Abstract
AIM: To determine the prevalence of celiac disease (CD) in children with idiopathic short stature (ISS) and the diagnostic value of immunoglobulin (Ig) A G antigliadin antibodies (AGA) and transglutaminase (TTG) antibodies for CD.
METHODS: A total of 104 children (49 male, 55 female) with ISS without a specific etiology were studied. Extensive endocrine investigations had shown no abnormalities in any subject. Anthropometric parameters and IgA AGA and IgA TTG antibodies were evaluated in this study group. These antibodies were measured by enzyme-linked immunosorbent assay. All patients were referred for an endoscopic intestinal biopsy. The biopsy samples were classified according to revised Marsh criteria (UEGW 2001).
RESULTS: We detected positive IgA TTG antibodies in 36 and IgA AGA in 35 of these patients. Thirty one IgA TTG antibody positive and 28 IgA AGA positive subjects showed histological abnormalities compatible with celiac disease (33.6%). Sensitivity, specificity, positive predictive value (PPV) and negative predictive value for IgA AGA were found to be 80%, 88.4%, 77.8% and 89.7%, respectively. Sensitivity, specificity and PPV for IgA TTG antibodies were 88.6%, 94.2% and 88.6%, respectively.
CONCLUSION: We conclude that the prevalence of celiac disease is high in patients with ISS and it is important to test all children with ISS for celiac disease by measuring serologic markers and performing an intestinal biopsy.
Keywords: Celiac disease, Growth disorders, Transglu-taminases, Antibodies, Gliadin
INTRODUCTION
Short stature is one of the most common causes for referrals to pediatric endocrinologists. Many of these patients have no identifiable medical abnormality and are classified as idiopathic short stature (ISS). For most of these patients, it is believed that genetic variations are the underlying cause. Short stature is a well-known feature of pediatric celiac disease (CD)[1]. In recent studies, CD was considered to be a more common cause of short stature in otherwise healthy children than growth hormone deficiency[2,3]. In other studies, CD has been found without typical gastrointestinal symptoms in some cases of short stature[4-26]. Moreover, some studies suggested an association between CD and growth hormone deficiency[27]. Diagnosis of CD depends on the demonstration of a flat or almost flat jejunal mucosa in biopsy specimens from the small intestine and regeneration of the mucosa after a gluten-free diet[5]. It has been suggested that patients with untreated CD have circulating antibodies against gliadin, and antiendomysium antibodies (anti-EMA) have proven to be a reliable screening test for CD, even in asymptomatic patients[6,7]. The immunofluorescence test is technically difficult to interpret, with large interobserver variability. In addition, esophageal tissue from monkeys is a common substrate, and the testing is time consuming. Transglutaminase (TTG) antibodies are also highly sensitive and specific and since IgA antibodies to TTG can be examined by enzyme-linked immunosorbent assay (ELISA), they are easier to use as screening antibodies compared with EMA testing[9]. The purpose of the present study was to evaluate prospectively the clinical, laboratory and histologic features of CD and the sensitivity, specificity and positive and negative predictive values (PPV and NPV, respectively) of antibodies against gliadin and TTG in 104 children with a diagnosis of ISS but with no specific etiology.
MATERIALS AND METHODS
A total of 104 children (55 female, 49 male) with ISS and height less than the 2nd percentile adjusted for age and sex, but without specific etiology were enrolled in the study from November 1, 2003 to September 1, 2005 at Ahwaz Jundishapour University Hospitals. Ages ranged from 2 to 18 years. The height, weight and weight for height measurements had been recorded for all patients at presentation and the patients and their parents answered a CD-specific questionnaire used for data collection. All children were being followed at the Department of Endocrinology of Golestan Hospital and had undergone an extensive negative endocrine investigation which included: concentrations of serum electrolytes and glucose, sweat test, total proteins and albumin, determination of immunoglobulin A (IgA), assessment of liver and renal function (determined by standard methods), and hormonal evaluation through the measurement of thyroid-stimulating hormone, free-thyroxin, and growth hormone.
All etiologic factors known to produce growth retardation had also been excluded, e.g, diabetes mellitus, hematological and liver disease, renal failure, fetal growth failure, diseases of bone metabolism, and chromosomal abnormalities. When no cause of the short stature was found, additional investigations were performed by measuring the serum levels of IgA anti-TTG antibodies and IgA antigliadin antibodies (AGA). AGA was measured by a commercial ELISA assay (ELISA-Biosystem, Madrid, Spain). A serum dilution of 1:100 was used and the results were reported in terms of arbitrary units (AU/mL). An IgA AGA ≥ 20 AU/mL was considered positive. A commercial ELISA. (Orgentec) kit was used to measure anti-TTG antibodies and a titer of more than 1/10 was considered positive. Intestinal biopsies were obtained from all 104 patients with endoscopic grasp forceps (who had negative or positive results for anti-TTG antibody). Four to six biopsy specimens were taken from the second and third parts of the duodenum. Formalin-fixed biopsy specimens stained with hematoxylin and eosin were studied with the use of light microscopy. The slides were examined and confirmed by a pathologist experienced in CD. Mucosal lesions were classified according to the criteria of Marsh[8] as: (1) type 0, normal mucosa, pre-infiltrative lesions; (2) type 1, normal mucosal architecture with epithelial lymphocyte infiltration, infiltrative lesions; (3) type 2, hypertrophic crypts with epithelial lymphocyte infiltration, hyperplastic lesions; and (4) type 3, typical flat mucosa, destructive lesions. The research protocol was reviewed and approved by the Medical Ethics Committee of the Ahwaz Jundishapour University Hospitals. Written informed consent was obtained from the children’s parents.
Statistical analysis
The results are reported as mean ± SD. Statistical analysis was performed by the unpaired Student t-test (GraphPad Prism Software Incorporated), with the level of significance set at P < 0.05.
RESULTS
The most frequent symptom was diarrhea (n = 13) followed by abdominal pain and distention (n = 3) in patients with CD and the patients affected by CD did not differ from those without CD in any of the symptoms. A family history of CD was detected in two patients (5.7%). At diagnosis, in the CD patient group, mean weight was 37.9 ± 13.1 and mean height was 137.6 ± 13.1. In this group, short stature of > 2 SD and > 3 SD was found in 30 patients (85.7%) and 5 patients (14.3%), respectively (P > 0.05, Table 1).
Table 1.
The age, weight, height, short stature and BMI of patients (mean ± SD)
| Group | Age | Weight | Height |
Short stature (n) |
BMI | |
| > 2 SD | > 3 SD | |||||
| Without CD | 16.6 ± 6.5 | 38.7 ± 13.4 | 140 ± 17.1 | 54 | 15 | 19 ± 3.5 |
| With CD | 16.9 ± 7.1 | 37.9 ± 13.1 | 137.6 ± 13.1 | 30 | 5 | 19.1 ± 3.1 |
| Total | 16.8 ± 6.7 | 38.5 ± 13.2 | 139.2 ± 17.3 | 84 | 20 | 19.1 ± 3.5 |
Small intestine biopsies were performed in all 104 patients with ISS. Duodenal mucosal histopathology was normal in 69 patients. Histopathologic analysis showed evidence of abnormalities compatible with CD in 35 cases (33.6%).
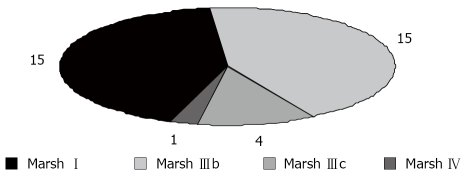
The following histological findings were obtained: (a) 15 of 35 patients had normal mucosal architecture with epithelial lymphocyte infiltration and (b) 15 cases had hypertrophic crypts with epithelial lymphocyte infiltration and partial villous atrophy and (c) five cases showed subtotal or total villous atrophy (Figure 1).
Figure 1.
Histological findings of celiac disease.
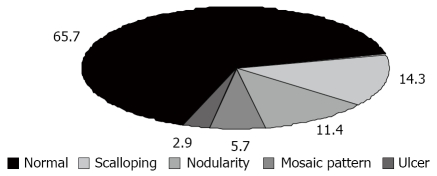
Therefore, the prevalence of properly diagnosed CD among patients with ISS in this study was 33.6% (35 of 104 patients). IgA AGA, and I IgA TTG antibodies were found in 80% (n = 28), and 88.6% (n = 31) of patients with ISS, respectively. Specificity and the positive predictive value (PPV) for TTG antibodies were found to be 94.2% and 88.6% for CD in the group of patients with ISS in this study. Table 2 shows the relationship between positive and negative IgA AGA, and IgA TTG antibodies and histological evidence of CD. IgA AGA: sensitivity 80%, specificity 88.4%, PPV: 77.8%, negative predictive value (NPV) 89.7%; IgA TTG antibodies: sensitivity 88.6%, specificity 94.2%, PPV 88.6%, NPV 94.2%. The endoscopic features are summarized in Figure 2.
Table 2.
Relationship between positive and negative IgA AGA, and IgA TTG antibodies and histological evidence of celiac disease (n)
| Lab group |
IgA AGA |
IgA TTG antibodies |
||
| Positive | Negative | Positive | Negative | |
| Without CD | 8 | 61 | 4 | 65 |
| With CD | 28 | 7 | 31 | 4 |
| Total | 36 | 68 | 35 | 69 |
Figure 2.
Endoscopic features.
DISCUSSION
Screening for CD in the general population indicates a prevalence of 1:300 to 1:100. About 50% of these children are completely symptomless but because of these figures some experts suggest CD screening for all adults[28] and children[29]. In a British population-based study on short stature, where CD was not specifically investigated, the prevalence of CD was 2:180. In children with short stature and no gastrointestinal symptoms who were investigated for CD, the prevalence increased to 2%-8%. When other (endocrine) causes for short stature are excluded, the prevalence might rise as high as 59%[30,31]. Although CD was once thought to be rare in Iran, several recent reports have cleared this misconception[23,24]. Dr. Shahbazkhani reported that the minimum prevalence of gluten sensitivity among apparently healthy urban Iranian blood donors is 1/166[25]. However, all of these reports deal with typical presentation of CD.
We performed a prospective study on newly diagnosed cases of CD in a group of short stature patients in the south-West of Iran in 2003-2005. Despite the presence of clinical signs of CD during childhood in more than one-third of the patients, the disease remained undiagnosed for many years. This late diagnosis may lead to short stature and low female fertility.
In the last 20 years the clinical picture of CD has changed considerably. The classic form of CD now accounts for a small and systematically shrinking percentage of cases, while atypical forms that present with few or no symptoms are the majority[11]. Short stature is a well-recognized complication of CD[12] although Cacciari et al[13] found that adult height is normal in patients who experienced their first symptoms of CD during adulthood. Adult height was shorter only in patients who had symptoms during childhood. This study demonstrated the prevalence and clinical features of CD (n = 35) in a group of 104 patients with ISS. Ages ranged from 2 to 18 years and the mean age of diagnosis was 16.9 years, similar to the results of the study by Mäki et al[14]. The age at onset of symptoms appeared to modify the clinical picture. Patients with an earlier onset of CD have a typical clinical picture, whereas patients with delayed onset have atypical presentation, such as short stature.
According to our findings, the prevalence of biopsy-proven CD was 33.6% in the group of ISS children, thereby justifying screening for this disease in all children with short stature. The proportion of CD in cases with ISS ranged from 18.6% to 59.1% in other studies[15,16]. The mechanism of growth retardation is not clearly understood in patients with CD; nutritional deficiencies especially zinc deficiency, low serum somatomedin activity and defects in growth hormone secretion have been proposed as underlying mechanisms[17-19]. An association between CD and autoimmune disorders, such as type I diabetes, autoimmune thyroid disease, and Sjögren’s syndrome, has been well documented in the literature[20]. These conditions were not detected in patients in the present study. Susceptibility to CD is determined by genetic factors, which is confirmed by the occurrence of multiple cases of CD in the same family. The prevalence of CD found among first degree relatives is approximately 10%[21]. Screening of siblings in the present study showed that only two siblings (5.7%) had CD. The tests used for CD in this study were IgAAGA and IgA TTG antibodies. The total IgA level was determined also, because CD is associated with IgA deficiency. Antiendomysial antibody and anti-TTG antibodies have been shown to have a high sensitivity and specificity for the diagnosis of CD and correlate well with villous atrophy in untreated patients[32], but false-negative results have been obtained for patients with IgA deficiency[22]. A jejunal biopsy remains the gold standard for the diagnosis of CD but both serologic and histopathologic parameters of CD were investigated in the patients in this study. The sensitivity and specificity of serologic tests were variable. The TTG antibody test has been shown to have a higher sensitivity and specificity for the diagnosis of CD in our patients. Our patients were also tested for IgA deficiency and all were found to have normal IgA values. However, negative results for these tests would not exclude CD. Shamir et al[10], using multiple serological strategies to diagnose silent CD, demonstrated that using any serological marker alone, including EMA antibodies detected by immunofluorescence, would underestimate the prevalence of CD[10]. We found that seven cases with partial villous atrophy had normal AGA, and four of this subgroup had normal anti-TTG antibodies also. Anti-TTG antibodies seem to be more specific but both measurements had limitations in the diagnosis of CD. Our data support the view that there is no single test or measurement that can identify all subjects with CD and ISS. Histological findings of CD showed a spectrum ranging from type 1 mucosal lesions to total villous atrophy type 4 in our study. Fifteen of 35 CD patients (42.9% of CD cases) had mild mucosal abnormality without villous atrophy, 15 (42.9% of CD cases) had partial villous atrophy, four (10.3% of CD cases) had subtotal villous atrophy and one (2.9% of CD cases) had total villous atrophy. According to histological findings, if we limited the diagnosis of CD to cases with villous atrophy, only 20 of 106 ISS cases in our study would have CD (19.3% of all cases) which is obviously more common than the general population.
In conclusion, the possibility of CD should be kept in mind as the prevalence of CD is high in patients with ISS. The patients affected by CD did not differ from those without CD in any of the symptoms. Patients with ISS should be evaluated for CD even in the absence of typical clinical symptoms. It is important to test all children with ISS for CD by measuring anti-EMA IgA or anti-TTG antibodies and performing an intestinal biopsy.
Clinical bottom line: In 33.6% patients with idiopathic short stature, CD may be the underlying cause. Investigation of CD is recommended in the diagnostic assessment of a short child with no endocrinological abnormality.
COMMENTS
Background
Short stature is one of the most common causes for referrals to pediatric endocrinologists. Many of these patients have no identifiable medical abnormality and are classified as idiopathic short stature (ISS). For most of these patients, it is believed that genetic variations are the underlying cause. Short stature is a well known feature of pediatric celiac disease (CD).
Research frontiers
The prevalence of CD is high in patients with ISS and it is important to test all children with ISS for CD by measuring serologic markers and performing an intestinal biopsy.
Peer review
This is a well written manuscript with a good abstract. In the text they mentioned jejunal biopsies as the gold standard for diagnosis.
Acknowledgments
It is our greate pleasure to thanks Dr. SP Payami for referring of some cases and Dr. T Rajabi for revewing of pathologic slides and Mr SA Latifi for statistical analysis of data. We greatly appreciate the cooperation and assistance we received from the nursing staff of Emam and Golestan Hospitals. The authors sincerely thank the children and their parents for their participation in this study. The authors also thank Mrs Shahnaz Shahid Zadeh for her excellent assistance.
Footnotes
Supported by A Grant from the Ahwaz Jondishapoor University of Medical Science, Ahwaz, Iran
Peer reviewer: Ian D Wallace, MD, Shakespeare Specialist Group, 181 Shakesperare Rd, Milford, Auckland 1309, New Zealand
S- Editor Li JL L- Editor Cant MR E- Editor Yin DH
References
- 1.Pasquino AM, Albanese A, Bozzola M, Butler GE, Buzi F, Cherubini V, Chiarelli F, Cavallo L, Drop SL, Stanhope R, et al. Idiopathic short stature. J Pediatr Endocrinol Metab. 2001;14 Suppl 2:967–974. doi: 10.1515/jpem-2001-s209. [DOI] [PubMed] [Google Scholar]
- 2.Cacciari E, Salardi S, Volta U, Biasco G, Lazzari R, Corazza GR, Feliciani M, Cicognani A, Partesotti S, Azzaroni D. Can antigliadin antibody detect symptomless coeliac disease in children with short stature? Lancet. 1985;1:1469–1471. doi: 10.1016/s0140-6736(85)92251-2. [DOI] [PubMed] [Google Scholar]
- 3.Cacciari E, Salardi S, Lazzari R, Cicognani A, Collina A, Pirazzoli P, Tassoni P, Biasco G, Corazza GR, Cassio A. Short stature and celiac disease: a relationship to consider even in patients with no gastrointestinal tract symptoms. J Pediatr. 1983;103:708–711. doi: 10.1016/s0022-3476(83)80462-4. [DOI] [PubMed] [Google Scholar]
- 4.Visakorpi JK, Maki M. Changing clinical features of coeliac disease. Acta Paediatr Suppl. 1994;83:10–13. doi: 10.1111/j.1651-2227.1994.tb13221.x. [DOI] [PubMed] [Google Scholar]
- 5.Misra S, Ament ME. Diagnosis of coeliac sprue in 1994. Gastroenterol Clin North Am. 1995;24:133–143. [PubMed] [Google Scholar]
- 6.Grodzinsky E, Franzen L, Hed J, Strom M. High prevalence of celiac disease in healthy adults revealed by antigliadin antibodies. Ann Allergy. 1992;69:66–70. [PubMed] [Google Scholar]
- 7.George EK, Mearin ML, Bouquet J, von Blomberg BM, Stapel SO, van Elburg RM, de Graaf EA, Hertzberger-ten Cate R, van Suijlekom-Smith LW, Reeser HM, et al. Screening for coeliac disease in Dutch children with associated diseases. Acta Paediatr Suppl. 1996;412:52–53. doi: 10.1111/j.1651-2227.1996.tb14251.x. [DOI] [PubMed] [Google Scholar]
- 8.Oberhuber G, Granditsch G, Vogelsang H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol. 1999;11:1185–1194. doi: 10.1097/00042737-199910000-00019. [DOI] [PubMed] [Google Scholar]
- 9.Vitoria JC, Arrieta A, Arranz C, Ayesta A, Sojo A, Maruri N, García-Masdevall MD. Antibodies to gliadin, endomysium, and tissue transglutaminase for the diagnosis of celiac disease. J Pediatr Gastroenterol Nutr. 1999;29:571–574. doi: 10.1097/00005176-199911000-00018. [DOI] [PubMed] [Google Scholar]
- 10.Shamir R, Lerner A, Shinar E, Lahat N, Sobel E, Bar-or R, Kerner H, Eliakim R. The use of a single serological marker underestimates the prevalence of celiac disease in Israel: a study of blood donors. Am J Gastroenterol. 2002;97:2589–2594. doi: 10.1111/j.1572-0241.2002.06028.x. [DOI] [PubMed] [Google Scholar]
- 11.Visakorpi JK, Maki M. Changing clinical features of coeliac disease. Acta Paediatr Suppl. 1994;83:10–13. doi: 10.1111/j.1651-2227.1994.tb13221.x. [DOI] [PubMed] [Google Scholar]
- 12.Bonamico M, Scire G, Mariani P, Pasquino AM, Triglione P, Scaccia S, Ballati G, Boscherini B. Short stature as the primary manifestation of monosymptomatic celiac disease. J Pediatr Gastroenterol Nutr. 1992;14:12–16. doi: 10.1097/00005176-199201000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Cacciari E, Corazza GR, Salardi S, Pascucci MG, Tacconi M, Cicognani A, Tassinari D, Biasco G, Volta U, Lazzari R. What will be the adult height of coeliac patients? Eur J Pediatr. 1991;150:407–409. doi: 10.1007/BF02093719. [DOI] [PubMed] [Google Scholar]
- 14.Mäki M, Holm K. Incidence and prevalence of coeliac disease in Tampere. Coeliac disease is not disappearing. Acta Paediatr Scand. 1990;79:980–982. doi: 10.1111/j.1651-2227.1990.tb11367.x. [DOI] [PubMed] [Google Scholar]
- 15.de Lecea A, Ribes-Koninckx C, Polanco I, Calvete JF. Serological screening (antigliadin and antiendomysium antibodies) for non-overt coeliac disease in children of short stature. Acta Paediatr Suppl. 1996;412:54–55. doi: 10.1111/j.1651-2227.1996.tb14252.x. [DOI] [PubMed] [Google Scholar]
- 16.Tumer L, Hasanoglu A, Aybay C. Endomysium antibodies in the diagnosis of celiac disease in short-statured children with no gastrointestinal symptoms. Pediatr Int. 2001;43:71–73. doi: 10.1046/j.1442-200x.2001.01327.x. [DOI] [PubMed] [Google Scholar]
- 17.Vanderschueren-Lodeweyckx M, Wolter R, Molla A, Eggermont E, Eeckels R. Plasma growth hormone in coeliac disease. Helv Paediatr Acta. 1973;28:349–357. [PubMed] [Google Scholar]
- 18.Lecornu M, David L, Francois R. Low serum somatomedin activity in celiac disease. A misleading aspect in growth failure from asymptomatic celiac disease. Helv Paediatr Acta. 1978;33:509–516. [PubMed] [Google Scholar]
- 19.Naveh Y, Lightman A, Zinder O. A prospective study of serum zinc concentration in children with celiac disease. J Pediatr. 1983;102:734–736. doi: 10.1016/s0022-3476(83)80248-0. [DOI] [PubMed] [Google Scholar]
- 20.Swinson CM, Slavin G, Coles EC, Booth CC. Coeliac disease and malignancy. Lancet. 1983;1:111–115. doi: 10.1016/s0140-6736(83)91754-3. [DOI] [PubMed] [Google Scholar]
- 21.Mäki M, Holm K, Lipsanen V, Hällström O, Viander M, Collin P, Savilahti E, Koskimies S. Serological markers and HLA genes among healthy first-degree relatives of patients with coeliac disease. Lancet. 1991;338:1350–1353. doi: 10.1016/0140-6736(91)92234-s. [DOI] [PubMed] [Google Scholar]
- 22.Hin H, Bird G, Fisher P, Mahy N, Jewell D. Coeliac disease in primary care: case finding study. BMJ. 1999;318:164–167. doi: 10.1136/bmj.318.7177.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shahbazkhani B, Faezi T, Akbari MR, Mohamadnejad M, Sotoudeh M, Rajab A, Tahaghoghi S, Malekzadeh R. Coeliac disease in Iranian type I diabetic patients. Dig Liver Dis. 2004;36:191–194. doi: 10.1016/j.dld.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 24.Malekzadeh R, Sachdev A, Fahid Ali A. Coeliac disease in developing countries: Middle East, India and North Africa. Best Pract Res Clin Gastroenterol. 2005;19:351–358. doi: 10.1016/j.bpg.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Shahbazkhani B, Malekzadeh R, Sotoudeh M, Moghadam KF, Farhadi M, Ansari R, Elahyfar A, Rostami K. High prevalence of coeliac disease in apparently healthy Iranian blood donors. Eur J Gastroenterol Hepatol. 2003;15:475–478. doi: 10.1097/01.meg.0000059118.41030.96. [DOI] [PubMed] [Google Scholar]
- 26.Queiroz MS, Nery M, Cancado EL, Gianella-Neto D, Liberman B. Prevalence of celiac disease in Brazilian children of short stature. Braz J Med Biol Res. 2004;37:55–60. doi: 10.1590/s0100-879x2004000100008. [DOI] [PubMed] [Google Scholar]
- 27.Bozzola M, Giovenale D, Bozzola E, Meazza C, Martinetti M, Tinelli C, Corazza GR. Growth hormone deficiency and coeliac disease: an unusual association? Clin Endocrinol (Oxf) 2005;62:372–375. doi: 10.1111/j.1365-2265.2005.02227.x. [DOI] [PubMed] [Google Scholar]
- 28.Collin P. Should adults be screened for celiac disease? What are the benefits and harms of screening? Gastroenterology. 2005;128:S104–S108. doi: 10.1053/j.gastro.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 29.Hoffenberg EJ. Should all children be screened for celiac disease? Gastroenterology. 2005;128:S98–S103. doi: 10.1053/j.gastro.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 30.van Rijn JC, Grote FK, Oostdijk W, Wit JM. Short stature and the probability of coeliac disease, in the absence of gastrointestinal symptoms. Arch Dis Child. 2004;89:882–883. doi: 10.1136/adc.2004.057851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dieterich W, Laag E, Schopper H, Volta U, Ferguson A, Gillett H, Riecken EO, Schuppan D. Autoantibodies to tissue transglutaminase as predictors of celiac disease. Gastroenterology. 1998;115:1317–1321. doi: 10.1016/s0016-5085(98)70007-1. [DOI] [PubMed] [Google Scholar]
- 32.Ahmed ML, Allen AD, Sharma A, Macfarlane JA, Dunger DB. Evaluation of a district growth screening programme: the Oxford Growth Study. Arch Dis Child. 1993;69:361–365. doi: 10.1136/adc.69.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]