Abstract
Non-human primates (NHPs) offer valuable animal models for basic research into human diseases and for the preclinical validation of new therapeutics. Detailed in situ examination of the involved cell types using immunohistochemistry is often hampered by the lack of cross-reactive antibodies (Abs). In the current study, we have tested a large panel of monoclonal antibodies raised against human leukocyte differentiation and activation markers for cross-reactivity on cryosections of lymphoid tissue from six NHP species. In total, we have tested 130 Abs against 69 antigens expressed in tissues from one great ape species (chimpanzee/Pan troglodytes), two Old World species (rhesus macaque/Macaca mulatta and cynomolgus macaque/Macaca fascicularis), and three New World species (common marmoset/Callithrix jacchus, cotton-top tamarin/Saguinus oedipus, and owl monkey/Aotus triviogatus). We have found a large panel of cross-reactive Abs: 93 of 102 (91%) in chimpanzee, 97 of 125 (78%) in rhesus macaque, 70 of 109 (64%) in cynomolgus macaque, 69 of 116 (60%) in common marmoset, 40 of 81 (49%) in cotton-top tamarin, and 35 of 80 (44%) in owl monkey. The availability of a reliable panel of cross-reactive markers is important to gaining further insight into immunological processes in disease-affected tissues from NHP species. (J Histochem Cytochem 57:1159–1167, 2009)
Keywords: immunology, toxicology, pathology, arthritis, multiple sclerosis, HIV-SIV
Non-human primates (NHPs) provide important experimental models of immune-mediated inflammatory disorders in biomedical research, because of the outbred nature and the phylogenetic proximity with humans. Recent milestones in biotechnology have resulted in the development of species-specific therapeutics, such as humanized antibodies (Abs) or fully human Abs (Lutterotti and Martin 2008), which need to be evaluated in relevant disease models before they can be tested in patients. In cases in which rodent models are precluded by the lack of sufficient cross-reactivity, NHP models provide a useful alternative ('t Hart et al. 2004).
A variety of NHP species are used in biomedicine. Although biomedical research in great apes has been banned from Europe for a few years, chimpanzees have been and still are productively used as models for human immunodeficiency virus (HIV) infection (Rutjens et al. 2003; Heeney et al. 2006) and for the development of certain vaccines, e.g., against hepatitis (Bukh 2004). Old World monkeys, such as rhesus macaques and cynomolgus macaques, are frequently used as experimental models in transplantation (Kean et al. 2006; Haanstra et al. 2007), tuberculosis (Capuano et al. 2003), malaria (Moreno et al. 2008), rheumatoid arthritis (Vierboom et al. 2007), and HIV infection (Ambrose et al. 2007). New World monkeys serve as important tools, for e.g., for Parkinson's disease (Eslamboli 2005), idiotypic colitis (Warren and Watkins 1994), malaria vaccine research (Herrera et al. 2002), and for modeling multiple sclerosis (MS) in experimental autoimmune encephalomyelitis (EAE) ('t Hart et al. 2000).
Studies of immunopathogenic mechanisms in each of these experimental models rely on the availability of reagents and techniques for ex vivo immunodiagnosis, such as flow cytometry analysis or immunohistochemistry. Previously, the cross-reactivity in flow cytometry of anti-human monoclonal antibodies (mAbs) with NHPs has been reported (Neubert et al. 1996; Brok et al. 2001). However, little is known about the cross-reactivity of mAbs on NHP tissue for usage in immunohistochemistry. In this study, we have investigated a set of 130 human-specific mAbs against 69 molecules used in immunohistochemistry for cross-reactivity with lymphoid tissue of six NHP species. The mAbs in this set were reactive against cluster of differentiation (CD) markers, cytokines, and other immunological markers, such as human leukocyte antigen (HLA) and Igs. The selection of CD markers included critical costimulatory molecules against which novel immunotherapeutics, such as CD40 (Laman et al. 1996,2002), are under development. Furthermore, the selection included markers distinguishing leukocyte cell types as T-cells, B-cells, natural killer cells, and macrophages. The selected cytokines, such as interferon-gamma (IFN-γ), interleukin (IL)-12, and IL-17A, play a central role in inflammatory responses. The latter is produced by the newly identified Th17 functional T-cell subset, which is presumed to be critical in several autoimmune diseases, including rheumatoid arthritis and MS (Ivanov and Linden 2009). Furthermore, IL-17A may be involved in neutrophil recruitment (Witowski et al. 2000; Yu et al. 2007).
Materials and Methods
Tissues
For the detection of cross-reactivity, lymph node and/or spleen samples were used. Material from the African chimpanzee (Pan troglodytes), as a representative species of the great apes, was used. The selected Old World species were the rhesus macaque (Macaca mulatta) and the cynomolgus macaque (Macaca fascicularis). As representatives of New World species, the common marmoset (Callithrix jacchus), the cotton-top tamarin (Saguinus oedipus), and the owl monkey (Aotus trigivatus) were chosen. All animals, except the cynomolgus macaques, were obtained from the outbred, genetically typed, breeding colonies at the Biomedical Primate Research Center (BPRC), Rijswijk, The Netherlands. The breeding policy is aimed at prevention of inbreeding using genetic typing and the regular introduction of new animals. Cynomolgus macaques were obtained from licensed breeders. Tissue samples were collected at necropsy and were snap frozen in liquid nitrogen and stored at −80C until use. Human tonsil served as positive control tissue.
mAbs
All mAbs described in this study are commercially available unless stated otherwise. Samples were kindly donated by the participating companies: Becton Dickinson (BD) Bioscience (San Jose, CA); Dako (Glostrup, Denmark); Sanquin (Amsterdam, The Netherlands); Diaclone (Fleming, France); Serotec (Düsseldorf, Germany); Abcam (Cambridge, MA); Cymbus Biotech (Hampshire, UK); Beckman Coulter (Fullerton, CA); Bender MedSystems (Vienna, Austria); Genzyme (Framingham, MA); Sanbio (Uden, The Netherlands); Innogenetics (Gent, Belgium); Santa Cruz Biotechnology (Santa Cruz, CA); U-Cytech (Utrecht, The Netherlands); Ebioscience (San Diego, CA); Nordic Labs (Tilburg, The Netherlands); Biosource (Nivelles, Belgium); and BMA Biomedicals (Augst, Switzerland). Other mAbs were kindly provided by Dr. D. Boraschi (Centro Ricerche Sclavo; Sienna, Italy), Dr. R. Noelle (Dartmouth Medical School; Lebanon, NH), and Prof. Dr. G. Opdenakker (Rega Institute; Leuven, Belgium). BD Bioscience and Cymbus Biotech provided isotype-matched Abs as controls. All specificities and clone names are listed in Table 1.
Table 1.
Cross-reaction of anti-human antibodies on cryosections of non-human primate lymphoid tissue
| Great ape |
Old World monkey |
New World monkey |
||||||
|---|---|---|---|---|---|---|---|---|
| Clone | Providera | Chimpanzee | Rhesus macaque | Cynomolgus macaque | Common marmoset | Cotton-top tamarin | Owl monkey | |
| CD marker | ||||||||
| CD2 | CLB-T11/1 (6G4) | 3 | + | + | + | − | − | − |
| RPA-2.10 | 1 | + | + | + | −/+ | − | −/+ | |
| S5.2 | 1 | + | + | + | + | + | − | |
| B-E2 | 4 | + | + | + | + | + | − | |
| LT2 | 5 | + | + | + | − | − | − | |
| CD3 | SK7 | 1 | + | |||||
| FN-18 | 6 | + | + | |||||
| CLB-T3/2 (16A9) | 3 | + | + | − | −/+ | + | − | |
| SP34 | 1 | + | + | −/+ | − | − | − | |
| CD4 | SK3 | 1 | − | − | − | |||
| MT477 | 1 | − | − | − | ||||
| CLB-T4/2 (6D10) | 3 | + | − | − | − | − | + | |
| SK3, SK4 | 1 | + | − | − | − | − | + | |
| MT310 | 2 | + | − | − | − | − | + | |
| CD5 | UCHT2 | 7 | + | + | + | + | −/+ | + |
| CD7 | B-F12 | 4 | + | + | −/+ | − | + | + |
| CD8 | DK25 | 2 | + | + | ||||
| SK1 | 1 | − | − | |||||
| LT8 | 5 | + | + | + | −/+ | − | + | |
| CLB-T8/4 (4H8) | 3 | + | + | + | + | − | + | |
| UCHT4 | 7 | + | + | + | − | + | − | |
| CD10 | ALB1 | 8 | + | − | − | + | + | + |
| CD11a | HI111 | 1 | + | + | + | + | + | + |
| G-25.2 | 1 | + | + | + | + | + | + | |
| 25.3 | 8 | + | + | + | + | + | + | |
| B-B15 | 4 | + | −/+ | + | + | + | + | |
| R7.1 | 9 | + | + | + | + | + | − | |
| CD11b | D12 | 1 | + | + | + | + | ||
| CD13 | B-F10 | 4 | + | + | −/+ | −/+ | + | + |
| CD14 | 322A-1 (MY4) | 8 | + | + | −/+ | + | ||
| B-A8 | 4 | + | −/+ | −/+ | − | −/+ | − | |
| CD16 | NKP15 | 1 | − | + | + | − | −/+ | − |
| 3G8 | 1 | + | + | + | + | − | + | |
| B-E16 | 4 | + | + | − | − | + | − | |
| CD18 | L130 | 1 | + | + | + | + | + | − |
| CLB-LFA-1/1 (54) | 3 | + | + | + | + | + | + | |
| 6.7 | 1 | + | + | + | + | + | + | |
| CD19 | SJ25C1 (FITC) | 1 | + | − | − | − | − | − |
| 4G7 | 1 | + | − | − | − | + | − | |
| CD20 | L26 | 2 | + | + | + | + | ||
| H299 (B1) | 8 | + | + | + | + | − | − | |
| B-H20 | 4 | + | −/+ | − | − | + | − | |
| CD21 | BL13 | 8 | + | + | + | + | + | + |
| B-LY4 | 1 | + | + | + | + | + | + | |
| B-E5 | 4 | + | + | + | + | + | + | |
| CD24 | SN3 | 5 | + | −/+ | −/+ | − | − | − |
| CD25 | B-B10 | 4 | + | + | − | − | − | − |
| Tu69 | 7 | − | − | − | − | − | − | |
| CLB-IL2R/1 (TB30) | 3 | + | + | − | − | − | − | |
| 2A3 | 1 | + | ||||||
| CD26 | BA5 | 2 | −/+ | + | + | − | − | + |
| L272 | 1 | + | − | − | − | − | − | |
| CD27 | L128 | 1 | + | − | −/+ | − | − | − |
| CD28 | CLB-CD28/1 (15E8) | 3 | + | + | ||||
| L293 | 1 | + | + | + | − | − | − | |
| B-T3 | 4 | + | + | + | + | + | −/+ | |
| CD29 | TDM29 | 7 | + | + | + | + | + | + |
| B-D15 | 4 | + | + | + | + | −/+ | + | |
| CD30L | M81 | 10 | + | |||||
| CD31 | 5.6E | 8 | + | + | + | − | + | − |
| CD33 | D3HL60.251 | 8 | + | |||||
| CD34 | QBEnd 10 | 3 | + | − | ||||
| CD35 | E-11 | 1 | + | + | + | + | + | + |
| CD38 | T16 | 8 | + | − | −/+ | − | + | − |
| HB7 | 1 | + | − | − | − | − | − | |
| CD40 | 5C3 | 1 | + | + | − | − | − | |
| CLB-14G7 | 3 | + | + | |||||
| B-B20 | 7 | + | + | + | + | + | + | |
| CD44 | B-F24 | 4 | + | + | − | −/+ | + | + |
| NKI-P2 | 3 | + | + | + | + | + | + | |
| F10-44-2 | 5 | + | − | − | + | + | + | |
| CD45 | B-A11 | 4 | + | + | −/+ | + | + | − |
| CD45RA | B-C15 | 4 | + | + | + | + | − | + |
| CD45RO | UCHL-1 | 1 | + | − | ||||
| CD49d | HP2/1 | 8 | + | + | + | − | −/+ | − |
| CD49e | SAM1 | 8 | + | + | + | + | − | − |
| CD55 | CLB-CD97L/1 | 3 | − | − | + | |||
| CD56 | 123C3 | 11 | + | + | ||||
| B-A19 | 4 | + | −/+ | − | − | − | − | |
| N901 (NKH-1) | 8 | − | − | + | + | + | − | |
| MEM188 | 7 | − | + | + | − | + | − | |
| NCAM16.2 | 1 | + | − | + | + | − | − | |
| CD58 | L306.4 | 1 | −/+ | − | − | − | − | − |
| 1C3 | 1 | + | + | + | + | + | + | |
| CD62L | FMC46 | 7 | + | − | − | − | − | − |
| DREG.55 | 9 | + | + | + | + | − | + | |
| CD64 | 10.1 | 1 | + | + | ||||
| CD68 | KP1 | 2 | + | + | + | + | ||
| CD70 | CLB-2F2 | 3 | + | + | −/+ | −/+ | ||
| CD80 | M24 | 12 | + | + | + | |||
| CD83 | HB15A | 8 | + | + | + | + | ||
| CD86 | 1G10 | 12 | + | + | + | + | ||
| B-T7 | 4 | + | + | −/+ | − | + | − | |
| IT2.2 | 1 | − | + | + | + | − | − | |
| CD95 | UB2 | 8 | + | + | ||||
| FAS6 | 3 | + | + | + | ||||
| APO1-1 | 9 | −/+ | −/+ | + | − | − | − | |
| DX2 | 1 | + | + | + | − | − | − | |
| BMS-140 | 9 | + | + | + | −/+ | − | − | |
| CD97 | CLB-CD97/1 | 3 | + | + | ||||
| CD138 | BB4 | 8 | + | + | + | − | − | − |
| CLB-1D4 | 3 | + | − | − | − | − | − | |
| CD154/CD40L | TRAP1 | 1 | + | + | + | +b | ||
| 24-31 | 13 | + | + | + | ||||
| CD169 | HSN1 (7D2) | 5 | + | + | + | |||
| Cytokines | ||||||||
| IFN-α | MC-16 | 14 | + | + | + | |||
| IFN-γ | MD-1 | 14 | + | |||||
| MD-2 | 14 | + | + | + | ||||
| TNF-α | 61E71 | 14 | − | + | −/+ | + | ||
| IL-1α | Vmp18 | 15 | + | + | ||||
| IL-1β | Vmp20 | 15 | + | + | ||||
| IL-2 | 80-3418-01 | 10 | +b | |||||
| IL-4 | QS-4 | 14 | + | + | + | |||
| 1842-01 | 10 | + | −/+ | + | ||||
| IL-6 | BE-8 | 15 | + | + | + | |||
| IL-10 | B-S10 | 16 | + | + | + | + | ||
| IL-12p40/p70 | C8.6 | 1 | + | −/+ | + | |||
| IL-17A | eBio64CAP17 | 17 | + | + | ||||
| Miscellaneous | ||||||||
| HLA-ABC | G46-2.6 | 1 | + | + | + | − | − | − |
| W6/32 | 7 | + | + | + | + | + | + | |
| HLA-DP, DQ, DR | CR3/43 | 2 | + | + | + | |||
| HLA-DR | TAL-1B5 | 2 | + | + | ||||
| B-F1 | 4 | + | + | + | + | + | + | |
| CLB-HLA-DR (1E5) | 3 | + | + | + | + | + | + | |
| L243 | 1 | + | + | + | + | + | + | |
| HLA-DQ | SPV-L3 | 7 | + | + | + | + | + | + |
| IgM | NI179 | 18 | + | + | ||||
| IgG | − | 19 | + | + | + | |||
| MMP-9 | 2D9 | 20 | + | + | ||||
| MRP 14 |
27E10 (early) |
21 |
+ |
+ |
+ |
|||
Providers: 1, Becton Dickinson; 2, Dako; 3, Sanquin; 4, Diaclone; 5, Serotec; 6, Abcam; 7, Cymbus Biotech; 8, Beckman Coulter; 9, Bender MedSystems; 10, Genzyme; 11, Sanbio; 12, Innogenetics; 13, gift from Dr. R. Noelle; 14, U-Cytech; 15, gift from Dr. D. Boraschi; 16, Santa Cruz; 17, Ebioscience; 18, Nordic Labs; 19, Biosource; 20, gift from Prof. Dr. G. Opdenakker; 21, BMA Biomedicals.
Only cross-reactive when paraformaldehyde is used as fixative.
+, cross-reactive; −, no specific staining; −/+, needs more investigation; blank boxes, not tested. CD, cluster of differentiation; HLA, human leukocyte antigen; IFN-γ interferon-gamma, IL, interleukin.
Immunohistochemistry
Immunohistochemistry was performed as described in detail previously (Laman et al. 1998). Briefly, 6-μm frozen spleen and lymph node sections were cut and thawed and mounted on glass slides. Slides were kept overnight at room temperature, which is below the optimum temperature for most proteolytic enzymes, in a humidified atmosphere to allow controlled drying of the tissue. After the slides were air dried for 1 hr, they were fixed either in fresh acetone or 4% paraformaldehyde in PBS (pH 7.4), both containing 0.02% (v/v) H2O2. Acetone-fixed slides were air dried for 10 min and subsequently washed in PBS, whereas paraformaldehyde-fixed slides were immediately washed in PBS. Tissue sections were incubated with primary Abs overnight at 4C in a humidified atmosphere. Abs were tested in a dose titration that was based on the recommended dilution for human tissue and included a 10× dilution of the provided Ab. The same dose titration was used for all species. If the staining in a given case was doubtful, a higher concentration was tested if sufficient Ab was available. Incubations with secondary and tertiary reagents were performed for 1 hr at room temperature. Between incubation steps, the slides were washed twice with PBS. Detection of primary unlabeled mouse anti-human Ab was followed by incubation with rabbit anti-mouse-Ig-horseradish peroxidase (HRP) (Dako), or in a three-step staining with anti-mouse-Ig-biotin (Dako) and HRP-labeled avidin-biotin-complex (Dako). HRP activity was revealed by incubation for 10 min at room temperature with 3-amino-9-ethyl-carbozole (Sigma, Zwijndrecht, The Netherlands), leading to a bright-red precipitate. Some positive stainings, i.e., IL-17A, IFN-γ, and CD40, were enhanced with the tyramide signal amplification kit (Invitrogen; Carlsbad, CA). Human tonsil was used as positive control tissue. Incubation with isotype-matched primary Abs of irrelevant specificity and omission of the primary Ab served as negative controls.
Immunohistochemistry Analysis
Cross-reactivity is expressed as follows: − = no specific staining; −/+ = doubtful and needs more investigation; + = specific staining.
Ethics
All NHP tissues that were used for this study were obtained from purpose-bred animals at the BPRC or purchased from licensed breeders. All tissues were from animals from other experimental studies; no monkeys were sacrificed solely for the purpose of this study. All study protocols were reviewed and approved according to Dutch law on animal experimentation.
Results
Table 1 shows the Abs clustered according to their reactivity with CD markers, cytokines, and miscellaneous markers, such as HLA and Igs. For most markers, we have tested Ab clones obtained from several companies. Cross-reactivity was scored as: no specific staining (−), doubtful (−/+), or specific staining (+). Doubtful means that some staining is observed, but the specificity is unclear. To further investigate the cross-reactivity of these Abs, the titration of the mAb should be extended or another staining method will be necessary, but this was outside the scope of the current study.
In total, we have tested 130 mAbs against 69 markers: 105 clones against 50 CD markers, 13 clones against 11 cytokines, and 12 clones against 8 miscellaneous markers. The isotype controls and primary Ab omission were negative on all tissues tested (Figure 1). All Abs were directed against human antigens, and, as expected, were positive on human control tissue (data not shown). All mAbs were first tested on acetone-fixed tissue. In addition, mAbs of particular interest that failed to stain cryosections were next tested on paraformaldehyde-fixed tissue. For example, an mAb against IL-2 that showed no cross-reactivity on acetone-fixed tissue did display cross-reactivity on paraformaldehyde-fixed common marmoset tissue.
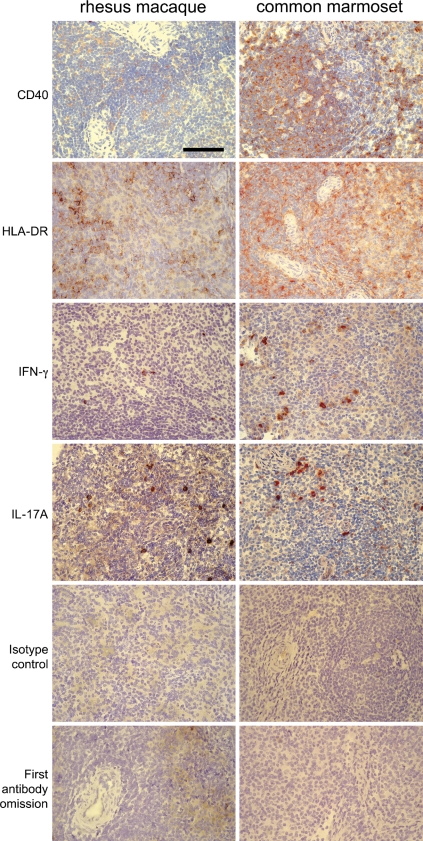
Figure 1.
Representative selection of cross-reactive monoclonal antibodies on spleen cryosections of rhesus macaque and common marmoset. Spleen sections were stained for CD40, HLA-DR, IFN-γ, and IL-17A. Controls included isotype control and first antibody omission. Bar = 100 mm.
Table 2 summarizes the number and percentages of cross-reactive mAbs tested per specificity per species. Predictably, the highest cross-reactivity was found in chimpanzees: 93 of 102 tested mAbs showed cross-reactivity (91%). A somewhat lower percentage of cross-reactive mAbs was found with the Old World species, i.e., 78% in rhesus macaques and 64% in cynomolgus macaques. An again lower, but still high, percentage of mAbs were found to cross-react with New World species. In the common marmoset, cotton-top tamarin, and owl monkey the cross-reactivity was, respectively, 60%, 49%, and 44%.
Table 2.
Number and percentage of cross-reactive monoclonal antibodies
| Great ape |
Old World monkey |
New World monkey |
||||
|---|---|---|---|---|---|---|
| Chimpanzee | Rhesus macaque | Cynomolgus macaque | Common marmoset | Cotton-top tamarin | Owl monkey | |
| CD markers | ||||||
| Tested (n) | 92 | 103 | 91 | 93 | 75 | 74 |
| + | 84 | 75 | 55 | 47 | 35 | 30 |
| + (%) | 91 | 73 | 60 | 51 | 47 | 41 |
| Cytokines | ||||||
| Tested (n) | 3 | 11 | 8 | 12 | ||
| + | 2 | 11 | 5 | 12 | ||
| + (%) | 67 | 100 | 63 | 100 | ||
| Miscellaneous | ||||||
| Tested (n) | 7 | 11 | 10 | 11 | 6 | 6 |
| + | 7 | 11 | 10 | 10 | 5 | 5 |
| + (%) | 100 | 100 | 100 | 91 | 83 | 83 |
| Total | ||||||
| Tested (n) | 102 | 125 | 109 | 116 | 81 | 80 |
| + | 93 | 97 | 70 | 69 | 40 | 35 |
| + (%) |
91 |
78 |
64 |
60 |
49 |
44 |
Figure 1 shows a selection of cross-reactive mAbs on the spleen of a rhesus macaque and a common marmoset, i.e., mAbs directed against CD40, HLA-DR, IFN-γ, and IL-17A.
Discussion
NHP models are invaluable models for research in biomedicine. For the study of immunopathological mechanisms in these models, mAbs for flow cytometry and immunohistochemistry are indispensable. A selection of mAbs directed against human antigens for flow cytometry analysis of NHP cells has previously been reported (Neubert et al. 1996; Brok et al. 2001). The purpose of the current study was to compose a panel of cross-reactive mAbs that could be used for immunohistochemical analysis of lymphoid tissues from six NHP species. Cross-reactive mAbs can be used for immunopathological examination of NHP disease models, which subsequently will increase our knowledge about NHP models and human diseases.
We have found a large panel of cross-reactive Abs for each species, with the highest level of cross-reactivity in chimpanzees, followed by monkeys of Old World origin and of New World origin. The decreasing level of cross-reactivity reflects the evolutionary distance, which has been estimated at 5 million years for chimpanzees, 25 million years for macaques, and 35 million years for marmosets (Enard and Pääbo 2004). We assessed the cross-reactivity in three categories, but not the intensity of the staining, although we did observe that some clones had more-intense staining than did other clones. We did not include this observation in the results, because a subjective scoring of intensity grades may be confusing. Furthermore, the intensity of a staining can be enhanced today by several techniques, and the intensity may vary between batches.
The markers shown in Figure 1 were chosen because CD40, HLA-DR, IFN-γ, and IL-17A are important in immune-mediated diseases. CD40 is a member of the tumor necrosis factor receptor family and is expressed on antigen-presenting cells, such as B-cells, macrophages, and dendritic cells, and also on endothelial cells and fibroblasts. CD40 is a costimulatory molecule that binds to CD154 (CD40L) expressed on T-cells. Binding of CD40L to CD40 on B-cells induces B-cell proliferation and isotype switching. Interaction of CD40L with CD40 on monocytes induces the production of cytokines and nitric oxide. Furthermore, CD40 activation can prevent apoptosis of B-cells and monocytes. CD40 is involved in several immune-mediated diseases (Laman et al. 1996; Grewal and Flavell 1998; Vogel and Noelle 1998). Blocking of CD40 prevents EAE in common marmosets (Boon et al. 2001; Laman et al. 2002) and kidney allograft rejection in rhesus macaques (Haanstra et al. 2003). HLA-DR is associated with several disease susceptibilities, such as rheumatoid arthritis and MS (Fernando et al. 2008). In the common marmoset, in which HLA-DR is called Caja-DR, Caja-DRB*W1201 is essential for the activation of Th1 cells that induce EAE (Brok et al. 2000). IL-17A and IFN-γ are both pro-inflammatory cytokines produced by T-cells. IFN-γ is also produced by natural killer cells, and IL-17A may also be produced by non-T-cells such as microglia (Kawanokuchi et al. 2008) and lymphoid tissue inducer-like cells (Takatori et al. 2009). IL-17A is suggested to be involved in several immune-mediated inflammatory diseases, such as rheumatoid arthritis, psoriasis, and MS (Ivanov and Linden 2009). The IL-17–producing T-cells, called Th17, are now being recognized as a functional CD4+ T-cell subset in addition to Th1 and Th2, as critically reviewed recently (Steinman 2007). In MS, IL-17A may play a role in blood–brain barrier transmigration of inflammatory cells (Kebir et al. 2007).
Some mAb clones cannot be used for immunohistochemistry, but can be used for flow cytometry analysis (Brok et al. 2001) or vice versa. For example, the anti-CD4 clones MT310 and SK3, as well as the anti-CD3 clone SP34, can be used for flow cytometry analysis of common marmoset mononuclear cells, but cannot be used for immunohistochemistry on common marmoset tissue. Vice versa, clone B-B15 against CD11a can be used for immunohistochemistry of common marmoset tissue, but not for flow cytometry analysis of common marmoset cells. Representation and accessibility of Ab epitopes on live cells in cell suspensions can be quite different from that in frozen sections of solid tissue subjected to fixation, even with relatively mild fixatives such as acetone (Laman et al. 1991). Another explanation is that the activation and differentiation status of leukocytes might differ between blood and lymphoid tissue.
Although rhesus macaques and cynomolgus macaques are closely related Old World species, differences in cross-reactivity can be found. For example, the anti-CD3 clone CLB-T3/2 showed cross-reactivity in rhesus macaque tissue, but not in cynomolgus macaque tissue. Differences between New World species were also observed. As an example, clone CLB-T8/4 against CD8 showed cross-reactivity in common marmoset and owl monkey tissue, but not in cotton-top tamarin tissue. In contrast, clone UCHT4 directed against CD8 showed cross-reactivity in cotton-top tamarin tissue, but not in common marmoset and owl monkey tissue. This demonstrates that although species are closely related, differences in epitope conservation do occur.
Seven of the 69 markers seem to be highly conserved between humans and NHPs. Antibodies directed against CD11a, CD18, CD21, CD29, CD35, HLA-DR, and HLA-DQ were cross-reactive with tissue of all six NHPs. CD21 is the complement receptor 2 and CD35 is the complement receptor 1, suggesting a conservation of complement receptors between humans and NHPs. CD21 is expressed on B-cells and is the receptor for Epstein-Barr virus (EBV). Conservation of CD21 suggests that these NHPs are susceptible to infection with EBV-related herpes viruses, which is indeed compatible with the literature (Johannessen and Crawford 1999). CD11a and CD18, which together form lymphocyte function–associated antigen-1, as well as the very late antigen-4 component, CD29, are expressed on leukocytes and are involved in adhesion. This may suggest a conservation in the adhesion system between humans and NHPs.
For some markers, no cross-reactive mAbs were found for a species. For example, no cross-reactive anti-CD3 or anti-CD4 mAb could be found for the common marmoset. This was expected because in earlier studies, it was observed that Abs against human CD3 did not cross-react with rhesus monkeys, necessitating the generation of a specific Ab, known as FN18 (Nooij et al. 1986). A polyclonal Ab that recognizes multiple epitopes may overcome the problem of no cross-reactivity with mAbs. For example, a polyclonal Ab against CD3 (Dako) can be used to stain common marmoset tissue. To obtain Abs that recognize these species-specific epitopes, new Abs are being developed. For instance, Ito and colleagues recently generated mAbs recognizing common marmoset lymphocytes (Ito et al. 2008).
In summary, we have identified a large panel of cross-reactive mAbs for in situ analysis of NHP lymphoid tissues and organs affected by inflammation, infection, or tumors. These mAbs will serve as important tools in future investigations of leukocyte differentiation and activation status in NHPs and will aid in dissecting the mechanisms underlying the efficacy of novel vaccination and immunotherapeutic regimens.
Acknowledgments
The authors thank the companies for donating the antibodies and Mr. Henk van Westbroek for the artwork.
References
- Ambrose Z, KewalRamani VN, Bieniasz PD, Hatziioannou T (2007) HIV/AIDS: in search of an animal model. Trends Biotechnol 25:333–337 [DOI] [PubMed] [Google Scholar]
- Boon L, Brok HP, Bauer J, Ortiz-Buijsse A, Schellekens MM, Ramdien-Murli S, Blezer E, et al. (2001) Prevention of experimental autoimmune encephalomyelitis in the common marmoset (Callithrix jacchus) using a chimeric antagonist monoclonal antibody against human CD40 is associated with altered B cell responses. J Immunol 167:2942–2949 [DOI] [PubMed] [Google Scholar]
- Brok HP, Hornby RJ, Griffiths GD, Scott LA, 't Hart BA (2001) An extensive monoclonal antibody panel for the phenotyping of leukocyte subsets in the common marmoset and the cotton-top tamarin. Cytometry 45:294–303 [DOI] [PubMed] [Google Scholar]
- Brok HP, Uccelli A, Kerlero De Rosbo N, Bontrop RE, Roccatagliata L, de Groot NG, Capello E, et al. (2000) Myelin/oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis in common marmosets: the encephalitogenic T cell epitope pMOG24-36 is presented by a monomorphic MHC class II molecule. J Immunol 165:1093–1101 [DOI] [PubMed] [Google Scholar]
- Bukh J (2004) A critical role for the chimpanzee model in the study of hepatitis C. Hepatology 39:1469–1475 [DOI] [PubMed] [Google Scholar]
- Capuano SV III, Croix DA, Pawar S, Zinovik A, Myers A, Lin PL, Bissel S, et al. (2003) Experimental Mycobacterium tuberculosis infection of cynomolgus macaques closely resembles the various manifestations of human M. tuberculosis infection. Infect Immun 71:5831–5844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enard W, Pääbo S (2004) Comparative primate genomics. Annu Rev Genomics Hum Genet 5:351–378 [DOI] [PubMed] [Google Scholar]
- Eslamboli A (2005) Marmoset monkey models of Parkinson's disease: which model, when and why? Brain Res Bull 68:140–149 [DOI] [PubMed] [Google Scholar]
- Fernando MM, Stevens CR, Walsh EC, De Jager PL, Goyette P, Plenge RM, Vyse TJ, et al. (2008) Defining the role of the MHC in autoimmunity: a review and pooled analysis. PLoS Genet 4:e1000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal IS, Flavell RA (1998) CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol 16:111–135 [DOI] [PubMed] [Google Scholar]
- Haanstra KG, Ringers J, Sick EA, Ramdien-Murli S, Kuhn EM, Boon L, Jonker M (2003) Prevention of kidney allograft rejection using anti-CD40 and anti-CD86 in primates. Transplantation 75:637–643 [DOI] [PubMed] [Google Scholar]
- Haanstra KG, Wubben JA, Korevaar SS, Kondova I, Baan CC, Jonker M (2007) Expression patterns of regulatory T-cell markers in accepted and rejected nonhuman primate kidney allografts. Am J Transplant 7:2236–2246 [DOI] [PubMed] [Google Scholar]
- Heeney JL, Dalgleish AG, Weiss RA (2006) Origins of HIV and the evolution of resistance to AIDS. Science 313:462–466 [DOI] [PubMed] [Google Scholar]
- Herrera S, Perlaza BL, Bonelo A, Arevalo-Herrera M (2002) Aotus monkeys: their great value for anti-malaria vaccines and drug testing. Int J Parasitol 32:1625–1635 [DOI] [PubMed] [Google Scholar]
- Ito R, Maekawa S, Kawai K, Suemizu H, Suzuki S, Ishii H, Tanioka Y, et al. (2008) Novel monoclonal antibodies recognizing different subsets of lymphocytes from the common marmoset (Callithrix jacchus). Immunol Lett 121:116–122 [DOI] [PubMed] [Google Scholar]
- Ivanov S, Linden A (2009) Interleukin-17 as a drug target in human disease. Trends Pharmacol Sci 30:95–103 [DOI] [PubMed] [Google Scholar]
- Johannessen I, Crawford DH (1999) In vivo models for Epstein-Barr virus (EBV)-associated B cell lymphoproliferative disease (BLPD). Rev Med Virol 9:263–277 [DOI] [PubMed] [Google Scholar]
- Kawanokuchi J, Shimizu K, Nitta A, Yamada K, Mizuno T, Takeuchi H, Suzumura A (2008) Production and functions of IL-17 in microglia. J Neuroimmunol 194:54–61 [DOI] [PubMed] [Google Scholar]
- Kean LS, Gangappa S, Pearson TC, Larsen CP (2006) Transplant tolerance in non-human primates: progress, current challenges and unmet needs. Am J Transplant 6:884–893 [DOI] [PubMed] [Google Scholar]
- Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, et al. (2007) Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med 13:1173–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laman JD, Claassen E, Noelle RJ (1996) Functions of CD40 and its ligand, gp39 (CD40L). Crit Rev Immunol 16:59–108 [DOI] [PubMed] [Google Scholar]
- Laman JD, Kors N, Heeney JL, Boersma WJ, Claassen E (1991) Fixation of cryo-sections under HIV-1 inactivating conditions: integrity of antigen binding sites and cell surface antigens. Histochemistry 96:177–183 [DOI] [PubMed] [Google Scholar]
- Laman JD, 't Hart BA, Brok H, Meurs M, Schellekens MM, Kasran A, Boon L, et al. (2002) Protection of marmoset monkeys against EAE by treatment with a murine antibody blocking CD40 (mu5D12). Eur J Immunol 32:2218–2228 [DOI] [PubMed] [Google Scholar]
- Laman JD, van Meurs M, Schellekens MM, de Boer M, Melchers B, Massacesi L, Lassmann H, et al. (1998) Expression of accessory molecules and cytokines in acute EAE in marmoset monkeys (Callithrix jacchus). J Neuroimmunol 86:30–45 [DOI] [PubMed] [Google Scholar]
- Lutterotti A, Martin R (2008) Getting specific: monoclonal antibodies in multiple sclerosis. Lancet Neurol 7:538–547 [DOI] [PubMed] [Google Scholar]
- Moreno A, Caro-Aguilar I, Yazdani SS, Shakri AR, Lapp S, Strobert E, McClure H, et al. (2008) Preclinical assessment of the receptor-binding domain of Plasmodium vivax Duffy-binding protein as a vaccine candidate in rhesus macaques. Vaccine 26:4338–4344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubert R, Foerster M, Nogueira AC, Helge H (1996) Cross-reactivity of antihuman monoclonal antibodies with cell surface receptors in the common marmoset. Life Sci 58:317–324 [DOI] [PubMed] [Google Scholar]
- Nooij FJ, Jonker M, Balner H (1986) Differentiation antigens on rhesus monkey lymphocytes. II. Characterization of RhT3, a CD3-like antigen on T cells. Eur J Immunol 16:981–984 [DOI] [PubMed] [Google Scholar]
- Rutjens E, Balla-Jhagjhoorsingh S, Verschoor E, Bogers W, Koopman G, Heeney J (2003) Lentivirus infections and mechanisms of disease resistance in chimpanzees. Front Biosci 8:d1134–1145 [DOI] [PubMed] [Google Scholar]
- Steinman L (2007) A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med 13:139–145 [DOI] [PubMed] [Google Scholar]
- Takatori H, Kanno Y, Watford WT, Tato CM, Weiss G, Ivanov II, Littman DR, et al. (2009) Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med 206:35–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 't Hart BA, Amor S, Jonker M (2004) Evaluating the validity of animal models for research into therapies for immune-based disorders. Drug Discov Today 9:517–524 [DOI] [PubMed] [Google Scholar]
- 't Hart BA, van Meurs M, Brok HP, Massacesi L, Bauer J, Boon L, Bontrop RE, et al. (2000) A new primate model for multiple sclerosis in the common marmoset. Immunol Today 21:290–297 [DOI] [PubMed] [Google Scholar]
- Vierboom MP, Jonker M, Tak PP, 't Hart BA (2007) Preclinical models of arthritic disease in non-human primates. Drug Discov Today 12:327–335 [DOI] [PubMed] [Google Scholar]
- Vogel LA, Noelle RJ (1998) CD40 and its crucial role as a member of the TNFR family. Semin Immunol 10:435–442 [DOI] [PubMed] [Google Scholar]
- Warren BF, Watkins PE (1994) Animal models of inflammatory bowel disease. J Pathol 172:313–316 [DOI] [PubMed] [Google Scholar]
- Witowski J, Pawlaczyk K, Breborowicz A, Scheuren A, Kuzlan-Pawlaczyk M, Wisniewska J, Polubinska A, et al. (2000) IL-17 stimulates intraperitoneal neutrophil infiltration through the release of GRO alpha chemokine from mesothelial cells. J Immunol 165:5814–5821 [DOI] [PubMed] [Google Scholar]
- Yu JJ, Ruddy MJ, Wong GC, Sfintescu C, Baker PJ, Smith JB, Evans RT, et al. (2007) An essential role for IL-17 in preventing pathogen-initiated bone destruction: recruitment of neutrophils to inflamed bone requires IL-17 receptor-dependent signals. Blood 109:3794–3802 [DOI] [PMC free article] [PubMed] [Google Scholar]