Abstract
Human small intestine accounts for 75% of the gastrointestinal (GI) length but for only 1–5% of GI tumors. The reason remains as yet unclearly understood. Our study was designed to examine whether increased apoptosis and expression of related genes/proteins, especially those of the Bcl-2 family, contribute to this difference. For this purpose, 77 samples from patients were examined by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling and immunohistochemistry, including 40 cases from normal small intestine (jejunum), 7 cases from jejunum and ileum adenocarcinomas, and 30 cases from normal colon. The results showed that a significantly higher level of enterocyte apoptosis was observed in normal small intestine compared with small intestinal adenocarcinomas and normal colon (median of apoptotic index, 15.2% vs 0.1% and 1.6%, p<0.01). A similar pattern was observed for Bax (expression-positive, 77.5% vs 28.6% and 53.3%, p<0.05) but not for Bcl-2 (42.5% vs 42.9% and 46.7%, p>0.05) or Bax/Bcl-2 ratio (percent of samples having a ratio ≥1, 45.0% vs 14.3% and 36.7%, p>0.05). In conclusion, increased apoptosis and expression of Bax, not Bcl-2 or the Bax/Bcl-2 ratio, may play some role in the relatively lower incidence of human small intestinal carcinomas. However, more studies are required for a better understanding of these changes. (J Histochem Cytochem 57:1139–1148, 2009)
Keywords: small intestine, adenocarcinoma, apoptosis, Bax, Bax/Bcl-2 ratio
Although human small intestine represents 75% of the length and over 90% of the mucosal surface of the alimentary tract, it is the site of only ∼2% of malignant tumors of the entire gastrointestinal (GI) tract (Brueckl et al. 2004; Singhal and Singhal 2007). Adenocarcinoma is the most common histological subtype, accounting for ∼40% of all malignant small intestinal tumors. The infrequent occurrence of small intestinal tumors is accompanied by nonspecific clinical symptoms when compared with malignancies of the stomach and colon (Singhal and Singhal 2007). Apoptosis, the major physiological mechanism of cell death, plays important roles in the maintenance of homeostasis in tissues. Decreased apoptosis disturbs the balance between cell proliferation and cell death, leading to the increased susceptibility to occurrence of tumors (Thompson 1995; Melino 2001). It is likely that increased apoptosis in human small intestine, especially the jejunum and ileum, may contribute to the decreased occurrence of tumors. Identifying the specific genes that are responsible for this difference may provide a new approach to the treatment of GI tumors.
Apoptosis is controlled by a large number of genes acting as death switches. Among them, the most crucial regulators are members of the Bcl-2 gene family. Bcl-2 was originally discovered as a chromosomal translocation in B-cell follicular lymphoma. It has the ability to block a wide variety of apoptotic signals (Adams and Cory 1998), and its expression has been reported in such neoplasms as breast, prostate, and thyroid carcinoma, and small-cell and large-cell carcinoma (LaPoint et al. 2007) of the lung. It has been validated that Bcl-2 can block the release of cytochrome C, a factor that is necessary for the activation of caspases that mediate apoptosis (Rosse et al. 1998).
Bax was the first protein to be isolated that showed homology with Bcl-2 throughout two highly conserved regions. It is also considered the representative pro-apoptotic protein/gene of the Bcl-2 family. Gene products of the Bcl-2 family can form homo- and heterodimers with each other. Bax can dimerize with itself or with Bcl-2 and, when overproduced, Bax homodimers promote apoptosis. In contrast, when Bcl-2 is in excess, Bcl-2 homodimers predominate and cells are protected from death (Oltvai et al. 1993). The ratio of Bax to Bcl-2 expression represents a cell death switch, which determines the life or death of cells in response to an apoptotic stimulus; an increased Bax/Bcl-2 ratio decreases the cellular resistance to apoptotic stimuli, leading to increased cell death and reduced incidence of tumors (Vaskivuo et al. 2002).
Given the very limited number of experimental studies on apoptosis in human small intestinal adenocarcinoma, especially from the jejunum and ileum, our study was designed to determine whether the increased apoptosis protects the human small intestine from the occurrence of cancers.
Materials and Methods
Patients and Tissue Sections
A total of 77 patients treated at our department (Peking University Third Hospital, Beijing, China) in the period January 2003 to September 2007 were included in our study, either hospitalized or treated on an outpatient basis, with a diagnosis indicating approximately normal or adenocarcinomas in the locations of small intestine (jejunum and ileum) and colon through clinical, biochemical, and pathological diagnosis, as well as radiodiagnosis and colonoscopy. This included patients who fulfilled the following criteria: (1) The specimens from small intestine (jejunum and ileum) or large intestine (colon) could be obtained through intubation of double-contrast small intestinal radiography or double-balloon enteroscopy, colonoscopy, or surgery that was only for carcinomas; (2) specimens that could only be acquired from duodenum, cecum, or rectum were excluded; and (3) absence of the following diseases, including any malignancy except for small intestinal adenocarcinomas: cardiovascular diseases, chronic pulmonary diseases, cerebrovascular diseases, rheumatological diseases, and serious renal diseases.
Our research was approved by the Peking University committee on human experiments and was carried out in accordance with the Helsinki Declaration. The final 77 specimens included 40 cases of normal jejunum mucosa, 7 cases of small intestinal (jejunum and ileum) adenocarcinomas, and 30 cases of normal colonic mucosa. The ages of patients with normal small intestine varied from 25 to 77 years (mean, 49.9 years). The clinical and pathological characteristics of patients with small intestinal carcinomas are described in detail in the Results section. Some formalin-fixed and paraffin-embedded (FFPE) tissue sections were provided by the pathology laboratory of the Gastroenterology Department, Peking University Third Hospital. All fresh tissues were fixed immediately in 10% neutral buffered formalin for 24 hr, dehydrated in ethanol, cleared in xylene, and embedded in paraffin blocks. Four-micron-thick representative tissue sections were cut, mounted on poly-lysine-coated slides, and dried at 60C in an oven for 1 hr before use.
Materials
Materials for the terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining assay, including the in situ cell death detection kit, proteinase K, and DNase I, were purchased from Roche Molecular Biochemicals (Mannheim, Germany). Monoclonal anti-Bax Ab-1, specially prepared for immunohistochemistry (IHC), was obtained from Lab Vision (Fremont, CA). Monoclonal anti-Bcl-2 was from Sigma (Saint Louis, MO), and a 1:600 dilution was used for IHC staining. The Ultravision detection system anti-mouse horseradish peroxidase/DAB was supplied by Lab Vision. Poly-l-lysine, phosphate-buffered saline (PBS) buffer, and all other routine reagents were from Zhongshan (Beijing, China). For the antigen retrieval of IHC, a microwave oven operating at a frequency of 2.45 GHz with six power level settings was used at the highest power setting (720 W).
TUNEL Staining
To identify the apoptotic cells in paraffin sections, TUNEL staining was carried out strictly according to the manufacturer's instructions using in situ cell death detection kits. Briefly, paraffin sections were dewaxed and rehydrated through xylene and an alcohol series. The permeability of cell membranes was increased by incubating the sections in 50 μl proteinase K (10 μg/ml) at 37C for 10 min. They were then incubated with 50 μl terminal deoxynucleotidyl transferase buffer at 37C for 60 min in a moist chamber after three rinses with PBS buffer. Incorporated fluorescein was detected immediately and directly by fluorescence microscopy. The sections that were pretreated with DNase I (3000 U/ml) served as positive control, and the sections stained without deoxynucleotide substrate served as negative control.
For each specimen, cells with positive nuclei staining from five to ten microscopic fields (×400) were counted. The total number of glandular cells was counted using light microscopy and ×400 magnification. Data were expressed as apoptotic index (AI), which is the mean of TUNEL-positive cells per microscopic field.
IHC
Paraffin sections were deparaffinized in xylene and rehydrated gradually through a series of graded alcohols. Pretreatment with diluted 3% hydrogen peroxide in methanol was omitted, following our previous publication, which showed that microwave oven heat–induced antigen retrieval can block the endogenous peroxidase in paraffin sections (Gao et al. 2008). IHC staining was performed following the manufacturer's instructions. The paraffin sections for positive control were provided by Lab Vision, and sections for negative control were identified in our earlier experiments. Another negative control was obtained by treating tissue sections in the absence of primary antibodies.
Immunostained sections were evaluated in a blind fashion by the authors, with the help of a pathologist. If scoring results diverged, agreement was reached in a joint session. Immunopositivity was scored from grade 2 to grade 7 by increasing the extent of the grade of immunostaining (1, <25%; 2, 25%–50%, 3, 50%–75%; 4, 75%–100%) through the addition of the staining intensity grade [1 (mild), 2 (moderate), and 3 (strong) immunoreactivity]. The final grades were expressed as 2–3 (mild or weak), 4–5 (moderate), and 6–7 (strong).
Statistical Analysis
For the AI, the medians and inter-quartile ranges were described in the three independent groups. For other variables, including age, sex, original location of biopsies, results of radiodiagnosis, colonoscopy, and pathological diagnosis, positive expression of two proteins, and percent of samples that have a Bax/Bcl-2 ratio ≥1 in each group, the data were presented as proportions or ratios. Comparisons between continuous variables were performed using the Mann-Whitney non-parametric U test. Comparisons between proportions or ratios were performed using Pearson's χ2 tests, continuity correction χ2 tests, or Fisher's exact tests, respectively, depending on the total samples and different conditions. For all tests, p<0.05 was considered statistically significant. The analysis was performed using SPSS version 13.0 (SPSS, Inc.; Chicago, IL).
Results
Baseline Characteristics of Patients
Seventy-seven patients following the inclusion criteria were enrolled in our study. The baseline characteristics of patients are presented, including age, sex, original location of biopsies, results of radiodiagnosis, colonoscopy, and pathologic diagnosis, in Table 1. No significant differences were observed in comparison of demographic data, including age and sex, between the three groups. For the results of pathological diagnosis, more mild inflammation appeared in the group of normal small intestine than in the normal colonic mucosa. The reason was inferred to be related to the tissues themselves, although the patients had been controlled strictly. The seven patients with small intestinal adenocarcinomas included five men and two women. The clinical and pathological characteristics were demonstrated, including gender, age, tumor location, tumor size, depth of tumor, serosal exposure, tumor nodes metastasis (TNM) stage, general classification, histological type, nodal metastasis, and distant metastasis (Table 2).
Table 1.
Baseline characteristics of patients
| Normal SI (n=40) | SI cancers (n=7) | Normal colon (n=30) | p value | |
|---|---|---|---|---|
| Age (years) | ||||
| ≥55 | 17 | 4 | 19 | >0.05b |
| <55 | 23 | 3 | 11 | — |
| Sex | ||||
| Male | 18 | 5 | 19 | >0.05b |
| Female | 22 | 2 | 11 | — |
| Location | ||||
| Jejunum | 40 | 6 | — | |
| Ileum | — | 1 | — | |
| Colona | — | — | 30 | |
| Radiodiagnosis | ||||
| Normal | 40 | — | — | |
| Neoplasma | — | 7 | — | |
| Colonoscopy | ||||
| Normal | — | — | 22 | |
| Polyp | — | — | 8 | |
| Pathological diagnosis | ||||
| Normal | 11 | — | 18 | 0.006b |
| Mild inflammation | 29 | — | 12 | — |
| Adenocarcinoma |
— |
7 |
— |
|
Thirty cases from the human colon included 10 cases of the ascending colon, 8 cases of the transverse colon, 7 cases of the descending colon, and 5 cases of the sigmoid colon.
For the comparisons of age and sex, statistical analysis was conducted among the three dependent groups, and the lowest p value was demonstrated in the above table. For the pathological diagnosis, only the normal small intestine group and normal colon group were analyzed; the small intestinal cancer group was excluded.
SI, small intestine or small intestinal.
Table 2.
Clinical and pathological characteristics of patients with small intestinal adenocarcinomas
| Patient | A | B | C | D | E | F | G |
|---|---|---|---|---|---|---|---|
| Gender | Male | Male | Male | Male | Female | Female | Male |
| Age (years) | 43 | 39 | 56 | 74 | 73 | 77 | 41 |
| Tumor location | Lower jejunum | Upper jejunum | Upper jejunum | Lower jejunum | Upper ileum | Lower jejunum | Upper jejunum |
| Tumor size (cm) | 10 × 8.0 × 1.7 | 4.0 | 6.0 | 4.0 | 6.0 | 5.0 × 4.5 × 2.0 | 8.0 × 5.0 × 4.0 |
| Depth of tumor | Full thickness | Full thickness | Full thickness | Full thickness | Full thickness | Full thickness | Full thickness |
| Serosal exposure | Yes | Yes | No | Yes | No | No | No |
| TNM stage | Stage III | Stage III | Stage II | Stage IV | Stage IV | Stage IV | Stage III |
| General classification | Ulcer type | Narrow type | Ulcer type | Ulcer type | Ulcer type | Protruded type | Protruded type |
| Histological type | PDA | PDA, some SRCA | MuA, some SRCA | MDTuA | MDA | MDA, some MuA | MDA, some MuA |
| Nodal metastasis (present/total) | 5/15 | 5/28 | 0/5 | 2/10 | 0/1 | 0/3 | 3/7 |
| Distant metastasis |
Absent |
Absent |
Absent |
Present |
Present |
Present |
Absent |
MDA, moderately differentiated adenocarcinoma; MDTuA, moderately differentiated tubular adenocarcinoma; MuA, mucinous adenocarcinoma; PDA, poorly differentiated adenocarcinoma; SRCA, signet-ring cell carcinoma; TNM, tumor nodes metastasis.
Cell Apoptosis: Significant Increased Level in Normal Small Intestine
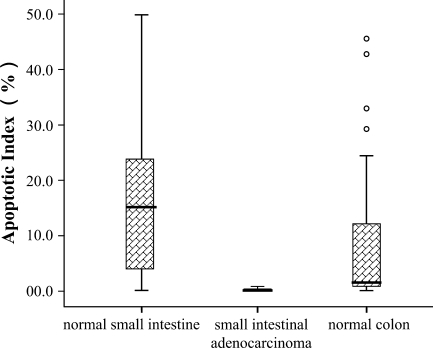
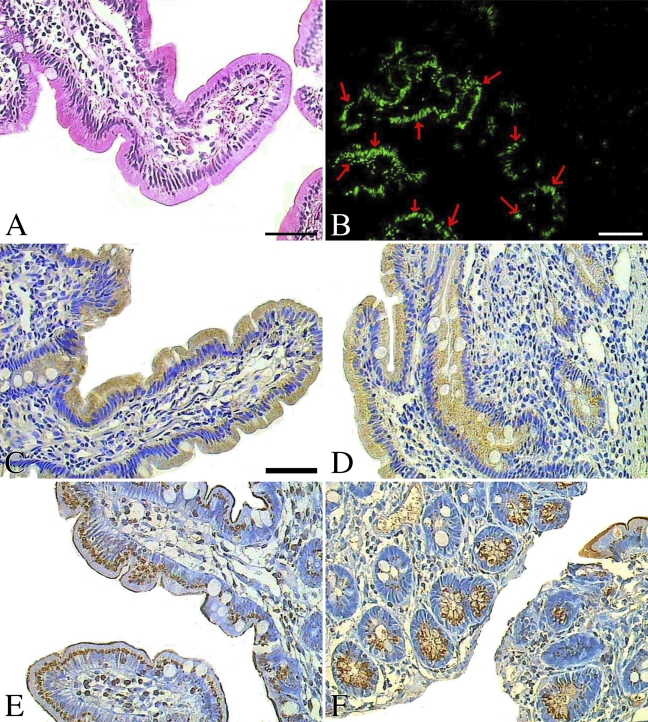
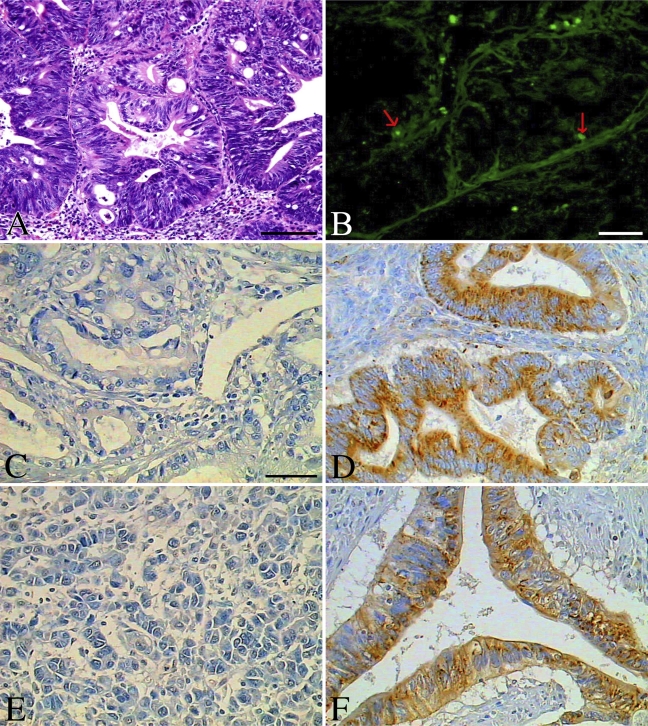
We found that more apoptotic cells (the median is 15.2% and inter-quartile is 20.9% for the AI) were demonstrated in normal human small intestine than in small intestinal adenocarcinoma (the median is 0.1% and inter-quartile is 0.4%) or in normal colonic mucosa (the median is 1.6% and inter-quartile is 12.3%) (Figure 1). The maximum AI in small intestinal adenocarcinoma was no more than 0.9%, whereas the AI in normal mucosal tissues of the small intestine and colon was 49.9% and 32.8%, respectively (Figures 1, 2B, 3B, and 4B). The number of apoptotic cells was increased significantly in normal human small intestinal mucosa, compared with small intestinal adenocarcinomas (p<0.01) or with normal colonic mucosa (p<0.01). For the small intestinal adenocarcinomas, very few positive cells could be observed, and they were scattered across different microscopic fields (×400) (Figure 3B). For the normal small intestinal tissues in which the AI was over 5%, apoptotic cells could be observed in almost all microscopic fields, including the surface epithelial cells of the villus and the lower part or base of the crypts (Figure 2B). However, in the normal small intestinal sections in which the AI was less than 5%, apoptotic cells were present mostly in the surface epithelial cells of the villus, especially in the tips of villus.
Figure 1.
Apoptotic index (median and inter-quartile range) in normal small intestine, small intestinal adenocarcinomas, and normal colon: p<0.01 for comparisons between normal small intestine and small intestinal adenocarcinomas or normal colon. Error bars indicate full ranges; circles above normal colon indicate the four exceptional values.
Figure 2.
Apoptosis by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) and related Bax and Bcl-2 by immunohistochemical analysis in normal human small intestine. (A) Hematoxylin-eosin staining of small intestine. (B) Apoptotic cells detected by the in situ cell death kits (red arrows indicate the representative apoptotic cells). (C) Bax was observed in the surface epithelial cells of the villus from the normal small intestine. (D) Bax was observed in both the villus and the crypts in some cases of normal small intestine. (E,F) Bcl-2 expression in the normal small intestine. Bars: B = 80 μm; A,C–F = 40 μm.
Figure 3.
Apoptosis by TUNEL and related Bax and Bcl-2 by immunohistochemistry in small intestinal adenocarcinomas. (A) Hematoxylin-eosin staining of one cancer case. (B) Apoptotic cells by TUNEL (red arrows indicate the representative apoptotic cells). (C) Bax was absent in most small intestinal cancers. (D) Bax was strong in a case of human small intestinal adenocarcinoma. (E) Bcl-2 was absent in most small intestinal adenocarcinomas. (F) Bcl-2 expression was strong in a case of small intestinal adenocarcinoma. Bars: A,B = 80 μm; C–F = 40 μm.
Figure 4.
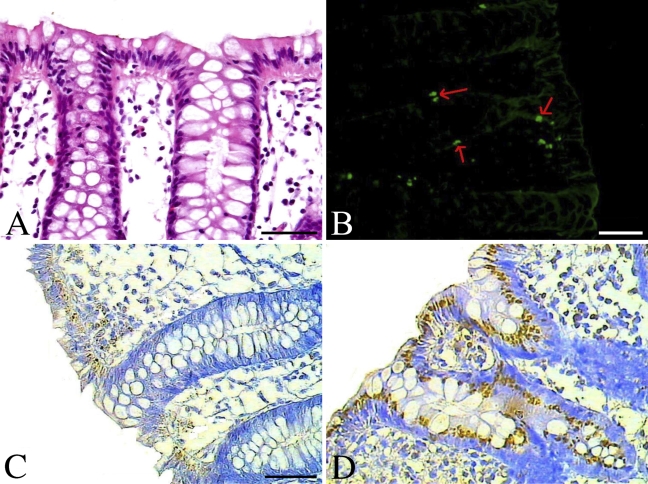
Apoptosis by TUNEL and related Bax and Bcl-2 by immunohistochemistry in normal colon. (A) Hematoxylin-eosin staining. (B) Apoptotic cells by TUNEL (red arrows indicate the representative apoptotic cells). (C) Expression of protein Bax in normal human colon. (D) Bcl-2 expression in normal colon. Bars: B = 80 μm; A,C,D = 40 μm.
Expression of Bax: Increased Significantly in Normal Small Intestine
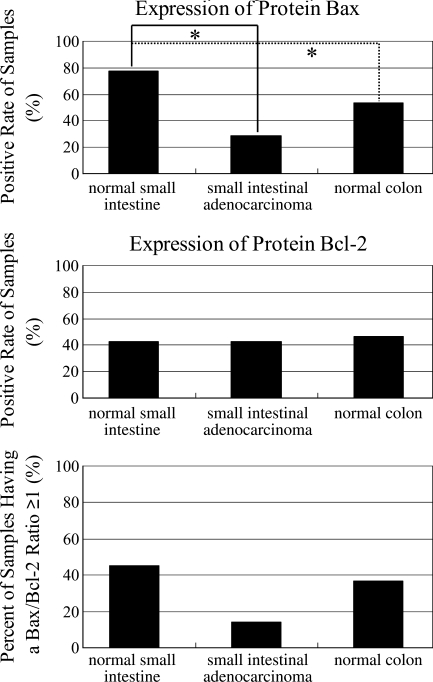
We found that Bax protein was detected in 31 cases of normal small intestine (31/40, 77.5%), 2 cases of small intestinal adenocarcinoma (2/7, 28.6%), and 16 cases of normal colon (16/30, 53.3%) (Figure 5A). Among the positive sections, the expression of Bax was moderate in 12 cases of normal small intestine (12/40, 30%), 0 case of small intestinal adenocarcinoma (0/7, 0%), and 7 cases of normal colon (7/30, 23.3%), whereas Bax was strong in 7 cases of normal small intestine (7/40, 17.5%), 1 case of small intestinal adenocarcinoma (1/7, 14.3%), and 3 cases of normal colon (3/30, 10%) (Table 3). Statistically, the expression of Bax was higher in normal small intestine than in small intestinal adenocarcinoma (p<0.05) and higher than in normal colon (p<0.05). For the intensity of expression, no significant differences were observed among the three groups (Table 3). The increased expression of protein Bax may play some role in the lower incidence of human small intestinal carcinoma.
Figure 5.
Proportions with positive expression of Bax and Bcl-2, and percent of samples that have a Bax/Bcl-2 ratio ≥1 in normal human small intestine, small intestinal adenocarcinoma, and normal colon. The asterisks represent statistically significant differences at p=0.05.
Table 3.
Expression of proteins Bax and Bcl-2
| Normal SI (n=40) |
SI cancers (n=7) |
Normal colon (n=30) |
p valuea |
|||
|---|---|---|---|---|---|---|
| A | B | C | A, B | A, C | B, C | |
| Bax | ||||||
| Negative | 9 | 5 | 14 | 0.031 | 0.033 | 0.405 |
| Weak | 12 | 1 | 6 | 0.690 | 0.343 | 1.000 |
| Moderate | 12 | 0 | 7 | — | 0.535 | — |
| Strong | 7 | 1 | 3 | 1.000 | 0.588 | 1.000 |
| Bcl-2 | ||||||
| Negative | 23 | 4 | 16 | 1.000 | 0.728 | 1.000 |
| Weak | 0 | 1 | 5 | — | — | 1.000 |
| Moderate | 1 | 0 | 7 | — | 0.020 | — |
| Strong |
16 |
2 |
2 |
0.879 |
0.002 |
0.155 |
Three groups were compared using Pearson χ2 tests, continuity correction χ2 tests, or Fisher's exact tests, respectively, depending on the total samples and different conditions. The analysis was omitted if one cell was zero.
We also found that expression of Bax was limited to the endoplasm of surface epithelial cells, mostly at the basal side of normal mucosal tissue sections from the colon (Figure 4C). In Bax-positive small intestinal adenocarcinoma, Bax was widely distributed in the endoplasm of carcinoma cells (Figures 3C and 3D). However, in the normal human small intestine, Bax protein was mostly located at the free edge of the endoplasm in the surface epithelial cells of the villus, especially in the tips of the villus (Figure 2C), although in some cases, Bax was detected in the endoplasm of the lower part or base of crypts, and the intensity was much lower than in the surface epithelial cells of the villus (Figure 2D).
Expression of Bcl-2: No Statistical Differences Were Observed
Bcl-2-positive cells were observed in 17 cases of normal small intestine (17/40, 42.5%), 3 cases of small intestinal adenocarcinoma (3/7, 42.9%), and 14 cases of normal colon (14/30, 46.7%) (Figure 5B). Bcl-2 expression was strong in 16 cases of normal small intestine (16/40, 40%), 2 cases of small intestinal adenocarcinoma (2/7, 28.6%), and 2 cases of normal colon (2/30, 6.7%), and moderate in 1 case of normal small intestine (1/40, 2.5%), 0 case of small intestinal adenocarcinoma (0/7, 0%), and 7 cases of normal colon (7/30, 23.3%) (Table 3). Although the intensity of expression seems stronger in normal small intestine than in small intestinal adenocarcinoma (Table 3), no significant difference for positive expression was observed statistically.
Bcl-2 protein was observed mostly in the endoplasm and partly in the nuclear envelope or cell membrane. Although Bcl-2 was mostly detected in the endoplasm of the lower part or base of crypts in normal small intestine, it was also observed in the surface epithelial cells of the villus and at the lower part or base of the crypts in some cases (Figures 2E and 2F). The expression of protein Bcl-2 in normal colon was similar to expression in normal small intestine and was found in cells of the surface epithelia and the crypts (Figure 4D). However, it was mostly at the basal side of the endoplasm, not at the free edge, as expression of Bax was. In expression-positive small intestinal adenocarcinoma, Bcl-2 could be detected widely in the endoplasm, nuclear envelope, and cell membrane of carcinoma cells (Figures 3E and 3F), but the intensity was relatively lower than that of protein Bax.
Significance of the Ratio of Protein Bax to Bcl-2
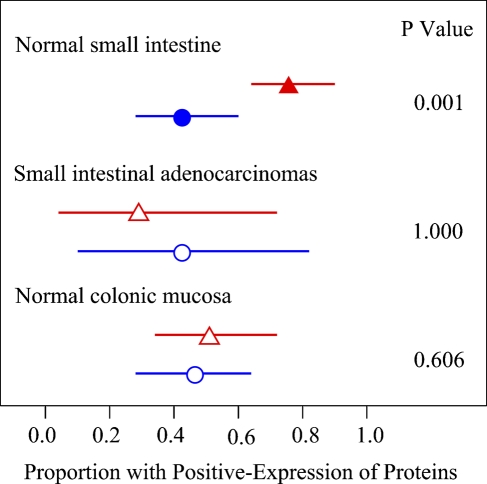
Because proteins Bax and Bcl-2 have opposite functions, we compared the rates of positive staining of two proteins in the three groups. The results showed that expression of protein Bax was stronger than that of Bcl-2 in the normal small intestine (p<0.01). However, no significant changes were shown in small intestinal adenocarcinoma and normal colon groups (Figure 6). This phenomenon also indicates that Bax may play some role in the relatively lower incidence of human small intestinal carcinoma.
Figure 6.
Expression of proteins Bax and Bcl-2 in each group of tissues. Observed proportions are shown by red triangles for the protein Bax and blue circles for Bcl-2. Horizontal lines show the 95% confidence interval. Solid symbols represent statistically significant differences at p=0.05.
Finally, the Bax/Bcl-2 ratio was compared among the three groups on the basis of the specific function of the ratio for apoptosis reported by previously published studies. Cases were categorized as either Bax/Bcl-2 ≥ 1 or Bax/Bcl-2 < 1 in each group. The numbers of cases of Bax/Bcl-2 ≥ 1 were 18 of normal small intestine (18/40, 45.0%), 1 of small intestinal adenocarcinoma (1/7, 14.3%), and 11 of normal colon (11/30, 36.7%) (Figure 5C). Although the raw data showed that the Bax/Bcl-2 ratio in human normal small intestine was higher than in small intestinal adenocarcinoma or normal colon, no significant difference was obtained statistically.
Discussion
Our study showed the appearance of apoptosis and expression of two related proteins, Bax and Bcl-2, in human small intestinal adenocarcinomas, especially from the jejunum. The onset and development of many carcinomas have been linked to the dysfunction of apoptosis. However, few studies have reported the changes of apoptosis and related proteins in small intestinal adenocarcinoma (Shibahara et al. 1995; Moss et al. 1996; Groos et al. 2001; Ciccocioppo et al. 2002). In our study, the samples from duodenum were excluded, although it is the most common site of small intestinal adenocarcinomas. From a purely anatomical point of view, the duodenum is part of the small intestine; nonetheless, it is functionally and pathophysiologically more closely related to the stomach, not to the jejunum and ileum. Recently, the American Gastroenterological Association Institute has recommended that the GI tract should be reclassified into three parts (upper, mid, and lower), instead of adhering to the traditional classification of upper and lower GI tract. The site above the ampulla of Vater is defined as upper GI tract; from the ampulla of Vater to the terminal ileum is defined as mid GI tract; and the left is defined as lower GI tract. It is thought that this change may be useful in improving our understanding of digestive diseases. For better understanding of the question posed and to achieve better results, the duodenum was excluded from our study.
Perhaps it is for this reason that we obtained better results, even though a very limited number of small intestinal adenocarcinomas were examined in our study. For the AI, a significant difference was observed when small intestinal cancer was compared with normal small intestine. For normal small intestine, the median and maximal values of the AI were 15.2% and 49.9%, respectively, whereas for adenocarcinomas, these values were 0.1% and 0.9%, respectively (Figures 1, 2B, and 3B). A similar pattern was observed for expression of Bax, which can be detected in 77.5% and 28.6% of samples for normal small intestine and adenocarcinomas, respectively (Figure 5A; Table 3). However, for expression of protein Bcl-2, no difference was observed, and the p value was 1.000 (Figure 5B; Table 3). Considering these results, more samples were thought to be unnecessary. Moreover, the human small intestinal adenocarcinoma is very rare, and it would be difficult to be improve the number greatly. We wish that these results could be validated in more samples.
In our study, we observed that most TUNEL-positive cells were located in the villus of normal small intestine, which was similar to findings in other studies, although the majority of those specimens were from duodenum (Shibahara et al. 1995; Groos et al. 2001), whereas ours were from the jejunum. However, some positive cells can be observed in the lower portion or base of crypts. It is thought that this may be related to pathological conditions, because the changes in apoptosis under certain pathological conditions have been investigated (Moss et al. 1996; Ciccocioppo et al. 2002; An et al. 2005). In a study on patients with active celiac disease, apoptotic cells were found to be distributed throughout the crypt–villus unit (Moss et al. 1996). In ischemia–reperfusion injury in animal experiments, apoptotic cells were increased in all parts of the intestine (villous epithelium, crypt epithelium, and stroma) after reperfusion (An et al. 2005). In a study in elderly subjects, apoptotic cells of duodenal enterocyte were increased significantly compared with other adults (15.3% vs 2.1%) (Ciccocioppo et al. 2002). The value of 15.3% was almost equal to the result from our experiments on the normal small intestine (15.9%; mean age was 49.9 years), although the majority of our specimens were from the jejunum, whereas theirs were from the duodenum. Taking these findings together, perhaps we can deduce that age may affect the AI and that apoptosis may be related to such conditions as age and pathological condition.
The conclusion from our studies, that increased apoptosis may play a role in the relatively lower incidence of human small intestinal adenocarcinomas, was also supported by published studies and reports on human large intestine (Bedi et al. 1995; Wincewicz et al. 2007) and animal small intestine. Results from human colorectal cancers show that decreased apoptosis may contribute to tumor growth and promote neoplastic progression, consistent with reports from experiments on animals and cultured cells (Keefe et al. 2000; Hong et al. 2005). In a study on intestinal crypts after chemotherapy, Keefe et al. (2000) found that apoptotic cells were increased significantly. Another study demonstrated that the small intestine may be more protected against cancer by having a more dynamic response to oxidative damage, whereas in large intestine, the response makes it more susceptible to loss of crypt architecture (Hong et al. 2005). Results from studies on animal ischemia–reperfusion injury also showed that the differing recovery capability of intestinal epithelium may account in part for the different incidence rate of large and small intestinal tumors.
Bax and Bcl-2 are the two representative proteins/genes of the Bcl-2 family. Although they have been tested in some tissues, studies on human small intestine, especially on carcinomas, are very limited (Uesugi et al. 1999; Bowen et al. 2005; Schulmann et al. 2005). Schulmann et al. (2005) showed that the positive expression of Bax was 59% in 32 cases of small bowel cancer associated with colorectal cancer. In one case report on small intestinal adenocarcinoma in a Japanese patient with Crohn's disease, no Bcl-2 expression was observed, which apparently supports our conclusion (Uesugi et al. 1999). Our results showed that no significant change for Bcl-2 was observed between small intestinal adenocarcinoma and normal mucosa, whereas Bax may play some role in the lower occurrence of small intestinal carcinomas. Our conclusion did not contradict these reported studies, because different tissues were examined in the different studies. Moreover, Bowen et al. (2005) found that increased expression of Bax and Bak, but not of other members of the Bcl-2 family, were associated with apoptosis in small intestinal crypts.
Our study failed to provide supporting evidence that the Bax/Bcl-2 ratio is a cell death switch in response to apoptotic stimuli, which has been proven by some studies (Vaskivuo et al. 2002; Lalier et al. 2007). Oltvai et al. (1993) first put forward the concept that the Bcl-2/Bax ratio appears to determine the survival or death of cells following an apoptotic stimulus. This was later proven by studies on different tumors, such as prostatic tumors, rectal cancer (Scopa et al. 2001), bladder cancer, acute myeloid leukemia, and oral cancer (Oshikawa et al. 2006). However, an opposing conclusion, that a high Bcl-2/Bax ratio correlates with a good prognosis, was drawn from a study on childhood acute lymphoblastic leukemia (Narayan et al. 2007). Results from our study provide a third relationship, that the Bax/Bcl-2 ratio may not play a role in the relatively lower incidence of human small intestinal adenocarcinomas.
The major limitation of our study, in addition to the very limited number of human small intestinal adenocarcinomas tested, was that our conclusion was reached based simply on the apoptotic level of tissues by TUNEL and expression of the apoptosis-related proteins Bax and Bcl-2 by immunohistochemistry. In designing the study, we hoped to validate the results using more strategies, especially molecular biological and cellular biological methods, including flow cytometry, immunoblotting, and RT-PCR, even in animal models or culture cell lines. However, no good animal models or cell lines for human small intestine or small intestinal cancers are available, and the fresh tissues from endoscopy were very small and limited and supplemented by FFPE tissue sections. But we did provide relatively sufficient evidence for the results and conclusion. We wish that these could be validated in more human samples and by more methods.
In conclusion, the increased level in the appearance of apoptosis and in the expression of Bax, but not Bcl-2 or the Bax/Bcl-2 ratio, may have some role in the relative rareness of human small intestinal adenocarcinoma. However, more studies are required for a better understanding of these changes.
Acknowledgments
This work was supported by grant 30571810 from the National Natural Science Foundation of China.
We thank Dr. Liang Chen, Departments of Pathology and Laboratory Medicine, Indiana University School of Medicine, for helpful suggestions, Dr. Ying-Zi Chang for English improvement, Dr. Zhu Jin for her help in evaluating the sections, and Ya-Jing Han and others in the pathology laboratory and endoscopic center for their kind help.
References
- Adams JM, Cory S (1998) The Bcl-2 protein family: arbiters of cell survival. Science 281:1322–1326 [DOI] [PubMed] [Google Scholar]
- An S, Hishikawa Y, Koji T (2005) Induction of cell death in rat small intestine by ischemia reperfusion: differential roles of Fas/Fas ligand and Bcl-2/Bax systems depending upon cell types. Histochem Cell Biol 123:249–261 [DOI] [PubMed] [Google Scholar]
- Bedi A, Pasricha PJ, Akhtar AJ, Barber JP, Bedi GC, Giardiello FM, Zehnbauer BA, et al. (1995) Inhibition of apoptosis during development of colorectal cancer. Cancer Res 55:1811–1816 [PubMed] [Google Scholar]
- Bowen JM, Gibson RJ, Keefe DM, Cummins AG (2005) Cytotoxic chemotherapy upregulates pro-apoptotic Bax and Bak in the small intestine of rats and humans. Pathology 37:56–62 [DOI] [PubMed] [Google Scholar]
- Brueckl WM, Heinze E, Milsmann C, Wein A, Koebnick C, Jung A, Croner RS, et al. (2004) Prognostic significance of microsatellite instability in curatively resected adenocarcinoma of the small intestine. Cancer Lett 203:181–190 [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Di-Sabatino A, Luinetti O, Rossi M, Cifone MG, Corazza GR (2002) Small bowel enterocyte apoptosis and proliferation are increased in the elderly. Gerontology 48:204–208 [DOI] [PubMed] [Google Scholar]
- Gao C, Wang AY, Han HY (2008) Microwave antigen retrieval blocks endogenous peroxidase activity in immunohistochemistry. Appl Immunohistochem Mol Morphol 16:393–399 [DOI] [PubMed] [Google Scholar]
- Groos S, Hunefeld G, Luciano L (2001) Epithelial cell turnover–extracellular matrix relationship in the small intestine of human adults. Ital J Anat Embryol 106(2 suppl 1):353–361 [PubMed] [Google Scholar]
- Hong MY, Turner ND, Carroll RJ, Chapkin RS, Lupton JR (2005) Differential response to DNA damage may explain different cancer susceptibility between small and large intestine. Exp Biol Med 230:464–471 [DOI] [PubMed] [Google Scholar]
- Keefe DM, Brealey J, Goland GJ, Cummins AG (2000) Chemotherapy for cancer causes apoptosis that precedes hypoplasia in crypts of the small intestine in humans. Gut 47:632–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalier L, Cartron PF, Juin P, Nedelkina S, Manon S, Bechinger B, Vallette FM (2007) Bax activation and mitochondrial insertion during apoptosis. Apoptosis 12:887–896 [DOI] [PubMed] [Google Scholar]
- LaPoint RJ, Bourne PA, Wang HL, Xu H (2007) Coexpression of c-kit and bcl-2 in small cell carcinoma and large cell neuroendocrine carcinoma of the lung. Appl Immunohistochem Mol Morphol 15:401–406 [DOI] [PubMed] [Google Scholar]
- Melino G (2001) The Sirens' song. Nature 412:23. [DOI] [PubMed] [Google Scholar]
- Moss SF, Attia L, Scholes JV, Walters JR, Holt PR (1996) Increased small intestinal apoptosis in coeliac disease. Gut 39:811–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan S, Chandra J, Sharma M, Naithani R, Sharma S (2007) Expression of apoptosis regulators Bcl-2 and Bax in childhood acute lymphoblastic leukemia. Hematology 12:39–43 [DOI] [PubMed] [Google Scholar]
- Oltvai ZN, Milliman CL, Korsmeyer SJ (1993) Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74:609–619 [DOI] [PubMed] [Google Scholar]
- Oshikawa T, Okamoto M, Ahmed SU, Tano T, Sato M (2006) The relationship between gene expression of Bcl-2 and Bax and the therapeutic effect in oral cancer patients. Gan To Kagaku Ryoho 33:1723–1725 [PubMed] [Google Scholar]
- Rosse T, Olivier R, Monney L, Rager M, Conus S, Fellay I, Jansen B, et al. (1998) Bcl-2 prolongs cell survival after Bax-induced release of cytochrome c. Nature 391:496–499 [DOI] [PubMed] [Google Scholar]
- Schulmann K, Brasch FE, Kunstmann E, Engel C, Pagenstecher C, Vogelsang H, Kruger S, et al. (2005) HNPCC-associated small bowel cancer: clinical and molecular characteristics. Gastroenterology 128:590–599 [DOI] [PubMed] [Google Scholar]
- Scopa CD, Vagianos C, Kardamakis D, Kourelis TG, Kalofonos HP, Tsamandas AC (2001) Bcl-2/bax ratio as a predictive marker for therapeutic response to radiotherapy in patients with rectal cancer. Appl Immunohistochem Mol Morphol 9:329–334 [DOI] [PubMed] [Google Scholar]
- Shibahara T, Sato N, Waguri S, Iwanaga T, Nakahara A, Fukutomi H, Uchiyama Y (1995) The fate of effete epithelial cells at the villus tips of the human small intestine. Arch Histol Cytol 58:205–209 [DOI] [PubMed] [Google Scholar]
- Singhal N, Singhal D (2007) Adjuvant chemotherapy for small intestine adenocarcinoma. Cochrane Database Syst Rev 3:CD005202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CB (1995) Apoptosis in the pathogenesis and treatment of disease. Science 267:1456–1462 [DOI] [PubMed] [Google Scholar]
- Uesugi H, Mitomi H, Sada M, Takahashi H, Kobayashi K, Igarashi M, Katsumata T, et al. (1999) A case of adenocarcinoma of the small intestine in a Japanese patient with Crohn disease: a report with immunohistochemical and oncogenic analyses. Scand J Gastroenterol 34:1162–1167 [DOI] [PubMed] [Google Scholar]
- Vaskivuo TE, Stenback F, Tapanainen JS (2002) Apoptosis and apoptosis-related factors Bcl-2, Bax, tumor necrosis factor-alpha, and NF-kappaB in human endometrial hyperplasia and carcinoma. Cancer 95:1463–1471 [DOI] [PubMed] [Google Scholar]
- Wincewicz A, Sulkowska M, Koda M, Kanczuga-Koda L, Witkowska E, Sulkowski S (2007) Significant coexpression of GLUT-1, Bcl-xL, and Bax in colorectal cancer. Ann N Y Acad Sci 1095:53–61 [DOI] [PubMed] [Google Scholar]