Abstract
Notch receptor regulates differentiation of almost all tissues and organs during animal development. Many mechanisms function at the protein level to finely regulate Notch activity. Here we provide evidence for Notch regulation at an earlier step - mRNA 3′ processing. Processing at the Notch consensus polyadenylation site appears by default to be suppressed in Drosophila embryos. Interference with this suppression, by a mutation, results in increased levels of polyadenylated Notch mRNA, excess Notch signaling, and severe developmental defects. We propose that Notch mRNA 3′ processing is negatively regulated to limit the production of Notch protein and render it a controlling factor in the generation of Notch signaling.
Introduction
Notch (N) signaling specifies binary cell fates and refines morphological patterns during differentiation of almost all tissues or organs in animals. N, a cell surface receptor, and Delta, a cell surface anchored ligand, mediate N signaling. N and Delta binding results in the release of the N intracellular domain (Nintra) from the cell surface. Nintra translocates to the nucleus and activates transcription of target genes. Cells that suppress N signaling commit to one developmental fate whereas cells that activate N signaling commit to the alternative developmental fate [1]–[9]. N signaling is very finely and tightly regulated. A mere 1.5–2X difference in gene dosage, or very low levels of constitutive activation, results in mutant phenotypes [10]–[20]. A number of mechanisms function at the level of N protein modification, trafficking, recycling, and degradation to regulate N activity [5]–[6], [21]–[26]. Whether N activity is regulated at the level of mRNA as well is uncertain.
Genetic screens indicate that many RNA binding proteins play important roles in N signaling [27]–[28]. We neither know their Notch pathway targets nor the mechanisms employed except for mushashi, which represses the translation of the numb mRNA [29]). Many of these RNA binding proteins are part of the basic mRNA 3′ processing machinery such as hiiragi that encodes a Poly(A) Polymerase in Drosophila melanogaster. Basic mRNA 3′ processing factors are required for processing of all poly(A) tailed mRNA but they show special interaction with N signaling, to the extent of even reproducing N mutant phenotypes [30]–[31]. It is not known why N signaling is particularly sensitive to changes in levels of basic mRNA 3′ processing factors and which among the more than 60 N signaling pathway genes is the target. Here we present evidence that the N gene itself is a target. N mRNA 3′ processing at the consensus poly(A) site appears to be usually suppressed in Drosophila embryos. A mutation in this poly(A) site increases the production of polyadenylated N mRNA, Nintra, and N signaling in association with severe developmental defects. Default suppression of mRNA 3′ processing at the N consensus poly(A) site might be important for limiting the production of the N protein, thereby enabling sensitive responses to developmental cues.
Materials and Methods
Fly Procedures
Wild type (y w), FM7a balancer, and DSC Nnd1 stocks were obtained from the Drosophila Stock Center. Nnd1/C(1)A/Y stock was obtained from Dr. Spyros Artavanis-Tsakonas (Harvard University). Standard Drosophila techniques [32] were used for generating iso-chromosomal lines and processing embryos for immuno-staining, northern blotting, and western blotting procedures. Embryos were staged according to reference [33].
Nnd1-dse is a temperature sensitive allele [34]. Nnd1-dse embryos are more or less wild type at the permissive temperature of 18°C and mutant at the restrictive temperature of 29°C. Embryonic mortality is about 60% at the restrictive temperature. Nnd1-dse and the wild type y, w embryos were collected at 18°C for 2–5 hours, aged to desired stages (taking into account the slower developmental rate at this temperature), and then shifted to 29°C for desired time periods before processing them for molecular procedures. Nnd1-dse and the wild type embryos were processed in parallel and in an identical manner. Embryos used for immuno-staining were transferred to the restrictive temperature half an hour before N signaling is used to specify neuronal and epidermal precursor cells (after about six hours of embryogenesis at 18°C).
Molecular Procedures
Procedures followed for DNA extraction, cloning, cDNA synthesis, immuno-staining, northern blotting, western blotting, and collection of staged embryos are described [18], [22], [25], [35], [and 36]. Northern blotting was used to check the levels of N and rp49 mRNA before proceeding with RT-PCR based analyses. For western blots, embryos were pulverized in 1X Laemmli buffer with β-marcaptoethanol and protease inhibitors. These blots were probed with N intracellular (NI; [18]) or hsp 70 antibodies (Sigma). Immuno-staining of embryos was performed with an anti-Hunchback antibody (Paul Macdonald) and signals developed with a HRP conjugated secondary antibody.
Primers used in PCR analysis for the isolation of the original Nnd-1 allele were: 5′ primer 1 (5′cggcggaggaggaggtggtggtggtggtgttgg3′); downstream 3′ primer 1 (5′aatcatccagatcacggtca3′); and deletion 3′ primer (5′ttcaggtccaagcccgctg3′). Primers flanking the deletion that were used for confirming the absence of the deletion in the original Nnd-1 allele were: 5′ primer 1 (5′cggcggaggaggaggtggtggtggtggtgttgg3′) and 3′ primer 2 (5′tatcgagggcggattcatttg3′). Sequencing was performed at the UVM Vermont Cancer Center Core Facility. PAT assays were done following the procedures described [37] [and 38]. The N specific primer used in these assays was 5 primer 2 (5′cacaaaaatcaccaatggaaacgtataagtc3′) and the rp49 specific primer used was 5′agtatctgatgcccaacatcg3′. Unprocessed (extended) N transcript analysis was done using 5′ primer 2 (5′cacaaaaatcaccaatggaaacgtataagtc3′) as the 5′ primer and 3′ primer 3, (5′cgggtttgtgtgtgtgtgtc3′) as the 3′ primer. Total RNA in the samples was assessed using rp49 primers 5′agtatctgatgcccaacatcg3′ and 5′ ttccgaccaggttacaagaac3′. High fidelity pfu turbo enzyme (Stratagene) was used for all PCR reactions. Megascript kit (Ambion) and poly(dT) 3′ primer with a 3-fold degenerate (A/G/C) 3′ end (for site of mRNA cleavage assay), poly(dT) 3′ primer (for PAT assay), or random hexamers (for extended transcript assay) were used to prepare cDNA. For northern blotting, PAT, and PCR assays embryos were collected 60–90 minutes after the flies were shifted to the restrictive temperature; for western blotting assay, embryos were collected after 120 minutes at the restrictive temperature.
For making actin promoter-GFPcoding-N3′UTR+DSE and actin promoter-GFPcoding-Nnd13′UTR+ Nnd1 DSE constructs, the KpnI-NotI GFP coding sequence fragment from pEGFP (Clontech) was inserted after the ∼2.7 kb EcoRI fragment containing actin 5C promoter in the pBluescript (pBS) plasmid. N3′UTR+N DSE and Nnd13′UTR+ Nnd1 DSE sequence were amplified with primers containing NotI sites, cloned into the NotI site at the end of the GFP coding sequence in pEGFP. Nnd13′UTR+N DSE was generated in a similar manner using a primer including the wild type DSE sequence and the Nnd13′UTR+ Nnd1 DSE template. Plasmids with the correct orientation and sequence were determined by sequencing and used to transiently transfect S2 cells. DNA was extracted from these cells and used to transfect bacteria for assessing transfection efficiency based on the number of bacterial colony forming units. We found the transfection efficiency to be the similar with different constructs (data not shown).
Images and figures were processed using Photoshop (Adobe) and Canvas (Deneba) programs. Any adjustments were applied to whole images.
Results
The Original Nnd-1 Allele Contains a Mutation in the Consensus Poly(A) Site of the N Gene
The original Nnd-1 mutation was mapped downstream of the DNA encoding Ankyrin repeats in the intracellular domain of the N protein [19, 34, 39; http://flybase.org). Initial reports that the Nnd-1 protein coding sequence downstream of Ankyrin repeats contains two amino acid polymorphisms were shown to be erroneous by later studies (http://flybase.org). All reports before the year 2000 showed that the coding sequence in the Nnd-1 allele is complete indicating that the Nnd-1 lesion lies in the 3′ UTR and the adjacent sequence important for mRNA 3′ processing (http://flybase.org). In 2007, Harding-Theobald et al. [40] reported that the Nnd-1 stock in the Drosophila Stock Center (DSC) contains a 41 base pair deletion within the coding region resulting in a frame-shift that would replace the terminal 129 amino acids of the N protein with a 63 amino acid-long novel sequence (http://flybase.org). Thus, the Harding-Theobald et al. (2007) report suggested the accumulation of a second mutation in the Nnd-1 allele.
To confirm our inference we obtained a culture of the Nnd-1 stock from Dr. Spyros Artavanis-Tsakonas (SAT) who was the source for the DSC Nnd-1 stock. We performed PCR analysis on the DSC and SAT Nnd-1 flies with a 5′ primer upstream of the deletion reported by Harding-Theobald et al. (2007) and two 3′ primers: one downstream of the deletion and one within the deleted sequence. From the DNA of wild-type flies we expected a 670 base pair (bp) product with the downstream 3′ primer 1 and a 290 bp product with the deletion 3′primer. The 290 bp-product was not expected from the DNA of flies carrying the deletion. Results showed that the DSC Nnd-1 fly stock does not yield the 290 bp product confirming the presence of the deletion ( Fig. 1A ). There was no evidence that the original Nnd-1 allele was present in this stock as increasing the number of PCR cycles, the amount of template, or the number of flies used for DNA extraction (up to 100) did not yield the 290 bp product. We always obtained the expected products from both the wild type and the DSC Nnd-1 DNA using PCR primers located outside the deletion (data not shown).
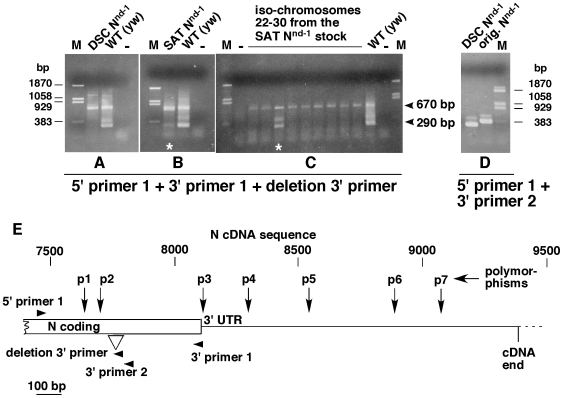
Figure 1. The original Nnd-1 allele is present in the SAT Nnd-1 stock.
(A) DSC Nnd-1 stock is homozygous for the Harding-Theobald et al. [40] deletion, as the deletion 3′ primer does not yield the 290 bp PCR product. (B) SAT Nnd-1 stock contains the original Nnd1 allele, as the 290 bp PCR product is present at a low level (lane with white asterisk). (C) In a sub-sample of 9 iso-chromosomal lines isolated from the SAT Nnd-1 stock one is revealed as the original Nnd-1 chromosome (lane with white asterisk). Photographs of ethidium bromide stained agarose gels are shown. PCR included one 5′ N primer (5′ primer 1) and two 3′ N primers; one located downstream of the deletion (3′ primer 1 that yields the 670 bp product) and one inside the deletion (deletion 3′ primer that yields the 290 bp product from alleles without the Theobald-Harding et al. deletion). (D) PCR primers flanking the Theobald-Harding et al. deletion (5′ primer 1 and 3′ primer 2) yield PCR products of different sizes indicating that this deletion is present in the DSC Nnd-1 allele but not in the original Nnd-1 allele. A 307 bp product was expected with the Theobald-Harding et al. deletion (from the DSC Nnd-1 allele) and a 348 bp product was expected without this deletion (from the original Nnd-1 allele). - = no template control; M = marker DNA. (E) Schematic representation of the Theobald-Harding et al., deletion (∇), primers used determine its presence or absence in fly lines (arrow heads), and polymorphisms (↓p1 – p5) detected in the study (p1 = CAA to CAG in the eighth Glutamine codon in the opa region; p2 = CAA to CAG in the last Glutamine codon in the opa region; p3 = G to C at position 8161; p4 = a T deletion at position 8300; and p5 = a T insertion at position 8544. Coding region polymorphisms p1 and p2 do not change the amino acid sequence, confirming the earlier report by others that there is no change in the amino acid sequence of the original nd1 allele (FlyBase).
The same assay on the SAT Nnd-1 fly stock showed that this stock contains the original Nnd-1 allele at a low frequency as we obtained a low level of the 290 bp PCR product ( Fig. 1B , lane with asterisk). To isolate this allele from the SAT Nnd-1 stock, we established 47 iso-chromosomal lines and assayed them for the deletion. Figure 1C shows a portion of our result in which one out of nine iso-chromosomes carries the original Nnd-1 allele indicated by the presence of the 290-bp product. The band above the 290 bp product is single-strand DNA produced by an imbalance in the activities of the primers (data not shown). Further experiments using primers that flanked the deletion confirmed that the original Nnd-1 chromosome does not contain the deletion, as PCR product obtained from this chromosome was longer than the product obtained from the DSC Nnd1 chromosome ( Fig. 1D ). Overall, we isolated six original Nnd-1 chromosomes.
We sequenced the entire DNA corresponding to the N intracellular domain, the 3′ UTR, and the downstream sequence (∼ 500 bp) in three iso-chromosomal lines with the deletion and three lines without the deletion. In the coding region of both types of lines, we found two polymorphisms that do not alter the amino acid sequence (p1 and p2 in Fig. 1E ). In the 3′ UTR region of both types of lines we discovered three classes of changes. The first class contained three polymorphisms that are also present in flies with the wild type phenotype (p3, p6, and p7 in Fig. 1E; http://flybase.org). The second class of change was either a T deletion within a run of 15 Ts or a T insertion within a run of 12 Ts in regions that are poorly conserved across the 12 sequenced Drosophila species, including changes in the number of Ts (p4 and p5 in Fig. 1E). In Drosophila S2 cells, the wild type N 3′ UTR and the Nnd-1 3′ UTR (with polymorphisms p3 to p7) linked to the actin promoter and the GFP coding sequence produced comparable levels of mRNA ( Fig. 2A ). This result was not surprising as these polymorphisms were too far upstream of the consensus poly(A) site (>290 bp) to affect mRNA 3′ processing. We conclude that the above two classes of changes are not the cause of the Nnd-1 phenotype.
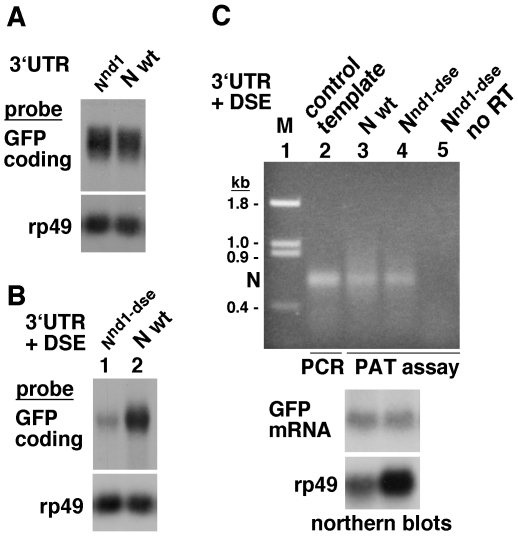
Figure 2. The mutation in the DSE and not polymorphisms or T deletion/insertion in the 3′ UTR of the original Nnd1 allele affects mRNA expression in Drosophila S2 cells.
(A) Northern blots showing that the 3′ UTR of the Nnd1 allele and 3′ UTR of the wild type N allele produce comparable levels of GFP mRNA. Both constructs contained the actin promoter, GFP coding region, and the wild type N DSE. (B) Northern blots showing that addition of the Nnd1 DSE mutation to the Nnd1 3′ UTR affects mRNA expression in Drosophila S2 cells. Nnd1-dse lane = actin promoter +GFP coding+ Nnd1 3′ UTR + Nnd1-dse; N wt lane = actin promoter +GFP coding+ N 3′ UTR + N dse. rp49 = RNA loading control. (C) Poly(A) Tail assay (PAT) of RNA samples used in Fig. 2B showing that polyadenylation of residual Nnd1-dse mRNA is comparable to the level of polyadenylation of wild type (wt) N mRNA (lanes 3 and 4). A 10∶1 ratio of Nnd1-dse:wt total RNA was used as it provided comparable levels of N RNA as determined by northern blots (bottom panel). PAT assay was performed using a N specific (5′ primer 2, see Fig. 4D) and an oligo d(T) primer that initiates DNA synthesis all along the length of the poly(A) tail, thereby revealing the level of polyadenylation of N mRNA. Lane 2 is the product of PCR using N cDNA template (control template) and an N primer (3′ primer 3, see Fig. 4D) ending at the N mRNA cleavage site to indicate the size of the fragment without the poly(A) tail. Ethidium bromide gel images are shown. no RT = reverse transcriptase omitted for cDNA synthesis.
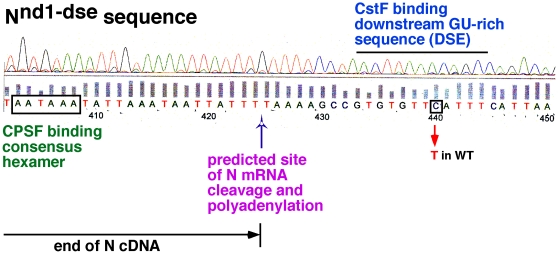
The third class of change was a T > C transition (position 9408 in N cDNA sequence) within the highly conserved GU-rich Downstream Sequence Element (DSE) of the N consensus polyadenylation (poly(A)) site ( Fig. 3 ). DSE is the binding site for mRNA 3′ processing factor Cleavage Stimulation Factor (CstF). CstF stimulates the activity of the basic mRNA 3′ processing complex that also contains Cleavage/Polyadenylation Specificity Factor (CPSF) and Poly(A) Polymerase (PAP). This complex binds mRNA in the region encompassing the AAUAAA hexamer and the GU-rich DSE, cleaves the nascent mRNA at a specific site called the cleavage site, and polyadenylates it. Polyadenylation is critical for mRNA stability, nuclear export, and translation [41]–[51]. Thus, the DSE mutation was expected to reduce mRNA levels.
Figure 3. Nnd1-dse mutation is in the GU-rich Down Stream Element (DSE) of the N consensus poly(A) site.
Important features of the poly(A) site are marked on the actual sequencing read-out of the Nnd1-dse sequence revealing the site of mutation.
To examine the possibility that the DSE mutation reduces mRNA levels, we cloned N 3′ UTR and downstream sequence, with and without the DSE mutation, after the GFP coding sequence linked to the actin 5C promoter. These constructs were expressed in S2 cells and levels of RNA assessed by northern blotting. Results of these studies confirmed that the DSE mutation suppresses mRNA expression in S2 cells ( Fig. 2B ). To determine if the DSE mutation affected polyadenylation as well, we first determined the ratio of total RNA amount that would contain approximately the same amount of GFP mRNA with and without the DSE mutation. We found it to be about 1∶10 (see the bottom panel in Fig. 2C ). We used this ratio of RNA in Poly(A) Tail (PAT) assays with a N 3′ UTR specific primer and poly(T) primer that would initiate cDNA synthesis all along the length of the poly(A) tail and reveal any difference in polyadenylation. We found comparable levels of polyadenylation in GFP mRNA with and without the DSE mutation ( Fig. 2C ). These data indicated that the T>C mutation in the N DSE is the cause of mutant phenotypes associated with the original Nnd-1 allele and suggested that it might reduce the stability of unprocessed N pre-mRNA in vivo leading to less mature mRNA. An effect on polyadenylation was not expected.
SAT Nnd-1 and DSC Nnd-1 Alleles Are Closely Related and Manifest Similar Mutant Phenotypes
Sequences from iso-chromosomal lines with the deletion (isolated from the SAT Nnd-1 stock) were identical to the sequence from the DSC Nnd-1 stock (which contains the same deletion). In other words, three sets of Nnd-1 lines (SAT without deletion, SAT with deletion, and DSC with deletion) can be grouped into two classes: Nnd-1 with or without the Harding-Theobald et al. (2007) deletion. These two classes manifest similar morphological and molecular phenotypes (data not shown). As all three sets of Nnd-1 lines share the DSE mutation but only two share the deletion mutation, the parsimonious conclusion is that the DSE mutation is the ancestral mutation. Thus, it appears that the deletion reported by Harding-Theobald et al. [40] originated in the SAT Nnd-1 fly stock and it is close to displacing the original Nnd-1 allele from this stock; the displacement is complete in the DSC Nnd-1 stock. From here onwards, we will focus on the ancestral (original) Nnd-1 allele, which will be referred to as Nnd1-dse.
The Nnd1-dse Allele Produces More Polyadenylated N mRNA and Nintra
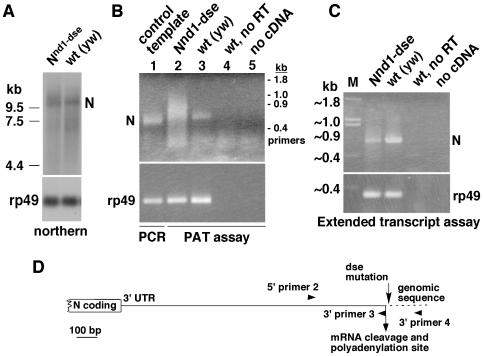
Northern blotting analysis showed that N mRNA in Nnd1-dse embryos generally ‘smears upwards’ suggesting increased mRNA length due to polyadenylation. A sample northern blot with comparable amounts of N mRNA in Nnd1-dse and wild type (y, w) embryos is shown in Figure 4A . An increase in the size of mRNA could be due to alteration in mRNA cleavage (the DSE mutation results in a CA sequence doublet that is frequently used for cleavage in the context of a poly(A) site), alteration in polyadenylation (fraction of mRNA polyadenylated or the length of ploy(A) tail), or increase in unprocessed (extended) transcripts. We examined these possibilities with the same RNA samples used in Figure 4A. First, we performed RT-PCR using a N specific 5′ primer and a poly(T) 3′ primer containing a degenerate base (A/G/C) at the 3′ end. The latter primer should initiate synthesis at the base preceding the poly(A) tail, thereby marking the site of mRNA cleavage. Sequencing of these RT-PCR fragments showed that mRNAs from both Nnd1-dse and wild type embryos were cleaved and poly(A) tailed at the predicted cleavage site (data not shown; see Figure 3 for the cleavage site). Next, we performed RT-PCR with an N-specific 5′ primer and a poly(T) 3′ primer, which would hybridize all along the length of the poly(A) tail and reflect the extent of poly(A) tails. Results from these experiments showed increased amounts of poly(A) tailed N mRNA in Nnd1-dse embryos ( Fig. 4B ). The same assay performed on the control rp49 mRNA in the same samples showed low and comparable levels between Nnd1-dse and wild-type embryos ( Fig. 4B , bottom panel). RT-PCR with one primer before the N mRNA cleavage site and one primer downstream of this site showed that the levels of unprocessed (extended) N transcripts were lower in Nnd1-dse embryos, which is consistent with an increased amount of the mature N mRNA in this sample ( Fig. 4C ). Thus, it appears that the DSE mutation increased processing of the N mRNA and reduced bypassing of the N consensus poly(A) site.
Figure 4. Nnd1-dse embryos produce higher levels of poly(A)-tailed N mRNA and Nintra.
(A) Northern blots showing comparable levels of N mRNA in Nnd1-dse and wild type embryos after 60 minutes at the restrictive temperature of 30°C. (B) PAT assay using N specific (5′ primer 2) or rp49 specific primer and an oligo d(T) primer that reveals the level of polyadenylation of N mRNA (top panel) and the control rp49 mRNA (bottom panel). The smear of fragments of heterogeneous lengths that is present only in the Nnd1-dse lane indicates that N mRNA is poly(A)-tailed to a higher level in Nnd1-dse embryos than in wild type embryos. Lane 1 is the product of PCR using N cDNA template (control template) and an N primer (3′ primer 3) ending at the N mRNA cleavage site. Ethidium bromide gel images are shown. no RT = reverse transcriptase omitted in the cDNA synthesis reaction. rp49 = rp49 PAT fragments amplified from the same samples that served as controls. (C) Unprocessed (extended) transcript assay using one primer upstream of the mRNA cleavage site (5′ primer 2) and one primer downstream of the cleavage site (3′ primer 4). Only N transcripts that bypass the consensus poly(A) site are expected to be amplified. To assess the level of total RNA in the reactions, primers located within the rp49 cDNA were used. Ethidium bromide gel images are shown. D. Schematic representation of the primers used for results presented in B and C.
Nnd1-dse Embryos Manifest Gain of N Signaling Phenotypes
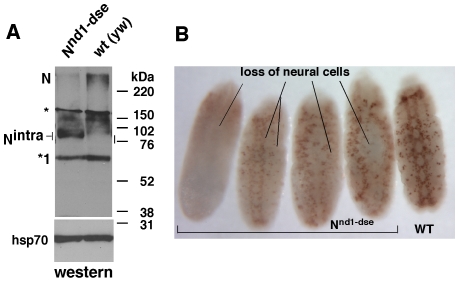
To determine if an increased level of mature N mRNA affected the production of either the full length N protein or Nintra, we performed western blotting analysis. Nnd1-dse embryo samples contained a much higher level of Nintra compared with the level in wild-type embryos ( Fig. 5A ). The lower level of full length N is possibly a consequence of rapid conversion to Nintra and negative regulation by Nintra (see discussion). Given the presence of higher amounts of Nintra, we predicted that Nnd1-dse embryos would manifest gain of N signaling phenotypes. To test this prediction, we studied neurogenesis where N functions are best understood. During neurogenesis, clusters of 12–20 cells first acquire the potential to become neuronal cells. These cells are called proneural cells. N signaling is inhibited in 1–2 proneural cells within each cluster to commit them to the neuronal fate. N signaling is increased in the remaining proneural cells in the cluster to commit them to the alternative epidermal fate. As a consequence, embryos with reduced N signaling manifest excess neuronal cells and embryos with increased N signaling manifest loss of neuronal cells [1], [13], [35]. We found that Nnd1-dse embryos manifest varying degrees of loss of neuronal cells ( Fig. 5B ). Comparison of Nnd1-dse and wild-type embryos at different stages of development indicate that about 50% of each stage of Nnd1-dse embryos manifested syndromes of defects consistent with increased N signaling (data not shown). These data confirmed our prediction from molecular analyses that the Nnd1-dse mutation results in a gain of N signaling. These results suggest that the level of Nintra is initially kept low via the suppression of mRNA 3′ processing at the consensus N poly(A) site. This suppression is disrupted by the Nnd1-dse mutation. As a consequence, N signaling is excessive and embryogenesis is severely disrupted.
Figure 5. Nnd1-dse embryos show gain-of-N signaling molecular and developmental phenotypes.
(A) Western blots showing that Nnd1-dse embryos overproduce Nintra. Statistical analysis of values standardized to the level of hsp70 showed that Nnd1-dse embryos produce 28.8X higher Nintra and 9.2X lower full length N compared to wild type embryos (p<0.01 for both, n = 3). N = full length N protein; * = non-specific band; *1 = the dominant-negative NΔCterm fragment [22], [25]. (B) Neuronal cells are lost to varying degrees in Nnd1-dse embryos, as expected with the gain in N signaling. Embryos were probed for the Hunchback protein, a neurogenesis marker.
Discussion
Our data show that the original Nnd1 allele, which is designated Nnd1-dse, is mutated in the DSE of the consensus poly(A) site of the N gene. DSE is well known to be required for mRNA 3′ processing and polyadenylation. Thus, a mutation in the N DSE was expected to reduce N mRNA 3′ processing and polyadenylation. We find the contrary result: N mRNA 3′ processing and polyadenylation is increased in Nnd1-dse embryos. Accordingly, N signaling is excessive in these embryos and embryogenesis is severely disrupted.
One possible explanation for our unexpected result is that the Nnd1-dse mutation in the DSE increases the activity of one of the component of the basic mRNA 3′ processing complex, for example CstF. If this were the case, we expected to have observed higher levels of mRNA and polyadenylation from DSE mutation constructs in cultured S2 cells as these cells contain all the basic mRNA 3′ processing factors. Instead, we observe a lower level of mRNA and no change in polyadenylation compared to the control construct. The lower level of mRNA from the mutated DSE construct is consistent with the interpretation that unprocessed transcripts are rapidly degraded. An alternative explanation for increased polyadenylation with the DSE mutation is based on the report from mammalian systems that the DSE can act as a binding site for a negative regulator of mRNA 3′ processing and polyadenylation [52]. Thus, poly(A) tailing of N mRNA in embryos might be normally kept low by a negative regulator that is not part of the basic mRNA 3′ processing complex, one that keeps poly(A) tailed N mRNA even lower than the level produced with impaired CstF function. In other words, the DSE mutation in the Nnd1-dse allele might reduce the activity of this negative regulator more than it affects the function of CstF, resulting in a net increase in N mRNA 3′ processing that in turn increases N mRNA polyadenylation and translation, Nintra production, and Notch signaling.
The higher level of Nintra in Nnd1-dse embryos is consistent with N mRNA polyadenylation being a limiting factor in the production of Nintra. Interestingly, the level of the full-length N protein (which is the substrate for Nintra production) is reduced rather than increased. One possibility is that the DSE mutation somehow affects the Nintra-producing N proteolysis mechanism operating at the cell surface (or in cytoplasmic vesicles). There is no known mechanism (nor can we imagine one) that links an mRNA 3′ processing mutation in the DSE to proteolysis of a protein at the cell surface or the cytoplasm (note that this mutation which lies outside the cleavage site is not expected to be in the polyadenylated mRNA transported to the cytoplasm for translation). We favor the alternative possibility that the reduction in the level of the full-length N protein in Nnd1-dse embryos is a combined effect of rapid conversion to Nintra and suppression due to Nintra over-expression. It is well known in the field that although the full length N protein is easily detected in western blots of wild type embryonic extract, Nintra is barely detectable indicating that the wild type N expression does not lead to promiscuous Nintra production. However, with even a mere 1.5 fold increases in endogenous N expression excess N signaling becomes apparent [11], [12], [14], [15]. In our experiments, we frequently detected mild accumulation of the full length N protein in Nnd1-dse embryos for a brief period (between 15 to 30 minutes) after transfer to the restrictive temperature and before the accumulation of Nintra (data not shown). These observations suggest that any increase in the level of the full-length N protein beyond the wild type level tips the balance towards increased processing of N to generate Nintra. Our previous studies have shown that increased Nintra production from a transgenic construct (that directly produces this molecule) suppresses the expression of the full length N protein from the endogenous gene in the background [22], [35], [36]. Thus, the loss of full length N protein in Nnd1-dse embryos could be a consequence of increased Nintra production.
We interpret our data as indicating that N mRNA 3′ processing and polyadenylation is subjected to strong negative regulation. This interpretation is supported by two well known and long-standing observations: (1) sensitivity of development to a mere 1.5–2X difference in N gene dosage or very small differences in the level of N signaling and (2) low, uniform expression of the Notch protein throughout the embryo [10]–[20]. The negative regulator could be one of the RNA binding proteins identified in genetic screens as a suppressor of N signaling [27], [28]. However, given the temperature sensitivity of the Nnd1-dse allele [34], it could also be the local structure of the N mRNA region encompassing the DSE. At this time, the mechanism is obscure but all indications are that it is unusual and novel, one that doesn't fit the known aspects of mRNA 3′ processing. The primary function of the N DSE appears to be prevention of mRNA 3′ processing rather than its promotion, which would explain the bypass of the consensus poly(A) site and production of extended transcripts. This mRNA 3′ processing mechanism could be an important regulator of N expression. The Notch promoter in Drosophila has remained elusive despite intense efforts by many laboratories and it appears that none of the many protein level regulatory mechanisms was able to check the abnormal molecular and morphological phenotypic effects of the Nnd1-dse mutation.
In summary, N is a basic regulator of development in all animals and a small difference in its function is frequently used during development to specify alternative cell fates, establish boundaries between tissues, or refine morphological patterns. Small perturbations in N activity lead to numerous human developmental defects and diseases including cancer, stroke, and dementia. As N activity is based on Nintra, our data showing that the loss of N DSE function results in a high level of Nintra production suggests that the mechanism regulating N mRNA 3′ processing is a critical regulator of N signaling. This mechanism appears to use mRNA 3′ processing elements for default suppression of N mRNA processing at the consensus poly(A) site. Our data and the Nnd1-dse embryo could be very useful for further studies aimed at dissecting this mechanism. They might also be useful for studies aimed at understanding how the many RNA binding proteins identified in genetic screens fit into the regulation of N signaling.
Acknowledgments
We thank the Drosophila Stock Center and Spyros Artavanis-Tsakonas for fly stocks; Paul McDonald for the Hunchback antibody; Greg Gilmartin and Krishnan Venkataraman for technical help and discussion; Uma Wesley, Chris Huston, and Justin Blau for comments on the manuscript. We thank the reviewers for their incisive comments that improved the quality of the manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by National Institutes of Health grant R01 NS43122; the Department of Microbiology and Molecular Genetics, University of Vermont; College of Medicine Bridge Funds, University of Vermont; and the National Institutes of Health COBRE grant P20 RR016435. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: Cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 2.Schweisguth F. Notch signaling activity. Curr Biol. 2004;14:R129–138. [PubMed] [Google Scholar]
- 3.Ahimou F, Mok L-P, Bardot B, Wesley CS. The adhesion force of Notch with Delta and the rate of Notch signaling. J Cell Bio. 2004;167:1217–1229. doi: 10.1083/jcb.200407100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lai EC. Notch signaling: control of cell communication and cell fate. Development. 2004;131:965–973. doi: 10.1242/dev.01074. [DOI] [PubMed] [Google Scholar]
- 5.Le Borgne R, Bardin A, Schweisguth F. The roles of receptor and ligand endocytosis in regulating Notch signaling. Development. 2005;132(8):1751–1762. doi: 10.1242/dev.01789. [DOI] [PubMed] [Google Scholar]
- 6.Herranz H, Milan M. Notch and affinity boundaries in Drosophila. BioEssays. 2006;28:113–116. doi: 10.1002/bies.20366. [DOI] [PubMed] [Google Scholar]
- 7.Baker NE. Patterning signals and proliferation in Drosophila imaginal discs. Curr Opin Genet Dev. 2007;17:287–293. doi: 10.1016/j.gde.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Carlson ME, Conboy IM. Regulating the Notch pathway in embryonic, adult, and old stem cells. Curr Opin Pharmacol. 2007;7:303–309. doi: 10.1016/j.coph.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Blank U, Karlsson G, Karlsson S. Signaling pathways governing stem-cell fate. Blood. 2008;111:492–503. doi: 10.1182/blood-2007-07-075168. [DOI] [PubMed] [Google Scholar]
- 10.Shellenbarger DL, Mohler JD. Temperature-sensitive periods and autonomy of pleiotropic effects of l(1)Nts1, a conditional Notch lethal in Drosophila. Dev Biol. 1978;62:432–446. doi: 10.1016/0012-1606(78)90226-9. [DOI] [PubMed] [Google Scholar]
- 11.De la Concha A, Dietrich U, Weigel D, Campos-Ortega J. Functional interactions of neurogenic genes of Drosophila melanogaster. Genetics. 1988;118:499–508. doi: 10.1093/genetics/118.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alton AK, Fechtel K, Kopczynski CC, Shepard SB, Kooh PJ, et al. Molecular genetics of Delta, a locus required for ectodermal differentiation in Drosophila. Dev Genetics. 1989;10:261–272. doi: 10.1002/dvg.1020100315. [DOI] [PubMed] [Google Scholar]
- 13.Cabrera CV. Lateral inhibition and cell fate during neurogenesis in Drosophila: the interactions between scute, Notch and Delta. Development. 1990;110:733–742. [PubMed] [Google Scholar]
- 14.Palka JC, Shubiger M, Schwaninger H. Neurogenic and antineurogenic effects from modifications at the Notch locus. Development. 1990;109:167–175. doi: 10.1242/dev.109.1.167. [DOI] [PubMed] [Google Scholar]
- 15.Heitzler P, Simpson P. The choice of cell fate in the epidermis of Drosophila. Cell. 1991;64:1083–1092. doi: 10.1016/0092-8674(91)90263-x. [DOI] [PubMed] [Google Scholar]
- 16.Fortini ME, Rebay I, Caron LA, Artavanis-Tsakonas S. An activated Notch receptor blocks cell-fate commitment in the developing Drosophila eye. Nature. 1993;365:555–557. doi: 10.1038/365555a0. [DOI] [PubMed] [Google Scholar]
- 17.Struhl G, Fitzgerald K, Greenwald I. Intrinsic activity of the Lin-12 and Notch intracellular domains in vivo. Cell. 1993;74:331–345. doi: 10.1016/0092-8674(93)90424-o. [DOI] [PubMed] [Google Scholar]
- 18.Lieber T, Kidd S, Alcamo E, Corbin V, Young MW. Antineurogenic phenotypes induced by truncated Notch proteins indicate a role in signal transduction and may point to a novel function for Notch in nuclei. Genes Dev. 1993;7:1949–1965. doi: 10.1101/gad.7.10.1949. [DOI] [PubMed] [Google Scholar]
- 19.Lyman D, Young MW. Further evidence for function of the Drosophila Notch protein as a transmembrane receptor. Proc Natl Acad Sci U S A. 1993;90:10395–10399. doi: 10.1073/pnas.90.21.10395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brennan K, Tateson R, Lewis K, Martinez-Arias A. A functional analysis of Notch mutations in Drosophila. Genetics. 1997;147:177–188. doi: 10.1093/genetics/147.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mishra-Gorur K, Rand MD, Perez-Villamil B, Artavanis-Tsakonas S. Down-regulation of Delta by proteolytic processing. J Cell Biol. 2002;159:313–324. doi: 10.1083/jcb.200203117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wesley CS, Mok L-P. Regulation of Notch signaling by a novel mechanism involving Suppressor of Hairless stability and carboxyl terminus-truncated Notch. Mol Cell Biol. 2003;23:5581–5593. doi: 10.1128/MCB.23.16.5581-5593.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sapir A, Assa-Kunik E, Tsruya R, Schejter E, Shilo B. Unidirectional Notch signaling depends on continuous cleavage of Delta. Development. 2005;132(1):123–32. doi: 10.1242/dev.01546. [DOI] [PubMed] [Google Scholar]
- 24.Emery G, Hutterer A, Berdnik D, Mayer B, Wirtz-Peitz F, et al. Asymmetric Rab11 Endosomes regulate Delta recycling and specify cell fate in the Drosophila nervous system. Cell. 2005;122:1–11. doi: 10.1016/j.cell.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 25.LeComte M, Wesley U, Mok L-P, Wesley CS. Evidence for involvement of dominant negative Notch molecules in the normal course of Drosophila development. Dev Dyn. 2006;235:411–426. doi: 10.1002/dvdy.20650. [DOI] [PubMed] [Google Scholar]
- 26.Zolkiewska A. ADAM proteases: ligand processing and modulation of Notch pathway. Cell Mol Life Sci. 2008;65:2056–2068. doi: 10.1007/s00018-008-7586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Norga KK, Gurganus MC, Dilda CL, Yamamoto A, Lyman RF, et al. Quantitative analysis of bristle number in Drosophila melanogaster mutants identifies genes involved in neural development. Curr Biol. 2003;13:1388–1397. doi: 10.1016/s0960-9822(03)00546-3. [DOI] [PubMed] [Google Scholar]
- 28.Kankel MW, Hurlbut GD, Upadhyay G, Yajnik V, Yedvobnick B, et al. Investigating the genetic circuitry of Mastermind in Drosophila, a Notch signal effector. Genetics. 2007;177:2493–2505. doi: 10.1534/genetics.107.080994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okano H, Imai T, Okabe M. Mushashi: a translational regulator of cell fate. J Cell Sci. 2002;115:1355–1359. doi: 10.1242/jcs.115.7.1355. [DOI] [PubMed] [Google Scholar]
- 30.Murata T, Nagaso H, Kashiwabara S, Baba T, Okano H, et al. The hiiragi gene encodes a poly(A) polymerase, which controls the formation of the wing margin in Drosophila melanogaster. Dev Biol. 2001;233(1):137–147. doi: 10.1006/dbio.2001.0205. [DOI] [PubMed] [Google Scholar]
- 31.Juge F, Zaessinger S, Temme C, Wahle E, Simonelig M. Control of poly(A) polymerase level is essential to cytoplasmic polyadenylation and early development in Drosophila. EMBO J. 2002;21(23):6603–13. doi: 10.1093/emboj/cdf633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ashburner M, Golic KG, Hawley RS. A Laboratory Handbook. 2nd Edition. New York, USA: Cold Spring Harbor Press; 2004. Drosophila. [Google Scholar]
- 33.Campos-Ortega JA, Hartenstein V. Springer; 1997. The embryonic development of Drosophila melanogaster. Second Edition. [Google Scholar]
- 34.Shellenbarger D, Mohler JD. Temperature-sensitive mutations of the Notch locus in Drosophila melanogaster. Genetics. 1975;81:143–162. doi: 10.1093/genetics/81.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bardot B, Mok L-P, Thayer T, Ahimou F, Wesley CS. The Notch amino terminus regulates protein levels and Delta induced clustering of Drosophila Notch receptors. Exp Cell Res. 2005;304:202–223. doi: 10.1016/j.yexcr.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 36.Wesley CS, Saez L. Analysis of Notch lacking the carboxyl terminus identified in Drosophila embryos. J Cell Biol. 2000;149:683–696. doi: 10.1083/jcb.149.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zanger RC, Hernandez M, Kocarek TA, Novak RF. Determination of the Poly(A) tail length of a single species in total hepatic RNA. BioTechniques. 1995;18:465–469. [PubMed] [Google Scholar]
- 38.Salles FJ, Richards WG, Strickland S. Assaying the polyadenylation state of mRNAs. Methods: A companion to Methods in Enzymology. 1999;17:38–45. doi: 10.1006/meth.1998.0705. [DOI] [PubMed] [Google Scholar]
- 39.Xu T, Rebay I, Fleming RJ, Scottgale N, Artavanis-Tsakonas S. The Notch locus and the genetic circuitry involved in early Drosophila neurogenesis. Genes Dev. 1990;4:464–475. doi: 10.1101/gad.4.3.464. [DOI] [PubMed] [Google Scholar]
- 40.Harding-Theobald ER, Ajagbe LA, Amina KK, Bajwa HS, Barnett CR, et al. N[nd-1] molecular lesion. 2007 http://flybase.org/reports/FBrf0195930.html. [Google Scholar]
- 41.Danckwardt S, Gehring NH, Neu-Yilik G, Hundsdoerfer P, Pforsich M, et al. The prothrombin 3′ end formation signal reveals a unique architecture that is sensitive to thrombophilic gain-of-function mutations. Blood. 2004;104:428–435. doi: 10.1182/blood-2003-08-2894. [DOI] [PubMed] [Google Scholar]
- 42.Zhang S, Ruiz-Echevarria MJ, Quan Y, Peltz SW. Identification and characterization of a sequence motif involved in nonsense-mediated mRNA decay. Mol Cell Biol. 1995;15(4):2231–2244. doi: 10.1128/mcb.15.4.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wells SE, Hillner PE, Vale RD, Sachs AB. Circularization of mRNA by eukaryotic translation initiation factors. Mol Cell. 1998;2(1):135–40. doi: 10.1016/s1097-2765(00)80122-7. [DOI] [PubMed] [Google Scholar]
- 44.Zhao J, Hyman L, Moore C. Formation of mRNA 3′ ends in eukaryotes: mechanism, regulation, and interrelationships with other steps in mRNA synthesis. Microbiology and Molecular Biology reviews. 1999;63:405–445. doi: 10.1128/mmbr.63.2.405-445.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hentze MW, Kulozik AE. A perfect message: RNA surveillance and nonsense-mediated decay. Cell. 1999;96:307–10. doi: 10.1016/s0092-8674(00)80542-5. [DOI] [PubMed] [Google Scholar]
- 46.Wilusz CJ, Gao M, Jones CL, Wilusz J, Peltz SW. Poly(A)-binding proteins regulate both mRNA deadenylation and decapping in yeast cytoplasmic extracts. RNA. 2001a;7(10):1416–1424. [PMC free article] [PubMed] [Google Scholar]
- 47.Wilusz CJ, Wormington M, Peltz SW. The cap-to-tail guide to mRNA turnover. Nat Rev Mol Cell Biol. 2001b;2(4):237–46. doi: 10.1038/35067025. [DOI] [PubMed] [Google Scholar]
- 48.Reed R, Hurt E. A conserved mRNA export machinery coupled to pre-mRNA splicing. Cell. 2002;108:523–31. doi: 10.1016/s0092-8674(02)00627-x. [DOI] [PubMed] [Google Scholar]
- 49.Kornblihtt AR, de la Mata M, Fededa JP, Munoz MJ, Nogues G. Multiple links between transcription and splicing. RNA. 2004;10(10):1489–98. doi: 10.1261/rna.7100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bentley DL. Rules of engagement: co-transcriptional recruitment of pre-mRNA processing factors. Curr Opin Cell Biol. 2005;17(3):251–6. doi: 10.1016/j.ceb.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 51.Garneau NL, Wilusz J, Wilusz CJ. The highways and byways of mRNA decay. Nature Reviews Mol Cell Biol. 2007;8:113–126. doi: 10.1038/nrm2104. [DOI] [PubMed] [Google Scholar]
- 52.Castelo-Branco P, Furger A, Wollerton M, Smith C, Moreira A, et al. Polypyrimidine tract binding protein modulates efficiency of polyadenylation. Mol Cell Biol. 2004;24:4174–4183. doi: 10.1128/MCB.24.10.4174-4183.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]