Abstract
The importance of the molecule cystic fibrosis transmembrane conductance regulator (CFTR) is reflected in the many physiological functions it regulates. It is known to be present in epithelial cells of the lungs, pancreas, sweat glands, gut, and other tissues, and gene mutations of CFTR cause cystic fibrosis (CF). We studied the expression and distribution of CFTR in the human brain with reverse transcriptase polymerase chain reaction, in situ hybridization, and immunohistochemistry. This study demonstrates widespread and abundant expression of CFTR in neurons of the human brain. Techniques of double labeling and evaluation of consecutive tissue sections localized CFTR protein and mRNA signals to the cytoplasm of neurons in all regions of the brain studied, but not to glial cells. The presence of CFTR in central neurons not only provides a possible explanation for the neural symptoms observed in CF patients, but also may lead to a better understanding of the functions of CFTR in the human brain. This manuscript contains online supplemental material at http://www.jhc.org. Please visit this article online to view these materials. (J Histochem Cytochem 57:1113–1120, 2009)
Keywords: brain, immunocytochemistry, in situ hybridization, neurons, chloride channel, cystic fibrosis
The cystic fibrosis transmembrane conductance regulator (CFTR) gene was first discovered in 1989 (Kerem et al. 1989; Riordan et al. 1989). Mutation of the CFTR gene causes cystic fibrosis (CF), which is one of the most common lethal genetic diseases in the Caucasian population. CF is characterized by abnormally thick mucus, with symptoms such as meconium ileus, pancreatic insufficiency, and progressive pulmonary failure. Abnormalities have been reported in the central nervous system (CNS) of patients with CF (Goldstein et al. 2000), including axonal dystrophy and detectable amyloid precursor protein. CNS complications occur in more than 50% of lung transplant recipients with CF, which is significantly higher than in other groups of lung transplant recipients (Goldstein et al. 2000). However, the mechanism of these abnormalities is not understood.
Functionally, CFTR is a cAMP-dependent chloride channel, and can also function as a regulator of outward-rectifying chloride and sodium channels (Egan et al. 1992). A variety of intracellular functions have been attributed to CFTR, including regulation of membrane vesicle trafficking and fusion, acidification of organelles, and small anion transport (Bradbury et al. 1992; Egan et al. 1992; Hasegawa et al. 1992; Lukacs et al. 1992; Smith and Welsh 1992; Bradbury 1999; Chandy et al. 2001). Recently, CFTR expression has been demonstrated in lysosomes of alveolar macrophages (Di et al. 2006), which suggests that CFTR can also regulate phagosome acidification (Di et al. 2006). Regulation of vacuolar hydrogen-ion concentration (pH) in intracellular organelles is related to receptor-mediated endocytosis, intracellular membrane trafficking, and secretion (Tsunoda et al. 1991; Clague et al. 1994; Reaves and Banting 1994; van Weert et al. 1995; Presley et al. 1997; Kawai et al. 2007). It is possible that some or all of these functions may be related to CNS physiological or pathological processes under some circumstances.
CFTR is known to be present in a variety of epithelial cells, including those in the lungs (Engelhardt et al. 1992; Cozens et al. 1994; Jiang and Engelhardt 1998; Nagayama et al. 1999), the intestine, the pancreas, the salivary glands (Trezise and Buchwald 1991; O'Riordan et al. 1995), the kidney (Todd-Turla et al. 1996), and the reproductive tract (Tizzano et al. 1994; Mularoni et al. 1995; Patrizio and Salameh 1998). This distribution of CFTR serves in part to explain the pathophysiology of CF in these organs. Mulberg and associates also found CFTR expressed in rat brain and human hypothalamus (Johannesson et al. 1997; Mulberg et al. 1998; Weyler et al. 1999; Lahousse et al. 2003). However, there has been no report on the presence of CFTR in other parts of the human brain in addition to the hypothalamus. In this study, we present evidence demonstrating that CFTR is expressed in the neurons of the entire human brain, by investigation of its distribution with IHC, ISH, and RT-PCR. The expression and distribution of CFTR in the human brain may help to explain the CNS abnormalities that have been observed in CF patients.
Materials and Methods
Tissue
Fresh human brain (n=5) tissue samples were snap-frozen immediately at autopsy in liquid nitrogen and stored at −80C until use. Ten cases of formalin-fixed paraffin-embedded human brain tissue were used for IHC and ISH. The case information is shown in supplementary Table 1. These tissues were normal clinically and pathologically. All of the various regions of the human brain, including all lobes of the telencephalon and diencephalon, together with brain stem and cerebellum, were investigated. The use of autopsy tissues in this study was approved by the Peking University ethics committee.
RNA Extraction and Real-time PCR
Real-time PCR analysis of total human brain RNA was performed to evaluate the presence and expression levels of CFTR mRNA in different brain regions. Total RNA was extracted from brain tissues in different regions using TRIzol reagent (Gibco Invitrogen; Carlsbad, CA) according to established protocols (Chomczynski and Sacchi 1987,2006). Briefly, tissues were cut into pieces and homogenized in TRIzol reagent with RNase-free homogenizers. Phenol and chloroform were then used to remove the DNA and protein. RNA was precipitated using 100% ethanol and washed with 75% ethanol.
The reverse transcription reactions were performed using a cDNA synthesis kit (#K1621, Fermentas; Lithuania) according to established protocols. Briefly, total RNA was extracted from the brain tissue and the oligo (dT) primers were used. Reverse transcription reactions were performed at 42C for 60 min and then finished at 74C for 10 min.
The CFTR mRNA sequence was obtained from GenBank (accession no. NM000492), and primers were designed for amplification of a specific fragment of CFTR mRNA. The product spanned two introns of the CFTR gene to help differentiate it from genomic DNA contamination.
PCR was performed for amplification of reverse transcription products with a real-time PCR kit (Tiangen; Beijing, China) following the manufacturer's protocol. The amplification procedure used 40 cycles at 94C for 30 sec, 54C for 30 sec, and 72C for 30 sec, and the final cycle was followed by a 72C extension for 5 min. The primers for PCR were: sense CFTR primer, 5′-AGGAGGAACGCTCTATCG-3′ and antisense CFTR primer, 5′-GCAGACGCCTGTAACAAC-3′. Real-time PCR and analysis were performed with a real-time PCR instrument (ABI 7700; Foster City, CA). The CFTR/actin mRNA ratios were calculated to evaluate the expression level in different brain regions.
Plasmid Construction and Complementary RNA (cRNA) Probe Preparation
To perform sequence analysis and generate the cRNA probe, we subcloned the RT-PCR product into a pGM-T vector (Tiangen; Beijing, China). The plasmid was then transfected into Escherichia coli XLl-Blue. X-gal/isopropyl-β-d-thiogalactopyranoside and ampicillin double selection were performed. Sequencing was carried out to determine identity with the CFTR gene. Plasmid extracted from the positive clone was linearized with either the SalI or the NcoI restriction enzyme, and an in vitro transcription reaction was performed to generate the cRNA probe. The probe was labeled with digoxigenin RNA labeling mix (Roche Molecular Biochemicals; Mannheim, Germany) for ISH.
IHC in Human Brain
IHC was performed to demonstrate and localize CFTR expression in human brain. Brain tissues from 10 autopsies of patients with no known CNS pathology were used for this study. The regions of the brain investigated are listed in Table 1. All tissues were fixed in 10% formalin and embedded in paraffin. Tissue sections (5-μm) were deparaffinized in xylene and rehydrated in ethanol. Antigen retrieval was then performed by heating the slides at 96C in sodium citrate buffer (pH 6.0) for 15 min, followed by cooling to room temperature (Kamo et al. 2007). Endogenous peroxidase activity was quenched with 3% hydrogen peroxide. Primary antibodies were used to identify neurons, glial cells, and CFTR-positive cells. Optimal contrast between the specific labeling and the background for each antigen was achieved using a PV9000 IHC kit containing polyperoxidase anti-mouse/rabbit IgG (Zymed Laboratories; South San Francisco, CA) and a SP9003 IHC kit containing polyperoxidase anti-goat IgG (Zymed Laboratories). After incubation with secondary antibody for 30 min at room temperature, DAB (Zymed Laboratories) or amino ethylcarbazole (Zymed Laboratories) was used to visualize the antigen. The IHC results were analyzed with Image-Pro Plus 5.0 (Media Cybernetics; Bethesda, MD). The IHC staining intensity of the positive signals in neurons was analyzed, and results are summarized in Table 1.
Table 1.
Examined regions of human brain
| Region studied | IHC staining intensity (OD) | ISH staining intensity(OD) |
|---|---|---|
| Telencephalon | ||
| Frontal lobe | 0.160487 ± 0.042237 | 0.201126 ± 0.055673 |
| Parietal lobe | 0.174368 ± 0.070239 | 0.215305 ± 0.088162 |
| Temporal lobe | 0.190205 ± 0.058752 | 0.216539 ± 0.038792 |
| Occipital lobe | 0.167337 ± 0.058732 | 0.186255 ± 0.063786 |
| Hippocampus | 0.218866 ± 0.047579 | 0.183419 ± 0.04575 |
| Corpus callosum | 0.159873 ± 0.052924 | 0.121803 ± 0.017576 |
| Basal ganglia gyrum | 0.170809 ± 0.078881 | 0.163657 ± 0.053808 |
| Diencephalon | ||
| Hypothalamus | 0.176616 ± 0.041705 | 0.162257 ± 0.040939 |
| Dorsal thalamus | 0.158755 ± 0.033423 | 0.174476 ± 0.065364 |
| Brain stem | ||
| Midbrain | 0.195819 ± 0.048257 | 0.179935 ± 0.042014 |
| Pons | 0.165708 ± 0.084874 | 0.190987 ± 0.077901 |
| Medulla oblongata | 0.200607 ± 0.040139 | 0.18986 ± 0.056757 |
| Cerebellum |
0.182268 ± 0.043918 |
0.217864 ± 0.09305 |
IHC and ISH staining intensity was analyzed with Image-Pro Plus 5.0 in the same areas of different brain regions. All neurons are positive for cystic fibrosis transmembrane conductance regulator in the analyzed regions. The average optical density (OD) of the positive signals inside the neurons of each region was analyzed. Data are given as mean ± standard deviations of OD.
To help confirm the specificity of the antibody reactions, two primary antibodies for CFTR [monoclonal CFTR M3A7 (Neomarker Labvision; Fremont, CA), 1:100; and polyclonal CFTR (Santa Cruz Biotechnology; Santa Cruz, CA), 1:200] were used in these experiments. Two negative controls and a positive control were used. In one negative control, the primary antibody was replaced with buffer without IgG, and in the other, the antibody was neutralized with antigenically specific blocking peptide prior to immunostaining (Santa Cruz Biotechnology). Thus, the absence of signal demonstrated that the antibody used was neutralized by the specific antigen and was therefore specific for CFTR. The concentrations of the antibody and blocking peptide were 1 μg/ml and 5 μg/ml, respectively.
ISH in Human Brain
To localize CFTR mRNA in human brain, ISH was performed on the same set of tissue samples used for IHC. All solutions were prepared with diethyl pyrocarbonate–treated water. After deparaffinization and rehydration, tissue sections were subjected to microwave heating for 10 min and incubation with 0.01 M HCl for 20 min to increase permeability. The sections were then incubated with hybridization cocktail containing CFTR probe at 45C for 18 hr. After serial washing with standard saline citrate and blocking with normal horse serum (1:100), sections were incubated with alkaline phosphatase–labeled anti-digoxigenin antibody (1:500) (Roche Diagnostics; Pensburg, Germany) for 1 hr, and the color was developed with nitro blue tetrazolium/5-bromo-4-choloro-3-indolyl phosphate (NBT/BCIP) (Promega Corp.; Madison, WI), resulting in a purple-blue signal. The images of ISH results were analyzed with Image-Pro Plus 5.0. The ISH intensity of the positive signals in neurons was examined, and results are summarized in Table 1. Signal specificity was assessed by substitution of an unrelated probe of similar nucleotide content and anti-sense probe length.
Double Labeling and Consecutive Tissue Sections Labeling
To identify the cell types expressing CFTR by ISH or IHC, both double labeling and staining consecutive tissue sections using either ISH or IHC combined with IHC were carried out. For double labeling on the same section, following the colorizing reaction step of ISH or IHC, the slides were heated at 96C in sodium citrate buffer (pH 6.0) for 10 min to remove the immune complexes and prevent any possible interference of the immune complex with the immunostaining of the second antigen (Lan et al. 1995; Kamo et al. 2007). New primary antibodies were then incubated on the same slide. In addition, consecutive sections were stained for CFTR or its mRNA and for cell markers, including neurofilament for neurons and glial fibrillary acidic protein (GFAP) for glial cells. The protocols used were as described above. Slides were examined with an Olympus Research light microscope (BX51; Tokyo, Japan). Photomicrographs of CFTR staining with CFTR-specific antibodies or mRNA probes in the same field were identified as neurons by positive staining with the specific marker neurofilament.
Results
CFTR mRNA Expression Was Detected in the Human Brain
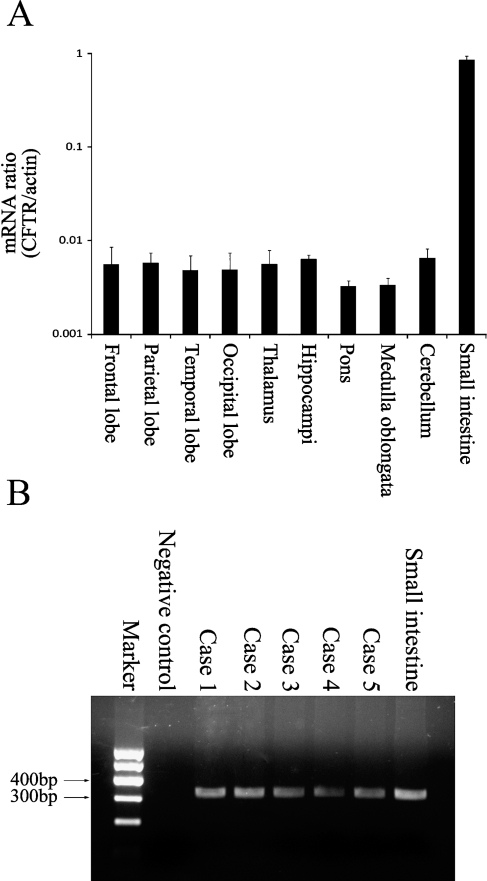
The results of RT-PCR analysis showed CFTR gene expression in human brain tissues (Figure 1). Figure 1A shows the positive results in various regions of human brain. No obvious differences were observed among the different regions of each case or among the different cases. An RT-PCR product of 328 base pairs was detected in total RNA extracts from human brain tissues (Figure 1B), indicating the presence of CFTR mRNA. PCR product sequence analysis was performed, and the results were applied for basic local alignment search tool (BLAST) in National Center for Biotechnology Information. BLAST results confirmed the expected CFTR mRNA sequence. The total CFTR mRNA contains 6132 nucleotides, and the PCR product is positioned from 473 to 800 nucleotides. PCR results of the five cases are shown in Figure 1B. Human small intestine tissue was used as the positive control for PCR and worked as expected. The negative controls yielded no signal.
Figure 1.
RT-PCR amplification of cystic fibrosis transmembrane conductance regulator (CFTR) from human brain tissues. Expression of CFTR in human brain tissues was demonstrated by amplifying CFTR mRNA. (A) Real-time PCR results in different regions of the human brain. CFTR expressions in different regions of brain are at a similar level. Error bars indicate mean ± SEM. (B) Agarose gel electrophoresis of the PCR products. A predicted product of ∼328 base pairs was observed. The product sequencing shows 100% identity with CFTR mRNA. This product was also observed when RT-PCR was performed on RNA extracted from human small intestine, which was used as a positive control. As a negative control, diethypyrocarbonate-treated water instead of RNA was used, which gave no band at the expected position.
Immunolocalization of CFTR in Human Brain With IHC
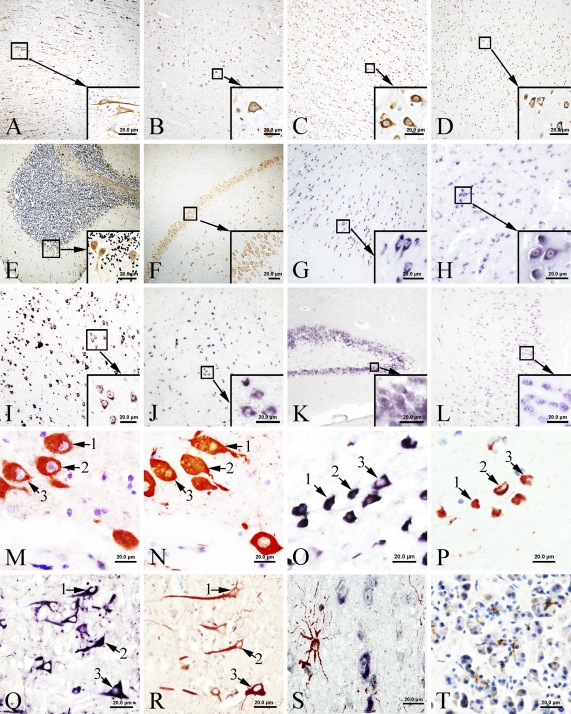
IHC was performed to localize CFTR immunoreactivity in the human brain. Widely distributed CFTR immunoreactivity signals were found in most areas of the human brain (Figures 1A–1F; Table 1). The localization of CFTR to neurons was confirmed by immunostaining of neurofilament and CFTR on consecutive tissue sections (Figures 1M and 1N). Neurofilament is a specific marker for neurons. All cells that were identifiable as neurons by neurofilament showed signals positive for CFTR. These signals were cytoplasmic and resided in somas, axons, and dendrites of these cells (Figure 2M). The signal strength varied among subgroups of neurons, with pyramidal neurons showing stronger staining than other types of neurons. In the cerebellum, signals were found in most neurons of the molecular and Purkinje layers (Figure 2E). Purkinje cells showed strong staining. CFTR-positive signals were absent in glial cells. The specificity of this IHC was established in parallel control experiments. No specific signal was observed when antigen neutralization was performed or when the primary antibodies were replaced by buffer without IgG.
Figure 2.
IHC and ISH for CFTR in human brain (A–F). (A) IHC results for CFTR in the frontal lobe. Lower right insert is the high-power image of area indicated by the small square. (B–F) IHC results for CFTR in the temporal lobe, parietal lobe, hypothalamus, cerebellum, and hippocampus, respectively. Lower right square insert in each figure shows the higher power image of indicated area of the lower power view. The brown positive signals developed with DAB show CFTR staining. CFTR-positive signals are localized to the cytoplasm of the neurons (Purkinje cells in E). (G–L) ISH results for CFTR mRNA in the frontal lobe (G). Lower right insert is the high-power view of area indicated by the small square. (H–L) ISH results for CFTR in the parietal lobe, temporal lobe, hypothalamus, hippocampus, and occipital lobe, respectively. Lower right insert in each figure shows the higher power view of indicated area in the lower power view. The purple-blue positive signals indicate CFTR mRNA. CFTR mRNA signals are mainly localized to cytoplasm of the neurons. Some nuclei also show positive staining. (M,N) IHC for CFTR and neurofilament (NF), respectively, on serial sections from the temporal lobe. The red positive signals were developed with AEC. The same neurons shown on two consecutive sections are indicated by arrows with the same numerical numbers. Results indicate that the CFTR-positive cells are also positive for NF, and are therefore neurons. (O,P) ISH for CFTR and IHC for NF on serial tissue sections in the basal nuclei. (O) ISH of CFTR mRNA. The purple signal was colorized by alkaline phosphatase and shows that CFTR mRNA is present in the cytoplasm. (P) Indicates that these positive cells are NF-positive neurons. The same cells on the two consecutive sections are labeled by arrows with the same numerical number. (Q) ISH of CFTR mRNA in the frontal lobe. The purple-blue signals were colorized with nitro blue tetrazolium/5-bromo-4-choloro-3-indolyl phosphate. Results demonstrate that CFTR mRNA is present in the cytoplasm. (R) Serial section of Q, which was immunostained with CFTR antibody. The same positive cells on consecutive sections are indicated by arrows with the same numerical numbers. Results demonstrate that cells expressing CFTR mRNA are also CFTR immunoreactive. (S) Double labeling combining ISH of CFTR mRNA (dark blue) and IHC of glial fibrillary acidic protein (GFAP) (brown) (glial cell marker) in the temporal lobe. Results demonstrate that CFTR mRNA is present in a cell population different from that of the GFAP-positive glial cells. (T) Immunohistochemical results of CFTR in human pancreas as a positive control. The brown positive signals indicate the existence of CFTR in the glandular tissue of pancreatic exocrine gland. Bar = 20 μm.
Detection and Localization of CFTR mRNA in Human Brain With ISH
Results obtained from ISH demonstrated extensive expression of CFTR mRNA in most areas of the human brain. The distribution pattern of mRNA signal was identical to that found by CFTR protein IHC (Figures 2G–2L). Positive ISH signals for CFTR mRNA were found in the cytoplasm of most neuronal cell types (Figures 2Q and 2R). Some nucleoli also show positive staining. To detect CFTR mRNA, we used an antisense CFTR mRNA probe. Positive signals were clearly demonstrated. However, when the sense CFTR mRNA probe was applied for ISH, we could also detect positive signals in neurons, but the signals were much weaker than those obtained with the antisense probe. Hybridization with a size-matched and guanine and cytosine content–matched unrelated probe gave no positive signal.
Labeling for CFTR mRNA (ISH) and Immunostaining With Anti-CFTR or With Anti-GFAP
Both immunostaining of CFTR protein and ISH of CFTR mRNA, combined with immunostaining for GFAP, demonstrated that CFTR protein and mRNA are present in a population of cells different from GFAP-positive cells (Figure 2O).
This result contrasted sharply with double staining of CFTR and neurofilament, which showed that both antibodies were positive in the same neuronal cells. However, because the glial cells are much smaller in size than the neurons and not all glial cells are positive for GFAP staining, we could not completely rule out the presence of CFTR in glial cells.
Discussion
In this study, the presence of CFTR in the human brain was demonstrated by real-time PCR, IHC, and ISH. Expression of CFTR in epithelial cells has been reported previously (Kerem et al. 1989; Riordan et al. 1989), and the function of CFTR in these cells has been extensively investigated and related to CF pathophysiology (Hasegawa et al. 1992; Bradbury 1999; Mehta 2005). However, expression of CFTR in non-epithelial cells was reported only recently (Fonknechten et al. 1992; Johannesson et al. 1997; Di et al. 2006; Painter et al. 2006; Vandebrouck et al. 2006), and this included expression of CFTR in neurons of the human hypothalamus (Mulberg et al. 1995,1998; Weyler et al. 1999). Compared with previous reports, the widespread expression of CFTR in this report was more identical with that in rats than in humans. In rat brain, the expression of CFTR has been confirmed in extensive regions, including cortex, hippocampus, nucleus, diencephalon, midbrain, pons, and medulla oblongata (Johannesson et al. 1997). However, in human brain, CFTR expression has been reported only in hypothalamus, not in any other regions.
In this study, the distribution of CFTR in human brain autopsy tissues was striking. CFTR expression was widespread and extensive in every region of the brain evaluated, including confirmation of previously reported hypothalamic expression, with localization to the cytoplasm of apparently most neurons (or all, based on visual impression). The CFTR immunohistochemistry staining results showed cytoplasmic distribution and, seemingly, Nissl body localization. The Nissl body is involved in protein synthesis, and this distribution provides a morphological basis for the possible synthesis of CFTR in neurons. In addition, we found CFTR protein signals in axons, where Nissl bodies do not exist. Therefore, CFTR seems not to be associated exclusively with Nissl bodies. There is also nucleolus staining for ISH. This might be explained by the fact that specific mRNA sequences might exist in the nucleolus regions and could be involved in mRNA export from the nucleus, as reported previously (Ideue et al. 2004). This widespread distribution highlights the potential significance of this protein in normal and abnormal states of the human CNS.
In view of the fact that CFTR is expressed in neurons throughout the entirety of the brain, together with the significance of its previously identified cellular functions, the implications of the CFTR distribution we have demonstrated here may be multiple. First, normal nervous system function could be affected by CFTR through several mechanisms, for example, by maintenance of the steady state of cellular electrolytes. CFTR has been found in epithelial cells as a phosphorylation-dependent epithelial chloride channel (Denning et al. 1992; Mohamed et al. 1997; Benharouga et al. 2003) regulating salt transport, fluid flow, and even transportation of large molecules, through its own direct function (Egan et al. 1992; Sheppard and Welsh 1999), by interaction with other membrane proteins (Stutts et al. 1995), or by modulating membrane traffic (Prince et al. 1994). In addition, CFTR itself could function as a neuromodulator and cell-signaling molecule. CFTR has ATP hydrolysis activity and can mediate nucleotide-dependent glutathione (GSH) flux. GSH is an important intra- and extracellular antioxidant that protects cells from reactive oxygen species (Kogan et al. 2003), and GSH can also function as a neurotransmitter or neuromodulator (Janaky et al. 1999). CFTR is also critical for cAMP-dependent regulation of membrane recycling (Bradbury et al. 1992), and has been associated with clathrin-coated vesicles in neurons of the hypothalmus of the bovine brain (Mulberg et al. 1994). Bradbury and associates reported that CFTR protein within subcellular compartments may play a role in neuropeptide transport or other molecular trafficking (Bradbury et al. 1994; Mulberg et al. 1994). It is not unreasonable to speculate that some or all of these functions that have been described largely in epithelia may also be associated with the CFTR, which is so widely distributed over neurons in the brain, and it is clear that such functions might profoundly affect neuronal physiology.
On the other hand, mutation or alteration of CFTR may induce CNS dysfunction as a part of CF or other neural diseases. For example, there may be accumulation of mutant CFTR, leading to blockage of molecular transmission and therefore to neuronal dysfunction. This mechanism has been demonstrated in epithelial cells (Gentzsch et al. 2007) and a similar mechanism may operate in neurons. Axonal dystrophy characterized by focal enlargement of the terminal region of the axon accompanied by detectable amyloid precursor protein has been found in the brain of CF patients (Cochran et al. 1991). This result suggests that CF can cause molecular accumulation in the brain and lends support to the mutant CFTR-blocking hypothesis. It is also of interest that subclinical extrapyramidal hemosiderosis has also been reported in CF (Wongmongkolrit et al. 1985), and seizures occur more frequently in lung transplant recipients with CF (Goldstein et al. 2000). This further supports the concept that mutation of CFTR affects neuronal function, and seizures in this context may be related to electrolyte disturbances caused by mutant CFTR (Castilla-Guerra et al. 2006). Although cyclosporine toxicity was hypothesized to be the cause of CNS complications in CF, changes in CFTR protein could not be ruled out (Goldstein et al. 2000). Facial nerve paralysis is an uncommon occurrence in the neonatal period. However, facial nerve palsy associated with hypovitaminosis A has been reported and occurs more frequently in CF patients (Keating and Feigin 1970; Cameron et al. 2007). Interestingly, pseudotumor cerebri were also reported in children with cystic fibrosis, but the exact mechanism is still poorly understood (Roach and Sinal 1989; Eid et al. 1990). Such changes appear to be distinctly different from the pathological changes in lungs and other organs in CF that are related to CFTR. However, it is not unlikely that CFTR intrinsic to the central neurons is involved in specific dysfunction unique to the CNS, and the strikingly widespread distribution of CFTR in neurons in the brain might account in part for these phenomena.
In summary, this study provides evidence for widespread expression and distribution of CFTR in neurons of the human brain, thus underscoring the probable importance of this molecule in CNS physiology. In addition, the distribution of CFTR identified in this study may help to provide an explanation for the neuronal symptoms associated with CF. The physiological and pathological significance of CFTR in central neurons therefore warrants further investigation.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant no. 30570686) and the 111 Project (grant no. B07001). The general support of LiFu Educational Foundation is acknowledged.
We are grateful to Ruishu Deng, Zhuo Li, Yan Dong, Yan Zhou, Peng Ding, Yingying Zhao, Baokai Yang, Qi Cao, and Lu Yao for their helpful discussions and technical assistance.
References
- Benharouga M, Sharma M, So J, Haardt M, Drzymala L, Popov M, Schwapach B, et al. (2003) The role of the C terminus and Na+/H+ exchanger regulatory factor in the functional expression of cystic fibrosis transmembrane conductance regulator in nonpolarized cells and epithelia. J Biol Chem 278:22079–22089 [DOI] [PubMed] [Google Scholar]
- Bradbury NA (1999) Intracellular CFTR: localization and function. Physiol Rev 79(suppl 1):175–191 [DOI] [PubMed] [Google Scholar]
- Bradbury NA, Cohn JA, Venglarik CJ, Bridges RJ (1994) Biochemical and biophysical identification of cystic fibrosis transmembrane conductance regulator chloride channels as components of endocytic clathrin-coated vesicles. J Biol Chem 269:8296–8302 [PubMed] [Google Scholar]
- Bradbury NA, Jilling T, Berta G, Sorscher EJ, Bridges RJ, Kirk KL (1992) Regulation of plasma membrane recycling by CFTR. Science 256:530–532 [DOI] [PubMed] [Google Scholar]
- Cameron C, Lodes MW, Gershan WM (2007) Facial nerve palsy associated with a low serum vitamin A level in an infant with cystic fibrosis. J Cyst Fibros 6:241–243 [DOI] [PubMed] [Google Scholar]
- Castilla-Guerra L, del Carmen Fernandez-Moreno M, Lopez-Chozas JM, Fernandez-Bolanos R (2006) Electrolytes disturbances and seizures. Epilepsia 47:1990–1998 [DOI] [PubMed] [Google Scholar]
- Chandy G, Grabe M, Moore HP, Machen TE (2001) Proton leak and CFTR in regulation of Golgi pH in respiratory epithelial cells. Am J Physiol Cell Physiol 281:C908–921 [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159 [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N (2006) The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: twenty-something years on. Nat Protoc 1:581–585 [DOI] [PubMed] [Google Scholar]
- Clague MJ, Urbe S, Aniento F, Gruenberg J (1994) Vacuolar ATPase activity is required for endosomal carrier vesicle formation. J Biol Chem 269:21–24 [PubMed] [Google Scholar]
- Cochran E, Bacci B, Chen Y, Patton A, Gambetti P, Autilio-Gambetti L (1991) Amyloid precursor protein and ubiquitin immunoreactivity in dystrophic axons is not unique to Alzheimer's disease. Am J Pathol 139:485–489 [PMC free article] [PubMed] [Google Scholar]
- Cozens AL, Yezzi MJ, Kunzelmann K, Ohrui T, Chin L, Eng K, Finkbeiner WE, et al. (1994) CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells. Am J Respir Cell Mol Biol 10:38–47 [DOI] [PubMed] [Google Scholar]
- Denning GM, Ostedgaard LS, Cheng SH, Smith AE, Welsh MJ (1992) Localization of cystic fibrosis transmembrane conductance regulator in chloride secretory epithelia. J Clin Invest 89:339–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di A, Brown ME, Deriy LV, Li C, Szeto FL, Chen Y, Huang P, et al. (2006) CFTR regulates phagosome acidification in macrophages and alters bactericidal activity. Nat Cell Biol 8:933–944 [DOI] [PubMed] [Google Scholar]
- Egan M, Flotte T, Afione S, Solow R, Zeitlin PL, Carter BJ, Guggino WB (1992) Defective regulation of outwardly rectifying Cl- channels by protein kinase A corrected by insertion of CFTR. Nature 358:581–584 [DOI] [PubMed] [Google Scholar]
- Eid NS, Shoemaker LR, Samiec TD (1990) Vitamin A in cystic fibrosis: case report and review of the literature. J Pediatr Gastroenterol Nutr 10:265–269 [PubMed] [Google Scholar]
- Engelhardt JF, Yankaskas JR, Ernst SA, Yang Y, Marino CR, Boucher RC, Cohn JA, et al. (1992) Submucosal glands are the predominant site of CFTR expression in the human bronchus. Nat Genet 2:240–248 [DOI] [PubMed] [Google Scholar]
- Fonknechten N, Chelly J, Lepercq J, Kahn A, Kaplan JC, Kitzis A, Chomel JC (1992) CFTR illegitimate transcription in lymphoid cells: quantification and applications to the investigation of pathological transcripts. Hum Genet 88:508–512 [DOI] [PubMed] [Google Scholar]
- Gentzsch M, Choudhury A, Chang XB, Pagano RE, Riordan JR (2007) Misassembled mutant DeltaF508 CFTR in the distal secretory pathway alters cellular lipid trafficking. J Cell Sci 120:447–455 [DOI] [PubMed] [Google Scholar]
- Goldstein AB, Goldstein LS, Perl MK, Haug MT, Arroliga AC, Stillwell PC (2000) Cystic fibrosis patients with and without central nervous system complications following lung transplantation. Pediatr Pulmonol 30:203–206 [DOI] [PubMed] [Google Scholar]
- Hasegawa H, Skach W, Baker O, Calayag MC, Lingappa V, Verkman AS (1992) A multifunctional aqueous channel formed by CFTR. Science 258:1477–1479 [DOI] [PubMed] [Google Scholar]
- Ideue T, Azad AK, Yoshida J, Matsusaka T, Yanagida M, Ohshima Y, Tani T (2004) The nucleolus is involved in mRNA export from the nucleus in fission yeast. J Cell Sci 117:2887–2895 [DOI] [PubMed] [Google Scholar]
- Janaky R, Ogita K, Pasqualotto BA, Bains JS, Oja SS, Yoneda Y, Shaw CA (1999) Glutathione and signal transduction in the mammalian CNS. J Neurochem 73:889–902 [DOI] [PubMed] [Google Scholar]
- Jiang Q, Engelhardt JF (1998) Cellular heterogeneity of CFTR expression and function in the lung: implications for gene therapy of cystic fibrosis. Eur J Hum Genet 6:12–31 [DOI] [PubMed] [Google Scholar]
- Johannesson M, Bogdanovic N, Nordqvist AC, Hjelte L, Schalling M (1997) Cystic fibrosis mRNA expression in rat brain: cerebral cortex and medial preoptic area. Neuroreport 8:535–539 [DOI] [PubMed] [Google Scholar]
- Kamo N, Yasuchika K, Fujii H, Hoppo T, Machimoto T, Ishii T, Fujita N, et al. (2007) Two populations of Thy1-positive mesenchymal cells regulate in vitro maturation of hepatic progenitor cells. Am J Physiol Gastrointest Liver Physiol 292:G526–534 [DOI] [PubMed] [Google Scholar]
- Kawai A, Uchiyama H, Takano S, Nakamura N, Ohkuma S (2007) Autophagosome-lysosome fusion depends on the pH in acidic compartments in CHO cells. Autophagy 3:154–157 [DOI] [PubMed] [Google Scholar]
- Keating JP, Feigin RD (1970) Increased intracranial pressure associated with probable vitamin A deficiency in cystic fibrosis. Pediatrics 46:41–46 [PubMed] [Google Scholar]
- Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, Chakravarti A, Buchwald M, et al. (1989) Identification of the cystic fibrosis gene: genetic analysis. Science 245:1073–1080 [DOI] [PubMed] [Google Scholar]
- Kogan I, Ramjeesingh M, Li C, Kidd JF, Wang Y, Leslie EM, Cole SP, et al. (2003) CFTR directly mediates nucleotide-regulated glutathione flux. EMBO J 22:1981–1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahousse SA, Stopa EG, Mulberg AE, de la Monte SM (2003) Reduced expression of the cystic fibrosis transmembrane conductance regulator gene in the hypothalamus of patients with Alzheimer's disease. J Alzheimers Dis 5:455–462 [DOI] [PubMed] [Google Scholar]
- Lan HY, Mu W, Nikolic-Paterson DJ, Atkins RC (1995) A novel, simple, reliable, and sensitive method for multiple immunoenzyme staining: use of microwave oven heating to block antibody crossreactivity and retrieve antigens. J Histochem Cytochem 43:97–102 [DOI] [PubMed] [Google Scholar]
- Lukacs GL, Chang XB, Kartner N, Rotstein OD, Riordan JR, Grinstein S (1992) The cystic fibrosis transmembrane regulator is present and functional in endosomes. Role as a determinant of endosomal pH. J Biol Chem 267:14568–14572 [PubMed] [Google Scholar]
- Mehta A (2005) CFTR: more than just a chloride channel. Pediatr Pulmonol 39:292–298 [DOI] [PubMed] [Google Scholar]
- Mohamed A, Ferguson D, Seibert FS, Cai HM, Kartner N, Grinstein S, Riordan JR, et al. (1997) Functional expression and apical localization of the cystic fibrosis transmembrane conductance regulator in MDCK I cells. Biochem J 322:259–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mularoni A, Beck L, Sadir R, Adessi GL, Nicollier M (1995) Down-regulation by progesterone of CFTR expression in endometrial epithelial cells: a study by competitive RT-PCR. Biochem Biophys Res Commun 217:1105–1111 [DOI] [PubMed] [Google Scholar]
- Mulberg AE, Resta LP, Wiedner EB, Altschuler SM, Jefferson DM, Broussard DL (1995) Expression and localization of the cystic fibrosis transmembrane conductance regulator mRNA and its protein in rat brain. J Clin Invest 96:646–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulberg AE, Weyler RT, Altschuler SM, Hyde TM (1998) Cystic fibrosis transmembrane conductance regulator expression in human hypothalamus. Neuroreport 9:141–144 [DOI] [PubMed] [Google Scholar]
- Mulberg AE, Wiedner EB, Bao X, Marshall J, Jefferson DM, Altschuler SM (1994) Cystic fibrosis transmembrane conductance regulator protein expression in brain. Neuroreport 5:1684–1688 [DOI] [PubMed] [Google Scholar]
- Nagayama S, Kai H, Okiyoneda T, Horikawa S, Miyata T (1999) Characterization of CFTR expression in a human pulmonary mucoepidermoid carcinoma cell line, NCI-H292 cells. FEBS Lett 455:215–218 [DOI] [PubMed] [Google Scholar]
- O'Riordan CR, Erickson A, Bear C, Li C, Manavalan P, Wang KX, Marshall J, et al. (1995) Purification and characterization of recombinant cystic fibrosis transmembrane conductance regulator from Chinese hamster ovary and insect cells. J Biol Chem 270:17033–17043 [DOI] [PubMed] [Google Scholar]
- Painter RG, Valentine VG, Lanson NA Jr, Leidal K, Zhang Q, Lombard G, Thompson C, et al. (2006) CFTR expression in human neutrophils and the phagolysosomal chlorination defect in cystic fibrosis. Biochemistry 45:10260–10269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrizio P, Salameh WA (1998) Expression of the cystic fibrosis transmembrane conductance regulator (CFTR) mRNA in normal and pathological adult human epididymis. J Reprod Fertil Suppl 53:261–270 [PubMed] [Google Scholar]
- Presley JF, Mayor S, McGraw TE, Dunn KW, Maxfield FR (1997) Bafilomycin A1 treatment retards transferrin receptor recycling more than bulk membrane recycling. J Biol Chem 272:13929–13936 [DOI] [PubMed] [Google Scholar]
- Prince LS, Workman RB Jr, Marchase RB (1994) Rapid endocytosis of the cystic fibrosis transmembrane conductance regulator chloride channel. Proc Natl Acad Sci USA 91:5192–5196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaves B, Banting G (1994) Vacuolar ATPase inactivation blocks recycling to the trans-Golgi network from the plasma membrane. FEBS Lett 345:61–66 [DOI] [PubMed] [Google Scholar]
- Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, et al. (1989) Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245:1066–1073 [DOI] [PubMed] [Google Scholar]
- Roach ES, Sinal SH (1989) Initial treatment of cystic fibrosis. Frequency of transient bulging fontanel. Clin Pediatr (Phila) 28:371–373 [DOI] [PubMed] [Google Scholar]
- Sheppard DN, Welsh MJ (1999) Structure and function of the CFTR chloride channel. Physiol Rev 79(suppl 1):23–45 [DOI] [PubMed] [Google Scholar]
- Smith JJ, Welsh MJ (1992) cAMP stimulates bicarbonate secretion across normal, but not cystic fibrosis airway epithelia. J Clin Invest 89:1148–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutts MJ, Canessa CM, Olsen JC, Hamrick M, Cohn JA, Rossier BC, Boucher RC (1995) CFTR as a cAMP-dependent regulator of sodium channels. Science 269:847–850 [DOI] [PubMed] [Google Scholar]
- Tizzano EF, Silver MM, Chitayat D, Benichou JC, Buchwald M (1994) Differential cellular expression of cystic fibrosis transmembrane regulator in human reproductive tissues. Clues for the infertility in patients with cystic fibrosis. Am J Pathol 144:906–914 [PMC free article] [PubMed] [Google Scholar]
- Todd-Turla KM, Rusvai E, Naray-Fejes-Toth A, Fejes-Toth G (1996) CFTR expression in cortical collecting duct cells. Am J Physiol 270:F237–244 [DOI] [PubMed] [Google Scholar]
- Trezise AE, Buchwald M (1991) In vivo cell-specific expression of the cystic fibrosis transmembrane conductance regulator. Nature 353:434–437 [DOI] [PubMed] [Google Scholar]
- Tsunoda T, Furui J, Yamada M, Eto T, Ura K, Matsumoto T, Segawa T, et al. (1991) Caroli's disease associated with hepatolithiasis: a case report and review of the Japanese literature. Gastroenterol Jpn 26:74–79 [DOI] [PubMed] [Google Scholar]
- Vandebrouck C, Melin P, Norez C, Robert R, Guibert C, Mettey Y, Becq F (2006) Evidence that CFTR is expressed in rat tracheal smooth muscle cells and contributes to bronchodilation. Respir Res 7:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Weert AW, Dunn KW, Gueze HJ, Maxfield FR, Stoorvogel W (1995) Transport from late endosomes to lysosomes, but not sorting of integral membrane proteins in endosomes, depends on the vacuolar proton pump. J Cell Biol 130:821–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyler RT, Yurko-Mauro KA, Rubenstein R, Kollen WJ, Reenstra W, Altschuler SM, Egan M, et al. (1999) CFTR is functionally active in GnRH-expressing GT1–7 hypothalamic neurons. Am J Physiol 277:C563–571 [DOI] [PubMed] [Google Scholar]
- Wongmongkolrit T, Wyszynski R, Hershey CO, Varnes AW (1985) Evidence of subclinical extrapyramidal hemosiderosis in cystic fibrosis. Acta Neuropathol 65:265–269 [DOI] [PubMed] [Google Scholar]