Abstract
Objective
To determine the risk of stroke in patients with rheumatoid arthritis (RA) and risk factors associated with stroke.
Methods
We performed nested case–control analyses within a longitudinal databank, matching up to 20 controls for age, sex, and time of cohort entry to each patient with stroke. Conditional logistic regression was performed as an estimate of the relative risk of stroke in RA patients compared with those with noninflammatory rheumatic disorders, and to examine severity and anti–tumor necrosis factor (anti-TNF) treatment effects in RA.
Results
We identified 269 patients with first-ever all-category strokes and 67 with ischemic stroke, including 41 in RA patients. The odds ratio (OR) for the risk of all-category stroke in RA was 1.64 (95% confidence interval [95% CI] 1.16–2.30, P = 0.005), and for ischemic stroke was 2.66 (95% CI 1.24–5.70, P = 0.012). Ischemic stroke was predicted by hypertension, myocardial infarction, low-dose aspirin, comorbidity score, Health Assessment Questionnaire score, and presence of total joint replacement, but not by diabetes, smoking, exercise, or body mass index. Adjusted for cardiovascular and RA risk factors, ischemic stroke was associated with rofecoxib (P = 0.060, OR 2.27 [95% CI 0.97–5.28]), and possibly with corticosteroid use. Anti-TNF therapy was not associated with ischemic stroke (P = 0.584, OR 0.80 [95% CI 0.34–1.82]).
Conclusion
RA is associated with increased risk of stroke, particularly ischemic stroke. Stroke is predicted by RA severity, certain cardiovascular risk factors, and comorbidity. Except for rofecoxib, RA treatment does not appear to be associated with stroke, although the effect of corticosteroids remains uncertain.
INTRODUCTION
There is evidence that cardiovascular and cerebrovascular diseases are more common in patients with rheumatoid arthritis (RA) than in patients without RA (1–10), and 2 studies have demonstrated an increased risk of stroke among patients with RA (1,2). However, to our knowledge no studies have addressed the effect of RA severity, RA therapy, or cardiovascular risk factors on the risk of stroke in patients with RA. A number of therapies are available that influence RA activity and severity and possible long-term outcome. These include methotrexate, which may have a positive effect on outcome (11); prednisone, which has uncertain effects (12,13); and anti–tumor necrosis factor (anti-TNF) therapy, for which effects have not yet been determined, but might be promising, because it may further reduce inflammatory activity and have direct cardiovascular effects.
There is increasing evidence that inflammation is implicated in the pathogenesis of stroke (14–16). An increased serum level of C-reactive protein, which is produced in response to TNF and interleukin-6, has been shown to be an independent risk factor of myocardial infarction and stroke (17–19). In addition to initiation of C-reactive protein synthesis, TNF may activate endothelial cells, converting them into procoagulant and prothrombotic states (14,17). TNF is a key cytokine involved in all phases of stroke pathogenesis, including initiation and progression, as well as repair and development of ischemic tolerance (20). In preclinical stroke models, TNF inhibition employing anti-TNF monoclonal antibodies and TNF-binding proteins showed significant neuroprotection in animals treated with these compounds (20). However, efficacy of TNF inhibition for stroke prevention or treatment in humans has not yet been demonstrated.
In addition to possible effects of treatment, the issue of whether cardiovascular risk factors such as obesity, diabetes, and exercise are also risk factors for stroke in RA has not been determined. In the current study, we used 3 data sets to investigate the risk of stroke in patients with RA compared with those with noninflammatory disorders, identify predictors of stroke in RA, and determine the effect of anti-TNF therapy on the risk of stroke.
Because RA treatment can be confounded by RA severity and comorbidity, we used covariate control in the analysis of treatment effect. We controlled for RA severity using duration of RA, presence or absence of total joint replacement (TJR), and Health Assessment Questionnaire (HAQ) scores (21); and we used a comorbidity index and the presence or absence of individual comorbid conditions to address comorbidity confounding.
PARTICIPANTS AND METHODS
We studied participants from the National Data Bank for Rheumatic Diseases (NDB) longitudinal study of rheumatic disease outcomes. NDB participants are recruited from the practices of US rheumatologists, and are followed prospectively with semiannual, detailed, 28-page questionnaires, as previously described (22,23). This study used NDB data from 22,131 adult participants in nested case–control analyses, 16,990 of whom had RA, and 5,141 of whom had a noninflammatory rheumatic disorder (NIRD). Of the 16,990 RA patients, 11,225 were not members of a safety registry. Safety registry patients are those enrolled at the time they started a specific therapy (e.g., infliximab) and may be biased by increased RA severity. All patients were enrolled continuously beginning in 1999 and completed ≥2 semiannual questionnaires between January 1999 and July 2006. Diagnoses were made by the patients’ rheumatologists. NIRDs included diagnoses such as osteoarthritis, back pain syndromes, fibromyalgia, tendinitis, etc.
Case–control analysis
Cases consisted of all patients ages 25–100 years with a first-ever stroke. We included patients who had a diagnosis of stroke recorded as the cause of death if the stroke occurred within 6 months of the patient’s last questionnaire. The index observation was the observation at which the stroke was noted. We matched up to 20 controls to each case at the index observation by 10 categories of age, calendar time, calendar time of entry into the cohort, and sex, using incidence density sampling (selecting controls without replacement from all persons at risk at the time of case occurrence, excluding the index case itself) (24). All controls were alive and NDB participants at the time their matched case had the stroke.
Assessment of exposure
We determined medication use based on patients’ self-report in their semiannual questionnaires. Patients were classified as using a therapy if they used that therapy for any time in the index period prior to the index event. We determined the use of each nonsteroidal antiinflammatory drug (NSAID) and low-dose aspirin. We also identified use of corticosteroids and disease-modifying antirheumatic drugs (DMARDs). We classified infliximab, etanercept, and adalimumab as anti-TNF antagonists. For each medication, we identified use within each semiannual assessment period. We calculated cumulative exposure time and determined use at baseline and within 6 months of the index period.
Case definition
Possible strokes were identified from study questionnaires, hospitalization records, physician reports, and death records. Only strokes that were confirmed by medical review or death records were recognized as strokes in this study. If hospital or death records were not available, we contacted the patient’s physician and/or interviewed the patient or family with a structured, preplanned interview designed to address the reported condition. Comparison of patient reports with medical records indicated agreement in >94% of cases. Death records, in which stroke was recorded, must have referred to deaths that occurred within 6 months of the last questionnaire to be included as a stroke in this study. Review of potential cases was performed by 2 trained, experienced NDB staff members. This review was followed by an independent physician review. All cases of ischemic stroke were based on hospital records.
International Classification of Diseases, Ninth Revision (ICD-9) codes were used for identification of stroke cases and were classified as follows: ischemic strokes included ICD-9 codes 433.01 through 433.80, provided the code indicated infarction, and ICD-9 codes 434 through 434.91. We combined ischemic stroke and unclassified stroke (ICD-9 code 436) into an “all-stroke” category. We excluded intracerebral, subarachnoid, subdural, and epidural hemorrhages, as well as transient ischemic attacks.
Covariates
Study variables were assessed at entry into the NDB and at every subsequent semiannual questionnaire. For use as covariates in this study, baseline and preindex (antecedent) observation values were analyzed. Demographic variables included age, sex, body mass index (BMI), and smoking history. Comorbidity was measured by a patient-reported composite comorbidity score (range 0–9) comprised of 11 present or past comorbid conditions, including pulmonary disorders, myocardial infarction, other cardiovascular disorders, stroke, hypertension, diabetes, spine/hip/leg fracture, depression, gastrointestinal ulcer, other gastrointestinal disorders, and cancer (25), and by identification of prior myocardial infarction, hypertension, and diabetes. We also identified the use of low-dose (81 mg) aspirin as an indicator of preexisting cardiovascular disease. An additional potential risk factor assessed was the amount of weekly exercise. As specific covariate measures of RA severity, we also included prior TJRs (26,27) and the HAQ disability index score (21). Duration of RA was added to assess the effect of long-term RA. Treatment variables studied included prednisone (corticosteroids), NSAIDs, and DMARDs.
Statistical analysis
In the study analyses, we used the full data set (n = 22,131) as the basis of the comparison of RA and NIRD patients for the risk of ischemic and all-cause stroke (Table 1); we used all RA patients from the full data set (n = 16,990) to form the basis of the analysis of nontreatment associations with ischemic stroke (Table 2); and we used a subset of the RA patients reduced by removal of participants in safety registries (n = 5,765) to examine the effect of treatment on ischemic stroke (Figure 1 and Figure 2). We used this last restriction to ensure absence of severity bias and immeasurable confounding.
Table 1.
Stroke risk in patients with RA*
| No. strokes |
No. patients |
RA cases/ noncases |
NIRD cases/ noncases |
OR (95% CI)† | P | |
|---|---|---|---|---|---|---|
| All strokes | 269 | 5,640 | 226/4,125 | 43/1,246 | 1.64(1.16–2.30) | 0.005 |
| Ischemic strokes | 67 | 1,405 | 59/996 | 8/342 | 2.66(1.24–5.70) | 0.012 |
RA = rheumatoid arthritis; NIRD = noninflammatory rheumatic disorder; OR = odds ratio; 95% CI = 95% confidence interval.
Comparison group consists of patients with NIRDs, matched for age, sex, and time of study entry.
Table 2.
The association of univariate baseline demographics, cardiovascular risk factors, and RA risk factors with ischemic stroke in RA (n = 1,230)*
| Value | OR (95% CI) | P | |
|---|---|---|---|
| Non-RA variables | |||
| Low-dose aspirin | 25.8 | 3.60 (2.09–6.22) | < 0.001 |
| Myocardial infarction | 6.9 | 2.58 (1.22–5.47) | 0.013 |
| Hypertension | 41.8 | 1.98 (1.16–3.37) | 0.012 |
| First comorbidity index (range 0–9), mean ± SD† | 1.6 ± 1.4 | 1.69 (1.44–1.99) | < 0.001 |
| Index comorbidity index (range 0–9), mean ± SD† | 1.7 ± 1.5 | 1.42 (1.20–1.64) | < 0.001 |
| College graduate | 24.1 | 1.42 (0.80–2.55) | 0.235 |
| Moderate or greater aerobic exercise | 9.6 | 1.29 (0.57–2.91) | 0.535 |
| Diabetes | 10.1 | 1.22 (0.54–2.76) | 0.636 |
| Any aerobic exercise | 21.8 | 1.12 (0.62–2.01) | 0.706 |
| BMI, mean ± SD kg/m2 | 26.9 ± 5.5 | 1.00 (0.95–1.05) | 0.825 |
| Ever smoked | 56.9 | 1.00 (0.58–1.72) | 0.888 |
| Current smoker | 10.8 | 0.96 (0.40–2.32) | 0.930 |
| Non-Hispanic white | 94.0 | 0.86 (0.30–2.45) | 0.777 |
| RA variables | |||
| Lifetime TJR | 17.5 | 2.13 (1.14–3.95) | 0.017 |
| Index HAQ score, mean ± SD (range 0–3) | 1.08 ± 0.73 | 2.04 (1.40–2.97) | < 0.001 |
| HAQ score, mean ± SD (range 0–3) | 1.04 ± 0.70 | 1.43 (0.98–2.11) | 0.067 |
| Disease duration, mean ± SD years | 15.9 ± 13.5 | 1.02 (1.00–1.03) | 0.100 |
Values are the percentage unless otherwise indicated. Variables represent baseline values except as described. RA = rheumatoid arthritis; OR = odds ratio; 95% CI = 95% confidence interval; BMI = body mass index; TJR = total joint replacement; HAQ = Health Assessment Questionnaire.
Conditions in the comorbidity index include pulmonary disorders, myocardial infarction, other cardiovascular disorders, stroke, hypertension, diabetes, spine/hip/leg fracture, depression, gastrointestinal ulcer, other gastrointestinal disorders, and cancer.
Figure 1.
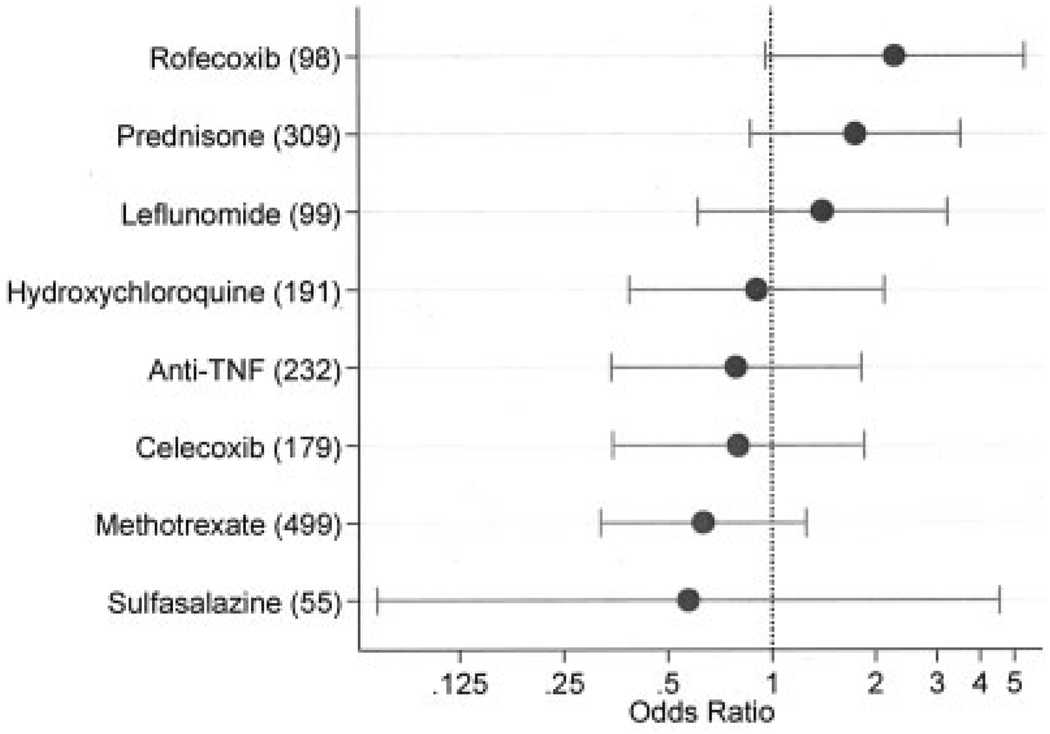
Multivariable ischemic stroke risk (odds ratios and 95% confidence intervals on log scale) associated with rheumatoid arthritis (RA) treatment in a 6-month window before the index stroke, adjusted for Health Assessment Questionnaire score, total joint replacement, RA duration, and for low-dose aspirin and comorbidity index immediately prior to index observation. Numbers in parentheses represent the number of patients using that therapy (cases and controls). Analysis included 41 RA patients with ischemic stroke and 791 RA patients without ischemic stroke. Anti-TNF = anti–tumor necrosis factor.
Figure 2.
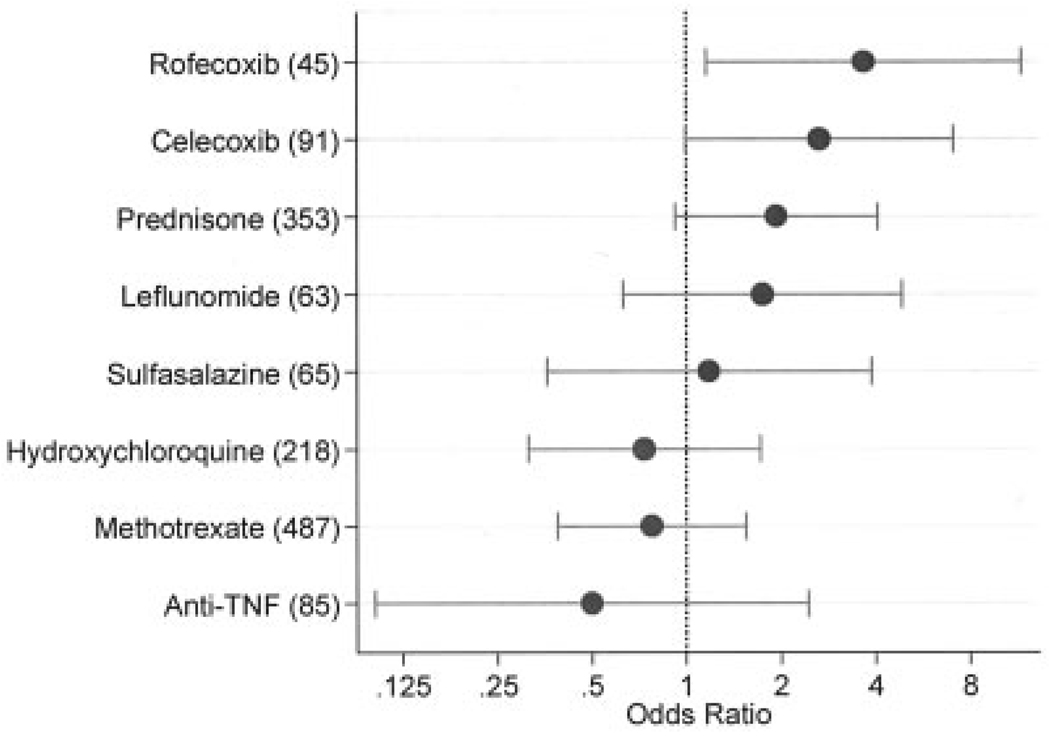
Multivariable ischemic stroke risk (odds ratios and 95% confidence intervals) associated with baseline rheumatoid arthritis (RA) treatment, adjusted for Health Assessment Questionnaire score, total joint replacement, RA duration, and for low-dose aspirin and comorbidity index at baseline. Numbers in parentheses represent the number of patients using that therapy in cases and controls. Analysis included 41 RA patients with ischemic stroke and 791 RA patients without ischemic stroke. Anti-TNF = anti–tumor necrosis factor.
Within each of these data sets, we used conditional logistic regression for individually matched case–control studies to derive odds ratios (ORs) with 95% confidence intervals (95% CIs) as a measure of relative risk. In the treatment effect analyses we fitted 2 models. The first model assessed variables with a 0–6-month window prior to the index time. The second model analyzed covariates obtained at the baseline observation (entry into the cohort). In these 2 models, we made adjustments for possible confounding effects of prior cardiovascular disease, co-morbidity, RA severity, and treatment variables. We used Stata statistical software, version 10.0 (StataCorp, College Station, TX) for all of the analyses. We selected a P value of 0.05 (2-tailed) to be significant, and we reported 95% CIs.
RESULTS
Risk of ischemic and all-cause stroke in RA compared with NIRD
From the NDB research database, we identified 269 cases with a first-ever stroke between the ages of 33 and 91 years (26.8% men). We also identified 67 cases of ischemic stroke between the ages of 35 and 90 years (25.4% men). We matched all cases by age, calendar time, and sex to up to 20 controls per case. For ischemic stroke analysis, the major outcome of this study, the mean time of databank followup until the index event was 3.9 years (interquartile range [IQR] 2.0–6.0). The mean ± SD age for cases and controls at the index event was 70.0 ± 9.6 and 69.5 ± 10.6 years, respectively (P = 0.704), and men constituted 25.4% of cases and 25.3% of controls (P = 0.541). As shown in Table 1, patients with RA were at increased risk for ischemic stroke (P = 0.012, OR 2.66 [95% CI 1.24–5.70]) and for all categories of stroke (P = 0.005, OR 1.64 [95% CI 1.16–2.30]).
Stroke in RA
From all RA patients, we identified 59 cases with a first-ever ischemic stroke between the ages of 35 and 84 years. We matched these cases by age, sex, and calendar time to up to 20 controls per case (mean 19.8). The mean time of databank followup was 3.9 years (IQR 2.0–6.0). The mean ± SD age for cases and controls at the index event time was 70.0 ± 9.8 and 69.1 ± 10.6 years, respectively (P = 0.521), and men constituted 27.1% of cases and 25.6% of controls (P = 0.514).
Factors associated with ischemic stroke in RA
A number of baseline and preindex observation characteristics were associated with future ischemic strokes in RA (Table 2). The risk of stroke increased with the number of comorbid conditions, a history of hypertension (OR 1.98, 95% CI 1.16–3.37), myocardial infarction (OR 2.58, 95% CI 1.22–5.47), and the use of low-dose aspirin (OR 3.60, 95% CI 2.09–6.22). BMI, diabetes, smoking history, the amount of weekly exercise, and duration of RA were not significantly associated with the risk of ischemic stroke. RA-related factors at baseline that were associated with the risk of ischemic stroke included TJR (OR 2.13, 95% CI 1.14–3.95), baseline HAQ score (P = 0.067, OR 1.43 [95% CI 0.98–2.11]), and immediately antecedent HAQ score (OR 2.04, 95% CI 1.40–2.97). The 2 RA measures, however, differed in that HAQ scores can be elevated by concurrent comorbidity, but total joint assessments will be unaltered by current comorbidity. In a multivariable assessment of stroke risk with antecedent total joint assessment, HAQ score, low-dose aspirin, and comorbidity as regressors, TJR was significantly associated with stroke (P = 0.021, OR 2.28 [95% CI 1.13–4.58]).
The association of RA therapy and ischemic stroke in RA
Among the non-safety registry RA patients, who constituted the subjects of the effect of RA treatment on stroke risk, we identified 41 cases with a first-ever ischemic stroke between the ages of 35 and 84 years. We matched these cases by age, sex, and calendar time to up to 20 controls per case (mean 19.3, n = 791). The mean time of databank followup was 4.0 years (IQR 2.0–6.0). The mean age for cases and controls at the index event time was 69.8 and 69.9 years, respectively (P = 0.956), and men constituted 26.8% of cases and 25.4% of controls (P = 0.855).
In multivariable conditional logistic regression analyses that were adjusted for HAQ disability index score, TJR, RA duration, low-dose aspirin, and comorbidity index at baseline for the baseline analyses and immediately prior to the index observation for the antecedent (current) analyses, we studied the simultaneous risk of common RA treatments (Figure 1 and Figure 2).
For the preindex multivariable analyses adjusted for covariates (Figure 1), rofecoxib was associated with an increased risk of stroke (P = 0.060, OR 2.28 [95% CI 0.97–5.38]). Other risk associations included prednisone (P = 0.114, OR 1.75 [95% CI 0.87–3.53]), methotrexate (P = 0.191, OR 0.63 [95% CI 0.32–1.26]), and anti-TNF therapy (P = 0.584, OR 0.79 [95% CI 0.34–1.82]). In univariate unadjusted analyses, rofecoxib and prednisone were significantly associated with stroke: the OR for rofecoxib was 2.32 (95% CI 1.05–5.13, P = 0.037), the OR for prednisone was 2.03 (95% CI 1.08–3.84, P = 0.029), the OR for methotrexate was 0.55 (95% CI 0.30–1.04, P = 0.066), and the OR for anti-TNF therapy was 0.80 (95% CI 0.38–1.70, P = 0.562).
We also conducted analyses to determine if length of treatment exposure during the study was associated with stroke. We found a univariate (but not multivariate) exposure-time effect for prednisone (P = 0.039, OR 1.19 [95% CI 1.00–1.40]), and no effect for any other treatment. However, prednisone dose at the preindex assessment was also associated with the risk of stroke. Compared with 576 patients not receiving prednisone, the OR for stroke for 1–5 mg/day was 1.68 (95% CI 0.76–3.73; n = 187), the OR for 6–10 mg/day was 4.36 (95% CI 1.60–11.90; n = 56), and the OR for >10 mg/day was 4.87 (95% CI 0.85–27.77, P = 0.075; n = 13).
For the baseline multivariable analyses adjusted for covariates (Figure 2), rofecoxib was associated with an increased risk of stroke (P = 0.027, OR 3.66 [95% CI 1.16–11.60]). Other risk associations included celecoxib (P = 0.051, OR 2.65 [95% CI 0.99–7.08]), prednisone (P = 0.083, OR 1.93 [95% CI 0.92–4.07]), methotrexate (P = 0.464, OR 0.77 [95% CI 0.39–1.54]), and anti-TNF therapy (P = 0.391, OR 0.50 [95% CI 0.10–2.44]). Univariate associations for the above variables were rofecoxib (P = 0.012, OR 3.73 [95% CI 1.34–10.38]), celecoxib (P = 0.019, OR 2.91 [95% CI 1.19–7.13]), prednisone (P = 0.014, OR 2.27 [95% CI 1.18–4.69]), methotrexate (P = 0.320, OR 0.73 [95% CI 0.39–1.36]), and anti-TNF therapy (P = 0.196, OR 0.37 [95% CI 0.18–1.66]). In evaluating the result of Figure 1 and Figure 2, it is worthwhile to note that the use of rofecoxib, celecoxib, and anti-TNF therapy increased substantially from the numbers using these treatments at baseline compared with the current use numbers ~4 years later on average.
DISCUSSION
This study contributes new information regarding pharmacologic and nonpharmacologic risk factors for stroke in RA. In addition, we provide another estimate of the risk of RA on subsequent stroke using a patient population where clinical information was available, complementing the administrative database estimates already available (1,2).
A number of studies have addressed cardiovascular risk among patients with RA (1–10). However, stroke has been the subject of only a few studies (1,2). A British Columbia administrative database cohort study of 25,385 adults with RA showed that the overall rate of stroke increased in patients with RA (rate ratio 1.9, 95% CI 1.7–2.1) (1), and an incidence sample of 527 women with RA demonstrated that the relative risk for stroke was 1.48 (95% CI 0.70–3.12) (2). The current study confirms these reports: our overall stroke risk ratio was 1.64 (95% CI 1.16–2.30), and the risk ratio for ischemic stroke was 2.66 (95% CI 1.24–5.70).
Risk factors for stroke can be separated into 3 parts: factors associated with stroke risk in all persons regardless of RA status, factors associated with RA severity, and factors associated with RA treatment. With respect to the non-RA risk factors for stroke that were available to this study, including hypertension, cardiac disease, diabetes, smoking, lack of exercise, and obesity (28,29), we confirmed the effect of hypertension and preexisting cardiac disease, but we found no evidence for an association of stroke with diabetes, smoking, and obesity. It is not entirely clear why diabetes, smoking, and obesity had no effect in our cohort of RA patients, and confirmation of these findings is required. However, it has been previously demonstrated that elevated BMI does not predict mortality in RA (30). In data not shown, we examined the relationship between BMI and ischemic stroke using fractional polynomial regression to observe possible nonlinear effects. Although this analysis was nonsignificant, a U-shaped association between stroke and BMI could be identified. Therefore, it seems likely that the standard concept of obesity compared with nonobesity may not be appropriate in RA (31). With respect to smoking in this sample of older subjects, current smokers tend to be healthier than ex-smokers. In the current data set, for example, current smokers had comorbidity scores that were 0.6 lower than ex-smokers, and 0.34 lower than those who never smoked (P < 0.05). With respect to exercise, few persons reported strenuous exercise, thereby limiting somewhat the inferences that can be drawn about exercise in our study sample. However, in an analysis of myocardial infarction in the NDB cohort (32), we found that smoking and diabetes were significant univariate risks for myocardial infarction and that obesity was a possible risk (P > 0.05, hazard ratio 1.3 [95% CI 1.0–1.6]), but exercise was not a significant univariate risk factor.
RA severity measures (HAQ and TJR) predict stroke, as might be expected given the increased risk of RA itself. Although we did not have laboratory measures in our study, the RA severity variables in this study are effective measures of cumulative damage: the results of RA severity (33–37). The 2 measures that we used differ, however, in that HAQ score effect can be confounded by concurrent comorbidity (38), but total joint assessments are unaltered by current comorbidity. Although not shown in Figure 1 and Figure 2, TJR remained a statistically significant predictor in both analyses.
RA severity and current activity are also represented by treatment choice, with patients using corticosteroids and leflunomide having greater severity, for example, and those using methotrexate having less severity (12). Practically, this means that inclusion of RA treatments in stroke risk models also adjusts for severity of RA. In addition to reflecting RA activity and severity, individual RA treatments might contribute to the risk of stroke by contributing to atherogenesis or clotting problems (39–43), while still being measures of severity: in effect, confounders.
When we compared the univariate and multivariate treatment effects, covariate adjustment resulted in ORs moving closer to 1.0 and in a loss of statistical significance in most instances. Considering the univariate data and the results shown in Figure 1 and Figure 2, we did not find evidence to support the association of RA treatment and stroke, with the exception of rofecoxib and possibly antecedent prednisone (P = 0.114, OR 1.75 [95% CI 0.87–3.53]). Cumulative corticosteroid dose has been shown to be associated with atherogenesis in RA (4). Corticosteroids alter lipid metabolism (42,44,45), increase the risk of hypertension (42), and increase and are linked to carotid artery abnormalities and metabolic syndrome in non-RA patients (46,47). But as we have indicated, the interpretation of the effect of corticosteroids in RA is confounded by RA severity (4,12), and it is likely that residual confounding remains even after the adjustments used in the current study. There are limited data on the effect of corticosteroids on other cardiovascular outcomes such as myocardial infarction. In a population-based study of 5,648 subjects, current use of inhaled corticosteroids was not associated with myocardial infarction risk, but a 32% reduction in risk was noted for dosages of 50–200 µg/day (28). In another study, corticosteroids had a protective association with myocardial infarction in persons with kidney transplantation, although confounding factors limited generalizability (30). Additional or larger studies will be needed to further elucidate the prednisone association with stroke.
There are a number of caveats. The number of ischemic strokes studied was relatively small. It is possible that nonsignificant associations noted for prednisone might be statistically significant with a larger sample. In addition, if residual confounding exists for treatment effect, the associations noted might be slightly weaker than those described here. However, that would be unlikely to change the study conclusion of no treatment effect except for rofecoxib. With respect to residual confounding, we also want to note that our data set did not contain markers for joint counts, radiographic erosions, inflammatory markers, and autoantibodies, and it is possible that such data could play a role in reducing residual confounding, if present.
There is also the possibility that the degree of confounding changed over time. For example, when initially prescribed, celecoxib was associated with more severe RA (48). With time, celecoxib was prescribed more generally and the association with severity was lost. This effect is suggested in the current study by the change in OR for baseline celecoxib compared with immediately antecedent celecoxib. In addition to using different time-related covariates to explore such differences, we used baseline values to explore the effect of treatment that patients may have received for long periods of time (prestudy enrollment), in order that we might better evaluate the association with methotrexate and prednisone. But no substantial differences were noted, as shown in Figure 1 and Figure 2.
In addition to the time-based approach we used to analyze the data, use of treatment exposure time is still another approach. However, if treatment immediately before the index time is central to detecting effects, as has been shown for rofecoxib (49,50), this method will fail to detect such effects, and the exposure-time method does not include time on therapy prior to enrollment. Many valid and confirmed epidemiologic associations have not shown dose-response relationships (51). As indicated in our results, we found a univariate (but not multivariate) exposure-time effect for prednisone (P = 0.039, OR 1.19 [95% CI 1.00–1.40]), and no effect for any other treatment.
In summary, RA is associated with increased risk of stroke, particularly ischemic stroke. Stroke is predicted by RA severity, hypertension, myocardial infarction, low-dose aspirin, and comorbidity. Diabetes, obesity, and smoking were not risk factors for stroke among patients with RA in this study. Except for rofecoxib, RA treatment does not appear to be associated with stroke, although the effect of corticosteroids remains uncertain.
Footnotes
The National Data Bank for Rheumatic Diseases has conducted safety registries for Centocor, Sanofi-Aventis, and Bristol-Myers Squibb, and has received research grants from Abbott, Amgen, Wyeth-Australia, Merck, and Pfizer.
Dr. Wolfe has received consultant fees (less than $10,000) from Bristol-Myers Squibb.
AUTHOR CONTRIBUTIONS
Dr. Wolfe had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study design. Nadareishvili, Hallenbeck, Wolfe.
Acquisition of data. Michaud, Wolfe.
Analysis and interpretation of data. Nadareishvili, Michaud, Hallenbeck, Wolfe.
Manuscript preparation. Nadareishvili, Wolfe.
Statistical analysis. Michaud, Wolfe.
REFERENCES
- 1.Solomon DH, Goodson NJ, Katz JN, Weinblatt ME, Avorn J, Setoguchi S, et al. Patterns of cardiovascular risk in rheumatoid arthritis. Ann Rheum Dis. 2006;65:1608–1612. doi: 10.1136/ard.2005.050377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Solomon DH, Karlson EW, Rimm EB, Cannuscio CC, Mandl LA, Manson JE, et al. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation. 2003;107:1303–1307. doi: 10.1161/01.cir.0000054612.26458.b2. [DOI] [PubMed] [Google Scholar]
- 3.Del Rincon I, Freeman GL, Haas RW, O’Leary DH, Escalante A. Relative contribution of cardiovascular risk factors and rheumatoid arthritis clinical manifestations to atherosclerosis. Arthritis Rheum. 2005;52:3413–3423. doi: 10.1002/art.21397. [DOI] [PubMed] [Google Scholar]
- 4.Del Rincon I, O’Leary DH, Haas RW, Escalante A. Effect of glucocorticoids on the arteries in rheumatoid arthritis. Arthritis Rheum. 2004;50:3813–3822. doi: 10.1002/art.20661. [DOI] [PubMed] [Google Scholar]
- 5.Del Rincon I, Williams K, Stern MP, Freeman GL, O’Leary DH, Escalante A. Association between carotid atherosclerosis and markers of inflammation in rheumatoid arthritis patients and healthy subjects. Arthritis Rheum. 2003;48:1833–1840. doi: 10.1002/art.11078. [DOI] [PubMed] [Google Scholar]
- 6.Del Rincon I, Williams K, Stern MP, Freeman GL, Escalante A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001;44:2737–2745. doi: 10.1002/1529-0131(200112)44:12<2737::AID-ART460>3.0.CO;2-%23. [DOI] [PubMed] [Google Scholar]
- 7.Krishnan E, Lingala VB, Singh G. Declines in mortality from acute myocardial infarction in successive incidence and birth cohorts of patients with rheumatoid arthritis. Circulation. 2004;110:1774–1779. doi: 10.1161/01.CIR.0000142864.83780.81. [DOI] [PubMed] [Google Scholar]
- 8.Goodson NJ, Wiles NJ, Lunt M, Barrett EM, Silman AJ, Symmons DP. Mortality in early inflammatory polyarthritis: cardiovascular mortality is increased in seropositive patients. Arthritis Rheum. 2002;46:2010–2019. doi: 10.1002/art.10419. [DOI] [PubMed] [Google Scholar]
- 9.Van Doornum S, Brand C, King B, Sundararajan V. Increased case fatality rates following a first acute cardiovascular event in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:2061–2068. doi: 10.1002/art.21932. [DOI] [PubMed] [Google Scholar]
- 10.Turesson C, Jarenros A, Jacobsson L. Increased incidence of cardiovascular disease in patients with rheumatoid arthritis: results from a community based study. Ann Rheum Dis. 2004;63:952–955. doi: 10.1136/ard.2003.018101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi HK, Hernan MA, Seeger JD, Robins JM, Wolfe F. Methotrexate therapy and mortality in patients with rheumatoid arthritis: a prospective study. Lancet. 2002;359:1173–1177. doi: 10.1016/S0140-6736(02)08213-2. [DOI] [PubMed] [Google Scholar]
- 12.Caplan L, Wolfe F, Russell AS, Michaud K. Corticosteroid use in rheumatoid arthritis: prevalence, predictors, correlates, and outcomes. J Rheumatol. 2007;34:696–705. [PubMed] [Google Scholar]
- 13.Boers M. Studying the benefit/risk ratio of glucocorticoids in rheumatoid arthritis. J Rheumatol. 2007;34:661–663. [PubMed] [Google Scholar]
- 14.Hallenbeck JM. Inflammatory reactions at the blood-endothelial interface in acute stroke. Adv Neurol. 1996;71:281–297. [PubMed] [Google Scholar]
- 15.Becker KJ. Targeting the central nervous system inflammatory response in ischemic stroke. Curr Opin Neurol. 2001;14:349–353. doi: 10.1097/00019052-200106000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Del Zoppo GJ. TIAs and the pathology of cerebral ischemia. Neurology. 2004;62 8 Suppl 6:S15–S19. doi: 10.1212/wnl.62.8_suppl_6.s15. [DOI] [PubMed] [Google Scholar]
- 17.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 18.Curb JD, Abbott RD, Rodriguez BL, Sakkinen P, Popper JS, Yano K, et al. C-reactive protein and the future risk of thromboembolic stroke in healthy men. Circulation. 2003;107:2016–2020. doi: 10.1161/01.CIR.0000065228.20100.F7. [DOI] [PubMed] [Google Scholar]
- 19.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 20.Hallenbeck JM. The many faces of tumor necrosis factor in stroke. Nat Med. 2002;8:1363–1368. doi: 10.1038/nm1202-1363. [DOI] [PubMed] [Google Scholar]
- 21.Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum. 1980;23:137–145. doi: 10.1002/art.1780230202. [DOI] [PubMed] [Google Scholar]
- 22.Wolfe F, Michaud K. The effect of methotrexate and anti-tumor necrosis factor therapy on the risk of lymphoma in rheumatoid arthritis in 19,562 patients during 89,710 person-years of observation. Arthritis Rheum. 2007;56:1433–1439. doi: 10.1002/art.22579. [DOI] [PubMed] [Google Scholar]
- 23.Wolfe F, Caplan L, Michaud K. Treatment for rheumatoid arthritis and the risk of hospitalization for pneumonia: associations with prednisone, disease-modifying antirheumatic drugs, and anti–tumor necrosis factor therapy. Arthritis Rheum. 2006;54:628–634. doi: 10.1002/art.21568. [DOI] [PubMed] [Google Scholar]
- 24.Robins JM, Gail MH, Lubin JH. More on “biased selection of controls for case-control analyses of cohort studies.”. Biometrics. 1986;42:293–299. [PubMed] [Google Scholar]
- 25.Michaud K, Wolfe F. The development of a rheumatic disease research comorbidity index for use in outpatients with RA, OA, SLE and fibromyalgia (FMS) [abstract] Arthritis Rheum. 2007;56 Suppl 9:S596. [Google Scholar]
- 26.Wolfe F, Zwillich SH. The long-term outcomes of rheumatoid arthritis: a 23-year prospective, longitudinal study of total joint replacement and its predictors in 1,600 patients with rheumatoid arthritis. Arthritis Rheum. 1998;41:1072–1082. doi: 10.1002/1529-0131(199806)41:6<1072::AID-ART14>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 27.Da Silva E, Doran MF, Crowson CS, O’Fallon WM, Matteson EL. Declining use of orthopedic surgery in patients with rheumatoid arthritis? Results of a long-term, population-based assessment. Arthritis Rheum. 2003;49:216–220. doi: 10.1002/art.10998. [DOI] [PubMed] [Google Scholar]
- 28.Whisnant JP. Modeling of risk factors for ischemic stroke: the Willis Lecture. Stroke. 1997;28:1840–1844. doi: 10.1161/01.str.28.9.1840. [DOI] [PubMed] [Google Scholar]
- 29.Lee CD, Folsom AR, Blair SN. Physical activity and stroke risk: a meta-analysis. Stroke. 2003;34:2475–2481. doi: 10.1161/01.STR.0000091843.02517.9D. [DOI] [PubMed] [Google Scholar]
- 30.Escalante A, Haas RW, del Rincon I. Paradoxical effect of body mass index on survival in rheumatoid arthritis: role of comorbidity and systemic inflammation. Arch Intern Med. 2005;165:1624–1629. doi: 10.1001/archinte.165.14.1624. [DOI] [PubMed] [Google Scholar]
- 31.Rexrode KM, Hennekens CH, Willett WC, Colditz GA, Stampfer MJ, Rich-Edwards JW, et al. A prospective study of body mass index, weight change, and risk of stroke in women. JAMA. 1997;277:1539–1545. doi: 10.1001/jama.1997.03540430051032. [DOI] [PubMed] [Google Scholar]
- 32.Wolfe F, Michaud K. The risk of myocardial infarction (MI) and pharmacologic and nonpharmacologic MI predictors in rheumatoid arthritis: a cohort and nested case-control analysis. Arthritis Rheum. doi: 10.1002/art.23811. In press. [DOI] [PubMed] [Google Scholar]
- 33.Drossaers-Bakker KW, Zwinderman AH, Vliet Vlieland TP, Van Zeben D, Vos K, Breedveld FC, et al. Long-term outcome in rheumatoid arthritis: a simple algorithm of baseline parameters can predict radiographic damage, disability, and disease course at 12-year followup. Arthritis Rheum. 2002;47:383–390. doi: 10.1002/art.10513. [DOI] [PubMed] [Google Scholar]
- 34.Drossaers-Bakker KW, de Buck M, van Zeben D, Zwinderman AH, Breedveld FC, Hazes JM. Long-term course and outcome of functional capacity in rheumatoid arthritis: the effect of disease activity and radiologic damage over time. Arthritis Rheum. 1999;42:1854–1860. doi: 10.1002/1529-0131(199909)42:9<1854::AID-ANR9>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 35.Kuper HH, van Leeuwen MA, van Riel PL, Prevoo ML, Houtman PM, Lolkema WF, et al. Radiographic damage in large joints in early rheumatoid arthritis: relationship with radiographic damage in hands and feet, disease activity, and physical disability. Br J Rheumatol. 1997;36:855–860. doi: 10.1093/rheumatology/36.8.855. [DOI] [PubMed] [Google Scholar]
- 36.James D, Young A, Kulinskaya E, Knight E, Thompson W, Ollier W, et al. Early Rheumatoid Arthritis Study Group (ERAS), UK. Orthopaedic intervention in early rheumatoid arthritis: occurrence and predictive factors in an inception cohort of 1064 patients followed for 5 years. Rheumatology (Oxford) 2004;43:369–376. doi: 10.1093/rheumatology/keh059. [DOI] [PubMed] [Google Scholar]
- 37.Young A, Dixey J, Cox N, Davies P, Devlin J, Emery P, et al. How does functional disability in early rheumatoid arthritis (RA) affect patients and their lives? Results of 5 years of follow-up in 732 patients from the Early RA Study (ERAS) Rheumatology (Oxford) 2000;39:603–611. doi: 10.1093/rheumatology/39.6.603. [DOI] [PubMed] [Google Scholar]
- 38.Talamo J, Frater A, Gallivan S, Young A. Use of the Short Form 36 (SF36) for health status measurement in rheumatoid arthritis. Br J Rheumatol. 1997;36:463–469. doi: 10.1093/rheumatology/36.4.463. [DOI] [PubMed] [Google Scholar]
- 39.Blackburn D, Hux J, Mamdani M. Quantification of the risk of corticosteroid-induced diabetes mellitus among the elderly. J Gen Intern Med. 2002;17:717–720. doi: 10.1046/j.1525-1497.2002.10649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gurwitz JH, Bohn RL, Glynn RJ, Monane M, Mogun H, Avorn J. Glucocorticoids and the risk for initiation of hypoglycemic therapy. Arch Intern Med. 1994;154:97–101. [PubMed] [Google Scholar]
- 41.Maxwell SR, Moots RJ, Kendall MJ. Corticosteroids: do they damage the cardiovascular system? Postgrad Med J. 1994;70:863–870. doi: 10.1136/pgmj.70.830.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sholter DE, Armstrong PW. Adverse effects of corticosteroids on the cardiovascular system. Can J Cardiol. 2000;16:505–511. [PubMed] [Google Scholar]
- 43.McEvoy CE, Niewoehner DE. Adverse effects of corticosteroid therapy for COPD: a critical review. Chest. 1997;111:732–743. doi: 10.1378/chest.111.3.732. [DOI] [PubMed] [Google Scholar]
- 44.Ettinger WH, Jr, Hazzard WR. Prednisone increases very low density lipoprotein and high density lipoprotein in healthy men. Metabolism. 1988;37:1055–1058. doi: 10.1016/0026-0495(88)90067-4. [DOI] [PubMed] [Google Scholar]
- 45.Ettinger WH, Goldberg AP, Applebaum-Bowden D, Hazzard WR. Dyslipoproteinemia in systemic lupus erythematosus: effect of corticosteroids. Am J Med. 1987;83:503–508. doi: 10.1016/0002-9343(87)90762-5. [DOI] [PubMed] [Google Scholar]
- 46.Pivonello R, Faggiano A, Lombardi G, Colao A. The metabolic syndrome and cardiovascular risk in Cushing’s syndrome. Endocrinol Metab Clin North Am. 2005;34:327–339. doi: 10.1016/j.ecl.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 47.Faggiano A, Pivonello R, Spiezia S, De Martino MC, Filippella M, Di Somma C, et al. Cardiovascular risk factors and common carotid artery caliber and stiffness in patients with Cushing’s disease during active disease and 1 year after disease remission. J Clin Endocrinol Metab. 2003;88:2527–2533. doi: 10.1210/jc.2002-021558. [DOI] [PubMed] [Google Scholar]
- 48.Wolfe F, Flowers N, Burke TA, Arguelles LM, Pettitt D. Increase in lifetime adverse drug reactions, service utilization, and disease severity among patients who will start COX-2 specific inhibitors: quantitative assessment of channeling bias and confounding by indication in 6689 patients with rheumatoid arthritis and osteoarthritis. J Rheumatol. 2002;29:1015–1022. [PubMed] [Google Scholar]
- 49.Solomon DH, Avorn J, Sturmer T, Glynn RJ, Mogun H, Schneeweiss S. Cardiovascular outcomes in new users of coxibs and nonsteroidal antiinflammatory drugs: high-risk subgroups and time course of risk. Arthritis Rheum. 2006;54:1378–1389. doi: 10.1002/art.21887. [DOI] [PubMed] [Google Scholar]
- 50.Hippisley-Cox J, Coupland C. Risk of myocardial infarction in patients taking cyclo-oxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs: population based nested case-control analysis. BMJ. 2005;330:1366. doi: 10.1136/bmj.330.7504.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosenbaum PR. Does a dose-response relationship reduce sensitivity to hidden bias? Biostatistics. 2003;4:1–10. doi: 10.1093/biostatistics/4.1.1. [DOI] [PubMed] [Google Scholar]