472B [(+)-1] in 77% yield (83% yield based on 92% conversion). Synthetic (+)-1 exhibited identical physical properties to those reported for the natural product and essentially the same optical rotation ( (c = 0.14 in CHCl3) = +411.4) as that of natural (+)-1 ( (c = 0.14 in CHCl3) = +403.1, and so did (–)-1, except for its optical rotation, which was of the opposite sign ( (c = 0.14 in CHCl3) = –417.1).26 Because our data for synthetic (+)-1 matched both the data reported by Rowley1 and in the Japanese patent,3 we concluded that all three compounds are identical, that is to say one and the same.
Scheme 17.
Final Steps of the Total Synthesis of BE-43472B [(+)-1]a
For the photolytically induced rearrangement of epoxy ketone 80 to BE-43472B [(+)-1] we propose the radical based mechanism shown in Scheme 17. Thus, it is presumed that initial n → π* photoexcitation of 80 forms transient excited state diradical 81, which suffers homolytic epoxide rupture as shown to generate diradical 82. The latter possesses an ideal geometry for a 1,4-hydrogen shift (see σ and n orbital arrangement, structure 82-o in box) as shown in Scheme 17 to give enol 83, whose facile and spontaneous tautomerization leads to the observed product [(+)-1]. The photolytically induced rearrangement of α,β-oxiranyl ketones to conjugated enol ketones was observed as early as 1918 by Bodfoss,29 and recently revisited by Jang, Park, and coworkers30 in a flash laser spectroscopic study that revealed a 1,4-hydrogen shift as a key element of its mechanism.
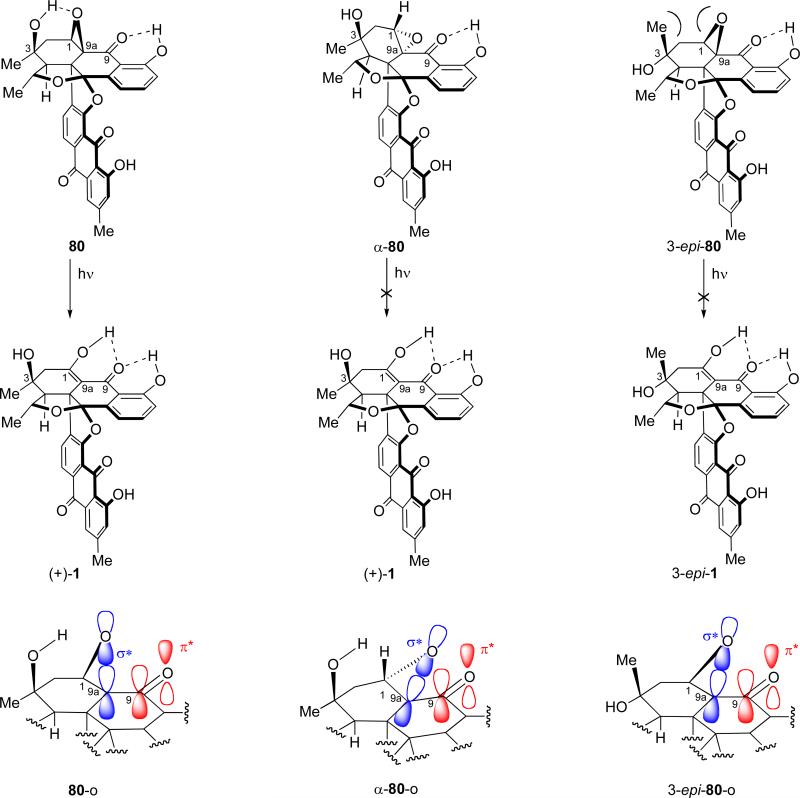
Having completed the total synthesis of both (+)-1 and (–)-1, we then attempted to construct the C-3 epimer of the natural product [(+)-1] starting from the C-3 epimeric enone 3-epi-75, which we obtained from the SeO2 mediated oxidation of 9a-epi-72/72 (see Scheme 16). As shown in Scheme 18, selective thioketalization of 3-epi-75 under the previously developed conditions (ethane-1,2-dithiol as solvent, 15 equiv BF3•OEt2) gave dithiolane 3-epi-78 in 66% yield. Reductive desulfurization of this compound (Raney-Ni 2400, MeOH; then aq. HCl) furnished enone 3-epi-79 (27% yield, unoptimized), which was subjected to epoxidation (t-BuOOH, DBU, –30 °C) to afford β-oxirane 3-epi-80 as a single isomer and in 97% yield. Unfortunately, irradiation of the latter oxirane under the same conditions (benzene, ambient temperature) used for the successful rearrangement of oxirane 80 (see Scheme 17) failed to induce any change, leading only to recovered starting material. This outcome did not change, neither by irradiation in benzene at 60 °C, nor by changing the solvent to MeOH (irradiation at ambient temperature). Interestingly, we observed the same reluctance of α-oxirane α-80 (Scheme 17) to undergo the photolytically induced rearrangement to the desired enol structure. These results may be explained by considering the frontier molecular orbitals of the epoxy ketone structural motif in each of the three substrates subjected to the photolytic conditions (80, α-80, and 3-epi-80), as illustrated in Figure 6. As seen, the structure of the oxiranyl ketone 80 allows almost perfect alignment of the C-9a–O bond and its associated σ* orbital with the π* orbital of the adjacent C=O bond as required for the oxirane rupture (see drawing 80-o, Figure 6). The indicated H-bonding between the C-3 hydroxyl group and the oxirane oxygen [downfield shift of the OH proton to δ = 3.37 ppm in the 1H NMR spectrum, CDCl3, 500 MHz; higher Rf value of oxiranyl ketone 80 (TLC, silica) as compared to that of enone 79] is presumed to play a subtle role in bringing about this alignment by pulling the oxirane oxygen atom closer to the C-3 hydroxyl group. In contrast to the situation with the β-oxirane 80, in the C-3 epimeric β-oxirane3-epi-80 the steric repulsion between the C-3 methyl group and the pseudoaxial oxirane moiety results in the bending of the latter out of the required orthogonal geometry to the adjacent carbonyl moiety, resulting in insufficient overlap of the C-9a–O σ* orbital with the π* orbital of the carbonyl group for fragmentation to occur (see drawing 3-epi-80, Figure 6). A similar situation exists for the α-oxirane, α-80 (which could have provided BE-43472B had the epoxide rearrangement occured) in which the stereoelectronics are even worse (see drawing α-80-o, Figure 6). Indeed, the preferred conformation of the cis-decalin system within which the epoxy ketone structural motif resides forces the oxirane into an equatorial position, resulting in inadequate orbital overlap between the relevant σ* and π* orbitals. Manual molecular modeling is sufficient to recognize these crucial stereoelectronic effects within the oxiranes discussed above.
Scheme 18.
Synthesis of the C-3 Epimeric Oxirane 3-epi-80 and Attempted Photolytic Rearrangementa
Figure 6.
Conformations of the diastereomeric oxiranes 80, α-80, and 3-epi-80 (oxirane orbitals are shown on a straight line for convenience).
Biological Evaluation of Both Enantiomers of Antibiotic BE-43472B and Related Compounds
The set of the synthesized compounds enabled us to biologically evaluate a selected number of them in order to obtain insights on structure-activity relationships (SARs) within the class. The results are listed in Table 1 (none of the compounds tested showed activity against E. Coli at 100 μM).
Table 1.
Biological Activities of (+)- and (–)-BE-43472B and Related Compounds
| Entry | Compound | MIC (μM)a | IC50 (μM)d | |||
|---|---|---|---|---|---|---|
| MRSAb | VREc | HCT-116e | HeLaf | MCF-7g | ||
| 1 | (+)-1 | 0.15–0.3 | 0.6–1.5 | 4.1 | 2.6 | 26 |
| 2 | (–)-1 | 0.10–0.2 | 0.3–2.0 | 3.8 | 2.2 | 40 |
| 3 | 55 | NA | NA | 7 | 5 | 15 |
| 4 | 72 | NA | NA | NA | NA | NA |
| 5 | 75 | NA | NA | 16 | 18 | NA |
| 6 | 3-epi-75 | NA | NA | 10 | 7.7 | NA |
| 7 | ent-75 | NA | NA | NA | NA | NA |
| 8 | ent-3-epi-75 | NA | NA | 16 | 14 | NA |
| 9 | 78 | NA | NA | NA | NA | NA |
| 10 | ent-3-epi-78 | NA | NA | 31 | 30 | NA |
| 11 | 79 | NA | NA | 32 | NA | NA |
| 12 | ent-79 | NA | NA | NA | 30 | NA |
| 13 | 80 | NA | NA | NA | NA | NA |
| 14 | α-80 | NA | NA | NA | NA | NA |
| 15 | 3-epi-79 | NA | NA | 8 | 9 | 40 |
| 16 | 3-epi-80 | NA | NA | NA | NA | NA |
Minimum Inhibitory Concentration.
Methicillin-resistant Staphylococcus aureus.
Vancomycin-resistant Enterococcus faecalis.
Concentration that causes 50% of cell growth inhibition. Experiments were done in triplicates as described in the supplementary information.
Human colon cancer cell line.
Human cervical cancer cell line.
Human breast cancer cell line.
NA: Not active at the highest concentration tested (100 μM for the antibacterial assay and 40 μM for the cytotoxicity assay). (Further information is available in the supplementary information).
The antimicrobial activities of both enantiomers of the bisanthraquinone BE-43472B [(+)-1 and (–)-1] and analogues thereof were determined against Gram-positive methicillin-resistant Staphylococus aureus (MRSA), vancomycin-resistant Enterococcus faecalis (VRE), and Gram negative Escherichia coli. In accord with the values reported by Rowley and coworkers1,2, (+)-1 (natural enantiomer) exhibited strong bactericidal activity against Gram-positive pathogens (MRSA and VRE). Given the architectural complexity of 1 it is notable that the unnatural enantiomer [(–)-1] displayed almost similar activity. The fact that all of the derivatives screened in these assay (55, 72, 75, 3-epi-75, ent-75, ent-3-epi-75, 78, ent-3-epi-78, 79, ent-79, 80, α-80, 3-epi-79, 3-epi-80) lack activity implies that the enol functionality (C–1 hydroxyl) is critical for antibacterial activity.
The initial cytotoxicity evaluation of 1 and derivatives thereof was performed across a panel of three cell lines of different histological origin (HCT-116, colon; MCF-7, breast; and HeLa, cervix). It is worth noting that both (+)-1 and (–)-1 displayed higher potency against HeLa and HCT-116 cells than against MCF-7 cells. While for HeLa and HCT-116 cells (–)-1 is slightly more active than its enantiomer (+)-1, in the case of MCF-7 cells the (+)-1 enantiomer exhibited an IC50 value almost two times higher than the (–)-1 enantiomer. Additionally, the simple dienophile 55 (for structure, see Scheme 15) displayed activity in all three tested cell lines with IC50 values in the same order of magnitude as (+)-1 and (–)-1 (entry 3, 7 μM against HCT-116, 5 μM against HeLa, and 15 μM against MCF-7). While none of the tested compounds exhibited significant antibacterial properties against the bacterial strains employed, a number of them showed notable activities against some, or all, of the tumor cell lines used. Those included enones 75 (entry 5, 16 μM against HCT-116 and 18 μM against HeLa), 3-epi-75 (entry 6, 10 μM against HCT-116 and 7.7 μM against HeLa), ent-3-epi-75 (entry 8, 16 μM against HCT-116 and 14 μM against HeLa), ent-3-epi-78 (entry 10, 31 μM against HCT-116 and 30 μM against HeLa), as well as alkenes 79 (entry 11, 32 μM against HCT-116), ent-79 (entry 12, 30 μM against HeLa), and 3-epi-79 (entry 15, 8 μM against HCT-116, 9 μM against HeLa, and 40 μM against MCF-7). The latter compound showed IC50 values against all three cell lines that are reminiscent of the natural product itself despite lacking the C–1 hydroxyl group, which appears to be necessary for the aforementioned antibacterial activity. In addition, it is interesting to note that epoxidation of the C–1/C–9a olefin leads to a complete loss of growth inhibition activity (72, 80, α-80, and 3-epi-80).
These initial investigations into the biology of 1 and related compounds led to the following conclusions: 1) the potencies of the natural and unnatural enantiomers of 1 against bacteria and tumor cells are similar despite their rigid polycyclic, but antipodal structures, 2) antibacterial activity is abolished with even slight modifications of the C-ring of the molecule, and 3) whereas the enol form of the 1,3-dicarbonyl function (C-1 hydroxyl) is critical for antibacterial activity, an α,β-unsaturated carbonyl C-1 hydrogen) is in some cases (i.e. 75, 3-epi-75, ent-3-epi-75, 79, ent-79, 3-epi-79) sufficient to retain cytotoxic activity. On the other hand, epoxidation of the enone functionality leads to complete loss of activity.
Conclusion
Based on a cascade sequence, initiated by a thermally induced Diels–Alder reaction, the described synthetic strategy led to an efficient total syntheses of (+)- and (–)-bisanthraquinone antibiotic BE-43472B [(+)-1 and (–)-1] and the assignment of the absolute configuration of this natural product as (+)-1. In addition to demonstrating the power of cascade reactions in total synthesis,26 the developed chemistry highlights the use of heat and light as tools for green and efficient chemistry. Biological evaluation of (+)-1 and (–)-1 revealed that both compounds are endowed with almost equipotent antibacterial properties and are less active as antitumor agents. Although the various derivatives and intermediates en route to 1 were not as potent antibacterial agents, important information has been gathered from the biological evaluation of these compounds regarding useful structure-activity relationships (SARs) to aid in the design of future analogs. With a synthetic pathway to the BE-43472B structure now available and initial biological data in hand, the design and synthesis of analogues of antibiotic BE-43472B as part of an effort to discover and develop new antibacterial agents is now feasible.
Supplementary Material
Figure 4.
X-Ray derived ORTEP drawing of quinone 7c.
Acknowledgements
Insightful discussions with Prof. A. Eschenmoser regarding the mechanistic aspects of this work are gratefully acknowledged. We thank Prof. D. C. Rowley and A. Socha for a sample of BE-43472B [(+)-1]. We also thank Dr. D. H. Huang and Dr. L. Pasterneck, Dr. G. Siuzdak, and Dr. R. Chadha for NMR spectroscopic, mass spectrometric, and X-ray crystallographic assistance, respectively. Financial support for this work was provided by Evonik Industries (Germany), the National Institutes of Health (USA), the National Science Foundation (CHE-0603217), the Skaggs Institute for Chemical Biology, the Alexander von Humboldt Foundation (Germany, postdoctoral fellowship to J.B.), A*STAR (Singapore, predoctoral fellowship to Y.H.L.), the Skaggs-Oxford scholarship program (predoctoral fellowship to Y.H.L.), the NSERC (Canada, postdoctoral fellowship to A.L.), and the Max Weber-Programm (Germany, student internship stipend to T.N.).
Footnotes
Supporting Information Available: Experimental procedures and full compound characterization. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Socha AM, Garcia D, Sheffer R, Rowley DC. J. Nat. Prod. 2006;69:1070. doi: 10.1021/np050449b. [DOI] [PubMed] [Google Scholar]
- 2.Socha AM, LaPlante KL, Rowley DC. Bioorg. Med. Chem. 2006;14:8446. doi: 10.1016/j.bmc.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 3.Kushida H, Nakajima S, Koyama T, Suzuki H, Ojiri K, Suda H. Antitumoric BE-43472 Manufacture with Streptomyces. 1996. JP 08143569.
- 4.For selected references, see: Nicolaou KC, Chen JS, Edmonds DJ, Estrada AA. Angew. Chem., Int. Ed. 2009;48:660. doi: 10.1002/anie.200801695.Roberts L, Simpson S. Science. 2008;321:355. doi: 10.1126/science.321.5887.355., and references therein. Walsh CT, Wright G. Chem. Rev. 2005;105:391. doi: 10.1021/cr030100y.Nicolaou KC, Boddy CNC, Bräse S, Winssinger N. Angew. Chem., Int. Ed. 1999;38:2096. doi: 10.1002/(sici)1521-3773(19990802)38:15<2096::aid-anie2096>3.0.co;2-f.
- 5.For previous synthetic studies inspired by BE-43472B, see: Takikawa H, Hikita K, Suzuki K. Angew. Chem., Int. Ed. 2008;47:9887. doi: 10.1002/anie.200801577.Suzuki K, Takikawa H, Hachisu Y, Bode JW. Angew. Chem., Int. Ed. 2007;46:3252. doi: 10.1002/anie.200605138.
- 6.For a preliminary communication on this work, see: Nicolaou KC, Lim YH, Becker J. Angew. Chem., Int. Ed. 2009;48:3444. doi: 10.1002/anie.200900058.
- 7.Highlight: Rowley DC. Nature Chemistry. 2009;1:110. doi: 10.1038/nchem.192.
- 8.Nicolaou KC, Lim YH, Piper JL, Papageorgiou CD. J. Am. Chem. Soc. 2007;129:4001. doi: 10.1021/ja0685708. [DOI] [PubMed] [Google Scholar]
- 9.Nicolaou KC, Lim YH, Papageorgiou CD, Piper JL. Angew. Chem., Int. Ed. 2005;44:7917. doi: 10.1002/anie.200503678. [DOI] [PubMed] [Google Scholar]
- 10.Reviews: Snieckus V. Chem. Rev. 1990;90:879.Whisler MC, MacNeil S, Snieckus V, Beak P. Angew. Chem., Int. Ed. 2004;43:2206. doi: 10.1002/anie.200300590.
- 11.Hauser FM, Rhee RP. J. Org. Chem. 1978;43:78.Hauser FM, Chakrapani S, Ellenberger WP. J. Org. Chem. 1991;56:5248.Mal D, Ray S, Sharma I. J. Org. Chem. 2007;72:4981. doi: 10.1021/jo062271j.. Review: Mal D, Pahari P. Chem. Rev. 2007;107:1892. doi: 10.1021/cr068398q.
- 12.For Diels–Alder reactions with juglone as dienophile, see: Trost BM, Ippen J, Vladuchick WC. J. Am. Chem. Soc. 1977;99:8116.Stork G, Hagedorn AA., III J. Am. Chem. Soc. 1978;100:3609.Boeckman RK, Jr., Dolak TM, Culos KO. J. Am. Chem. Soc. 1978;100:7098.
- 13.The synthesis of dienes 33a and 33b was inspired by the work of McDougal et al., see: McDougal PG, Jump JM, Rojas C, Rico JG. Tetrahedron Lett. 1989;30:3897.McDougal PG, Rico JG. J. Org. Chem. 1987;52:4817.
- 14.Denmark SE, Jones TK. J. Org. Chem. 1982;47:4595. [Google Scholar]
- 15.Review: Fürstner A. Chem. Rev. 1999;99:991. doi: 10.1021/cr9703360. For an example where the Z-iodoalkene retains its stereochemistry after coupling, see: Nicolaou KC, Baran PS, Zhong Y-L, Barluenga S, Hunt KW, Kranich R, Vega JA. J. Am. Chem. Soc. 2002;124:2233. doi: 10.1021/ja012126h.
- 16.Overman LE, Rishton GM. Org. Synth., Coll. 1998;IX:139. [Google Scholar]; Overman LE, Rishton GM. Org. Synth. 1993;71:56. [Google Scholar]
- 17.Isomers 32a and 32b exhibited similar reactivities in this elimination reaction on exposure to s-BuLi as noted in their reactions either as individual compounds, or as a mixture. Partial elimination of MeOH from the terminal MEM group of diene 33a to form the corresponding triene was observed on prolonged reaction times or when excessive amounts of base were used.
- 18.Reviews: Farina V, Krishnamurthy V, Scott WK. Org. React. Vol. 50. Wiley-VCH; New York: 1997. p. 1.Fugami K, Kosugin M. Top. Curr. Chem. 2002;219:1.Mitchell TN. In: Metal-Catalyzed Cross-Coupling Reactions. 2nd. ed. de Meijere A, Diederich F, editors. Wiley-VCH; Weinheim: 2004. p. 125.
- 19.Echavarren AM, Tamayo N, Cárdenas DJ. J. Org. Chem. 1994;59:6075. [Google Scholar]
- 20.Grunwell JR, Karipides A, Wigal CT, Heinzman SW, Parlow J, Surso JA, Clayton L, Fleitz FJ, Daffner M, Stevens JE. J. Org. Chem. 1991;56:91. [Google Scholar]
- 21.a Tietze LF, Gericke KM, Singidi RR, Schuberth I. Org. Biomol. Chem. 2007;5:1191. doi: 10.1039/b700838d. [DOI] [PubMed] [Google Scholar]; b Boeckman RK, Jr., Dolak TM, Culos KO. J. Am. Chem. Soc. 1978;100:7098. [Google Scholar]; c Kelly TR, Montury M. Tetrahedron Lett. 1978;45:4311. [Google Scholar]; d Trost BM, Ippen J, Vladuchick WC. J. Am. Chem. Soc. 1977;99:8116. [Google Scholar]
- 22.For Diels–Alder reactions with similar chiral 1,3-dienes, see: Barriault L, Thomas JDO, Clément R. J. Org. Chem. 2003;68:2317. doi: 10.1021/jo020664m.Carreño MC, Garciá-Cerrada S, Urbano A, Di Vitta C. J. Org. Chem. 2000;65:4355. doi: 10.1021/jo000210u.. (c) Ref. 12(a).
- 23.Rozeboom MD, Tegmo-Larsson I-M, Houk KN. J. Org. Chem. 1981;46:2338. [Google Scholar]
- 24.Marshall JA, Yanik MM, Adams ND, Ellis KC, Chobanian HR. Org. Synth. 2005;81:157. [Google Scholar]
- 25.a Nahm S, Weinreb SM. Tetrahedron Lett. 1981;22:3815. [Google Scholar]; b Sibi MP. Org. Prep. Proced. Int. 1993;25:15. [Google Scholar]; c Mentzel M, Hoffmann HMR. J. Prakt. Chem. 1997;339:517. [Google Scholar]; d Singh J, Satyamurthi N, Aidhen IS. J. Prakt. Chem. 2000;342:340. [Google Scholar]
- 26.Reviews: Nicolaou KC, Edmonds DJ, Bulger PG. Angew. Chem. Int. Ed. 2006;45:7134. doi: 10.1002/anie.200601872.Tietze LF, Brasche G, Gericke K. Domino Reactions in Organic Synthesis. Wiley-VCH; Weinheim: 2006. p. 672.Nicolaou KC, Montagnon T, Snyder SA. Chem. Commun. 2003:551. doi: 10.1039/b209440c.
- 27.Since epimers 72 and epi-72 are hardly separable and because the subsequent intermediates 73 and 74 were also shown to epimerize under the reaction conditions employed for their generation, it was more expedient and efficient to carry the mixture of 72 and epi-72 through the three-step sequence and separate the obtained enones (75 and its C-3 epimer epi-75) by chromatography.
- 28.Tsuji J, Nagashima H, Hori K. Chem. Lett. 1980:257. [Google Scholar]
- 29.Bodforss S. Ber. deut. chem. Ges. 1918;51:214. [Google Scholar]; b Jeger O, Schaffner K. Pure Appl. Chem. 1970;21:247. doi: 10.1351/pac197021020247. [DOI] [PubMed] [Google Scholar]
- 30.Kim H, Kim TG, Hahn J, Jang D-J, Chang DJ, Park BS. J. Phys. Chem. A. 2001;105:3555. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.