Abstract
Endoscopic ultrasonography (EUS) is a highly sensitive diagnostic method for the detection of small pancreatic carcinomas. Recently, there have been some reports describing the utility of contrast-enhanced harmonic EUS (CEH-EUS) which uses sonographic contrast agent for differentiation of a pancreatic mass. This report describes a case of small adenocarcinoma of the pancreas distinct from branch duct intraductal papillary mucinous neoplasm (IPMN) in which investigation by EUS took place every 6 mo and diagnosis was made accurately by additional CEH-EUS during the follow-up of the branch duct IPMN. A 68-year-old female was admitted to our hospital because of a branch duct IPMN in the pancreatic body. She had been followed-up by EUS every 6 mo. However, after 2 years EUS demonstrated a low echoic area distinct from the branch duct IPMN which was vaguely discernible by EUS, and accurate sizing and differential diagnosis were considered difficult on the EUS imaging. CH-EUS with Sonazoid revealed a hypovascular tumor and we suspected small pancreatic carcinoma. The histopathological diagnosis was adenocarcinoma (10 mm) in the pancreatic tail, distinct from the branch duct IPMN of the pancreatic body. EUS and CEH-EUS may play an important role in the correct diagnosis of small pancreatic tumors, including synchronous and metachronous occurrence of IPMN and ductal adenocarcinoma of the pancreas.
Keywords: Intraductal papillary mucinous adenoma, Small pancreatic cancer, Endoscopic ultrasonography, Contrast-enhanced harmonic endoscopic ultrasonography, Pancreatic cancer, Pancreas, Contrast enhanced endoscopic ultrasonography
INTRODUCTION
Endoscopic ultrasonography (EUS) is now commonly used worldwide and is more sensitive for the detection of small pancreatic lesions when compared with transabdominal ultrasonography (US) or contrast-enhanced computed tomography (CE-CT)[1,2]. Recently, there have been some reports describing the utility of contrast-enhanced EUS (CE-EUS) and contrast-enhanced harmonic EUS (CEH-EUS) with sonographic contrast agent for differentiation of pancreatic masses[3-7].
Intraductal papillary mucinous neoplasm (IPMN) of the pancreas is a unique clinicopathological entity characterized by cystic dilatation of the main or branch pancreatic duct, mucus production and intraductal papillary growth[8-11]. Metachronous occurrence of IPMN and ductal carcinoma of the pancreas has been recently reported[12-15]. These authors concluded that special attention should be paid to the development of ductal carcinoma of the pancreas during the follow-up of IPMN. However, the detection of ductal carcinoma at an early stage is still difficult, even under close surveillance by imaging examinations such as abdominal US and computed tomography (CT) during the follow-up of IPMN.
Here, we report a patient with small invasive ductal carcinoma of the pancreas that is distinct from branch duct IPMN. The lesion in this patient was detected by EUS and the diagnosis was established accurately by contrast-enhanced harmonic EUS during the follow-up of the branch duct IPMN.
CASE REPORT
A 68-year-old female was admitted to our hospital because of a branch duct IPMN in the pancreatic body that was detected by contrast-enhanced computed tomography (CE-CT) (Figure 1). Magnetic resonance cholangiopancreatography (MRCP) (Figure 2) and EUS revealed a dilatation of the branch pancreatic ducts in the pancreatic body (22 mm × 9 mm, 8 mm × 5 mm); however, no nodules were found in the cyst and in any other parts of the pancreas. The patient was followed up by performing EUS every six months, as well as MRCP and CE-CT once a year. The size of the branch ducts was unchanged one year after the last MRCP (Figure 3). EUS performed at two years revealed a low echoic area that was distinct from the dilatation of the branch duct IPMN. This finding was vague and the area had unclear margins with a low echoic area, as assessed by EUS (Figure 4A). Thus, it was very difficult to determine the accurate size of the area and perform a differential diagnosis based on the EUS imaging. We performed CEH-EUS using an Olympus GF-UE260P apparatus (Olympus Medical Systems Co. Ltd., Tokyo, Japan) for endoscopy, Extended Pure Harmonic Detection (ExPHD) mode of ALOKA Prosound α-10 (ALOKA Co. Ltd., Tokyo, Japan) for image analysis and Sonazoid (15 μL/kg intravenously, Daiichi Sankyo, Tokyo, Japan; GE Healthcare, Milwaukee, Wisc., USA) as a contrast agent. CEH-EUS revealed the presence of a 10 mm hypovascular tumor, which led us to suspect a small carcinoma of the pancreas (Figure 4B). Pancreatoduodenectomy was performed. The histopathological diagnosis established was a moderately differentiated tubular adenocarcinoma in the 10 mm range at the maximum length of the pancreatic tail, which was distinct from the branch duct intraductal papillary mucinous adenoma of the pancreatic body (Figures 5 and 6).
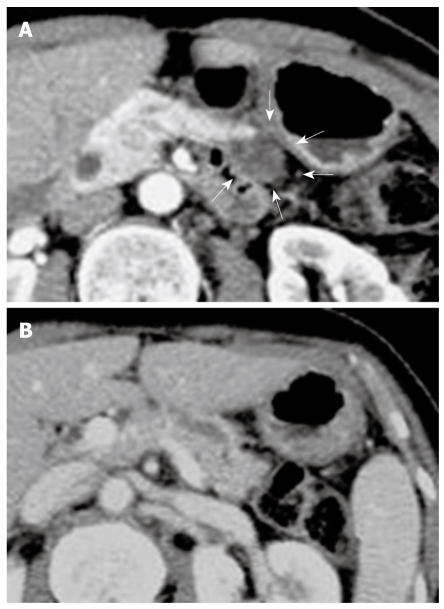
Figure 1.
Contrast-enhanced computed tomography (CE-CT) scan of pancreas. A: A cystic lesion in pancreatic body (arrows); B: A dilatation of the main duct in the pancreatic tail. Solid masses were not observed.
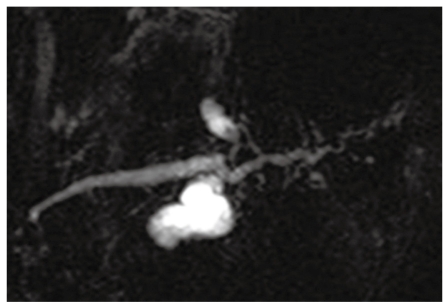
Figure 2.
Magnetic resonance cholangiopancreatography (MRCP) at initial examination showing dilatation of the branch duct.
Figure 3.
The size of the branch ducts was unchanged one year after the last MRCP.
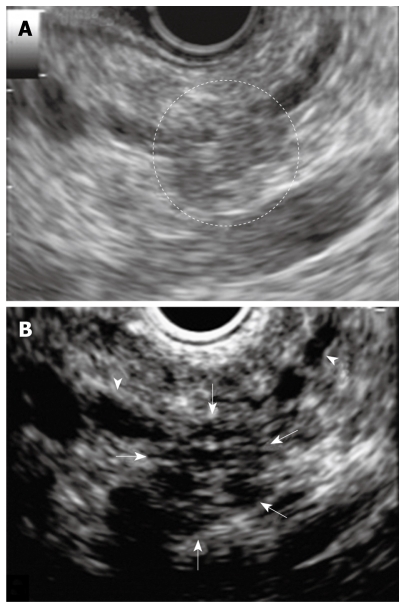
Figure 4.
Endoscopic ultrasonography (EUS) at two years later examination showing pancreatic tail. A: EUS showing low echoic lesion which is vague and the area had unclear margins in the pancreatic tail; B: Contrast-enhanced harmonic EUS showing a clear margin of 10 mm in diameter and hypovascular nodule compared with surrounding pancreatic tissue (arrows). MPD: Main pancreatic duct (arrowheads).
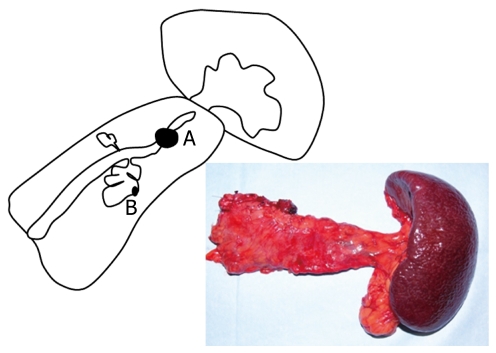
Figure 5.
Scheme showing the pancreatic body and tail by pancreatoduodenectomy. A: Intraductal papillary tumor of the pancreatic body; B: Invasive adenocarcinoma of the pancreatic tail.
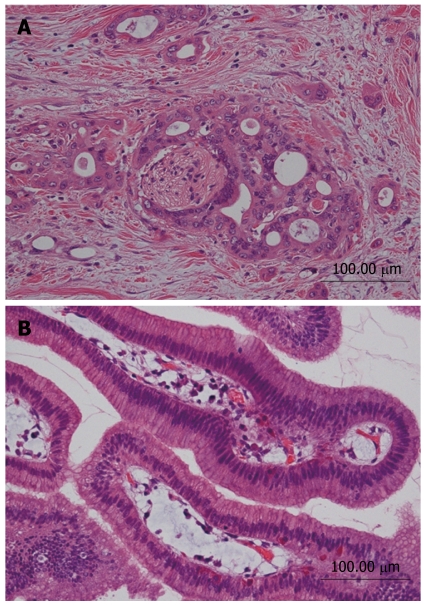
Figure 6.
Histological appearance of two lesions (HE). A: Intraductal papillary mucinous adenoma; B: Moderately differentiated tubular adenocarcinoma.
DISCUSSION
We report here the discovery of independent development of a ductal carcinoma of the pancreas during follow-up of an IPMN, similar to the metachronous occurrence of IPMN and ductal carcinoma of the pancreas which has been recently reported[13-15].
Uehara et al[13] reported results from a follow-up study of 60 patients with branch duct IPMNs over an average period of 87 mo, and the follow-up was performed by US every three or six months. These authors detected ductal pancreatic carcinomas that were distinct from IPMN in 5 of 60 patients (8%). Four of the 5 ductal carcinomas detected during follow-up were resectable, but all of them were advanced cancer.
Tada et al[14] followed-up 197 cystic lesions of the pancreas, including 80 branch duct IPMNs and 117 non-IPMN cysts, for an average period of 3.8 years, and found 5 ductal carcinomas between 14 and 60 mo after the diagnosis of the cystic lesions. In two patients, ductal carcinomas were < 2 cm in size, arising in apparently different sites from the cystic lesion. In one patient, the ductal carcinoma developed around the pre-existing cystic lesion. In the remaining two patients, the tumors were too large to evaluate any local relationship between carcinoma and cystic lesion.
These results indicate that the detection of ductal carcinoma at an early stage was still difficult even under close surveillance imaging examination such as US.
EUS is a highly sensitive diagnostic method for detection of pancreatic carcinoma, especially small pancreatic carcinomas. In the case reported here, the small pancreatic mass was not detected by CE-MDCT, US or MRCP. EUS was the only imaging technique that detected this mass. During the follow-up of branch duct IPMN, special attention should be paid to the occurrence of ductal carcinoma distinct from IPMN as detected by EUS. Recent reports have described the utility of contrast-enhanced EUS (CE-EUS) and CEH-EUS using sonographic contrast agent for the differentiation of pancreatic masses[3-7]. In the case reported here, CEH-EUS imaging using Sonazoid clearly distinguished the margin of the tumor and accurately measured the size of the lesion. Moreover, CH-EUS revealed that the mass was hypovascular when compared with surrounding tissue, which suggested an adenocarcinoma of the pancreatic tail. Therefore, CEH-EUS should be used for the accurate diagnosis of small pancreatic tumors, including the synchronous and metachronous occurrence of IPMN and ductal adenocarcinoma of the pancreas.
Here, we reported a patient with a very small ductal carcinoma of the pancreas distinct from a branch duct IPMN. The performance of EUS every six months coupled with CEH-EUS imaging during the follow-up of the branch duct IPMN allowed the establishment of an accurate diagnosis of the disease.
Footnotes
Supported by The Japan Society for Promotion of Science; The Research and Development Committee Program of The Japan Society of Ultrasonics in Medicine; Japan Research Foundation for Clinical Pharmacology; Japanese Foundation for Research and Promotion of Endoscopy
Peer reviewer: Yuk-Tong Lee, MD, Department of Medicine and Therapeutics, Prince of Wales Hospital, Shatin, New Territories, Hong Kong, China
S- Editor Wang JL L- Editor Logan S E- Editor Lin YP
References
- 1.Rösch T, Lorenz R, Braig C, Feuerbach S, Siewert JR, Schusdziarra V, Classen M. Endoscopic ultrasound in pancreatic tumor diagnosis. Gastrointest Endosc. 1991;37:347–352. doi: 10.1016/s0016-5107(91)70729-3. [DOI] [PubMed] [Google Scholar]
- 2.Snady H, Cooperman A, Siegel J. Endoscopic ultrasonography compared with computed tomography with ERCP in patients with obstructive jaundice or small peri-pancreatic mass. Gastrointest Endosc. 1992;38:27–34. doi: 10.1016/s0016-5107(92)70326-5. [DOI] [PubMed] [Google Scholar]
- 3.Sakamoto H, Kitano M, Suetomi Y, Maekawa K, Takeyama Y, Kudo M. Utility of contrast-enhanced endoscopic ultrasonography for diagnosis of small pancreatic carcinomas. Ultrasound Med Biol. 2008;34:525–532. doi: 10.1016/j.ultrasmedbio.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Kitano M, Sakamoto H, Matsui U, Ito Y, Maekawa K, von Schrenck T, Kudo M. A novel perfusion imaging technique of the pancreas: contrast-enhanced harmonic EUS (with video) Gastrointest Endosc. 2008;67:141–150. doi: 10.1016/j.gie.2007.07.045. [DOI] [PubMed] [Google Scholar]
- 5.Kitano M, Kudo M, Maekawa K, Suetomi Y, Sakamoto H, Fukuta N, Nakaoka R, Kawasaki T. Dynamic imaging of pancreatic diseases by contrast enhanced coded phase inversion harmonic ultrasonography. Gut. 2004;53:854–859. doi: 10.1136/gut.2003.029934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becker D, Strobel D, Bernatik T, Hahn EG. Echo-enhanced color- and power-Doppler EUS for the discrimination between focal pancreatitis and pancreatic carcinoma. Gastrointest Endosc. 2001;53:784–789. doi: 10.1067/mge.2001.115007. [DOI] [PubMed] [Google Scholar]
- 7.Ding H, Kudo M, Onda H, Suetomi Y, Minami Y, Chung H, Kawasaki T, Maekawa K. Evaluation of posttreatment response of hepatocellular carcinoma with contrast-enhanced coded phase-inversion harmonic US: comparison with dynamic CT. Radiology. 2001;221:721–730. doi: 10.1148/radiol.2213010358. [DOI] [PubMed] [Google Scholar]
- 8.Yamaguchi K, Ohuchida J, Ohtsuka T, Nakano K, Tanaka M. Intraductal papillary-mucinous tumor of the pancreas concomitant with ductal carcinoma of the pancreas. Pancreatology. 2002;2:484–490. doi: 10.1159/000064716. [DOI] [PubMed] [Google Scholar]
- 9.Loftus EV Jr, Olivares-Pakzad BA, Batts KP, Adkins MC, Stephens DH, Sarr MG, DiMagno EP. Intraductal papillary-mucinous tumors of the pancreas: clinicopathologic features, outcome, and nomenclature. Members of the Pancreas Clinic, and Pancreatic Surgeons of Mayo Clinic. Gastroenterology. 1996;110:1909–1918. doi: 10.1053/gast.1996.v110.pm8964418. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K, Matsuno S. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 11.Yamaguchi K, Sugitani A, Chijiiwa K, Tanaka M. Intraductal papillary-mucinous tumor of the pancreas: assessing the grade of malignancy from natural history. Am Surg. 2001;67:400–406. [PubMed] [Google Scholar]
- 12.Obara T, Maguchi H, Saitoh Y, Itoh A, Arisato S, Ashida T, Nishino N, Ura H, Namiki M. Mucin-producing tumor of the pancreas: natural history and serial pancreatogram changes. Am J Gastroenterol. 1993;88:564–569. [PubMed] [Google Scholar]
- 13.Uehara H, Nakaizumi A, Ishikawa O, Iishi H, Tatsumi K, Takakura R, Ishida T, Takano Y, Tanaka S, Takenaka A. Development of ductal carcinoma of the pancreas during follow-up of branch duct intraductal papillary mucinous neoplasm of the pancreas. Gut. 2008;57:1561–1565. doi: 10.1136/gut.2007.145631. [DOI] [PubMed] [Google Scholar]
- 14.Tada M, Kawabe T, Arizumi M, Togawa O, Matsubara S, Yamamoto N, Nakai Y, Sasahira N, Hirano K, Tsujino T, et al. Pancreatic cancer in patients with pancreatic cystic lesions: a prospective study in 197 patients. Clin Gastroenterol Hepatol. 2006;4:1265–1270. doi: 10.1016/j.cgh.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Allen PJ, D'Angelica M, Gonen M, Jaques DP, Coit DG, Jarnagin WR, DeMatteo R, Fong Y, Blumgart LH, Brennan MF. A selective approach to the resection of cystic lesions of the pancreas: results from 539 consecutive patients. Ann Surg. 2006;244:572–582. doi: 10.1097/01.sla.0000237652.84466.54. [DOI] [PMC free article] [PubMed] [Google Scholar]