Abstract
AIM: To investigate the protective effect of melatonin on liver after intestinal ischemia-reperfusion injury in rats.
METHODS: One hundred and fifty male Wistar rats, weighing 190-210 g, aged 7 wk, were randomly divided into melatonin exposure group, alcohol solvent control group and normal saline control group. Rats in the melatonin exposure group received intraperitoneal (IP) melatonin (20 mg/kg) 30 min before intestinal ischemia-reperfusion (IR), rats in the alcohol solvent control group received the same concentration and volume of alcohol, and rats in the normal saline control group received the same volume of normal saline. Serum samples were collected from each group 0.5, 1, 6, 12, and 24 h after intestinal IR. Levels of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured with an auto-biochemical analyzer. Serum TNF-α was tested by enzyme-linked immunosorbent assay (ELISA). Malondialdehyde (MDA) in liver was detected by colorimetric assay. Pathological changes in liver and immunohistochemical straining of ICAM-1 were observed under an optical microscope.
RESULTS: The levels of ALT measured at various time points after intestinal IR in the melatonin exposure group were significantly lower than those in the other two control groups (P < 0.05). The serum AST levels 12 and 24 h after intestinal IR and the ICAM-1 levels (%) 6, 12 and 24 h after intestinal IR in the melatonin exposure group were also significantly lower than those in the other two control groups (P < 0.05).
CONCLUSION: Exotic melatonin can inhibit the activity of ALT, AST and TNF-α, decrease the accumulation of MDA, and depress the expression of ICAM-1 in liver after intestinal IR injury, thus improving the liver function.
Keywords: Melatonin, Intestinal ischemia-reperfusion injury, Liver, TNF-α
INTRODUCTION
Bowel transplantation is still the only definite therapy for bowel dysfunction although it is more difficult than other organ transplantations at the final stage of bowel dysfunction[1]. Small intestine with high immunogenicity has the highest reject reaction rate in all organ transplantations[2]. Although more and more powerful nonspecific immunosuppressive agents have been used in recent years, the rate of graft rejecting reaction is still higher than 50% in intestine[3]. Current clinical data show that combined liver and small intestine transplantation decreases both acute and chronic rejection compared with simple small intestine transplantation[4]. Small intestine transplantation, the best therapy for bowel dysfunction at present, cannot replace total parenteral nutrition (TPN) due to its severe rejecting reaction. Long term TPN therapy may induce liver injury and some patients with intestinal dysfunction can accompany hepatic diseases. Since combined liver and intestine transplantation can prevent further liver injuries[5], systematic research should be done to identify the mechanism of hepatic injury caused by intestinal ischemia-reperfusion (IR) and detect the possible intervention which can lessen the injury. Melatonin can protect liver from intestinal IR injury due to its powerful ability to clear free radicals[6]. The objective of this study was to determine the possible protective effect and mechanisms of melatonin in intestinal IR models and provide evidence for its clinical application in spare-part surgery.
MATERIALS AND METHODS
Materials
One hundred and fifty healthy male Wistar rats, weighing 190 to 210 g, aged 6-7 wk, were randomly allocated into melatonin disposure group, alcohol solvent control group and normal saline (NS) control group. All rats were raised for at least 1 wk before operation in a 12 h dark and 12 h light cycle, with free access to food and water. One gram of melatonin (Sigma Company, USA) was dissolved in alcohol solvent (40%) and kept at a sub-ambient temperature. Rats in the melatonin exposure group received 20 mg/kg intra-peritoneal melatonin diluted by NS to 1/10 of the incipient concentration 30 min before intestinal IR. Rats in the alcohol solvent control group received the same concentration of alcohol and NS. Thiopental sodium (40 mg/kg) was injected into biceps femoris of rats 45 min before the intestinal IR model was established. A model of pan-small intestinal IR injury was established by occlusion of the superior mesenteric artery (SMA) for 30 min. Serum samples were collected from rats in each group after reperfusion for 0.5, 1, 6, 12 and 24 h. A paraformaldehyde (PFA, 40 g/L) phosphate buffer (0.1 mol/L, pH = 7.3) was provided by Laboratory of Shengjing Hospital, China Medical University (Shenyang, China). Correlated biochemical agents were bought from Boehringer Mannheim Company in Germany. MDA kits were purchased from Nanjing Jiancheng Biological Engineering Research Center. TNF-α kit was bought from Shenzhen Jingmei Biological Engineering Limited Company (China). ICAM-1 kit was purchased from Serotech Company in UK. OLYMPUS-BX60 optical microscope and photograph system, HITACHI-7600A automatic biochemistry analyzer and SAKURA paraffin section cutter, were provided by Laboratory of China Medical University (Shenyang, China).
Methods
Blood was obtained by trans-diaphragmatic cardiac puncture, each sample was centrifuged for 5 min at 3000 r/min with the clear supernatant remained. ALT and AST were detected with an automatic biochemistry analyzer. Serum TNF-α was determined by ELISA. MDA in liver tissue homogenate obtained from the central part of medial lobes was assayed by colorimetry. ICAM-1 in liver cells was tested by immunohistochemistry and observed under an optical microscope. Cells (including endotheliocytes in liver sinusoid and hepatocytes) with fine yellow particles in cytoplasm were defined as positive. The number of ICAM-1 positive cells per one high power field was calculated, the mean value was expressed as mean ± SD. All parameters were analyzed by variance analysis and SNK test using SPSS 13.0.
RESULTS
Serum ALT
After reperfusion for 12 and 24 h, the levels of ALT in the exposure group at each time point were significantly lower than those in the alcohol solvent and NS control groups (P < 0.05), while there was no obvious difference between alcohol solvent and NS control groups (Table 1).
Table 1.
ALT, AST, TNF-α, MDA and ICAM-1 in rats with ischemia-reperfusion injury (mean ± SD)
| Group | Time (h) | Serum ALT (μkat/L) | Serum AST (μkat/L) | Serum TNF-α (pg/mL) | MDA in liver (nmol/g) | ICAM-1 in liver (%) |
| Melatonin | 0.5 | 1.8 ± 0.6a | 1.8 ± 0.6a | 8.2 ± 2.9 | 23.7 ± 4.3 | 2.9 ± 1.2 |
| 1 | 1.6 ± 0.5a | 1.6 ± 0.5a | 10.9 ± 3.0 | 28.3 ± 5.1 | 3.0 ± 0.9 | |
| 6 | 1.5 ± 0.5a | 1.5 ± 0.5a | 14.8 ± 3.8a | 42.0 ± 5.2a | 4.0 ± 1.4a | |
| 12 | 1.4 ± 0.4a | 1.4 ± 0.4a | 18.0 ± 3.3a | 55.2 ± 5.4a | 11.8 ± 3.3a | |
| 24 | 1.3 ± 0.4a | 1.3 ± 0.4a | 15.7 ± 3.3 | 82.7 ± 6.1a | 6.9 ± 2.7a | |
| Alcohol | 0.5 | 3.1 ± 0.3 | 3.1 ± 0.3 | 8.8 ± 2.5 | 27.7 ± 5.9 | 2.4 ± 1.3 |
| 1 | 3.0 ± 0.6 | 3.0 ± 0.6 | 11.8 ± 3.7 | 33.2 ± 6.3 | 2.5 ± 0.7 | |
| 6 | 2.5 ± 0.4 | 2.5 ± 0.4 | 20.9 ± 4.3 | 54.3 ± 6.5 | 7.0 ± 2.3 | |
| 12 | 2.4 ± 0.4 | 2.4 ± 0.4 | 23.5 ± 4.3 | 79.1 ± 6.2 | 22.4 ± 4.3 | |
| 24 | 2.1 ± 0.5 | 2.1 ± 0.5 | 17.7 ± 3.9 | 106.2 ± 7.1 | 16.0 ± 3.2 | |
| NS | 0.5 | 3.3 ± 0.4 | 3.3 ± 0.4 | 9.4 ± 2.8 | 29.5 ± 6.3 | 2.8 ± 0.8 |
| 1 | 2.9 ± 0.5 | 2.9 ± 0.5 | 11.6 ± 3.3 | 34.1 ± 5.4 | 2.6 ± 1.3 | |
| 6 | 2.6 ± 0.4 | 2.6 ± 0.4 | 21.4 ± 5.0 | 53.8 ± 6.1 | 6.8 ± 2.2 | |
| 12 | 2.5 ± 0.4 | 2.5 ± 0.4 | 24.4 ± 4.9 | 83.3 ± 5.2 | 21.4 ± 5.4 | |
| 24 | 2.2 ± 0.4 | 2.2 ± 0.4 | 16.3 ± 3.6 | 108.5 ± 9.8 | 14.3 ± 2.8 |
P < 0.05 vs alcohol solvent and NS control groups.
Serum AST
After reperfusion for 12 and 24 h, the levels of AST in the melatonin exposure group were significantly lower than those in the alcohol solvent and NS control groups (P < 0.05), while there was no obvious difference between the alcohol solvent and NS control groups (Table 1).
Serum TNF-α
After reperfusion for 12 and 24 h, the levels of TNF-α in the melatonin exposure group were significantly lower than those in the alcohol solvent and NS control groups (P < 0.05), while there was no obvious difference between the alcohol solvent and NS control groups (Table 1).
MDA in liver tissue homogenate
After reperfusion for 6, 12 and 24 h, MDA in the melatonin exposure group was significantly lower than that in the alcohol solvent and NS control groups (P < 0.05), while there was no obvious difference between the alcohol solvent and NS control groups (Table 1).
ICAM-1 stained cells in liver tissue
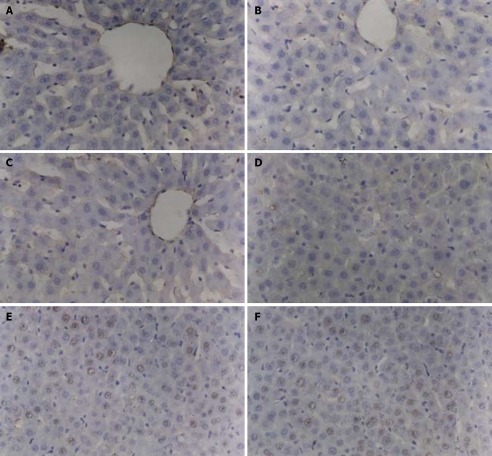
After reperfusion for 6, 12 and 24 h, the positive rate of ICAM-1-stained cells in the melatonin exposure group was significantly lower than that in the alcohol solvent and NS control groups (P < 0.05), while there was no obvious difference between the alcohol solvent and NS control groups. The positive cells were mainly liver parenchymal cells located near the sinus hepaticus and sinusoid endothelial cells of liver (Figure 1A-F). The number of positive cells in the alcohol solvent and NS control groups increased gradually and reached its peak at 12 h, and then decreased. The similar trend occurred in the melatonin exposure group, but the extent was much lower than that in the other two control groups (Table 1).
Figure 1.
Positive cells are mainly hepatic parenchymal cells located near the sinus hepaticus and sinusoid endothelial cells of liver in melatonin exposure group (A, D), alcohol solvent control group (B, E), NS control group (C, F). The positive cell rate of two control groups increased gradually and reached its peak 12 h after reperfusion, then decreased. This condition also occurred in melatonin exposure group, but the extent was much lower than that in the control groups.
DISCUSSION
Serum ALT and AST levels are generally accepted as the most sensitive indexes of acute hepatic injury and AST appears at the later phase[7]. After reperfusion, the ALT levels in the three groups gradually decreased with the time and were lower in the melatonin exposure group than in the other two control groups (P < 0.05), while AST level gradually increased with the time and maintained at its original level in the melatonin exposure group (P < 0.05, Table 1). Hepatic injury occurred at the early stage of pan-intestinal IR SMA was blocked for 30 min. The possible reason is that indirect clipping SMA reduced the portal blood flow. The accrescence of ALT indicates the hepatic injury, but the ischemic time is too short to lead to prominent AST changes. After the block of SMA was released, the hepatic injury due to reduced blood flow was ameliorated, but a great amount of active substances, such as free radicals, TNF-α, ICAM-1, etc, entered hepatic tissues via superior mesenteric and portal veins leading to secondary hepatic injuries, which can explain the variable curves of ALT and AST in the other two control groups. Compared with the other two control groups, exogenous melatonin could ameliorate the hepatic function after intestinal IR injury at the early or late phase. Recent studies about the protective effect of melatonin on IR injury in various organs showed that melatonin can eliminate free radicals, inhibit release of inflammatory media and apoptosis[8-10]. On the other hand, many factors can result in IR injury, such as free radicals[11], overload of calcium[12], inflammation of white blood cells and vascular endothelial cells[13] and apoptosis, etc[14]. Therefore, we detected serum TNF-α, MDA and ICAM-1-stained cells in liver tissues in this study. TNF-α gradually increased during the first 12 h after reperfusion and then decreased when it reached its peak between 12 and 24 h in the three groups. However, the TNF-α level was lower in the melatonin exposure group than in the other two control groups 12 and 24 h after reperfusion (P < 0.05), suggesting that TNF-α increases in systemic circulation after pan-intestinal IR, which can be effectively inhibited by exogenous melatonin[15-17]. Furthermore, TNF-α is generally accepted as an important inflammatory medium which has the same position as the interleukin family, and participates in almost all inflammatory reactions[18,19]. According to the curves of TNF-α and AST in the first 12 h systemic circulation after reperfusion, the secondary hepatic injury after pan-intestinal reperfusion is related to the concentration of TNF-α in systemic circulation. MDA is a final product of lipid peroxide, and its concentration in tissues can directly reflect the extent of lipid peroxide injury[20]. The concentration of MDA in each group gradually increased after reperfusion, which is generally considered the result of generous release of free radicals and accumulation of lipid peroxide products after pan-intestinal IR[21]. However, the concentration of MDA was obviously lower in the melatonin exposure group than in the other two control groups 6, 12 and 24 h after reperfusion, indicating that melatonin can relieve the IR injury by eliminating free radicals[22]. It was reported that ICAM-1 is directly correlated with the inflammation of white blood cells and vascular endothelial cells[23]. In this study, ICAM-1 gradually increased after reperfusion and decreased after reaching its peak between 12 and 24 h, which was in accordance with the concentration of TNF-α in systemic circulation. However, ICAM-1 was obviously lower in the melatonin exposure group than in the other two control groups. Analysis of the data showed that after clipping SMA for 30 min, (1) the first hepatic injury was relatively mild and reversible while the secondary injury was permanent and severe; (2) a large number of free radicals induced the expression of inflammatory factors such as TNF-α and ICAM-1 leading to hepatic injury through the portal vein, and free radicals directly caused peroxide injury after reperfusion; (3) exogenous melatonin protected liver from intestinal IR injury by inhibiting the production of free radicals, reducing the concentration of TNF-α in systemic circulation, and suppressing the expression of ICAM-1 in liver, etc. It has been recently shown that liver is the final metabolic place of melatonin and melatonin protects liver from intestinal IR injury[24-26]. Studies have shown that melatonin has no side effect when a large dose is used[27,28]. Intestine can absorb nutrients and has immune functions, and its vascular anatomy is specifically related with liver, the biggest digestive gland in human body. Since the blood in intestinal vein goes through the portal vein to the liver, intestinal IR inevitably affects the normal physical function of liver. During IR, a great amount of xanthic dehydrogenase in intestinal mucosa would change into xanthic oxydase, and xanthic oxydase can induce the production of free radicals which enhance the expression of adherence factors such as ICAM-1. These adherence factors are the main etiological agents of the secondary hepatic injury[29]. Up to now, rejecting reaction, graft-versus-host disease (GVHD) and infection are the restrained factors for intestine transplantation[30].
In conclusion, IR is the key mechanism underlying liver injuries at the early stage of organ transplantation.
COMMENTS
Background
Common intestinal ischemic diseases, intestinal resection caused by various etiologies, intestinal transplantation due to short bowel syndrome and long-term intravenous nutrition induce various hepatic injuries. On the other hand, combined liver and intestine transplantation has some immune dominance compared with simple intestinal transplantation, and acute or chronic rejecting reaction occurred in combined liver and intestine transplantation obviously decreases. Thus, it is necessary to study the mechanism of hepatic injury caused by intestine ischemia-reperfusion (IR) and discuss the injury relieving therapies.
Research frontiers
Melatonin is an effective free radical scavenger in liver, kidney, pancreas, digestive tract, etc. It is generally accepted that melatonin plays a role in protecting liver from intestinal IR injury by eliminating free radicals in cytoplasm. Thus, melatonin secreted by conarium is an endocrine hormone and has a very complicated mechanism of action and its receptors are distributed in almost all human organs. At present, researchers are paying their attention to the modulating function of melatonin in various inflammatory media.
Innovations and breakthroughs
Melatonin interferes with hepatic injury after intestinal reperfusion. The possible protective mechanism of melatonin by producing and releasing inflammatory agents was discussed. Melatonin was found to be able to reduce the concentration of serum TNF-α and inhibit the expression of ICAM-1.
Applications
Exogenous melatonin can protect liver from intestinal IR injury after intestinal ischemic-reperfusion by eliminating free radicals, reducing the production and release of inflammatory media, etc. As an effective and safe clinical medicine, it can be extensively used in surgery.
Terminology
Melatonin is an endocrine hormone secreted by conarium. Endogenous melatonin plays an important role in organic biorhythm, developing cycle, internal environmental stabilization and anti-caducity. TNF-α is an important inflammatory medium and participates in almost all inflammatory reactions. ICAM-1 is a member of the adhesive molecule family and is closely related to adhesion of neutrophile granulocytes and vascular endothelial cells in inflammatory reaction.
Peer review
This is a well written and interesting manuscript. The authors demonstrated that melatonin can protect liver from intestinal IR injury by abating the concentration of serum TNF-α and inhibiting the expression of ICAM-1 in liver cells.
Acknowledgments
The authors thank Dr. Wei-Guo Zhang for his helpful discussion and proof reading.
Footnotes
Supported by The Natural Science Foundation of Liaoning Province, No. 20042064
Peer reviewer: Adrian G Cummins, MD, Department of Gastroenterology and Hepatology, 28 Woodville Road, Woodville South, 5011, South Australia, Australia
S- Editor Zhu WL L- Editor Wang XL E- Editor Yin DH
References
- 1.DeLegge M, Alsolaiman MM, Barbour E, Bassas S, Siddiqi MF, Moore NM. Short bowel syndrome: parenteral nutrition versus intestinal transplantation. Where are we today? Dig Dis Sci. 2007;52:876–892. doi: 10.1007/s10620-006-9416-6. [DOI] [PubMed] [Google Scholar]
- 2.Harada E, Ito H, Murakami M, Li TS, Enoki T, Noshima S, Hamano K. Small bowel transplantation tolerance achieved by costimulatory blockade leading to mixed chimerism. Front Biosci. 2007;12:3017–3023. doi: 10.2741/2292. [DOI] [PubMed] [Google Scholar]
- 3.O'Keefe SJ, Emerling M, Koritsky D, Martin D, Stamos J, Kandil H, Matarese L, Bond G, Abu-Elmagd K. Nutrition and quality of life following small intestinal transplantation. Am J Gastroenterol. 2007;102:1093–1100. doi: 10.1111/j.1572-0241.2007.01125.x. [DOI] [PubMed] [Google Scholar]
- 4.Buchman AL, Iyer K, Fryer J. Parenteral nutrition-associated liver disease and the role for isolated intestine and intestine/liver transplantation. Hepatology. 2006;43:9–19. doi: 10.1002/hep.20997. [DOI] [PubMed] [Google Scholar]
- 5.Troxell ML, Higgins JP, Kambham N. Evaluation of C4d staining in liver and small intestine allografts. Arch Pathol Lab Med. 2006;130:1489–1496. doi: 10.5858/2006-130-1489-EOCSIL. [DOI] [PubMed] [Google Scholar]
- 6.Cay A, Imamoglu M, Unsal MA, Aydin S, Alver A, Akyol A, Sarihan H. Does anti-oxidant prophylaxis with melatonin prevent adverse outcomes related to increased oxidative stress caused by laparoscopy in experimental rat model? J Surg Res. 2006;135:2–8. doi: 10.1016/j.jss.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 7.Zhang WH, Li JY, Zhou Y. Melatonin abates liver ischemia/reperfusion injury by improving the balance between nitric oxide and endothelin. Hepatobiliary Pancreat Dis Int. 2006;5:574–579. [PubMed] [Google Scholar]
- 8.Wang WZ, Fang XH, Stephenson LL, Khiabani KT, Zamboni WA. Melatonin reduces ischemia/reperfusion-induced superoxide generation in arterial wall and cell death in skeletal muscle. J Pineal Res. 2006;41:255–260. doi: 10.1111/j.1600-079X.2006.00361.x. [DOI] [PubMed] [Google Scholar]
- 9.Duan Q, Wang Z, Lu T, Chen J, Wang X. Comparison of 6-hydroxylmelatonin or melatonin in protecting neurons against ischemia/reperfusion-mediated injury. J Pineal Res. 2006;41:351–357. doi: 10.1111/j.1600-079X.2006.00374.x. [DOI] [PubMed] [Google Scholar]
- 10.Munoz-Casares FC, Padillo FJ, Briceno J, Collado JA, Munoz-Castaneda JR, Ortega R, Cruz A, Tunez I, Montilla P, Pera C, et al. Melatonin reduces apoptosis and necrosis induced by ischemia/reperfusion injury of the pancreas. J Pineal Res. 2006;40:195–203. doi: 10.1111/j.1600-079X.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- 11.He SQ, Zhang YH, Venugopal SK, Dicus CW, Perez RV, Ramsamooj R, Nantz MH, Zern MA, Wu J. Delivery of antioxidative enzyme genes protects against ischemia/reperfusion-induced liver injury in mice. Liver Transpl. 2006;12:1869–1879. doi: 10.1002/lt.21001. [DOI] [PubMed] [Google Scholar]
- 12.Li SZ, Wu F, Wang B, Wei GZ, Jin ZX, Zang YM, Zhou JJ, Wong TM. Role of reverse mode Na+/Ca2+ exchanger in the cardioprotection of metabolic inhibition preconditioning in rat ventricular myocytes. Eur J Pharmacol. 2007;561:14–22. doi: 10.1016/j.ejphar.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 13.Hsieh YH, Huang SS, Wei FC, Hung LM. Resveratrol attenuates ischemia - reperfusion-induced leukocyte - endothelial cell adhesive interactions and prolongs allograft survival across the MHC barrier. Circ J. 2007;71:423–428. doi: 10.1253/circj.71.423. [DOI] [PubMed] [Google Scholar]
- 14.Kovacevic M, Simic O, Jonjic N, Stifter S. Apoptosis and cardiopulmonary bypass. J Card Surg. 2007;22:129–134. doi: 10.1111/j.1540-8191.2006.00355.x. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez-Reynoso S, Leal C, Portilla E, Olivares N, Muniz J. Effect of exogenous melatonin on hepatic energetic status during ischemia/reperfusion: possible role of tumor necrosis factor-alpha and nitric oxide. J Surg Res. 2001;100:141–149. doi: 10.1006/jsre.2001.6185. [DOI] [PubMed] [Google Scholar]
- 16.Gitto E, Romeo C, Reiter RJ, Impellizzeri P, Pesce S, Basile M, Antonuccio P, Trimarchi G, Gentile C, Barberi I, et al. Melatonin reduces oxidative stress in surgical neonates. J Pediatr Surg. 2004;39:184–189; discussion 184-189. doi: 10.1016/j.jpedsurg.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Gitto E, Reiter RJ, Cordaro SP, La Rosa M, Chiurazzi P, Trimarchi G, Gitto P, Calabro MP, Barberi I. Oxidative and inflammatory parameters in respiratory distress syndrome of preterm newborns: beneficial effects of melatonin. Am J Perinatol. 2004;21:209–216. doi: 10.1055/s-2004-828610. [DOI] [PubMed] [Google Scholar]
- 18.Lopez-Neblina F, Toledo-Pereyra LH. Anti-ischemic effect of selectin blocker through modulation of tumor necrosis factor-alpha and interleukin-10. J Surg Res. 2007;138:275–283. doi: 10.1016/j.jss.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 19.Cavriani G, Domingos HV, Oliveira-Filho RM, Sudo-Hayashi LS, Vargaftig BB, de Lima WT. Lymphatic thoracic duct ligation modulates the serum levels of IL-1beta and IL-10 after intestinal ischemia/reperfusion in rats with the involvement of tumor necrosis factor alpha and nitric oxide. Shock. 2007;27:209–213. doi: 10.1097/01.shk.0000238068.84826.52. [DOI] [PubMed] [Google Scholar]
- 20.Bolcal C, Yildirim V, Doganci S, Sargin M, Aydin A, Eken A, Ozal E, Kuralay E, Demirkilic U, Tatar H. Protective effects of antioxidant medications on limb ischemia reperfusion injury. J Surg Res. 2007;139:274–279. doi: 10.1016/j.jss.2006.10.043. [DOI] [PubMed] [Google Scholar]
- 21.Ozacmak VH, Sayan H, Igdem AA, Cetin A, Ozacmak ID. Attenuation of contractile dysfunction by atorvastatin after intestinal ischemia reperfusion injury in rats. Eur J Pharmacol. 2007;562:138–147. doi: 10.1016/j.ejphar.2007.01.061. [DOI] [PubMed] [Google Scholar]
- 22.Kiarostami V, Samini L, Ghazi-Khansari M. Protective effect of melatonin against multistress condition induced lipid peroxidation via measurement of gastric mucosal lesion and plasma malondialdehyde levels in rats. World J Gastroenterol. 2006;12:7527–7531. doi: 10.3748/wjg.v12.i46.7572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monson KM, Dowlatshahi S, Crockett ET. CXC-chemokine regulation and neutrophil trafficking in hepatic ischemia-reperfusion injury in P-selectin/ICAM-1 deficient mice. J Inflamm (Lond) 2007;4:11. doi: 10.1186/1476-9255-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chegaev K, Lazzarato L, Rolando B, Marini E, Tosco P, Cena C, Fruttero R, Bertolini F, Reist M, Carrupt PA, et al. NO-donor melatonin derivatives: synthesis and in vitro pharmacological characterization. J Pineal Res. 2007;42:371–385. doi: 10.1111/j.1600-079X.2007.00429.x. [DOI] [PubMed] [Google Scholar]
- 25.Sutken E, Aral E, Ozdemir F, Uslu S, Alatas O, Colak O. Protective role of melatonin and coenzyme Q10 in ochratoxin A toxicity in rat liver and kidney. Int J Toxicol. 2007;26:81–87. doi: 10.1080/10915810601122893. [DOI] [PubMed] [Google Scholar]
- 26.Xu J, Sun S, Wei W, Fu J, Qi W, Manchester LC, Tan DX, Reiter RJ. Melatonin reduces mortality and oxidatively mediated hepatic and renal damage due to diquat treatment. J Pineal Res. 2007;42:166–171. doi: 10.1111/j.1600-079X.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- 27.Cheung RT, Tipoe GL, Tam S, Ma ES, Zou LY, Chan PS. Preclinical evaluation of pharmacokinetics and safety of melatonin in propylene glycol for intravenous administration. J Pineal Res. 2006;41:337–343. doi: 10.1111/j.1600-079X.2006.00372.x. [DOI] [PubMed] [Google Scholar]
- 28.Pignone AM, Rosso AD, Fiori G, Matucci-Cerinic M, Becucci A, Tempestini A, Livi R, Generini S, Gramigna L, Benvenuti C, et al. Melatonin is a safe and effective treatment for chronic pulmonary and extrapulmonary sarcoidosis. J Pineal Res. 2006;41:95–100. doi: 10.1111/j.1600-079X.2006.00326.x. [DOI] [PubMed] [Google Scholar]
- 29.Xiaoqiao Z, Rong M, Zhigang Y, Yong D, Xihong F, Jingzhong S. Protective effect of ulinastatin against ischemia-reperfusion injury in rat small bowel transplantation. Transplant Proc. 2004;36:1564–1566. doi: 10.1016/j.transproceed.2004.05.059. [DOI] [PubMed] [Google Scholar]
- 30.Ruiz P, Kato T, Tzakis A. Current status of transplantation of the small intestine. Transplantation. 2007;83:1–6. doi: 10.1097/01.tp.0000232694.80537.d5. [DOI] [PubMed] [Google Scholar]