Abstract
Rationale: Severe pulmonary arterial hypertension (PAH) is characterized by the formation of plexiform lesions and concentric intimal fibrosis in small pulmonary arteries. The origin of cells contributing to these vascular lesions is uncertain. Endogenous endothelial progenitor cells are potential contributors to this process.
Objectives: To determine whether progenitors are involved in the pathobiology of PAH.
Methods: We performed immunohistochemistry to determine the expression of progenitor cell markers (CD133 and c-Kit) and the major homing signal pathway stromal cell–derived factor-1 and its chemokine receptor (CXCR4) in lung tissue from patients with idiopathic PAH, familial PAH, and PAH associated with congenital heart disease. Two separate flow cytometric methods were employed to determine peripheral blood circulating numbers of angiogenic progenitors. Late-outgrowth progenitor cells were expanded ex vivo from the peripheral blood of patients with mutations in the gene encoding bone morphogenetic protein receptor type II (BMPRII), and functional assays of migration, proliferation, and angiogenesis were undertaken.
Measurements and Main Results: There was a striking up-regulation of progenitor cell markers in remodeled arteries from all patients with PAH, specifically in plexiform lesions. These lesions also displayed increased stromal cell–derived factor-1 expression. Circulating angiogenic progenitor numbers in patients with PAH were increased compared with control subjects and functional studies of late-outgrowth progenitor cells from patients with PAH with BMPRII mutations revealed a hyperproliferative phenotype with impaired ability to form vascular networks.
Conclusions: These findings provide evidence of the involvement of progenitor cells in the vascular remodeling associated with PAH. Dysfunction of circulating progenitors in PAH may contribute to this process.
Keywords: pulmonary hypertension, endothelial progenitor
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Although cells bearing markers suggesting a progenitor phenotype have been described in patients with pulmonary arterial hypertension (PAH), the role of endothelial progenitor cells (EPCs) in the pathobiology of PAH is controversial.
What This Study Adds to the Field
Dysfunctional endothelial progenitor cells in mutations in bone morphogenetic protein type II receptor are associated with PAH, and engraftment of EPCs can be found in vascular lesions of these patients. These observations are of importance for ongoing work examining potential roles for EPCs in the pathogenesis of lung vascular disease in PAH.
Severe pulmonary arterial hypertension (PAH) is a rare but devastating condition with a poor prognosis. The conventional model of pulmonary endothelial dysfunction in PAH includes endothelial cell damage, failure of repair, and compromise of barrier integrity (1). Two types of vascular lesions predominate in small pulmonary arteries: concentric intimal lesions consisting of myofibroblast proliferation and smooth muscle hypertrophy with resultant luminal narrowing, and plexiform lesions characterized by disorganized focal proliferation of endothelial channels (2). The vascular injury has been hypothesized to lead to the emergence of apoptosis-resistant endothelial cell clones, and Lee and coworkers (3) have suggested that plexiform lesions, at least in the idiopathic form of PAH, are monoclonal in origin. The appearance of plexiform lesions is thought to signify a poor prognosis (4), but little is understood about the evolution of these focal areas of neoangiogenesis. Their contribution to increased vascular resistance is unclear, although three-dimensional reconstruction studies suggest almost complete obliteration of the vessel lumen and a role in upstream vascular remodeling (5).
With a possible role in vascular homeostasis and angiogenesis, endothelial progenitor cells (EPCs) are being investigated as a potential treatment modality. The field is complicated by the controversy over how to identify circulating progenitors and what exactly their contribution is to angiogenesis. Despite this controversy ex vivo–expanded endothelial-like progenitor cells have shown therapeutic benefit in short-term studies in animal models of PAH (6, 7) and in a randomized controlled trial in humans with idiopathic PAH (8). Although enthusiasm for the therapeutic use of these cells in PAH is gaining momentum, there is little work detailing the function of endogenous human EPCs in PAH pathophysiology.
Reports provide conflicting data on the levels of “circulating progenitors” in PAH (9–11). It has been demonstrated that blood-derived cells from patients with idiopathic PAH and Eisenmenger syndrome are deficient in their ability to contribute to vascular network formation (9–11). These observations have been confined to the colony-forming endothelial-like cells that are derived from the so-called early-outgrowth fraction of mononuclear cells. However, these early-outgrowth cells lack the proliferative capacity characteristic of true progenitor cells and fail to form vascular networks on their own. The beneficial effects of these cells in animal models may be due to paracrine release of growth factors. There have been in vitro descriptions of a highly proliferative, polyclonally expanded EPC, generally termed an endothelial colony-forming cell or late-outgrowth EPC (12). This late-outgrowth EPC demonstrates proliferative potential and an increased capacity to form vascular networks (12). In addition, it has been shown that bone morphogenetic proteins (BMPs) are critical in late-outgrowth EPC differentiation, regulation, and angiogenic capacity (13). This is of clear relevance to pulmonary hypertension given the prominence of dysfunction of the bone morphogenetic protein receptor type II (BMPRII) pathway in familial and idiopathic forms of PAH (14, 15).
To assess the possible contribution of EPCs to the pathology of vascular lesions in human forms of PAH we studied the expression of progenitor markers in lung tissue sections from patients with familial and idiopathic PAH, and PAH associated with congenital heart disease and demonstrate increased expression of EPC markers and homing signals, particularly in plexiform lesions. In addition, we found an increased circulating fraction of angiogenic cells across the spectrum of causes of PAH. Furthermore, functional studies using late-outgrowth progenitors from patients with PAH with BMPRII mutations and from healthy control subjects demonstrated a hyperproliferative phenotype with an impaired ability to form vascular networks. Taken together our data suggest that circulating EPCs contribute to impaired vascular homeostasis.
METHODS
Human Lung Tissue
The local research ethics committee approved these studies. Tissues were obtained from the Papworth NHS Foundation Trust Hospital Tissue Bank (Papworth Everard, UK). Paraffin wax–embedded lung samples (n = 18) and unfixed frozen tissue were used from patients with familial PAH (n = 6), idiopathic PAH (n = 6), and congenital heart disease–associated PAH (n = 6) and from control lung (n = 4). BMPRII mutations were detected as previously described (16). Control tissue comprised cases of unused donor lung (n = 1, smoke inhalation injury), and tissue (n = 3) from pneumonectomy specimens resected for malignancy, but distant from the site of tumor.
Paraffin-embedded Tissue Immunostaining
Tissue blocks were sectioned (4 μm) with a microtome (Leica Microsystems, Milton Keynes, UK), placed onto poly-l-lysine–coated slides, dried at 60°C for 1 hour, and then dewaxed and dehydrated through graded alcohols. Slides were microwaved for 30 minutes in a Milestone microwave (Surgipath, Peterborough, UK) in sodium citrate buffer (0.4 mol/L) at pH 6.0 and incubated in proteinase K (Dako, Ely, UK) for 10 minutes. Endogenous tissue peroxidase was bound with hydrogen peroxidase blocking solution (Dako). All primary antibodies were incubated for 1 hour at room temperature. The primary antibodies used were polyclonal rabbit anti-human CD133 (Abcam, Cambridge, UK), stromal cell–derived factor (SDF)-1 (Abcam), CXCR4 and c-Kit (Dako), and monoclonal mouse anti-human CD31, proliferating cell nuclear antigen (PCNA), and Ki-67 (Dako). The specificity of immunostaining for CD133 was demonstrated by the absence of signal in sections incubated with antibody after absorption with peptide against which the antibody was raised (Abcam). In antibodies for which peptide was not available the primary was omitted. Secondary detection was done according to a streptABC peroxidase technique (ChemMate; Dako) and visualized with 3,3′-diaminobenzidine hydrochloride substrate. An automated immunostainer (TechMate 500; Dako) ensured consistency of incubation periods and substrate development. Sections were counterstained in Carazzi's hematoxylin, mounted in DPX (VWR/Merck, Lutterworth, UK) and examined by light microscopy as previously described (17). Semiquantification of CD133 expression was undertaken by two blinded observers; interobserver variability was less than 10%. In control tissue (n = 4), 32 vessels were counted. In PAH tissue from 18 subjects, 98 concentric lesions and 33 plexiform lesions were counted. Endothelial cells were manually counted in a manner similar to that previously described for smooth muscle cells (14) and results were expressed as a percentage of the total number of endothelial cells per vessel or lesion.
Immunofluorescence Staining of Frozen Tissue
For fluorescence immunostaining frozen sections (6 μm thick) were permeabilized in absolute methanol at −20°C for 5 minutes, washed, and incubated at 4°C overnight in primary antibody and subsequently in secondary antibody; goat anti-rabbit Texas red, horse anti-mouse fluorescein isothiocyanate (FITC) (Vector Laboratories Ltd, Peterborough, UK), and then in TO-PRO-3 iodide (Molecular Probes, Eugene, OR) for nuclear staining, before mounting with VECTASHIELD as previously described (18). Isotype controls were employed to verify specificity of staining. Slides were viewed with a Leica confocal laser scanning microscope.
Flow Cytometry
Two distinct methods were employed for the quantification of circulating cells in two independent centers, Giessen, Germany and Cambridge, United Kingdom. Studies were approved by the local internal review boards, all patients gave written consent, and diagnosis was made on the basis of standard Venice criteria (19). In Giessen, 23 patients with severe PAH were enrolled. The patients had IPAH (n = 10), PAH from other causes (n = 9), and chronic thromboembolic disease (n = 4). Detailed patient characteristics are given in Table E1 in the online supplement. In the Giessen group hemodynamic data were obtained at the same time as central venous blood sampling, allowing analysis of potential correlations. Venous blood was also taken from 11 healthy donors without any clinical signs of pulmonary hypertension. In Cambridge, seven subjects with IPAH and four with mutations in BMPRII were compared with age- and sex-matched healthy control samples. All subjects had no concomitant disease and were in New York Heart Association class II or III (see Table E2 in the online supplement).
CD133 Purification of Circulating Angiogenic Progenitors in Samples from Giessen Subjects
Venous blood (50 ml) was collected in heparinized syringes for EPC quantification. Blood was diluted 1:2 with phosphate-buffered saline (PBS)−2 mM ethylenediaminetetraacetic acid (EDTA) (Life Technologies, Karlsruhe, Germany) and mononuclear cells were isolated by density gradient centrifugation over Ficoll-Hypaque 1077 (Pharmacia, Uppsala, Sweden) in 50-ml tubes as previously described (20). The mononuclear cells were washed three times in PBS−0.5% bovine serum albumin−2 mM EDTA. Erythrocytes were lysed by addition of lysis buffer (Becton Dickinson, Heidelberg, Germany). Cells were counted and 2 × 108 cells were incubated and separated twice with anti-CD133–conjugated superparamagnetic microbeads, using MS+ columns and a VarioMACS device according to the guidelines of the manufacturer (Miltenyi Biotec, Bergisch Gladbach, Germany). Purified CD133+ cells were eluted from the column, counted, and further analyzed by flow cytometry.
CD133 Fractionated Flow Cytometric Analysis of Samples from Giessen Subjects
Half the purified CD133+ cells were incubated for 30 minutes on ice with phycoerythrin (PE)-labeled CD133 antibody (Miltenyi Biotec) and an FITC-conjugated anti-vascular endothelial growth factor receptor-2 (VEGFR2) antibody (clone 6.64, provided by ImClone Corporation; labeled with a FluorLink MAb FluorX labeling kit [Amersham, Braunschweig, Germany]). The other half of the eluted cells was incubated with FITC- and PE-labeled mouse isotype immunoglobulin (Becton Dickinson) to serve as negative control. To exclude unspecific staining of dead cells, 7-amino-actinomycin D (7-(AAD) was used as previously reported (21). The cells were analyzed with a FACScan flow cytometer (Becton Dickinson). Settings were adjusted to exclude 7-AAD+ cells from the analysis. At least 5,000 cells were analyzed. The percentage of CD133+VEGFR2+ cells was determined and the absolute number of CD133+VEGFR2+ cells per milliliter of peripheral blood was calculated on the basis of the initial blood volume, the total mononuclear cell number after Ficoll separation, the cell number after magnetic column elution, and the percentage of CD133+ cells in the eluent.
Whole Blood Flow Cytometry of Samples from Cambridge Subjects
Whole blood from recumbent, resting subjects was collected in EDTA tubes and stained within 15 minutes of venipuncture. After a red cell lysis step (Versalyse; Beckman Coulter, Fullerton, CA) samples were analyzed on a Beckman Coulter FC200. Antibodies used as per the manufacturer instructions were as follows: PE-conjugated anti-CD34, APC-VEGFR2, and PC7-CD45 (Beckman Coulter), FITC-CD34 and PE-CD133 (Miltenyi Biotec). A minimum of 200,000 events was recorded and a strict gating strategy included only high CD34- and CD133-expressing cells in the mononuclear gate. VEGFR2 expression was determined using isotype controls and events were included as positive only if exclusively triple positive (CD34+CD133+VEGFR2+) as determined with flow cytometric analysis software (PRISM; Beckman Coulter).
Late-outgrowth Progenitor Isolation and Culture
Peripheral blood mononuclear cells were isolated from 80 ml of venous blood by Ficoll density gradient centrifugation and plated onto type 1 rat tail collagen (BD Biosciences, Bedford, MA)–coated flasks in endothelial selective medium (EGM2; Lonza Biologics, Slough, UK) supplemented with 10% fetal calf serum (FCS) and additional growth factors (EGM2 bullet kit; Lonza Biologics) in an adapted method similar to the original work by Ingram and colleagues (12). Medium was changed every 48 hours. Late-outgrowth progenitors appeared after 2–3 weeks and were passaged when confluent. This resulted in polyclonal outgrowth cells that were studied at passage 4–8. Cells were isolated from three subjects with BMPRII mutations and associated PAH. These patients harbored the following mutations: W9X, C347R, and S399X, as previously described (22). Control cells were taken from healthy nonsmoking age-matched subjects. For immunostaining cells were fixed in methanol and stained as previously described (18). The additional primary antibodies were monoclonal mouse anti-human CD146 and von Willebrand factor (Abcam). Isotype controls were used for specificity of staining. Slides were mounted in VECTASHIELD with 4′,6-diamidino-2-phenylindole nuclear staining. For flow cytometry the same antibodies and isotype controls were used as described previously for the Cambridge protocol. Cells were incubated for 30 minutes with primary labeled antibodies and then washed twice with PBS and analyzed with a Beckman Coulter flow cytometer.
EPC Migration
After 1 hour of quiescence in 0.1% FCS, 30,000 EPCs in a total volume of 250 μl of 0.1% medium supplemented with FCS were seeded into a Transwell insert (Falcon; Becton Dickinson) and inserted into a 24-well plate (Falcon). Medium (750 μl) supplemented with 0.1% FCS and the chemoattractant SDF-1 (R&D Systems, Abingdon, UK) was placed in the lower chamber of the 24-well plate. Each experiment was performed in quadruplicate. After a 12-hour incubation period cells on the bottom of the insert were fixed in methanol and stained with a Diff-Quik staining kit (Fisher Scientific, Hampton, NH) and visualized by light microscopy. Images were acquired in four random fields and the mean number of migrating cells was determined.
EPC Proliferation
Late-outgrowth EPCs were seeded into 24-well plates (Falcon). Cells (30,000 per well) were suspended in 1 ml of medium supplemented with 10% FCS with additional growth factors (Lonza Biologics). Experiments were performed in quadruplicate. Medium was changed every 48 hours. Cells were stained with methylene blue, trypsinized, and counted on Days 2 and 7 with a hemocytometer.
EPC Vascular Network Formation
Matrix gel (angiogenesis assay kit; Chemicon, Temecula, CA) was seeded into the wells of a 96-well plate and incubated at 37°C for 1 hour. Cells (10,000) were suspended in 250 μl of medium supplemented with 10% FCS and seeded onto the gel plugs. All experiments were performed in quadruplicate. After 4 hours cells were visualized by phase-contrast light microscopy. Network formation was analyzed in four random fields, using a semiquantitative scale (see the online supplement). Data were averaged per high-power field.
Statistical Analysis
All values are given as means ± SEM. Analysis of variance or an unpaired t test was performed for evaluation of intergroup differences as appropriate. To assess correlations of cell number and hemodynamic parameters, the Pearson r correlation coefficient was used. Significance was set at P < 0.05.
RESULTS
Increased Expression of EPC Markers and Homing Signals in PAH Lung
In control lung we observed widespread vascular endothelial expression of CD31 as expected, but minimal expression of CD133 (Figures 1A and 1B). In contrast, increased expression of CD133 was observed in the endothelium of plexiform lesions in all cases of PAH studied, although with heterogeneity within individual samples (P = 0.0001; Figures 1E and 1F). No significant difference was seen in concentric lesions (P = 0.46). Confocal immunofluorescence microscopy of CD133 confirmed endothelial staining colocalizing with CD31 (Figures 2B, 2E, and 2H) within plexiform lesions. In addition, confocal immunofluorescence demonstrated colocalization of c-Kit, the receptor for the cytokine stem cell factor, and therefore highly expressed on hematopoietic stem and progenitor cells (23) with CD31 (Figures 2C, 2F, and 2I). There were isolated cells staining positive in the interstitium as previously reported by Majka and colleagues (24). Endothelial cell expression of the chemokine homing signal SDF-1 was up-regulated in plexiform lesions (Figures 3F and 3H). There were generally increased levels of CXCR4 in PAH lung tissue although not confined to the endothelium (Figures 3A, 3C, 3E, and 3G). Plexiform lesions expressed elevated levels of CXCR4 in the endothelial cells lining vascular channels, although the intensity of staining varied between lesions (Figures 3E and 3G). Staining for the proliferation markers Ki-67 and PCNA showed minimal evidence of endothelial cell proliferation within plexiform or concentric intimal lesions (see Figure E1 in the online supplement).
Figure 1.
(A–F) Representative photomicrographs of serial sections of lung tissue from control lung and a patient with familial pulmonary arterial hypertension (PAH); samples were immunostained for the endothelial marker CD31, and for CD133. Panels show staining in (A and B) a normal artery, (C and D) a concentric lesion, and (E and F) a plexiform lesion. (G) Semiquantitative analysis of CD133 expression in control arteries and in the vascular lesions of PAH. Box plots show median values, and upper and lower quartiles with standard error bars. Scale bars: 50 μm. ***P = 0.001.
Figure 2.
Representative photomicrographs of confocal immunofluorescence staining of peripheral lung tissue in a patient with idiopathic pulmonary arterial hypertension (PAH): (A–C) CD31 conjugated with fluorescein isothiocyanate (green) in (A) peripheral lung and (B and C) plexiform lesions. In the same sections (D) demonstrates CD133 in peripheral lung with isolated nonendothelial cells staining only in red (Texas red). (E) shows a plexiform lesion with CD133 staining the endothelial cell surface. (F) demonstrates a similar pattern of endothelial cell surface expression of c-Kit (Texas red). (G–I) demonstrate that CD31 colocalizes with (H) CD133 and (I) c-Kit on the endothelial cell surface in both plexiform lesions when compared with nonplexiform peripheral lung tissue. TO-PRO-3 iodide counterstained for nuclear staining (blue). Scale bars: 50 μm.
Figure 3.
Representative photomicrographs of peripheral lung tissue from (A and B) normal control lung and (C–H) a patient with pulmonary arterial hypertension (PAH); samples were immunostained for CXCR4 and stromal cell–derived factor (SDF)-1. Minimal staining is seen in normal lung. (C and D) In PAH lung low-level staining was observed in concentric intimal lesions. (E and G) CXCR4 expression was generally increased in the lung parenchyma of patients with PAH, but was also present in the endothelium of plexiform lesions. (F and H) SDF-1 was less prevalent but showed clear staining of the endothelium of plexiform lesions. Scale bars: 50 μm.
Increased Numbers of Circulating Angiogenic Progenitors in PAH
By the whole blood method of flow cytometric analysis used in Cambridge, circulating levels of CD133+CD34+VEGFR2+ cells were demonstrated to be increased: IPAH, 1,168 ± 648 cells/ml (P = 0.006) and BMPRII-associated PAH, 708 ± 315 (P = 0.03) versus control, 218 ± 231 cells/ml (Figure 4). The levels of CD34+CD133+VEGFR2− progenitor cells were similar in each group at 2,503 ± 2,007 cells/ml in IPAH, 2,538 ± 902 cells/ml in BMPRII-associated PAH, and 2,750 ± 2,379 cells/ml in control subjects, demonstrating a selective increase in the VEGFR2-differentiated cell population. There was no significant increase in overall CD34 or CD133 fractions (Figure E1).
Figure 4.
Gating strategy for flow cytometry of CD133+CD34+VEFGR2+ cells by direct analysis of whole blood. A minimum of 200,000 events was recorded. (A) In a control subject a gate was drawn around the mononuclear cells. (B) Subsequently gates were drawn around the high-expressing distinct population of CD34+ cells, and (C) triple-positive events for CD34, CD133, and VEGFR2 were determined. (D–F) Representative gates in a subject with idiopathic pulmonary arterial hypertension (IPAH). Events were included as positive only if exclusively triple positive on PRISM (Beckman Coulter analysis software). (G) Numbers of circulating CD133+CD34+VEGFR2+ cells per milliliter of blood in control subjects (n = 7), patients with IPAH (n = 7), and patients with PAH with bone morphogenetic protein receptor type II (BMPRII) mutations (n = 4). (H) Undifferentiated CD133+CD34+VEGFR2− circulating cells per milliliter of blood. Data are shown as means ± SEM (*P < 0.05).
In Giessen patients CD133+ cells were successfully and reproducibly purified from peripheral blood. Circulating CD133+VEGFR2+ cell numbers were 166 ± 55 cells/ml in IPAH (P = 0.009 vs. control), 125.8 ± 32 cells/ml in secondary PAH (P = 0.002 vs. control), and 29 ± 5 cells/ml in healthy control subjects (Figure 5). In both sets of experiments the numbers of CD133+VEGFR2+ cells were not related to targeted PAH therapy (Tables E1 and E2). In the Giessen samples, which were taken at the time of right-heart catheterization, the number of CD133+VEGFR2+ cells did not correlate with pulmonary arterial pressure, cardiac index, or pulmonary vascular resistance (Table E1).
Figure 5.
Flow cytometric quantification of circulating putative endothelial progenitor cells (EPCs), using the purified CD133 fraction. Mononuclear cells were purified for CD133 antigen expression by magnetic cell sorting and double stained for CD133 and VEGFR2 (KDR). (A) Gating of EPCs after magnetic cell sorting. (B) Dead cells were excluded from analysis by their uptake of 7-amino-actinomycin D (7-AAD). (C) Regions for fluorescence analysis were set with the negative control cells, and a maximum of 0.1% CD133+KDR+ cells was accepted in the negative control figure. (D) Cell preparation with 94.28% CD133-positive cells and a subset of 3.12% EPCs. (E) Quantification of circulating CD133+VEGFR2+ cells in the peripheral blood of control subjects (n = 11), patients with idiopathic pulmonary arterial hypertension (IPAH) (n = 10), and patients with secondary pulmonary arterial hypertension (SPAH, n = 13). Data are shown as means ± SEM (**P < 0.01).
Dysfunction of Late-outgrowth EPCs in Patients with PAH with BMPRII Mutations
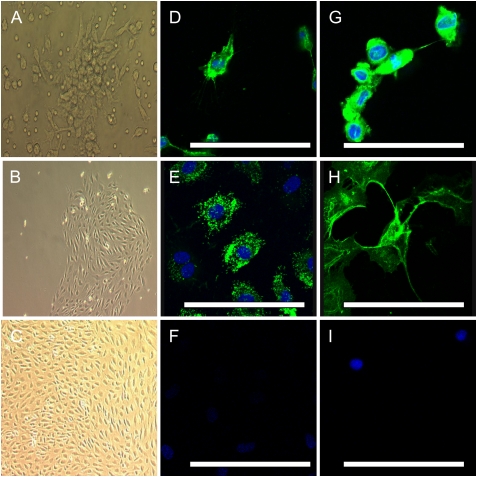
Late-outgrowth EPCs from patients and control subjects were observed to derive from single colonies and were grown to confluence before passaging. These cells maintained a cobblestone appearance consistent with endothelial morphology through numerous passages (Figures 6A–6C). Cells stained positive for CD34, CD146, and von Willebrand factor, with the occasional cell positive for CD133 (Figures 6D–6H). CD133-positive cells were present at 4.7% by flow cytometric analysis (data not shown).
Figure 6.
Phase-contrast photomicrographs of cultured late-outgrowth endothelial progenitor cells (EPCs) showing (A) a colony-forming unit at 3 days, and a late-outgrowth colony at (B) 2 weeks and (C) 3 weeks. Confocal immunofluorescence images using conjugated fluorescein isothiocyanate (green) demonstrate that occasional cells were positive for (D) CD133 and that the majority of cells were positive for (E) von Willebrand factor, (G) CD34, and (H) CD146. Nuclear counterstaining was performed with 4′,6-diamidino-2-phenylindole (blue). (F and I) Isotype controls for anti-mouse and anti-rabbit secondary antibodies. Scale bars: 50 μm.
In migration studies, SDF-1 stimulated migration of EPCs derived from control subjects and subjects with PAH. No difference in the number of migrating cells was observed between groups (Figure 7B). In proliferation studies over several days, cells cultured from the PAH group grew at a consistently faster rate in 10% FCS when compared with control subject cells (Figure 7A). Furthermore, angiogenesis assays revealed that the formation of vascular networks in matrix plugs was markedly impaired in the mutation group (Figures 7C–7E).
Figure 7.
Phenotypic analysis of late-outgrowth endothelial progenitor cells (EPCs) from control subjects and patients with pulmonary arterial hypertension (PAH) associated with bone morphogenetic protein receptor type II (BMPRII) mutations. (A) Growth curves demonstrate that cells from patients with BMPRII-associated PAH are hyperproliferative, compared with those from control subjects, when grown in 10% fetal calf serum supplemented with additional growth factors. (B) Migration assays demonstrate that stromal cell–derived factor (SDF)-1 stimulated similar migration of cells from control subjects and subjects with BMPRII-associated PAH. Shaded columns, control subjects; solid columns, subjects with BMPRII mutation. HPF = high-power field. (C and D) Angiogenesis assays clearly demonstrate that (C) control EPCs form intact vascular networks, whereas (D) PAH BMPRII mutant EPCs are markedly deficient. (E) Semiquantitative analysis of vascular network formation. Experiments were performed in quadruplicate, n = 3 control and mutation cell lines. Data are shown as means ± SEM (*P < 0.05, ***P < 0.001).
DISCUSSION
This study describes several findings supporting a contribution of progenitor cells in the pathobiology of PAH. First, we have demonstrated increased circulating numbers of angiogenic progenitors in PAH and independently confirmed this finding in a second PAH cohort, using a different methodological approach. In addition, we provide strong evidence for the presence of cells expressing progenitor markers, particularly within the plexiform lesions associated with severe PAH. This is accompanied by the expression of progenitor homing signals in these lesions. Furthermore, we have isolated late-outgrowth EPCs from patients with familial PAH and healthy control subjects and demonstrated a hyperproliferative phenotype in patient-derived cells and a reduced capacity of these cells to form vascular networks.
The endothelium is increasingly viewed as critical in the initiation and progression of disease. Loss of endothelial cell integrity has been suggested to initiate vascular remodeling and contribute to intimal, medial, and adventitial hypertrophy (1). Histopathological and molecular findings in lungs of patients with IPAH suggest a key role for dysregulated endothelial proliferation and misguided angiogenesis in small pulmonary arteries (25, 26). Within this vascular remodeling, plexiform lesions are found predominantly at vascular bifurcations (5). Endothelial cells at these sites are prone to high shear stress and dislocation. Clusters of endothelial cells in plexiform lesions of patients with IPAH have been shown to be of clonal origin, with different clones in different regions of the same lung (3). Progenitor expansion would fit this oligoclonal model of lesion generation. Work looking at cell fusion in PAH tissue has demonstrated increased CD133-staining cells in IPAH and familial PAH plexiform lesions (24). We confirm this finding and demonstrate staining for c-Kit and CXCR4, both of which are associated with the progenitor phenotype. In addition, we have shown the same pattern of staining in congenital heart disease–associated PAH. A common etiology of these vascular lesions is perhaps expected given their ubiquity across the various etiologies of PAH.
That lack of evidence of cell fusion leaves two possible explanations for increased progenitors in these lesions, namely the expansion of stem cell populations either tissue derived or peripherally recruited. In ex vivo studies of pulmonary vascular repair, the peripheral recruitment model of progenitors is supported by the demonstration of CD133+ cells migrating and incorporating into human pulmonary arteries in the context of chronic obstructive pulmonary disease (27). Data from the hypoxic mouse model of pulmonary hypertension show that SDF-1 mobilizes progenitors from the bone marrow in response to hypoxia (28). In our study we have demonstrated increased expression of SDF-1 in PAH lung and in plexiform lesions. Given the degree of inflammatory cells in PAH lung this is not surprising and SDF-1 is well described to be involved in the homing of neutrophils (29) and T cells (30). SDF-1 therefore is not specific to bone marrow progenitors. Our subsequent experiments, however, demonstrated increased circulating levels of angiogenic progenitors. This up-regulation was reproducible by two distinct and different methods of quantifying angiogenic progenitors. In peripheral blood, the measurement of progenitor markers is an exercise in rare event analysis. Techniques used to measure EPC numbers can involve cell separation, delayed sample analysis, and centrifugation, all of which may affect the expression of cell surface markers. The whole blood technique was adapted to minimize cell and epitope loss and most likely accounts for the higher levels seen across the three groups when compared with the cell fractionation method. The definition of markers for “circulating EPCs” remains highly controversial. There is still no generally agreed-on standardized method for their enumeration or indeed agreement on the correct markers to use. It is likely that the disparity of results so far in the literature reflects this lack of standardization. Nevertheless, at least according to the criteria adopted here it would appear that circulating angiogenic cell fractions are increased in patients with PAH.
An alternative source of progenitor cells is the expansion of a resident stem cell population. Rat pulmonary microvascular endothelial cells postulated to arise from stem cells have demonstrated the capacity to reconstitute the entire proliferative hierarchy of the microvasculature (31). In humans a fraction of human aortic endothelial cells has long been known to demonstrate proliferative characteristics strikingly similar to those of stem cells (32) and more pertinently a hyperproliferative phenotype in idiopathic PAH endothelial cells has been identified (33). Despite this, and despite previous work to the contrary (3, 26, 34), we found little evidence of ongoing proliferation in the plexiform lesions studied. This is in agreement with more recent work looking at proliferation in end-stage tissue (24). This lack of markers of proliferation may be a reflection of the end-stage nature of the tissue obtained from patients undergoing heart–lung transplantation and resident stem cells remain an attractive candidate.
To our knowledge we present the first functional characterization of late-outgrowth EPCs from patients with PAH. It is emerging that BMPs have a major role to play in adult stem cell regulation and we provide the first evidence that alterations in the BMPRII pathway have functional implications in a translational model. The late-outgrowth cells isolated from patients with BMPRII mutations demonstrated clear differences in angiogenic capacity and proliferation in response to serum. Interestingly, this is similar to the phenotypic descriptions of alterations in pulmonary microvascular endothelial cells that have been previously described in PAH (25, 31).
Potential criticisms of this work include the use of abnormal tissue to control progenitor tissue expression. Access to completely normal human lung tissue is for obvious reasons limited but the impact in particular of malignancy on progenitor cells is unclear. In addition, we acknowledge that our observations in circulating cells are not directly linked to the tissue expression, or to the cultured late-outgrowth cells. Although all these cell types share progenitor markers we are not able to draw any definitive conclusions about the relationship between them. Of interest, previous work in a nude mouse hind limb model has suggested that differing cell populations (in this case early- and late-outgrowth cells from humans) can have synergistic effects in vivo (35). We provide these observations together as accumulating evidence that endogenous progenitor cells may have a role in disease progression. Despite intense interest and investigation the definition and role of EPCs in general vascular homeostasis are far from clear and the fundamental questions about the role they play in vessel development and repair in health and disease remain to be fully elucidated. In PAH, given the emergence of EPCs as a potential treatment modality, it is important that their function in vascular remodeling be clarified to better understand the long-term implications of therapeutic administration.
Supplementary Material
Supported by a British Heart Foundation Fellowship (M.T.). This work was further funded by the Medical Research Council and received financial support from by the European Commission under the 6th Framework Programme (contract no. LSHM-CT-2005-018725, PULMOTENSION). Additional support was provided by an NIHR Biomedical Research Centre Award.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200810-1662OC on July 23, 2009
Conflict of Interest Statement: M.T. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.V. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.A.-L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. L.S.G.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.Y. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. E.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.E. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. Z.Z. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. U.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. W.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.P.-Z. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. N.W.M. holds a research grant from Novartis.
References
- 1.Voelkel NF, Cool C, Taraceviene-Stewart L, Geraci MW, Yeager M, Bull T, Kasper M, Tuder RM. Janus face of vascular endothelial growth factor: the obligatory survival factor for lung vascular endothelium controls precapillary artery remodeling in severe pulmonary hypertension. Crit Care Med 2002;30:S251–S256. [DOI] [PubMed] [Google Scholar]
- 2.Wagenvoort CA, Wagenvoort N. Primary pulmonary hypertension: a pathologic study of the lung vessels in 156 clinically diagnosed cases. Circulation 1970;42:1163–1184. [Google Scholar]
- 3.Lee SD, Shroyer KR, Markham NE, Cool CD, Voelkel NF, Tuder RM. Monoclonal endothelial cell proliferation is present in primary but not secondary pulmonary hypertension. J Clin Invest 1998;101:927–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palevsky HI, Schloo BL, Pietra GG, Weber KT, Janicki JS, Rubin E, Fishman AP. Primary pulmonary hypertension: vascular structure, morphometry, and responsiveness to vasodilator agents. Circulation 1989;80:1207–1221. [DOI] [PubMed] [Google Scholar]
- 5.Cool CD, Stewart JS, Werahera P, Miller GJ, Williams RL, Voelkel NF, Tuder RM. Three-dimensional reconstruction of pulmonary arteries in plexiform pulmonary hypertension using cell-specific markers: evidence for a dynamic and heterogeneous process of pulmonary endothelial cell growth. Am J Pathol 1999;155:411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao YD, Courtman DW, Deng Y, Kugathasan L, Zhang Q, Stewart DJ. Rescue of monocrotaline-induced pulmonary arterial hypertension using bone marrow–derived endothelial-like progenitor cells: efficacy of combined cell and eNOS gene therapy in established disease. Circ Res 2005;96:442–450. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi M, Nakamura T, Toba T, Kajiwara N, Kato H, Shimizu Y. Transplantation of endothelial progenitor cells into the lung to alleviate pulmonary hypertension in dogs. Tissue Eng 2004;10:771–779. [DOI] [PubMed] [Google Scholar]
- 8.Wang XX, Zhang FR, Shang YP, Zhu JH, Xie XD, Tao QM, Zhu JH, Chen JZ. Transplantation of autologous endothelial progenitor cells may be beneficial in patients with idiopathic pulmonary arterial hypertension: a pilot randomized controlled trial. J Am Coll Cardiol 2007;49:1566–1571. [DOI] [PubMed] [Google Scholar]
- 9.Diller GP, van ES, Okonko DO, Howard LS, Ali O, Thum T, Wort SJ, Bedard E, Gibbs JS, Bauersachs J, et al. Circulating endothelial progenitor cells in patients with Eisenmenger syndrome and idiopathic pulmonary arterial hypertension. Circulation 2008;117:3020–3030. [DOI] [PubMed] [Google Scholar]
- 10.Junhui Z, Xingxiang W, Guosheng F, Yunpeng S, Furong Z, Junzhu C. Reduced number and activity of circulating endothelial progenitor cells in patients with idiopathic pulmonary arterial hypertension. Respir Med 2008;102:1073–1079. [DOI] [PubMed] [Google Scholar]
- 11.Asosingh K, Aldred MA, Vasanji A, Drazba J, Sharp J, Farver C, Comhair SA, Xu W, Licina L, Huang L, et al. Circulating angiogenic precursors in idiopathic pulmonary arterial hypertension. Am J Pathol 2008;172:615–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ingram DA, Mead LE, Tanaka H, Meade V, Fengolio A, Mortell K, Pollock K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel heirarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood 2004;104:2752–2760. [DOI] [PubMed] [Google Scholar]
- 13.Smadja DM, Bieche I, Silvestre JS, Germain S, Cornet A, Laurendeau I, Duong-Van-Huyen JP, Emmerich J, Vidaud M, Aiach M, et al. Bone morphogenetic proteins 2 and 4 are selectively expressed by late outgrowth endothelial progenitor cells and promote neoangiogenesis. Arterioscler Thromb Vasc Biol 2008;28:2137–2143. [DOI] [PubMed] [Google Scholar]
- 14.Yang X, Long L, Southwood M, Rudarakanchana N, Upton PD, Jeffery TK, Atkinson C, Chen H, Trembath RC, Morrell NW. Dysfunctional Smad signaling contributes to abnormal smooth muscle cell proliferation in familial pulmonary arterial hypertension. Circ Res 2005;96:1053–1063. [DOI] [PubMed] [Google Scholar]
- 15.Atkinson C, Stewart S, Imamura T, Trembath RC, Morrell NW. Immunolocalisation of BMPR-II and TGF-β type I and II receptors in primary plexogenic pulmonary hypertension. J Heart Lung Transplant 2001;20:149. [DOI] [PubMed] [Google Scholar]
- 16.Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA III, Loyd JE, Nichols WC, Trembath RC; International PPH Consortium. Heterozygous germline mutations in BMPR2, encoding a TGF-β receptor, cause familial primary pulmonary hypertension. Nat Genet 2000;26:81–84. [DOI] [PubMed] [Google Scholar]
- 17.Atkinson C, Stewart S, Upton PD, Machado R, Thomson JR, Trembath RC, Morrell NW. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation 2002;105:1672–1678. [DOI] [PubMed] [Google Scholar]
- 18.Al-Lamki RS, Wang J, Vandenabeele P, Bradley JA, Thiru S, Luo D, Min W, Pober JS, Bradley JR. TNFR1- and TNFR2-mediated signaling pathways in human kidney are cell type–specific and differentially contribute to renal injury. FASEB J 2005;19:1637–1645. [DOI] [PubMed] [Google Scholar]
- 19.Simonneau G, Galie N, Rubin LJ, Langleben D, Seeger W, Domenighetti G, Gibbs S, Lebrec D, Speich R, Beghetti M, et al. Clinical classification of pulmonary hypertension. J Am Coll Cardiol 2004;43:5S–12S. [DOI] [PubMed] [Google Scholar]
- 20.Gehling UM, Ergun S, Schumacher U, Wagener C, Pantel K, Otte M, Schuch G, Schafhausen P, Mende T, Kilic N, et al. In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood 2000;95:3106–3112. [PubMed] [Google Scholar]
- 21.Schmid I, Uittenbogaart CH, Keld B, Giorgi JV. A rapid method for measuring apoptosis and dual-color immunofluorescence by single laser flow cytometry. J Immunol Methods 1994;170:145–157. [DOI] [PubMed] [Google Scholar]
- 22.Machado RD, Aldred MA, James V, Harrison RE, Patel B, Schwalbe EC, Gruenig E, Janssen B, Koehler R, Seeger W, et al. Mutations of the TGF-β type II receptor BMPR2 in pulmonary arterial hypertension. Hum Mutat 2006;27:121–132. [DOI] [PubMed] [Google Scholar]
- 23.Edling CE, Hallberg B. c-Kit: a hematopoietic cell essential receptor tyrosine kinase. Int J Biochem Cell Biol 2007;39:1995–1998. [DOI] [PubMed] [Google Scholar]
- 24.Majka SM, Skokan M, Wheeler L, Harral J, Gladson S, Burnham E, Loyd JE, Stenmark KR, Varella-Garcia M, West J. Evidence for cell fusion is absent in vascular lesions associated with pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 2008;295:L1028–L1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tuder RM, Groves B, Badesch DB, Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol 1994;144:275–285. [PMC free article] [PubMed] [Google Scholar]
- 26.Voelkel NF, Tuder RM. Cellular and molecular biology of vascular smooth muscle cells in pulmonary hypertension. Pulm Pharmacol Ther 1997;10:231–241. [DOI] [PubMed] [Google Scholar]
- 27.Peinado VI, Ramirez J, Roca J, Rodriguez-Roisin R, Barbera JA. Identification of vascular progenitor cells in pulmonary arteries of patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 2006;34:257–263. [DOI] [PubMed] [Google Scholar]
- 28.Satoh K, Fukumoto Y, Nakano M, Sugimura K, Nawata J, Demachi J, Karibe A, Kagaya Y, Ishii N, Sugamura K, et al. Statin ameliorates hypoxia-induced pulmonary hypertension associated with down-regulated stromal cell–derived factor-1. Cardiovasc Res 2009;81:226–234. [DOI] [PubMed] [Google Scholar]
- 29.Christopher MJ, Link DC. Regulation of neutrophil homeostasis. Curr Opin Hematol 2007;14:3–8. [DOI] [PubMed] [Google Scholar]
- 30.Oberlin E, E., A. Amara A, F. Bachelerie F, C. Bessia C, J. L. Virelizier JL, Renzana-Seisdedos F, Schwartz O, Heard JM, Clark-Lewis I, Legler DF, et al. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line–adapted HIV-1. Nature 1996;382:833–835. [DOI] [PubMed] [Google Scholar]
- 31.Alvarez DF, Huang L, King JA, ElZarrad MK, Yoder MC, Stevens T. Lung microvascular endothelium is enriched with progenitor cells that exhibit vasculogenic capacity. Am J Physiol Lung Cell Mol Physiol 2008;294:L419–L430. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz SM, Benditt EP. Clustering of replicating cells in aortic endothelium. Proc Natl Acad Sci USA 1976;73:651–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masri FA, Xu W, Comhair SA, Asosingh K, Koo M, Vasanji A, Drazba J, Nand-Apte B, Erzurum SC. Hyperproliferative apoptosis-resistant endothelial cells in idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 2007;293:L548–L554. [DOI] [PubMed] [Google Scholar]
- 34.Cool CD, Kennedy D, Voelkel NF, Tuder RM. Pathogenesis and evolution of plexiform lesions in pulmonary hypertension associated with scleroderma and human immunodeficiency virus infection. Hum Pathol 1997;28:434–442. [DOI] [PubMed] [Google Scholar]
- 35.Yoon CH, Hur J, Park KW, Kim JH, Lee CS, Oh IY, Kim TY, Cho HJ, Kang HJ, Chae IH, et al. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late-outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation 2005;112:1618–1627. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.