Abstract
Somatostatin released from capsaicin-sensitive sensory nerves of the lung during endotoxin-induced murine pneumonitis inhibits inflammation and hyperresponsiveness, presumably via somatostatin receptor subtype 4 (sst4). The goal of the present study was to identify sst4 receptors in mouse and human lungs and to reveal its inflammation-induced alterations with real-time quantitative PCR, Western blot, and immunohistochemistry. In non-inflamed mouse and human lungs, mRNA expression and immunolocalization of sst4 are very similar. They are present on bronchial epithelial, vascular endothelial, and smooth-muscle cells. The sst4 receptor protein in the mouse lung significantly increases 24 hr after intranasal endotoxin administration as well as in response to 3 months of whole-body cigarette smoke exposure, owing to the infiltrating sst4-positivite mononuclear cells and neutrophils. In the chronically inflamed human lung, the large number of activated macrophages markedly elevate sst4 mRNA levels, although there is no change in acute purulent pneumonia, in which granulocytes accumulate. Despite mouse granulocytes, human neutrophils do not show sst4 immunopositivity. We provide the first evidence for the expression, localization, and inflammation-induced alterations of sst4 receptors in murine and human lungs. Inasmuch as tissue distribution of this receptor is highly similar, extrapolation of murine experimental results to human conditions might be possible. (J Histochem Cytochem 57:1127–1137, 2009)
Keywords: airway inflammation, endotoxin exposure, immunohistochemistry, mouse and human lung pathology, pulmonary macrophage, reverse transcriptase polymerase chain reaction, somatostatin, Western blot
We have recently provided several lines of evidence that in the murine model of endotoxin lipopolysaccharide (LPS)-induced airway inflammation, somatostatin [somatotropin release-inhibiting factor (SRIF)] is released from capsaicin-sensitive sensory nerves of the lung. Somatostatin gets into the systemic circulation and inhibits the progress of the inflammatory reaction as well as bronchial hyperreactivity (Helyes et al. 2007). This potent somatostatin-mediated counter-regulatory mechanism in the airways was very similar to that observed in several other inflammation and pain models (Szolcsányi et al. 1998a,b; Helyes et al. 2004; Pintér et al. 2006).
Somatostatin is widely distributed throughout the body in 14- and 28-amino acid–containing forms (Gamse et al. 1981; Patel et al. 1995; ten Bokum et al. 2000) and exerts a wide range of actions (ten Bokum et al. 2000; Pintér et al. 2006). These effects are mediated via five different somatostatin receptor subtypes (sst1–sst5). In general, the somatostatin receptor family can be divided into two main groups on the basis of their sequence similarities and binding profile of synthetic analogs: the SRIF1 group comprises sst2, sst3, and sst5 receptors, whereas the SRIF2 group contains sst1 and sst4 receptors (Hoyer et al. 1995; Reisine and Bell 1995). Several data indicate that receptors in the SRIF1 group mediate the endocrine and anti-proliferative effects of somatostatin. Our previous results revealed that the SRIF2 group is likely to be responsible for the anti-inflammatory and anti-nociceptive actions (Helyes et al. 2000,2001; Szolcsányi et al. 2004). SRIF2-selective agonists such as the heptapeptide TT-232 (Helyes et al. 2001,2003,2004) or the highly sst4-selective peptidomimetic ligand J-2156 (Helyes et al. 2006) proved to be effective inhibitors of inflammatory and nociceptive processes (Pintér et al. 2006). The sst4 receptor, similarly to the other four sst subtypes, belongs to the G protein–coupled receptor family (Hoyer et al. 1995); its activation inhibits adenylate cyclase and, consequently, cAMP/protein kinase A via pertussis toxin–sensitive guanosine triphosphate binding proteins (Gi proteins) (Patel et al. 1995), opens various K+ channels, and inhibits voltage-gated Ca2+ channels. In addition, sst4 receptor agonists enhance signaling through mitogen-activated protein kinase, phospholipase C and phospholipase A2, and activate/inhibit phosphotyrosine phosphatases (Weckbecker et al. 2003).
Although previous results have suggested that the anti-inflammatory actions of endogenous somatostatin are mediated predominantly via sst4 receptors in various tissues, including the airways (Elekes et al. 2008), direct evidence supporting this view has not been provided, owing to the lack of selective sst4 antagonists. Furthermore, as compared with other receptor subtypes, there are relatively few data in the literature on the localization of sst4 receptors in general. In the rat, sst4 mRNA can be detected in the posterior iris epithelium and ciliary body (Mori et al. 1997), in several brain regions (Fehlmann et al. 2000), on peripheral blood mononuclear cells and thymocytes (ten Bokum et al. 1999; Lichtenauer-Kaligis et al. 2004), in vascular smooth-muscle cells, and in the lung (Schloos et al. 1997; Pintér et al. 2006). In humans, its immunohistochemical localization was found on exocrine gland cells and lymphocytes (Taniyama et al. 2005), as well as in the cytoplasm of tubular epithelial cells, podocytes, and mesangial cells in the kidney, where a marked upregulation was seen in response to inflammation (Bhandari et al. 2008). Sst4 mRNA was detected on human vascular endothelial cells (Curtis et al. 2000). In the mouse, however, its expression has only been shown on peripheral blood mononuclear cells, in the spleen, thymus, and bone marrow (Lichtenauer-Kaligis et al. 2004). We are lacking direct evidence for the localization of sst4 receptors in the lung, and it is also unknown how sst4 expression correlates with pulmonary inflammation.
Inasmuch as the function of sst4 during inflammation in the human lung cannot be studied directly, a model is essential to understanding the pathophysiological role of this receptor in inflammatory processes. Mouse models appear to be the most versatile and easily accessible, for example, with the help of creating genetically manipulated animals. However, animal models can only be useful if data gained in the experiments can be extrapolated to human conditions.
Because there is only limited information in the present literature concerning the presence and cellular distribution of the sst4 receptor in pulmonary tissues, the first aim of the present study was to determine quantitatively and qualitatively the expression and localization patterns of sst4 in mouse and human lungs. For this purpose, immunohistochemical and Western blot analysis, as well as RT-PCR for measuring sst4 mRNA, were used. Second, by comparing the alterations of sst4 receptor expression in experimentally induced acute and chronic murine lung inflammation and inflamed human pulmonary tissues, we aimed to investigate whether results obtained in airway inflammation models in mice can be reliably extrapolated to humans.
Materials and Methods
Animal Studies, Airway Inflammation Models
Six-week-old male C57BL/6 mice (20–25 g; n=6–10/group) were used for both models and were bred and kept in the animal house of the department of pharmacology and pharmacotherapy at the University of Pécs. The animals were provided with standard rodent chow and water ad libitum.
Endotoxin-induced Subacute Pneumonitis in Mice
Subacute airway inflammation was evoked by 60 μl intranasally applied Escherichia coli (serotype: 083) LPS (167 μg/ml dissolved in sterile PBS; Sigma, St. Louis, MO) and mice were sacrificed 24 hr later. Data showing that intranasal administration of this LPS dose evoked maximal inflammation (neutrophil accumulation and inflammatory cytokine production) 24 hr after its instillation served as the basis for choosing this time point. The same volume of sterile PBS was administered to control mice regarded as intact (non-inflamed) animals (Elekes et al. 2007,2008; Helyes et al. 2007,2009).
Cigarette Smoke–induced Chronic Bronchitis in Mice
Mice were exposed to 3R4F research cigarette smoke (College of Agriculture, University of Kentucky; Lexington, KY) with the help of a manual smoking system (TE2; Teague Enterprises, Woodland, CA). Whole-body smoke exposure was performed twice a day for 40 min in the closed chamber of the equipment (two cigarettes per occasion for six mice in the cage) for 3 months. Cigarettes burned down within 10 min; then the ventilator was switched off for 30 min, and the chamber was ventilated afterward for 10 min. The average concentration of the total suspended particles in the chamber was 70 mg/m3.
At the end of both experiments, the mice were anesthesized with ketamine-xylazine (5 mg/kg intraperitoneally-100 mg/kg intramuscularly), and killed by cervical dislocation, and the lungs were excised. Each lung was cut into three parts; the upper half of the left lung was put into RNAlater (Qiagen; Hilden, Germany) for molecular biological studies, the lower half was placed in 1 ml lysis buffer for Western blot analysis, and the whole right lung sample was fixed in 4% buffered formaldehyde (Szkarabeusz Ltd.; Pécs, Hungary) for immunohistochemical examination.
Human Samples
Samples were obtained from 11 patients undergoing lung operations from the intact and inflamed parts surrounding the tumor. The surgical procedure was lobectomy in eight cases, pulmectomy in one case, and excision in two cases (non-malignant diseases). The non-inflamed specimens were obtained from a distant part of the resected lung lobe (or excised area) and appeared macroscopically to be obviously healthy, and subsequently proven to be so microscopically. Perifocal biopsies were performed to obtain inflamed but non-tumor lung samples; this was verified by subsequent histopathological examinations. Permission was obtained from the local ethical committee, and patients signed the informed consent form. Tissues were processed and stored similarly to the protocol described for the mouse samples.
Determination of Sst4 Receptor mRNA in the Lung
Lung samples placed into 1 ml RNAlater solution were stored at −80C if not processed immediately. Total RNA was isolated using the GenElute Mammalian Total RNA Miniprep Kit (Sigma). RNA yield purity was determined by spectrophotometry (Nanodrop; Bio-Science Ltd., Budapest, Hungary) and electrophoresis on 1% agarose gel. Then cDNA was prepared from 1 μg total RNA with MuMLV reverse transcriptase (Abgene Ltd.; Epsom, UK), which has been proven to introduce no bias into the original balance of different RNA sequences.
Amounts of cDNAs encoding the sst4 receptor and the housekeeping endogenous control 18S rRNA for comparison were measured by quantitative real-time PCR using TaqMan assays (Applied Biosystems, Inc., Foster City, CA; mouse sst4 assay ID: Mm00436710_s1, human sst4 assay ID: Hs012566620_s1, and eukaryotic 18S RNA: 4352930E). Each PCR reaction was performed in triplicate using Taqman Universal PCR Master Mix on an ABI 7500 real-time PCR system (Applied Biosystems). The ABI 7500 PCR program consisted of an initial step of 50C for 2 min, then 95C for 10 min, followed by a denaturation step at 95C for 15 sec and 40 cycles of amplification for 15 sec at 95C and 1 min at 60C. To confirm the purity and size of the PCR products, the reactions were analyzed by electrophoresis, and the results were evaluated by QuantityOne software (BioRad; Hercules, CA). TaqMan data were analyzed by the standard curve method according to the manufacturer's recommendation (ABI 7500 manual). Serial dilutions of total mRNA isolated from the lung (33 ng, 66.5 ng, 125 ng, 250 ng per reaction) were run on 96-well RT-PCR plates (Applied Biosystems) for all TaqMan probes in three parallels. Standard curves were generated for each transcript, and expression levels were normalized to the housekeeping gene of S18 mRNA of the same sample. The parameters of the standard curves (slope, intercept, and R2), as well as the mean mRNA quantities in the samples, were calculated automatically by the 7500 system SDS software (Applied Biosystems).
Measurement of the Sst4 Receptor Protein in the Lung With Western Blot
The mouse lung was homogenized in ice-cold lysis buffer (50 mM HEPES, 10 mM sodium pyrophosphate, 10 mM EDTA, 100 mM sodium fluoride, 10% glycerol, and 1% Triton X-100) containing protease inhibitor cocktail (1:100) (Sigma). Whole-cell lysates were resolved by SDS-PAGE on 10% acrylamide gels after adding equal amounts of 2× sample buffer (125 mM Tris, 4% SDS, 10% glycerol, 0.006% bromo-phenol blue, and 10% mercaptoethanol). The gels were blotted for 2 hr to nitrocellulose membranes using a wet transfer procedure on BioRad MiniProtean electrophoresis equipment. To saturate nonspecific binding sites, the blots were soaked in blocking buffer (3% dry milk, 10 mM Tris, 100 mM sodium chloride, and 0.1% Tween 20, pH 7.4). For washing the blots, washing buffer (TBS-T) was used (10 mM Tris, 100 mM sodium chloride, and 0.1% Tween-20, pH 7.4). The membranes were incubated for 2 hr at room temperature with 500× dilution of the primary sst4 antibody [goat polyclonal anti-sst4 (P-15) antibody, Lot# G-121; Santa Cruz Biotechnology, Santa Cruz, CA], then washed three times for 5 min with TBS-T. Specific binding of the primary antibody was detected with horseradish peroxidase (HRP)-conjugated donkey anti-goat IgG (Lot# E-3106; Santa Cruz Biotechnology). Western blot visualization was performed by enhanced chemiluminescence as described in the manufacturer's instructions (SuperSignal West Pico Chemiluminescent substrate; Pierce, Rockford, IL) by exposure to XAR-5 X-ray film (Kodak Laboratories; New York, NY). For stripping the blots, Restore Stripping Buffer (Pierce) was used.
To prove the equal protein loading we used 10,000× dilution of monoclonal mouse anti-β-actin antibody Lot# 097K4834 (Sigma-Aldrich, St. Louis, MO) and 2000× dilution of HRP-conjugated goat anti-mouse IgG (Lot#0001; Hunnavix Ltd., Nagygörbő, Hungary). Blots were analyzed with densitometry with the QuantityOne software (BioRad).
Immunohistochemical Detection of the Sst4 Receptor Protein in the Lung
Lung specimens were fixed in 4% buffered formaldehyde for 24 hr and embedded in paraffin, and 5–7-μm sections were made with a microtome. The antigen was revealed by incubating the slides in acidic (pH 6) citrate buffer in a microwave oven three times for 5 min (750 W). The endogenous peroxidase activity of the tissue was inhibited by a 20-min incubation with 3% hydrogen peroxide. For the prevention of nonspecific binding of the secondary antibody, preincubation was performed with normal goat serum for 20 min. The slides were incubated with a 1:100 dilution of rabbit polyclonal anti-sst4 antibody (for the mouse lung, ASR-004 Lot#: AN-01, Alomone Labs Ltd., Jerusalem, Israel; for the human lung, LSA4148, MBL International Corporation, Woburn, MA) for 1 hr and then with the secondary HRP-conjugated EnVision anti-rabbit secondary antibody (Dako-Cytomation; Dako North America, Inc., Carpinteria, CA) for 1 hr. Finally, immunolocalization of the sst4 receptor was detected by DAB development for 10 min, and nuclear staining was performed with hematoxylin. The specificity of the immunohistochemical reaction was confirmed by the lack of staining after preincubation of the primary antibody with an equal amount of the control peptide antigen included in the package of the primary antibody (Lot#AG-01; Alomone Labs), as well as by lack of immunopositivity after using only the secondary antibody without the primary antibody. Furthermore, staining was not observed in tissues of sst4 receptor knockout mice.
Statistical Analysis
Each RT-PCR and Western blot analysis was repeated at least three times, and the data were averaged. Values for all measurements were expressed as means ± SEM of n=6–11 samples in both series of studies. In the mouse experiments, two-tailed Student's t-tests, and in the human samples, one-tailed Student's t-tests were used for unpaired and paired comparisons, respectively, to perform statistical analyses. In all cases, p<0.05 was considered to be significant.
Ethical Considerations
All animal experimental procedures were carried out according to the 1998/XXVIII Act of the Hungarian Parliament on Animal Protection and Consideration Decree of Scientific Procedures of Animal Experiments (243/1988) and complied with the recommendations of the Helsinki Declaration. The studies were approved by the ethics committee on animal research of the University of Pécs according to the ethical codex of animal experiments, and permission was given (license no. BA 02/2000-11-2006). For the examination of the human lungs, permission was given by the ethical committee of the University of Pécs, and all patients signed the informed consent forms.
Results
Studies on Mouse Pulmonary Tissues
Expression of Sst4 Receptor mRNA in Total Extracts of Intact and Inflamed Mouse Lung Tissues
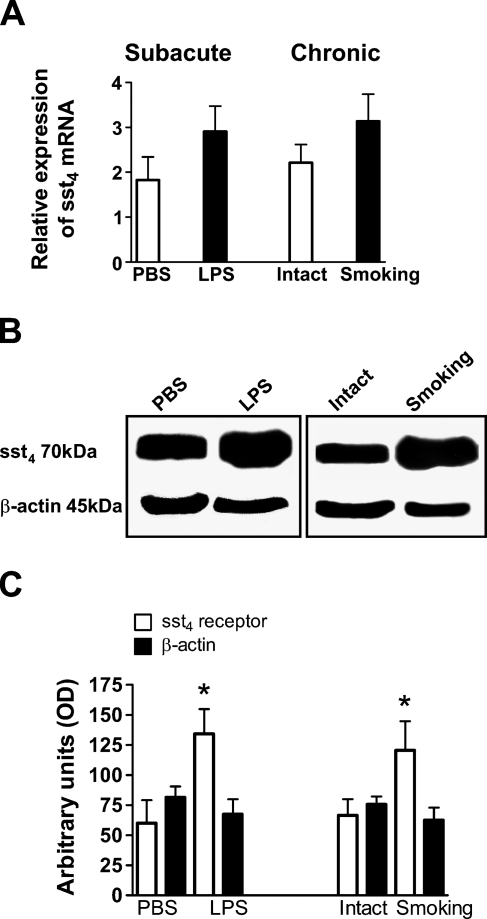
Real-time quantitative PCR results have shown that sst4 receptor mRNA is present in total mouse lung extracts. Subacute interstitial pulmonary inflammation 24 hr after intranasal LPS administration and three months of whole-body cigarette smoke exposure slightly, but not significantly, increased sst4 mRNA concentration, compared with its basal expression measured in non-inflamed lungs (Figure 1A).
Figure 1.
Expression of the sst4 receptor in the mouse lung. (A) Somatostatin receptor subtype 4 (sst4) receptor mRNA expression in the non-inflamed and inflamed mouse lung normalized to the housekeeping 18S rRNA. (B) Representative Western blot membranes and (C) their densitometric analysis showing increased sst4 receptor protein expression in both subacutely and chronically inflamed pulmonary tissues. At the left of each panel are shown the results obtained 24 hr after intranasal lipopolysaccharide (LPS) administration compared with PBS-treated animals (subacute inflammation); on the right of each panel are shown the data of mice exposed to cigarette smoke for 3 months compared with controls (intact; chronic inflammation). Columns represent means ± SEM of 6 (smoking model), 8 (Western blot, LPS model) and 10 (PCR, LPS model) mice in each group. Student's t-test for unpaired comparison was used for analysis. *p<0.05.
Expression of the Sst4 Receptor Protein in Total Extracts of Intact and Inflamed Mouse Lung Tissues
Detection of the sst4 receptor at the protein level confirmed its presence in the mouse lung. Western blot analysis revealed that both subacute and chronic inflammatory reactions induced by intranasal LPS administration and chronic cigarette smoke exposure, respectively, significantly increased the amount of sst4 receptors in the lung, compared with non-inflamed controls (Figures 1B and 1C).
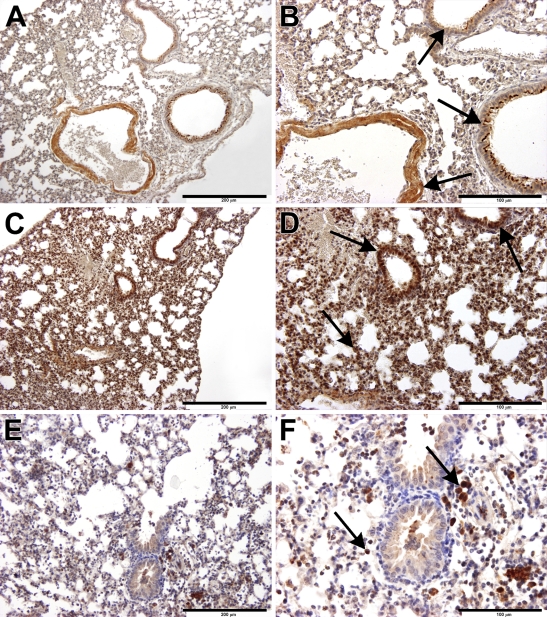
Tissue- and Cell-specific Localization of Sst4 Receptors in the Mouse Lung
Immunolocalization of the sst4 receptor protein in the intact lung was clearly detected on the bronchial epithelial cells, particularly on their luminal surfaces, on vascular as well as bronchial/bronchiolar smooth-muscle cells, vascular endothelial cells, and fibrocytes in the interalveolar septal regions (Figures 2A and 2B). One day after intranasal endotoxin treatment, a marked infiltration of mononuclear cells and neutrophils was observed predominantly in the peribronchial/perivascular spaces, indicating the development of subacute interstitial pneumonitis. Both accumulating mononuclear cell types (activated macrophages and lymphocytes), as well as neutrophils, stained for the sst4 receptor (Figures 2C and 2D). In the lungs of mice exposed to cigarette smoke for 3 months (for 40 min twice a day), typical signs of chronic bronchitis/bronchiolitis were seen. The interalveolar septum was desintegrated, the bronchiolar epithelial layer was irregular, and epithelial damage and hyperplasia could simultaneously be observed. Large numbers of sst4 receptor–expressing activated macrophages infiltrated the alveolar spaces and dominated the picture, but there were also sst4-positive lymphocytes and a few granulocytes (Figures 2E and 2F).
Figure 2.
Immunohistochemical localization of sst4 receptors in the mouse lung. Arrows indicate sst4 receptor positivity on epithelial/endothelial and smooth muscle cells, as well as on activated macrophages. (A,B) In the non-inflamed lung, sst4 immunopositivity is observed in bronchial epithelial cells, particularly on the luminal surfaces, vascular endothelial cells, and vascular and bronchial smooth-muscle cells in the interalveolar septal regions. (C,D) In the inflamed lung, 24 hr after intranasal LPS administration, there is a marked accumulation of sst4-immunopositive mononuclear cells, predominantly macrophages but also neutrophils and lymphocytes, in the peribronchial/perivascular spaces. (E,F) Three months of whole-body cigarette smoke exposure induces infiltration of sst4 receptor–expressing activated macrophages in the intraalveolar regions; there are also lymphocytes and a few granulocytes. Bars: A,C,E = 200 μm; B,D,F = 100 μm.
Studies on Human Pulmonary Tissues
Expression of Sst4 Receptor mRNA in Intact and Inflamed Human Lungs
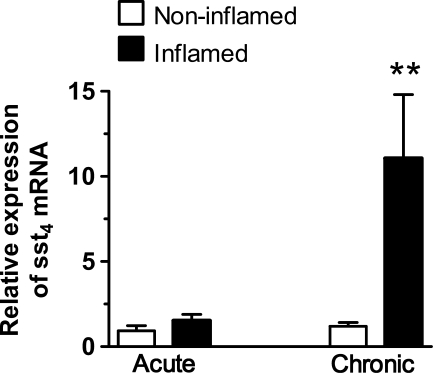
Lung samples were obtained both from macroscopically inflamed and intact sites of 11 patients undergoing operations: nine tumor resections and two non-malignant cases (aspergilloma and tuberculoma). RT-PCR data have revealed that the sst4 receptor mRNA is present in the non-inflamed human lung. Its relative expression, compared with the housekeeping S18 mRNA, was 1.9 ± 0.19, which was an expression level very similar to that found in mice.
A significant increase in sst4 receptor mRNA levels (ranging between 2.78-fold and 30.07-fold) was observed in the inflamed specimens compared with the respective intact tissue counterparts of eight patients. Clinical history, preoperative bronchoscopy, and histological diagnosis of the inflamed pulmonary samples of these patients have shown characteristic features of chronic bronchitis. These patients (except Patient 7) were heavy smokers undergoing surgery for relatively small peripheral primary adenocarcinomas (Patients 4, 6, 8, and 10), metastatic tumors (Patient 1, hepatocellular carcinoma; Patient 5, renal carcinoma), or non-neoplastic diseases (Patient 7, aspergilloma; Patient 11, tuberculoma). The presence of chronic inflammation was supported by a large number of infiltrating mononuclear cells (desquamating interstitial pneumonitis, follicular bronchiolitis). The extent of sst4 mRNA increase in the inflamed lung was in good correlation with the severity of the chronic inflammatory reaction seen on histology. Results of the two patients without malignancies revealed changes in sst4 expression very similar to those found in the other cases of chronic inflammation.
In contrast, in the inflamed pulmonary samples obtained from Patients 2, 3, and 9, who had acute/subacute pneumonia with a marked accumulation of granulocytes, no change was observed in sst4 receptor mRNA expression in the inflamed and intact sites. Patients 2 and 3 underwent surgery for large planocellular cancers in the central lung regions; Patient 9 had mixed-type (95% planocellular cancer, 5% adenocarcinoma) in the right lower lobe. Acute purulent inflammation was observed in the peripheral sites where atelectasia developed (Figure 3; Table 1).
Figure 3.
Expression of sst4 receptor mRNA in the human lung. Sst4 receptor mRNA in the non-inflamed and inflamed regions of human lung samples normalized to the housekeeping 18S rRNA. Columns represent means ± SEM of three patients with acute/subacute and eight with chronic inflammation. Student's t-test for paired comparison was used for analyzing the results of the two regions of the same lung. **p<0.01 vs respective intact samples.
Table 1.
Expression and inflammation-induced alteration of the sst4 receptor mRNA in the human lung
| Expression of the sst4 receptor mRNA normalized to the 18S ribosomal mRNA |
||||
|---|---|---|---|---|
| Patient | Type of inflammation: histopathological description | Intact site | Inflamed site | Fold change of relative sst4 mRNA expression compared to the respective intact site |
| 1 | Chronic inflammation with a great number of activated macrophages accumulated in the alveolar spaces (desquamating interstitial pneumonitis), lymphocyte infiltration, peribronchial fibrosis, and black granular depositions; respiratory bronchiolitis | 1.21 | 36.39 | 30.07 |
| 2 | Acute inflammatory reaction on the basis of bronchiectasis with predominant granulocyte, mostly neutrophil, accumulation | 1.52 | 1.86 | 1.22 |
| 3 | Acute pneumonia with interstitial edema and marked neutrophil accumulation | 0.60 | 0.78 | 1.3 |
| 4 | Chronic inflammatory reaction, respiratory bronchiolitis, macrophage and lymphocyte infiltration without inflammatory signs in the lung parenchyma, centroacinar emphysema | 1.88 | 9.62 | 5.11 |
| 5 | Chronic inflammation with predominant lymphocyte infiltration, respiratory bronchiolitis with activated macrophages, smoking-induced pigment granule depositions | 1.21 | 4.09 | 3.38 |
| 6 | Chronic inflammation with groups of pigmented intraalveolar macrophages, destruction of the respiratory bronchioles, infiltration of lymphocytes, atelectasia | 0.42 | 8.24 | 19.62 |
| 7 | Chronic follicular bronchiolitis with interstitial lymphocyte infiltration, macrophage accumulation, abscess formation | 0.61 | 7.35 | 12.05 |
| 8 | Chronic obliterating bronchiolitis with lymphocytic predominance, anthracosis | 0.69 | 1.92 | 2.78 |
| 9 | Subacute inflammatory reaction on the basis of bronchiectasis with predominant granulocyte, mostly neutrophil, accumulation | 1.79 | 2.70 | 1.51 |
| 10 | Chronic inflammatory reaction with marked intraalveolar macrophage accumulation, excessive mucus secretion | 1.91 | 9.07 | 4.75 |
| 11 |
Chronic inflammation with classical granulomatosus tuberculoid reaction with Langerhans-type giant cells, central necrosis, and epitheloid cells |
0.54 |
11.01 |
20.39 |
Sst4, somatostatin receptor subtype 4. Data show sst4 receptor mRNA expression normalized to the 18S ribosomal mRNA as a housekeeping gene in non-inflamed and inflamed lung specimens of 11 patients. The fold changes in the inflamed areas compared to the respective intact sites as well as the histopathological diagnosis are also described.
Localization of Sst4 Receptor Protein in Intact and Inflamed Human Lungs
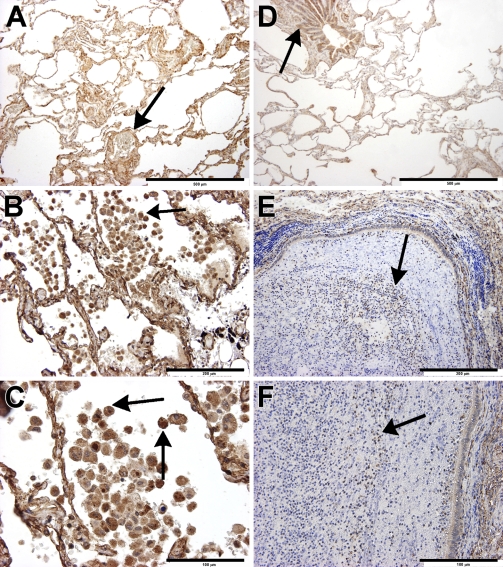
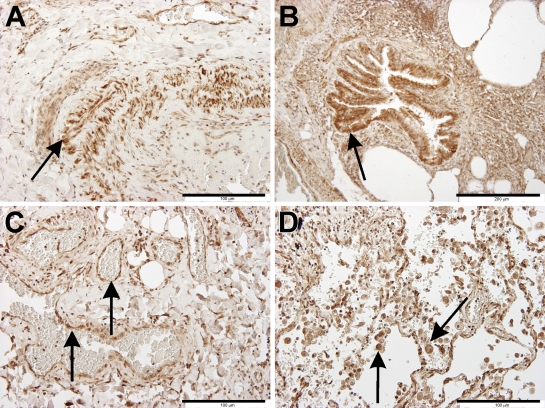
Localization of the sst4 receptor protein in the intact human lung (Figures 4A and 4D) using immunohistochemistry could be detected in smooth-muscle cells (Figures 5A and 5C), bronchial and bronchiolar epithelial cells (Figure 5B), and vascular endothelial cells (Figure 5C), very similar to that observed in mice. The left three panels of Figure 4 show sst4 receptor immunostaining of Patient 1, who underwent surgery for a relatively small solitary metastasis of a liver cancer in the peripheral region of the lung. Compared with the intact site (Figure 4A), a large number of sst4-immunopositive activated macrophages and some lymphocytes can be seen in the intra-alveolar region of the chronically inflamed lung (Figures 4B and 4C and Figure 5D). This image is in perfect correlation with the 30.07-fold elevation of sst4 mRNA detected in this sample. In the lung specimens obtained from Patient 2, in whom bronchiectasia and a severe acute purulent pneumonia were diagnosed, the bronchial lumen is completely filled with neutrophils that do not express the sst4 receptor, and there are also a few sst4-positive lymphocytes (Figures 4E and 4F). The small 1.86-fold increase of sst4 receptor mRNA found in this patient is explained well by this morphological finding.
Figure 4.
Immunohistochemical localization and inflammation-induced alterations of sst4 receptors in the human lung. Arrows indicate sst4 receptor positivity on epithelial/endothelial and smooth muscle cells, as well as on activated macrophages and lymphocytes. Immunolocalization of the sst4 receptor in the intact (A,D) and inflamed (B,C,E,F) lung specimens of Patient 1 and Patient 2, respectively. (A,D) In the non-inflamed lung samples of both patients, sst4 immunopositivity can be detected in bronchial epithelial and vascular endothelial cells, smooth-muscle cells, and fibrocytes in the interalveolar septal regions, similar to that observed in mice. (B,C) In the chronically inflamed lung of Patient 1, the interalveolar regions are full of sst4-immunopositive activated macrophages and include some lymphocytes. (E,F) In the inflamed lung specimens obtained from Patient 2, in whom bronchiectasy and severe acute purulent pneumonia were diagnosed, the bronchial lumen is completely filled with neutrophils that do not express the sst4 receptor, but there are also a few sst4-positive lymphocytes. Bars: A,D = 500 μm; B,E = 200 μm; C,F = 100 μm.
Figure 5.
Sst4 receptor immunopositivity in the human lung. Sst4 receptor immunopositivity is shown in (A) vascular smooth-muscle cells, (B) bronchial epithelial and smooth-muscle cells, (C) vascular endothelial cells and fibrocytes in the septal regions, as well as (D) activated macrophages accumulated in the intraalveolar sites (desquamating intraalveolar pneumonitis, respiratory bronchiolitis). Bars: A,C,D = 100 μm; B = 200 μm.
Discussion
The existence of sst4 receptors in mammalian tissues was first identified in the rat lung (Schloos et al. 1997). This article provided molecular biological evidence that sst4 was the predominant form of somatostatin receptors found in the rat airways. At that time, subtype-specific antibodies and agonists were not available, and therefore, neither the cell types exhibiting sst4, nor its role could be determined. However, the authors emphasized the great need for analyzing the localization and function of sst4 in the airways (Schloos et al. 1997; Fehlmann et al. 2000). The present results provide the first immunohistochemical, molecular biological, and Western blot evidence for the expression of sst4 receptors in mouse and human pulmonary tissues. In intact, non-inflamed lungs, the relative expression of sst4 mRNA and the localization of this receptor protein are very similar in mice and humans. In both species, sst4 is predominantly localized on bronchial epithelial and vascular endothelial cells, as well as on bronchial/vascular smooth-muscle cells and fibrocytes in the interalveolar septal regions. Because most human samples were obtained from patients with malignancies, the influencing effect of the tumor on sst4 expression even in the macroscopically and microscopically intact distant parts of the removed sections certainly cannot be excluded. However, sst4 expression in the intact parts was rather similar in different patients with different cancer types as well as in the two patients without neoplastic disease.
Endotoxin-induced murine pneumonitis is a widely used subacute interstitial lung inflammation model with a well-defined mechanism. Mononuclear cells and granulocytes infiltrating predominantly into the peribronchial and perivascular regions secrete inflammatory cytokines, mainly TNF-α and IL-1β (Savov et al. 2002; Elekes et al. 2007,2008; Helyes et al. 2007). The most-relevant model of chronic bronchitis/bronchiolitis is cigarette smoke–evoked experimental inflammation, in which the histopathological changes are very similar to those in human conditions (Wright et al. 2008). In these models, alterations of sst4 receptor expression were found to be different at the mRNA and protein levels. The amount of the sst4 protein markedly increased in the inflamed lung 24 hr after intranasal LPS administration, as well as in response to 3 months of whole-body cigarette smoke exposure, and can be explained by the infiltration of sst4-positive macrophages, lymphocytes, and neutrophils. Despite the infiltration of sst4-expressing inflammatory cells, which is in correlation with the data obtained at the protein level, the sst4 mRNA concentration did not increase significantly in either subacute or chronic experimental inflammatory conditions. Potential explanations for this discrepancy might be the enhanced mRNA degradation during inflammation, the differences in the turnover rate of the RNA and the protein, several posttranscriptional factors modifying mRNA processing, and/or the fact that the receptors are already on the accumulating inflammmatory cells when they infiltrate the lung. Such virtual contradictions are often found; proteomic studies have demonstrated that the levels of several proteins rarely correlate with the mRNA content of the tissues (Chen et al. 2002).
We have previously shown in several inflammation models that somatostatin is released from activated capsaicin-sensitive peptidergic sensory nerve endings in response to inflammatory/nociceptive stimuli, and that it can reach the circulation to exert systemic anti-inflammatory actions (Szolcsányi et al. 1998a,b,2004; Helyes et al. 2004). More recently, this somatostatin-mediated counter-regulatory mechanism was also proven in the endotoxin-induced lung inflammation model (Helyes et al. 2007,2009). The wide range of inhibitory actions of somatostatin derived from sensory nerves were suggested to be mediated via sst4 receptor activation (Szolcsányi et al. 2004; Pintér et al. 2006; Helyes et al. 2007) on the basis of potent anti-inflammatory and anti-hyperreactivity effects of synthetic sst4 receptor agonists in both LPS-induced acute pneumonitis and ovalbumine-evoked chronic asthma models in mice (Elekes et al. 2008). The present data strongly support these findings; we show a direct increase of sst4 receptors in response to inflammatory stimuli.
The main difference found between mouse and human tissues is the lack of sst4 receptors on human neutrophils. This is in agreement with results shown on human peripheral blood granulocytes (Hiruma et al. 1990), as well as with our unpublished findings on human synovium, colon, and peripheral blood neutrophils. The presence of sst4 receptors in human macrophages and lymphocytes, and its remarkable upregulation during chronic inflammatory conditions, suggest its potential therapeutic significance.
The presence of somatostatin and sst4 receptors in the lung is of great pathophysiological importance. Somatostatin has been shown to effectively inhibit the release of pro-inflammatory sensory neuropeptides (Helyes et al. 2001,2003; Pintér et al. 2006) and to modulate the immune system by inhibiting monocyte-macrophage functions (Krantic 2000), immunoglobulin production of B lymphocytes, proliferation and cytokine production of T lymphocytes, and fibrosis (Kolasinski et al. 1992; Pintér et al. 2006; Borie et al. 2008). Furthermore, upregulation of somatostatin binding sites in several immune-mediated and inflammatory diseases (ten Bokum et al. 1999,2000; Pintér et al. 2006) shows a direct link with the regulation of inflammatory processes.
The present study, which describes a remarkable upregulation of sst4 receptors during chronic inflammatory conditions in humans, supports the conclusions obtained in mouse experiments (Helyes et al. 2007; Elekes et al. 2008; Helyes et al. 2009) and suggests the potential therapeutic significance of synthetic sst4 receptor agonists as novel tools for the treatment of inflammatory diseases of the airways.
Acknowledgments
This work was supported by Hungarian grants OTKA K73044 and NK78059. Z.H. is supported by a János Bolyai postdoctoral research fellowship.
The authors thank Dr. Ágnes Kemény for her help with the photographic images.
References
- Bhandari S, Watson N, Long E, Sharpe S, Zhong W, Xu SZ, Atkin SL (2008) Expression of somatostatin and somatostatin receptor subtypes 1-5 in human normal and diseased kidney. J Histochem Cytochem 56:733–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borie R, Fabre A, Prost F, Marchal-Somme J, Lebtahi R, Marchand-Adams S, Aubier M, et al. (2008) Activation of somatostatin receptors attenuates pulmonary fibrosis. Thorax 63:251–258 [DOI] [PubMed] [Google Scholar]
- Chen G, Gharib TG, Huang C-C, Taylor JMG, Misek DE, Kardia SLR, Giordano TJ, et al. (2002) Discordant protein and mRNA expression in lung adenocarcinomas. Mol Cell Proteomics 1:304–313 [DOI] [PubMed] [Google Scholar]
- Curtis SB, Hewitt J, Yakubovitz S, Anzarut A, Hsiang YN, Buchan AM (2000) Somatostatin receptor subtype expression and function in human vascular tissue. Am J Physiol Heart Circ Physiol 278:1815–1822 [DOI] [PubMed] [Google Scholar]
- Elekes K, Helyes Z, Kereskai L, Sándor K, Pintér E, Pozsgai G, Tékus V, et al. (2008) Inhibitory effects of synthetic somatostatin receptor subtype 4 agonists on acute and chronic airway inflammation and hyperreactivity in the mouse. Eur J Pharmacol 578:313–322 [DOI] [PubMed] [Google Scholar]
- Elekes K, Helyes Z, Németh J, Sándor K, Pozsgai G, Kereskai L, Börzsei R, et al. (2007) Role of capsaicin-sensitive afferents and sensory neuropeptides in endotoxin-induced airway inflammation and consequent bronchial hyperreactivity in the mouse. Regul Pept 141:44–54 [DOI] [PubMed] [Google Scholar]
- Fehlmann D, Langenegger D, Schuepbach E, Siehler S, Feuerbach D, Hoyer D (2000) Distribution and characterisation of somatostatin receptor mRNA and binding sites in the brain and periphery. J Physiol (Paris) 94:265–281 [DOI] [PubMed] [Google Scholar]
- Gamse R, Lackner D, Gamse G, Leeman SE (1981) Effect of capsaicin pretreatment on capsaicin-evoked release of immunoreactive somatostatin and substance P from primary sensory neurons. Naunyn Schmiedebergs Arch Pharmacol 316:38–41 [DOI] [PubMed] [Google Scholar]
- Helyes Z, Elekes K, Németh J, Pozsgai G, Sándor K, Kereskai L, Börzsei R, et al. (2007) Role of transient receptor potential vanilloid 1 receptors in endotoxin-induced airway inflammation in the mouse. Am J Physiol Lung Cell Mol Physiol 292:L1173–1181 [DOI] [PubMed] [Google Scholar]
- Helyes Z, Pintér E, Németh J, Kéri Gy, Thán M, Oroszi G, Horváth A, et al. (2001) Anti-inflammatory effect of synthetic somatostatin analogues in the rat. Br J Pharmacol 134:1572–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helyes Z, Pintér E, Németh J, Sándor K, Elekes K, Szabó Á, Pozsgai G, et al. (2006) Effects of the somatostatin receptor subtype 4 selective agonist J-2156 on sensory neuropeptide release and inflammatory reactions in rodents. Br J Pharmacol 149:405–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helyes Z, Pintér E, Németh J, Szolcsányi J (2003) Pharmacological targets for the inhibition of neurogenic inflammation. Curr Med Chem AIAAA 2:191–218 [Google Scholar]
- Helyes Z, Pintér E, Sándor K, Elekes K, Bánvölgyi Á, Keszthelyi D, Szőke É, et al. (2009) Impaired defense mechanism against inflammation, hyperalgesia, and airway hyperreactivity in somatostatin 4 receptor gene-deleted mice. Proc Natl Acad Sci USA 106:13088–13093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helyes Z, Szabó Á, Németh J, Jakab B, Pintér E, Bánvölgyi Á, Kereskai L, et al. (2004) Antiinflammatory and analgesic effects of somatostatin released from capsaicin-sensitive sensory nerve terminals in a Freund's adjuvant-induced chronic arthritis model in the rat. Arthritis Rheum 50:1677–1685 [DOI] [PubMed] [Google Scholar]
- Helyes Z, Thán M, Oroszi G, Pintér E, Németh J, Kéri G, Szolcsányi J (2000) Anti-nociceptive effect induced by somatostatin released from sensory nerve terminals and by synthetic somatostatin analogues in the rat. Neurosci Lett 278:185–188 [DOI] [PubMed] [Google Scholar]
- Hiruma K, Koike T, Nakamura H, Sumida T, Maeda T, Tomioka H, Yoshida S, et al. (1990) Somatostatin receptors on human lymphocytes and leukaemia cells. Immunology 71:480–485 [PMC free article] [PubMed] [Google Scholar]
- Hoyer D, Bell GI, Berelowitz M, Epelbaum J, Feniuk W, Humphrey PP, O'Carroll AM, et al. (1995) Classification and nomenclature of somatostatin receptors. Trends Pharmacol Sci 16:86–88 [DOI] [PubMed] [Google Scholar]
- Kolasinski SL, Haines KA, Siegel EL, Cronstein BN, Abramson SB (1992) Neuropeptides and inflammation. A somatostatin analog as a selective antagonist of neutrophil activation by substance P. Arthritis Rheum 35:369–375 [DOI] [PubMed] [Google Scholar]
- Krantic S (2000) Peptides as regulators of the immune system: emphasis on somatostatin. Peptides 21:1941–1964 [DOI] [PubMed] [Google Scholar]
- Lichtenauer-Kaligis EG, Dalm VA, Oomen SP, Mooij DM, van Hagen PM, Lamberts SW, Hofland LJ (2004) Differential expression of somatostatin receptor subtypes in human peripheral blood mononuclear cell subsets. Eur J Endocrinol 150:565–577 [DOI] [PubMed] [Google Scholar]
- Mori M, Aihara M, Shimizu T (1997) Differential expression of somatostatin receptors in the rat eye: SSTR4 is intensely expressed in the iris/ciliary body. Neurosci Lett 223:185–188 [DOI] [PubMed] [Google Scholar]
- Patel YC, Greenwood MT, Panetta R, Demchyshyn L, Niznik H, Srikant CB (1995) The somatostatin receptor family. Life Sci 57:1249–1265 [DOI] [PubMed] [Google Scholar]
- Pintér E, Helyes Zs, Szolcsányi J (2006) Inhibitory effect of somatostatin on inflammation and nociception. Pharmacol Ther 112:440–456 [DOI] [PubMed] [Google Scholar]
- Reisine T, Bell GI (1995) Molecular properties of somatostatin receptors. Neuroscience 67:777–790 [DOI] [PubMed] [Google Scholar]
- Savov JD, Gavett SH, Brass DM, Costa DL, Schwartz DA (2002) Neutrophils play a critical role in development of LPS-induced airway disease. Am J Physiol Lung Cell Mol Physiol 283:L952–962 [DOI] [PubMed] [Google Scholar]
- Schloos J, Raulf F, Hoyer D, Bruns C (1997) Identification and pharmacological characterization of somatostatin receptors in rat lung. Br J Pharmacol 121:963–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szolcsányi J, Helyes Z, Oroszi G, Németh J, Pintér E (1998a) Release of somatostatin and its role in the mediation of the anti-inflammatory effect induced by antidromic stimulation of sensory fibres of rat sciatic nerve. Br J Pharmacol 123:936–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szolcsányi J, Pintér E, Helyes Z (2004) Sensocrine function of capsaicin-sensitive nociceptors mediated by somatostatin regulates against inflammation and hyperalgesia. In Handwerker HO, Brune K, eds. Hyperalgesia: Molecular Mechanisms and Clinical Implications. Seattle, IASP Press, 113–128
- Szolcsányi J, Pintér E, Helyes Z, Oroszi G, Németh J (1998b) Systemic anti-inflammatory effect induced by counter-irritation through a local release of somatostatin from nociceptors. Br J Pharmacol 125:916–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniyama Y, Suzuki T, Mikami Y, Moriya T, Satomi S, Sasano H (2005) Systemic distribution of somatostatin receptor subtypes in human: an immunohistochemical study. Endocr J 52:605–611 [DOI] [PubMed] [Google Scholar]
- ten Bokum AM, Hofland LJ, van Hagen PM (2000) Somatostatin and somatostatin receptors in the immune system: a review. Eur Cytokine Netw 11:161–176 [PubMed] [Google Scholar]
- ten Bokum AM, Lichtenauer-Kaligis EG, Melief MJ, van Koetsveld PM, Bruns C, van Hagen PM, Hofland LJ, et al. (1999) Somatostatin receptor subtype expression in cells of the rat immune system during adjuvant arthritis. J Endocrinol 161:167–175 [DOI] [PubMed] [Google Scholar]
- Weckbecker G, Lewis I, Albert R, Schmid HA, Hoyer D, Bruns C (2003) Opportunities in somatostatin research: biological, chemical and therapeutic aspects. Nat Rev Drug Discov 2:999–1017 [DOI] [PubMed] [Google Scholar]
- Wright JL, Cosio M, Churg A (2008) Animal models of chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol 295:L1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]