Abstract
BACKGROUND
The genetic causes of nonsyndromic ovarian insufficiency are largely unknown. A nuclear receptor, NR5A1 (also called steroidogenic factor 1), is a key transcriptional regulator of genes involved in the hypothalamic–pituitary–steroidogenic axis. Mutation of NR5A1 causes 46,XY disorders of sex development, with or without adrenal failure, but growing experimental evidence from studies in mice suggests a key role for this factor in ovarian development and function as well.
METHODS
To test the hypothesis that mutations in NR5A1 cause disorders of ovarian development and function, we sequenced NR5A1 in four families with histories of both 46,XY disorders of sex development and 46,XX primary ovarian insufficiency and in 25 subjects with sporadic ovarian insufficiency. None of the affected subjects had clinical signs of adrenal insufficiency.
RESULTS
Members of each of the four families and 2 of the 25 subjects with isolated ovarian insufficiency carried mutations in the NR5A1 gene. In-frame deletions and frameshift and missense mutations were detected. Functional studies indicated that these mutations substantially impaired NR5A1 transactivational activity. Mutations were associated with a range of ovarian anomalies, including 46,XX gonadal dysgenesis and 46,XX primary ovarian insufficiency. We did not observe these mutations in more than 700 control alleles.
CONCLUSIONS
NR5A1 mutations are associated with 46,XX primary ovarian insufficiency and 46,XY disorders of sex development.
Primary ovarian insufficiency, also termed premature ovarian failure, is a condition characterized by the arrest of normal ovarian function before the age of 40 years. The disorder occurs in 1% of all women.1,2 Although causes such as autoimmunity, monosomy X, and environmental factors play a role in primary ovarian insufficiency, the cause of the majority of cases remains unknown.1-3 A genetic basis for ovarian insufficiency is supported by the observation that a substantial minority of cases are familial4 and that the prevalence of the condition varies according to ancestral origin.5
The identification of genetic causes of ovarian insufficiency has proved to be elusive. For example, mutations in the APECED, FOXL2, EIF2B, and GALT genes are associated with syndromic forms of primary ovarian insufficiency.3,6-8 In nonsyndromic forms, rare mutations in the follicle-stimulating-hormone and luteinizing-hormone receptor genes and in BMP15, POF1B, and NOBOX have been described.3,9-11 Mutations affecting these genes account for a small minority of all cases of ovarian dysfunction, which suggests that there are additional factors that remain to be identified.
NR5A1, also termed Ad4 binding protein (Ad4BP) or steroidogenic factor 1 (SF-1), is a nuclear receptor and a key transcriptional regulator of genes involved in the hypothalamic–pituitary–steroidogenic axis.12-14 Newborn mice that are deficient in Nr5a1 lack both gonads and adrenal glands, have impaired expression of pituitary gonadotropins, and have structural anomalies of the ventromedial hypothalamic nucleus.13 NR5A1 is expressed in fetal and adult adrenal cortex and Sertoli and Leydig cells of the testis.12-15 The protein regulates the transcription of key genes involved in sexual development and reproduction, including STAR (encoding steroidogenic acute regulatory protein), CYP17A1 (encoding 17-alpha-hydroxylase), CYP11A1 (encoding cytochrome P-450 cholesterol side-chain cleavage), LHB (encoding the beta subunit of luteinizing hormone), AMH (encoding antimüllerian hormone), CYP19A1 (encoding aromatase), and INHA (encoding inhibin alpha subunit).16-22 NR5A1 is expressed in multiple cell types in the fetal, postnatal, prepubertal, and mature ovary.12,13,23 The inactivation of Nr5a1 specifically in mouse granulosa cells causes infertility associated with hypoplastic ovaries.23 Nr5a1-/- ovaries have follicles but lack corpora lutea, a finding that indicates impaired ovulation.15 These data establish a key role for Nr5a1 in ovarian development and function in the mouse.
There are 18 known mutations of the NR5A1 gene in humans.24-34 Three of these were originally identified in patients with adrenal insufficiency.24-26 Subsequent analyses have revealed pathogenic mutations in an additional 15 patients (all 46,XY),27-34 each of whom presented with various anomalies of testis development and no evidence of adrenal failure. In this study, we show that mutations in NR5A1 are associated with impairment of ovarian development and function.
METHODS
PATIENTS
We studied four families with a history of 46,XY disorders of sex development and 46,XX ovarian insufficiency and 25 subjects with 46,XX sporadic ovarian insufficiency. Control samples were obtained from a panel of 1465 subjects of various ancestral origins, selected to match those of the affected subjects carrying putative NR5A1 mutations. Details regarding mutational screening and functional studies of NR5A1 are described in the Supplementary Appendix, available with the full text of this article at NEJM.org. We obtained written informed consent from all patients, family members, and control subjects who participated in the study. Consent forms were approved by local ethics committees.
RESULTS
FAMILY 1
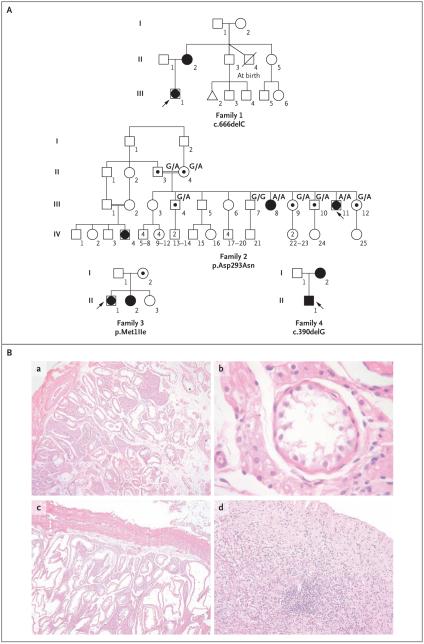
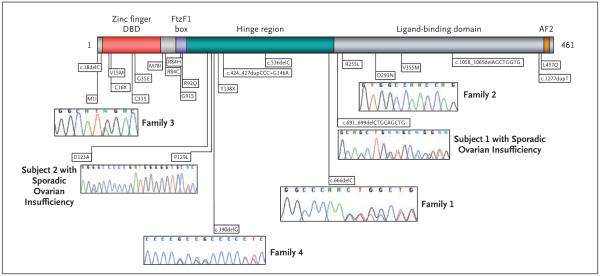
The proband in Family 1 (Subject III-1), who was of European descent, presented at the age of 17 years with primary amenorrhea and an absence of secondary sex characteristics. Gonadal histologic analysis revealed homogeneous fibrous tissue, and 46,XY complete gonadal dysgenesis was diagnosed (Fig. 1A and Table 1). The mother of the proband (Subject II-2) had a history of irregular menstrual cycles and had become pregnant at the age of 23 years. After giving birth, she had anovulatory cycles that were treated for 2 years with clomiphene citrate with no improvement. When she was 35 years of age, her condition was diagnosed as 46,XX primary ovarian insufficiency. Sequence analysis of the NR5A1 gene from the proband and her mother revealed a heterozygous frameshift mutation, c.666delC, in codon 222 (Fig. 2). This mutation is predicted to alter the protein sequence and create a premature termination codon in the messenger RNA (mRNA) at codon 295, truncating the normal protein from 461 to 295 amino acids (Fig. 2 in the Supplementary Appendix). This mutation was not observed in 350 control subjects of European descent.
Figure 1. Pedigrees of Four Families with 46,XY Disorders of Sex Development or 46,XX Ovarian Insufficiency and Histologic Analysis of Samples from Two Affected Subjects in Family 2.
In Panel A, squares represent male family members and circles represent female family members. Solid squares represent affected 46,XY subjects who were raised as boys, and solid circles represent affected 46,XX subjects. Squares containing solid circles represent affected 46,XY subjects who were raised as girls. Symbols containing a black dot represent apparently unaffected carriers of the mutation. The triangle in Family 1 represents miscarriage, and the symbol with a slash represents a deceased twin. Numbers within symbols indicate multiple siblings. The index patient is indicated with an arrow in each family. Genotyping information is provided for Family 2. The genotypes of the parents of the proband are inferred, whereas all others have been determined by molecular analysis. Panel B shows gonadal histologic analysis of affected members of Family 2. Subpanels a, b, and c show dysgenetic pubertal testis with Leydig-cell hyperplasia and aplasia of germ cells in the proband (Subject III-11), who had been raised as a girl. Subpanel a shows cells from the right testis, which are shown under higher magnification in subpanel b; cells from the left testis are shown in subpanel c. Subpanel d shows a sample from the proband’s sister (Subject III-8), who had 46,XX primary amenorrhea, showing a dysgenetic gonad with fibrovascular tissue and without germ cells.
Table 1.
Phenotypes, Genotypes, and Investigation of Gonadal Function in 10 Case Subjects with Mutations in the NR5A1 Gene.*
| Variable | Family 1 | Family 2 | Family 3 | Family 4 | Sporadic Ovarian Insufficiency | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| c.666delC Heterozygous | p.Asp293Asn Homozygous | p.Met1Ile Heterozygous | c.390delG Heterozygous | p.Leu231_Leu233del Heterozygous |
p.Gly123Ala p.Pro129Leu Heterozygous |
|||||
| Family member number | II-2 | III-1 | III-11 | III-8 | II-1 | II-2 | II-1 | I-2 | Subject 1 | Subject 2 |
| Sex of rearing | Female | Female | Female | Female | Female | Female | Male | Female | Female | Female |
| Karyotype | 46,XX | 46,XY | 46,XY | 46,XX | 46,XY | 46,XX | 46,XY | 46,XX | 46,XX | 46,XX |
| Age at presentation | 35 yr | 17 yr | 18 yr | 19 yr | 12 yr | 16 yr | 21 day | 29 yr | 12.5 yr | 4 mo |
| Diagnosis | Primary ovarian in- sufficiency |
46,XY complete go- nadal dysgenesis |
46,XY disorder of sex de- velopment |
Primary ovarian in- sufficiency |
46,XY partial gonadal dysgenesis |
Primary ovarian insufficiency |
46,XY disorder of sex development |
Primary ovarian insufficiency |
Primary ovarian insuffi- ciency |
Hypertrophic cli- toris |
| Phenotype | Female | Primary amenor- rhea, absence of secondary sex characteristics |
Primary amenorrhea and signs of virilization |
Primary amenorrhea with female-type adult pubic hair and breasts, small uterus |
Primary amenorrhea, small breasts and uterus |
Secondary amen- orrhea, small breasts, no pubic hair |
Ambiguous external genitalia |
Female | Intrauterine growth re- tardation, eczema, no development of breasts or pubic hair |
Female, hypertro- phic clitoris |
| External genitalia | Female | Female | Phallus length, 7.5 cm; sin- gle perineal opening; fused labioscrotal folds |
Female | Female, hypertrophic cli- toris |
Female | Ambiguous male genitalia |
Female | Female | Female, hypertro- phic clitoris |
| Gonadal position | Pelvic | Pelvic | Inguinal | Pelvic, only right gonad |
Pelvic, very small | Pelvic | Inguinal testes | Pelvic, small gonads |
Pelvic, only small right gonad |
Pelvic |
| Gonadal histologic analysis | NA | Homogeneous fi- brous tissue |
Fibrous tissue, germ-cell aplasia, Leydig-cell hy- perplasia |
Fibrous tissue, no follicles |
Fibrous tissue, disorga- nized tubules, Leydig- cell hyperplasia |
Fibrous tissue, no follicles |
NA | Fibrous tissue, no follicles |
Fibrous tissue, no fol- licles |
NA |
| Luteinizing hormone (U/liter) | ||||||||||
| Value | 18.0 | 56.7 | 18.0 | 18.0 | 21.7 | 106.0 | 0.3 | 18.0 | 19.0 | 11.0 |
| Normal range | 2.1–14.9 | 2.4–13.0 | 2.4–13.0 | 2.1–14.9 | ND–4.3 | 2.1–14.9 | ND–4.0 | 2.1–14.9 | ND–5.0 | ND–2.0 |
| Follicle-stimulating hormone (U/liter) | ||||||||||
| Value | 76.0 | 13.7 | 36.3 | 51.1 | 96.9 | 30.0 | 1.8 | 48.0 | 76.0 | 44.0 |
| Normal range | 2.0–15.0 | ND–13.5 | ND–13.5 | 2.0–15.0 | ND–4.6 | 2.0–15.0 | ND–3.9 | 2.0–15.0 | ND–4.6 | ND–12.5 |
| Testosterone (ng/ml) | ||||||||||
| Value | NA | 0.30 | 0.42 | NA | 1.85 | 0.07 | 0.23 | NA | <0.07 | 0.49 |
| Normal range | 2.70–9.00 | 2.70–9.00 | 0.20–2.00 | <0.50–0.80 | 0.20–0.50 | <0.20 | <0.20 | |||
| Estradiol (pg/ml) | ||||||||||
| Value | 23 | NA | NA | 12 | NA | NA | NA | 25 | <10 | NA |
| Normal range | 17–290 | 17–290 | 17–290 | 7–60 | ||||||
Normal range refers to the range of basal levels in control subjects matched according to age and chromosomal sex with the case subjects. To convert the values for testosterone to nanomoles per liter, multiply by 3.467. To convert the values for estradiol to picomoles per liter, multiply by 3.671. NA denotes not available, and ND not detectable.
Figure 2. Distribution of NR5A1 Mutations in Relation to the Protein.
The functional domains of the NR5A1 protein are shown, indicating the position of known NR5A1 mutations, including the six mutations described in this study. Representative chromatograms are shown with each mutation. The DNA-binding domain (DBD) containing two zinc-finger motifs is indicated. The FtzF1 box stabilizes protein binding to DNA. The hinge region is important for stabilizing the ligand-binding domain and interacts with other proteins that control NR5A1 transcriptional activity. The AF2 domain recruits cofactors necessary for NR5A1 transactivating activity.
FAMILY 2
The proband in Family 2 (Subject III-11) presented at the age of 18 years with primary amenorrhea and signs of virilization (Fig. 1A and Table 1). The diagnosis was a 46,XY disorder of sex development (Fig. 1B, subpanels a through c). A sister of the proband (Subject III-8) presented at the age of 19 years with primary amenorrhea, and the diagnosis was 46,XX primary ovarian insufficiency (Table 1 and Fig. 1B, subpanel d). Mutational analysis of the NR5A1 gene revealed a homozygous c.877G→A transition, predicted to cause a p.Asp293Asn amino acid change (Fig. 2, and Fig. 3A in the Supplementary Appendix). The parents of the two daughters (Subjects II-3 and II-4), who were of Brazilian origin, were first cousins (Fig. 1A). DNA and hormonal studies were performed on five of the eight fertile siblings of the proband (Table 1 in the Supplementary Appendix). Four of the siblings were heterozygotes, and a brother did not carry the mutation. The parents were inferred to be heterozygotes. Further investigation of the family revealed a female family member (Subject IV-4) with 46,XY complete gonadal dysgenesis (DNA unavailable). The parents of Subject IV-4 (Subjects III-1 and III-2) were also first cousins. This mutation was not observed in 782 control subjects (133 West Africans, 27 South or East Africans, 100 North Africans, 357 Europeans, 63 Mexicans or Colombians, and 102 Brazilians).
FAMILY 3
A French proband in Family 3 (Subject II-1) presented at the age of 12 years with signs of virilization. The diagnosis was 46,XY partial gonadal dysgenesis (Fig. 1A and Table 1). A sister of the proband (Subject II-2) presented at the age of 16 years with secondary amenorrhea. Menarche had begun at 14 years with irregular cycles that ended at 15 years. The diagnosis was 46,XX primary ovarian insufficiency (Fig. 1A and Table 1). The third sibling (Subject II-3) was seen at 11 years of age for systematic evaluation. She showed age-appropriate onset of puberty, normal female external genitalia, and hormonal data (Table 1 in the Supplementary Appendix). The karyotype was 46,XX. The mother was 46 years of age, and menstruation was reportedly normal. The two affected siblings and their mother carried a heterozygous c.3G→A transition in the first codon of NR5A1 that predicts a p.Met1Ile change (Fig. 2, and Fig. 2 in the Supplementary Appendix). This mutation was not present in the unaffected sibling or in the father, and it was not observed in 350 unaffected French control subjects.
FAMILY 4
Another French proband, in Family 4 (Subject II-1), presented at birth with ambiguous external genitalia. His condition was diagnosed as a 46,XY disorder of sex development, and he was raised as a boy (Fig. 1A and Table 1). After his birth, his mother took oral contraceptives for 2 years, until she was 29 years old, after which her menstrual cycles did not reappear. The mother’s diagnosis was 46,XX primary ovarian insufficiency (Fig. 1A and Table 1). A heterozygote frameshift mutation, c.390delG, was detected in NR5A1 in both the proband and his mother (Fig. 2). This mutation is predicted to alter the protein sequence and create a premature termination codon in the mRNA at codon 295 (Fig. 2 in the Supplementary Appendix). This mutation was absent in 350 unaffected French control subjects.
GENETIC ANALYSIS OF SPORADIC OVARIAN INSUFFICIENCY
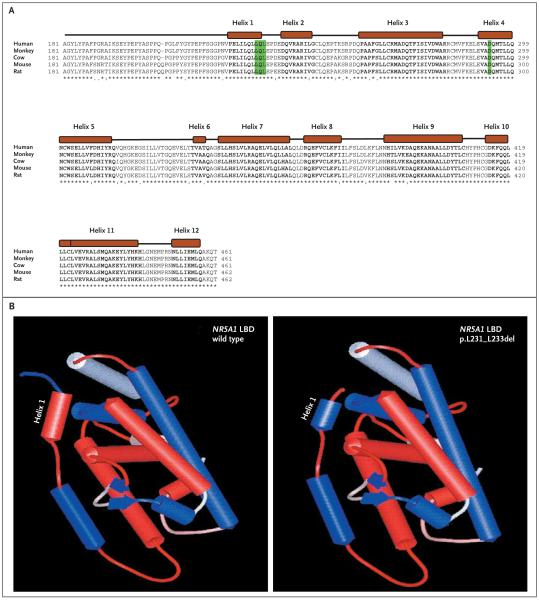
We then analyzed samples from 25 women with sporadic ovarian insufficiency. One girl, of Roma origin, presented at the age of 12.5 years because of short stature (Table 1). She had been born at term (38.5 weeks) by cesarean section (birth length, 44 cm [17.3 in.]; weight, 2.4 kg [5.3 lb]). She had generalized seizures on day 7. She grew at −2 SD for height until the age of 1.9 years and then at −3 SD. At 12.5 years, her bone age was 9 years, her height was 130 cm (51.2 in.), and her weight was 30 kg (66.1 lb). Known causes of short stature were excluded by measuring peak growth hormone after stimulation, plasma insulin-like growth factor I, creatinine, thyrotropin, thyroxine, and erythrocyte sedimentation rate. Her hormonal profile, before and 1 hour after stimulation with synthetic adrenocorticotropic hormone, showed low levels of 17-OH progesterone (0.16 to 1.10 ng per milliliter) and delta 4-androstenedione (0.27 to 0.49 ng per milliliter) and normal levels of cortisol (110 to 320 ng per milliliter). Her karyotype was 46,XX, and the diagnosis was ovarian failure (Table 1). Analysis of the NR5A1 gene revealed a heterozygous in-frame 9-bp deletion that results in the loss of three amino acids (p.Leu231_Leu233del) in the N-terminal region of the ligand-binding domain (Fig. 2 and 3A). In silico analysis predicted a change in hydrophobicity of helix 1 of the ligand-binding domain, which may result in altered protein conformation or function (Fig. 3B). The deletion was not observed in 800 control alleles, including samples from 69 unaffected subjects of Roma origin and 56 unaffected subjects from an Indian Gujarati population.
Figure 3. Comparison of Sequence Alignment of Portions of the Human NR5A1 Protein with Those of Other Mammals and 3-D Models of Wild-Type and Mutated NR5A1 Proteins.
In Panel A, sequence alignment of the distal portion of the hinge and the ligand-binding domain (LBD) of human NR5A1 protein is compared with those of other mammals. The 12 predicted alpha helixes in the ligand-binding domain of NR5A1 are indicated as solid boxes, and amino acids in bold text. The position of the p.Asp293Asn missense mutations and the deletions of three amino acids (p.Leu231_Leu233) are highlighted. Both mutations fall either in the highly conserved Helix 1 (p.Leu231_Leu233) or in Helix 4 (p.Asp293Asn) of the ligand-binding domain. In Panel B, three-dimensional models of the ligand-binding domain of both the wild-type and p.Leu231_Leu233del mutated NR5A1 proteins were obtained with the use of the Web-based interface 3D-JIGSAW (for details, see the Supplementary Appendix). The hydrophobic helixes are shown in red, and the hydrophilic helixes in blue. Note the change of Helix 1 from hydrophobic to hydrophilic in the p.Leu231_Leu233 deletion mutant.
A second girl, of West African (Senegalese) origin, presented at 4 months of age with hypertrophy of the clitoris (Table 1). The child, born to nonconsanguineous parents, had elevated levels of follicle-stimulating hormone, a finding that indicates ovarian insufficiency. Analysis of the NR5A1 gene revealed two closely linked heterozygous mutations — c.368G→C (p.Gly123Ala) and c.386C→T (p.Pro129Leu) — in the hinge domain of the protein (Fig. 2, and Fig. 2 in the Supplementary Appendix). Enzymatic digestion of the amplicon revealed that both mutations were present on the same chromosome (data not shown). The DNA of her parents was unavailable for study. Neither mutation was observed in 479 unaffected control subjects (113 Senegalese, 111 other West Africans, 100 North Africans, 27 South or East Africans, and 128 other subjects).
We observed no other nonsynonymous variants in NR5A1 in any of the four families. Both subjects who had sporadic primary ovarian insufficiency were homozygous for the p.Gly146Ala (c.437G→C) polymorphism that has previously been demonstrated to have approximately 80% of the activity of the wild-type protein.35 To further assess the degree of genetic variability, we sequenced the open reading frame of NR5A1 in 140 subjects with normal sperm levels (>20×106 sperm per milliliter), of whom 46 had fathered at least one child. In this panel, we detected the previously characterized p.Gly146Ala as the only nonsynonymous change (allelic frequencies, G 0.843 and C 0.157).
FUNCTIONAL ASSAYS OF NR5A1 ACTIVITY
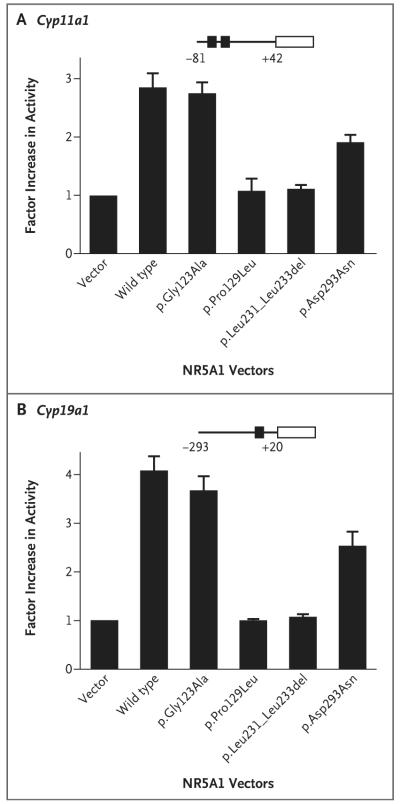
We observed a quantitative reduction in the transactivation of both CYP11A1 and CYP19A1 when testing the effect of each of the NR5A1 mutations on protein function, using embryonic kidney tsa201 cells (Fig. 4A and 4B). Proteins with mutations at p.Pro129Leu, c.666delC, and p.Leu231_Leu233del showed severe loss of activation, whereas the p.Gly123Ala mutant had activity more similar to the wild-type protein. This finding shows that the p.Pro129Leu variant is pathologic in the patient who carries both variants. The construct containing the p.Asp293Asn mutation that was detected in Family 2 partially activated the CYP11A1 and CYP19A1 promoters. We obtained similar results in transient gene-expression assays using Chinese hamster ovary cells (for details, see the Supplementary Appendix).
Figure 4. Assays of NR5A1 Transcriptional Activity.
The transcriptional activity of wild-type NR5A1 (SF1) and variants associated with ovarian insufficiency was studied with the use of Cyp11a1 (Panel A) and Cyp19a1 (Panel B) promoters in tsa201 cells. NR5A1 activity is shown as the factor increase above baseline, which is defined as the activity observed in transfection with empty vector. Data represent the mean of three independent experiments, each performed in triplicate. The T bars represent standard errors.
DISCUSSION
We identified a series of missense, in-frame, and frameshift mutations in the NR5A1 gene in association with anomalies of ovarian development and function. In four of the probands, whose condition was diagnosed as 46,XY disorders of sex development, the mutation was familial. In each of these families, another member of the family with primary ovarian insufficiency also harbored the mutation. We identified two additional heterozygous mutations by screening 25 female subjects with primary ovarian insufficiency. In all subjects, there was no evidence of adrenal dysfunction, a finding that is consistent with reported mutations in NR5A1 in patients with 46,XY disorders of sex development with apparently normal adrenal function.27-34 Taken together, these data indicate that mutations in NR5A1 can cause ovarian insufficiency.
The subjects we describe show variations in expressivity of the phenotype, penetrance, and modes of inheritance. Families 1, 3, and 4 show a dominant mode of inheritance, whereas Family 2 shows a recessive mode. Families 1 and 4 carry heterozygous frameshift mutations that generate a premature termination codon predicted to result in truncated proteins. Nonsense-mediated decay usually results in the degradation of mRNAs containing a premature termination codon at least 50 nucleotides upstream of the last exon–exon boundary.36 The two mutations, c.666delC and c.390delG, result in a premature termination codon at amino acid position 295, predicted to be recognized by the nonsense-mediated decay surveillance complexes and degraded. Our functional analyses indicated that even if a truncated protein were produced, it would have severely impaired transcriptional activity (data not shown).
In Family 3, both XY and XX affected family members harbored the same heterozygous c.3G→A transition as their apparently unaffected mother. This mutation abolishes the Kozak consensus sequence for translation initiation, which may either abolish translation or result in a defect in translation. An alternative initiation codon is located downstream at codon position 78. Translation initiation at this codon is predicted to truncate the N-terminal of the protein, eliminating the DNA-binding domain. The absence of a phenotype in the mother carrying the same mutation as the affected family members suggests incomplete penetrance of the mutant allele, owing to a variable translation defect, the existence of modifier genes, or environmental effects. This is consistent with incomplete penetrance of NR5A1 mutations present in 46,XY dizygotic twins with disorders of sex development: one boy presented with anorchia, and the other had normal testicular development.33
In six reported patients with 46,XY disorders of sex development, the NR5A1 mutation was inherited from the mother, who had apparently normal ovarian function.30-34 These cases may be explained by incomplete penetrance of the mutant allele or by a progressive failure of ovarian function. In one published familial case, the mother of a 46,XY boy with anorchia who also harbored a p.Val355Met mutation in NR5A1 underwent left ovariectomy and homolateral fallopian tube ablation for ovarian cysts at the age of 22 years and subsequently had two spontaneous miscarriages, an outcome that suggests impaired ovarian function.33
In Family 2, it is simplest to interpret the mode of inheritance as recessive. The two affected family members had a homozygous p.Asp293Asn mutation in a region of the gene that encodes the ligand-binding domain of NR5A1. Unaffected family members who were genotyped were either heterozygous or lacked the mutation. A recessive mode of inheritance for this family can be explained by the residual activity of the NR5A1 p.Asp293Asn protein, which was about half as active as wild-type NR5A1.
During further screening of isolated cases of ovarian insufficiency, we detected heterozygous NR5A1 mutations in two subjects. The mutation in Subject 1 was predicted to result in the deletion of three amino acids (p.Leu231_Leu233) in the ligand-binding domain of NR5A1. These three amino acids are highly conserved in subclass V of nuclear receptors and overlap with the first helix (Helix 1) of the ligand-binding domain. Helix 1 contains an activation function domain (AFH1) that is essential for maximal receptor function through interaction with coactivators.37 Disruption of this structure is predicted to dramatically alter NR5A1 stability and activity, as demonstrated by the failure of mutant NR5A1 (p.Leu231_Leu233del) to transactivate the CYP11A1 and CYP19A1 promoters. We observed two changes on a single chromosome in the second subject with isolated primary ovarian insufficiency (data not shown). One of these changes, p.Pro129Leu, is probably pathogenic, since it severely impairs transcriptional activity.
We have shown that mutations in NR5A1 are associated with human ovarian insufficiency, an observation consistent with the hypoplastic ovaries and infertility of mice lacking Nr5a1 in their granulosa cells.23 NR5A1 plays an important role in human ovarian development and function. During early human embryonic development, NR5A1 is expressed in the bipotential gonad in both sexes and is not sexually dimorphic after sex determination. A broad range of expression persists in the developing human fetal ovary, as well as in the testis.38,39 In cycling human ovaries, NR5A1 is expressed in the undeveloped follicles during the preantral phase.38 It is also expressed in theca interna cells, in luteinized and nonluteinized granulosa cells, and in corpus luteum during the luteal phase, as well as in both atretic follicles and degenerating corpora lutea.38
In both theca and granulosa cells, NR5A1 regulates genes required for ovarian steroidogenesis and follicle growth and maturation, including, STAR, CYP11A1, CYP17A1, CYP19A1, LHCGR (encoding luteinizing hormone receptor), and INHA.12,14,15,17-21 Dysregulation of any of the proteins encoded by these genes could lead to ovarian dysfunction. Our data show that mutated forms of NR5A1, detected in subjects with anomalies of ovarian development and function, show quantitative impairment in the transactivation of two of these factors (CYP11A1 and CYP19A1).
Our data suggest that mutated NR5A1 is associated with a progressive loss of ovarian reproductive capacity. A diagnostic genetic test could aid in counseling for the possibility of familial recurrence and in evaluation of prospects for treatment of primary ovarian insufficiency.
Supplementary Material
Acknowledgments
Supported by grants from the Agence Nationale de la Recherche-GIS Institut des Maladies Rares (to Dr. McElreavey and Ms. Lourenço); by a research grant (1-FY07-490) from the March of Dimes Foundation (to Dr. McElreavey); by the National Institute for Health Research (to UCL Institute of Child Health Biomedical Research Centre); and a Wellcome Trust Senior Research Fellowship in Clinical Science (079666, to Dr. Achermann).
We dedicate this manuscript to the late Professor Keith L. Parker, chief of the Division of Endocrinology and Metabolism at UT Southwestern, Dallas. His studies of the mechanisms that regulate steroidogenesis led to the isolation of NR5A1.
We thank Joelle Bignon-Topalovic for providing technical assistance; Dr. Stephen Lortat-Jacob of Hôpital Necker, Paris, for performing a surgical procedure on the proband of Family 4; Dr. Alexandre Barbier of Service de Médecine Néonatale de Port Royal, Paris, for referring Subject 2 with isolated ovarian insufficiency; Dr. David Comas of Universitat Pompeu Fabra, Barcelona, for providing Roma DNA samples; Dr. Anavaj Sakuntabhai of the Institut Pasteur, Paris, for providing Senegalese DNA samples; and Drs. Ron Evans, Meera Ramayya, Masafumi Ito, and J. Larry Jameson for providing plasmids.
Footnotes
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Nelson LM. Primary ovarian insufficiency. N Engl J Med. 2009;360:606–14. doi: 10.1056/NEJMcp0808697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coulam CB, Stringfellow S, Hoefnagel D. Evidence for a genetic factor in the etiology of premature ovarian failure. Fertil Steril. 1983;40:693–5. doi: 10.1016/s0015-0282(16)47433-9. [DOI] [PubMed] [Google Scholar]
- 3.Laissue P, Vinci G, Veitia RA, et al. Recent advances in the study of genes involved in non-syndromic premature ovarian failure. Mol Cell Endocrinol. 2008;282:101–11. doi: 10.1016/j.mce.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 4.van Kasteren YM, Hundscheid RD, Smits AP, Cremers FP, van Zonneveld P, Braat DD. Familial idiopathic premature ovarian failure: an overrated and underestimated genetic disease? Hum Reprod. 1999;14:2455–9. doi: 10.1093/humrep/14.10.2455. [DOI] [PubMed] [Google Scholar]
- 5.Luborsky JL, Meyer P, Sowers MF, Gold EB, Santoro N. Premature menopause in a multi-ethnic population study of the menopause transition. Hum Reprod. 2003;18:199–206. doi: 10.1093/humrep/deg005. [DOI] [PubMed] [Google Scholar]
- 6.Finnish-German APECED Consortium An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat Genet. 1997;17:399–403. doi: 10.1038/ng1297-399. [DOI] [PubMed] [Google Scholar]
- 7.Leslie ND. Insights into the pathogenesis of galactosemia. Annu Rev Nutr. 2003;23:59–80. doi: 10.1146/annurev.nutr.23.011702.073135. [DOI] [PubMed] [Google Scholar]
- 8.Fogli A, Gauthier-Barichard F, Schiffmann R, et al. Screening for known mutations in EIF2B genes in a large panel of patients with premature ovarian failure. BMC Womens Health. 2004;4:8. doi: 10.1186/1472-6874-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin Y, Choi Y, Zhao H, Simpson JL, Chen ZJ, Rajkovic A. NOBOX homeobox mutation causes premature ovarian failure. Am J Hum Genet. 2007;81:576–81. doi: 10.1086/519496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toniolo D, Rizzolio F. X chromosome and ovarian failure. Semin Reprod Med. 2007;25:264–71. doi: 10.1055/s-2007-980220. [DOI] [PubMed] [Google Scholar]
- 11.Lacombe A, Lee H, Zahed L, et al. Disruption of POF1B binding to nonmuscle actin filaments is associated with premature ovarian failure. Am J Hum Genet. 2006;79:113–9. doi: 10.1086/505406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lavorgna G, Ueda H, Clos J, Wu C. FTZ-F1, a steroid hormone receptor-like protein implicated in the activation of fushi tarazu. Science. 1991;252:848–51. doi: 10.1126/science.1709303. [DOI] [PubMed] [Google Scholar]
- 13.Luo X, Ikeda Y, Parker KL. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994;77:481–90. doi: 10.1016/0092-8674(94)90211-9. [DOI] [PubMed] [Google Scholar]
- 14.Lin L, Achermann JC. Steroidogenic factor-1 (SF-1, Ad4BP, NR5A1) and disorders of testis development. Sex Dev. 2008;2:200–9. doi: 10.1159/000152036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao H, Li Z, Cooney AJ, Lan ZJ. Orphan nuclear receptor function in the ovary. Front Biosci. 2007;12:3398–405. doi: 10.2741/2321. [DOI] [PubMed] [Google Scholar]
- 16.Shen W-H, Moore CCD, Ikeda Y, Parker KL, Ingraham HA. Nuclear receptor steroidogenic factor 1 regulates the müllerian inhibiting substance gene: a link to the sex determination cascade. Cell. 1994;77:651–61. doi: 10.1016/0092-8674(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 17.Keri RA, Nilson JH. A steroidogenic factor-1 binding site is required for activity of the luteinizing hormone beta subunit promoter in gonadotropes of transgenic mice. J Biol Chem. 1996;271:10782–95. doi: 10.1074/jbc.271.18.10782. [DOI] [PubMed] [Google Scholar]
- 18.Sugawara T, Kiriakidou M, McAllister JM, Kallen CB, Strauss JF., III Multiple steroidogenic factor 1 binding elements in the human steroidogenic acute regulatory protein gene 5′-flanking region are required for maximal promoter activity and cyclic AMP responsiveness. Biochemistry. 1997;36:7249–55. doi: 10.1021/bi9628984. [DOI] [PubMed] [Google Scholar]
- 19.Hanley NA, Rainey WE, Wilson DI, Ball SG, Parker KL. Expression profiles of SF-1, DAX1, and CYP17 in the human fetal adrenal gland: potential interactions in gene regulation. Mol Endocrinol. 2001;15:57–68. doi: 10.1210/mend.15.1.0585. [DOI] [PubMed] [Google Scholar]
- 20.Gurates B, Amsterdam A, Tamura M, et al. WT1 and DAX-1 regulate SF-1-mediated human P450arom gene expression in gonadal cells. Mol Cell Endocrinol. 2003;208:61–75. doi: 10.1016/s0303-7207(03)00198-9. [DOI] [PubMed] [Google Scholar]
- 21.Weck J, Mayo KE. Switching of NR5A proteins associated with the inhibin alpha-subunit gene promoter after activation of the gene in granulosa cells. Mol Endocrinol. 2006;20:1090–103. doi: 10.1210/me.2005-0199. [DOI] [PubMed] [Google Scholar]
- 22.Mendelson CR, Kamat A. Mechanisms in the regulation of aromatase in developing ovary and placenta. J Steroid Biochem Mol Biol. 2007;106:62–70. doi: 10.1016/j.jsbmb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeyasuria P, Ikeda Y, Jamin SP, et al. Cell-specific knockout of steroidogenic factor 1 reveals its essential roles in gonadal function. Mol Endocrinol. 2004;18:1610–9. doi: 10.1210/me.2003-0404. [DOI] [PubMed] [Google Scholar]
- 24.Achermann JC, Ito M, Ito M, Hindmarsh PC, Jameson JL. A mutation in the gene encoding steroidogenic factor-1 causes XY sex reversal and adrenal failure in humans. Nat Genet. 1999;22:125–6. doi: 10.1038/9629. [DOI] [PubMed] [Google Scholar]
- 25.Biason-Lauber A, Schoenle EJ. Apparently normal ovarian differentiation in a prepubertal girl with transcriptionally inactive steroidogenic factor 1 (NR5A1/SF-1) and adrenocortical insufficiency. Am J Hum Genet. 2000;67:1563–8. doi: 10.1086/316893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Achermann JC, Ozisik G, Ito M, et al. Gonadal determination and adrenal development are regulated by the orphan nuclear receptor steroidogenic factor-1, in a dose-dependent manner. J Clin Endocrinol Metab. 2002;87:1829–33. doi: 10.1210/jcem.87.4.8376. [DOI] [PubMed] [Google Scholar]
- 27.Correa RV, Domenice S, Bingham NC, et al. A microdeletion in the ligand binding domain of human steroidogenic factor 1 causes XY sex reversal without adrenal insufficiency. J Clin Endocrinol Metab. 2004;89:1767–72. doi: 10.1210/jc.2003-031240. [DOI] [PubMed] [Google Scholar]
- 28.Mallet D, Bretones P, Michel-Calemard L, Dijoud F, David M, Morel Y. Gonadal dysgenesis without adrenal insufficiency in a 46,XY patient heterozygous for the nonsense C16X mutation: a case of SF1 haploinsufficiency. J Clin Endocrinol Metab. 2004;89:4829–32. doi: 10.1210/jc.2004-0670. [DOI] [PubMed] [Google Scholar]
- 29.Hasegawa T, Fukami M, Sato N, et al. Testicular dysgenesis without adrenal insufficiency in a 46,XY patient with a heterozygous inactive mutation of steroidogenic factor-1. J Clin Endocrinol Metab. 2004;89:5930–5. doi: 10.1210/jc.2004-0935. [DOI] [PubMed] [Google Scholar]
- 30.Lin L, Philibert P, Ferraz-de-Souza B, et al. Heterozygous missense mutations in steroidogenic factor 1 (SF1/Ad4BP, NR5A1) are associated with 46,XY disorders of sex development with normal adrenal function. J Clin Endocrinol Metab. 2007;92:991–9. doi: 10.1210/jc.2006-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coutant R, Mallet D, Lahlou N, et al. Heterozygous mutation of steroidogenic factor-1 in 46,XY subjects may mimic partial androgen insensitivity syndrome. J Clin Endocrinol Metab. 2007;92:2868–73. doi: 10.1210/jc.2007-0024. [DOI] [PubMed] [Google Scholar]
- 32.Reuter AL, Goji K, Bingham NC, Matsuo M, Parker KL. A novel mutation in the accessory DNA-binding domain of human steroidogenic factor 1 causes XY gonadal dysgenesis without adrenal insufficiency. Eur J Endocrinol. 2007;157:233–8. doi: 10.1530/EJE-07-0113. [DOI] [PubMed] [Google Scholar]
- 33.Philibert P, Zenaty D, Lin L, et al. Mutational analysis of steroidogenic factor 1 (NR5a1) in 24 boys with bilateral anorchia: a French collaborative study. Hum Reprod. 2007;22:3255–61. doi: 10.1093/humrep/dem278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Köhler B, Lin L, Ferraz-de-Souza B, et al. Five novel mutations in steroidogenic factor 1 (SF1, NR5A1) in 46,XY patients with severe underandrogenization but without adrenal insufficiency. Hum Mutat. 2008;29:59–64. doi: 10.1002/humu.20588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.WuQiang F, Yanase T, Wei L, et al. Functional characterization of a new human Ad4BP/SF-1 variation, G146A. Biochem Biophys Res Commun. 2003;311:987–94. doi: 10.1016/j.bbrc.2003.10.096. [DOI] [PubMed] [Google Scholar]
- 36.Khajavi M, Inoue K, Lupski JR. Nonsense-mediated mRNA decay modulates clinical outcome of genetic disease. Eur J Hum Genet. 2006;14:1074–81. doi: 10.1038/sj.ejhg.5201649. [DOI] [PubMed] [Google Scholar]
- 37.Desclozeaux M, Krylova IN, Horn F, Fletterick RJ, Ingraham HA. Phosphorylation and intramolecular stabilization of the ligand binding domain in the nuclear receptor steroidogenic factor 1. Mol Cell Biol. 2002;22:7193–203. doi: 10.1128/MCB.22.20.7193-7203.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takayama K, Sasano H, Fukaya T, et al. Immunohistochemical localization of Ad4-binding protein with correlation to steroidogenic enzyme expression in cycling human ovaries and sex cord stromal tumors. J Clin Endocrinol Metab. 1995;80:2815–21. doi: 10.1210/jcem.80.9.7673429. [DOI] [PubMed] [Google Scholar]
- 39.Hanley NA, Ball SG, Clement-Jones M, et al. Expression of steroidogenic factor 1 and Wilms’ tumour 1 during early human gonadal development and sex determination. Mech Dev. 1999;87:175–80. doi: 10.1016/s0925-4773(99)00123-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.